Abstract
Background
Primary sclerosing cholangitis (PSC) and autoimmune sclerosing cholangitis (AISC) are related, but distinct chronic liver diseases. PSC is associated with a high prevalence of ulcerative colitis while the intestinal inflammation associated with AISC is less well characterised.
Aims
To assess and contrast aspects of intestinal inflammation in patients with AISC and PSC and compare the clinical features with those of patients with ulcerative colitis and Crohn's disease.
Methods
23 and 22 patients with AISC and PSC, respectively, underwent review of colonoscopy and biopsy findings, capsule enteroscopy and assessment of clinical and inflammatory (faecal calprotectin) disease activity, which was compared with that of patients with ulcerative colitis and Crohn's disease (n = 55 each).
Findings
Five and 6 patients with AISC and PSC, respectively, had normal colonoscopy and faecal calprotectin levels of 34.4 ± 8.3 and 39.7 ± 8.4 μg/g, respectively (normal < 50 μg/g), whereas 18 and 16, respectively, had identical variably severe, right sided colitis with frequent rectal sparing, consistent with ulcerative colitis. Mean (± SD) faecal calprotectin levels did not differ significantly (p > 0.05) between patients with intestinal inflammation in AISC (588 ± 549 μg/g), PSC (421 ± 351 μg/g), ulcerative colitis (501 ± 656 μg/g) or Crohn's disease (476 ± 571 μg/g). Capsule enteroscopy showed that 7 of 18 (39%) (p < 0.03) of those with AISC had small bowel mucosal breaks whereas no patient with PSC had these findings.
Interpretation
Collectively these findings lend support to the suggestion that the chronic inflammatory bowel disease associated with PSC and in particular AISC may represent a distinct nosologic entity different from classic ulcerative colitis and Crohn's disease.
Keywords: Inflammatory bowel disease, Ulcerative colitis, Crohn's disease, Primary sclerosing cholangitis, Autoimmune sclerosing cholangitis, Capsule enteroscopy, Chronic liver disease, Enteropathy
Highlights
-
•
The ulcerative colitis (UC) associated with autoimmune and primary sclerosing cholangitis behaves differently to classic UC.
-
•
Patients with autoimmune and primary sclerosing cholangitis have similar pattern of colitis, resembling ulcerative colitis.
-
•
39% of those with autoimmune sclerosing cholangitis had small intestinal mucosal breaks resembling Crohn’s disease.
-
•
The colitis associated with these chronic liver diseases differs distinctively from classic inflammatory bowel disease.
Most patients with the chronic liver disease primary sclerosing cholangitis have a colitis, which is classified as ulcerative colitis. Here we show that patients with a related liver disease, namely autoimmune sclerosing cholangitis, have an identical colitis, but 39% also have small bowel erosions or ulcers which is more suggestive of Crohn's disease. Intestinal inflammatory activity was similar in these patients as compared with patients with ulcerative colitis and Crohn's disease. Collectively these findings and the contrasting natural history of the colitis of chronic liver suggests that this represents a separate nosologic entity from classic inflammatory bowel disease.
1. Introduction
The association between hepatobiliary disease and inflammatory bowel disease (IBD) is well documented (Bjarnason et al., 1995). It is most obviously illustrated in the high prevalence (70–90%) of colitis in patients with primary sclerosing cholangitis (PSC) (Saich and Chapman, 2008), which falls within the spectrum of ulcerative colitis (UC) (Saich and Chapman, 2008). Conversely PSC is an extra-intestinal manifestation of IBD with approximately 5% and 3.5% of patients with UC and Crohn's disease (CD), respectively, developing the disease (Saich and Chapman, 2008, Schrumpf et al., 1980, Olsson et al., 1991). However the colitis in patients with and without PSC differs. The former is characteristically associated with a more indolent clinical course and a more severe proximal colonic involvement with rectal sparing (60–70%) (Loftus et al., 2005) and there is an unexplained higher incidence of colorectal cancer (Shetty et al., 1999, Soetikno et al., 2002). Collectively these observations suggest that PSC-colitis may represent a distinct disease entity from classic UC (Loftus et al., 2005, Lundqvist and Broomé, 1997).
Patients with PSC may have clinical, biochemical and serological features resembling other liver diseases, especially that of autoimmune hepatitis (Vogel et al., 2002), the latter having a lower prevalence of colitis (about 16%) (Saich and Chapman, 2008). Furthermore autoimmune sclerosing cholangitis (AISC) (Floreani et al., 2005), proposed as a distinctive disease entity in 2001 (Gregorio et al., 2001), has clinical overlap features with PSC and autoimmune hepatitis, but patients are typically a younger age at presentation and the autoimmune serology differs (Gregorio et al., 2001, Bogdanos et al., 2008). The colitis of AISC has not been examined in detail.
Here we characterise the intestinal inflammation associated with AISC and compared the findings with that seen in patients with PSC-associated colitis. Intestinal inflammatory activity and clinical relapse rates were compared with that of patients with classic IBD.
2. Materials & Methods
The liver unit at King's College Hospital runs a transition clinic for adolescents and young adults with chronic liver disease, whereby care is transferred from paediatric to adult liver and gastroenterology services. Such patients undergo a protocol-driven evaluation and assessment to determine the presence, severity, type and extent of intestinal inflammation. The diagnosis of AISC (Gregorio et al., 2001) and PSC had been based on standardised criteria following consideration of conventional clinical features and a number of test results, including a panel of auto-antibodies, endoscopic retrograde or magnetic resonance cholangiopancreatography (all patients had one or the other, ranging in number from 1 to 17) and liver biopsy (all patients had a liver biopsy, ranging in number from 1 to 7) that had been assessed by the Liver Unit histopathologists.
During the period of January 2009 to July 2013 twenty three patients with AISC were transferred to the King's IBD adult clinic. Five of these did not have colitis on previous colonoscopy with biopsy. Their demographic details are shown in Table 1.
Table 1.
Demographic details of the patients with AISC, PSC, UC and CD.
| AISC | AISC + IBD | PSC | PSC + IBD | UC | Crohn's disease | |
|---|---|---|---|---|---|---|
| Number | 5 | 18 | 6 | 16 | 55 | 55 |
| Age at diagnosis (years)⁎ | 10.8 ± 1.7 | 12.3 ± 1.0 | 41.7 ± 5.9 | 30.9 ± 2.9 | 38.6 ± 17.1 | 33.2 ± 15.9 |
| Age at study (years)⁎ | 18.0 ± 0.8 | 19.4 ± 0.6 | 47.5 ± 5.6 | 42.9 ± 3.7 | 44.6 ± 18.6 | 38.7 ± 17.2 |
| Duration of colitis⁎ | 4.5 ± 0.9 | 8.2 ± 1.7 | ||||
| Treatment | ||||||
| Prednisolone (2.5–10 mg/day) | 3/5 (60%) | 14/18 (78%) | 5/6 (83%) | 11/16 (69%) | 0 | 1/55 (1.8%) |
| Azathioprine (25–125 mg) | 3/5 (60%) | 16/18 (89%) | 4/6 (67%) | 14/16 (88%) | 11/55 (20%)⁎⁎ | 16/55 (29%)⁎⁎ |
| Ursodeoxycholic acid (UDCA) | 4/5 (80%) | 17/18 (94%) | 6/6 (100%) | 15/16 (94%) | 0 | 0 |
| Tacrolimus | 1/5 (20%) | 5/18 (28%) | 0/6 (0%) | 9/16 (56%) | 0 | 0 |
| Mycophenolate | 0/5 (0%) | 1/18 (6%) | 0/6 (0%) | 3/16 (19%) | 0 | 0 |
| Mesalazine | 0/5 (0%) | 15/18 (83%) | 0/6 (0%) | 13/16 (81%) | 49/55 (89%) | 32/55 (58%) |
| Faecal calprotectin (microg/g)⁎ | 34.4 ± 8.3 | 588 ± 549 | 39.7 ± 8.4 | 428 ± 351 | 501 ± 656 | 476 ± 571 |
AISC = Autoimmune sclerosing cholangitis.
PSC = Primary sclerosing cholangitis.
UC = Ulcerative colitis.
UDCA = Ursodeoxycholic acid.
Mean ± SD.
Azathioprine dose in UC and Crohn's disease ranged from 100 to 225 mg (2–21/2 mg per kg bodyweight).
During the same time, 22 patients with PSC with and without colitis (16 and 6, respectively) were seen in the IBD clinic. Their demographic details are shown in Table 1.
Patients with AISC and PSC and a diagnosis of CD (n = 4) were specifically excluded from this study, as were patients that had undergone colectomy.
None of the patients with liver disease had received topical therapy for colitis prior to colonoscopy and all had mild to moderate (≤ 6 point scores) clinical disease activity as assessed by the Truelove–Witts criteria (Truelove and Witts, 1955). Liver biochemical profiles showed all but 3 to have stabile or quiescent liver disease. These 3 (one with AISC and 2 with PSC) had jaundice with raised bilirubin and raised transaminase levels, but their other results did not differ or stand out in any major way from the other patients.
Clinical notes were reviewed along with a face-to-face consultation. Each patient underwent routine laboratory tests, faecal calprotectin (providing a quantitative measure of intestinal inflammation) and video capsule enteroscopy as part of a standardised protocol.
Control groups for the faecal calprotectin studies comprised 55 UC patients (26 (47%) with procto-sigmoiditis, 20 (36%) with left sided colitis and 9 (16%) with pan-colitis) and 55 with CD (34 (62%) with and 21 (38%) without small bowel involvement). These were identified in the IBD clinics as routine follow-up or ‘walk-ins’ at King's College Hospital appointments, during the same time period. They were chosen on the basis that they had no more than moderate clinical disease activity; 4–≤ 6 point score for UC (Truelove and Witts, 1955), and Harvey–Bradshaw disease score < 16 for CD (Harvey and Bradshaw, 1980). All patients attending these clinics have a faecal calprotectin measurement as part of their standard of care one week prior to clinic attendance. Their demographic details are shown in Table 1.
2.1. Colonoscopy and Histopathology
The colonoscopy reports, images and biopsies from the patients with AISC and PSC were re-examined (colonoscopy was not clinically indicated or performed at the time of study). The histopathologic examination was carried out without knowledge of the underlying disease and the diagnosis of colitis was made using conventional criteria.
2.2. Faecal Calprotectin
Faecal calprotectin test was carried out as previously described (Maiden et al., 2005), using an ELISA method (Buhlmann Laboratories AG, Schonenbuch, Switzerland). The upper normal value for the test (< 50 μg/g) is based on over 250 normal subjects (Department of Clinical Biochemistry at King's College Hospital).
2.3. Capsule Enteroscopy
Small bowel capsule enteroscopy was carried out using PillCam SB1 or 2 (Given Imaging Ltd.; Yoqneam, Israel) and processed as previously described (Maiden et al., 2005, Maiden et al., 2007). The digital image stream was assessed and interpreted by two experienced staff. The local database includes studies on over 200 healthy volunteer controls.
There are no widely accepted criteria for presenting capsule enteroscopy results, but for the purpose of this study we combined lesions that encompassed erosions and ulcers into a single group “mucosal breaks” as we and other groups have done previously (Maiden et al., 2005, Maiden et al., 2007, Goldstein et al., 2005, Goldstein et al., 2007). The reason for not distinguishing between the two is that the images do not allow a reliable assessment of the depth of the lesions, which is a prerequisite for a reliable diagnosis of an ulcer.
These data were collected as a part of an audit of gastroenterology services, approved by the King's College Hospital Clinical Effectiveness Department. All patients gave informed written consent to video capsule enteroscopy.
3. Results
Patients with AISC were significantly younger than those with PSC and IBD (Table 1). Immunosuppressants and ursodeoxycholic acid were commonly used in the liver patients, but not in the IBD groups, but otherwise the demographic details of patients with AISC and PSC at the time of study did not differ significantly.
3.1. Colonoscopy and Histopathology in AISC and PSC
Five and 6 patients with AISC and PSC, respectively, had normal colonoscopy with biopsy. The rest had undergone a number of colonoscopies (AISC = 2 to 5 and PSC 2–11) at various stages of their disease. The macroscopic appearances did not differ between the two groups and ranged from that of classical ulcerative colitis (recto-sigmoid to pan-colitis) to that of right-sided ulcerative colitis with rectal sparing. The macroscopic rectal sparing was variable given an individual and the activity of disease, but overall at some time evident in 70%.
The microscopic findings of the colonic mucosa were identical between patients with AISC and PSC. The inflammation was almost invariably more pronounced in the right colon and while there were no features suggestive of CD (granulomas, giant cells, fissures, transmural inflammation), the microscopic changes (branching of glands, goblet cell depletion, cryptitis, etc.) that had suggested a diagnosis of UC were often mild, so much so as to give the overall impression of a chronic non-specific indeterminate colitis although clearly favouring UC over CD.
3.2. Faecal Calprotectin
Table 1 shows that the faecal calprotectin concentrations from the patients without colitis were normal while those from patients with AISC- and PSC-colitis, UC and CD were increased and did not differ significantly (p > 0.05) between the groups.
3.3. Capsule Enteroscopy
The 11 patients with AISC and PSC without colitis all had normal small bowel capsule studies.
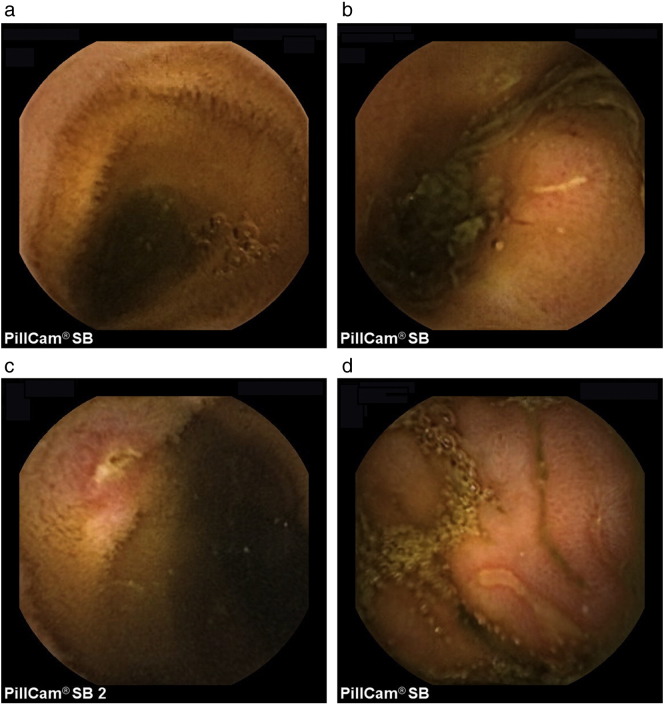
Seven of the capsule enteroscopy studies in the patients with AISC were abnormal with mucosal breaks (ranged in number from 3 to over 20) most pronounced in the distal ileum. Representative images are shown in Fig. 1. There were also some changes indicative of inflammation and although difficult to quantitate, these were mild, patchy and equally found in the mid small bowel and in the distal ileum.
Fig. 1.
a: A representative capsule enteroscopy ileal image from a normal subject.
b: A capsule enteroscopy ileal image demonstrating a linear mucosal break in a patient with AISC.
c: A capsule enteroscopy ileal image demonstrating an aphthous like mucosal break in a patient with AISC.
d: A capsule enteroscopy ileal image demonstrating two linear mucosal breaks in a patient with AISC.
All of the capsule enteroscopy studies in the PSC patients were normal, apart from one that was suggestive of backwash ileitis without mucosal breaks and a further 3 that had very mild inflammatory changes in the mid small bowel, consistent with small bowel bacterial overgrowth.
4. Discussion
This study shows that patients with AISC have a colitis that is indistinguishable from that seen in patients of PSC, which, by convention, represents UC (Saich and Chapman, 2008, Loftus et al., 2005). Certain clinical and histopathology aspects of the disease conform to what is already described, however, we have also demonstrated that over a third of patients with AISC have small bowel findings similar to that seen in CD. Thus, placing these colitides under the umbrella of UC may need revision.
In this report, all patients with AISC and PSC had initially been diagnosed with liver disease prior to the diagnosis of colitis. The colonoscopic and histopathologic features of the colitis associated with AISC and PSC conforms to that already described (Saich and Chapman, 2008, Loftus et al., 2005). As colonoscopies were carried out at varying times and prior to this study it is not possible to correlate the functional (calprotectin) and morphological (colonic biopsies) inflammatory activity in these patients. Most studies show a good correlation between levels of faecal calprotectin and intestinal inflammation in patients with UC (Roseth et al., 1999, Schoepfer et al., 2009, Sipponen et al., 2008a) and somewhat more variable, albeit significant, in CD (Sipponen et al., 2008b, D'Incà et al., 2007, Costa et al., 2003, Vieira et al., 2009 Oct 29). The histopathology features in the colitis associated with AISC and PSC are less severe than in classical IBD, yet, these patients have comparable levels of intestinal inflammation as assessed by faecal calprotectin. This apparent discrepancy may be due to the fact that the inflammation was predominantly right sided in AISC and PSC and thus not associated with the common symptoms of proctitis, evident in patients with classic ulcerative colitis, and they may hence have been ‘undertreated’. Furthermore histology represents a static picture whereas faecal calprotectin levels represent the total flux of neutrophils entering the bowel over time. The speed by which neutrophils pass through the colonic mucosa differs from disease to disease depending on the site of the principal neutrophil chemoattractant (Teahon and Bjarnason, 1993), and if particularly rapid may not be so evident on biopsy.
A most significant finding is that of approximately one third of the patients with AISC having mucosal breaks on capsule enteroscopy. A possible confounding factor, however, is that these patients were frequently on immunosuppressive agents that have been implicated in small bowel damage (Gabe et al., 1998, Bjarnason et al., 2004, Bjarnason et al., 2006), although if this was the case then this should have been equally evident in PSC as they were receiving the same drugs.
Capsule enteroscopy images of mucosal breaks (erosions and ulcers) are not pathognomonic for any disease and have numerous causes including use of NSAIDs, cocaine, certain chemotherapeutic agents and are also described in patients with HIV, CD, Behçet's syndrome, spondyloarthropathy and radiation enteropathy (Bjarnason et al., 2006, Oette et al., 2009). Most of these can be and were excluded as a cause for the small bowel findings in AISC. Mucosal breaks are not seen in small bowel bacterial overgrowth, but bacterial overgrowth and or immunosuppressant drugs could be responsible for the mild small intestinal inflammatory changes seen in some patients with AISC and PSC.
The combination of colitis and small bowel mucosal breaks in the patients with AISC might represent CD. However, it is unusual to see histopathological changes consistent with UC in Crohn's disease of the small bowel. There is a case report of 4 patients with well established pan-ulcerative colitis that had problematic diffuse upper small bowel inflammation (Valdez et al., 2000) but this inflammation seems to be somewhat different in location to the damage we observed at capsule enterosocpy and it is otherwise firmly accepted that patients with ulcerative have the disease confined to the large bowel. Small bowel CD frequently progresses to structuring requiring surgery, something which is not in evidence clinically (Gregorio et al., 2001) nor as evidenced by the capsule enteroscopy results in AISC.
Further points of distinction between the colitis of IBD and PSC (data on AISC is lacking) are the differences in the prevalence of pouchitis after restorative ileo-anal (rectal) pouch anastomosis after colectomy, being 2–3 times higher in PSC than UC (Pavlides et al., 2014) and quite comparable if not higher than that in CD (Achkar and Shen, 2001). Lastly genome wide association studies have identified a number of susceptibility genes in IBD and PSC, but most of these differ significantly between the two groups of patients (Eaton et al., 2013).
In conclusion, collectively our findings and previous studies suggest that the colitis associated with PSC, and AISC is distinctively different from classical IBD. Furthermore, the small bowel mucosal breaks in AISC are incompatible with a diagnosis of UC. These findings reinforce the idea that the inflammatory bowel disease associated with AISC and PSC should be considered as distinct nosologic entities separate from UC and CD, namely chronic IBD associated with chronic liver disease.
Funding
None.
Author Contributions
-
1.
Substantial contribution to conception of the work
-
a)
Acquisition of data
-
b)
Analyses of data
-
c)
Interpretation of data
-
2.
Drafting the work or revising it for important intellectual content
-
3.
Final approval of the version to be published
-
4.
Accountable for all aspects of the work
Ingvar Bjarnason: 1 a,b,c, 2, 3 and 4.
Bu Hayee: 1 b,c, 2, 3 and 4.
Polychronis Pavlidis: 1 b,c, 2, 3 and 4.
Charlott Kvasnovsky: 1 b, c, 2, 3 and 4.
Astrid Scalori: 1 a,b,c, 2, 3 and 4.
Guy Sisson: 1 b,c, 2, 3 and 4.
Annika Charlesworth: 1 a,b,c, 2, 3 and 4.
Hizbullah Shaikh: 1 b,c, 2, 3 and 4.
Einar Bjornsson: 1 b,c, 2, 3 and 4.
Michael A Heneghan: 1 b,c, 2, 3 and 4.
References
- Bjarnason I., Macpherson A.J.M., Hollander D. Intestinal permeability: an overview. gastroenterology. 1995;108:1566–1581. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- Saich R., Chapman R. Primary sclerosing cholangitis, autoimmune hepatitis and overlap syndromes in inflammatory bowel disease. World J. Gastroenterol. 2008;14:331–337. doi: 10.3748/wjg.14.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrumpf E., Elgjo K., Fausa O., Gjone E., Kolmannskog F., Ritland S. Sclerosing cholangitis in ulcerative colitis. Scand. J. Gastroenterol. 1980;15:689–697. doi: 10.3109/00365528009181516. [DOI] [PubMed] [Google Scholar]
- Olsson R., Danielsson A., Järnerot G. Prevalence of primary sclerosing cholangitis in patients with ulcerative colitis. Gastroenterology. 1991;100:1319–1323. [PubMed] [Google Scholar]
- Loftus E.V., Jr., Harewood G.C., Loftus C.G. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91–96. doi: 10.1136/gut.2004.046615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty K., Rybicki L., Brzezinski A., Carey W.D., Lashner B.A. The risk for cancer or dysplasia in ulcerative colitis patients with primary sclerosing cholangitis. Am. J. Gastroenterol. 1999;94:1643–1649. doi: 10.1111/j.1572-0241.1999.01156.x. [DOI] [PubMed] [Google Scholar]
- Soetikno R.M., Lin O.S., Heidenreich P.A., Young H.S., Blackstone M.O. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest. Endosc. 2002;56:48–54. doi: 10.1067/mge.2002.125367. [DOI] [PubMed] [Google Scholar]
- Lundqvist K., Broomé U. Differences in colonic disease activity in patients with ulcerative colitis with and without primary sclerosing cholangitis: a case control study. Dis. Colon Rectum. 1997;40:451–456. doi: 10.1007/BF02258391. [DOI] [PubMed] [Google Scholar]
- Vogel A., Wedemeyer H., Manns M.P., Strassburg C.P. Autoimmune hepatitis and overlap syndromes. J. Gastroenterol. Hepatol. 2002;17(Suppl. 3):S389–S398. doi: 10.1046/j.1440-1746.17.s3.33.x. [DOI] [PubMed] [Google Scholar]
- Floreani A., Rizzotto E.R., Ferrara F. Clinical course and outcome of autoimmune hepatitis/primary sclerosing cholangitis overlap syndrome. Am. J. Gastroenterol. 2005;100:1516–1522. doi: 10.1111/j.1572-0241.2005.41841.x. [DOI] [PubMed] [Google Scholar]
- Gregorio G.V., Portmann B., Karani J. Autoimmune hepatitis/sclerosing cholangitis overlap syndrome in childhood: a 16-year prospective study. Hepatology. 2001;33:544–553. doi: 10.1053/jhep.2001.22131. [DOI] [PubMed] [Google Scholar]
- Bogdanos D.P., Invernizzi P., Mackay I.R., Diego V.D. Autoimmune liver serology: current diagnostic and clinical challenges. World J. Gastroenterol. 2008;14:3374–3387. doi: 10.3748/wjg.14.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truelove S.C., Witts L.J. Cortisone in ulcerative colitis. Br. Med. J. 1955;2:1041–1048. doi: 10.1136/bmj.2.4947.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R.F., Bradshaw J.M. A simple index of Crohn's disease activity. Lancet. 1980;1:514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- Maiden L., Thjodleifsson B., Theodors A., Gonzalez J., Bjarnason I. A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology. 2005;128:1172–1178. doi: 10.1053/j.gastro.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Maiden L., Thjodleifsson B., Seigal A. Long-term effects of nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 selective agents on the small bowel: a cross-sectional capsule enteroscopy study. Clin. Gastroenterol. Hepatol. 2007;5:1040–1045. doi: 10.1016/j.cgh.2007.04.031. [DOI] [PubMed] [Google Scholar]
- Goldstein J.L., Eisen G.M., Lewis B., Gralnek I.M., Zlotnick S., Fort J.G. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin. Gastroenterol. Hepatol. 2005;3:133–141. doi: 10.1016/s1542-3565(04)00619-6. [DOI] [PubMed] [Google Scholar]
- Goldstein J.L., Eisen G.M., Lewis B. Small bowel mucosal injury is reduced in healthy subjects treated with celecoxib compared with ibuprofen plus omeprazole, as assessed by video capsule endoscopy. Aliment. Pharmacol. Ther. 2007;25:1211–1222. doi: 10.1111/j.1365-2036.2007.03312.x. [DOI] [PubMed] [Google Scholar]
- Roseth A.G., Schmidt P.N., Fagerhol M.K. Correlation between faecal excretion of indium-111-labelled granulocytes and calprotectin, a granulocyte marker protein, in patients with inflammatory bowel disease. Scand. J. Gastroenterol. 1999;34:50–54. doi: 10.1080/00365529950172835. [DOI] [PubMed] [Google Scholar]
- Schoepfer A.M., Beglinger C., Straumann A., Trummler M., Renzulli P., Seibold F. Ulcerative colitis: correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm. Bowel Dis. 2009;15:1851–1858. doi: 10.1002/ibd.20986. [DOI] [PubMed] [Google Scholar]
- Sipponen T., Kärkkäinen P., Savilahti E. Correlation of faecal calprotectin and Lactoferrin with an endoscopic score for Crohn's disease and histological findings. Aliment. Pharmacol. Ther. 2008;28:1221–1229. doi: 10.1111/j.1365-2036.2008.03835.x. [DOI] [PubMed] [Google Scholar]
- Sipponen T., Savilahti E., Kolho K.L., Nuutinen H., Turunen U., Färkkilä M. Crohn's disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn's disease activity index and endoscopic findings. Inflamm. Bowel Dis. 2008;14:40–46. doi: 10.1002/ibd.20312. [DOI] [PubMed] [Google Scholar]
- D'Incà R., Dal Pont E., Di Leo V. Calprotectin and lactoferrin in the assessment of intestinal inflammation and organic disease. Int. J. Color. Dis. 2007;22:429–437. doi: 10.1007/s00384-006-0159-9. [DOI] [PubMed] [Google Scholar]
- Costa F., Mumolo M.G., Bellini M. Role of faecal calprotectin as non-invasive marker of intestinal inflammation. Dig. Liver Dis. 2003;35:642–647. doi: 10.1016/s1590-8658(03)00381-5. [DOI] [PubMed] [Google Scholar]
- Vieira A., Fang C.B., Rolim E.G. Inflammatory bowel disease activity assessed by fecal calprotectin and lactoferrin: correlation with laboratory parameters, clinical, endoscopic and histological indexes. BMC Res. Notes. 2009 Oct 29;2:221. doi: 10.1186/1756-0500-2-221. (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teahon K., Bjarnason I. Comparison of leukocyte excretion and blood loss in inflammatory disease of the bowel. gut. 1993;34:1535–1538. doi: 10.1136/gut.34.11.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabe S., Bjarnason I., Redger J.M. The effects of tacrolimus on mitochondrial and intestinal barrier function. Gastroenterology. 1998;115:67–74. doi: 10.1016/s0016-5085(98)70366-x. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Takeuchi K., Bjarnason A., Adler S.N., Teahon K. The G.U.T. of gut. Scand. J. Gastroenterol. 2004;39:807–815. doi: 10.1080/00365520410003326. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Adler S.N., Maiden L. Physical-chemical small bowel injury. In: Keuchil M., Hagenmuller F., Fleischer D.E., editors. Atlas of video capsule endoscopy. Springer Medizin Verlag; Heidelberg: 2006. pp. 114–148. [Google Scholar]
- Oette M., Stelzer A., Göbels K. Wireless capsule endoscopy for the detection of small bowel diseases in HIV-1-infected patients. Eur. J. Med. Res. 2009;14:191–194. doi: 10.1186/2047-783X-14-5-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez R., Appelman H.D., Bronner M.P., Greenson J.K. Diffuse duodenitis associated with ulcerative colitis. Am. J. Surg. Pathol. 2000;24:1407–1413. doi: 10.1097/00000478-200010000-00011. [DOI] [PubMed] [Google Scholar]
- Pavlides M., Cleland J., Rahman M. Outcomes after ileal pouch anal anastomosis in patients with primary sclerosing cholangitis. J. Crohns Colitis. 2014;8:662–670. doi: 10.1016/j.crohns.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Achkar J.P., Shen B. Medical management of postoperative complications of inflammatory bowel disease: pouchitis and Crohn's disease recurrence. Curr. Gastroenterol. Rep. 2001;3:484–490. doi: 10.1007/s11894-001-0069-5. [DOI] [PubMed] [Google Scholar]
- Eaton J.E., Talwalkar J.A., Lazaridis K.N., Gores G.J., Lindor K.D. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology. 2013;145:521–536. doi: 10.1053/j.gastro.2013.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]