Abstract
Background
Cognitive difficulties are the most common neurological complications in neurofibromatosis type 1 (NF1) patients. Recent animal models proposed increased GABA-mediated inhibition as one underlying mechanism directly affecting the induction of long-term potentiation (LTP) and learning. In most adult NF1 patients, apparent cognitive and attentional deficits, tumors affecting the nervous system and other confounding factors for neuroscientific studies are difficult to control for. Here we used a highly specific group of adult NF1 patients without cognitive or nervous system impairments. Such selected NF1 patients allowed us to address the following open questions: Is the learning process of acquiring a challenging motor skill impaired in NF1 patients? And is such an impairment in relation to differences in intracortical inhibition?
Methods
We used an established non-invasive, double-pulse transcranial magnetic stimulation (dp-TMS) paradigm to assess practice-related modulation of intracortical inhibition, possibly mediated by gamma-minobutyric acid (GABA)ergic-neurotransmission. This was done during an extended learning paradigm in a group of NF1 patients without any neuropsychological deficits, functioning normally in daily life and compared them to healthy age-matched controls.
Findings
NF1 patients experienced substantial decline in motor skill acquisition (F = 9.2, p = 0.008) over five-consecutives training days mediated through a selective reduction in the early acquisition (online) and the consolidation (offline) phase. Furthermore, there was a consistent decrease in task-related intracortical inhibition as a function of the magnitude of learning (T = 2.8, p = 0.014), especially evident after the early acquisition phase.
Interpretations
Collectively, the present results provide evidence that learning of a motor skill is impaired even in clinically intact NF1 patients based, at least partially, on a GABAergic-cortical dysfunctioning as suggested in previous animal work.
Keywords: TMS, NF1, Motor Learning, SICI
Highlights
-
•
Learning of a fine motor skill is altered even in normal intelligent NF1-individuals well integrated in daily professional and social life.
-
•
The decline in motor learning is mediated by a reduction in fast-online and offline learning.
-
•
Decline in learning was associated with an impairment of the modulation of inhibitory intracortical neurotransmission
1. Introduction
Alterations in the balance of excitatory-inhibitory neurotransmission might underlie the cognitive and learning deficits found in several neurodevelopmental conditions (Ramamoorthi and Lin, 2011). Neurofibromatosis type 1 (NF1) is a common single gene disease affecting the human nervous system, inherited in autosomal dominant manner (Friedman and Birch, 1997). Besides cutaneous and musculoskeletal manifestations, cognitive problems resulting in learning disabilities are the most challenging complication, impacting the quality of life of the affected individuals (Krab et al., 2008). In addition, NF1 patients exhibit motor skill impairments. Johnson and colleagues investigated motor proficiency in NF1 children (n = 26, age = 4–15 years) using the Bruininks-Oseretsky Test (BOT 2) instrument. Patients presented significant impairments in a composite score including fine manual control, manual coordination, body coordination, strength and agility (Johnson et al., 2010). In a complementary study, Feldmann and colleagues showed in their cohort, which also covered adult NF1 patients (n = 100, age = 6–37 years), impaired fine motor skills. Furthermore, patients with focal areas of high signal intensity on T2-weighted MRI scored worse in cognitive and fine motor performance (Feldmann et al., 2003).
Until now, little is known whether NF1-adults without cognitive and attention deficits experience difficulties in learning abilities, such as acquiring a new skill. The interest of this study was to detect possible deficits, which usually go under the radar of standard assessments. Whereas, in most adult NF1-patients cognitive and attentional deficits, as well as tumors might be contributing confounding factors.
NF1 occurs by mutation of the Nf1-gene that encodes the Neurofibromin-protein, a negative regulator of the RAS signaling cascade. Animal studies have revealed that the Neurofibromin-protein modulates gamma-Aminobutyric acid(GABA)ergic neurotransmission leading to enhanced inhibitory activity directly affecting the induction of long-term potentiation (LTP) and learning (Costa et al., 2002). Recent studies using magnetic resonance spectroscopy measured the levels of GABA and glutamate + glutamine in the medial frontal cortex and the occipital cortex in NF1 patients. The GABA levels in patients were reduced in the medial frontal and occipital cortex when compared to controls. The glutamate + glutamine levels were normal, pointing to an abnormal inhibition/excitation balance in NF1. The medial frontal GABA levels correlates with intellectual abilities and inhibitory control. Interestingly, NF1 patients presented a reversed pattern, with higher GABA being associated with faster responses (Ribeiro et al., 2015). In this context, recent evidence supports the view that modulation of tonic GABA is essential for LTP-like plastic changes e.g., within the motor cortex (M1), (Stagg et al., 2011, Floyer-Lea et al., 2006) and further pharmacological studies demonstrated that GABA-agonist medication might suppress M1 plasticity and learning in healthy individuals (Butefisch et al., 2000).
In the present study, we investigated a well-defined and selected group of NF1 patients fully active in daily life, with normal intelligence and without motor or cognitive impairments carrying the NF1 mutation and compared them to age-matched controls. Participants were investigated over an extended course while learning a novel and challenging motor skill. In addition, by applying a well-established double-pulse transcranial magnetic stimulation (dp-TMS) protocol, intracortical (GABAergic) inhibition in the contralateral M1 was non-invasively assessed (Ziemann et al., 1996, Mainberger et al., 2013), during resting and movement-related states, to determine underlying pathophysiological mechanisms (Heise et al., 2013, Hummel et al., 2009, Liuzzi et al., 2014). We hypothesized, that NF1 patients show an impairment in motor skill acquisition, and that these deficits will be paralleled by impaired modulation of inhibitory neurotransmission in the M1.
2. Methods
2.1. Subjects
NF1 patients (aged 35·8 ± 11·0SD, range 25–58 years, 5 female) were carefully selected from a database of 1·200 NF1 patients. Out of the database, a selection was made according to local eligibility (metropolitan area of Hamburg, Germany). Based on this procedure, approximately 200 patients were determined. 35 patients were then identified according to the inclusion and exclusion criteria, of which 9 agreed to be enrolled in the study. All patients were genetically tested; in seven out of the 9 patients the diagnosis was genetically confirmed (see supplementary Table 1). Additionally, nine healthy subjects (aged 30·11 ± 13·2SD, range 24–65 years, 6 female) were included as a control group. Participants were assessed to be right handed by the Edinburgh handedness inventory (Oldfield, 1971), none of them had reported any history of serious neurological or psychiatric diseases or any contraindications for TMS, as probed by standardized questionnaire (Rossi et al., 2009). NF1 patients were included based on the following criteria: (1) absence of any cognitive impairments determined by a detailed neuropsychological testing including the mini–mental state examination (MMSE), and the German version of Wechsler adult intelligence scale (WAIS-III) (see Table 1). The patients showed no abnormalities in the test of variables of attention (TOVA), the Wender Utah rating scale (WURS-k), the ADHD self rating scale (ADHS-SB), and the hospital anxiety and depression scale (HADS) (see Table 1), (2) absence of visual impairment or any musculoskeletal dysfunction compromising normal finger movements, (3) normal neurological examination and normal clinical MRI, and (4) fulfilling the NIH clinical diagnostic criteria for NF1 (Gutmann et al., 1997). The 5–15 [FTF] was performed in the NF1 patients. The FTF is an established, free and validated questionnaire, covering development and behavior of children in ages 5 to 15 years (Korkman et al., 2004, Trillingsgaard et al., 2004). In regards to our purpose; we used only the motor skill development part (points 1–17) of the questionnaire. Furthermore, none of the participants took any CNS active medication during the course of the study. Besides age, healthy controls were also matched for the educational level. Participants were naïve to the experimental purpose and none of them were professional piano players or trained typists. Local institutional ethics committee approved the study and participants gave their written informed consent according to the ethical declaration of Helsinki (http://www.wma.net/en/30publications).
Table 1.
Characteristics of NF1 patients. M = male; F = female; MMSE = mini–mental state examination; IQ = intelligence quotient (mean = 100, SD = 15), measured with the German version of Wechsler Adult Intelligence Scale (WAIS-III). TOVA = Test of Variables of Attention, RTV = response time variability, RT = response time (mean = 100, SD = 15); WURS-k = Wender Utah rating scale, short-version for the assessment of the attention-deficit hyperactivity disorder in childhood (cut-off ≥ 30); ADHS-SB = ADHD Self Rating Scale (cut-off ≥ 15); HADS = Hospital Anxiety and Depression Scale (cut-off ≥ 8).
| NF1 Patients | Age | Gender | Profession | MMSE | Full IQ | Verbal IQ | Performance IQ | TOVA RTV/RT |
WURS-k | HADS-D anxiety |
HADS-D depression |
ADHS-SB |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NF1-01 | 36 | F | Teacher | 30/30 | 108 | 108 | 108 | 109/115 | 4 | 6 | 1 | 10 |
| NF1-02 | 32 | M | Architect | 30/30 | 132 | 138 | 118 | 113/119 | 4 | 2 | 4 | 7 |
| NF1-03 | 40 | F | Baker instructor | 30/30 | 95 | 92 | 101 | 118/126 | 13 | 4 | 2 | 8 |
| NF1-04 | 20 | M | Student | 30/30 | 113 | 120 | 102 | 93/119 | 7 | 0 | 4 | 8 |
| NF1-05 | 25 | F | Teacher | Missinga | 102 | 102 | 104 | 112/131 | 2 | 6 | 2 | 4 |
| NF1-06 | 44 | F | Nurse | 30/30 | 127 | 121 | 127 | 119/123 | 2 | 6 | 2 | 6 |
| NF1-07 | 58 | M | Fireman | 30/30 | 111 | 112 | 108 | 124/157 | 6 | 3 | 2 | 7 |
| NF1-08 | 35 | F | Tax-consultant | 30/30 | 92 | 91 | 93 | 102/112 | 3 | 7 | 7 | 6 |
| NF1-09 | 33 | M | Graphic designer | 30/30 | 92 | 96 | 89 | 107/115 | 21 | 6 | 3 | 13 |
| Mean +/− SD | 35,8 +/− 11 | 30/30 |
All participants presented normal or above normal IQ. None of the NF1 participants exhibit attention deficits, depression or anxiety.
One patient refused to do the MMSE.
2.2. Motor Task and Study Design
Skill learning was tested using an adapted version of the sequential finger-tapping task (Zimerman et al., 2013). Participants had to repeatedly tap the explicitly provided sequence on a four-button electronic keyboard with their non-dominant hand. They were instructed to tap as precisely and quickly as possible, according to the written instruction. During the study, subjects were comfortably seated in front of a 20-in. screen; all sessions were performed at the same time of the day in each participant. Before training, participants were first familiarized with the task and then performed a warm-up block. The training period consisted of five sessions divided in 5 consecutive days (20 min each). Motor performance was re-tested after 20 days of the initial training (long-term retention). A personal computer with Presentation (Neurobehavioural System, Albany, USA) was used to present the instructions, to display the numeric sequence at all time points and to record the total amount of keys pressed.
Each training session composed of seven 90s blocks with 90s break in-between. During each block, an instructive nine-element sequence in a non-consecutive, non-repetitive order (i.e. 2–3–5–4–2–5–4–3–4, numbers 2 to 5 indicating index-to-small finger) appeared on the screen to eliminate working memory components (Censor et al., 2010). An advancing cue, indicated the actual position within the sequence without any other feedback (e.g. errors), was displayed under each number. Aside from the training sequence (t-seq), an additional five random sequences (r-seq1-5) of the same length, number of repetition and complexity, assessed by Kolmogorov-complexity-index (Value = 1·41) (Lempel and Ziv, 1976), were pseudo-randomly assigned to each training day to avoid subjects' anticipation and served as a measure of general motor performance. Skill learning was assessed by the number of correctly played t-seq normalized to the average number of the correctly played r-seq1-5. The rationale behind the present design with r-seq1-5 intermingled was to individually characterize motor performance levels as a potential confounder independent of the trained sequence (Spencer et al., 2007). This procedure is especially relevant, as an impairment of fine motor skills in NF1 patients has been suggested (Johnson et al., 2010).
Remarkably, the present design allowed us to assess the temporal components of skill acquisition as follows: online gains were calculated between the last and the first block of each session; and offline effects were assessed by contrasting the first block of the following and the last block of the previous session (Robertson et al., 2005, Reis et al., 2009). Furthermore, long-term retention defined as a re-evaluation of the t-seq after 20 days, and savings defined as the delta between the first block of the retention session and the initial training (Krakauer, 2009) were studied in a subset of eight NF1 patients. Participants reported their hours of sleep, the quality of sleep by using a visual analog scale questionnaire (VAS) and completed the Stanford sleepiness scale (Hoddes et al., 1973). During every training block the level of attention towards the task, perception of fatigue and the level of discomfort, tiredness of the practicing hand were characterized.
2.3. Intracortical Inhibition (GABA-mediated) Determined by dp-TMS
Training-related changes in corticospinal excitability and short interval intracortical inhibition during rest (SICIrest) as well as task-related (SICImov), were evaluated in the contralateral M1 with well-established single and dp-TMS protocols (Heise et al., 2013, Kujirai et al., 1993a). SICI is a complex phenomenon representing the balance between inhibition and facilitation (Berardelli et al., 2008). The current understanding is, that the conditioning pulse activates short-lasting IPSPs in corticospinal neurons via stimulation of a low-threshold cortical inhibitory circuit. This inhibits action potential generation in these neurons by the suprathreshold second pulse (Hanajima et al., 1998) (Kujirai et al., 1993b) (for review see e.g., (Ziemann, 2013)). This hypothesis was further substantiated by combined TMS drug studies applying e.g., benzodiazepines as modulators of the GABAA-receptors, leading to an enhancement of SICI, as suggested in several studies (Florian et al., 2008). The current evidence, although indirectly determined by TMS drug studies, supports strongly the view that SICI is to a relevant part directed by GABA-related mechanisms in the motor cortex (for a detailed discussion, please see e.g., (Ziemann, 2013)). Based on this, the measurement of SICI provides an opportunity of evaluating the GABA system in the motor cortex noninvasively and in-vivo.
Two Magstim 2002 magnetic stimulators connected via a Bistim module (Magstim Company, Whitland, Dyfed, UK) have been used to deliver the conditioning and test stimuli through one figure of eight coil with a 70 mm wing diameter. At each time point the following adjustments were performed: the coil was placed over the representation of the hand motor area of the right M1 (opposite to the training hand) in an orientation inducing a posterior-anterior current perpendicular to the central sulcus at 45° angle from the midline. Optimal scalp position to elicit consistently the largest MEPs in the first dorsal interosseus muscle with slight suprathreshold stimulator intensity was considered the motor hot-spot. Resting motor threshold (RMT) was defined as the intensity of stimulator output to produce MEPs amplitudes of at least 50 μV peak-to-peak in 5 out of 10 consecutive trials (Rossini et al., 1999). In line with previous studies, we used an interstimulus interval of 3 ms to evaluate SICI (Heise et al., 2013). Conditioning stimulus (CS) was set at 80% of RMT and test stimulus (TS) was adjusted to elicit unconditioned MEP amplitudes of 1 mV peak-to-peak (Ziemann et al., 1996). EMG activity was recorded using disposable Ag/AgCl surface electrodes placed over the left FDI muscle in a belly-tendon montage. EMG signals were amplified (CED-1902 amplifier) then bandpass filtered (50 Hz to 1 kHz), digitized and stored offsite. Data acquisition and processing was performed using Signal software 4.05 (Cambridge Electronic Design, Cambridge, UK).
For evaluation of SICIrest, a total of 32 trials (16 single and 16 dp-TMS) were collected during rest at each evaluation day. During evaluation of SICImov, participants were asked to perform a simple reaction time (RT) task as described previously (Heise et al., 2013, Hummel et al., 2009, Liuzzi et al., 2014). Single and dp-TMS were implemented at the early (~ 20%) and the late (~ 90%) RT phase. A total of 36 trials (18 single and 18 dp-TMS) were tested at each time point. MEP amplitudes were measured peak-to-peak and SICI was normalized to the corresponding unconditioned MEP at either SICIrest or during SICImov after exclusion of trials with pre-activation (Heise et al., 2013, Hummel et al., 2009, Liuzzi et al., 2014). One NF1-patient declined to perform the TMS measurements, thus eight NF1 patients and eight healthy controls entered the analyses. The sessions were distributed as follows: before training (baseline), immediately after day-1, after day-3 and after day-5.
2.4. Statistics
Normality of distribution was confirmed for all variables through Kolmogorov–Smirnov tests (see supplementary Table 2). Repeated-measure analyses of variance (ANOVARM) were performed for behavioral and SICI analysis and the Greenhousee-Geisser correction were used to correct for non-sphericity. Unpaired t-test (two-tailed) for comparisons between groups and Scheffé post-hoc test was used to control for multiple comparisons. Pearson's correlation analysis was performed for testing behavior-physiology relationships. All statistical analyses were conducted with SPSS 21.0 (SPSS, Chicago, IL). The level of significance was set at p < 0·05.
3. Results
3.1. Behavioral Results
Analysis of r-seq1-5 demonstrated similar motor performance between GROUPNF1-healthy (F[1·16] = 2·2, p = 0·187) for the number of correct sequences. Furthermore, r-seq1-5 remained stable over the training DAYS1-5 (F[4·64] = 1·6, p = 0·196), indicating that there were no unspecific general effects influencing performance over time. The stable performance level allowed normalizing the analyses of the acquired skill to the individual performance level for a direct comparison by factorizing differences in skill level among groups.
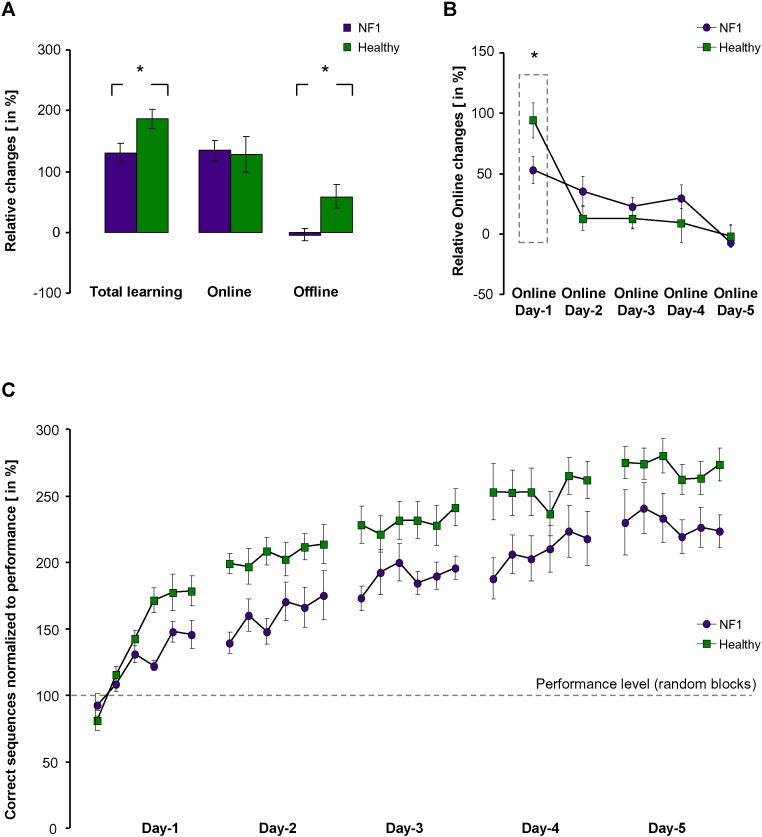
The analyses of the acquired skill during the five training days revealed that both NF1 and controls were able to improve across the training BLOCKS1-30 (F[29·464] = 25·4, p < 0·001) with a significant GROUPNF1-healthy effect (F[1·16] = 9·2, p = 0·008, Fig. 1C). Furthermore, a significant interaction between factors BLOCKS1-30 and GROUPNF1-healthy (F[29·464] = 3·9, p = 0·017) was evident. Dissecting the skill acquisition process in its temporal components revealed a reduction in the offline processes in NF1 (offlineNF1 = − 4·2% ± 10·8%, offlinehealthy = 59·9% ± 18·1%; T(Oldfield, 1971)=2·9, p = 0·009), without a significant difference in total online improvements (onlineNF1 = 134·7% ± 16·7%, onlinehealthy = 132·5% ± 30·4%; T(Oldfield, 1971)=0·6, p = ns). Remarkably, the present impairment in offline impairment in NF1, was stable across the training DAYS1-5 (F[4·48] = 0·5, p = 0·595). The analysis of online improvements revealed a GROUPNF1-healthy by DAYS1-5 interaction (F[4·64] = 3·1, p = 0·042) and Posthoc-Scheffé testing confirmed a significant difference between NF1 and controls at day-1 (fast-online learning, Fig. 1B), contributing to explain 74·8% of the total online-improvement in healthy and 40·1% in NF1 patients. It is of note that there were no group differences between NF1 and controls for factors that might influence skill acquisition and motor performance such as hours and quality of sleep and recovery (see supplementary Table 3). Attention, fatigue, and hand fatigue were assessed by visual analog scale questionnaires (VAS, 0–10) before and after each training block. Scales included the following: attention with 0 = highest level of attention, 10 = no attention; fatigue with 0 = no fatigue, 10 = highest level of fatigue and hand fatigue with 0 = no hand fatigue, 10 = highest level of hand fatigue. This data was analyzed by ANOVArm. Factor GROUPS (NF1 vs. Healthy), did not reveal any significant differences (attention: F = 0.08, p = 0.78, fatigue: F = 0.36, p = 0.56, hand fatigue: F = 3.28, p = 0.09). The trend towards hand fatigue was driven by increased hand fatigue in healthy controls. Taken together unspecific factors such as attention, fatigues and hand fatigue did not show significant differences between both groups and can therefore not explain the behavioral and electrophysiological differences between NF1 and controls.
Fig. 1.
Behavioral results. (A) Sum of the temporal components of skill acquisition. Online (within-session) and offline (between-days) effects and total learning (online + offline) in NF1 patients (purple bars) and in healthy (green bars) participants are shown. Note the significantly greater magnitude of total learning in the healthy control group predominantly driven by greater offline effects. (B) Online effects across training days revealed a reduction in the early acquisition phase (fast-online learning) in NF1 patients. (C) Summary of the motor skill acquisition during the whole training period (5 days). Data show mean ± SEM, * indicates p < 0.05.
The FTF revealed in four patients moderate peculiarities, such as difficulties in ball sports or in tying shoelaces (Table 2).
Table 2.
Questionnaire performed orally from memory for early childhood regarding abilities in comparison to children of the same age. Patients were asked if the statements: did not apply (1); did apply sometimes, to some extend (2); did apply (3); or missing memory (x). In 1 subjects we have missing data (−). Additionally the subjects were asked if they received occupational (A) or physical (B) therapy that was not related to rehabilitation of musculoskeletal problems in context of their NF1.
| NF1-01 | NF1-02 | NF1-03 | NF1-04 | NF1-05 | NF1-06 | NF1-07 | NF1-08 | NF1-09 | |
|---|---|---|---|---|---|---|---|---|---|
| Gross motor skills | |||||||||
| Difficulty acquiring motor skills, e.g.to skate, swim, cycle | 1 | 2 | 2 | 3 | 1 | 1 | 1 | - | 2 |
| Difficulty throwing and catching a ball | 1 | 2 | 1 | 2 | 1 | 3 | 1 | - | 2 |
| Difficulty running fast and smoothly | 2 | 2 | 1 | 2 | 1 | 2 | 1 | - | 1 |
| Difficulties didn't like game sports e.g. soccer, hockey | 3 | 3 | x | 1 | x | 2 | 1 | - | 1 |
| Balance problems; for instance standing on one leg | 3 | 2 | 1 | 2 | 1 | 2 | 1 | - | 2 |
| Often stumbled and fell | 1 | 1 | 1 | 2 | 1 | 1 | 1 | - | 2 |
| Clumsy or awkward movements | 2 | 1 | 1 | 2 | 1 | 1 | 1 | - | 1 |
| Fine motor skills | |||||||||
| Did not like to draw or paint | 1 | 1 | 1 | 2 | 2 | 1 | 2 | - | 1 |
| Difficulty handling, assembling, manipulating small objects | 1 | 1 | 1 | 2 | 2 | 1 | 1 | - | 1 |
| Difficulty pouring water into a glass without spilling | 1 | 1 | 1 | 1 | 1 | 1 | 1 | - | 1 |
| Often spilled food onto clothes or table when eating | 1 | 1 | 1 | 1 | 1 | 1 | 1 | - | 1 |
| Difficulty using knife and fork | 1 | 1 | 1 | 1 | 1 | 1 | 1 | - | 1 |
| Difficulty buttoning or tying shoe-laces | 1 | 1 | 1 | 3 | 3 | 1 | 1 | - | 1 |
| Difficulty using a pen (e.g., pressed too hard) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | - | 1 |
| Hadn't developed clear hand preference right away | 1 | 1 | 1 | 1 | 1 | 1 | 1 | - | 2 |
| Writing was slow and laborious | 1 | 1 | 2 | 1 | 2 | 1 | 2 | - | 1 |
| Immature pencil-grip, held the pen in an unusual manner | 1 | 1 | 1 | 1 | 2 | 1 | 1 | - | 1 |
| Therapy | |||||||||
| Occupational(A), Physical(B) | None | None | None | B | None | None | None | - | None |
Long-term retention, revealed a persistent difference in the motor skill between both GROUPNF1-healthy (day-20NF1 = 225·1% ± 24·1%, day-20healthy = 303·2% ± 17·9%; F(Ramamoorthi and Lin, 2011, Hummel et al., 2009)=7·1, p = 0·018) with reduced saving of the present skill in NF1 compared to healthy subjects (savingNF1 = 129·8% ± 27·7%, savinghealthy = 224·2% ± 21·5%; T(Hummel et al., 2009)=2·7, p = 0·016).
3.2. Intracortical Inhibition (GABA-mediated) Determined by dp-TMS
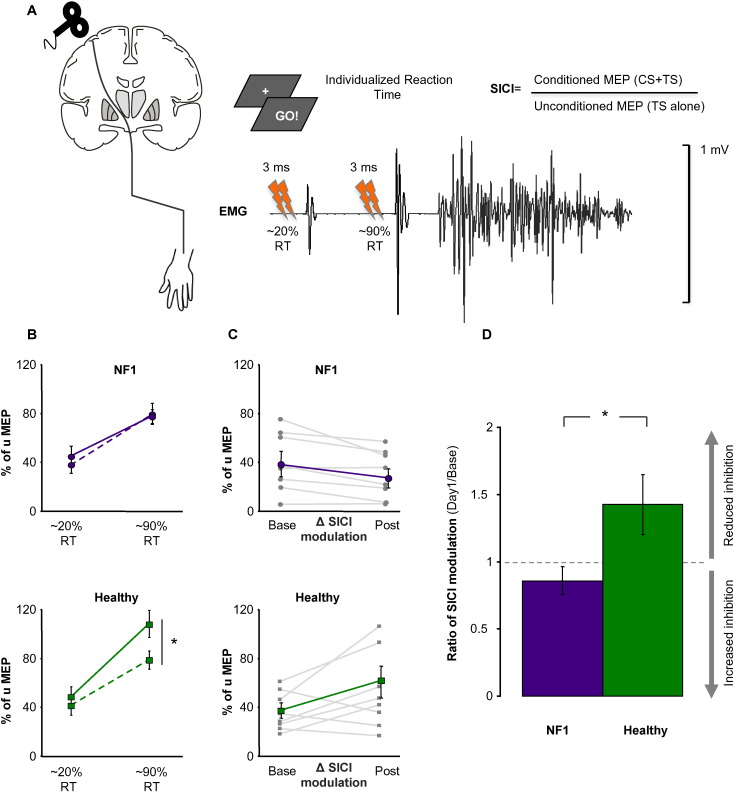
At baseline, SICIrest (T(Hummel et al., 2009)=6·7, p = 0·514) and SICImov modulation (T(Hummel et al., 2009)=0·1, p = 0·879), expressed by the Δ between SICImov at the beginning of movement preparation (at 20% of individual RT) and just before movement onset (at 90% of individual RT, Fig. 2A), were comparable between NF1 and controls. Directly after training, healthy subjects demonstrated an increase in SICImov modulation, that is a stronger release of inhibition in comparison to NF1 (T(Hummel et al., 2009)=2·8, p = 0·014). Strikingly, the present pattern of stronger release of inhibition was maintained across the whole training period in healthy participants (F[1·14] = 5·7, p = 0·031). On the other hand, NF1 did not reveal any changes in SICImov modulation, nor after day-1 (T(Ribeiro et al., 2015)=3·8, p = 0·714), nor after consecutive training days. In general, NF1 exhibited reduced SICImov modulation with persistent inhibition at movement onset compared to healthy participants (Fig. 2B and C). The analysis of SICIrest revealed no group differences during baseline (NF1-SICIrest 30·6% ± 5·1%, healthy-SICIrest 36·1% ± 6·4%; T(Hummel et al., 2009)=0·6, p = 0·514) as well as during the process of training the motor skill, GROUPNF1-healthy (F[1·14] = 2·1, p = 0·181).
Fig. 2.
Intracortical inhibition determined by dp-TMS. (A) Task-related paradigm. Based on individual reaction times (RT) during the preparation of a simple finger abduction movement, determined before the TMS experiment, unconditioned and conditioned dp-TMS pulses were applied randomly at early (~ 20%) or at late (~ 90%) phase of the individual RT. (B) SICImov modulation after day-1 training. There was a training specific modulation of inhibition (towards disinhibition) present in healthy subjects (green solid line) close to the movement onset compared to baseline measurements (dashed line), a pattern that was absent in NF1 patients. (C) Compared to healthy participants, there was a reduction of Δ SICI modulation in NF1 after training (gray line: single subject data; purple line: group average). As a result of that, (D) a significantly greater SICImov modulation (ratio Day1/Base) in healthy participants compared to NF1 patients has been observed, mainly driven by enhanced task-related disinhibition after training in healthy participants. Data show mean ± SEM, * indicates p < 0.05.
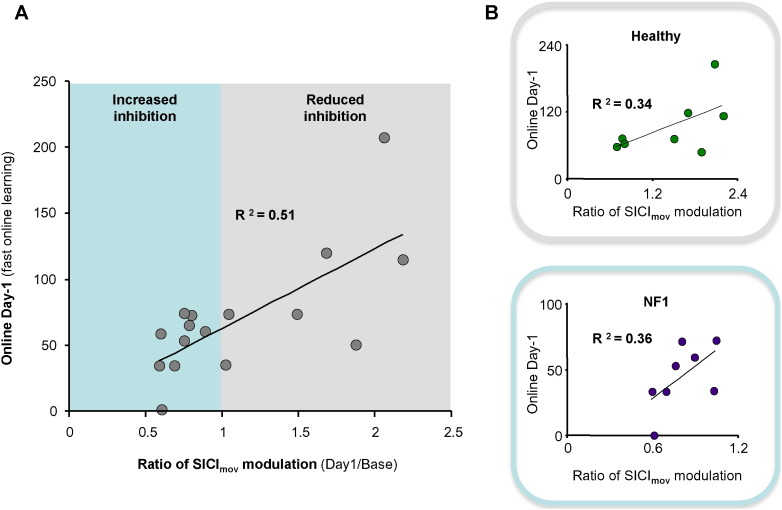
Remarkably, the ratio of SICImov modulation observed due to training on day-1 displayed a significant positive correlation with the behavioral improvement in the fast-online learning phase (R2 = 0·51, p = 0·002, Fig. 3). Thus indicating that the higher the behavioral improvement, the more pronounced was the disinhibition close to the movement onset. Additionally, this level of modulation was further associated with the total amount of learning within the whole training period (R2 = 0·37, p = 0·012). Interestingly, applying such a correlative approach independently for both of the groups revealed that controls mainly showed values for the ratio of SICImov modulation above 1, indicating enhanced modulation towards disinhibition. In contrast, the NF1 mainly showed values below 1, pointing to modulation towards more inhibition.
Fig. 3.
Association between fast online learning and SICImovmodulation. (A) The ratio of SICImov modulation observed after training on day-1 was positively correlated with the early training phase. Indicating that the higher the behavioral improvement, the more pronounced the disinhibition (reduced inhibition) close to the movement onset over the whole group of participants was. (B) Single group correlations revealed the same trend. Interestingly, both groups differed in the range of inhibition levels. NF1 patients mainly showed values below 1 for the ratio of SICImov modulation, indicating a trend for increased inhibition after learning. The healthy controls mainly showed values above 1, indicting an opposite pattern.
4. Discussion
In daily life, both the acquisition and long-term retention of a motor skill play a crucial role for the appropriate implementation of motor acts, such as practicing sports or using modern communication tools. The present study demonstrated a decline in motor skill acquisition in normal intelligent NF1-individuals well integrated in daily professional and social life. Additionally, a persistent impairment of the modulation of inhibitory intracortical neurotransmission as a function of learning has been observed in these patients.
Nf1 mutations lead to an increased risk for memory and attention problems. In addition, NF1-children display impairments in fine motor precision, upper limb coordination and fine motor integration (Johnson et al., 2010). Over 40% of these patients receive occupational therapy at some stage during their childhood to alleviate the developmental delays in motor skill (Krab et al., 2008). Recently, there have been groundbreaking advances in our understanding of the molecular, cellular, and neural systems underpinning NF1-associated cognitive deficits. Hyperactivation of the RAS signaling cascade resulting in increased GABA-mediated activity during periods of high-frequency neural stimulation in the hippocampus (Cui et al., 2008), medial prefrontal cortex and striatum (Shilyansky et al., 2010) have been proposed as the main mechanisms underlying Nf1+/− mice learning impairment. Interestingly, alteration in inhibition/excitation balance has been recently non-invasively demonstrated in the occipital and medial-frontal cortex of NF1 patients at rest, using MR-spectroscopy (Ribeiro et al., 2015, Violante et al., 2013). This suggests a region-specific abnormal GABAergic physiology in NF1 patients. As discussed in the paper of (Violante et al., 2013), MR-spectroscopy provides information about the overall concentration of GABA mainly including the cytosolic, extracellular and vesicular pools. GABA bound to macromolecules, such as to GABA receptors cannot be detected by this technique. dp-TMS allows to address non-invasively aspects of inhibitory (GABA-ergic) neurotransmission, not only in a static, resting state mode, but also during the performance of a task. This event-related approach provides information about fast dynamic changes in inhibitory neurotransmission, which can be associated with behavioral measures. Thus, although difficult to establish a direct parallelism with aforementioned studies and the present study due to technical aspects, the present results add on and extend the findings regarding the role of impaired inhibitory neurotransmission in NF1-patients. This small, but well defined study, adds to the understanding of the neuropathological mechanisms of NF1 by suggesting that not only the static GABA-ergic differences, but especially the impairments in dynamic GABA-ergic neurotransmission might underlie cognitive restrictions, such as demonstrated here within a motor skill acquisition task.
Learning a procedural task is a cognitive process that leads to the acquisition of complex goal-oriented movements with practice. Based on behavioral studies, distinct stages for the process of acquiring a skill were proposed: an early stage, in which considerable fast improvement occurs within a single training session, and a late one, characterized by slow changes in performance that can be observed across several sessions including time- and sleep-dependent consolidation processes (Doyon and Benali, 2005). In this context, M1 has been demonstrated as one key brain structure engaged not only in the fast acquisition but also in consolidation and re-consolidation processes of a motor memory trace (Censor et al., 2010, Muellbacher et al., 2002). Increasing evidence supports the view that reduction in tonic GABA is essential for the induction of LTP-like plastic changes within M1 (Stagg et al., 2011). These processes are most likely based on ‘unmasking’ of existing horizontal connections within the cortex, an essential mechanism that underpins the rapid remodeling of motor representations seen in the early stages of plasticity and learning (Xu et al., 2009). To determine intracortical (GABAergic) inhibitory processes, we used a well-established dp-TMS paradigm (Kujirai et al., 1993a). A method with high spatial and temporal resolution to non-invasively explore changes in cortical excitability, inhibitory, and facilitatory neurotransmisson in the motor cortical system (Ziemann et al., 1996, Heise et al., 2013, Kujirai et al., 1993a). In addition, a recent study proposed dp-TMS as a rapid way of evaluating treatment response to Lovastatin in NF1 patients (Mainberger et al., 2013). For instance, while resting state evaluation is dominated by an inhibitory tone within M1, task-related evaluation shows a task specific modulation of inhibition (towards disinhibition) in healthy subjects associated with higher levels of skill acquisition. In contrast, NF1 patients did not show this pattern. Task-related evaluation of SICI was unaltered with a tendency towards less modulation, which was associated with reduced levels of skill acquisition (Fig. 3B).
The present behavioral findings in NF1 patients were characterized by a decline of skill acquisition compared to controls, driven by two factors: (1) a reduction in fast-online learning, but also (2) by a prominent decrement in offline improvements between training sessions. The manifestation of NF1-related GABAergic dysfunctions in the inhibitory motor cortical system might be one mechanism involved in the present deficits. Even though intracortical inhibition is mainly associated with the early acquisition phase, it is likely the neural processes leading to successful consolidation start to operate during practice and evolve in the time after training. Support to this concept comes from the finding that facilitatory anodal tDCS, an intervention known to decrease GABAergic neurotransmission in the M1 (Stagg et al., 2009), applied concurrently with training, can enhance offline learning in healthy subjects (Reis et al., 2009).
The NF1 patients included in the present study were carefully selected to make sure that all of them were clinically asymptomatic, with a normal IQ and neuropsychological testing, and well integrated in daily life. Even then the patients showed clear deficits compared to healthy controls within the present complex motor skill acquisition paradigm. As the underlying mechanisms of the disease are apparent since birth, one can speculate that they have well adapted to their deficits in skill acquisition on a behavioral level, relevant in daily life. A similar pattern, present in the controlled environment of this study, is a reduced fast-online learning in the patients. While overall online learning over the five training days was not different from controls, indicating a distinct time course of online learning in these patients. Nevertheless, they still showed a reduction in offline learning leading to an overall difference in the magnitude of skill acquisition. This impairment seen in a complex motor task did not prevent the patients from being integrated into normal professional and private lives. Rather, the patients may have had to adapt for these deficits, e.g., through longer periods of practice to acquiring skills, a question that has to be addressed in upcoming studies. Strikingly, some of these patients reported that they might have had slight delays in motoric development and have received physio- or occupational therapy during childhood (Table 2).
A recent study by Omrani et al. (2015) suggests an alternative mechanism of inhibition in a mouse model: a weakening of hyperpolarization-activated cyclic nucleotide-gated (HCN) current might be the cause for increased inhibition in NF1 patients.
The present results are subject to some limitations. Although matched for age and educational level, no detailed neuropsychological examination and scales or motor development questionnaires were acquired for the control group. Offline learning effects are also related to sleep-dependent consolidation processes, as reported within explicit motor sequence learning paradigms (Walker et al., 2002). Despite the fact that there were no differences in sleep questionnaires between both groups, we cannot rule out an influence in alterations of sleep parameters (such as the architecture) in NF1 patients, as suggested in children with NF1 (Licis et al., 2013). It is of note that although there is evidence for cognitive and attentional deficits in NF1 children, the sample of patients who participated in the present study were carefully selected and did not show impairments in cognitive or attentional abilities to avoid potential confounders (e.g. attention deficit disorder or cognitive impairment). Lastly, a factor limiting interpretation of the results might be the small sample size of the study, due to the highly selective nature of the patient group to remove confounding factors.
In conclusion, the present study provides evidence that the acquisition of skills might be impaired even in clinically and neuropsychologically intact NF1 adults. One potential mechanism contributing to the explanaition of the functional impairments of this phenotype is the alteration of intracortical circuits related to GABAergic neurotransmission and learning in the motor cortex.
5. Panel: Research in context
Neurofibromatosis type 1 (NF1) is a neurological disease affecting the human nervous system. Besides cutaneous and musculoskeletal manifestations, cognitive problems resulting in learning disabilities are the most challenging complication. We assessed motor skill acquisition in normal intelligent NF1-individuals and found a decline of acquisition in fast motor learning. Furthermore, an impairment of the modulation of inhibitory intracortical neurotransmission as a function of learning has been observed in these patients.
Author Contributions
Máximo Zimerman: Design of the study. Acquisition, analysis and interpretation of data. Drafting the article.
Maximilian J. Wessel: Design of the study. Acquisition, analysis and interpretation of data. Drafting the article.
Jan. E. Timmermann: Design of the study. Acquisition and interpretation of data. Drafting the article.
Sofia Granström: Design of the study. Acquisition and interpretation of data. Drafting the article.
Christian Gerloff: Interpretation of data. Revising the article critically for important intellectual content.
Victor F. Mautner: Interpretation of data. Revising the article critically for important intellectual content.
Friedhelm C. Hummel: Conception and design of the study. Interpretation of data. Revising the article critically for important intellectual content.
Acknowledgements
This work has been supported by the German Research Foundation (DFG), SFB 936 „Multi-Site Communication in the Brain“, project C4 to F.C.H. and by the Bundesverband Neurofibromatose. The funders did not have any role in study design, data collection, data analysis, interpretation, writing of the report.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2015.08.036.
Appendix A. Supplementary data
Supplementary material
References
- Berardelli A., Abbruzzese G., Chen R. Consensus Paper on Short-Interval Intracortical Inhibition and Other Transcranial Magnetic Stimulation Intracortical Paradigms in Movement Disorders. Brain Stimul. 2008;1(3):183–191. doi: 10.1016/j.brs.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Butefisch C.M., Davis B.C., Wise S.P. Mechanisms of use-Dependent Plasticity in the Human Motor Cortex. Proc. Natl. Acad. Sci. U. S. A. 2000;97(7):3661–3665. doi: 10.1073/pnas.050350297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Censor N., Dimyan M.A., Cohen L.G. Modification of Existing Human Motor Memories is Enabled by Primary Cortical Processing During Memory Reactivation. Curr. Biol. 2010;20(17):1545–1549. doi: 10.1016/j.cub.2010.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa R.M., Federov N.B., Kogan J.H. Mechanism for the Learning Deficits in a Mouse Model of Neurofibromatosis Type 1. Nature. 2002;415(6871):526–530. doi: 10.1038/nature711. [DOI] [PubMed] [Google Scholar]
- Cui Y., Costa R.M., Murphy G.G. Neurofibromin Regulation of ERK Signaling Modulates GABA Release and Learning. Cell. 2008;135(3):549–560. doi: 10.1016/j.cell.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J., Benali H. Reorganization and Plasticity in the Adult Brain During Learning of Motor Skills. Curr. Opin. Neurobiol. 2005;15(2):161–167. doi: 10.1016/j.conb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Feldmann R., Denecke J., Grenzebach M., Schuierer G., Weglage J. Neurofibromatosis Type 1: Motor and Cognitive Function and T2-Weighted MRI Hyperintensities. Neurology. 2003;61(12):1725–1728. doi: 10.1212/01.wnl.0000098881.95854.5f. [DOI] [PubMed] [Google Scholar]
- Florian J., Muller-Dahlhaus M., Liu Y., Ziemann U. Inhibitory Circuits and the Nature of Their Interactions in the Human Motor Cortex a Pharmacological TMS Study. J. Physiol. 2008;586(2):495–514. doi: 10.1113/jphysiol.2007.142059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyer-Lea A., Wylezinska M., Kincses T., Matthews P.M. Rapid Modulation of GABA Concentration in Human Sensorimotor Cortex During Motor Learning. J. Neurophysiol. 2006;95(3):1639–1644. doi: 10.1152/jn.00346.2005. [DOI] [PubMed] [Google Scholar]
- Friedman J.M., Birch P.H. Type 1 Neurofibromatosis: ADescriptive Analysis of the Disorder in 1,728 Patients. Am. J. Med. Genet. 1997;70(2):138–143. doi: 10.1002/(sici)1096-8628(19970516)70:2<138::aid-ajmg7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Gutmann D.H., Aylsworth A., Carey J.C. The Diagnostic Evaluation and Multidisciplinary Management of Neurofibromatosis 1 and Neurofibromatosis 2. JAMA. 1997;278(1):51–57. [PubMed] [Google Scholar]
- Hanajima R., Ugawa Y., Terao Y. Paired-Pulse Magnetic Stimulation of the Human Motor Cortex: Differences Among I Waves. J. Physiol. 1998;509(Pt 2):607–618. doi: 10.1111/j.1469-7793.1998.607bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise K.F., Zimerman M., Hoppe J., Gerloff C., Wegscheider K., Hummel F.C. The Aging Motor System as a Model for Plastic Changes of GABA-Mediated Intracortical Inhibition and Their Behavioral Relevance. J. Neurosci. 2013;33(21):9039–9049. doi: 10.1523/JNEUROSCI.4094-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoddes E., Zarcone V., Smythe H., Phillips R., Dement W.C. Quantification of Sleepiness: A new Approach. Psychophysiology. 1973;10(4):431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Hummel F.C., Steven B., Hoppe J. Deficient Intracortical Inhibition (SICI) During Movement Preparation After Chronic Stroke. Neurology. 2009;72(20):1766–1772. doi: 10.1212/WNL.0b013e3181a609c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B.A., MacWilliams B.A., Carey J.C., Viskochil D.H., D'Astous J.L., Stevenson D.A. Motor Proficiency in Children with Neurofibromatosis Type 1. Pediatr. Phys. Ther. 2010;22(4):344–348. doi: 10.1097/PEP.0b013e3181f9dbc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkman M., Jaakkola M., Ahlroth A., Pesonen A.E., Turunen M.M. Screening of Developmental Disorders in Five-Year-Olds Using the FTF (Five to Fifteen) Questionnaire: AValidation Study. Eur. Child Adolesc. Psychiatry. 2004;13(Suppl 3):31–38. doi: 10.1007/s00787-004-3005-z. [DOI] [PubMed] [Google Scholar]
- Krab L.C., Aarsen F.K., de Goede-Bolder A. Impact of Neurofibromatosis Type 1 on School Performance. J. Child Neurol. 2008;23(9):1002–1010. doi: 10.1177/0883073808316366. [DOI] [PubMed] [Google Scholar]
- Krakauer J.W. Motor Learning and Consolidation: The Case of Visuomotor Rotation. Adv. Exp. Med. Biol. 2009;629:405–421. doi: 10.1007/978-0-387-77064-2_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T., Caramia M.D., Rothwell J.C. Corticocortical Inhibition in Human Motor Cortex. J. Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, et al. Corticocortical Inhibition in Human Motor Cortex. J. Physiol. Lond. 1993b; 471(501): 501–19. [DOI] [PMC free article] [PubMed]
- Lempel A., Ziv J. On the Complexity of Finite Sequences. IEEE Trans. Inf. Theory. 1976;22:75–81. [Google Scholar]
- Licis A.K., Vallorani A., Gao F. Prevalence of Sleep Disturbances in Children With Neurofibromatosis Type 1. J. Child Neurol. 2013;28(11):1400–1405. doi: 10.1177/0883073813500849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzi G., Horniss V., Lechner P. Development of Movement-Related Intracortical Inhibition in Acute to Chronic Subcortical Stroke. Neurology. 2014;82(3):198–205. doi: 10.1212/WNL.0000000000000028. [DOI] [PubMed] [Google Scholar]
- Mainberger F., Jung N.H., Zenker M. Lovastatin Improves Impaired Synaptic Plasticity and Phasic Alertness in Patients with Neurofibromatosis Type 1. BMC Neurol. 2013;13:131. doi: 10.1186/1471-2377-13-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muellbacher W., Ziemann U., Wissel J. Early Consolidation in Human Primary Motor Cortex. Nature. 2002;415(6872):640–644. doi: 10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The Assessment and Analysis of Handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Omrani A., van der Vaart T., Mientjes E. HCN channels are a novel therapeutic target for cognitive dysfunction in Neurofibromatosis type 1. Mol. Psychiatry. 2015 doi: 10.1038/mp.2015.48. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthi K., Lin Y. The Contribution of GABAergic Dysfunction to Neurodevelopmental Disorders. Trends Mol. Med. 2011;17(8):452–462. doi: 10.1016/j.molmed.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J., Schambra H.M., Cohen L.G. Noninvasive Cortical Stimulation Enhances Motor Skill Acquisition over Multiple Days Through an Effect on Consolidation. Proc. Natl. Acad. Sci. U. S. A. 2009;106(5):1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro M.J., Violante I.R., Bernardino I., Edden R.A., Castelo-Branco M. Abnormal Relationship Between GABA, Neurophysiology and Impulsive Behavior in Neurofibromatosis Type 1. Cortex. 2015;64:194–208. doi: 10.1016/j.cortex.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson E.M., Press D.Z., Pascual-Leone A. Off-Line Learning and the Primary Motor Cortex. J. Neurosci. 2005;25(27):6372–6378. doi: 10.1523/JNEUROSCI.1851-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S., Hallett M., Rossini P.M., Pascual-Leone A. Safety, Ethical Considerations, and Application Guidelines for the use of Transcranial Magnetic Stimulation in Clinical Practice and Research. Clin. Neurophysiol. 2009;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini P.M., Berardelli A., Deuschl G. Applications of Magnetic Cortical Stimulation. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999;52:171–185. [PubMed] [Google Scholar]
- Shilyansky C., Karlsgodt K.H., Cummings D.M. Neurofibromin Regulates Corticostriatal Inhibitory Networks During Working Memory Performance. Proc. Natl. Acad. Sci. U. S. A. 2010;107(29):13141–13146. doi: 10.1073/pnas.1004829107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer R.M., Gouw A.M., Ivry R.B. Age-Related Decline of Sleep-Dependent Consolidation. Learn. Mem. 2007;14(7):480–484. doi: 10.1101/lm.569407. [DOI] [PubMed] [Google Scholar]
- Stagg C.J., Best J.G., Stephenson M.C. Polarity-Sensitive Modulation of Cortical Neurotransmitters by Transcranial Stimulation. J. Neurosci. 2009;29(16):5202–5206. doi: 10.1523/JNEUROSCI.4432-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg C.J., Bachtiar V., Johansen-Berg H. The Role of GABA in Human Motor Learning. Curr. Biol. 2011;21(6):480–484. doi: 10.1016/j.cub.2011.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trillingsgaard A., Damm D., Sommer S. Developmental Profiles on the Basis of the FTF (Five to Fifteen) Questionnaire-Clinical Validity and Utility of the FTF in a Child Psychiatric Sample. Eur. Child Adolesc. Psychiatry. 2004;13(Suppl. 3):39–63. doi: 10.1007/s00787-004-3006-y. [DOI] [PubMed] [Google Scholar]
- Violante I.R., Ribeiro M.J., Edden R.A. GABA Deficit in the Visual Cortex of Patients with Neurofibromatosis Type 1: Genotype-Phenotype Correlations and Functional Impact. Brain. 2013;136(Pt 3):918–925. doi: 10.1093/brain/aws368. [DOI] [PubMed] [Google Scholar]
- Walker M.P., Brakefield T., Morgan A., Hobson J.A., Stickgold R. Practice with Sleep Makes Perfect: Sleep-Dependent Motor Skill Learning. Neuron. 2002;35(1):205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- Xu T., Yu X., Perlik A.J. Rapid Formation and Selective Stabilization of Synapses for Enduring Motor Memories. Nature. 2009;462(7275):915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U. Pharmaco-Transcranial Magnetic Stimulation Studies of Motor Excitability. Handb Clin. Neurol. 2013;116:387–397. doi: 10.1016/B978-0-444-53497-2.00032-2. [DOI] [PubMed] [Google Scholar]
- Ziemann U., Lonnecker S., Steinhoff B.J., Paulus W. The Effect of Lorazepam on the Motor Cortical Excitability in man. Exp. Brain Res. 1996;109(1):127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
- Zimerman M., Nitsch M., Giraux P., Gerloff C., Cohen L.G., Hummel F.C. Neuroenhancement of the Aging Brain: Restoring Skill Acquisition in old Subjects. Ann. Neurol. 2013;73(1):10–15. doi: 10.1002/ana.23761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material