Abstract
Limb-girdle muscular dystrophy type 1D (LGMD1D) is caused by dominantly inherited missense mutations in DNAJB6, an Hsp40 co-chaperone. LGMD1D muscle has rimmed vacuoles and inclusion bodies containing DNAJB6, Z-disc proteins and TDP-43. DNAJB6 is expressed as two isoforms; DNAJB6a and DNAJB6b. Both isoforms contain LGMD1D mutant residues and are expressed in human muscle. To identify which mutant isoform confers disease pathogenesis and generate a mouse model of LGMD1D, we evaluated DNAJB6 expression and localization in skeletal muscle as well as generating DNAJB6 isoform specific expressing transgenic mice. DNAJB6a localized to myonuclei while DNAJB6b was sarcoplasmic. LGMD1D mutations in DNAJB6a or DNAJB6b did not alter this localization in mouse muscle. Transgenic mice expressing the LGMD1D mutant, F93L, in DNAJB6b under a muscle-specific promoter became weak, had early lethality and developed muscle pathology consistent with myopathy after 2 months; whereas mice expressing the same F93L mutation in DNAJB6a or overexpressing DNAJB6a or DNAJB6b wild-type transgenes remained unaffected after 1 year. DNAJB6b localized to the Z-disc and DNAJB6b-F93L expressing mouse muscle had myofibrillar disorganization and desmin inclusions. Consistent with DNAJB6 dysfunction, keratin 8/18, a DNAJB6 client also accumulated in DNAJB6b-F93L expressing mouse muscle. The RNA-binding proteins hnRNPA1 and hnRNPA2/B1 accumulated and co-localized with DNAJB6 at sarcoplasmic stress granules suggesting that these proteins maybe novel DNAJB6b clients. Similarly, hnRNPA1 and hnRNPA2/B1 formed sarcoplasmic aggregates in patients with LGMD1D. Our data support that LGMD1D mutations in DNAJB6 disrupt its sarcoplasmic function suggesting a role for DNAJB6b in Z-disc organization and stress granule kinetics.
Introduction
Missense mutations in DNAJB6 cause a dominantly inherited progressive muscle disease termed limb-girdle muscular dystrophy type 1D (LGMD1D) (1,2). DNAJB6 is a ubiquitously expressed HSP40 co-chaperone that facilitates HSP70 functionality via its J domain (3). DNAJB6 like other DNAJB family members has a canonical J domain, a C terminal region and a glycine and phenylalanine rich region or G/F domain (3). All reported LGMD1D mutations reside within this G/F domain (1,2,4–6). Patients with LGMD1D have insidious onset of weakness in the second to sixth decade of life that affects their ability to ambulate (1,2,4,5,7). LGMD1D patient muscle tissue is characteristically myopathic with myofibrillar protein aggregates and rimmed vacuoles (1,2).
Several muscle diseases or ‘chaperonopathies’ are associated with dominantly inherited mutations in chaperone proteins. Mutations in CRYAB (αB-crystallin) and BCL2-associated athanogene 3 (BAG3) both cause a myofibrillar myopathy (MFM) (8,9). The pathologic features of a myofibrillar myopathy are similar to LGMD1D with protein inclusions that contain Z-disc elements and rimmed vacuoles. Studies suggest that αB-crystallin mediates desmin stability whereas BAG3 mediates the autophagic degradation of filamin C (10,11). DNAJB6 has been reported to localize to the Z-disc in both normal and LGMD1D patient muscle (2). Whether DNAJB6 has a clear myofibrillar client protein that mediates pathology in skeletal muscle is unclear.
DNAJB6 is expressed as two isoforms with distinct cellular localizations (12). DNAJB6a localizes to the nucleus and DNAJB6b localizes to both the cytoplasm and nucleus in tissue culture cells. In cell culture, DNAJB6 is a potent inhibitor of expanded polyglutamine (polyQ) aggregation and also rescues protein aggregate toxicity in human embryonic kidney cells (12). LGMD1D mutations in DNAJB6b have been shown to disrupt this function leading to reduced disaggregation of expanded polyQ-containing huntingtin protein (2). Upon heat shock, DNAJB6b redistributes from the cytoplasm to the nucleus where it co-localizes with TDP-43 positive nuclear stress granules (13). Overexpression of DNAJB6b facilitates the dissolution of TDP-43 granules upon heat shock recovery in Hela cells (13). Moreover, LGMD1D mutant DNAJB6b expression enhances TDP-43 aggregate formation and slows its disaggregation following heat shock (13). However, the role of DNAJB6 in differentiated skeletal muscle and the effect of LGMD1D mutations in DNAJB6 on muscle protein homeostasis is unknown.
Knockdown of DNAJB6b in zebrafish showed a muscle fiber detachment phenotype consistent with a role for DNAJB6b in myofiber integrity (2). Expression of LGMD1D mutant DNAJB6b in zebrafish recapitulated this loss-of-function phenotype. Interestingly, expression of LGMD1D mutant DNAJB6a (nuclear isoform) was not associated with a muscle phenotype in zebrafish (2). However, it is important to note that zebrafish only express one DNAJB6 isoform, DNAJB6b which can be complemented by human DNAJB6b but not human DNAJB6a (2). Therefore whether LGMD1D mutant toxicity is due to dysfunction of DNAJB6b, DNAJB6a or both isoforms is not established.
Over the past 3 years, the genetic etiology of autosomal dominantly inherited limb-girdle muscular dystrophies has more than doubled (14). Specifically, next generation sequencing and proteomic approaches have identified mutations in DNAJB6 (LGMD1D), DES (LGMD1), TNPO3 (LGMD1F) and HNRPDL (LGMD1G) (1,2,5–18). Our study begins to explore the molecular mechanism and develop a pre-clinical model for LGMD1D.
Results
Skeletal muscle expresses both DNAJB6 isoforms
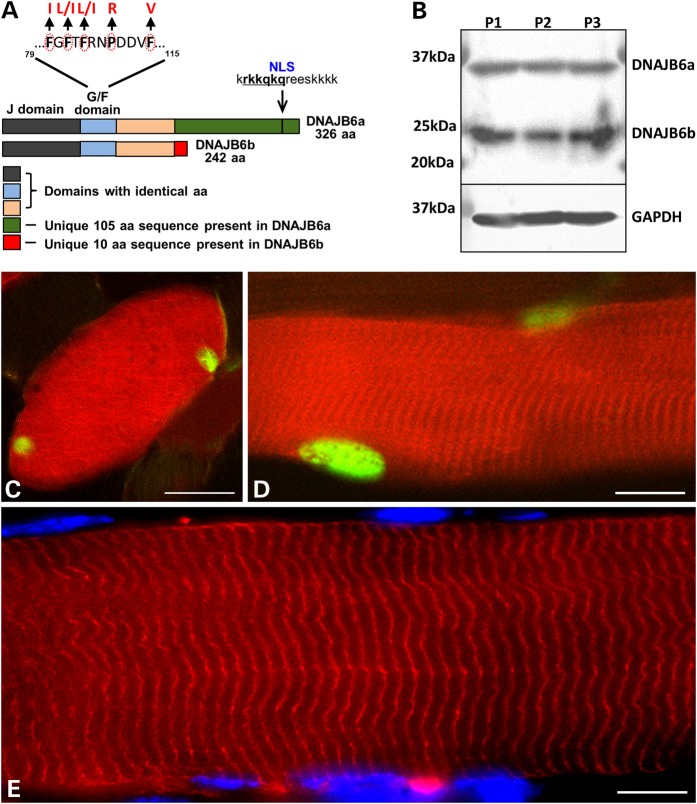
DNAJB6 is expressed as two alternatively spliced isoforms, an A isoform that is 326 amino acids long and a shorter B isoform (242 amino acids). Both isoforms contain residues mutated in LGMD1D within the G/F domain suggesting that each isoform may confer pathogenicity (Fig. 1A). To determine which DNAJB6 isoform was expressed in skeletal muscle, we immunoblotted lysates of human skeletal muscle with an antibody to DNAJB6. Consistent with both isoforms being expressed, two bands were present migrating at ∼37 kDa and 25 kDa as previously reported (4) (Fig. 1B). To further evaluate the cellular localization of each isoform in myofibers, we co-electroporated a GFP-tagged DNAJB6a and an mCherry tagged DNAJB6b into mouse tibialis anterior skeletal muscle. Similar to results previously observed in tissue culture cells, DNAJB6a was nuclear and DNAJB6b sarcoplasmic in mouse skeletal muscle (12) (Fig. 1C and D). In addition, DNAJB6b was incorporated into sarcomeric structures consistent with previous reports suggesting that it is a component of the Z-disc (2) (Fig. 1E).
Figure 1.
DNAJB6a and DNAJB6b expression in skeletal muscle. (A) Schematic of DNAJB6a and DNAJB6b isoforms. Note that all five LGMD1D mutant residues reside within the G/F domain that is common to both isoforms. (B) DNAJB6 immunoblot from three different human skeletal muscle lysates showing a DNAJB6a and DNAJB6b isoform. GAPDH is loading control. (C–E) Mouse tibialis anterior muscle was electroporated with constructs expressing both mCherry-DNAJB6b and GFP-DNAJB6a (C and D) or mCherry-DNAJB6b alone (E). DNAJB6b (red) is sarcoplasmic and associates with sarcomeric structures whereas DNAJB6a (green) is exclusively myonuclear. Bar (C and D), 20 µm and Bar (E), 10 µm.
DNAJB6b confers LGMD1D pathogenicity
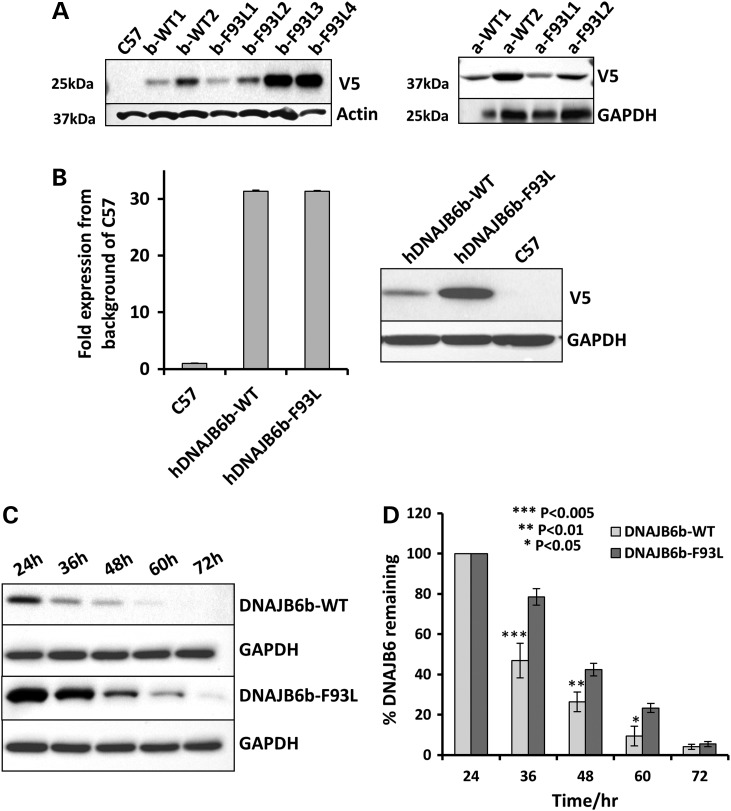
To establish a mouse model of LGMD1D and determine which DNAJB6 isoform (a or b) is pathogenic in the context of an LGMD1D missense mutation, we generated four independent transgenic mouse expression constructs. Two constructs contained wild-type (WT) human DNAJB6 either hDNAJB6a-WT or hDNAJB6b-WT and two constructs contained hDNAJB6 with the most common LGMD1D mutation, F93L, hDNAJB6a-F93L or hDNAJB6b-F93L with N-terminal V5 tags under a muscle-specific muscle creatine kinase (MCK) promoter. Using these constructs, we established 15 founder lines of transgenic mice that expressed transgenic V5-DNAJB6 at varying levels (some of the expressing lines are shown in Fig. 2A). First generation hybrid mice were evaluated for levels of protein expression and selected lines were backcrossed at least five generations to C57B6 mice in order maintain similar backgrounds. All studies were performed using two independent transgenic lines of the same construct with the exception of hDNAJB6b-F93L for which three independent lines were maintained with varied levels of protein expression. For ease of readership, lines will be referred to as hDNAJB6a-WT, hDNAJB6b-WT, hDNAJB6a-F93L or hDNAJB6b-F93L.
Figure 2.
LGMD1D mutant DNAJB6b is more stable than DNAJB6b-WT in vivo. (A) Immunoblot of skeletal muscle lysates from control (C57), V5-hDNAJB6b-WT (b-WT), V5-hDNAJB6b-F93L (b-F93L), V5-hDNAJB6a-WT (a-WT) and V5-hDNAJB6a-F93L (a-F93L) expressing mice using a V5 antibody. Actin and GAPDH are loading controls. (B) Quantitative PCR expression matched V5-hDNAJB6b-WT and V5-hDNAJB6b-F93L lines and corresponding V5 immunoblot from the same lysate. Note that hDNAJBb-F93L is more stable. (C) Tetracycline inducible isogenic 293 cell lines expressing V5-DNAJB6b-WT or V5-DNAJB6b-F93L were induced for 24 h and then tetracycline was removed and lysates were immunoblotted for V5 and GAPDH at the indicated times. (D) Graphical representation of the results in C from three independent experiments.
Surprisingly, hDNAJB6b-F93L protein levels were consistently higher than hDNAJB6b-WT protein levels as visualized via V5 immunoblot (Fig. 2A). To determine if this was due to an increase in transgene expression or an increase in hDNAJB6b-F93L protein stability, we performed qPCR on the hDNAJB6b transgene (Fig. 2B). Even when matched for expression via qPCR, hDNAJB6b-F93L protein levels were greater as assessed by immunoblot utilizing a V5-specific antibody suggesting that hDNAJB6b-F93L is more stable than hDNAJB6b-WT (Fig. 2B). To further asses this, we evaluated the rate of DNAJB6b degradation via immunoblot in cells. Using lysates from isogenic tetracycline inducible 293 cells stably expressing V5-hDNAJB6b-WT and V5-hDNAJB6b-F93L following 2 days of tetracycline induction and subsequent removal of tetracycline, we immunoblotted for V5 (Fig. 2C and D). Similar to a previous report, hDNAJB6b-F93L is degraded more slowly as compared with hDNAJB6b-WT (2).
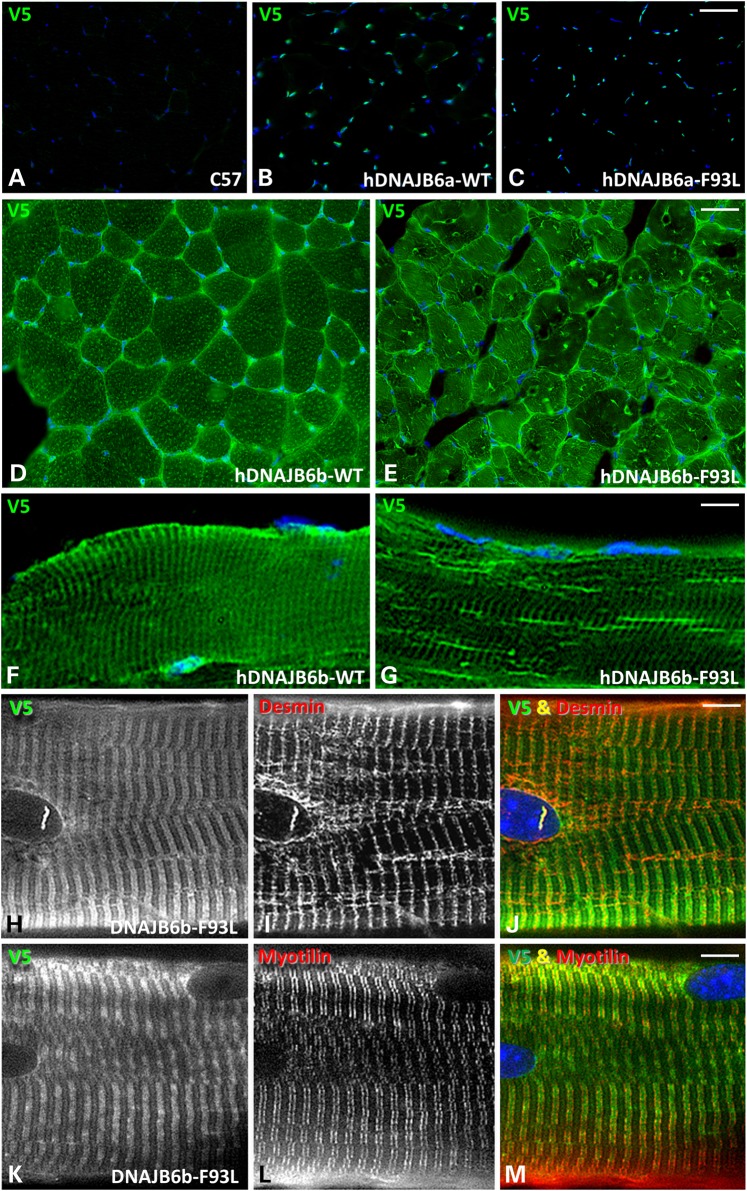
To evaluate the localization of transgenic expressed V5-hDNAJB6, we sacrificed 4-month old hDNAJB6a-WT, hDNAJB6b-WT, hDNAJB6a-F93L and hDNAJB6b-F93L mice and performed immunohistochemistry for V5 on isolated tibialis anterior, gastrocnemius and quadriceps. As expected hDNAJB6a-WT and hDNAJB6a-F93L were predominantly myonuclear (Fig. 3A–C). In contrast, hDNAJB6b-WT and hDNAJB6b-F93L were sarcoplasmic and integrated into sarcomeric structures (Fig. 3D–G). Teased fiber preparations from both hDNAJB6b-WT and hDNAJB6b-F93L confirmed that DNAJB6b co-localizes with desmin and myotilin at the Z-disc (Fig. 3H–M and Supplementary Material, Fig. S1A). The localization of hDNAJB6b-F93L was more disorganized and may relate to changes in myofiber organization since it was present in linear inclusions that co-localized with desmin on longitudinal sections and teased fibers (Fig. 3G and H–J).
Figure 3.
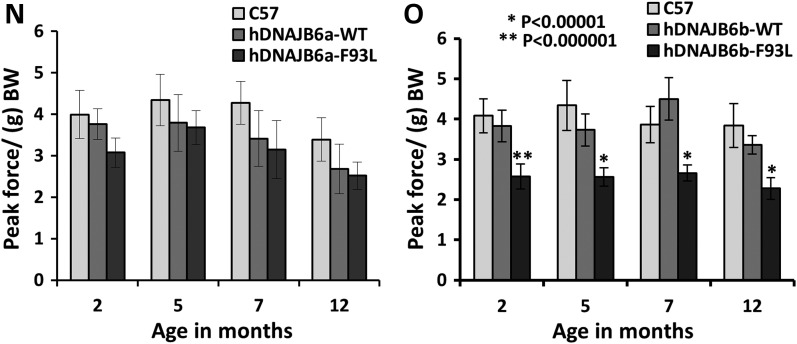
DNAJB6b-F93L transgenic develop muscle weakness. (A–C) Frozen sections of tibialis anterior from 4-month old control (C57), hDNAJB6a-WT or hDNAJB6a-F93L immunostained with V5 (green) and DAPI (blue). hDNAJB6a is localized to myonuclei. Bar, 100 µm. (D–G) Frozen sections of tibialis anterior from 4-month old hDNAJB6b-WT and hDNAJBb-F93L expressing mice using an V5 antibody. Note that hDNAJB6b is localized to the sarcomere. Bar (D and E), 100 µm and Bar (F–G), 20 µm. (H–M) Teased fibers from V5-hDNAJB6b-F93L mice co-stained with V5 and desmin or myotilin. Note that hDNAJB6b localizes to the Z-disc. Bar, 10 µm. (N) Forelimb grip strength measurements from littermate control (C57), hDNAJB6a-WT and hDNAJB6a-F93L mice at 2, 5, 7 and 12 months of age. (O) Forelimb grip strength measurements from littermate control (C57), hDNAJB6b-WT and hDNAJB6b-F93L mice at 2, 5, 7 and 12 months of age. Note that hDNAJB6b-F93L mice develop persistent weakness at 2 months of age.
To determine if hDNAJB6 expression resulted in muscle weakness, we performed forelimb grip strength analysis on hDNAJB6a-WT, hDNAJB6b-WT, hDNAJB6a-F93L or hDNAJB6b-F93L mice at 2, 5, 7 and 12 months of age (Fig. 3N and O). Surprisingly, only hDNAJB6b-F93L mice developed progressive muscle weakness that was detectable at 2 months of age (Fig. 3O). hDNAJB6a-F93L failed to develop measurable weakness (Fig. 3N) and further characterization of these mice was not performed.
Muscle-specific expression of hDNAJB6b-F93L recapitulates an LGMD1D phenotype
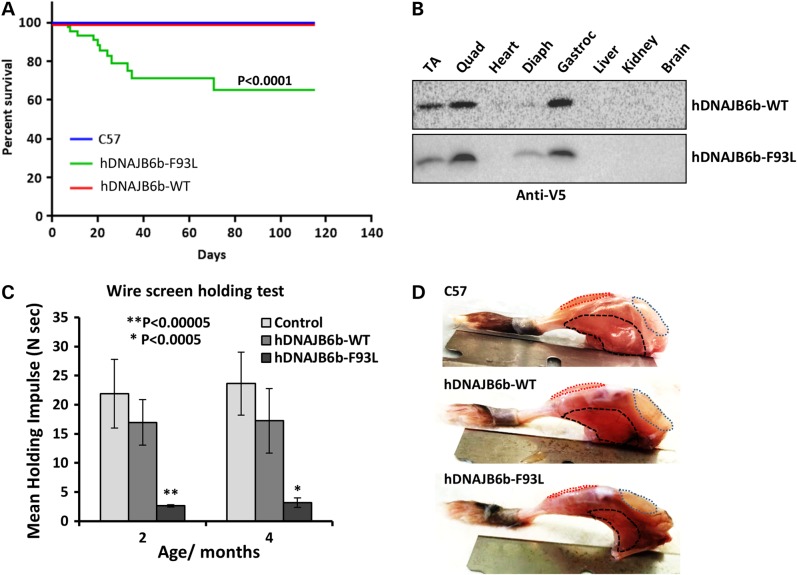
Interestingly, ∼30% of hDNAJB6b-F93L mice died within the first 40 days due to profound muscle weakness (Fig. 4A). To confirm that the increase in mortality was not due to hDNAJB6b-F93L expression in tissues other than skeletal muscle, we performed an immunoblot using a V5 antibody on skeletal muscle, heart, liver, kidney and brain tissue lysates (Fig. 4B). As expected the hDNAJB6b transgene was expressed exclusively in skeletal muscle. Further evaluation of strength demonstrated that hDNAJB6b-F93L mice were significantly weaker as assessed using the inverted wire screen holding test at 2 months of age (Fig. 4C).
Figure 4.
DNAJB6b-F93L transgenic mice have early lethality. (A) Kaplan–Meier survival plot of control (C57), hDNAJB6b-WT and hDNAJB6b-F93L expressing transgenic mice. Notably, ∼30% of hDNAJB6b-F93L mice die within the first 2 months. (B) Immunoblot for V5 from skeletal muscle (Tibialis anterior, Quadriceps, Diaphragm and Gastrocnemius); heart, liver, kidney and brain lysates of hDNAJB6b-WT and hDNAJB6b-F93L mice. Note that transgenic expression of hDNAJB6b is restricted to skeletal muscle. (C) Inverted wire screen holding test of control, hDNAJB6b-WT and hDNAJB6b-F93L at 2 and 4 months of age. (D) Images of hindlimb musculature from C57, hDNAJB6b-WT and hDNAJB6b-F93L at 4 months of age. Quadriceps outlined in blue, Tibialis anterior in red and Gastrocnemius in black.
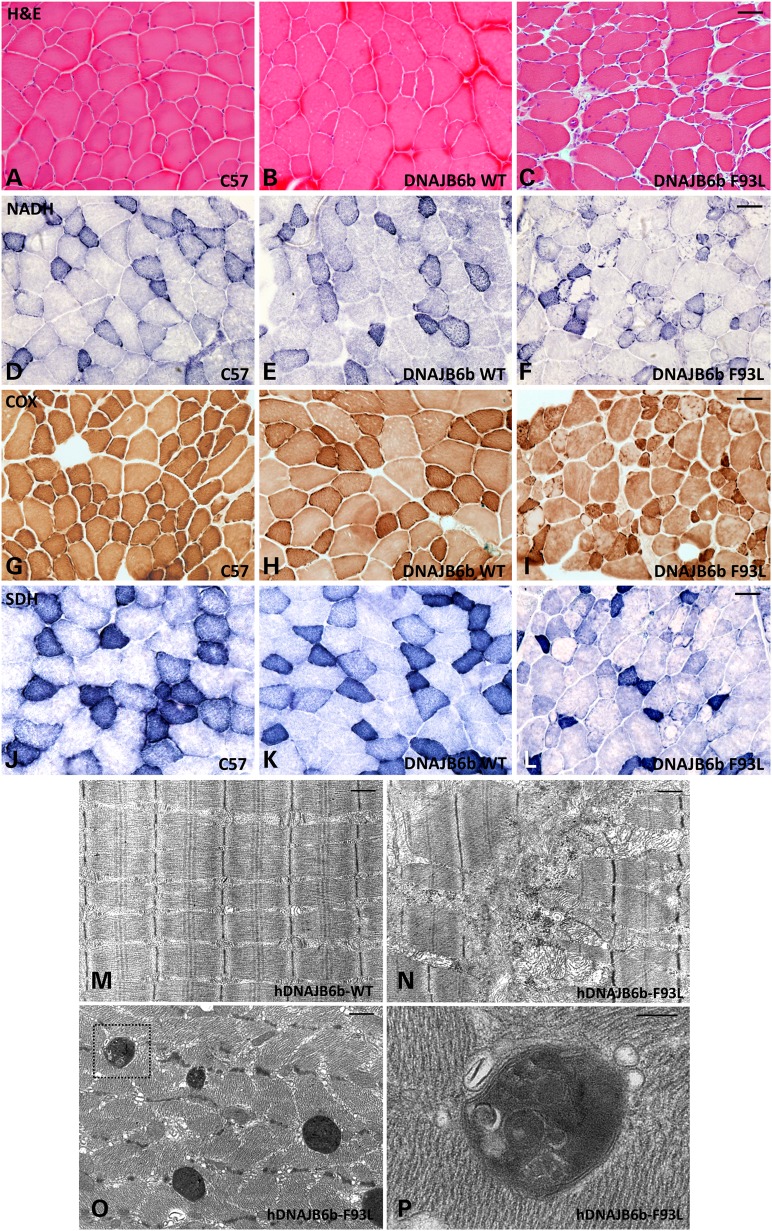
We performed routine histochemical analysis on skeletal muscle of 4-month old control, hDNAJB6b-WT and hDNAJB6b-F93L mice. Gross inspection found the most pronounced pathology in the hindlimb musculature and sections were performed on the tibialis anterior, quadriceps and gastrocnemius (Fig. 4D). hDNAJB6b-F93L mice had obvious myopathic features with internal nuclei, variation in fiber size and increased endomysial connective tissue (Fig. 5A–C). Other histochemical stains highlighted coarse internal architecture on NADH and fibers with ‘rubbed out’ appearance on SDH and COX staining (Fig. 5D–L). Electron microscopy further demonstrated alterations in myofibrillar organization, enlarged and swollen mitochondria and autophagic/lysosomal vacuoles (Fig. 5M–P).
Figure 5.
DNAJB6b-F93L transgenic mice develop a myopathy. (A–C) Hematoxylin and Eosin; (D–F) nicotinamide adenine dinucleotide diaphorase (NADH); (G–I) Cytochrome oxidase (COX) and (J–L) Succinate Dehydrogenase (SDH) histochemical analysis of tibialis anterior muscle from 4 months old control (C57), hDNAJB6b-WT or hDNAJB6b-F93L expressing mice. Bar (A–L), 100 µm. (M–P) Electron micrographs of tibialis anterior muscle from hDNAJB6b-WT or hDNAB6b-F93L expressing mice. Note disorganized myofibrils (N) and the presence of large autophagic structures in hDNAJB6-F93L mice (O). (P) Inset of these autophagic structures. Bar (M–O), 1 µm and Bar (P), 200 nm.
LGMD1D mutant mice accumulate desmin and keratin
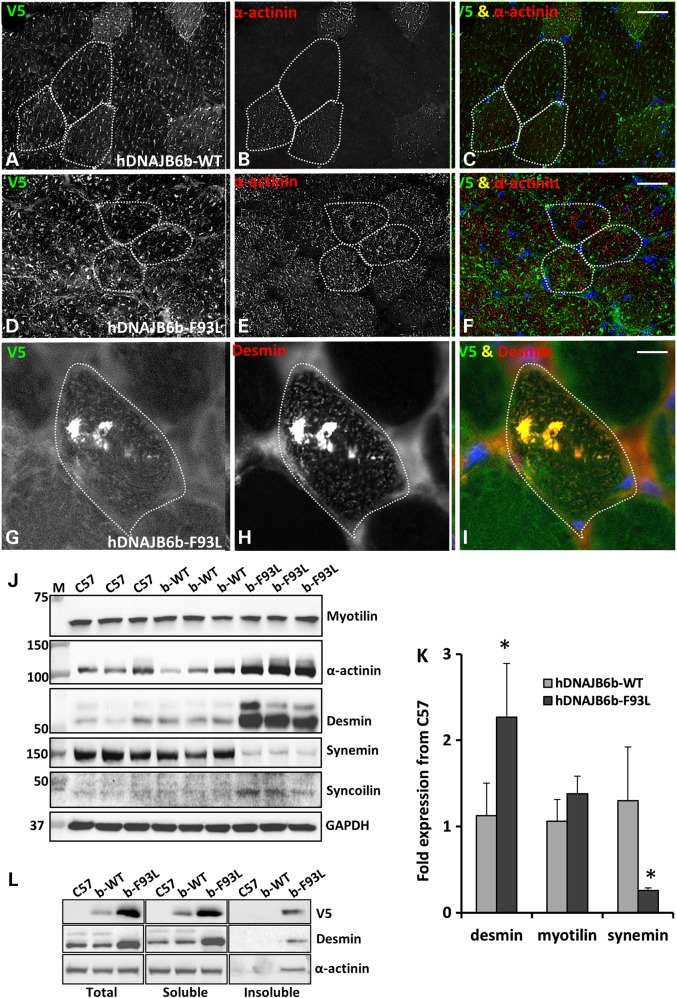
Immunostaining for the Z-disc components further revealed alterations in their expression and localization with an increase α-actinin signal intensity and decreased organization (Fig. 6A–F). Other features included large desmin inclusions that co-localized with hDNAJB6b-F93L in some fibers (Fig. 6G–I). In the case of desmin mutations, it has become increasingly clear that the levels of other structural proteins including other intermediate filaments are coordinately dysregulated (19). To analyze this, we evaluated the total levels of the Z-disc proteins desmin, α-actinin, myotilin, synemin and syncoilin from lysates of control, hDNAJB6b-WT and hDNAJB6b-F93L mouse muscle via immunoblot (Fig. 6J). Desmin, α-actinin and syncolin were increased whereas myotilin was unchanged and synemin was decreased in hDNAJB6b-F93L mice as compared with control and hDNAJB6b-WT expressing mice (Fig. 6J). Quantitative PCR demonstrated that desmin expression was increased by >2 fold as compared with C57 controls and hDNAJB6b-WT transgenic mice (Fig. 6K). In contrast, synemin expression was ∼25% of that seen in C57 controls and hDNAJB6b-WT transgenic mice (Fig. 6K). By separating tissue lysates into a total, soluble and insoluble fraction via differential centrifugation, we demonstrated that hDNAJB6b-V5, desmin and α-actinin were enriched in the insoluble pellet in hDNAJB6b-F93L mouse muscle, consistent with them being aggregated (Fig. 6L).
Figure 6.
Z-disc proteins accumulate in DNAJB6b-F93L transgenic mouse muscle. (A–I) Dual immunofluorescence staining of 4-month old hDNAJB6b-WT or hDNAJB6b-F93L tibialis anterior muscle using an antibody to V5 that recognizes transgenic hDNAJB6b protein and an antibody to actinin (A–F) or desmin (G–I). Bar (A–F), 50 µm and Bar (G–I), 20 µm. (J) Immunoblot of tibialis anterior muscle lysates from three different 4-month old C57, DNAJBb-WT and DNAJB6b-F93L mice using antibodies to Z-disc proteins myotilin, α-actinin, desmin, syncolin and synemin. GAPDH is loading control. (K) qPCR analysis of desmin, myotilin and synemin expression in 4-month old hDNAJB6b-WT or hDNAJB6b-F93L mice. Data are presented as a fold change as compared with C57 controls. * represent P-value <0.01. (L) Tibialis anterior muscle lysates from 4-month old C57, DNAJB6b-WT and DNAJB6b-F93L mice were separated into a total, soluble and insoluble fraction. Fractions were then immunoblotted for V5 (to detect hDNAJB6b), desmin and α-actinin.
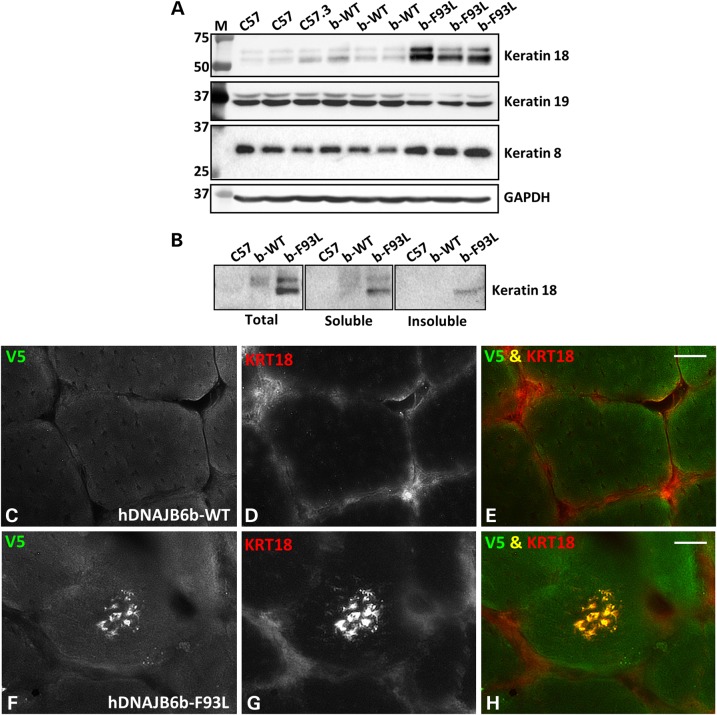
Previous studies have demonstrated that DNAJB6b may interact with keratin 8/18 in HeLa cells (20). To address this, we evaluated steady state levels of keratin 8, 18 and 19 from lysates of control, hDNAJB6b-WT and hDNAJB6b-F93L mouse muscle via immunoblot (Fig. 7A). Consistent with hDNAJB6b-F93L dysfunction in regulating keratin intermediate filament stability, keratin 8 and 18 were increased while keratin 19 was modestly decreased. Similar to desmin and α-actinin, keratin 18 accumulated in the insoluble pelleted muscle lysate fraction from hDNAJB6b-F93L mice (Fig. 7B). In addition, hDNAJB6b-F93L mouse muscle showed the presence of keratin 18 positive inclusions that co-localized with hDNAJB6b in the center of some muscle fibers (Fig. 7F–H). DNAJB6b-WT expressing mouse muscle had no keratin 18 positive inclusions (Fig. 7C–E).
Figure 7.
Keratin 8/18 accumulates in DNAJB6b-F93L transgenic mouse muscle. (A) Immunoblot of tibialis anterior muscle lysates from three different 4-month old C57, DNAJBb-WT and DNAJB6b-F93L mice using antibodies to keratin 18, keratin 19 and keratin 8. (B) Tibialis anterior muscle lysates from 4-month old C57, DNAJB6b-WT and DNAJB6b-F93L mice were separated into a total, soluble and insoluble fraction. Fractions were then immunoblotted for keratin 18. (C–H) Immunofluorescence staining of 4-month old hDNAJB6b-WT or hDNAJB6b-F93L tibialis anterior muscle using an antibody to keratin 18. Bar, 20 µm.
LGMD1D mutant mice and patients accumulate the RNA-binding proteins hnRNPA1 and hnRNPA2/B1
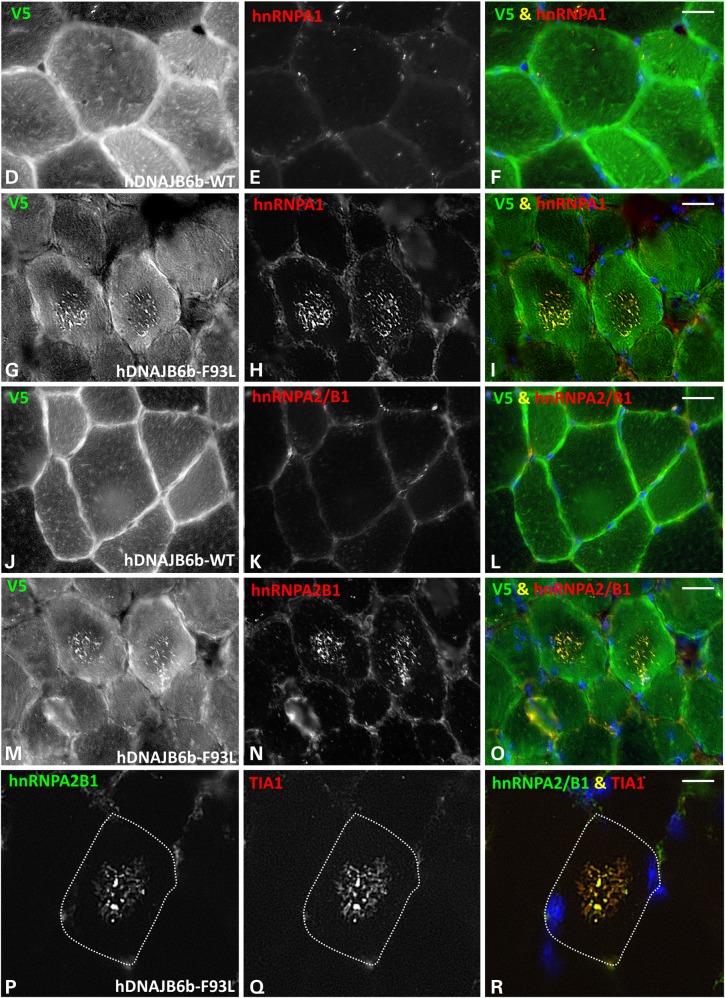
We have previously reported that the RNA-binding protein TDP-43 aggregates in LGMD1D patient muscle (1). Moreover, TDP-43 interacts with DNAJB6b, and LGMD1D mutations disrupt the disaggregation of TDP-43 positive stress granules in cells, suggesting that RNA-binding proteins may be client proteins of DNAJB6 (13,21). We explored whether TDP-43 or other RNA-binding proteins implicated in protein aggregate myopathies such as hnRNPA2/B1, hnRNPA1 and TIA1 accumulated in hDNAJB6b-F93L mouse muscle (22,23). To do this, we immunoblotted skeletal muscle lysates from control, hDNAJB6b-WT and hDNAJB6b-F93L mouse muscle using antibodies for TDP-43, hnRNPA2/B1, hnRNPA1 and TIA1 (Fig. 8A). Surprisingly, we found a dramatic increase in both hnRNPA1 and hnRNPA2/B1 (Fig. 8A). Similar to other aggregated proteins, hnRNPA2/B1 and hnRNPA1 partitioned into the insoluble pelleted muscle lysate fraction of hDNAJB6b-F93L mice (Fig. 8B). Levels of hnRNPA2/B1 and hnRNPA1 mRNA in hDNAJB6b-F93L mice, as assessed by quantitative PCR, were similar to that observed in C57 control and hDNAJB6b-WT expressing mice suggesting that their accumulation is not due to an increase in protein expression (Fig. 8C). Immunohistochemistry of mouse muscle for these same proteins found that TDP-43 was present in myonuclei of control, hDNAJB6b-WT and hDNAJB6b-F93L mouse muscle but did not accumulate in the sarcoplasm (not shown). In contrast, both hnRNPA2/B1 and hnRNPA1 accumulated as small puncta within the center of scattered myofibers from only hDNAJB6b-F93L mouse muscle. Serial sections of hDNAJB6b-F93L mouse muscle demonstrated that both hnRNPA2/B1 and hnRNPA1 accumulate within the same myofibers and likely co-localize (Fig. 8G–I and M–O). In addition, co-immunostaining with an antibody to V5 (to stain for V5-hDNAJB6b) and hnRNPA2/B1 or hnRNPA1 demonstrated that hDNAJB6b-F93L co-aggregates with these RNA-binding proteins (Fig. 8G–I and M–O). These same structures also co-localized with the stress granule protein TIA1, suggesting that they may indeed be stress granules (Fig. 8P–R and Supplementary Material, Figure S1B).
Figure 8.
RNA-binding proteins accumulate in DNAJB6b-F93L transgenic mouse muscle. (A) Immunoblot of tibialis anterior muscle lysates from three different 4-month old C57, DNAJBb-WT and DNAJB6b-F93L mice using antibodies to the RNA-binding proteins TDP-43, hnRNPA1, hnRNPA2/B1, TIA1 and G3BP1. GAPDH is loading control. (B) Tibialis anterior muscle lysates from 4-month old C57, DNAJB6b-WT and DNAJB6b-F93L mice were separated into a total, soluble and insoluble fraction. Fractions were then immunoblotted for hnRNPA2/B1 and hnRNPA1. (C) qPCR analysis of hnRNPA2/B1 and hnRNPA1 expression in 4-month old hDNAJB6b-WT or hDNAJB6b-F93L mice. Data are presented as a fold change as compared with C57 controls. (D–R) Dual immunofluorescence staining of 4-month old hDNAJB6b-WT or hDNAJB6b-F93L tibialis anterior muscle using an antibody to V5 that recognizes transgenic hDNAJB6b protein and an antibody to hnRNPA1 (D–I); hnRNPA2/B1 (J–O) or hnRNPA2/B1 and TIA1 (P–R). Bar (D–O), 20 µm and Bar (P–R), 10 µm.
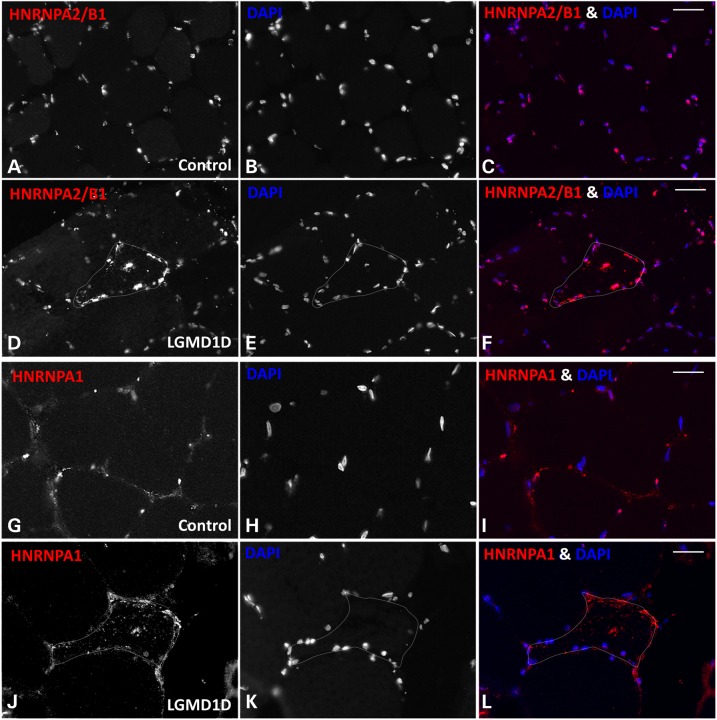
To see if the accumulation of hnRNPA1 and hnRNPA2/B1 inclusions was also a feature of human LGMD1D patient pathology, we immunostained control and LGMD1D patient muscle. Similar to our hDNAJB6b-F93L expressing transgenic mouse line, both hnRNPA1 and hnRNPA2/B1 accumulated in scattered myofibers of LGMD1D muscle (Fig. 9D–F and J–L) but not in control patient muscle (Fig. 9A–C and G–I).
Figure 9.
The RNA binding proteins hnRNPA2/B1 and hnRNPA1 accumulate in LGMD1D patient muscle. (A-C and G-I) Control and (D-F and J-L) LGMD1D patient skeletal muscle tissue was immunostained for (A-F) hnRNPA2/B1 and (G-L) hnRNPA1.
Discussion
Our study demonstrates that disease mutations in the sarcoplasmic/cytoplasmic isoform of DNAJB6 (DNAJB6b) are responsible for the pathogenesis of LGMD1D. Although both DNAJB6 isoforms are expressed in human skeletal muscle, only skeletal muscle-specific expression of DNAJB6b-F93L and not DNAJB6a-F93L led to muscle weakness and pathologic features consistent with LGMD1D. DNAJB6a localized to myonuclei whereas DNAJB6b localized to the sarcomere and, more specifically, to the Z-disc. Consistent with its Z-disc localization, DNAJB6b-F93L expressing muscle accumulated desmin aggregates and had myofibrillar disarray. Other pathologic findings included aggregation of the RNA-binding proteins hnRNPA1 and hnRNPA2/B1 and the presence of autophagic vacuoles. Our mouse model demonstrates pathologic features most consistent with an MFM in which myofibrillar disorganization is the prominent pathologic feature and is visualized best with routine histochemistry and desmin staining. Large protein aggregates visualized via immunohistochemistry and rimmed vacuoles are striking pathologic features but less frequent in myofibers. Thus transgenic muscle expression of DNAJB6b-F93L recapitulates many of the pathologic features seen in LGMD1D patient muscle and may serve as a pre-clinical model of LGMD1D.
Identifying the DNAJB6 isoform that confers pathogenicity is an essential first step in understanding LGMD1D pathogenesis. By defining the site of DNAJB6 dysfunction, either myonuclear or sarcoplasmic, one can begin to identify client proteins that may be dysregulated by LGMD1D mutant DNAJB6. However, it is conceivable that an LGMD1D mutation in the nuclear isoform of DNAJB6a may contribute to LGMD1D pathogenesis while not recapitulating disease pathology when expressed alone. Whether a mouse model that expresses an LGMD1D mutation that is present in DNAJB6a and DNAJB6b generates a more severe phenotype remains to be established.
Gene knockout of DNAJB6 in mice is lethal due to a failure in chorioallantoic fusion at embryonic day 8.5 (24). These mice lack all DNAJB6 isoforms, thus making it unclear whether lethality is due to nuclear or cytosolic function of DNAJB6. However, the embryonic lethality has been proposed to be due to the accumulation of toxic cytosolic keratin aggregates in placentally-derived trophoblasts (25). These studies, along with others, established keratin 8/18 as a potential DNAJB6 client protein (20,25). Keratin 8/18 and keratin 8/19 are paired type I and type II intermediate filaments or cytokeratins. While keratins are predominantly expressed in epithelial cells, cytokeratins are also present in skeletal muscle and localize to the Z-disc (26). In addition, keratin 8/19 associates with sarcolemmal proteins critical in organizing costameres in striated muscle (27). Our studies find that keratin 8/18 accumulates in LGMD1D transgenic mouse muscle. Indeed, keratin 18 has been previously demonstrated to accumulate in LGMD1D patient muscle (2).
The fact that overexpression of LGMD1D mutant DNAJB6b is sufficient to recapitulate disease pathology supports that LGMD1D is not due to DNAJB6 haploinsufficiency. However, our studies do not clarify whether the DNAJB6b-F93L mutation behaves in a dominant negative or a toxic gain of function manner. The finding that a G/F domain mutation in DNAJB6a fails to cause a myopathy supports the contention that DNAJB6b-F93L behaves selectively in a dominant negative manner within the sarcoplasmic compartment. Future studies will be needed to address this question.
Z-disc associated chaperone proteins such as CRYAB, HSP27 and BAG3 are important in the remodeling of muscle-specific intermediate filaments such as desmin and other Z-disc proteins such as FLNC (10,28). It is becoming clearer that the destabilization of one Z-disc protein such as desmin leads to destabilization of other associated proteins. For example desminopathy mice that are homozygous for the most common human desmin mutation, R350P, have a decrease in total desmin levels and another intermediate filament, syncolin (19). These data make it difficult to infer a true chaperone-client relationship between proteins that accumulate and are reduced in our hDNAJB6b-F93L mice. Regardless, it is intriguing that desmin and keratin 8/18 accumulated whereas other intermediate filaments seemed reduced or unaffected.
The role of RNA-binding proteins with aggregation prone domains or prion-like domains (PrLDs) in protein aggregate myopathies is emerging. TDP-43 aggregation is a sensitive and specific marker of sporadic inclusion body myositis and is present in other rimmed vacuolar myopathies including LGMD1D (1,29,30). Mutations in four other RNA-binding proteins with PrLDs also cause autosomal dominant rimmed vacuolar myopathies, these include hnRNPA1, hnRNPA2/B1, hnRNPDL and TIA1 (15,22,23). In fact some of these RNA-binding proteins aggregate in other vacuolar myopathies (23,31,32). We have previously demonstrated that DNAJB6b localizes to TDP-43 positive stress granules upon heat shock in HeLa cells (21). In addition, either siRNA knockdown of DNAJB6 or expression of LGMD1D mutations in DNAJB6b enhanced TDP-43 granule formation and slowed their dissolution post-heat shock recovery (13,21). TDP-43 RNA granule formation requires its C-terminal PrLD and this domain can be substituted by other aggregation prone domains, including yeast prion domains from the yeast prions Sup35 and Rnq1 (21,33). Yeast have several DNAJ family members one yeast DNAJB-like protein, Sis1, is required for the propagation of the yeast prions Sup35 and Rnq1 (34). This function requires the G/F domain of Sis1 (13,35). Previously, we analyzed the G/F domain missense mutations that are associated with LGMD1D in the context of Sis1. We found that the mutations abrogate its function in prion propagation (13). In addition, Sis1-LGMD1D mutants, abrogate the propagation of select [RNQ+] prion conformers, suggesting that LGMD1D mutations in DNAJB6 may affect its action on only select client protein conformers (13).
The development of small animal models of muscular dystrophies is the first step toward identifying a potential therapy. Importantly, our mouse model of hDNAJB6b-F93L expression recapitulates many of the pathologic features of LGMD1D. The therapeutic modulation of the HSP chaperone network has been proposed as a pharmacologic target in muscular dystrophy. In fact, Hsp72 overexpression or pharmacologic induction of Hsp70 is protective in mdx mouse models (36). Moreover, the first in human proof of concept studies utilizing a heat shock response modulator, arimoclomol, in the treatment of sporadic inclusion body myositis were recently reported (37). Future studies will be necessary to see if similar strategies are effective in other protein aggregate myopathies.
Materials and Methods
qPCR
Total RNA was isolated from TA muscle with SV Total RNA isolation kit (Promega, Z3100) according to the manufacturer's instructions. The concentration and quality of the total RNA isolated was determined using a Nanodrop spectrophotometer. cDNA was synthesized using the Transcriptor first strand cDNA synthesis kit (Roche, 04379012001). Gene expression levels were analyzed by real-time PCR on an Applied Biosystems model 7500 (software v2.0.5) using the Faststart universal SYBR Green master ROX qPCR mastermix (Roche, 04913850001).
Quantitative polymerase chain reaction was performed with primers to RNA-binding proteins (hnRNPA1 and hnRNPA2/B1), hDNABJB6 and intermediate filaments (Desmin, Myotilin and Synemin). The values were normalized to GAPDH and represented as fold change. Primer sequences are in Supplemental Material.
Antibodies
Antibodies used were the following: anti-rabbit GAPDH (Cell Signaling, 2118), anti-V5 HRP Invitrogen (B96125), anti-mouse Desmin (Dako, M0760), anti-rabbit Desmin (Abcam, ab8592), anti-goat TIA-1 (Santa cruz, sc175), anti-rabbit TDP43 (Proteintech, 10782-2-AP), anti-mouse DNAJB6 (Novus, H00010049-M01), anti-rabbit Actin (Sigma, A2066), anti-mouse hnRNPA2/B1 (Sigma, R4653), anti-rabbit Synemin (Bioss, bs-8555R), anti-goat Syncoilin (Santa cruz, sc-240950), anti-rabbit Actinin (Abcam ab68167), anti-rabbit Myotilin (Abcam, ab78492), anti-mouse Keratin 18 (Abcam, ab668), anti-rabbit Keratin 8 (Abcam, ab53280), anti-rabbit Keratin 19 (Abcam, ab15463) and anti-mouse hnRNPA1 (Abcam, ab5832). Secondary antibodies include anti-mouse HRP (Pierce), anti-rabbit HRP (cell signaling), anti-goat HRP (Santa cruz), anti-mouse AlexaFluor (555 and 488) and anti-rabbit AlexaFluro (555 and 488).
WB
Muscle tissues and cultured cells were homogenized using RIPA lysis buffer (50 mm Tris–HCl, pH 7.4, 150 mm NaCl, 1% NP-40, 0.25% Na-deoxycholate and 1 mm EDTA) supplemented with protease inhibitor cocktail (Sigma-Aldrich), and lysates were centrifuged at 13 000 rpm for 10 min. Protein concentrations were determined using a BCA protein assay kit (Thermo Fisher Scientific). Aliquots of lysates were solubilized in Laemmli sample buffer and equal amounts of proteins were separated on 12% SDS-PAGE gels. Proteins were transferred to nitrocellulose membrane and the membrane was blocked with 5% nonfat dry milk in PBS with 0.1% Tween-20 for 1 h. The membrane was then incubated with primary antibodies, specific to the protein of interest, in 5% nonfat dry milk overnight at 4°C. After incubation with the appropriate secondary antibody conjugated with horseradish peroxidase, enhanced chemiluminescence (GH Healthcare, UK) was used for protein detection. Immunoblots were obtained using the G:Box Chemi XT4, Genesys Version 1.1.2.0 (Syngene). Densitometry was measured with imageJ software (National Institute of Health).
Solubility assays
Fresh TA muscle was collected and flash frozen in liquid nitrogen. Tissue (20 mg) was homogenized in 250 µl of 2% SDS-radioimmunoprecipitation assay buffer (50 mm Tris–Cl (pH 8.0), 150 mm NaCl, 1% NP-40, 0.5% sodium deoxycholate and 2% SDS) and protease inhibitor cocktail (Sigma-Aldrich). Homogenates were precleared with a 30-s low speed spin and an aliquot of the supernatant was collected and named the total fraction. The additional supernatant was centrifuged at 100 000 × g for 30 min at 4°C and this supernatant was collected and named the soluble fraction. The pellet was then sonicated on ice after the addition of 150 µl of 5 M guanidine–-HCl and re-centrifuged at 100 000 × g for 30 min at 4°C. This supernatant was removed and named the insoluble fraction. The insoluble fraction was precipitated by adding an equal volume of 20% trichloroacetic acid (Sigma-Aldrich) and incubated on ice for 20 min. Samples were then centrifuged at 10 000 × g for 15 min at 4°C and the resulting pellet was washed twice with ice-cold acetone. Residual acetone was removed by drying tubes at 95°C and the samples were resuspended in 50 µl of 5% SDS in 0.1N NaOH. The protein concentrations of all samples were determined using a BCA protein assay kit (Pierce). Each sample (30 µg) was analyzed by Western blotting for each fraction.
Wire screen holding and grip test
Grip strength testing consisted of five separate measurements using a trapeze bar attached to a force transducer that recorded peak-generated force (Stoelting, WoodDale, IL, USA). Mice have the tendency to grab the bar with their forepaws and continue to hold while being pulled backwards by the tail, releasing only when unable to maintain grip. The resulting measurement was recorded and the average of the highest three measurements was determined to give the strength score. For every time point and strain, at least five animals were used. P-values were determined by a paired Student's t-test. To validate our results, another quantitative strength measurement was performed by wire screen holding test. Mice were placed on a grid where it stood using all four limbs. Subsequently, the grid was turned upside down 15 cm above a cage. Latency for the mouse to release the mesh is recorded and the average hanging time of three trials was used as an outcome measure.
Animal and experimental protocols
Human DNAJB6a-WT and DNAJB6b-WT were obtained via Addgene and the F93L point mutation was generated via QuickChange Site-directed mutagenesis by changing the cDNA position 277T>C. All four cDNAs were subcloned into a 1256MCK-CAT transgenic targeting vector obtained from Dr Stephen Hauschka, University of Washington. The promoter and coding sequence were confirmed by DNA sequence analysis. A linear fragment containing the MCK-V5-hDNAJB6 sequence was isolated by digesting the targeting vector with HindIII and KpnI and subsequent gel purification. This fragment was sent to the mouse genetics core facility at Washington University for transgenic animal production. Animals were screened for transgene insertion using PCR amplification of tail DNA. Animals were bred to C57/B6 (Jackson Laboratories) to at least the F5 generation for phenotypic analysis. Control animals were non-transgenic littermates. All animal experimental protocols were approved by the Animal Studies Committee of Washington University School of Medicine. Mice were housed in a temperature-controlled environment with 12 h light–dark cycles where they received food and water ad libitum.
Mice were euthanized and skeletal muscle was dissected. For western blot analysis, muscle was flash frozen in liquid nitrogen and stored at −80°C. For immunohistochemistry, muscle was mounted on a cork with tragacanth gum and quickly cooled in liquid nitrogen-cooled isopentane for 20 s and then stored at −80°C.
For survival study, animals were inspected daily for health issues and deaths were recorded for each animal. Moribund animals were euthanized by CO2 asphyxiation and recorded. Every animal found dead or euthanized was necropsied. Criteria for euthanasia were based on an independent assessment by a veterinarian according to AAALAC guidelines and only cases where the condition of the animal was considered incompatible with continued survival are represented as deaths in the curves. Animals removed at sacrifice were considered as censored deaths.
Electroporation
For electroporation, mice were anesthetized using inhaled isoflurane. The skin overlying the TA muscle was shaved, and the animals were injected with a 50 µg endotoxin-free expression plasmid (mCherry-DNAJB6b-WT and GFP-DNAJB6a-WT) diluted in sterile phosphate-buffered saline (PBS) by using a 0.5 ml syringe fitted with a 29-gauge needle. Two-needle array electrodes (450 121) (Harvard Apparatus, Holliston, MA, USA) were inserted into the muscle immediately after DNA delivery for electroporation. The distance between the electrodes was 5 mm, and the array was inserted longitudinally relative to the muscle fibers. In vivo electroporation parameters were the following: voltage, 100 V; pulse length, 50 ms; number of pulses, six; pulse interval, 200 ms; desired field strength, 200 V/cm, given by a BTX ECM830 Electro Square Porator. Animals were allowed to recover for 7 days prior to muscle isolation.
Cell culture
Flp-In T-REX 293 Cells (Invitrogen) expressing pcDNA5/FRT/TO constructs V5-DNAJB6b-WT and V5-DNAJB6b-F93L were cultured in DMEM containing 4 mm l-glutamine (Invitrogen; 11965-084), 10% FBS (Atlanta Biologicals; S10350) and penicillin (50 IU)/streptomycin (50 µg/ml) (Invitrogen; 15 140), 50 µg/ml hygromycin B (Invitrogen; 10687-010), 50 µg/ml blasticidin (life technologies; R21001) and induced with 1 µg/ml tetracycline hydrochloride (Sigma; T76600) 48 h prior to experimentation. Cells were maintained in 5% CO2 at 37°C in 60 mm tissue culture-treated plates until the cells were 80–85% confluent.
Histochemistry, immunohistochemistry and electron microscopy
Isolated muscle was mounted using tragacanth gum (Sigma, G1128) and quick frozen in liquid nitrogen-cooled 2-methylbutane. Samples were stored at −80°C until sectioning into 10-µm sections. Hematoxylin and eosin (H&E), nicotinamide adenine dinucleotide diaphorase (NADH), cytochrome oxidase (COX) and succinate dehydrogenase staining was performed as previously described (23). For immunohistochemistry, the sections were blocked in PNB (PerkinElmer), incubated with primary antibody followed by the appropriate secondary antibody. Briefly, muscle sections were affixed to slides incubated for 10 min in ice-cold acetone, mounted with Mowiol 4–88 (sigma) + DAPI and examined using a fluorescence microscope (Nikon 80i upright+ and Roper Scientific EZ monochrome CCD camera with deconvolution software analysis [NIS Elements, Nikon]). Nonfluoresent images were taken with a 5 megapixel color CCD (Nikon).
For electron microscopy, TA muscle was harvested, rinsed briefly in PBS and then placed immediately in Karnovsky fixative at 4°C for 24 h. Fixed muscle was embedded in plastic and sectioned for EM imaging. Images were taken using a Jeol 1400 and a Gatan Orius 832 Digital Camera. Image processing and analysis were done with (NIS Elements 4.0) software and Adobe Photoshop.
Human subjects
Tissue samples of skeletal muscle derived from a diagnostic muscle biopsy of patients with a heterozygous F93L DNAJB6 mutation were obtained from the neuromuscular clinic at Washington University and the diagnosis of LGMD1D was made by that patient's physician.
All participants gave their written informed consent, and all study procedures were approved by the Human Studies Committee at Washington University.
Supplementary Material
Funding
Funding was provided via NIH AG031867 (C.C.W.), AG042095 (C.C.W.), AR068797 (C.C.W. and H.L.T.), the Muscular Dystrophy Association (C.C.W.), the Myositis Association (C.C.W.), the Hope Center for Neurological Disorders (C.C.W. and H.L.T.).
Supplementary Material
Acknowledgments
Conflict of Interest statement. None declared.
References
- 1.Harms M.B., Sommerville R.B., Allred P., Bell S., Ma D., Cooper P., Lopate G., Pestronk A., Weihl C.C., Baloh R.H. (2012) Exome sequencing reveals DNAJB6 mutations in dominantly-inherited myopathy. Ann. Neurol., 71, 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarparanta J., Jonson P.H., Golzio C., Sandell S., Luque H., Screen M., McDonald K., Stajich J.M., Mahjneh I., Vihola A. et al. (2012) Mutations affecting the cytoplasmic functions of the co-chaperone DNAJB6 cause limb-girdle muscular dystrophy. Nat. Genet., 44, 450–455, S451–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kampinga H.H., Craig E.A. (2010) The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat. Rev. Mol. Cell Biol., 11, 579–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruggieri A., Brancati F., Zanotti S., Maggi L., Pasanisi M.B., Saredi S., Terracciano C., Antozzi C., MR D.A., Sangiuolo F. et al. (2015) Complete loss of the DNAJB6 G/F domain and novel missense mutations cause distal-onset DNAJB6 myopathy. Acta Neuropathol. Commun., 3, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmio J., Jonson P.H., Evila A., Auranen M., Straub V., Bushby K., Sarkozy A., Kiuru-Enari S., Sandell S., Pihko H. et al. (2015) Novel mutations in DNAJB6 gene cause a very severe early-onset limb-girdle muscular dystrophy 1D disease. Neuromuscul. Disord., http://dx.doi.org/doi:10.1016/j.nmd.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Sato T., Hayashi Y.K., Oya Y., Kondo T., Sugie K., Kaneda D., Houzen H., Yabe I., Sasaki H., Noguchi S. et al. (2013) DNAJB6 myopathy in an Asian cohort and cytoplasmic/nuclear inclusions. Neuromuscul. Disord., 23, 269–276. [DOI] [PubMed] [Google Scholar]
- 7.Sandell S., Huovinen S., Sarparanta J., Luque H., Raheem O., Haapasalo H., Hackman P., Udd B. (2010) The enigma of 7q36 linked autosomal dominant limb girdle muscular dystrophy. J. Neurol. Neurosurg. Psychiatry, 81, 834–839. [DOI] [PubMed] [Google Scholar]
- 8.Vicart P., Caron A., Guicheney P., Li Z., Prevost M.C., Faure A., Chateau D., Chapon F., Tome F., Dupret J.M. et al. (1998) A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat. Genet., 20, 92–95. [DOI] [PubMed] [Google Scholar]
- 9.Selcen D., Muntoni F., Burton B.K., Pegoraro E., Sewry C., Bite A.V., Engel A.G. (2009) Mutation in BAG3 causes severe dominant childhood muscular dystrophy. Ann. Neurol., 65, 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ulbricht A., Eppler F.J., Tapia V.E., van der Ven P.F., Hampe N., Hersch N., Vakeel P., Stadel D., Haas A., Saftig P. et al. (2013) Cellular mechanotransduction relies on tension-induced and chaperone-assisted autophagy. Curr. Biol., 23, 430–435. [DOI] [PubMed] [Google Scholar]
- 11.Perng M.D., Wen S.F., van den I.P., Prescott A.R., Quinlan R.A. (2004) Desmin aggregate formation by R120G alphaB-crystallin is caused by altered filament interactions and is dependent upon network status in cells. Mol. Biol. Cell., 15, 2335–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hageman J., Rujano M.A., van Waarde M.A., Kakkar V., Dirks R.P., Govorukhina N., Oosterveld-Hut H.M., Lubsen N.H., Kampinga H.H. (2010) A DNAJB chaperone subfamily with HDAC-dependent activities suppresses toxic protein aggregation. Mol. Cell., 37, 355–369. [DOI] [PubMed] [Google Scholar]
- 13.Stein K.C., Bengoechea R., Harms M.B., Weihl C.C., True H.L. (2014) Myopathy-causing mutations in an HSP40 chaperone disrupt processing of specific client conformers. J. Biol. Chem., 289, 21120–21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nigro V., Savarese M. (2014) Genetic basis of limb-girdle muscular dystrophies: the 2014 update. Acta Myol., 33, 1–12. [PMC free article] [PubMed] [Google Scholar]
- 15.Vieira N.M., Naslavsky M.S., Licinio L., Kok F., Schlesinger D., Vainzof M., Sanchez N., Kitajima J.P., Gal L., Cavacana N. et al. (2014) A defect in the RNA-processing protein HNRPDL causes limb-girdle muscular dystrophy 1G (LGMD1G). Hum. Mol. Genet., 23, 4103–4110. [DOI] [PubMed] [Google Scholar]
- 16.Torella A., Fanin M., Mutarelli M., Peterle E., Del Vecchio Blanco F., Rispoli R., Savarese M., Garofalo A., Piluso G., Morandi L. et al. (2013) Next-generation sequencing identifies transportin 3 as the causative gene for LGMD1F. PLoS One, 8, e63536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melia M.J., Kubota A., Ortolano S., Vilchez J.J., Gamez J., Tanji K., Bonilla E., Palenzuela L., Fernandez-Cadenas I., Pristoupilova A. et al. (2013) Limb-girdle muscular dystrophy 1F is caused by a microdeletion in the transportin 3 gene. Brain, 136, 1508–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenberg S.A., Salajegheh M., Judge D.P., Feldman M.W., Kuncl R.W., Waldon Z., Steen H., Wagner K.R. (2012) Etiology of limb girdle muscular dystrophy 1D/1E determined by laser capture microdissection proteomics. Ann. Neurol., 71, 141–145. [DOI] [PubMed] [Google Scholar]
- 19.Clemen C.S., Stockigt F., Strucksberg K.H., Chevessier F., Winter L., Schutz J., Bauer R., Thorweihe J.M., Wenzel D., Schlotzer-Schrehardt U. et al. (2015) The toxic effect of R350P mutant desmin in striated muscle of man and mouse. Acta Neuropathol., 129, 297–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izawa I., Nishizawa M., Ohtakara K., Ohtsuka K., Inada H., Inagaki M. (2000) Identification of Mrj, a DnaJ/Hsp40 family protein, as a keratin 8/18 filament regulatory protein. J. Biol. Chem., 275, 34521–34527. [DOI] [PubMed] [Google Scholar]
- 21.Udan-Johns M., Bengoechea R., Bell S., Shao J., Diamond M.I., True H.L., Weihl C.C., Baloh R.H. (2014) Prion-like nuclear aggregation of TDP-43 during heat shock is regulated by HSP40/70 chaperones. Hum. Mol. Genet., 23, 157–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hackman P., Sarparanta J., Lehtinen S., Vihola A., Evila A., Jonson P.H., Luque H., Kere J., Screen M., Chinnery P.F. et al. (2012) Welander distal myopathy is caused by a mutation in the RNA-binding protein TIA1. Ann. Neurol., 73, 500–9. [DOI] [PubMed] [Google Scholar]
- 23.Kim H.J., Kim N.C., Wang Y.D., Scarborough E.A., Moore J., Diaz Z., MacLea K.S., Freibaum B., Li S., Molliex A. et al. (2013) Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature, 495, 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunter P.J., Swanson B.J., Haendel M.A., Lyons G.E., Cross J.C. (1999) Mrj encodes a DnaJ-related co-chaperone that is essential for murine placental development. Development, 126, 1247–1258. [DOI] [PubMed] [Google Scholar]
- 25.Watson E.D., Geary-Joo C., Hughes M., Cross J.C. (2007) The Mrj co-chaperone mediates keratin turnover and prevents the formation of toxic inclusion bodies in trophoblast cells of the placenta. Development, 134, 1809–1817. [DOI] [PubMed] [Google Scholar]
- 26.Stone M.R., O'Neill A., Catino D., Bloch R.J. (2005) Specific interaction of the actin-binding domain of dystrophin with intermediate filaments containing keratin 19. Mol. Biol. Cell., 16, 4280–4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Neill A., Williams M.W., Resneck W.G., Milner D.J., Capetanaki Y., Bloch R.J. (2002) Sarcolemmal organization in skeletal muscle lacking desmin: evidence for cytokeratins associated with the membrane skeleton at costameres. Mol. Biol. Cell., 13, 2347–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perng M.D., Cairns L., van den I.P., Prescott A., Hutcheson A.M., Quinlan R.A. (1999) Intermediate filament interactions can be altered by HSP27 and alphaB-crystallin. J. Cell. Sci., 112(Pt 13), 2099–2112. [DOI] [PubMed] [Google Scholar]
- 29.Weihl C.C., Temiz P., Miller S.E., Watts G., Smith C., Forman M., Hanson P.I., Kimonis V., Pestronk A. (2008) TDP-43 accumulation in inclusion body myopathy muscle suggests a common pathogenic mechanism with frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry, 79, 1186–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salajegheh M., Pinkus J.L., Taylor J.P., Amato A.A., Nazareno R., Baloh R.H., Greenberg S.A. (2009) Sarcoplasmic redistribution of nuclear TDP-43 in inclusion body myositis. Muscle Nerve., 40, 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinkus J.L., Amato A.A., Taylor J.P., Greenberg S.A. (2014) Abnormal distribution of heterogeneous nuclear ribonucleoproteins in sporadic inclusion body myositis. Neuromuscul. Disord., 24, 611–616. [DOI] [PubMed] [Google Scholar]
- 32.Klar J., Sobol M., Melberg A., Mabert K., Ameur A., Johansson A.C., Feuk L., Entesarian M., Orlen H., Casar-Borota O. et al. (2013) Welander distal myopathy caused by an ancient founder mutation in TIA1 associated with perturbed splicing. Hum. Mutat., 34, 572–577. [DOI] [PubMed] [Google Scholar]
- 33.Wang I.F., Chang H.Y., Hou S.C., Liou G.G., Way T.D., James Shen C.K. (2012) The self-interaction of native TDP-43 C terminus inhibits its degradation and contributes to early proteinopathies. Nat. Commun., 3, 766. [DOI] [PubMed] [Google Scholar]
- 34.Sondheimer N., Lopez N., Craig E.A., Lindquist S. (2001) The role of Sis1 in the maintenance of the [RNQ+] prion. Embo. J., 20, 2435–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez N., Aron R., Craig E.A. (2003) Specificity of class II Hsp40 Sis1 in maintenance of yeast prion [RNQ+]. Mol. Biol. Cell., 14, 1172–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gehrig S.M., van der Poel C., Sayer T.A., Schertzer J.D., Henstridge D.C., Church J.E., Lamon S., Russell A.P., Davies K.E., Febbraio M.A. et al. (2012) Hsp72 preserves muscle function and slows progression of severe muscular dystrophy. Nature, 484, 394–398. [DOI] [PubMed] [Google Scholar]
- 37.Rose M.R., Jones K., Leong K., Walter M.C., Miller J., Dalakas M.C., Brassington R., Griggs R. (2015) Treatment for inclusion body myositis. Cochrane Database Syst. Rev., 6, CD001555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.