Abstract
Please cite this paper as: Li et al. (2012) Immunogenicity and safety of a 2009 pandemic influenza A (H1N1) monovalent vaccine in Chinese infants aged 6–35 months: a randomized, double‐blind, controlled phase I clinical trial. Influenza and Other Respiratory Viruses DOI: 10.1111/irv.12028.
Objectives The goal of this double‐blind, randomized, controlled clinical trial was to assess the safety and immunogenicity of two different doses of a monovalent split‐virion 2009 pandemic influenza A/H1N1 vaccine without adjuvant in Chinese infants aged 6‐35 months.
Design and setting Subjects were randomly assigned to receive either a 2009 pandemic (H1N1) vaccine containing 7.5 or 15 μg haemagglutinin (HA) or a seasonal influenza vaccine. 2 doses of the H1N1 vaccines or the seasonal influenza vaccine were given 21 days apart in younger infants aged 6‐23 months or older infants aged 24‐35 months.
Sample Serum samples were collected immediately before the first injection and before and 21 days after the second injection.
Main outcome measures Primary outcomes were haemagglutinin inhibition (HI) antibody responses 21 days following each vaccination. Safety was monitoring throughout the study.
Results The first vaccination of 7.5 μg and 15 μg H1N1 vaccine induced seroprotective antibody titers (HI titers ≥ 1: 40) in 42.9‐57.4% of younger infants and 49.1‐61.0% older infants. Immune responses after completion of the two dose schedule were comparable in both age groups with seroprotective rates of 91‐98% in each vaccine and age group and GMTs of 173‐263. The H1N1 vaccine elicited similar rates of local and systemic adverse reactions as the seasonal influenza vaccine.
Conclusions The 2009 pandemic influenza A /H1N1 vaccine were highly immunogenic in infants aged 6‐35 months, and displayed a safety and reactogenicity profile similar to the seasonal influenza vaccine.
Trial registration ClinicalTrial.gov identifier: NCT01047202
Keywords: Chinese infant, H1N1, pandemic influenza vaccine, seasonal influenza vaccine
Introduction
In April 2009, a novel influenza A (H1N1) virus was identified in humans in Mexico and the United States and was followed by rapid global spread, resulting in the World Health Organization (WHO) declaration of an influenza pandemic in June. 1 This virus, which can be transmitted from person to person, 2 has spread worldwide to more than 214 countries and overseas territories 3 and co‐circulated with seasonal influenza viruses. 4 Pandemic influenza was introduced to mainland China on May 2009 5 and then spread across the whole country. By the end of 2009, more than 120 000 confirmed cases were reported to the Chinese Center for Disease Control and Prevention (CCDC), including 648 deaths (http://www.moh.gov.cn/publicfiles/business/htmlfiles/mohwsyjbgs/s7863/200912/44826.htm, http://www.moh.gov.cn/publicfiles/business/htmlfiles/mohwsyjbgs/s7863/201001/45434.htm).
It is well known that children under the age of 5 years are at increased risk for complications and death during epidemic seasonal influenza. In an outbreak of 2009 influenza A (H1N1) infection, children have been a primary source of illness in community, as indicated by the association between outbreaks in schools and influenza activity in the community. 6 In addition, serological studies suggested that children had no measurable immunity against H1N1 prior to the outbreak. 7 , 8 Children also have developed severe influenza A (H1N1)‐related complications more frequently than was usually seen for seasonal influenza. 9 In the United States, 60% of confirmed cases of novel H1N1 infection were reported in persons 18 years of age or younger, and the rates of hospitalization were highest among children 0–4 years of age. 10 , 11 Based on seasonal influenza vaccine studies, on July 29, 2009, the Advisory Committee on Immunization Practices concluded that children under 9 years of age may need to be given two doses of vaccine to elicit sufficient immunogenic reactivity against the 2009 H1N1 virus. 12
In China, in response to the pandemic, a number of novel monovalent inactivated vaccines containing 7·5 or 15 μg hemagglutinin per dose, even without adjuvant, against the virus strain A/California/07/2009 (H1N1) has been developed and was approved for sale, and younger children were included among the subjects for priority vaccination. In the general population, these vaccines were shown to be immunogenic, safe, and well tolerated. 13 , 14 , 15 , 16 Children were included in some trials, and analyses of the pediatric data indicated >90% seroprotection and seroconversion rates after two doses. 13 , 14 , 15 , 16 However, as in the case of seasonal vaccines, the response of younger children was less than that of older children or adults. Moreover, no data relating to Chinese infants younger than 3 years were obtained. It is therefore still unclear whether Chinese infants at the highest risk of influenza‐related complications can have sufficient immune response to the novel pandemic influenza and whether the vaccines are safe and well tolerated by very young subjects.
The aim of this study was to evaluate the immunogenicity and tolerability of a specific monovalent split‐virion 2009 pandemic influenza A/H1N1 vaccine without adjuvant developed by Sinovac Biotech Co., Ltd, in such subjects. Seasonal influenza vaccine was chosen because of ethical considerations.
Methods
Vaccines
The investigational vaccine was a monovalent inactivated, split‐virion, 2009 pandemic influenza A /H1N1 vaccine, containing 15 μg hemagglutinin (HA) antigen per dose without adjuvant. The seed vaccine virus was reassortant strain NYMC X‐179A (A/California/07/2009) prepared by New York Medical College (Westchester Country, NY, USA) using classic reassortment technology. The strain was recommended by WHO for the development of 2009 pandemic H1N1 vaccines and distributed by US Centers for Disease Control and Prevention and other institutions. The virus was propagated in embryonated chicken eggs, inactivated, and split according to the process used to produce a trivalent seasonal influenza vaccine (TIV), Anflu®, licensed in China for persons aged >6 months. The vaccine was supplied as single‐dose vials without preservative (0·5 ml/vial, batch No. 20091043). For subjects assigned to 7·5 or 15 μg pandemic influenza A /H1N1 vaccine group, the volume injected was 0·25 or 0·5 ml per dose, respectively.
The control vaccine, Anflu®, contained 7·5 μg of each of the WHO reference strains recommended for 2009–2010 northern hemisphere influenza season [A/Brisbane/59/2007 (H1N1), A/Uruguay/716/2007 (H3N2), and B/Brisbane/60/2008]. The vaccine was also supplied as single‐dose vials without preservative (0·25 ml/vial, batch No. 20090843), and the volume injected was 0·25 ml per dose.
All formulations of the vaccines were qualified and quantified by National Institutes for Food and Drug Control, China (NIFDC), in Beijing. All vaccines were supplied in coded, identical‐appearing single‐dose vials.
Clinical study design
The study was conducted as a randomized, double‐blind, controlled clinical trial in the Lingchuan County, Guilin, Guangxi Zhuang Autonomous Region, China. The purpose of the study was to evaluate the safety and immunogenicity of 2009 pandemic influenza A /H1N1 vaccine administered in two‐dose regimen in infants aged 6–35 months. NIFDC and the Center for Disease Control and Prevention of the Guangxi Zhuang Autonomous Region (Guangxi CDC) designed the clinical trial. Guangxi CDC performed inoculation, data collection, statistical analysis, and the storage of the raw data and files. Serum samples assay was performed by NIFDC. The study was sponsored by Sinovac Biotech Co., Ltd.
Approval for the study protocol was obtained from the ethics committee of Guangxi CDC. The clinical trials were conducted in accordance with the principles of the Declaration of Helsinki, the standards of Good Clinical Practice (as defined by the International Conference on Harmonization), and the Chinese regulatory requirements, as stipulated by State Food and Drug Administration (SFDA). Before participation, written informed consent was obtained from each volunteer’s legal representative.
Healthy infants (full‐term: 37–42 weeks; birth weight ≥2500 g) were recruited and screened by medical history inquiring and physical examination. Volunteers meeting one or more of the following criteria were excluded: any case or cured case of influenza A (H1N1) virus or close contacts with H1N1 cases, history of H1N1 vaccine or 2009 seasonal influenza vaccine administration, allergic to any ingredient of vaccine, autoimmune disease or immunodeficiency, active malignancy, bleeding disorder, seizure disorder, treatment with cytotoxic or immunosuppressive drugs within the past 6 months, receipt of blood products within the past 3 months, administration of any other investigational research agents or live attenuated vaccine within 30 days, administration of subunit or inactivated vaccines within 14 days, and axillary temperature over 37·0°C at the time of vaccination.
Eligible infant subjects were stratified by age: 6–23 months (younger infant) and 24–35 months (older infant). Within each age group, subjects were randomized in a 2:2:1 ratio to receive 7·5 or 15 μg pandemic influenza A /H1N1 vaccine or a seasonal influenza vaccine. All subjects received two doses of vaccines 21 days apart.
The randomization code was prepared by a statistician, using sas software (version 9.1 SAS Institute Inc., Cary, NC, USA), based on the predefined block size. Randomization‐code numbers were assigned to subjects in chronological order by the investigators. During the whole study, only the study nurses responsible for the injections of the vaccines could be allowed to access the individual randomization code. All participants, investigators, and other site personnel involved in clinical assessments were blinded to treatment allocations. Vaccines were injected into the upper arm deltoid muscle (25 gauge needle, 3/4 inch length).
After an on‐site safety observation of 30 minutes duration, subjects or their guardians were asked to record axillary temperature and data on injection‐site reactions and systemic reactions for three consecutive days, in a diary card provided by the investigators. Subjects returned on Day 4 for review of the diary card, concomitant medications and medical history, and examination of the vaccination site. Subjects also returned approximately 21 days after vaccination for collection of safety data and next vaccination. During visits, the investigator determined the causality of systemic adverse events and local adverse events with the vaccination.
Laboratory tests
Serum samples were collected immediately before the first injection and before and 21 days after the second injection for hemagglutination inhibition (HI) antibody titration against the strain NYMC X‐179A (A/California/07/2009). The immunogenicity of the vaccines was evaluated by the standard HI assay using 4 hemagglutination units of viruses and 0·5% turkey erythrocytes. Briefly, serum samples were treated with receptor‐destroying enzyme (cholera filtrate; Sigma‐Aldrich, Beijing, China) at 36·0°C for 16 h before titration to remove non‐specific inhibitors of agglutination. Samples were tested in twofold dilution starting with 1:10 dilution. The titers were expressed as the reciprocal of the highest dilution that showed complete inhibition of hemagglutination. All samples were tested in duplicate under blinded conditions and double‐checked by two persons.
Surveillance for influenza
During this study, parents or legal guardians were asked to report an influenza‐like illness (ILI, defined as temperature ≥38·0°C and at least one of the following symptoms: cough or pharyngodynia). Subjects were instructed to return to the clinic for illness evaluations if they observed any acute respiratory tract symptoms or fever. During illness visits, symptoms were reviewed, a brief physical examination was conducted, and nasopharyngeal or pharyngeal swabs were collected for the detection of the virus.
Swab samples for virus detection were stored at −70°C and shipped on dry ice to NIFDC, where real‐time reverse transcriptase–polymerase chain reaction (RT‐PCR) assay was performed according to the Influenza Diagnostic Criteria and influenza virus laboratory testing methods (Chinese National Influenza Center, version May 3, 2009).
Statistical analysis
The planned sample size of 50 infants per dosage group per age cohort was estimated to provide >80% power to detect a seroconversion rate of more than 70%. We summarized results with point estimates and 95% CIs. We summarized safety data in terms of the number and proportion of individuals who had reactions in each group and used chi‐square test to compare groups when relevant. Immunogenicity data were summarized using geometric mean titer (GMT), geometric mean titer ratio (GMTR), seroprotection rate (defined as % of subjects with titers ≥1:40), and seroconversion rate (defined as % of subjects with a pre‐vaccination titer <1:10 and a post‐vaccination titer ≥1:40, or with a pre‐vaccination titer ≥1:10 and ≥4‐fold increase after vaccination). For the purpose of calculation, HI titers below 1:10 were assigned a value of 1:5.
All reported P values are two‐sided. All data manipulations and statistical computations were performed with sas (version 9.1).
Results
From December 1, 2009 to March 30, 2010, 310 healthy infants (147 male and 163 female) were selected from 339 volunteers. They were randomized in a stratified manner and vaccinated with H1N1 vaccine or a seasonal influenza vaccine, Anflu®. There were no significant differences in age, height, weight, or sex between the different study groups within each age group (Table 1). Of these, 283 received a second injection 21 days after the first vaccination and 280 attended the Day 42 visit. Blood samples were obtained from 299 infants who attended the Day 21 visit, while 280 infants provided a blood sample on Day 42 (Figure 1). During the study, 18 did not receive a second injection because of unresolved adverse events (AEs) or serious adverse events (SAEs), which were considered to be unrelated to the vaccine by the investigator, and 12 dropped out due to lost to follow‐up or withdrawal of consent.
Table 1.
Demographic characteristics of study subjects
| Age group | H1N1 vaccine | Seasonal influenza vaccine | P | |
|---|---|---|---|---|
| 7·5 μg* | 15 μg* | |||
| Intention‐to‐treat cohort | ||||
| 6–23 months | ||||
| Subjects (N) | 68 | 62 | 37 | |
| Age in months (mean ± SD) | 14·85 ± 5·09 | 14·98 ± 4·82 | 15·05 ± 5·32 | 0·463 |
| Height in cm (mean ± SD) | 75·81 ± 4·88 | 76·53 ± 6·09 | 75·23 ± 9·99 | 0·644 |
| Weight in kg (mean ± SD) | 9·92 ± 1·40 | 9·90 ± 1·46 | 12·39 ± 11·64 | 0·292 |
| Male/female | 39/29 | 28/34 | 20/17 | 0·367 |
| 24–35 months | ||||
| Subjects (N) | 56 | 61 | 26 | |
| Age in months (mean ± SD) | 30·98 ± 3·81 | 30·33 ± 4·16 | 30·76 ± 3·86 | 0·667 |
| Height in cm (Mean ± SD) | 89·30 ± 4·31 | 89·03 ± 4·17 | 86·67 ± 11·19 | 0·175 |
| Weight in kg (mean ± SD) | 13·10 ± 1·54 | 13·13 ± 1·69 | 12·94 ± 1·47 | 0·882 |
| Male/female | 27/29 | 22/39 | 11/15 | 0·413 |
| ATP cohort | ||||
| 6–23 months | ||||
| Subjects (N) | 61 | 54 | 27 | |
| Age in months (mean ± SD) | 14·50 ± 5·08 | 15·00 ± 5·03 | 16·26 ± 4·82 | 0·322 |
| Height in cm (Mean ± SD) | 75·50 ± 5·01 | 76·60 ± 6·37 | 75·56 ± 11·09 | 0·682 |
| Weight in kg (mean ± SD) | 9·89 ± 1·44 | 9·92 ± 1·52 | 12·96 ± 13·49 | 0·318 |
| Male/female | 35/26 | 22/32 | 13/14 | 0·203 |
| 24–35 months | ||||
| Subjects (N) | 55 | 59 | 24 | |
| Age in months (mean ± SD) | 31·02 ± 3·84 | 30·49 ± 4·12 | 30·92 ± 3·98 | 0·766 |
| Height in cm (Mean ± SD) | 89·35 ± 4·33 | 89·04 ± 4·23 | 86·75 ± 11·69 | 0·218 |
| Weight in kg (mean ± SD) | 13·12 ± 1·55 | 13·16 ± 1·71 | 13·04 ± 1·47 | 0·961 |
| Male/female | 27/28 | 22/37 | 11/13 | 0·432 |
SD, standard deviation.
*Infants in 7·5‐μg H1N1 vaccine group received the hemagglutinin (HA) content in a volume of 0·25 ml per vaccination and those in 15‐μg H1N1 vaccine group received the HA content in a volume of 0·5 ml per vaccination.
Figure 1.
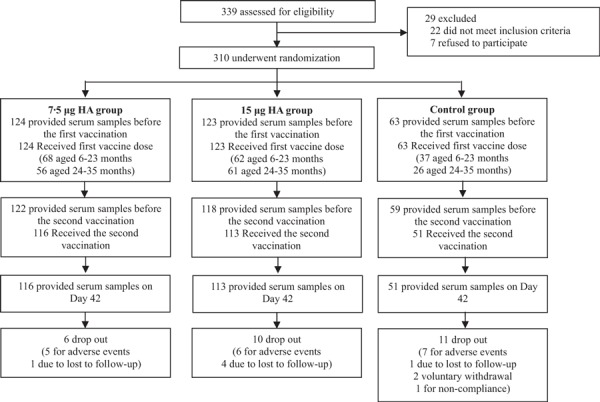
Flow chart of immunogenicity and safety of the vaccine of participants through the trail.
Safety
No immediate unsolicited adverse reactions, vaccine‐related SAE, adverse events of special interest, or new onset of chronic illness occurred after either vaccination.
The proportion of infants experiencing adverse reactions within 3 days after at least one of the two injections is reported separately for infants 6–23 months and 24–35 months and summarized in Table 2. Most adverse reactions were mild to moderate. Few injection‐site reactions were observed for vaccine or control recipients in both subject ages. Fever and gastrointestinal disorders were the most commonly reported systemic reactions across all age and vaccine groups, with no indication of increased frequency in subjects receiving the vaccine compared with the control recipients. Severe adverse reactions were reported by 0·81%, 3·25%, and 3·17% of participants in the 7·5‐μg group, the 15‐μg group, and the control group, respectively. The most common severe reactions in infants were fever and diarrhea. Considering reactogenicity of the first and second injections separately, reaction rates were lower after the second injection (7·5 μg HA: 12·7%; 15 μg HA: 10·6%) than after the first (7·5 μg HA: 22·6%; 15 μg HA: 18·7%).
Table 2.
Percentage of participants in each age group with adverse reactions within 3 days after the first or the second injection*
| 7·5 μg HA | 15 μg HA | Seasonal influenza vaccine | ||||
|---|---|---|---|---|---|---|
| Any grade** | Grade 3** | Any grade** | Grade 3** | Any grade** | Grade 3** | |
| 6–23 months | n = 68 | n = 62 | n = 37 | |||
| Injection‐site reactions, No.(%, 95% CI) | ||||||
| Swelling | 0 (0·0, 0·0–6·7) | 0 (0·0, 0·0–6·7) | 1 (1·6, 0·1–9·9) | 0 (0·0, 0·0–7·3) | 0 (0·0, 0·0–11·7) | 0 (0·0, 0·0–11·7) |
| Pain | 1 (1·5, 0·1–9·0) | 0 (0·0, 0·0–6·7) | 1 (1·6, 0·1–9·9) | 0 (0·0, 0·0–7·3) | 0 (0·0, 0·0–11·7) | 0 (0·0, 0·0–11·7) |
| Redness | 0 (0·0, 0·0–6·7) | 0 (0·0, 0·0–6·7) | 0 (0·0, 0·0–7·3) | 0 (0·0, 0·0–7·3) | 0 (0·0, 0·0–11·7) | 0 (0·0, 0·0–11·7) |
| Rash | 0 (0·0, 0·0–6·7) | 0 (0·0, 0·0–6·7) | 0 (0·0, 0·0–7·3) | 0 (0·0, 0·0–7·3) | 0 (0·0, 0·0–11·7) | 0 (0·0, 0·0–11·7) |
| Systemic reactions, No.(%, 95% CI) | ||||||
| Fever | 14 (20·6, 12·1–32·5) | 0 (0·0, 0·0–6·7) | 16 (25·8, 15·9–28·8) | 1 (1·6, 0·1–9·9) | 7 (18·9, 8·6–35·9) | 1 (2·7, 0·1–15·9) |
| Diarrhea | 7 (10·3, 4·6–20·7) | 0 (0·0, 0·0–6·7) | 6 (9·7, 4·0–20·6) | 0 (0·0, 0·0–7·3) | 5 (13·5, 5·1–29·7) | 0 (0·0, 0·0–11·7) |
| Allergy | 0 (0·0, 0·0–6·7) | 0 (0·0, 0·0–6·7) | 1 (1·6, 0·1–9·9) | 0 (0·0, 0·0–7·3) | 0 (0·0, 0·0–11·7) | 0 (0·0, 0·0–11·7) |
| Decreases in activity levels | 2 (2·9, 0·5–11·2) | 0 (0·0, 0·0–6·7) | 2 (3·2, 0·6–12·2) | 1 (1·6, 0·1–9·9) | 1 (2·7, 0·1–15·9) | 0 (0·0, 0·0–11·7) |
| Cough | 3 (4·4, 1·1–13·2) | 0 (0·0, 0·0–6·7) | 3 (4·8, 1·3–14·4) | 0 (0·0, 0·0–7·3) | 0 (0·0, 0·0–11·7) | 0 (0·0, 0·0–11·7) |
| Irritability | 5 (7·4, 2·7–17·1) | 0 (0·0, 0·0–6·7) | 3 (4·8, 1·3–14·4) | 0 (0·0, 0·0–7·3) | 1 (2·7, 0·1–15·9) | 0 (0·0, 0·0–11·7) |
| Nausea or vomiting | 1 (1·5, 0·1–9·0) | 0 (0·0, 0·0–6·7) | 4 (6·5, 2·1–16·5) | 0 (0·0, 0·0–7·3) | 3 (8·1, 2·1–23·1) | 1 (2·7, 0·1–15·9) |
| Headache | 0 (0·0, 0·0–6·7) | 0 (0·0, 0·0–6·7) | 0 (0·0, 0·0–7·3) | 0 (0·0, 0·0–7·3) | 0 (0·0, 0·0–11·7) | 0 (0·0, 0·0–11·7) |
| Loss of appetite | 2 (2·9, 0·5–11·2) | 0 (0·0, 0·0–6·7) | 2 (3·2, 0·6–12·2) | 1 (1·6, 0·1–9·9) | 0 (0·0, 0·0–11·7) | 0 (0·0, 0·0–11·7) |
| Other | 2 (2·9, 0·5–11·2) | 0 (0·0, 0·0–6·7) | 3 (4·8, 1·3–14·4) | 0 (0·0, 0·0–7·3) | 0 (0·0, 0·0–11·7) | 0 (0·0, 0·0–11·7) |
| 24–35 months | n = 56 | n = 61 | n = 26 | |||
| Injection‐site reactions, No.(%, 95% CI) | 0 (0·0, 0·0–16·1) | 0 (0·0, 0·0–16·1) | ||||
| Swelling | 0 (0·0, 0·0–8·0) | 0 (0·0, 0·0–8·0) | 0 (0·0, 0·0–7·4) | 0 (0·0, 0·0–7·4) | 0 (0·0, 0·0–16·1) | 0 (0·0, 0·0–16·1) |
| Pain | 0 (0·0, 0·0–8·0) | 0 (0·0, 0·0–8·0) | 0 (0·0, 0·0–7·4) | 0 (0·0, 0·0–7·4) | 0 (0·0, 0·0–16·1) | 0 (0·0, 0·0–16·1) |
| Redness | 0 (0·0, 0·0–8·0) | 0 (0·0, 0·0–8·0) | 1 (1·6, 0·1–10·0) | 0 (0·0, 0·0–7·4) | 0 (0·0, 0·0–16·1) | 0 (0·0, 0·0–16·1) |
| Rash | 0 (0·0, 0·0–8·0) | 0 (0·0, 0·0–8·0) | 0 (0·0, 0·0–7·4) | 0 (0·0, 0·0–7·4) | 1 (3·8, 0·2–21·7) | 0 (0·0, 0·0–16·1) |
| Systemic reactions, No.(%, 95% CI) | ||||||
| Fever | 9 (16·1, 8·1–28·9) | 0 (0·0, 0·0–8·0) | 8 (13·1, 6·2–24·8) | 1 (1·6, 0·1–10·0) | 6 (23·1, 9·8–44·4) | 0 (0·0, 0·0–16·1) |
| Diarrhea | 4 (7·1, 2·3–18·2) | 1 (1·8, 0·1–10·8) | 1 (1·6, 0·1–10·0) | 0 (0·0, 0·0–7·4) | 0 (0·0, 0·0–16·1) | 0 (0·0, 0·0–16·1) |
| Allergy | 1 (1·8, 0·1–10·8) | 0 (0·0, 0·0–8·0) | 0 (0·0, 0·0–7·4) | 0 (0·0, 0·0–7·4) | 1 (3·8, 0·2–21·7) | 0 (0·0, 0·0–16·1) |
| Decreases in activity levels | 0 (0·0, 0·0–8·0) | 0 (0·0, 0·0–8·0) | 0 (0·0, 0·0–7·4) | 0 (0·0, 0·0–7·4) | 1 (3·8, 0·2–21·7) | 0 (0·0, 0·0–16·1) |
| Cough | 2 (3·6, 0·6–13·4) | 0 (0·0, 0·0–8·0) | 1 (1·6, 0·1–10·0) | 0 (0·0, 0·0–7·4) | 0 (0·0, 0·0–16·1) | 0 (0·0, 0·0–16·1) |
| Irritability | 1 (1·8, 0·1–10·8) | 0 (0·0, 0·0–8·0) | 0 (0·0, 0·0–7·4) | 0 (0·0, 0·0–7·4) | 0 (0·0, 0·0–16·1) | 0 (0·0, 0·0–16·1) |
| Nausea or vomiting | 1 (1·8, 0·1–10·8) | 0 (0·0, 0·0–8·0) | 0 (0·0, 0·0–7·4) | 0 (0·0, 0·0–7·4) | 0 (0·0, 0·0–16·1) | 0 (0·0, 0·0–16·1) |
| Headache | 2 (3·6, 0·6–13·4) | 0 (0·0, 0·0–8·0) | 0 (0·0, 0·0–7·4) | 0 (0·0, 0·0–7·4) | 0 (0·0, 0·0–16·1) | 0 (0·0, 0·0–16·1) |
| Loss of appetite | 0 (0·0, 0·0–8·0) | 0 (0·0, 0·0–8·0) | 0 (0·0, 0·0–7·4) | 0 (0·0, 0·0–7·4) | 0 (0·0, 0·0–16·1) | 0 (0·0, 0·0–16·1) |
| Other | 1 (1·8, 0·1–10·8) | 0 (0·0, 0·0–8·0) | 3 (4·9, 1·3–14·6) | 0 (0·0, 0·0–7·4) | 1 (3·8, 0·2–21·7) | 0 (0·0, 0·0–16·1) |
CI, confidence interval; HA, hemagglutinin.
*Values are for participants who received at least one dose of vaccine with 1 post‐vaccination safety measure.
**Coded with three grades of severity for redness, swelling, and induration across both age cohorts: mild (<10 mm), moderate (≥10 mm to ≤30 mm), or severe (>30 mm). Pain was graded as mild (minor reaction on touch), moderate (cries or protests on touch), or severe (cries when limb is moved or spontaneously painful). Coding for mild, moderate, and severe was the same for all systemic reactions in both age cohorts: mild (transient or mild discomfort (< 48 hours); no medical intervention/therapy required), moderate (mild to moderate limitation in activity – some assistance may be needed; no or minimal medical intervention/therapy required), or severe (marked limitation in activity, some assistance usually required; medical intervention/therapy required, hospitalizations possible). For fever, the grades were mild (≥37·1 to ≤37·5°C), moderate (≥37·6 to ≤39·0°C), and severe (>39·0°C).
Immunogenicity
At baseline, detectable hemagglutination inhibition antibody (titer ≥1:10) was seen in 12 (7·2%) of 167 infants aged 6–23 months and 10 (7·0%) of 143 infants aged 24–35 months; seroprotective concentrations of the antibody (titer ≥1:40) were seen in 3·0% (5/167) infants in the younger age group and 2·1% (3/143) infants in the older age group.
After the first vaccination with either dose of 7·5 and 15 μg H1N1 vaccine, titers increased approximately from 5·8‐ to 7·1‐fold in younger infants and 5·9‐ to 6·3‐fold in the older infants. The first vaccination induced seroprotective antibody titers (HI titers ≥40) in 42·9–57·4% of younger infants and 49·1–61·0% older infants. As measured on Day 42, two vaccinations elicited a 30·2‐ to 41·9‐fold increase in titers in younger infants and 25·8‐ to 26·8‐fold increase in titers in older infants (Table 3). Immune responses after completion of the two‐dose schedule were comparable in both age groups with seroprotective rates of 91–98% in each vaccine and age group and GMTs of 173–263. In the control (seasonal influenza vaccine) group, 18 infants seroconverted during the study (13 younger infants and 5 older infants), bringing the seroprotection rate for the control group to 48·1% in younger infants and 20·8% in older infants (Table 3).
Table 3.
Hemagglutination inhibition antibody against H1N1 influenza virus 21 days after the first and second injections of H1N1 vaccine or seasonal influenza vaccine
| Age and vaccine group | ||||||
|---|---|---|---|---|---|---|
| 6–23 months | 24–35 months | |||||
| 7·5 μg HA n = 61 | 15 μg HA n = 54 | Control n = 27 | 7·5 μg HA n = 55 | 15 μg HA N = 59 | Control n = 24 | |
| Pre‐vaccination | ||||||
| GMT (95% CI) | 5·73 (4·86–6·76) | 6·30 (5·15–7·70) | 5·54 (4·80–6·39) | 5·19 (4·97–5·42) | 5·89 (5·06–6·85) | 5·15 (4·86–5·46) |
| First injection | ||||||
| Seroprotection D21, N (%) | 30 (49·2) | 31 (57·4) | 7 (25·9) | 27 (49·1) | 36 (61·0) | 2 (8·3) |
| Seroconversion D21/D0, N (%) | 29 (47·5) | 30 (55·6) | 6 (22·2) | 27 (49·1) | 35 (59·3) | 2 (8·3) |
| GMT (95% CI) | 33·35 (23·18–47·97) | 44·90 (29·25–68·92) | 14·70 (9·59–22·53) | 30·70 (24·22–38·92) | 37·28 (29·87–46·53) | 8·17 (5·95–11·22) |
| GMTR D21/D0 (95% CI) | 5·82 (4·17–8·13) | 7·13 (4·99–10·19) | 2·65 (1·78–3·95) | 5·91 (4·62–7·56) | 6·32 (5·10–7·82) | 1·59 (1·20–2·11) |
| Second injection | ||||||
| Seroprotection D42, N (%) | 56 (91·8) | 53 (98·1) | 13 (48·1) | 51 (92·7) | 55 (93·2) | 5 (20·8) |
| Seroconversion D42/D0, N (%) | 56 (91·8) | 53 (98·1) | 13 (48·1) | 51 (92·7) | 55 (93·2) | 5 (20·8) |
| GMT (95% CI) | 173·25 (121·10–247·85) | 263·96 (195·81–355·83) | 36·10 (23·35–55·82) | 134·12 (100·53–178·93) | 158·13 (117·51–212·80) | 17·31 (10·88–27·54) |
| GMTR D42/D0 (95% CI) | 30·23 (21·19–43·13) | 41·90 (31·74–55·31) | 6·51 (4·35–9·75) | 25·83 (19·25–34·66) | 26·83 (19·68–36·58) | 3·36 (2·17–5·19) |
Seroprotection: number and proportion (%) with titers ≥40; seroconversion: number and proportion (%) with either a Day 0 titer <10 and a Day 21 titer ≥40, or a Day 0 titer ≥10 and ≥4‐fold rise by Day 21.
GMT, geometric mean titer; GMTR, geometric mean of post‐/pre‐vaccination titer ratio; N, number of participants; CI: confidence interval; D, day; HA, hemagglutinin.
Among infants with antibody titer <1:10 pre‐vaccination, following the first injection, the seroprotection rate for the pandemic (H1N1) 2009 virus was observed in 45·6–52·1% younger infants and 50·0–58·5% older infants, respectively (Table 4). The GMTs of antibody titers were 29·2–31·8 and 31·5–34·2, and factor increases from the baseline were 5·8–6·4 and 6·3–6·8, respectively. Following the second injection, the seroprotection rate increased to 91–98% in the both age groups. The GMTs were 163·9–226·3 and 136·4–153·8, and factor increases from the baseline were 32·8–45·3 and 27·3–30·8, respectively. Overall, responses were similar in the younger age group compared with the older group, and no statistically significant differences in antibody titers were detected between the 7·5 and 15 μg H1N1 vaccine.
Table 4.
Hemagglutination inhibition antibody of infants with antibody titer <1:10 pre‐vaccination against H1N1 influenza virus 21 days after the first and second injections of H1N1 vaccine or seasonal influenza vaccine
| Age and vaccine group | ||||||
|---|---|---|---|---|---|---|
| 6–23 months | 24–35 months | |||||
| 7·5 μg HA n = 57 | 15 μg HA n = 48 | Control n = 25 | 7·5 μg HA n = 52 | 15 μg HA n = 53 | Control n = 23 | |
| Pre‐vaccination | ||||||
| GMT (95% CI) | 5·00 (5·00–5·00) | 5·00 (5·00–5·00) | 5·00 (5·00–5·00) | 5·00 (5·00–5·00) | 5·00 (5·00–5·00) | 5·00 (5·00–5·00) |
| First injection | ||||||
| Seroprotection D21, N (%) | 26 (45·6) | 25 (52·1) | 5 (20·0) | 26 (50·0) | 31 (58·5) | 1 (4·3) |
| Seroconversion D21/D0, N (%) | 26 (45·6) | 25 (52·1) | 5 (20·0) | 26 (50·0) | 31 (58·5) | 1 (4·3) |
| GMT (95% CI) | 29·16 (20·44–41·62) | 31·75 (22·52–44·77) | 13·20 (8·58–20·31) | 31·47 (24·60–40·26) | 34·19 (27·44–42·59) | 7·40 (5·71–9·59) |
| GMTR D21/D0 (95% CI) | 5·83 (4·09–8·32) | 6·35 (4·50–8·95) | 2·64 (1·72–4·06) | 6·29 (4·92–8·05) | 6·84 (5·49–8·52) | 1·48 (1·14–1·92) |
| Second injection | ||||||
| Seroprotection D42, N (%) | 52 (91·2) | 47 (97·9) | 11 (44·0) | 48 (92·3) | 49 (92·4) | 4 (17·4) |
| Seroconversion D42/D0, N (%) | 52 (91·2) | 47 (97·9) | 11 (44·0) | 48 (92·3) | 49 (92·4) | 4 (17·4) |
| GMT (95% CI) | 163·94 (113·37–237·06) | 226·27 (168·51–303·83) | 32·04 (20·71–49·57) | 136·35 (100·64–184·72) | 153·84 (111·96–211·39) | 15·25 (10·16–22·90) |
| GMTR D21/D0 (95% CI) | 32·79 (22·68–47·42) | 45·26 (33·71–60·78) | 6·41 (4·14–9·92) | 27·27 (20·13–36·95) | 30·77 (22·39–42·28) | 3·05 (2·03–4·58) |
Seroprotection: number and proportion (%) with titers ≥40; seroconversion: number and proportion (%) with either a Day 0 titer <10 and a Day 21 titer ≥40, or a Day 0 titer ≥10 and ≥4‐fold rise by Day 21.
GMT, geometric mean titer; GMTR, geometric mean of post‐/pre‐vaccination titer ratio; N, number of participants; CI, confidence interval; D, day; HA, hemagglutinin.
Protection against influenza illness
A total of 64 ILI episodes were reported during the period between receipt of the first and second vaccine dose, with incidences of 30·2%, 21·0%, and 15·4% in TIV group, 7·5‐μg H1N1 vaccine group, and 15‐μg H1N1 vaccine group, respectively. Of these ILI cases, pharyngeal or nasopharyngeal swab samples were collected from eight cases for detection of the virus. As a result, all three positive cases of 2009 influenza A/H1N1 virus were detected in the TIV group, and four positive cases of seasonal influenza A virus were only detected in the H1N1 vaccine groups (7·5‐μg group: two cases; 15‐μg group: two cases). One case in the 15‐μg H1N1 vaccine group showed negative result (Table 5).
Table 5.
The results of real‐time RT‐PCR of swab samples from infants with influenza‐like illness (ILI) post‐vaccination
| Vaccine group | Subject code | Sex | Age (months) | Day of ILI onset (after the first vaccination) | Day of swabs collection (after ILI onset) | Results | |
|---|---|---|---|---|---|---|---|
| Seasonal influenza A | 2009 influenza A/H1N1 | ||||||
| 7·5 μg HA | B080 | Male | 14 | 21 | 3 | Positive | Negative |
| B209 | Male | 35 | 21 | 3 | Positive | Negative | |
| 15 μg HA | B009 | Female | 8 | 21 | 5 | Negative | Negative |
| B094 | Male | 13 | 20 | 3 | Positive | Negative | |
| B246 | Female | 32 | 23 | 1 | Positive | Negative | |
| Control | B158 | Female | 21 | 22 | 1 | Negative | Positive |
| B280 | Female | 34 | 24 | 2 | Negative | Positive | |
| B300 | Female | 29 | 18 | 2 | Negative | Positive | |
HA, hemagglutinin.
Discussion
Previously, many preliminary safety and immunogenicity studies of non‐adjuvant, split‐virion, 2009 pandemic influenza A (H1N1) monovalent vaccines have been performed in Chinese children aged 3 years or older and in adults including elderly. 13 , 14 , 15 , 16 It was found that one injection of a non‐adjuvant split‐virion vaccine containing 7·5, 15, or 30 μg was well tolerated and highly immunogenic in individuals aged 3–12 years old and was able to induce protective levels of antibody, with seroprotection rates of 74–87%. 13 , 14 Similarly, in Australia, a single 15‐μg dose of an non‐adjuvant, split‐virion, influenza A/H1N1 vaccine can elicit significant increases in influenza‐specific antibody in more than 90% of healthy infants and children aged 6 months to <9 years. 17 The author suggested that a single 15‐μg dose of non‐adjuvant split‐virion vaccine might be promoted as the formulation choice against 2009 pandemic influenza A H1N1 for children. However, many other clinical trials showed different results. In a study carried out in Korea, 5·9% of the subjects between 6 months and <3 years of age receiving one 7·5‐μg dose of an inactivated split‐virus influenza A/H1N1 vaccine had HI titer of 1:40 or more after the first dose. 18 Another US study suggested that the first dose of either 7·5 or 15 μg influenza A/H1N1 vaccine formulation was more immunogenic in children older than 3 years than in younger children. About 45–50% of children aged 6–35 months and 69–75% of children aged 3–9 years attained HI titers of ≥1:40. 19 But, until now, the immunogenicity and tolerability of this vaccine formulation in Chinese infants younger than 3 years have not been demonstrated.
Our study demonstrated that a single 7·5‐ or 15‐μg dose of the inactivated, split‐virion, 2009 pandemic influenza A (H1N1) monovalent vaccine did not provide sufficient immunogenicity in Chinese infants. About 42·9–57·4% of younger infants (aged 6–23 months) and 49·1–61·0% of older infants (aged 24–35 months) had HI titers of 40 or more after the first dose, which was consistent with the antibody response in US children aged 6–35 months. 19 After the second injections, seroprotection and seroconversion rates of 91–98% elicited by either the 7·5‐μg HA or 15‐μg HA vaccine formulation satisfied the US Food and Drug Administration (FDA) immunogenicity requirement. 20
Possible explanation for the above variations in vaccine response in infant populations includes the differences in the baseline serostatus. In this study, before vaccination, 7·1% (22/310) subjects had detectable antibody against 2009 pandemic H1N1 virus (antibody titer ≥1:10). Additionally, approximately 2% had seroprotective concentration of antibody. Comparable results have been reported in the previous serologic survey in China’s Guangxi Province (1·7%) 21 and the study in US children (3%). 19 However, the results were higher than that reported in Korea (0%) 18 and lower than that reported in Australia (9–13%). 17
In addition, interestingly, among previously unexposed infants with antibody titer <1:10, the 7·5 μg of the inactivated, split‐virion, 2009 pandemic influenza A (H1N1) monovalent vaccine induced significantly different immune response between Chinese infants and Korean infants. In our study, the proportion with an HI titer of 1:40 or more was 49–61% of the subjects when measured 3 weeks after the first dose and 91–97% of the subjects after the second dose. The study carried out in Korea showed that the proportion with an HI titer of 1:40 or more was 5·9% of the subjects after the first dose and 55·9% of the subjects after the second dose. 18 The two following limitations in this trial might account for the differences in vaccine immune response. Firstly, the study design was not randomized or controlled for the various antigen doses. Secondly, the trial was conducted in a limited setting and with a small group of subjects (n = 34). Furthermore, during the outbreak of ILI episodes, none of positive cases of 2009 influenza A/H1N1 virus were detected in the H1N1 vaccine group. The results supported that a single injection of the inactivated 2009 pandemic influenza A (H1N1) vaccine could confer some degree of, although not sufficient, protection in infants.
Currently, the serum HI threshold of 1:40 is the most widely used predictor for efficacy of inactivated influenza vaccines. However, because the acceptance criteria for clinical development of influenza vaccines are in place for adults but not for children, the correlation between cutoff HI titer of ≥1:40 and the level of protection in immunologically naïve children is questioned. An efficacy trial of an adjuvanted and a non‐adjuvanted seasonal influenza vaccines showed that a cutoff of 1:40 was only associated with a protection rate of 22%, while the ≥80% efficacy of the adjuvanted vaccine corresponded to a titer of around 1:320. 22 But, there are limitations to the use of these data. Firstly, the derived correlate of protection has been derived using only an H3N2 strain, but the correlate for influenza B and/or A strains, especially for H1N1 strain, was not evaluated. In addition, there are some differences in the protective correlate for adjuvanted and non‐adjuvanted vaccines. And, the estimates in the study were derived from a European population with a specific age distribution and influenza exposure history. It is possible that the correlate may vary by population and ethnicity. Then, limited data from clinical studies about the relationship between HI antibody titer and clinical protection from influenza in young children were obtained. Therefore, although the relationship defined in adults largely based on controlled challenge studies may not be generalizable to children, seroprotection has still been defined as a HI titer of 40 or greater in the great majority of immunogenicity and/or efficacy trials of influenza vaccines.
In this study, no marked difference in the reactogenicity between the 2009 pandemic H1N1 vaccine and the seasonal influenza vaccine was found. In particular, similar local and systemic adverse reaction rates occurred in each of the three groups. The results also showed that the 2009 pandemic H1N1 vaccine was associated with an acceptable safety profile for infants, which was similar to that in children, adolescents, and adults. 13 , 14 , 15 , 16
There is debate regarding the effectiveness of seasonal influenza vaccination in providing protection against the 2009 pandemic strains, with different studies reporting varying results. Interim analysis of pandemic influenza (H1N1) 2009 in Australia estimates that seasonal vaccination may be 3% effective in preventing 2009 pandemic H1N1 influenza. 23 Another study documented that 12% of adults aged 18–40 years who were immunized with the 2008/2009 seasonal influenza vaccine revealed seroconversion against A/California/05/2009. 7 In our study, for seasonal influenza vaccine group, only 5·9% (3/51) infants showed baseline HI titers ≥1:10 against 2009 pandemic H1N1 virus, and nobody had seroprotective concentration of the antibody (≥1:40). Following two seasonal influenza vaccinations, 35·3% (18/51) subjects achieved antibody titers of 1:40 or greater, albeit much lower than the matched vaccine strain (data not shown). Considering 19 (30·2%) of ILI episodes reported in seasonal influenza vaccine group during the period between receipt of the first and second vaccine dose, it seemed to be reasonable that the TIV showed a modest effect of 5% in preventing 2009 pandemic H1N1 influenza.
Also, the question of whether seasonal influenza vaccines are effective at all for priming of immunity in young children is concerned. Some studies showed that influenza vaccines had no or limited efficacy in those 6–24 months of age. 24 , 25 , 26 On the other hand, however, some researchers believed that the dose of the non‐adjuvant vaccine may be critical for its efficacy in this age group. In a cohort study with virologically confirmed outcomes, the use of 0·5‐ml (instead of 0·25‐ml) doses for children under the age of 36 months showed a 79% vaccine effectiveness against matched strains of influenza and an overall effectiveness of 66% against any strains of influenza, even in children under the age of 2 years. 27 In another recent study, the higher dose significantly increased children’s antibody responses without any increase in reactogenicity. 28 And, in the efficacy trial by Vesikari et al., 26 the vaccine effectiveness of 0·5 ml of TIV was 66% (95% CI, 29–84) against all circulating influenza strains in children under the age of 36 months; however, this finding fell down to 43% (95% CI, 15–61) for TIV in children under the age of 72 months in which 0·25‐ml doses were used. Therefore, further research into the effectiveness of doses of non‐adjuvant seasonal influenza vaccines that are higher than traditional doses for young children seems warranted.
Our study suffers from several limitations. First, ILIs were not actively monitored throughout the subsequent influenza season. Although a total of 64 ILI episodes were reported during the period between receipt of the first and second vaccine dose, only eight pharyngeal or nasopharyngeal swab samples were collected. Second, the study did not address long‐term immunogenicity of 2009 pandemic influenza A (H1N1) vaccine in infants. Third, as children <3 years of age are considered immunologically naive, HI assay may lead to overestimation of the immune responses induced by influenza vaccines in infants. Virus neutralization assays are gaining popularity, because they detect a broader range of functional antibodies and show enhanced sensitivity for detecting antibody to some viruses. In the present study, the NT was not used for the immunogenicity assay due to interlaboratory variation.
Therefore, the results confirmed the necessity of a second dose of the inactivated, split‐virion, 2009 pandemic influenza A (H1N1) monovalent vaccine in infants to induce a protective immune response. And, the safety and reactogenicity of the vaccine, at either 7·5‐ or 15‐μg dosage, were acceptable and similar to those seen historically with seasonal inactivated seasonal influenza vaccines after both the first and second vaccinations.
Addendum
Guo‐Min Zhang, BS, Chinese Center for Disease Control and Prevention, Beijing, China; Li‐Rong Huang, BS, Guangxi Centers for Diseases Control and Prevention, Nanning, China; Qiao Li, BS, Guangxi Centers for Diseases Control and Prevention, Nanning, China; Jun‐Yu Wu, MD, PhD, Sinovac Biotech Co. Ltd, Beijing, China; Han‐Hua Fang, BS, National Institutes for Food and Drug Control, Beijing, China; Xiang Zhong, MD, PhD, Sinovac Biotech Co. Ltd, Beijing, China; Jiang‐Ting Chen, BS, Sinovac Biotech Co. Ltd, Beijing, China; Da‐Peng Yin, PhD, Chinese Center for Disease Control and Prevention, Beijing, China; Rui‐Ping Su, BS, Lingchuan Center for Disease Control and Prevention, Guilin, China; Xiao‐Mei Zhang, BS, Sinovac Biotech Co. Ltd, Beijing, China.
Conflict of interest
Yan Liu, Jun‐Yu Wu, Xiang Zhong, Jiang‐Ting Chen, Xiao‐Mei Zhang, and Wei‐Dong Yin are employed by Sinovac.
Acknowledgements
We would like to express our thanks to the Lingchuan CDC staffs who contributed the field work and to the member of the immunization program. This project was funded in whole with National High Technology Research and Development Program 863 Funds from the Center for Disease Control and Prevention, China, under Contract No. 2010AA022908.
References
- 1. WHO . New influenza A (H1N1) virus: global epidemiological situation, June 2009. Wkly Epidemiol Rec 2009; 84:249–257. [PubMed] [Google Scholar]
- 2. Uyeki TM. 2009 H1N1 virus transmission and outbreaks. N Engl J Med 2010; 362:2221–2223. [DOI] [PubMed] [Google Scholar]
- 3. Girard MP, Tam JS, Assossou OM, Kieny MP. The 2009 A (H1N1) influenza virus pandemic: a review. Vaccine 2010; 28:4895–4902. [DOI] [PubMed] [Google Scholar]
- 4. Kelly HA, Grant KA, Williams S, Fielding J, Smith D. Epidemiological characteristics of pandemic influenza H1N1 2009 and seasonal influenza infection. Med J Aust 2009; 191:146–149. [DOI] [PubMed] [Google Scholar]
- 5. Bin C, Xingwang L, Yuelong S et al. Clinical and epidemiologic characteristics of 3 early cases of influenza A pandemic (H1N1) virus 2009 infection, People’s Republic of China, 2009. Emerg Infect Dis 2009; 15:1418–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention (CDC) . Swine‐origin influenza A (H1N1) virus infections in a school – New York City, April 2009. MMWR Morb Mortal Wkly Rep 2009; 58:470–472. [PubMed] [Google Scholar]
- 7. Hancock K, Veguilla V, Lu X et al. Cross‐reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med 2009; 361:1945–1952. [DOI] [PubMed] [Google Scholar]
- 8. Plennevaux E, Sheldon E, Blatter M, Reeves‐Hoche MK, Denis M. Immune response after a single vaccination against 2009 influenza A H1N1 in USA: a preliminary report of two randomised controlled phase 2 trials. Lancet 2009; 375:41–48. [DOI] [PubMed] [Google Scholar]
- 9. Dawood FS, Jain S, Finelli L et al. Emergence of a novel swine‐origin influenza A (H1N1) virus in humans. N Engl J Med 2009; 360:2605–2615. [DOI] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention . Update: influenza activity, United States, August 30, 2009‐March 27, 2010, and composition of the 2010‐11 influenza vaccine. MMWR Morb Mortal Wkly Rep 2010; 59:423–430. [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention (CDC) . FluView: week 48 ending December 5, 2009. Available at http://www.cdc.gov/flu/weekly/ (Accessed 11 December 2009).
- 12. National Center for Immunization and Respiratory Diseases , Centers for Disease Control and Prevention . Use of influenza A (H1N1) 2009 monovalent vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2009; 58:1–8. [PubMed] [Google Scholar]
- 13. Zhu FC, Wang H, Fang HH et al. A novel influenza A (H1N1) vaccine in various age groups. N Engl J Med 2009; 361:2414–2423. [DOI] [PubMed] [Google Scholar]
- 14. Liang XF, Wang HQ, Wang JZ et al. Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: a multicentre, double‐blind, randomised, placebo‐controlled trial. Lancet 2010; 375:56–66. [DOI] [PubMed] [Google Scholar]
- 15. Wu J, Xu F, Lu L et al. Safety and effectiveness of a 2009 H1N1 vaccine in Beijing. N Engl J Med 2010; 363:2416–2423. [DOI] [PubMed] [Google Scholar]
- 16. Wu J, Li W, Wang HQ et al. A rapid immune response to 2009 influenza A (H1N1) vaccines in adults: a randomized, double‐blind, controlled trial. J Infect Dis 2010; 202:675–680. [DOI] [PubMed] [Google Scholar]
- 17. Nolan T, McVernon J, Skeljo M et al. Immunogenicity of a monovalent 2009 influenza A (H1N1) vaccine in infants and children: a randomized trial. JAMA 2010; 303:37–46. [DOI] [PubMed] [Google Scholar]
- 18. Oh CE, Lee J, Kang JH et al. Safety and immunogenicity of an inactivated split‐virus influenza A/H1N1 vaccine in healthy children from 6 months to <18 years of age: a prospective, open‐label, multi‐center trial. Vaccine 2010; 28:5857–5863. [DOI] [PubMed] [Google Scholar]
- 19. Plennevaux E, Blatter M, Cornish MJ et al. Influenza A (H1N1) 2009 two‐dose immunization of US children: an observer‐blinded, randomized, placebo‐controlled trial. Vaccine 2011; 29:1569–1575. [DOI] [PubMed] [Google Scholar]
- 20. CBER/FDA . Regulatory Considerations Regarding the Use of Novel Influenza A (H1N1) Virus Vaccines: United States Food and Drug Administration, 2009. [Google Scholar]
- 21. Chen H, Wang Y, Liu W et al. Serologic survey of pandemic (H1N1) 2009 virus, Guangxi Province, China. Emerg Infect Dis 2009; 15:1849–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Black S, Nicolay U, Vesikari T et al. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr Infect Dis J 2011; 30:1081–1085. [DOI] [PubMed] [Google Scholar]
- 23. Kelly H, Grant K. Interim analysis of pandemic influenza (H1N1) 2009 in Australia: surveillance trends, age of infection and effectiveness of seasonal vaccination. Euro Surveill 2009; 14:pii: 19288. [DOI] [PubMed] [Google Scholar]
- 24. Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta‐analysis. Lancet Infect Dis 2012; 12:36–44. [DOI] [PubMed] [Google Scholar]
- 25. Jefferson T, Rivetti A, Harnden A, Di Pietrantonj C, Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev 2008; 2:CD004879. [DOI] [PubMed] [Google Scholar]
- 26. Vesikari T, Knuf M, Wutzler P et al. Oil‐in‐water emulsion adjuvant with influenza vaccine in young children. N Engl J Med 2011; 365:1406–1416. [DOI] [PubMed] [Google Scholar]
- 27. Heinonen S, Silvennoinen H, Lehtinen P et al. Effectiveness of inactivated influenza vaccine in children aged 9 months to 3 years: an observational cohort study. Lancet Infect Dis 2011; 11:23–29. [DOI] [PubMed] [Google Scholar]
- 28. Skowronski DM, Hottes TS, Chong M et al. Randomized controlled trial of dose response to influenza vaccine in children aged 6 to 23 months. Pediatrics 2011; 128:e276–e289. [DOI] [PubMed] [Google Scholar]


