Abstract
Muscle weakness and exercise intolerance are hallmark symptoms in mitochondrial disorders. Little is known about the mechanisms leading to impaired skeletal muscle function and ultimately muscle weakness in these patients. In a mouse model of lethal mitochondrial myopathy, the muscle-specific Tfam knock-out (KO) mouse, we previously demonstrated an excessive mitochondrial Ca2+ uptake in isolated muscle fibers that could be inhibited by the cyclophilin D (CypD) inhibitor, cyclosporine A (CsA). Here we show that the Tfam KO mice have increased CypD levels, and we demonstrate that this increase is a common feature in patients with mitochondrial myopathy. We tested the effect of CsA treatment on Tfam KO mice during the transition from a mild to terminal myopathy. CsA treatment counteracted the development of muscle weakness and improved muscle fiber Ca2+ handling. Importantly, CsA treatment prolonged the lifespan of these muscle-specific Tfam KO mice. These results demonstrate that CsA treatment is an efficient therapeutic strategy to slow the development of severe mitochondrial myopathy.
Introduction
Mitochondrial myopathies are genetically heterogeneous metabolic disorders, originating from the dysfunction of one or more mitochondrial metabolic pathways. They can be caused by either mitochondrial (mt) or nuclear DNA mutations that alter expression or function of proteins in the mitochondrial respiratory chain, leading to defective oxidative phosphorylation (1,2). Mitochondrial Ca2+ homeostasis has been shown to be of critical importance for cell function and is finely tuned. Indeed, limited and transient mitochondrial Ca2+ uptake contributes to the elaborate control of cell metabolism, whereas prolonged and excessive uptake can activate cell death pathways (3–5). The recently identified mitochondrial Ca2+ uniporter (MCU) has been shown to be the main site of Ca2+ entry to the mitochondrial matrix (6,7), but the presence of Ca2+ in mitochondria from MCU−/− mice suggests that alternative mechanisms exist for Ca2+ entry (8).
Mice with skeletal muscle-specific disruption of the mitochondrial transcription factor A [Tfam; Tfam knock-out (KO) mice] develop severe mtDNA depletion with many phenotypic features also observed in human pathology (9,10). Thus, similar to patients suffering from various forms of mitochondrial myopathy, Tfam KO mice have a respiratory chain enzyme complex deficiency, including abnormally shaped mitochondria, and atrophic, ragged-red and a mosaic pattern of COX-deficient muscle fibers (2,11). Tfam KO muscle fibers demonstrated an excessive mitochondrial Ca2+ uptake during repeated contractions, which was partially inhibited by cyclosporine A (CsA) (12). CsA is widely used in clinical practice as an immunosuppressant to prevent the rejection of organ transplants and for treatment of various autoimmune diseases (13). CsA binds to the mitochondrial protein cyclophilin D (14,15) (CypD; encoded by the Ppif gene in the mouse, previously also called cyclophilin F) and has been shown to counteract the opening of the mitochondrial permeability transition pore (16–19). In this study, we assessed the involvement of CypD in the disease process of mitochondrial myopathies first by measuring the expression of CypD in muscles of mitochondrial myopathy patients and Tfam KO mice and second by testing whether CsA treatment counteracted the disease progression and muscle weakness in Tfam KO mice.
Results
Increased CypD expression in patients as well as mice with mitochondrial myopathy
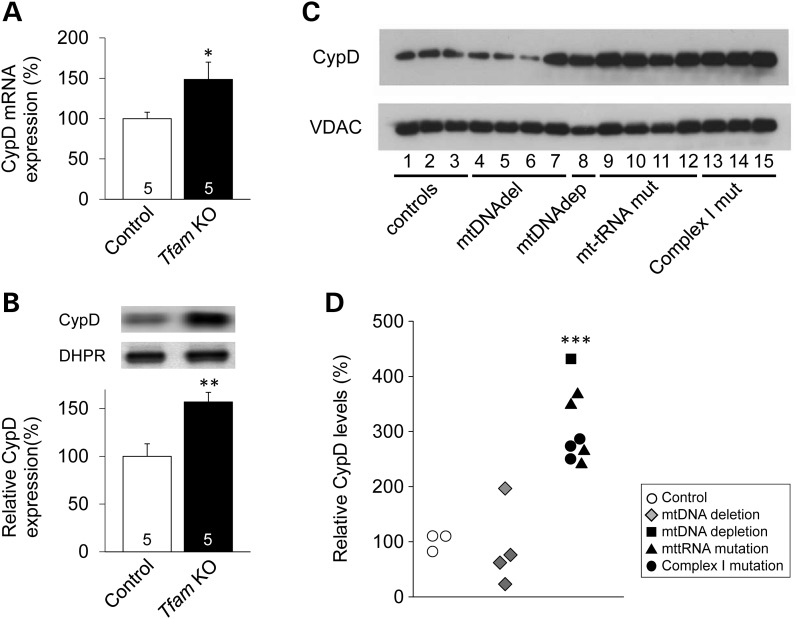
To investigate whether CypD is important in mitochondrial muscle pathophysiology, we measured CypD mRNA and/or protein levels in skeletal muscle from Tfam KO mice as well as patients with diagnosed mitochondrial myopathy (Supplementary Material, Table S1). In skeletal muscle from the Tfam KO mice, CypD mRNA and protein levels were significantly increased (∼60 and ∼55%, respectively; Fig. 1A and B). The patients were selected based on OXPHOS dysfunction and were grouped according to their mtDNA integrity in skeletal muscle. These groups were as follows: patients with mtDNA deletions (n = 4); mtDNA depletion (n = 1); mt-tRNA mutations (n = 4) and mutations in mitochondrial encoded RC complex I subunits (n = 3). Figure 1C and D show that patients with mtDNA depletion or mtDNA mutations all have markedly higher (∼200%) CypD levels than the control group. The only patient group with predominantly unchanged CypD levels had mtDNA deletions (Fig. 1C and D).
Figure 1.
CypD expression is increased in mitochondrial myopathy mice and patients. (A) CypD mRNA (normalized to B2M) and (B) protein levels (normalized to DHPR) of control and skeletal muscle-specific Tfam KO mice. Data are presented as mean ± SEM, and the mean expression in control muscles was set to 100%. Number at the bottom of the bars = number of mice. (C) Representative CypD western blot and (D) individual values of relative CypD protein expression relative to VDAC. Blots were performed on mitochondrial preparations isolated from muscle biopsies of control subjects and mitochondrial myopathy patients. The mean value in control subjects was set to 100%. Significantly different from control *, ** and ***P < 0.05, 0.01 and 0.001, respectively.
CsA treatment leads to extended lifespan and prevents muscle weakness in mitochondrial myopathy mice
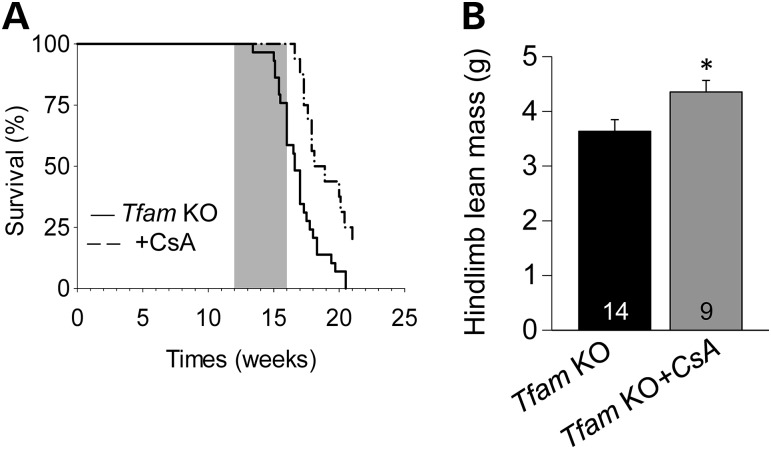
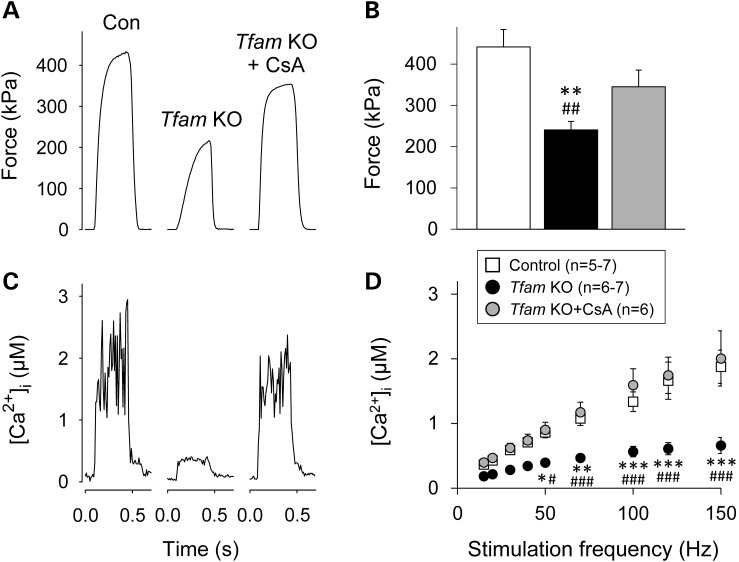
We next examined the effect of 4 weeks of CsA treatment (120 µg/day) on the Tfam KO mice. The treatment spanned from 12 to 16 weeks of age, when the disease progresses from moderate to terminal. CsA treatment resulted in a significant increase in lifespan of the Tfam KO mice: all treated mice were still alive at the end of the treatment period at 16 weeks of age, whereas ∼40% of the untreated Tfam KO mice were dead (Fig. 2A). CsA treatment also resulted in an ∼15% higher hindlimb lean mass in treated than in untreated Tfam KO mice (Fig. 2B), while no difference was observed for hindlimb fat mass (Supplementary Material, Fig. S1). In 16-week-old Tfam KO mice, CsA treatment significantly increased extensor digitorum longus (EDL) specific force at all stimulation frequencies, whereas it did not affect EDL force production in control mice (Supplementary Material, Fig. S2). The increased contractile strength of CsA-treated Tfam KO mice was confirmed by force measurements in intact single flexor digitorum brevis (FDB) fibers (Fig. 3A and B).
Figure 2.
Four weeks of CsA treatment prevents early death and loss of muscle mass in skeletal muscle-specific Tfam KO mice. (A) Survival curves of untreated (n = 18) and CsA-treated (n = 12) Tfam KO mice obtained with Kaplan–Meier survival analysis with the Log-Rank test (P < 0.001). Gray box indicates treatment period. (B) Lean mass of the hindlimbs of Tfam KO mice with and without CsA treatment. Number at the bottom of the bars = number of mice.
Figure 3.
Four weeks of CsA treatment prevents muscle weakness and maintains Ca2+ release in skeletal muscle-specific Tfam KO mice. Representative force records (A) and mean (±SEM) data (B) from 100 Hz contraction in control, untreated and CsA-treated Tfam KO intact single FDB fibers. (C) [Ca2+]i transients associated with the force records shown in (A). (D) Mean [Ca2+]i (±SEM) in response to 10–150 Hz stimulation. n = number of fibers. Significantly different from control *, ** and ***P < 0.05, 0.01 and 0.001, respectively. Significantly different from CsA-treated Tfam KO #, ## and ###P < 0.05, 0.01 and 0.001, respectively.
Preserved sarcoplasmic reticulum Ca2+ release in CsA-treated Tfam KO muscle fibers
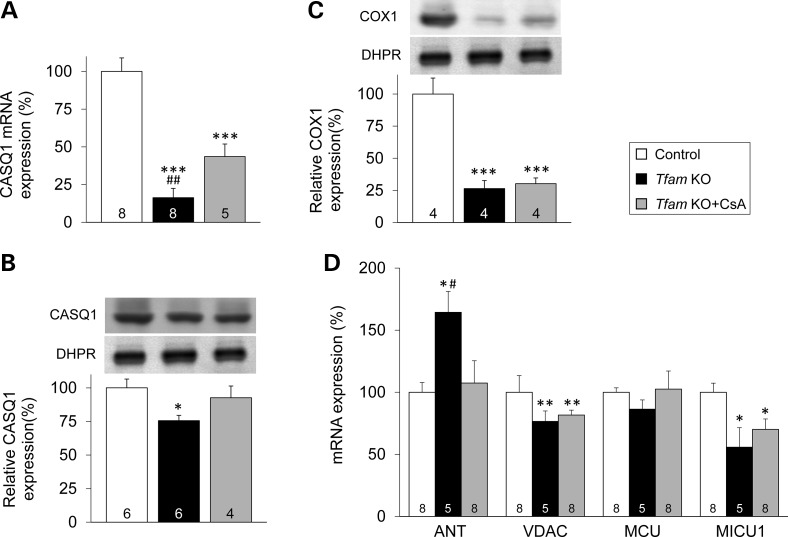
CsA treatment completely prevented the decrease in free cytosolic [Ca2+] ([Ca2+]i) during contractions in Tfam KO FDB fibers (Fig. 3C and D). Tfam KO muscles displayed a markedly decreased expression both at the transcript (to ∼15% of the control value; Fig. 4A) and protein (to ∼75% of the control value; Fig. 4B) level of the major sarcoplasmic reticulum (SR) Ca2+ buffer, calsequestrin 1 (CASQ1). CsA treatment partially prevented the decrease in CASQ1 mRNA (Fig. 4A) and fully prevented the decrease in protein expression (Fig. 4B). The decrease in CASQ1 expression in Tfam KO muscles was specific as shown by unchanged mRNA and protein levels of the t-tubular voltage sensor (the dihydropyridine receptor, DHPR, Supplementary Material, Fig. S3A and S3B).
Figure 4.
CsA treatment prevents the decrease of the CASQ1 in Tfam KO muscle. (A) CASQ1 mRNA level. Representative western blots and protein level of CASQ1 (B) and COX1 (C) protein expression relative to DHPR. (D) Expression of genes related mitochondrial proteins. (A–D) Data are presented as the mean ± SEM, and the mean expression in control muscles was set to 100%. Number at the bottom of the bars = number of mice. Significantly different from control *, ** and ***P < 0.05, 0.01 and 0.001, respectively. Significantly different from CsA-treated Tfam KO #P < 0.05 and ##P < 0.01.
No signs of CsA protecting mitochondrial energy metabolism in Tfam KO muscle
The protein expression of the mtDNA-encoded cytochrome c oxidase subunit 1 (COX1) was severely decreased in Tfam KO mice either with or without CsA treatment (Fig. 4C). Conversely, the activity of the nuclear DNA-encoded protein citrate synthase was not affected in Tfam KO muscles, either with or without CsA treatment (Supplementary Material, Fig. S4).
We measured the mRNA expression levels of several proteins suggested to be involved in mitochondrial Ca2+ handling (Fig. 4D): (i) the adenine nucleotide translocator (ANT), implicated in the modulation of the mitochondrial permeability transition pore (20), (ii) the voltage-dependent anion channel (VDAC), proposed to conduct Ca2+ through the mitochondrial outer membrane (21), (iii) the MCU (6,7,22) and one of its regulator (iv) the mitochondrial calcium uptake 1 (MICU1) (22,23). The expression of mRNA ANT transcripts was markedly increased in Tfam KO muscle, and this was fully prevented by CsA treatment (Fig. 4D). We observed decreases in the mRNA expression of MICU1 (to ∼55% of the control value; P = 0.03) and VDAC (to ∼75% of the control value; P = 0.007) in untreated Tfam KO muscle, and their mean expression was slightly higher with CsA treatment, but the differences did not reach statistical significance (Fig. 4D). MCU mRNA expression was unchanged in untreated and CsA-treated Tfam KO muscle.
We also measured gene expression of another tentative target of CsA, the Ca2+-calmodulin-dependent phosphatase calcineurin (CaN), and observed no change in mRNA in Tfam KO muscles either with or without CsA treatment (Supplementary Material, Fig. S5).
MCU is not a mitochondrial Ca2+ entry site during repeated contractions in Tfam KO fibers
We measured mitochondrial [Ca2+] in response to 50 tetanic stimulations induced in control solution, or during exposure to either CsA or the MCU inhibitor Ru360 (6) in FDB muscle fibers from Tfam KO mice (Supplementary Material, Fig. S6). In control solution, the repeated contractions resulted in more than a doubling of mitochondrial [Ca2+]. Exposure of Tfam KO fibers to 1.6 µm CsA led to a significantly reduced mitochondrial [Ca2+] increase uptake during the stimulation protocol (to ∼50% of the control value; P = 0.029). On the other hand, when fibers were exposed to Ru360 the increase in mitochondrial [Ca2+] was similar to that in the control solution.
Discussion
Mitochondrial myopathies refer to a group of genetic disorders mainly characterized by an impaired respiratory chain activity and muscle weakness. So far, no effective treatment for mitochondrial myopathies is available (2,24). Here we present data supporting usage of the CypD inhibitor CsA to prevent the deteriorating contractile function in mitochondrial myopathies.
We found that CypD expression was upregulated in muscles of both a mouse model and patients with mitochondrial myopathy. We observed markedly increased CypD levels in all patients with mtDNA depletion or mtDNA mutations, whereas the CypD level was unchanged in patients with mtDNA deletions. The lack of CypD upregulation in patients with mtDNA deletions might, in principle, be related to a low level of deletion or a low level of respiratory chain dysfunction. However, all patients with mtDNA deletion presented a clear mitochondrial dysfunction with decreased respiratory chain enzyme activities and mitochondrial ATP production rate (Supplementary Material, Table S1). Moreover, the mutation level was >50% for patients with mtDNA deletion or mt-DNA/tRNA mutations, i.e. patients showing no change or an upregulation of CypD level, respectively. It should also be pointed out that the patient with mtDNA depletion, who showed the largest increase in CypD, is the only one to have a mutation level <50% (Supplementary Material, Table S1). Thus, our results suggest that the increase in CypD expression depends on the type of mtDNA mutation rather than the level of mutation or the extent of respiratory chain dysfunction.
We previously reported that the mitochondrial matrix of Tfam KO but not control muscle fibers accumulates Ca2+ during repeated tetanic contractions, and this mitochondrial Ca2+ uptake was reduced by acute exposure to CsA (12). Furthermore, in muscle fibers of young Tfam KO mice (i.e. before overt signs of muscle dysfunction) mitochondrial [Ca2+] decreased promptly when contractions were stopped, whereas it remained elevated in muscle fibers of end-stage Tfam KO mice. Thus, the transient increase in mitochondrial [Ca2+] in the young Tfam KO mice might be beneficial by improving mitochondrial respiration, whereas the subsequent prolonged increase is likely to be detrimental (3–5). In this study, we exposed Tfam KO fibers to either CsA or Ru360 during 50 tetanic stimulation. As previously reported, CsA significantly reduced the mitochondrial Ca2+ uptake during the repeated contractions (12), whereas Ru360 had no effect. Although MCU is generally considered as the main mitochondrial Ca2+ entry site (6,7), our results indicate that MCU is not the entrance pathway for Ca2+ in Tfam KO fibers during repeated contractions. Interestingly, our results are in accordance with a recent study describing mitochondrial Ca2+ entry in ischemic rabbit cardiomyocytes that was partially inhibited by CsA; this mitochondrial Ca2+ uptake was reduced when mitochondria were moderately depolarized and the authors proposed that CsA might act on the mitochondrial permeability transition pore in a low-conductance mode (25). Furthermore, it has been previously demonstrated that endoplasmic reticulum and mitochondria interact through the VDAC1/Grp75/IP3R1 complex (26). CypD was subsequently found to be in close contact with this complex and genetic or pharmacological inhibition of CypD reduced mitochondrial Ca2+ uptake and decreased interactions between the proteins of the complex, thereby suggesting that CypD controls the Ca2+ transfer from the SR to mitochondria (27). Considering that we previously showed that CsA-sensitive mitochondrial Ca2+-uptake in the Tfam KO muscle fibers was not accompanied by any detectable decrease in the mitochondrial membrane potential (12), one may suggest that CsA inhibits, at least in part, CypD at the SR–mitochondria interface, thereby preventing mitochondrial Ca2+ overload during periods of repeated contractions.
Considering the decreased mitochondrial Ca2+ uptake in the Tfam KO fibers when exposed to CsA, we examined the effect of 4 weeks of CsA treatment of Tfam KO mice, commencing at 12 weeks of age when the disease starts to progress from moderate to terminal. CsA treatment resulted in a significantly prolonged lifespan of the Tfam KO mice, and it counteracted the decrease in muscle mass and force production. Our previous results link the muscle weakness in Tfam KO muscle fibers to decreased SR Ca2+ release during contractions due to reduced concentration of the SR Ca2+-buffering protein CASQ1 and hence decreased SR Ca2+ storage (12). Importantly, the decreases in CASQ1 protein expression and SR Ca2+ release during contraction in Tfam KO muscle were prevented by CsA treatment. Therefore, the markedly improved contractile performance in the CsA-treated Tfam KO mice can be explained by the greater SR Ca2+ release during contraction due to better Ca2+ storage (see Fig. 3). However, the signaling pathway(s) linking mitochondrial Ca2+ loading to the expression of CASQ1, and hence SR Ca2+ storage, is unknown and was not experimentally addressed in this study. One might speculate that excessive mitochondrial Ca2+ loading in untreated Tfam KO muscle triggers signaling to reduce the cellular Ca2+ stores. This would limit the mitochondrial Ca2+ load, but the drawback is decreased SR Ca2+ release during contractions resulting in progressive muscle weakness. CsA treatment then prevents the excessive mitochondrial Ca2+ uptake in Tfam KO muscles, which results in maintained SR Ca2+ storage and preserved force production.
Tfam KO muscle displays a progressive respiratory chain dysfunction due to decreases in mtDNA-encoded proteins (11). CsA treatment did not prevent the decrease in the mtDNA-encoded COX1 protein expression, nor did it affect the activity of the nuclear DNA-encoded citrate synthase. Thus, we observed no signs of CsA treatment having a beneficial effect on the deteriorating mitochondrial energy production in Tfam KO muscle. Still, CsA treatment prolonged the lifespan of the Tfam KO mice, which implies that the dominating problem in this model of mitochondrial myopathy is the progressive muscle weakness rather than the energy deficiency.
CsA also targets the CaN (28). However, several findings indicate that CaN is not the key target of CsA in relation to its beneficial effects on disorders with mitochondrial involvement. First, CsA was found to have beneficial effects in collagen VI myopathies and in muscular dystrophy where mitochondrial dysfunction is a key aberration (29–33). Second, CsA analogs that do not affect CaN counteracted the mitochondrial dysfunctions in collagen VI myopathies (29,34). Third, mitochondrial defects do not occur in mice deficient of collagen VI when these mice are crossed with CypD-deficient mice (35). Fourth, our results show no difference in CaN mRNA expression between control muscle and CsA-treated and untreated Tfam KO muscle. Fifth, acute exposure to CsA inhibits mitochondrial Ca2+ uptake in Tfam KO fibers during repeated contractions (12). Thus, in conditions with mitochondrial dysfunction, CsA most likely exerts its positive effects by acting directly on the mitochondria rather than via CaN, although the latter alternative cannot be excluded.
In conclusion, our results emphasize CsA treatment as a promising therapy for patients with mitochondrial myopathies and tentatively other disorders with critical mitochondrial involvement.
Materials and Methods
Human subjects
All subjects included in the study were referred to the Center of Inherited Metabolic Disease (CMMS) at the Karolinska University Hospital for muscle biopsy and investigation of mitochondrial disease. Subject characteristics are summarized in Supplementary Material, Table S1. Respiratory chain function was assessed by measuring ATP production and respiratory chain enzyme activities in mitochondria isolated from small muscle biopsy samples (36). The three control subjects were selected based on normal mitochondrial biochemistry, normal muscle histology and no signs of mitochondrial dysfunction in skeletal muscle. In contrast, all patients had confirmed mitochondrial disease and were grouped according to whether they had mtDNA deletion, mtDNA depletion, mt-tRNA mutation or mutations in mitochondrial subunits of respiratory chain enzyme complex I. Further, all patients presented with either single or a combined respiratory chain enzyme complex dysfunction in skeletal muscle. The Regional Ethics Committee at Karolinska Institutet approved the use of patient material in this study.
Animals
Skeletal muscle-specific Tfam KO mice and control littermates were used for the experiments (11). Mice were housed in an environment-controlled facility (12–12 h light–dark cycle, 22°C) and received water and standard food ad libitum until the end of experiments. Tfam KO mice were treated with CsA (120 µg/day), which was administered via osmotic pumps implanted subcutaneously on the back under anesthesia. In order to address the potential adverse effects of pump implantation, an identical procedure but without addition of CsA was conducted on the control littermates. The duration of the CsA treatment spanned from 12 to 16 weeks of age, covering the period when the myopathy goes from mild to terminal. Experiments on muscles of CsA-treated mice were performed at the end of the treatment period. Untreated Tfam KO mice would frequently not survive to 16 weeks of age. Experiments on untreated Tfam KO mice were therefore performed either when they entered the end stage, which involves a rapid loss of body weight (20% loss in maximum body weight), or when they reached 16 weeks of age. Mice were euthanized by rapid neck disarticulation, and the muscles were excised. The survival curve was designed according to the Kaplan–Meier estimate. All animal experiments were approved by the Stockholm North Local Animal Ethics Committee.
Force and [Ca2+]i measurements
Fast-twitch single FDB fibers were isolated by mechanical dissection and suspended between an adjustable hook and an Akers AE801 force transducer in the perfusion channel of a muscle bath placed on the stage of an inverted microscope. The fiber was superfused at room temperature (25°C) in Tyrode solution (in mm): NaCl, 121; KCl, 5.0; CaCl2, 1.8; MgCl2, 0.5; NaH2PO4, 0.4; NaHCO3, 24.0; EDTA, 0.1; glucose, 5.5; 0.2% fetal calf serum. The solution was bubbled with 95% O2–5% CO2. Fibers were stimulated with supramaxima current pulses with a pulse duration of 0.5 ms delivered via platinum plate electrodes lying parallel to the fiber. Tetanic force was measured as the mean over 100 ms, where it was maximal and expressed relative to the fiber cross-sectional area.
[Ca2+]i was measured with the fluorescent Ca2+ indicator indo-1 (Molecular Probes/Invitrogen). Indo-1 was mixed in a buffer (150 mm KCl, 10 mm HEPES, pH 7.1) to a final concentration of 10 mm and microinjected into fibers. The dye was excited with light at 360 ± 5 nm, and the light emitted at 405 ± 5 and 495 ± 5 nm was measured with two photomultiplier tubes. The 405/495 ratio (R) was translated to [Ca2+]i using the following equation:
where KD is the apparent dissociation constant of the dye, β the ratio of the 495 nm signals at very low and saturating [Ca2+]i, and Rmin and Rmax the ratios at very low and saturating [Ca2+]i, respectively (37). The [Ca2+]i–frequency and force–frequency relationships were obtained by stimulating fibers with 350 ms duration tetani at 10–150 Hz at 1 min intervals.
The force–frequency relationship was measured in isolated whole EDL muscles. These were mounted at optimal length in a stimulation chamber filled with Tyrode solution (30°C) bubbled with 95% O2–5% CO2. Muscles were stimulated with 300 ms trains of current pulses at 10–150 Hz at 1 min intervals and force was measured. Muscle length was measured, and muscles were then removed from the chamber, tendons were cut off and muscle weight was measured. The specific force (i.e. force per cross-sectional area) produced by each muscle was calculated from the absolute force, muscle weight and muscle length, assuming a density of 1.056 g/ml.
Measurements of mitochondrial [Ca2+]
Mechanically dissected Tfam KO FDB fibers were incubated in 5 µm Rhod-2 AM (Invitrogen, Molecular Probes) for 20 min at room temperature and then washed for 20 min. Measurements were performed using a Bio-Rad MRC 1024 confocal unit with a krypton–argon mixed gas laser attached to a Nikon Diaphot 200 inverted microscope. Rhod-2 was excited with 568 nm light, and the emitted light was collected through a 585 nm long-pass filter. Confocal images were obtained before and immediately after a series of 50 repeated tetani (70 Hz, 350 ms stimulation trains given at 2 s interval). Images were analyzed using ImageJ, and data are expressed as F/F0, i.e. the fluorescence intensity after and before the repeated contractions, respectively.
To investigate the potential sites of Ca2+ entry into mitochondria, fibers were exposed to either CsA or Ru360. CsA (1.6 µm) was applied to the fibers for 5 min before and during the stimulation protocol. The MCU inhibitor Ru360, a tribasic compound consisting of two ruthenium amine–formate nuclei bridged with an oxygen atom, was shown to inhibit Ca2+ uptake into isolated mitochondria (IC50 = 0.184 nM) but not isolated SR (38,39). Moreover, the addition of Ru360 for 30 min was shown to block mitochondrial Ca2+ uptake into intact cardiomyctes, albeit with a much higher IC50 than for isolated mitochondria (38,39). In initial experiments, we superfused the FDB fibers with 1 µm Ru360 but found no effect on the increase in mitochondrial Ca2+ uptake during repeated tetanic contractions. Therefore, we adopted a procedure of first injecting 1 mm Ru360 (dissolved in 150 mm KCl and 10 mm HEPES, pH 7) into the fiber, reaching an estimated cytosolic concentration of Ru360 of ≥ 1 µm, and thereafter superfused the fiber with 1 µm Ru360 for 20 min before starting the stimulation protocol.
RNA isolation and quantitative RT-PCR
Total RNA was isolated from EDL muscle, using the ToTALLY RNA kit (Ambion, Life Technologies) and quantified with a Qubit fluorometer (Life Technologies). Reverse transcription was performed using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Life Technologies). Quantitative RT-PCR (qRT-PCR) was performed on a ViiA 7 system (Life Technologies), using the TaqMan Universal Master Mix II, with UNG and TaqMan assays (Life Technologies, assay number given in brackets) or Platinum SYBR Green qPCR supermix-UDG (Life Technologies) (for CypD). Expression of transcripts encoding for proteins involved in SR Ca2+ handling [CASQ1 (Mm01293333_m1) and DHPR (Mm01306805_m1)], mitochondrial Ca2+ handling [CypD (CypD-for: aaggatggcaaggattgaaa, CypD-rev: ctttaagcaattctgcctgga), ANT (Mm00846873_g1), VDAC (Mm00834272_m1), MCU (Mm01168773_m1), MICU1 (Mm00522778_m1)] and CaN (Mm01317674_m1) was measured. TaqMan assays were normalized to β2-microglobulin (B2M) (Mm00437762_m1), while the SyBr green assay was normalized to 18S rRNA transcript levels (18S-for: caacttcttagagggacaagtgg, 18S-rev: cggacatctaagggcatcac).
Western blotting
Western blot analysis of CyD and VDAC levels in patients were performed on mitochondrial preparations isolated from skeletal muscle biopsies, as previously described (36). Equal amounts of protein were loaded into each lane on the gel. VDAC expression did not differ between the groups and was used as the loading control.
Muscles from both CsA-treated and untreated control and Tfam KO mice were homogenized in ice-cold lysis buffer (in mm): HEPES, 20; NaCl, 150; EDTA, 5; KF, 25; Na3VO4, 1; glycerol (20% v/v); Triton X-100 (0.5% v/v); protease inhibitor cocktail (Roche) (pH 7.6) and centrifuged at 700g for 10 min. Supernatant protein content was determined using Bradford assay (Bio-Rad). Samples were diluted 1:1 with Laemmli sample buffer (Bio-Rad) containing 5% β-mercaptoethanol and heated to 70°C for 10 min. Further, 20 µg protein were separated on a NuPAGE Novex 4–12% Bis–Tris Gels (NP0336PK2, NuPAGE, Invitrogen) and transferred onto Immobilon-FL PVDF membrane (IPFL00010, Millipore). Membranes were blocked with LI-COR blocking buffer (927-40000, LI-COR), followed by overnight incubation at 4°C with primary antibodies diluted in blocking buffer: mouse anti-DHPR (ab2864, Abcam), rabbit anti-CASQ1 (ab3516, Abcam), mouse anti-oxphos complex IV, subunit 1 (COX1; #459600, Invitrogen) and mouse anti-cyclophilin D (CypD; ab110324, Abcam). Membranes were then washed and incubated with secondary antibody IRDye 680-conjugated donkey-anti-mouse IgG and IRDye 800-conjugated donkey-anti-rabbit IgG (926-68072, 926-32213, LI-COR). Immunoreactive bands were visualized using infrared fluorescence (IR-Odyssey scanner, LI-COR Biosciences), and band densities were measured using Image Studio v 2.0.38 (LI-COR Biosciences). DHPR was used as the loading control given that no difference in protein level was observed between control and Tfam KO groups (see Supplementary Material, Fig. S3).
Enzyme activity assay
EDL muscles were homogenized with a motor-driven ground glass homogenizer in ice-cold buffer (50 µl/mg wet weight) consisting of (mm): KH2PO4, 50; EDTA, 1; Triton X-100, 0.05% v/v (pH 7.5). The homogenate was centrifuged at 1400g for 1 min at 4°C. The supernatant was used for analysis of CS activity using standard spectrophotometric techniques (40). Activities were measured at room temperature under conditions that yielded linearity with respect to extract volume and time. The supernatant protein content was determined using the Bradford assay (Bio-Rad, Hemel Hempstead, UK), and activities were adjusted for protein content.
Body composition analysis
Dual-emission X-ray absorptiometry (DXA) studies were performed to determine body composition. Anesthesia was induced and maintained by 2.2% isoflurane (Forene; Abbot Scandinavia AB, Solna, Sweden) in air. Whole-body scans were performed using a Lunar PIXImus™ densitometer (GE Medical-Lunar, Madison, WI). The instrument was calibrated with an aluminum/lucite phantom, according to the manufacturer's instructions. The phantom was analyzed before animal testing for quality control purposes, and scans were analyzed with the software provided by the manufacturer. The results are presented as g for hindlimb lean and fat mass. CsA-treated mice were tested at the end of the treatment period, and untreated mice were tested at the same age or when they entered the end stage.
Statistics
Statistical analyses were performed with SigmaPlot 13 (Systat Software Inc.). Student's unpaired t tests were used to compare CsA-treated versus untreated Tfam KO mice for lean and fat body mass, control versus Tfam KO mice and control subjects versus patients for CypD protein levels. One-way analysis of variance (ANOVA) was used to compare tetanic force, mRNA, CS activity and protein expression level. Two-factor (group × stimulation frequency) ANOVAs with repeated measures on stimulation frequency were used to compare force production or [Ca2+]i. When a main effect or a significant interaction was found, Newman–Keuls post-hoc analysis was used. Significance was accepted when P < 0.05. Data are presented as mean ± SEM, except for Figure 1D that shows the individual values of each subject.
Supplementary Material
Supplementary Material is available at HMG online.
Conflict of Interest statement. None declared.
Funding
This work was supported by grants from the Swedish Research Council, the Stockholm County Council (SLL20130090), the Swedish National Center for Sports Research, Association Française contre les Myopathies (AFM-TELETHON; to C.G.). Research reported in this publication was supported by The National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number 1F32AR057619 (NIAMS; to A.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. A.W. is supported by the Swedish Foundation for Strategic Research (SSF) and is a Ragnar Söderberg Fellow.
Supplementary Material
References
- 1.Schmiedel J., Jackson S., Schafer J., Reichmann H. (2003) Mitochondrial cytopathies. J. Neurol., 250, 267–277. [DOI] [PubMed] [Google Scholar]
- 2.Larsson N.G., Oldfors A. (2001) Mitochondrial myopathies. Acta Physiol. Scand., 171, 385–393. [DOI] [PubMed] [Google Scholar]
- 3.Brookes P.S., Yoon Y., Robotham J.L., Anders M.W., Sheu S.S. (2004) Calcium, ATP, and ROS: a mitochondrial love–hate triangle. Am. J. Physiol. Cell Physiol., 287, C817–C833. [DOI] [PubMed] [Google Scholar]
- 4.Duchen M.R. (2000) Mitochondria and calcium: from cell signalling to cell death. J. Physiol., 529, 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizzuto R., De Stefani D., Raffaello A., Mammucari C. (2012) Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol., 13, 566–578. [DOI] [PubMed] [Google Scholar]
- 6.Baughman J.M., Perocchi F., Girgis H.S., Plovanich M., Belcher-Timme C.A., Sancak Y., Bao X.R., Strittmatter L., Goldberger O., Bogorad R.L. et al. (2011) Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature, 476, 341–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Stefani D., Raffaello A., Teardo E., Szabo I., Rizzuto R. (2011) A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature, 476, 336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan X., Liu J., Nguyen T., Liu C., Sun J., Teng Y., Fergusson M.M., Rovira I.I., Allen M., Springer D.A. et al. (2013) The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat. Cell Biol., 15, 1464–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor R.W., Turnbull D.M. (2005) Mitochondrial DNA mutations in human disease. Nat. Rev. Genet., 6, 389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallace D.C., Fan W. (2009) The pathophysiology of mitochondrial disease as modeled in the mouse. Genes Dev., 23, 1714–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wredenberg A., Wibom R., Wilhelmsson H., Graff C., Wiener H.H., Burden S.J., Oldfors A., Westerblad H., Larsson N.G. (2002) Increased mitochondrial mass in mitochondrial myopathy mice. Proc. Natl. Acad. Sci. USA, 99, 15066–15071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aydin J., Andersson D.C., Hanninen S.L., Wredenberg A., Tavi P., Park C.B., Larsson N.G., Bruton J.D., Westerblad H. (2009) Increased mitochondrial Ca2+ and decreased sarcoplasmic reticulum Ca2+ in mitochondrial myopathy. Hum. Mol. Genet., 18, 278–288. [DOI] [PubMed] [Google Scholar]
- 13.Azzi J.R., Sayegh M.H., Mallat S.G. (2013) Calcineurin inhibitors: 40 years later, can't live without. J. Immunol., 191, 5785–5791. [DOI] [PubMed] [Google Scholar]
- 14.Connern C.P., Halestrap A.P. (1992) Purification and N-terminal sequencing of peptidyl–prolyl cis–trans-isomerase from rat liver mitochondrial matrix reveals the existence of a distinct mitochondrial cyclophilin. Biochem. J., 284, 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giorgio V., Soriano M.E., Basso E., Bisetto E., Lippe G., Forte M.A., Bernardi P. (2010) Cyclophilin D in mitochondrial pathophysiology. Biochim. Biophys. Acta, 1797, 1113–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crompton M., Ellinger H., Costi A. (1988) Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem. J., 255, 357–360. [PMC free article] [PubMed] [Google Scholar]
- 17.Broekemeier K.M., Dempsey M.E., Pfeiffer D.R. (1989) Cyclosporin A is a potent inhibitor of the inner membrane permeability transition in liver mitochondria. J. Biol. Chem., 264, 7826–7830. [PubMed] [Google Scholar]
- 18.Halestrap A.P., Davidson A.M. (1990) Inhibition of Ca2+-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl–prolyl cis–trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem. J., 268, 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernardi P. (2013) The mitochondrial permeability transition pore: a mystery solved? Front. Physiol., 4, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kokoszka J.E., Waymire K.G., Levy S.E., Sligh J.E., Cai J., Jones D.P., MacGregor G.R., Wallace D.C. (2004) The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature, 427, 461–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schein S.J., Colombini M., Finkelstein A. (1976) Reconstitution in planar lipid bilayers of a voltage-dependent anion-selective channel obtained from paramecium mitochondria. J. Membr. Biol., 30, 99–120. [DOI] [PubMed] [Google Scholar]
- 22.Marchi S., Pinton P. (2014) The mitochondrial calcium uniporter complex: molecular components, structure and physiopathological implications. J. Physiol., 592, 829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perocchi F., Gohil V.M., Girgis H.S., Bao X.R., McCombs J.E., Palmer A.E., Mootha V.K. (2010) MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nature, 467, 291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moggio M., Colombo I., Peverelli L., Villa L., Xhani R., Testolin S., Di Mauro S., Sciacco M. (2014) Mitochondrial disease heterogeneity: a prognostic challenge. Acta Myol., 33, 86–93. [PMC free article] [PubMed] [Google Scholar]
- 25.Seidlmayer L.K., Juettner V.V., Kettlewell S., Pavlov E.V., Blatter L.A., Dedkova E.N. (2015) Distinct mPTP activation mechanisms in ischaemia–reperfusion: contributions of Ca2+, ROS, pH, and inorganic polyphosphate. Cardiovasc. Res., 106, 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szabadkai G., Bianchi K., Varnai P., De Stefani D., Wieckowski M.R., Cavagna D., Nagy A.I., Balla T., Rizzuto R. (2006) Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol., 175, 901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paillard M., Tubbs E., Thiebaut P.A., Gomez L., Fauconnier J., Da Silva C.C., Teixeira G., Mewton N., Belaidi E., Durand A. et al. (2013) Depressing mitochondria-reticulum interactions protects cardiomyocytes from lethal hypoxia-reoxygenation injury. Circulation, 128, 1555–1565. [DOI] [PubMed] [Google Scholar]
- 28.Liu J., Farmer J.D. Jr., Lane W.S., Friedman J., Weissman I., Schreiber S.L. (1991) Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell, 66, 807–815. [DOI] [PubMed] [Google Scholar]
- 29.Angelin A., Tiepolo T., Sabatelli P., Grumati P., Bergamin N., Golfieri C., Mattioli E., Gualandi F., Ferlini A., Merlini L. et al. (2007) Mitochondrial dysfunction in the pathogenesis of Ullrich congenital muscular dystrophy and prospective therapy with cyclosporins. Proc. Natl. Acad. Sci. USA, 104, 991–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hicks D., Lampe A.K., Laval S.H., Allamand V., Jimenez-Mallebrera C., Walter M.C., Muntoni F., Quijano-Roy S., Richard P., Straub V. et al. (2009) Cyclosporine A treatment for Ullrich congenital muscular dystrophy: a cellular study of mitochondrial dysfunction and its rescue. Brain, 132, 147–155. [DOI] [PubMed] [Google Scholar]
- 31.Irwin W.A., Bergamin N., Sabatelli P., Reggiani C., Megighian A., Merlini L., Braghetta P., Columbaro M., Volpin D., Bressan G.M. et al. (2003) Mitochondrial dysfunction and apoptosis in myopathic mice with collagen VI deficiency. Nat. Genet., 35, 367–371. [DOI] [PubMed] [Google Scholar]
- 32.Merlini L., Angelin A., Tiepolo T., Braghetta P., Sabatelli P., Zamparelli A., Ferlini A., Maraldi N.M., Bonaldo P., Bernardi P. (2008) Cyclosporin A corrects mitochondrial dysfunction and muscle apoptosis in patients with collagen VI myopathies. Proc. Natl. Acad. Sci. USA, 105, 5225–5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Millay D.P., Sargent M.A., Osinska H., Baines C.P., Barton E.R., Vuagniaux G., Sweeney H.L., Robbins J., Molkentin J.D. (2008) Genetic and pharmacologic inhibition of mitochondrial-dependent necrosis attenuates muscular dystrophy. Nat. Med., 14, 442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tiepolo T., Angelin A., Palma E., Sabatelli P., Merlini L., Nicolosi L., Finetti F., Braghetta P., Vuagniaux G., Dumont J.M. et al. (2009) The cyclophilin inhibitor Debio 025 normalizes mitochondrial function, muscle apoptosis and ultrastructural defects in Col6a1−/− myopathic mice. Br. J. Pharmacol., 157, 1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palma E., Tiepolo T., Angelin A., Sabatelli P., Maraldi N.M., Basso E., Forte M.A., Bernardi P., Bonaldo P. (2009) Genetic ablation of cyclophilin D rescues mitochondrial defects and prevents muscle apoptosis in collagen VI myopathic mice. Hum. Mol. Genet., 18, 2024–2031. [DOI] [PubMed] [Google Scholar]
- 36.Wibom R., Hagenfeldt L., von Dobeln U. (2002) Measurement of ATP production and respiratory chain enzyme activities in mitochondria isolated from small muscle biopsy samples. Anal. Biochem., 311, 139–151. [DOI] [PubMed] [Google Scholar]
- 37.Andrade F.H., Reid M.B., Allen D.G., Westerblad H. (1998) Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J. Physiol., 509, 565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Jesus Garcia-Rivas G., Guerrero-Hernandez A., Guerrero-Serna G., Rodriguez-Zavala J.S., Zazueta C. (2005) Inhibition of the mitochondrial calcium uniporter by the oxo-bridged dinuclear ruthenium amine complex (Ru360) prevents from irreversible injury in postischemic rat heart. FEBS J., 272, 3477–3488. [DOI] [PubMed] [Google Scholar]
- 39.Matlib M.A., Zhou Z., Knight S., Ahmed S., Choi K.M., Krause-Bauer J., Phillips R., Altschuld R., Katsube Y., Sperelakis N. et al. (1998) Oxygen-bridged dinuclear ruthenium amine complex specifically inhibits Ca2+ uptake into mitochondria in vitro and in situ in single cardiac myocytes. J. Biol. Chem., 273, 10223–10231. [DOI] [PubMed] [Google Scholar]
- 40.Bass A., Brdiczka D., Eyer P., Hofer S., Pette D. (1969) Metabolic differentiation of distinct muscle types at the level of enzymatic organization. Eur. J. Biochem., 10, 198–206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.