Abstract
Objectives The HPAI H5N2 strain that caused an outbreak in ostriches of the Eastern Cape Province, South Africa in 2004 was characterized.
Design Haemagglutination inhibition (HI) and agar gel immunodiffusion (AGID) were performed on sera from ostrich farms in the outbreak region, and intravenous pathogenicity (IVPI) tests, reverse‐transcriptase‐polymerase‐chain reaction (RT‐PCR), nucleic acid sequencing and phylogenetic comparisons were performed on the HPAI H5N2 virus isolated during the outbreak.
Results The deduced amino acid sequence at the HA0 cleavage site determined by RT‐PCR and nucleotide sequencing was PQREKRRKKRGLF and thus the virus fell within the definition of a highly pathogenic virus, but in an IVPI test in chickens on the virus isolated from the index case and a value of 0·63 was recorded, which is below the criterion for highly pathogenic viruses in this in vivo test. After a further passage in embryonated eggs a second IVPI was carried out and an elevated value of 1·19 was obtained. Cloacal swabs were taken from the initial IVPI birds, inoculated into embryonated chickens eggs and a third IVPI was then performed on the resulting haemagglutinating, infective allantoic fluid. An index of 2·73 was recorded.
Conclusions HI tests appeared to be the more sensitive test compared to AGID when testing for antibodies to avian influenza in sera. An ostrich‐derived virus with a virulent HA0 cleavage site was not initially virulent in chickens but after passage in the latter the virulence increased. Phylogenetic analyses demonstrated the link between AI viruses carried by wild ducks and those infecting ostriches.
Keywords: Highly pathogenic avian influenza, H5N2, intravenous pathogenicity index test, ostrich, phylogeny
Introduction
The transmissibility and virulence of any avian influenza virus may vary between different species and the clinical signs may be different in different hosts complicating the initial diagnosis. 1 , 2 , 3 To date only subtypes H5 and H7 have been reported as causing the severe disease in chickens and other species termed highly pathogenic avian influenza (HPAI) although not all H5 and H7 subtype viruses cause HPAI.
An avian influenza virus infection of ostriches was first diagnosed in South Africa in 1991 4 when high mortality was seen in flocks of young birds, but only an avian influenza virus with low virulence for chickens of subtype of H7N1 was isolated. Subsequent infections of ostriches with low pathogenicity avian influenza (LPAI) viruses of subtypes H5 and H7 were experienced in South Africa, The Netherlands, and Zimbabwe and in birds imported into Denmark and The Netherlands. 5 , 6 , 7 Low pathogenicity subtypes H6N8, 8 H10N1 and H9N2 have also been isolated in ostriches in South Africa and reports from the USA cite H3N2, H4N2, H4N6, H10N4, H10N7 in rheas and emus as well as subtypes of H5N2, H5N9, H7N1 and H7N3. 9 The first report of a natural infection of HPAI virus in ratites came from Italy in 2000. 10 This occurred during an epidemic of HPAI H7N1 in which hundreds of commercial poultry flocks became infected. The ostriches exhibited nervous signs, brilliant green urine, loss of appetite and depression before death.
Objectives
In July 2004, unexplained deaths in birds showing similar clinical signs to the Italian ostriches occurred in ostrich farms in the Eastern Cape Province of South Africa. This paper reports the characterisation of the causative agent involved in the 2004 outbreak.
Methods
Viruses
Low pathogenicity avian influenza virus A/Egyptian goose/South Africa/04 (H5N2) (EgZA04) was detected by reverse‐transcription polymerase‐chain reaction (RT‐PCR) during active surveillance conducted prior to the HPAI outbreak in the Oudtshoorn area of the Western Cape Province adjacent to the Eastern Cape Province where the HPAI ostrich outbreaks occurred. 11 Egyptian geese (Alopochen aegypticus) are resident, locally nomadic birds in South Africa that frequently come into contact with migratory birds in lagoons, water treatment works, dams and rivers but also have direct contact with farmed ostriches when sharing the same pasture. 11
Virus isolation and identification
Virological examination of tissue and swab material was carried out by the inoculation of 9‐ to 10‐day‐old specific pathogen free (SPF) embryonated fowls’ eggs into the allantoic cavity. The alantoic fluids of all eggs were checked for haemagglutination activity using standard techniques. 12 , 13 The virulence of the isolates obtained was determined by the intravenous pathogenicity index test (IVPI) and the derived amino acid sequence at the cleavage site of the precursor haemagglutinin molecule (HA0). The IVPI test was done following standard procedures 13 in ten 6‐week‐old white leghorn chickens hatched from eggs obtained from a commercial SPF flock and reared at the Veterinary Laboratories Agency (VLA), Weybridge, UK, under SPF conditions. Each bird was inoculated intravenously with 0·1ml of infective allantoic fluid from SPF fowls’ eggs inoculated with the virus. Birds were recorded as normal, sick, very sick or dead each day for 10 days. Serological techniques for the detection of antibodies to avian influenza were carried out using agar gel immunodiffusion for specific influenza A antibodies and haemagglutination inhibition (HI) tests for antibodies specific to the H5 subtype using the H5N2 virus from the primary isolation as an antigen and standard techniques. 12 , 13 Ostrich sera were heat inactivated at 56°C for 30 minutes and adsorbed with packed chicken red blood cells.
RNA isolation and RT‐PCR
Viral RNA was extracted from harvested allantoic fluids using the QIAquick RNA Extraction Kit (Qiagen, Hilden, Germany) or a MagnaPure system (Roche). The HA1 fragment was amplified using gene‐specific primers S1 (5′‐AGCAGGGGTATAATCTCTCA‐3′) and B2a (5′‐TTTTGTCAATGATTGAGTTGACCTTATTGG‐3′) using One‐step RT‐PCR Kits (Qiagen, Germany). Gene‐specific primers and procedures used to amplify and sequence internal genes of Egyptian goose (EgZA04) genes are described elsewhere. 14 Polymerase chain reaction amplicons were visualised by ethidium bromide staining after 2% w/v agarose gel (Invitrogen, UK) electrophoresis and purified using QIAquick Gel Extraction Kits (Qiagen, Germany).
Sequencing and phylogenetic analysis
Reverse‐transcriptase‐polymerase‐chain reaction products were sequenced by cycle‐sequencing reactions using ABI Terminator cycle sequencing V1·1 chemistry on a Perkin‐Elmer ABI PrismTM 310 DNA sequencer (Applied Biosystems, Foster City, USA). Sequence data was assembled and edited using the Lasergene version 7 seqman software (DNASTAR Inc.). Sequence alignments were performed using the Lasergene version 7 megalign software (DNASTAR Inc). Maximum Likelihood trees were generated using Phylogeny Inference Package (phylip) software version 3·57c 15 with the transition to transversion ratio for the data set calculated using tree‐puzzle v5·2. 16 Nucleotide distance matrices were generated using the Lasergene version 7 megalign software (DNASTAR Inc). For phylogenetic tree construction and comparison, sequences generated from isolates submitted to VLA Weybridge as part of its EU/OIE/FAO Reference Laboratory status or obtained from the Influenza Sequence Database (ISD), Los Alamos, USA 17 were included in the trees. Sequences submitted to GenBank were assigned the accession numbers FJ519978–FJ519989.
Results
Disease investigation and diagnosis
Respiratory signs, swelling and exudates from the eyes, fluorescent green diarrhoea, depression, emaciation, collapse and death were some of the clinical signs observed in ostriches in the flock from which virus was isolated. On post‐mortem examination some cases revealed prominent liver damage comprising multifocal to diffuse necrosis and degeneration often together with haemorrhagic lesions of the heart, lungs, kidney, pancreas and intestine. In some cases miliary granulomas in the lungs and fungal growth on the airsac membranes due to infection with Aspergillus fumigatus were observed. Preliminary laboratory results indicated infection with an HPAI virus and sero surveillance was undertaken in the immediate area.
Further virus isolation attempts were carried out resulting in the isolation of an influenza A virus of subtype H5N2.
An IVPI test was done on the virus isolated from the index case (supplied by Stellenbosch Provincial Veterinary Laboratory) at VLA Weybridge and an index of 0·63 was recorded. After a further passage in embryonated eggs a second IVPI was carried out and an elevated value of 1·19 was obtained. Cloacal swabs were taken from the initial IVPI birds, inoculated into embryonated fowls’ eggs and a third IVPI was then performed on the resulting haemagglutinating, infective allantoic fluid. An index of 2·73 was recorded. In each of the first two IVPI tests the chickens that survived had exhibited marked cyanosis of wattles, combs and legs and became depressed, but by the end of the ten day test period the birds had returned to an apparently normal clinical state. The deduced amino acid sequence at the HA0 cleavage site determined by RT‐PCR and nucleotide sequencing revealed a virulent cleavage site containing multi basic amino acids PQREKRRKKRGLF for all three samples giving the different IVPI values.
No clinical signs or raised antibody levels were seen in the local domestic poultry population during the outbreak or during a surveillance exercise carried out in the infected area. However, avian influenza antibodies were detected in samples taken from ostriches, HI titres ranged from <21 to 211. The results of the agar gel precipitin tests in ostriches were not always comparable to the HI titres, in fact in this instance where the HA subtype of the infecting virus was known the HI test appeared to be the more sensitive test when testing for antibodies to avian influenza in sera obtained from the ostriches.
The initial testing of serological samples from ostriches on farms in the Eastern Cape was carried out using agar gel precipitin testing (AGPT). Serum samples from three farms, under the same ownership, out of a total of four farms sampled produced specific lines of identity for influenza A antibodies in the AGPT. Further confirmation of AGPT positives was carried out using HI testing with H5 homologous antigen and at least two of four farms had detectable titres of >24 with titres ranging from 24 to 28. Follow‐up serology on 71 serum samples from the three original AI‐positive farms resulted in only seven testing positive by AGPT whereas 24 out of 71 sera gave HI titres of >24.
Unfortunately due to only small amounts of sera available it was not possible to test and compare all sera by the two different serological tests. (See Table 1).
Table 1.
Comparative serology on ostrich sera using HI and AGP tests
| HI | ||
|---|---|---|
| Positive* | Negative | |
| AGPT | ||
| Positive | 6 | 1 |
| Negative | 18 | 46 |
AGPT, agar gel precipitin test; HI, haemagglutination inhibition.
*A positive serum is one with a titre of 24 or more. 13 .
Diagnostic sensitivity 25%.
Diagnostic specificity 97·9%.
Further random testing of ostrich sera from the Western Cape were analysed by HI for antibodies to H5, seven farms recorded titres of 24 and above. All positive titres were further analysed using additional H5 antigen with a heterologous neuraminidase subtype to confirm the antibody response was specific to the H5 subtype.
Phylogenetic analysis
Phylogenetic trees were generated using a 971 nucleotide length of HA1 from H5 sequences (excluding the haemagglutinin cleavage site) which placed the ostrich South African 2004 isolate A/Ostrich/South Africa/N227/04 (H5N2) (osZA04) within the Eurasian H5 lineage (Figure 1). osZA04 was incorporated into a cluster containing isolates from Italy in 2004; (A/duck/Italy/1349/2004,‐dkIT04), Denmark in 2003/2004; (A/duck/Denmark/1356/2003)‐maDK03), (A/duck/Denmark/150/2004‐maDK04) and Germany in 2003; (A/mallard/Germany/Wv1349/03‐maDE03). osZA04 was shown to be most closely related to the low pathogenic isolate, dkIT04, with a nucleotide sequence identity of 95·9%.
Figure 1.
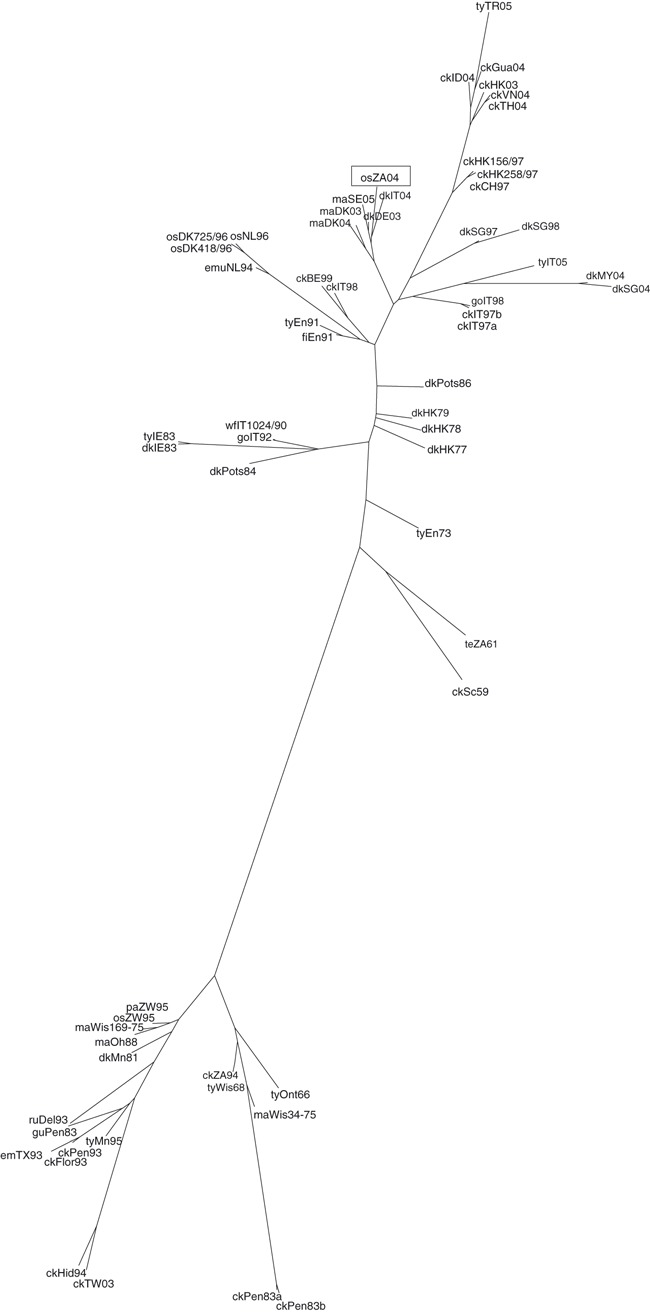
Maximum likelihood phylogenetic tree of partial H5 sequences, generated using Phylogeny Inference Package (phylip) software and including sequences generated from isolates submitted to VLA Weybridge as part of its EU/OIE/FAO Reference Laboratory status or obtained from the Influenza Sequence Database (ISD).
Genotyping
Genotyping phylogenetic trees were generated from partial internal protein gene sequences produced from swine, avian and human isolates submitted to VLA, Weybridge or obtained from the Influenza Sequence Database (ISD) (data not shown). Phylogenetic analyses of osZA04 internal protein genes placed osZA04 for all gene segments in the Eurasian influenza lineages within clusters containing H7 isolates from Italy in 1999/2001; A/turkey/Italy/4603/1999 (H7N1), A/mallard/Italy/43/01 (H7N3) and A/mallard/Italy/33/01 (H7N3): an H1 virus from Denmark in 2002; A/dk/Denmark/74–68 425 1–3/02 (H1N3).
Ostrich ZA 2004 compared to Egyptian goose ZA 2004
To determine the similarity between the Egyptian goose LPAI virus and the HPAI virus, HA, NA, NS and M sequences amplified from the A/Egyptian goose/South Africa/AI23/04 (H5N2) (EgZA04) were compared to the sequences obtained for osZA04. Partial HA1/HA2 amino acid alignments between sequences obtained for osZA04 and EgZA04 identified two amino acid differences (D227N and P333L) and the insertion of basic amino acids to generate a HP cleavage site in the ostrich isolate.
A 316 nucleotide partial matrix gene alignment of osZA04 with EgZA04 showed a 98·5% identity between the two isolates. A 457 nucleotide partial non‐structural (NS) gene alignment of osZA04 with EgZA04 showed a 98·7% identity between the two isolates.
Conclusions
In 2004, South Africa recorded its first isolation of HPAI virus in 43 years when an H5N2 strain caused increased mortalities in ostrich flocks of the Eastern Cape Province. Due to contrasting reports from the field and from past experimental data further assessments for the pathogenicity of avian influenza in ratites have been carried out in an attempt to clarify the role of these viruses in ratite hosts, 3 , 18 but the contribution of viral infection to morbidity remains unclear as it is possible that under field conditions secondary bacterial or fungal contamination may have exacerbated the clinical disease picture (A Olivier, personal communication).
The variable values obtained in the IVPI tests were somewhat unusual and emphasise the importance of using both in vivo pathogenicity tests and, for AI viruses of H5 and H7 subtype, molecular estimations of potential virulence as required by the Office International des Epizooties (OIE) definition of HPAI. 13 The initial IVPI value and that obtained after one further passage in eggs were below the HPAI qualifying value of 1·2, although these viruses would still have been considered HPAI for control purposes because of their HA0 cleavage site deduced amino acid sequences. This being confirmed by the IVPI value of 2·73 obtained after this virus had undergone one passage in chickens. It seems likely that passage in ostriches had for some reason resulted in a virus that was attenuated for chickens, but that passages in chickens restored the virulence for chickens produced a ‘chicken‐adapted’ strain with higher virulence. Further investigations into this host adaptation effect on virulence would be valuable.
During active surveillance conducted prior to the outbreak, an LPAI H5N2 virus was detected in an Egyptian goose in the adjacent Western Cape Province. Phylogenetic analysis of the H5 genes confirmed that the LPAI H5N2 Egyptian goose virus was the closest relative to the HPAI H5N2 Eastern Cape ostrich outbreak strain. Some of the internal protein genes of the ostrich virus (PB2, PB1 and PA) were closely‐related to other wild duck isolates (LP H5N1, H3N8 and H4N8) isolated in South Africa during the same period from another province. 19 This demonstrates the link between AI viruses carried by wild ducks and those infecting ostriches. Southern African ducks and geese are short‐to‐medium distance migrants and are thus unlikely to be directly involved in the initial introduction of LPAI from Eurasia into southern Africa. However, about two dozen palearctic migrant species of the orders Ciconiiformes and Charadriiformes overwinter annually in Southern Africa. 20 Viruses shed by migrants into the waterbodies could thus be ingested by local ducks and geese and amplified in these reservoir hosts, which move extensively throughout the region. The majority of ostrich farms in South Africa are located in the Karoo area of the Western Cape Province, a semi‐arid region with sparse natural grazing and low annual rainfall. Ostrich farms are often concentrated around rivers and riverine areas, and are used by the ostrich farmers in cultivating irrigated lucerne pastures for ostriches and sheep. Wild indigenous and migratory birds are found in abundance in this area and accumulate in vast numbers on ostrich farms, where they graze alongside ostriches and concentrate in great numbers around the watering troughs and feeders, contaminating ostrich water and feed with faecal material. 21 After the 2004 HPAI H5N2 outbreaks, changes in farm management practices were implemented to minimise the contact between free‐ranging ostriches and wild waterfowl, for example by fencing off rivers on ostrich farms, preventing contact with wild birds at ostrich feed points and by regular disinfection of feed and water troughs (Adriaan Olivier, personal communication). Hopefully these biosecurity measures will help to minimise the risk of infection, but again the importance of improved surveillance for AIV in wild birds is highlighted. Following the detection of the HPAI H5N2 virus disease control and eradication programme resulted in the culling of over 26 000 ostriches from 38 farms in the Eastern Cape Province. Monitoring of depopulated ostrich farms that were subsequently restocked did not reveal any seropositive birds indicating that the virus was successfully eliminated by the measures taken. However, caution must be taken in determining the most sensitive test for detecting antibodies in ratite serum given the results obtained when the field sera were tested. Despite the absence of high mortality in ostriches, caution must still be exercised when moving these possible carriers of HPAI in an attempt to prevent the spread of disease from apparently clinically normal birds and flocks.
Acknowledgements
James Kitching and Magdalene Dreyer are acknowledged for the original Stellenbosch isolate of the Eastern Cape HPAI H5N2 ostrich virus. Josephine Mitchell performed RNA isolation on the Egyptian goose tissues.
References
- 1. Alexander DJ, Allan WH, Parsons D, Parsons G. The pathogenicity of four avian influenza viruses for chickens, turkeys and ducks. Res Vet Sci 1978; 24:242–247. [PubMed] [Google Scholar]
- 2. Alexander DJ, Parsons G, Manvell RJ. Experimental assessment of the pathogenicity of eight avian influenza A viruses of H5 subtype for chickens, turkeys, ducks and quail. Avian Pathol 1986; 15:647–662. [DOI] [PubMed] [Google Scholar]
- 3. Manvell RJ, Jorgensen PH, Nielsen OL, Alexander DJ. Experimental assessment of the pathogenicity of two avian influenza A H5 viruses in ostrich chicks (Struthio camelus) and chickens. Avian Pathol 1998; 27:400–404. [DOI] [PubMed] [Google Scholar]
- 4. Allwright DM, Burger WP, Geyer A, Terblanche AW. Isolation of an influenza A virus from ostriches (Struthio camelus). Avian Pathol 1993; 22:59–65. [DOI] [PubMed] [Google Scholar]
- 5. Koch G. Report of disease incidence of avian influenza in The Netherlands in 1994 in press (ed): Proceedings of the Joint Second Annual Meeting of the National Newcastle Disease and Avian Influenza Laboratories of Countries of the European Union. Brussels: 1995:11–12. [Google Scholar]
- 6. Jorgensen PH, Nielsen OL, Hansen HC, Manvell RJ, Banks J, Alexander DJ. Isolation of influenza A virus, subtype H5N2, and avian paramyxovirus type 1 from a flock of ostriches in Europe. Avian Pathol 1998; 27:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. OIE Disease Information. Available at: (http://www.oie.int/eng/info/hebdo/AIS_42.HTM#Sec9) [accessed 2 December 2005].
- 8. Pfitzer S, Verwoerd DJ, Gerdes GH, Labuschagne AE, Erasmus A, Manvell RJ, Grund C. Newcastle disease and avian influenza A virus in wild waterfowl in South Africa. Avian Dis 2000; 44:655–660. [PubMed] [Google Scholar]
- 9. Panigrahy B, Senne DA, Pearson JE. Presence of avian influenza virus (AIV) subtypes H5N2 and H7N1 in emus (Dromaius novaehollandiae) and rheas (Rhea americana): virus isolation and serologic findings. Avian Dis 1995; 39:64–67. [PubMed] [Google Scholar]
- 10. Capua I, Mutinelli F, Bozza MA, Terregino C, Cattoli G. Highly pathogenic avian influenza (H7N1) in ostriches (Struthio Camelus). Avian Pathol 2000; 29:643–646. [DOI] [PubMed] [Google Scholar]
- 11. Olivier AJ. Ecology and epidemiology of avian influenza in ostriches. Proceedings of the OIE/FAO International Conference on Avian Influenza. Schudel A, Lombard M (eds): Dev Biol 2006; 124:51–57. [PubMed] [Google Scholar]
- 12. CEC . Council Directive 91/40/EEC, 19 May 1992, introducing community measures for the control of avian influenza. Official Journal of the European Union 1992; L167:1–16. [Google Scholar]
- 13. Alexander DJ. Avian influenza. Chapter 2.07.12. Manual for Diagnostic Tests and Vaccines for Terrestrial Animals. 5th edn Paris: OIE; Available at: (http://www.oie.int/eng/normes/MMANUAL/A_00037.htm), 2005. [accessed 30 January 2009]. [Google Scholar]
- 14. Abolnik C. Molecular epidemiology of Newcastle disease and avian influenza in South Africa [dissertation], South Africa: University of Pretoria; 2007. Available at: (http://upetd.up.ac.za/UPeTD.htm) [accessed 30 January 2009]. [Google Scholar]
- 15. Felsenstein J. PHYLIP (Phylogeny Inference Package) version 3.57c. Distributed by the author. Department of Genome Sciences. Seattle: University of Washington, 1993. [Google Scholar]
- 16. Schmidt HA, Strimmer K, Vingron M, Von Haeseler A. TREE‐PUZZLE: maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics 2002; 18:502–504. [DOI] [PubMed] [Google Scholar]
- 17. Macken C, Lu H, Goodman J, Boykin L. The value of a database in surveillance and vaccine selection; in Osterhaus ADME, Cox N, Hampson AW. (eds): Options for the Control of Influenza IV. Amsterdam: Elsevier Science; 2001. [Google Scholar]
- 18. Clavejo A, Riva J, Copps J, Robinson Y, Zhou E. Assessment of the pathogenicity of an emu‐origin influenza A H5 in ostriches (Struthio camelus). Avian Pathol 2001; 30:83–89. [DOI] [PubMed] [Google Scholar]
- 19. Abolnik C, Cornelius E, Bisschop SPR, Romito M, Verwoerd D. Phylogenetic analysis of genes from South African LPAI viruses isolated in 2004 from wild aquatic birds suggests introduction by Eurasian migrants. Dev Biol 2006; 124:189–199. [PubMed] [Google Scholar]
- 20. Underhill LG, Tree AJ, Oschadleus HD, Parker V. Review of ring recoveries of waterbirds in southern Africa. Avian Demography Unit, University of Cape Town, Cape Town; 1999. [Google Scholar]
- 21. Sinclair M, Brückner GK, Kotze JJ. Avian Influenza in Ostriches: Epidemiological Investigation into the Western Cape Province of South Africa. Western Cape: Elsenburg Journal, Department of Agriculture, 2005. [PubMed] [Google Scholar]


