Abstract
Naegleria fowleri is a thermophilic free-living ameba that causes primary amebic meningoencephalitis. Infections are nearly always fatal. We present the third well-documented survivor of this infection in North America. Survival most likely resulted from a combination of early identification and treatment, use of a combination of antimicrobials including miltefosine and management of elevated intracranial pressure based on traumatic brain injury principles.
Keywords: Naegleria fowleri, Primary Amebic Meningoencephalitis, miltefosine, hypothermia
Naegleria fowleri is a thermophilic free-living ameba found in warm freshwater. Infection is rare and occurs when water containing the ameba enters the nose and subsequently invades the brain. Infection with N. fowleri causes primary amebic meningoencephalitis (PAM), resulting in destruction of brain tissue and cerebral edema. There have been two well-documented survivors in North America: one in California in 19781–2 and one in Mexico in 2003.3 We present the third documented survivor of PAM in North America.
Case
The patient, a previously healthy 12-year-old female, presented to the emergency department with a two-day history of headache and a one-day history of fever (39.4°C), along with nausea, vomiting and somnolence. She had a normal neurologic exam. She reported swimming at an outdoor water park seven days prior to symptom onset. Initial laboratory evaluation included peripheral white blood cell count of 18.4 cells/μL (77% segmented, 13% banded neutrophils). Cerebrospinal fluid (CSF) revealed a white blood cell count of 3675 cells/μL (86% segmented neutrophils), red blood cell count of 53 cells/μL, protein of 374 mg/dL and glucose of 22 mg/dL. The Giemsa-Wright stain of the CSF revealed amebae consistent with N. fowleri. The initial computed tomography scan of her brain was normal.
She was admitted to the pediatric intensive care unit on July 19, 2013 and started on the following: conventional amphotericin B 1.5mg/kg/day intravenously in two divided doses, fluconazole 10mg/kg/day, rifampin 10mg/kg/day and azithromycin 10mg/kg/day.1, 3 Dexamethasone was initiated concurrently. After three days, the amphotericin B was decreased to 1mg/kg/day.1 Approximately 36 hours after admission, she was started on miltefosine 50mg every eight hours. Consent was obtained from the family prior to administering miltefosine.
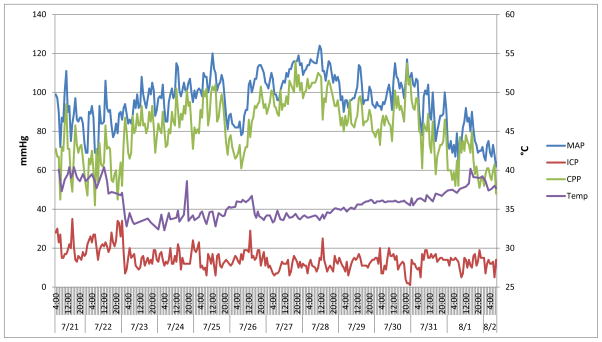
Almost 24 hours after admission, she developed a right-sided abducens nerve palsy. An extraventricular drain (EVD) was placed in the operating room, and initial intracranial pressure (ICP) was approximately 50 mmHg. Intrathecal amphotericin B was started at a dose of 1.5mg daily for two days followed by a dose of 1mg every other day for eight days.1 On the third day of hospitalization, she developed worsening intracranial pressure. Management of her cerebral edema (goal ICP below 20 mmHg) included: drainage of CSF, hyperosmolar therapy with mannitol and 3% saline, moderate hyperventilation (goal pCO2 of 30–35 mmHg), and induced hypothermia (32–34°C). Cerebral edema resolved after approximately two weeks. (Figure 1)
Figure 1. The Relationship Between Mean Arterial Pressure, Cerebral Perfusion Pressure, Intracranial Pressure and Core Body Temperature During the Management of a Twelve-Year-Old Female with Naegleria fowleri Primary Amebic Meningoencephalitis.
MAP is mean arterial pressure. ICP is intracranial pressure. CPP is cerebral perfusion pressure. Temp is temperature. The graph above illustrates our management strategy of maintaining CPP above 60mmHg and ICP below 20mmHg. With induction of hypothermia (7/22 22:00), ICPs were sustained under 20mmHg. Please note early attempts to rewarm the patient on 7/24 and 7/26 led to elevations of ICP. After 5 days of cooling, rewarming the patient was met with minimal ICP elevations.
Her initial CSF specimen grew N. fowleri on culture and was positive for N. fowleri by polymerase chain reaction (PCR). By day three, her CSF specimen was culture negative for N. fowleri. Serial CSF specimens showed decreasing white blood cells and decreasing amounts of N. fowleri DNA on PCR.
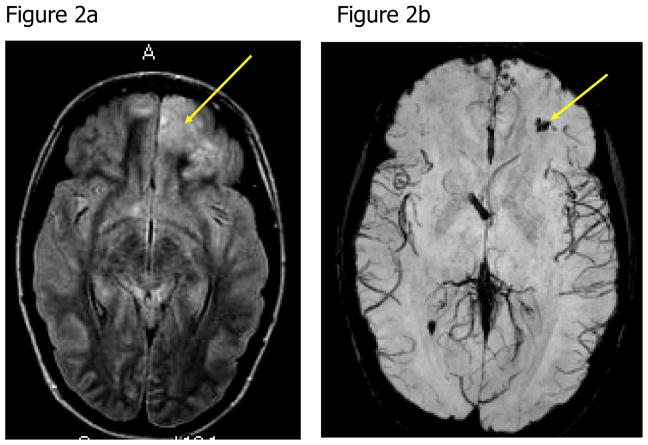
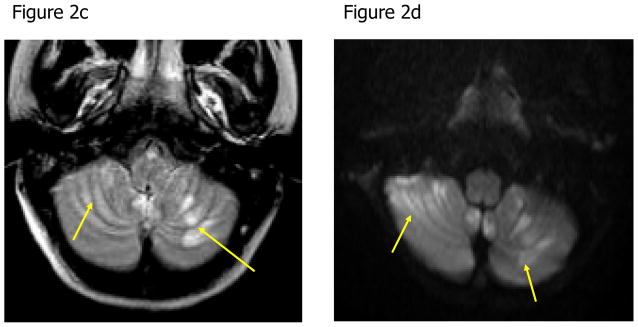
A magnetic resonance image (MRI) of her brain two weeks into her illness revealed blood in the frontal lobes and multiple areas of restricted diffusion primarily in the cerebellum, right internal capsule and corpus collosum (Figure 2). A repeat MRI of her brain one week later showed improvement. She completed 26 days of a planned 28-day course of antimicrobials (miltefosine, azithromycin, rifampin and fluconazole), which were stopped early due to nausea and vomiting. Upon transfer to the rehabilitation unit, the patient had left-sided weakness, dysarthria and dysphagia. After 55 days of hospitalization, she was discharged home. At six months post infection, her level of functioning was normal and without residual deficits.
Figure 2. Noncontrast Axial Magnetic Resonance Images of the Brain of a Patient with Naegleria fowleri Primary Amebic Meningoencephalitis.
Figure 2a: The axial fluid attenuated inversion recovery (FLAIR) image shows focal edema in the left frontal lobe (arrow). Figure 2b: Axial susceptibility-weighted image shows hemorrhage within the left frontal edematous lesion (arrow). Figure 2c: Axial FLAIR image demonstrates multiple areas of edema in the cerebellum bilaterally (arrows). Figure 2d: Axial diffusion-weighted image shows areas of restricted diffusion consistent with acute cerebellitis (arrows).
Discussion
We report the third documented survivor of N. fowleri PAM in North America. Her survival is likely the result of a combination of factors: early diagnosis and treatment, use of a combination of antimicrobials including miltefosine4 and management of elevated ICP based on traumatic brain injury principles. This is the first report of a patient with PAM successfully treated with a regimen that included miltefosine. This is also the first report to document successful use of induced hypothermia (32–34°C) in the management of PAM.
Between 1962 and 2013, 132 cases of N. fowleri PAM were reported to the Centers for Disease Control and Prevention (CDC).5–6 The number of infections reported each year (0–8 infections) remains stable.5–6 The majority of these infections occurred in southern-tier states. Roughly 75% of infections are associated with swimming in warm freshwater lakes and rivers.5–6 The median age of infection is 11 years (range 8 months to 66 years).5–6 Recently the epidemiology of N. fowleri PAM has changed. Since 2010, two cases were reported in Minnesota6–7 as well as single cases identified in Indiana and Kansas.6 This suggests that the geographic range of this thermophilic organism may be expanding. In addition, infections have been reported in patients exposed to nonsterile tap water that was used for sinus irrigation8 or ritual ablution.9
The time from exposure to N. fowleri to the onset of symptoms is approximately 5 days (range 1 to 7 days).5 The initial symptoms of PAM are indistinguishable from bacterial meningitis. Patients experience rapid deterioration with death resulting from brain injury and edema occurring within about 5 days (range 1 to 12 days).5 In the two weeks prior to symptom onset, our patient swam in a number of locations. Epidemiologic investigation by the state health department suggested that a local water park was the likely source of infection. Water samples from only this site tested positive for N. fowleri. Our patient developed symptoms seven days after this exposure. She presented to the hospital approximately 30 hours after initial symptoms and was started on recommended therapy within 36 hours of symptom onset. By comparison, the median time from symptom onset to hospital presentation for patients with PAM is two days, and the median time from symptom onset to initiation of recommended therapy is three days (CDC unpublished data). Because N. fowleri PAM is rare and not often considered as a diagnosis, pre-mortem identification of the ameba is often delayed or not attempted, precluding the timely initiation of recommended therapy. Early identification and initiation of recommended therapy seems critical for survival; however, this timeframe is likely impacted by multiple factors including strain virulence, inoculum and host immune response.
The only documented survivor in the United States received amphotericin and miconazole intravenously and intrathecally and intravenous rifampin.1–2 The survivor from Mexico received intravenous amphotericin, fluconazole and rifampin.3 Conventional amphotericin has a lower minimum inhibitory concentration compared to the liposomal formulation and is thus preferred even though the liposomal form has better CSF penetration.10 Other medications such as fluconazole, voriconazole and azithromycin have shown activity against N. fowleri.10–12 Studies suggest that azithromycin may have a synergistic effect when used in combination with amphotericin.13 Recently, miltefosine, an antiparasitic agent, has shown in vitro effectiveness against N. fowleri and other clinically important free-living amebae.11 Although in recent years miltefosine has been used to successfully treat patients with other free-living amebae infections14–15, our patient is the first to be successfully treated with miltefosine for PAM. Miltefosine is available directly from CDC for treatment of infections caused by free-living amebae in the United States.4
Current neuroprotective management principles following brain injury are based on maintaining adequate cerebral perfusion, tempering oxygen consumption, and limiting intracranial pressure.16 Data suggests that mild-moderate hypothermia (32–34°C) may have neuroprotective effects including: lowering ICP, reducing production of reactive oxygen and nitrogen species, reduction of proinflammatory cytokine levels, and preventing neuronal apoptosis.16–18 Clinical studies evaluating the effects of mild-moderate hypothermia in patients with traumatic brain injury and bacterial meningitis have shown conflicting results.19–22
N. fowleri initially causes direct damage to surrounding neuronal and other cells through direct cell-to-cell interaction as well as release of a number of cytotoxic proteins.2 In addition, cytotoxic proteins released by N. fowleri and debris from lysed neuronal and other cells generate a cascade of proinflammatory cytokines resulting in hyperinflammation and further injury.23 It is possible that the beneficial effects of hypothermia seen in patients with traumatic brain injury and bacterial meningitis may also attenuate the inflammatory response that occurs in patients with N. fowleri PAM. Cytokine levels were not measured in our patient, so it is unclear whether the use of hypothermia impacted the host inflammatory response. Although N. fowleri is a thermophilic organism, it is unclear whether the degree of hypothermia used in our patient resulted in reduced pathogenicity of the ameba.
The changing epidemiology of N. fowleri necessitates its inclusion in the differential of patients with meningoencephalitis, particularly those who report a recent history of swimming in warm freshwater, regardless of geographic location, or use of nonsterile water for nasal irrigation or ritual ablution. Since early diagnosis and therapy are critical, laboratory technicians must be able to quickly identify amebae on CSF specimens. Prompt initiation of a regimen including conventional amphotericin, fluconazole, azithromycin and rifampin is recommended (Table 1). Miltefosine should be added to this regimen as soon as possible. In the presence of signs of increased ICP, we recommend placement of an EVD and administration of intrathecal amphotericin (Table 1). Elevated ICP should be aggressively managed, maintaining an ICP below 20 mmHg. Administer dexamethasone concurrently with the antimicrobials as it was utilized in all three documented survivors, and has shown benefit in central nervous system insults due to infectious etiologies.1–3, 24 Consider also lowering the patient’s core body temperature (32–34°C) as this may have beneficial effects beyond lowering ICP. Additional data are needed to determine adequate length of therapy and to determine the effects of hypothermia in the management of PAM. Ongoing surveillance and continued reporting of PAM cases are needed to continue to learn the best way to manage these infections.
Table 1.
Recommended Treatment Regimen for Naegleria fowleri Primary Amebic Meningoencephalitis
| Medication | Dose | Route | Maximum Dose | Duration | Comments |
|---|---|---|---|---|---|
| Amphotericin B*1 | 1.5 mg/kg/day in 2 divided doses | IV | 1.5 mg/kg/day | 3 days | |
| then | 1 mg/kg/day once daily | IV | 11 days | 14-day course | |
| Amphotericin B*1 | 1.5 mg once daily | Intrathecal | 1.5 mg/day | 2 days | |
| then | 1 mg/day every other day | Intrathecal | 8 days | 10-day course | |
| Azithromycin12 | 10 mg/kg/day once daily | IV/PO | 500 mg/day | 28 days | |
| Fluconazole3 | 10 mg/kg/day once daily | IV/PO | 600 mg/day | 28 days | |
| Rifampin1,3 | 10 mg/kg/day once daily | IV/PO | 600 mg/day | 28 days | |
| Miltefosine13 | Weight < 45 kg: 50 mg BID | ||||
| Weight > 45 kg: 50 mg TID | PO | 2.5 mg/kg/day | 28 days | 50 mg tablets | |
| Dexamethasone3,24 | 0.6mg/kg/day in 4 divided doses | IV | 0.6mg/kg/day | 4 days |
Conventional amphotericin preferred.
All medications should be started in combination as soon as the diagnosis is suspected. Intrathecal amphotericin B should be initiated if the patient develops signs/symptoms of increased intracranial pressure. Miltefosine should be started once available.
Acknowledgments
Funding/Support: None
Abbreviations
- PAM
Primary amebic meningoencephalitis
- CSF
Cerebrospinal fluid
- EVD
Extraventricular drain
- ICP
Intracranial pressure
- PCR
Polymerase chain reaction
- MRI
Magnetic resonance image
- CDC
Centers for Disease Control and Prevention
Footnotes
Financial Disclosure: All authors have indicated they have no financial relationships relevant to this article to disclose.
Conflict of Interest Disclosures: All authors have indicated they have no potential conflicts of interest to disclose.
Contributors’ Statement:
W. Matthew Linam: Dr. Linam provided medical care for the patient, drafted the initial manuscript, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Mubbasheer Ahmed, Craig Chu, and Jerril Green: Drs. Ahmed, Chu and Green provided medical care for the patient, critically reviewed the manuscript, and approved the final manuscript as submitted.
Jennifer R. Cope: Dr. Cope advised the medical care of the patient, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Govinda S. Visvesvara, Alexandre J. da Silva, and Yvonne Qvarnstrom: Drs. Visvesvara, da Silva, and Qvarnstrom performed diagnostic studies and interpretation of results, critically reviewed the manuscript, and approved the final manuscript as submitted.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent those of the Centers for Disease Control and Prevention.
Additional Contributions: We would like to acknowledge the incredible teamwork and communication of the countless members of the healthcare team that each played a role in this patient’s survival. We also thank Dr. Charles M. Glasier for his assistance in providing the MRI images for this case. We are also grateful for the diligent work of the CDC staff whose contributions ensured timely access to miltefosine only days before it was needed in this case. Finally, we dedicate this report to Dr. Govinda Visvesvara, who diagnosed the first U.S. survivor of Naegleria infection 35 years ago and has worked tirelessly to ensure that there would be another. None of the individuals received compensation besides their salaries.
References
- 1.Seidel JS, Harmatz P, Visvesvara GS, Cohen A, Edwards J, Turner J. Successful treatment of primary amebic meningoencephalitis. N Engl J Med. 1982;306(6):346–348. doi: 10.1056/NEJM198202113060607. [DOI] [PubMed] [Google Scholar]
- 2.Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol. 2007;50(1):1–26. doi: 10.1111/j.1574-695X.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- 3.Vargas-Zepeda J, Gomez-Alcala AV, Vasquez-Morales JA, Licea-Amaya L, De Jonckheere JF, Lares-Villa F. Successful treatment of Naegleria fowleri meningoencephalitis by using intravenous amphotericin B, fluconazole and rifampicin. Arch Med Res. 2005;36(1):83–86. doi: 10.1016/j.arcmed.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Investigational drug available directly from CDC for the treatment of infections with free-living amebae. MMWR Morb Mortal Wkly Rep. 2013;62(33):666. [PMC free article] [PubMed] [Google Scholar]
- 5.Yoder JS, Eddy BA, Visvesvara GS, Capewell L, Beach MJ. The epidemiology of primary amoebic meningoencephalitis in the USA, 1962–2008. Epidemiol Infect. 2010;138(7):968–975. doi: 10.1017/S0950268809991014. [DOI] [PubMed] [Google Scholar]
- 6. [Accessed July 11, 2014];Naegleria fowleri - Primary Amebic Meningoencephalitis (PAM) http://www.cdc.gov/parasites/naegleria/infection-sources.html. Updated April 14, 2014.
- 7.Kemble SK, Lynfield R, DeVries AS, et al. Fatal Naegleria fowleri infection acquired in Minnesota: possible expanded range of a deadly thermophilic organism. Clin Infect Dis. 2012;54(6):805–809. doi: 10.1093/cid/cir961. [DOI] [PubMed] [Google Scholar]
- 8.Yoder JS, Straif-Bourgeois S, Roy SL, et al. Primary amebic meningoencephalitis deaths associated with sinus irrigation using contaminated tap water. Clin Infect Dis. 2012;55(9):e79–85. doi: 10.1093/cid/cis626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Notes from the field: primary amebic meningoencephalitis associated with ritual nasal rinsing--St. Thomas, U.S. Virgin islands, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(45):903. [PMC free article] [PubMed] [Google Scholar]
- 10.Goswick SM, Brenner GM. Activities of azithromycin and amphotericin B against Naegleria fowleri in vitro and in a mouse model of primary amebic meningoencephalitis. Antimicrob Agents Chemother. 2003;47(2):524–528. doi: 10.1128/AAC.47.2.524-528.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuster FL, Guglielmo BJ, Visvesvara GS. In-vitro activity of miltefosine and voriconazole on clinical isolates of free-living amebas: Balamuthia mandrillaris, Acanthamoeba spp., and Naegleria fowleri. J Eukaryot Microbiol. 2006;53(2):121–126. doi: 10.1111/j.1550-7408.2005.00082.x. [DOI] [PubMed] [Google Scholar]
- 12.Tiewcharoen S, Junnu V, Chinabut P. In vitro effect of antifungal drugs on pathogenic Naegleria spp. Southeast Asian J Trop Med Public Health. 2002;33(1):38–41. [PubMed] [Google Scholar]
- 13.Soltow SM, Brenner GM. Synergistic activities of azithromycin and amphotericin B against Naegleria fowleri in vitro and in a mouse model of primary amebic meningoencephalitis. Antimicrob Agents Chemother. 2007;51(1):23–27. doi: 10.1128/AAC.00788-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez DY, Seas C, Bravo F, et al. Successful treatment of Balamuthia mandrillaris amoebic infection with extensive neurological and cutaneous involvement. Clin Infect Dis. 2010;51(2):e7–11. doi: 10.1086/653609. [DOI] [PubMed] [Google Scholar]
- 15.Aichelburg AC, Walochnik J, Assadian O, et al. Successful treatment of disseminated Acanthamoeba sp. infection with miltefosine. Emerg Infect Dis. 2008;14(11):1743–1746. doi: 10.3201/eid1411.070854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irazuzta JE, Pretzlaff R, Rowin M, Milam K, Zemlan FP, Zingarelli B. Hypothermia as an adjunctive treatment for severe bacterial meningitis. Brain Res. 2000;881(1):88–97. doi: 10.1016/s0006-8993(00)02894-8. [DOI] [PubMed] [Google Scholar]
- 17.Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37(7 Suppl):S186–202. doi: 10.1097/CCM.0b013e3181aa5241. [DOI] [PubMed] [Google Scholar]
- 18.Xu L, Yenari MA, Steinberg GK, Giffard RG. Mild hypothermia reduces apoptosis of mouse neurons in vitro early in the cascade. J Cereb Blood Flow Metab. 2002;22(1):21–28. doi: 10.1097/00004647-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Lepur D, Kutlesa M, Barsic B. Induced hypothermia in adult community-acquired bacterial meningitis--more than just a possibility? J Infect. 2011;62(2):172–177. doi: 10.1016/j.jinf.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Clifton GL, Miller ER, Choi SC, et al. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344(8):556–563. doi: 10.1056/NEJM200102223440803. [DOI] [PubMed] [Google Scholar]
- 21.McIntyre LA, Fergusson DA, Hebert PC, Moher D, Hutchison JS. Prolonged therapeutic hypothermia after traumatic brain injury in adults: a systematic review. JAMA. 2003;289(22):2992–2999. doi: 10.1001/jama.289.22.2992. [DOI] [PubMed] [Google Scholar]
- 22.Mourvillier B, Tubach F, van de Beek D, et al. Induced Hypothermia in Severe Bacterial Meningitis: A Randomized Clinical Trial. JAMA. 2013 doi: 10.1001/jama.2013.280506. [DOI] [PubMed] [Google Scholar]
- 23.Rojas-Hernandez S, Jarillo-Luna A, Rodriguez-Monroy M, Moreno-Fierros L, Campos-Rodriguez R. Immunohistochemical characterization of the initial stages of Naegleria fowleri meningoencephalitis in mice. Parasitol Res. 2004;94(1):31–36. doi: 10.1007/s00436-004-1177-6. [DOI] [PubMed] [Google Scholar]
- 24.van de Beek D, de Gans J, McIntyre P, Prasad K. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst Rev. 2007;(1):CD004405. doi: 10.1002/14651858.CD004405.pub2. [DOI] [PubMed] [Google Scholar]