Abstract
Background
Novel therapies are needed for children with relapsed or refractory leukemia. We therefore tested the safety and feasibility of haploidentical natural killer cell therapy in this patient population.
Procedure
Twenty-nine children who had relapsed or refractory leukemia were treated with chemotherapy followed by the infusion of haploidentical NK cells. Cohort 1 included 14 children who had not undergone prior allogeneic hematopoietic cell transplantation (HCT), whereas Cohort 2 included 15 children with leukemia that had relapsed after HCT.
Results
Twenty-six (90%) NK donors were KIR mismatched (14 with one KIR and 12 with 2 KIRs). The peak NK chimerism levels were >10% donor in 87% of the evaluable recipients. In Cohort 1, 10 had responsive disease and 12 proceeded to HCT thereafter. Currently, 5 (36%) are alive without leukemia. In Cohort 2, 10 had responsive disease after NK therapy and successfully proceeded to second HCT. At present, 4 (27%) are alive and leukemia-free. The NK cell infusions and the IL-2 injections were well-tolerated.
Conclusions
NK cell therapy is safe, feasible, and should be further investigated in patients with chemotherapy-resistant leukemia.
Keywords: NK cell, childhood leukemia, relapse, cell therapy
INTRODUCTION
Children with newly diagnosed acute lymphoblastic leukemia (ALL) or acute myeloid leukemia (AML) experience long-term disease free survival rates greater than 80% and 60%, respectively, when treated on contemporary clinical trials.[1–9] However, the outcome of patients with refractory or relapsed disease remains poor, with survival rates of less than 40%.[10–14] Except for the small group of patients with late recurrences of B-lineage ALL, children with relapsed ALL or AML are unlikely to be cured with chemotherapy alone, and hematopoietic cell transplantation (HCT) is the treatment of choice.[15, 16]
The development of novel methods of graft preparation has led to high success rates of HCT in children regardless of matched donor availability.[17–19] In addition, modern supportive care measures have reduced the risk of death related to regimen toxicity, infection, and graft-versus-host disease (GVHD) to less than 15%. Nevertheless, HCT is not always successful, as leukemia relapse remains common. In this regard, the most powerful predictor of relapse after HCT is the level of minimal residual disease (MRD) at the time of transplantation.[20–22] In this high risk, often heavily pretreated pediatric oncology population, additional attempts at MRD reduction with chemotherapy combinations prior to HCT or dose escalation of conditioning chemotherapy during HCT are unlikely to improve outcome and may contribute to overall morbidity and mortality. Therefore, new therapeutic strategies that have non-cross resistant mechanisms of action and no overlapping toxicities are desirable.[23] One such approach is immunotherapy with the use of allogeneic natural killer (NK) cells.
NK cells can target and kill leukemia cells without prior exposure to those cells.[24] In the setting of allogeneic HCT, several studies have demonstrated the powerful effect of NK cells against leukemia.[25–30] Recently, we demonstrated that infusions of haploidentical NK cells in patients with AML were well tolerated and void of GVHD.[31] In the present study, we infused haploidentical NK cells from parental donors to 29 patients with relapsed or refractory childhood leukemia. The safety, feasibility and outcome strongly support further investigations into the use of NK cells in frontline leukemia treatments.
METHODS
Patients
Two cohorts of patients were treated with haploidentical NK cells. The first cohort included patients who had refractory leukemia and had not received prior HCT. The second cohort had leukemia that had relapsed after prior allogeneic HCT. Availability of matched donors was not an exclusion criterion; these patients were eligible to proceed to HCT with a matched donor if they had stable or responsive disease after the haploidentical NK cell therapy.
Conditioning and NK cell purification
Patients were enrolled on the St. Jude NKAML or NKHEM protocols (ClinicalTrials.gov NCT00697671 and NCT00187096) and received clofarabine 40 mg/m2, etoposide 100 mg/m2, and cyclophosphamide 400 mg/m2 on days-6 through -2 before NK cell infusion.[32] On alternate days, 1 million units/m2 of IL-2 was administered subcutaneously for 6 doses starting on day –1 to activate and expand circulating donor NK cells. The donors underwent apheresis on day-1 and the product was purified for CD3− CD56+ cells in our Human Applications Laboratory by a 2-step procedure using magnetic activated cell sorting.[33] First, the CliniMACS system (Miltenyi Biotec, Cambridge, MA) was used to deplete T cells from the mononuclear cell product obtained by leukapheresis by using anti-CD3 antibody-coated beads. Second, the CD3-depleted product was enriched for CD56+ cells by incubation with anti-CD56 antibody-coated beads. The entire cell-purification process lasted less than 12 hours and the final products were infused immediately without in vitro exposure to IL-2 or other cytokines.
Response criteria
MRD was studied by flow cytometry as previously described.[34, 35] Briefly, bone marrow mononuclear cells were labeled with various combinations of monoclonal antibodies conjugated to fluorescein isothiocyanate, phycoerythrin, peridinin chlorophyll protein, and allophycocyanin directed against surface antigens, or isotype-matched nonreactive monoclonal antibodies which served as a control for non-specific binding to Fc receptors. After 10 minutes incubation at 20°C in the dark, the 4-color stained cell preparations were washed in PBSA twice, fixed in 0.5% paraformaldehyde, and analyzed with a dual laser-FACSCalibur flow cytometer with Cell Quest Pro software (Becton Dickinson, San Jose, CA). Results were reported as percentage of nucleated cells with the leukemia-associated immunophenotype.
For this study, patients were considered to have attained complete remission (CR) if bone marrow blasts were less than 5% with evidence of trilineage hematopoietic recovery. Complete remission with incomplete blood count recovery (CRi) was defined as less than 5% bone marrow blasts without trilineage hematopoietic recovery. Patients achieved a partial response (PR) if the bone marrow contained 5% to 25% blasts and a decrease of at least 50% in blast percentage. All other patients were considered to have no response (NR).
Statistics
Summary statistics were provided for baseline variables at the time of NK cell therapy and for cell composition before and after NK cell purification. Survival rates were estimated by the method of Kaplan-Meier. All reported P values are two sided and are considered significant if <.05. Statistical analyses were performed with SAS software (version 9.2, Cary, NC).
RESULTS
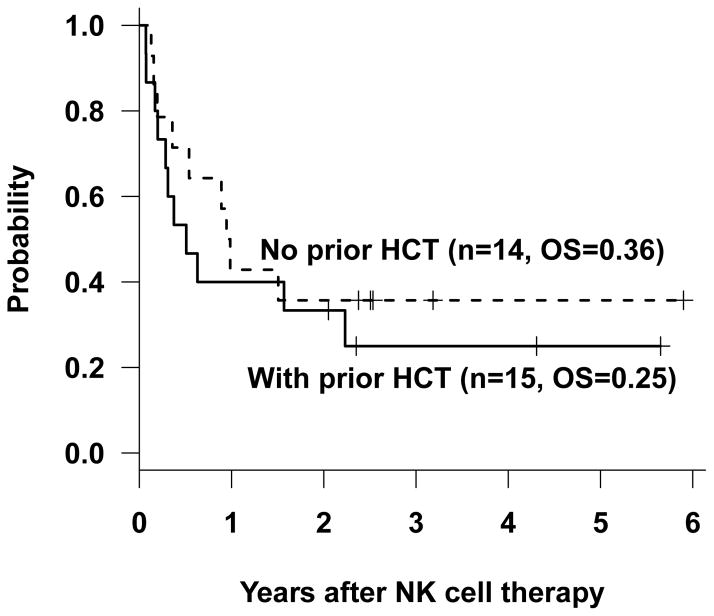
The 29 patients who received haploidentical NK cell infusions included 14 children with relapsed or refractory leukemia (no prior HCT), and 15 children with leukemia that had relapsed after HCT (Table I). Among the 14 children with relapsed or refractory leukemia who had not undergone prior HCT (8 with ALL and 6 with AML), 1 was in morphologic remission, but with detectable MRD, whereas 4 had 5% to 25% blasts, and 9 had more than 25% blasts in the marrow at the time of NK cell therapy (Table II). Following NK cell therapy, 7 patients achieved CR or CRi, 3 attained PR, and 4 had NR; 12 patients proceeded to subsequent HCT. Currently, 5 patients (36%) are alive with no evidence of disease, 6 died of progressive disease, and 1 died from HCT-related toxicity (Fig. 1).
Table I.
Patient summary
| Characteristic | No prior HCT N |
Prior HCT N |
|---|---|---|
| Number of patients | 14 | 15 |
|
| ||
| Age (years) | ||
| <10 | 8 | 10 |
| >10 | 7 | 5 |
|
| ||
| Sex | ||
| Male | 9 | 9 |
| Female | 5 | 6 |
|
| ||
| Race | ||
| White | 9 | 15 |
| Non-white | 5 | 0 |
|
| ||
| Disease | ||
| ALL | 8 | 9 |
| AML | 6 | 6 |
|
| ||
| Leukemia blast % in BM at the time of NK therapy | ||
| <0.01% | 0 | 0 |
| 0.01% – 5% | 1 | 1 |
| 5% – 25% | 5 | 4 |
| >25% | 8 | 10 |
|
| ||
| Haploidentical NK donor | ||
| Parent | 13 | 15 |
| Other adult relatives | 1 | 0 |
|
| ||
| Current report HCT donor | ||
| Matched unrelated | 5 | 6 |
| Haploidentical | 7 | 4 |
Table II.
Patient details
| Patient | Prior HCT | Diagnosis | Relapse | Peak Chimerism (%) | Pre NK Blasts (%) | Post NK Blasts (%) | Response | HCT after NK | Status |
|---|---|---|---|---|---|---|---|---|---|
| 1 | No | ALL | 1 | 0 | 40 | 18 | PR | yes | Dead |
| 2 | No | ALL | 1 | 1 | 2 | Neg | CR | yes | Alive |
| 3 | No | AML | 1 | 12 | 80 | 91 | NR | yes | Dead |
| 4 | No | ALL | 1 | 17 | 30 | Neg | CR | yes | Alive |
| 5 | No | ALL | 1 | 82 | 97 | Neg | CR | yes | Dead |
| 6 | No | ALL | 1 | 100 | 15 | Neg* | CRi | no | Dead |
| 7 | No | AML | 1 | 100 | 80 | 14 | PR | no | Alive |
| 8 | No | ALL | 1 | 100 | 37 | 6 | PR | yes | Dead |
| 9 | No | ALL | 1 | 100 | 92 | 100 | NR | yes | Dead |
| 10 | No | ALL | 1 | 100 | 20 | 96 | NR | yes | Dead |
| 11 | No | AML | 1 | 100 | 40 | 31 | NR | yes | Dead |
| 12 | No | AML | 1 | 100 | 56 | 2 | CR | yes | Dead |
| 13 | No | AML | 1 | 100 | 12 | 0.3 | CR | yes | Alive |
| 14 | No | AML | 1 | QNS | 15 | Neg* | CRi | yes | Alive |
| 15 | Yes | ALL | 2 | 0 | 100 | 100 | NR | yes | Dead |
| 16 | Yes | ALL | 2 | 24 | 30 | Neg | CR | yes | Dead |
| 17 | Yes | AML | 2 | 49 | 10 | Neg* | CRi | yes | Dead |
| 18 | Yes | ALL | 2 | 98 | 90 | 90 | NR | no | Dead |
| 19 | Yes | ALL | 2 | 100 | 14 | Neg | CR | no | Dead |
| 20 | Yes | ALL | 2 | 100 | 100 | 100 | NR | no | Dead |
| 21 | Yes | ALL | 2 | 100 | 95 | 95 | NR | yes | Dead |
| 22 | Yes | ALL | 2 | 100 | 97 | Neg* | CRi | yes | Alive |
| 23 | Yes | ALL | 2 | 100 | 14 | 3 | PR | yes | Alive |
| 24 | Yes | AML | 2 | 100 | 90 | 36 | PR | yes | Alive |
| 25 | Yes | AML | 1 | 100 | 93 | Neg* | CRi | yes | Alive |
| 26 | Yes | AML | 1 | 100 | 13 | 4 | PR | yes | Dead |
| 27 | Yes | AML | 1 | QNS | 11 | Neg* | CRi | no | Dead |
| 28 | Yes | AML | 2 | QNS | 52 | NE | NE | no | Dead |
| 29 | Yes | ALL | 2 | QNS | 90 | Neg* | CRi | yes | Dead |
Neg, negative; Neg*, no detectable blasts, but hypocellular marrow; PR, partial response; CR, complete remission; CRi, complete remission with incomplete count recovery; QNS, quantity not sufficient; NE, not evaluable.
Figure 1.
Survival probability after NK cell therapy and HCT.
Among the 15 patients (9 with ALL and 6 with AML) whose leukemia had relapsed after prior allogeneic HCT, all had detectable leukemia at the time of NK cell therapy, including 10 patients with greater than 25% blasts (Tables I and II). Despite having undergone prior HCT and having high leukemic burdens at the time of NK cell therapy, 10 of these 15 had responsive disease (7 CR/CRi and 3 PR) and successfully proceeded to second HCT. At last follow-up, 4 (27%) are alive and leukemia-free, 3 died of progressive leukemia after the second HCT and 3 died of HCT-related toxicity (Fig 1).
All NK cell donors underwent apheresis without complications. Patients received a median of 18.6 × 106/kg NK cells (range, 3.5 to 103 × 106/kg), with undetectable or minimal contamination of products by B or T cells (Table III). Among the 29 donors, 26 (90%) were KIR mismatched (14 with one KIR and 12 with 2 KIRs; Table IV). The NK cell infusions and the IL-2 injections were well-tolerated with no attributable side effects. Specially, none of the patients developed GVHD, cytokine storm, or effusion syndrome. Among the 29 patients, 23 had sufficient numbers of NK cells to perform chimerism studies. The donor chimerism levels peaked between Day 7 and Day 14, reaching 100% donor in 16 recipients, 12–98% in 4 recipients, 1% in one recipient, and 0% in two recipients.
Table III.
Cell populations before and after NK cell purification
| Cells | Before cell processing Median (range) | After cell processing Median (range) |
|---|---|---|
| TNC/kg (106) | 401 (46 – 2044) | 18.8 (3.8 – 104) |
| CD56+ NK cell % | 10.1 (4.47 – 24.5) | 98.4 (84.7 – 99.6) |
| CD56+ cells/kg (106) | 49.9 (6.75 – 248) | 18.6 (3.5 – 103) |
| CD3+56− T cell % | 56.1 (15.5 – 69.2) | 0 (0 – 0.14) |
| CD3+56− cells/kg (106) | 202 (26.3 – 990) | 0 (0 – 0.01) |
| CD19+ B cell % | 10.2 (3.13 – 21.4) | 0.31 (0 – 1.69) |
| CD19+ cells/kg (106) | 40.1 (4.85 – 239) | 0.04 (0 – 0.71) |
TNC; total nucleated cells.
Table IV.
KIR mismatch between NK cell donor and recipient
| Characteristic | No prior HCT N |
Prior HCT N |
|---|---|---|
| Number of patients | 14 | 15 |
|
| ||
| Mismatched KIR(s) | ||
| 2DL1 | 4 | 5 |
| 3DL1 | 3 | 2 |
| 2DL1, 3DL1 | 3 | 3 |
| 2DL2, 2DL3 | 3 | 3 |
| 2DL3, 3DL1 | 0 | 0 |
| None | 1 | 2 |
DISCUSSION
The outcome of children with primary refractory or relapsed ALL or AML remains poor, with survival rates approximately 30%.[10, 11] Although allogeneic HCT is potentially curative for patients who have relapsed, short and long-term treatment-related morbidity remain significant problems. In addition, the risk of subsequent relapse is high for patients who undergo HCT with high disease burden.[22] Clearly, alternative therapies, such as NK cell therapy, are needed for patients with chemotherapy resistant disease.
In the setting of allogeneic HCT, the beneficial effects of donor NK cells have been well documented.[25, 26, 30] We and others have also demonstrated the safety and feasibility of NK cell infusions in the absence of HCT.[31, 36–38] The key determinant of NK cell activity is the KIR gene family. As KIRs are highly polymorphic in gene alleles and cell surface expression, KIR typing is essential for donor selection.[24, 39–41] Better outcomes were observed in pediatric leukemia if the donor NK cells expressed a certain KIR that the cognate ligand was absent in the recipient.[42] In the present study, 20 of the 29 patients achieved at least a PR, including 11 of 17 patients with ALL and 9 of 12 with AML. We attribute the favorable responses in part to the preferential use of KIR mismatched donors. KIR typing did not delay the NK cell therapy as the results were available before donor infectious evaluations were completed in all recipients.
Although this report includes the largest number of NK recipients published to date, our study is still limited by relatively small number of patients. In addition, the contribution of the NK cell infusions cannot be determined, since all patients received intensive chemotherapy. Randomized studies are needed to address this issue. Moreover, future studies are needed to determine the optimal timing of NK cell therapy, the best preparative regimen, and the most effective methods to enhance NK cell activity. In this regard, we have established methods to generate large numbers of activated NK cells for clinical use.[43, 44] Addition of RxRγ agonists or lineage-specific antibodies such as those against CD19 or CD33 may further augment the donor NK cell activity.[45, 46] Enhancement of NK activity by using anti-KIR antibodies to block inhibitory KIRs is another attractive approach; this strategy has been proven to be safe in patients with AML and was shown to enhance NK-cell-mediated cytotoxicity against CD20+ lymphomas.[47, 48] A more potent reagent to overcome inhibitory signals and enhance NK activity may be bispecific killer cell engagers, antibodies that are designed to bind both NK cell and target antigens. Recently, a novel bispecific antigen that activates NK cells through CD16 and binds AML cells via CD33 was shown to enhance the antileukemic effects of NK cells.[49] An alternative approach is to deplete host regulatory T cells, which may inhibit the proliferation of donor NK cells. The results of a recent clinical trial demonstrated that depletion of host regulatory T cells by an IL-2 diphtheria toxin fusion protein may improve the efficacy of NK cell therapy for AML.[50]
The present study demonstrates that haploidentical NK cell therapy can be readily administered even in patients with rapidly progressive leukemia, is well-tolerated, and may be useful in reduction of disease burden prior to HCT. Among the 29 patients with relapsed or refractory leukemia who received clofarabine, etoposide, cyclophosphamide, and NK cells, 22 proceeded to HCT and 9 are alive. Although the small patient numbers and potential differences in patient characteristics preclude formal comparisons, only 2 of 15 patients previously treated at our institution with the same chemotherapy, but without NK cells, are alive.[32] The results from this study, together with those described above, should encourage future investigations of NK therapy for patients with refractory leukemias, as well as for those with newly diagnosed high-risk disease.
Acknowledgments
Supported in part by the National Institutes of Health Cancer Center Support (CORE) grant P30 CA021765-30, U01 GM092666, and RO1 CA115422, a Center of Excellence Grant from the State of Tennessee, the Assisi Foundation of Memphis, and the American Lebanese Syrian Associated Charities (ALSAC). C-H Pui is an American Cancer Society Professor.
We thank Elaine Coustan-Smith and Dario Campana for performing minimal residual disease analyses; our clinical, laboratory, and research office colleagues for data collection; and the many patients and parents who participated in the transplantation and cellular therapy research program.
Footnotes
Disclosure of Conflict of Interest: The authors declare no conflicts of interest.
AUTHORSHIP CONTRIBUTIONS
W. L., H.I., and J.R. designed the study, analyzed and interpreted data, and wrote the paper. G.L. performed statistical analyses. W.L., H.I., K.G., C.H., B.M.T., M.D., D.S., T.G., and C-H.P provided study material and patient information, and contributed to the interpretation of data. W.L., H.I., and J.R drafted the paper. All authors contributed to the revisions of the draft and approval of the final manuscript. The authors declare no conflicts of interest.
References
- 1.Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC, Ribeiro RC, Rubnitz JE, Raimondi SC, Onciu M, Coustan-Smith E, Kun LE, Jeha S, Cheng C, Howard SC, Simmons V, Bayles A, Metzger ML, Boyett JM, Leung W, Handgretinger R, Downing JR, Evans WE, Relling MV. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaynon PS, Angiolillo AL, Carroll WL, Nachman JB, Trigg ME, Sather HN, Hunger SP, Devidas M. Long-term results of the children’s cancer group studies for childhood acute lymphoblastic leukemia 1983–2002: a Children’s Oncology Group Report. Leukemia. 2010;24:285–297. doi: 10.1038/leu.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moricke A, Zimmermann M, Reiter A, Henze G, Schrauder A, Gadner H, Ludwig WD, Ritter J, Harbott J, Mann G, Klingebiel T, Zintl F, Niemeyer C, Kremens B, Niggli F, Niethammer D, Welte K, Stanulla M, Odenwald E, Riehm H, Schrappe M. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia. 2010;24:265–284. doi: 10.1038/leu.2009.257. [DOI] [PubMed] [Google Scholar]
- 4.Silverman LB, Stevenson KE, O’Brien JE, Asselin BL, Barr RD, Clavell L, Cole PD, Kelly KM, Laverdiere C, Michon B, Schorin MA, Schwartz CL, O’Holleran EW, Neuberg DS, Cohen HJ, Sallan SE. Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985–2000) Leukemia. 2010;24:320–334. doi: 10.1038/leu.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell C, Richards S, Harrison CJ, Eden T. Long-term follow-up of the United Kingdom medical research council protocols for childhood acute lymphoblastic leukaemia, 1980–2001. Leukemia. 2010;24:406–418. doi: 10.1038/leu.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubnitz JE, Inaba H, Dahl G, Ribeiro RC, Bowman WP, Taub J, Pounds S, Razzouk BI, Lacayo NJ, Cao X, Meshinchi S, Degar B, Airewele G, Raimondi SC, Onciu M, Coustan-Smith E, Downing JR, Leung W, Pui CH, Campana D. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010;11:543–552. doi: 10.1016/S1470-2045(10)70090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Creutzig U, Zimmermann M, Bourquin JP, Dworzak MN, Fleischhack G, Graf N, Klingebiel T, Kremens B, Lehrnbecher T, von NC, Ritter J, Sander A, Schrauder A, von SA, Stary J, Reinhardt D. Randomized trial comparing liposomal daunorubicin with idarubicin in induction for pediatric acute myeloid leukemia: results from Study AML-BFM 2004. Blood. 2013;122:37–43. doi: 10.1182/blood-2013-02-484097. [DOI] [PubMed] [Google Scholar]
- 8.Gibson BE, Webb DK, Howman AJ, de Graaf SS, Harrison CJ, Wheatley K. Results of a randomized trial in children with Acute Myeloid Leukaemia: medical research council AML12 trial. Br J Haematol. 2011;155:366–376. doi: 10.1111/j.1365-2141.2011.08851.x. [DOI] [PubMed] [Google Scholar]
- 9.Abrahamsson J, Forestier E, Heldrup J, Jahnukainen K, Jonsson OG, Lausen B, Palle J, Zeller B, Hasle H. Response-guided induction therapy in pediatric acute myeloid leukemia with excellent remission rate. J Clin Oncol. 2011;29:310–315. doi: 10.1200/JCO.2010.30.6829. [DOI] [PubMed] [Google Scholar]
- 10.Bhojwani D, Pui CH. Relapsed childhood acute lymphoblastic leukaemia. Lancet Oncol. 2013;14:e205–e217. doi: 10.1016/S1470-2045(12)70580-6. [DOI] [PubMed] [Google Scholar]
- 11.Kaspers GJ, Zimmermann M, Reinhardt D, Gibson BE, Tamminga RY, Aleinikova O, Armendariz H, Dworzak M, Ha SY, Hasle H, Hovi L, Maschan A, Bertrand Y, Leverger GG, Razzouk BI, Rizzari C, Smisek P, Smith O, Stark B, Creutzig U. Improved outcome in pediatric relapsed acute myeloid leukemia: results of a randomized trial on liposomal daunorubicin by the International BFM Study Group. J Clin Oncol. 2013;31:599–607. doi: 10.1200/JCO.2012.43.7384. [DOI] [PubMed] [Google Scholar]
- 12.Schrappe M, Hunger SP, Pui CH, Saha V, Gaynon PS, Baruchel A, Conter V, Otten J, Ohara A, Versluys AB, Escherich G, Heyman M, Silverman LB, Horibe K, Mann G, Camitta BM, Harbott J, Riehm H, Richards S, Devidas M, Zimmermann M. Outcomes after induction failure in childhood acute lymphoblastic leukemia. N Engl J Med. 2012;366:1371–1381. doi: 10.1056/NEJMoa1110169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tallen G, Ratei R, Mann G, Kaspers G, Niggli F, Karachunsky A, Ebell W, Escherich G, Schrappe M, Klingebiel T, Fengler R, Henze G, von SA. Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: results of trial ALL-REZ BFM 90. J Clin Oncol. 2010;28:2339–2347. doi: 10.1200/JCO.2009.25.1983. [DOI] [PubMed] [Google Scholar]
- 14.Ko RH, Ji L, Barnette P, Bostrom B, Hutchinson R, Raetz E, Seibel NL, Twist CJ, Eckroth E, Sposto R, Gaynon PS, Loh ML. Outcome of patients treated for relapsed or refractory acute lymphoblastic leukemia: a Therapeutic Advances in Childhood Leukemia Consortium study. J Clin Oncol. 2010;28:648–654. doi: 10.1200/JCO.2009.22.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliansky DM, Camitta B, Gaynon P, Nieder ML, Parsons SK, Pulsipher MA, Dillon H, Ratko TA, Wall D, McCarthy PL, Jr, Hahn T. Role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of pediatric acute lymphoblastic leukemia: update of the 2005 evidence-based review. Biol Blood Marrow Transplant. 2012;18:505–522. doi: 10.1016/j.bbmt.2011.12.585. [DOI] [PubMed] [Google Scholar]
- 16.Oliansky DM, Rizzo JD, Aplan PD, Arceci RJ, Leone L, Ravindranath Y, Sanders JE, Smith FO, III, Wilmot F, McCarthy PL, Jr, Hahn T. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute myeloid leukemia in children: an evidence-based review. Biol Blood Marrow Transplant. 2007;13:1–25. doi: 10.1016/j.bbmt.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 17.Leung W, Campana D, Yang J, Pei D, Coustan-Smith E, Gan K, Rubnitz JE, Sandlund JT, Ribeiro RC, Srinivasan A, Hartford C, Triplett BM, Dallas M, Pillai A, Handgretinger R, Laver JH, Pui CH. High success rate of hematopoietic cell transplantation regardless of donor source in children with very high-risk leukemia. Blood. 2011;118:223–230. doi: 10.1182/blood-2011-01-333070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eapen M, Rubinstein P, Zhang MJ, Stevens C, Kurtzberg J, Scaradavou A, Loberiza FR, Champlin RE, Klein JP, Horowitz MM, Wagner JE. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369:1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 19.Klingebiel T, Cornish J, Labopin M, Locatelli F, Darbyshire P, Handgretinger R, Balduzzi A, Owoc-Lempach J, Fagioli F, Or R, Peters C, Aversa F, Polge E, Dini G, Rocha V. Results and factors influencing outcome after fully haploidentical hematopoietic stem cell transplantation in children with very high-risk acute lymphoblastic leukemia: impact of center size: an analysis on behalf of the Acute Leukemia and Pediatric Disease Working Parties of the European Blood and Marrow Transplant group. Blood. 2010;115:3437–3446. doi: 10.1182/blood-2009-03-207001. [DOI] [PubMed] [Google Scholar]
- 20.Leung W, Pui CH, Coustan-Smith E, Yang J, Pei D, Gan K, Srinivasan A, Hartford C, Triplett BM, Dallas M, Pillai A, Shook D, Rubnitz JE, Sandlund JT, Jeha S, Inaba H, Ribeiro RC, Handgretinger R, Laver JH, Campana D. Detectable minimal residual disease before hematopoietic cell transplantation is prognostic but does not preclude cure for children with very-high-risk leukemia. Blood. 2012;120:468–472. doi: 10.1182/blood-2012-02-409813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bader P, Kreyenberg H, Henze GH, Eckert C, Reising M, Willasch A, Barth A, Borkhardt A, Peters C, Handgretinger R, Sykora KW, Holter W, Kabisch H, Klingebiel T, von SA. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM Study Group. J Clin Oncol. 2009;27:377–384. doi: 10.1200/JCO.2008.17.6065. [DOI] [PubMed] [Google Scholar]
- 22.Campana D, Leung W. Clinical significance of minimal residual disease in patients with acute leukaemia undergoing haematopoietic stem cell transplantation. Br J Haematol. 2013;162:147–161. doi: 10.1111/bjh.12358. [DOI] [PubMed] [Google Scholar]
- 23.Grupp SA, Verneris M, Sondel PM, Cooper LJ. Immunotherapy for pediatric cancer. Biol Blood Marrow Transplant. 2008;14:33–43. doi: 10.1016/j.bbmt.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leung W. Infusions of allogeneic natural killer cells as cancer therapy. Clin Cancer Res. 2014;20:3390–3400. doi: 10.1158/1078-0432.CCR-13-1766. [DOI] [PubMed] [Google Scholar]
- 25.Velardi A, Ruggeri L, Mancusi A. Killer-cell immunoglobulin-like receptors reactivity and outcome of stem cell transplant. Curr Opin Hematol. 2012;19:319–323. doi: 10.1097/MOH.0b013e32835423c3. [DOI] [PubMed] [Google Scholar]
- 26.Leung W. Use of NK cell activity in cure by transplant. Br J Haematol. 2011;155:14–29. doi: 10.1111/j.1365-2141.2011.08823.x. [DOI] [PubMed] [Google Scholar]
- 27.Venstrom JM, Pittari G, Gooley TA, Chewning JH, Spellman S, Haagenson M, Gallagher MM, Malkki M, Petersdorf E, Dupont B, Hsu KC. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N Engl J Med. 2012;367:805–816. doi: 10.1056/NEJMoa1200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooley S, Weisdorf DJ, Guethlein LA, Klein JP, Wang T, Marsh SG, Spellman S, Haagenson MD, Saeturn K, Ladner M, Trachtenberg E, Parham P, Miller JS. Donor killer cell Ig-like receptor B haplotypes, recipient HLA-C1, and HLA-C mismatch enhance the clinical benefit of unrelated transplantation for acute myelogenous leukemia. J Immunol. 2014;192:4592–4600. doi: 10.4049/jimmunol.1302517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oevermann L, Michaelis SU, Mezger M, Lang P, Toporski J, Bertaina A, Zecca M, Moretta L, Locatelli F, Handgretinger R. KIR B haplotype donors confer a reduced risk for relapse after haploidentical transplantation in children with ALL. Blood. 2014;124:2744–2747. doi: 10.1182/blood-2014-03-565069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knorr DA, Bachanova V, Verneris MR, Miller JS. Clinical utility of natural killer cells in cancer therapy and transplantation. Semin Immunol. 2014;26:161–172. doi: 10.1016/j.smim.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, Pui CH, Leung W. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28:955–959. doi: 10.1200/JCO.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inaba H, Bhojwani D, Pauley JL, Pei D, Cheng C, Metzger ML, Howard SC, Rubnitz JE, Sandlund JT, Ribeiro RC, Leung W, Campana D, Pui CH, Jeha S. Combination chemotherapy with clofarabine, cyclophosphamide, and etoposide in children with refractory or relapsed haematological malignancies. Br J Haematol. 2012;156:275–279. doi: 10.1111/j.1365-2141.2011.08847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iyengar R, Handgretinger R, Babarin-Dorner A, Leimig T, Otto M, Geiger TL, Holladay MS, Houston J, Leung W. Purification of human natural killer cells using a clinical-scale immunomagnetic method. Cytotherapy. 2003;5:479–484. doi: 10.1080/14653240310003558. [DOI] [PubMed] [Google Scholar]
- 34.Inaba H, Coustan-Smith E, Cao X, Pounds SB, Shurtleff SA, Wang KY, Raimondi SC, Onciu M, Jacobsen J, Ribeiro RC, Dahl GV, Bowman WP, Taub JW, Degar B, Leung W, Downing JR, Pui CH, Rubnitz JE, Campana D. Comparative analysis of different approaches to measure treatment response in acute myeloid leukemia. J Clin Oncol. 2012;30:3625–3632. doi: 10.1200/JCO.2011.41.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coustan-Smith E, Song G, Clark C, Key L, Liu P, Mehrpooya M, Stow P, Su X, Shurtleff S, Pui CH, Downing JR, Campana D. New markers for minimal residual disease detection in acute lymphoblastic leukemia. Blood. 2011;117:6267–6276. doi: 10.1182/blood-2010-12-324004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, DeFor TE, Burns LJ, Orchard PJ, Blazar BR, Wagner JE, Slungaard A, Weisdorf DJ, Okazaki IJ, McGlave PB. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 37.Curti A, Ruggeri L, D’Addio A, Bontadini A, Dan E, Motta MR, Trabanelli S, Giudice V, Urbani E, Martinelli G, Paolini S, Fruet F, Isidori A, Parisi S, Bandini G, Baccarani M, Velardi A, Lemoli RM. Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood. 2011;118:3273–3279. doi: 10.1182/blood-2011-01-329508. [DOI] [PubMed] [Google Scholar]
- 38.Locatelli F, Moretta F, Brescia L, Merli P. Natural killer cells in the treatment of high-risk acute leukaemia. Semin Immunol. 2014;26:173–179. doi: 10.1016/j.smim.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Leung W, Iyengar R, Triplett B, Turner V, Behm FG, Holladay MS, Houston J, Handgretinger R. Comparison of killer Ig-like receptor genotyping and phenotyping for selection of allogeneic blood stem cell donors. J Immunol. 2005;174:6540–6545. doi: 10.4049/jimmunol.174.10.6540. [DOI] [PubMed] [Google Scholar]
- 40.Bari R, Bell T, Leung WH, Vong QP, Chan WK, Das GN, Holladay M, Rooney B, Leung W. Significant functional heterogeneity among KIR2DL1 alleles and a pivotal role of arginine 245. Blood. 2009;114:5182–5190. doi: 10.1182/blood-2009-07-231977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bari R, Rujkijyanont P, Sullivan E, Kang G, Turner V, Gan K, Leung W. Effect of donor KIR2DL1 allelic polymorphism on the outcome of pediatric allogeneic hematopoietic stem-cell transplantation. J Clin Oncol. 2013;31:3782–3790. doi: 10.1200/JCO.2012.47.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leung W, Iyengar R, Turner V, Lang P, Bader P, Conn P, Niethammer D, Handgretinger R. Determinants of antileukemia effects of allogeneic NK cells. J Immunol. 2004;172:644–650. doi: 10.4049/jimmunol.172.1.644. [DOI] [PubMed] [Google Scholar]
- 43.Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, Eldridge P, Leung WH, Campana D. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69:4010–4017. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rujkijyanont P, Chan WK, Eldridge PW, Lockey T, Holladay M, Rooney B, Davidoff AM, Leung W, Vong Q. Ex vivo activation of CD56(+) immune cells that eradicate neuroblastoma. Cancer Res. 2013;73:2608–2618. doi: 10.1158/0008-5472.CAN-12-3322. [DOI] [PubMed] [Google Scholar]
- 45.Leung WH, Vong QP, Lin W, Janke L, Chen T, Leung W. Modulation of NKG2D ligand expression and metastasis in tumors by spironolactone via RXRgamma activation. J Exp Med. 2013;210:2675–2692. doi: 10.1084/jem.20122292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan WK, Kung SM, Li Y, Zalevsky J, Schell S, Leung W. Antibody-dependent cell-mediated cytotoxicity overcomes NK cell resistance in MLL-rearranged leukemia expressing inhibitory KIR ligands but not activating ligands. Clin Cancer Res. 2012;18:6296–6305. doi: 10.1158/1078-0432.CCR-12-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vey N, Bourhis JH, Boissel N, Bordessoule D, Prebet T, Charbonnier A, Etienne A, Andre P, Romagne F, Benson D, Dombret H, Olive D. A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood. 2012;120:4317–4323. doi: 10.1182/blood-2012-06-437558. [DOI] [PubMed] [Google Scholar]
- 48.Kohrt HE, Thielens A, Marabelle A, Sagiv-Barfi I, Sola C, Chanuc F, Fuseri N, Bonnafous C, Czerwinski D, Rajapaksa A, Waller E, Ugolini S, Vivier E, Romagne F, Levy R, Blery M, Andre P. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood. 2014;123:678–686. doi: 10.1182/blood-2013-08-519199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiernik A, Foley B, Zhang B, Verneris MR, Warlick E, Gleason MK, Ross JA, Luo X, Weisdorf DJ, Walcheck B, Vallera DA, Miller JS. Targeting natural killer cells to acute myeloid leukemia in vitro with a CD16 x 33 bispecific killer cell engager and ADAM17 inhibition. Clin Cancer Res. 2013;19:3844–3855. doi: 10.1158/1078-0432.CCR-13-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bachanova V, Cooley S, DeFor TE, Verneris MR, Zhang B, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lewis D, Hippen K, McGlave P, Weisdorf DJ, Blazar BR, Miller JS. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood. 2014;123:3855–3863. doi: 10.1182/blood-2013-10-532531. [DOI] [PMC free article] [PubMed] [Google Scholar]