INTRODUCTION
Iron serves important functions in many biochemical processes including the development of the central nervous system, and it is essential to neural myelination, neurotransmitter function, neuronal energy metabolism and neurite differentiation (1). The requirement for iron is particularly high during periods of rapid growth and differentiation, e.g. during the last trimester of pregnancy and during infancy when the brain experiences its growth spurt. Ineffective iron homeostasis during these periods may therefore result in delayed neurodevelopment and cognitive functions (2). Many studies have shown an association between iron deficiency anemia (IDA) and poor neurodevelopment in infants that lasts beyond the period of deficiency (3). Iron supplementation reduces the risk of developing anemia in children at risk of developing iron deficiency (ID) and IDA. On the other hand, excessive iron supplementation of infants may lead to increased risk of infection, impaired growth and disturbed absorption or metabolism of other minerals (4). Iron is also a known pro-oxidant, and non-protein bound iron may cause formation of free oxygen radicals. Taken together, it is important to find optimal strategies to prevent ID and avoid iron overload and its potential adverse effects. Hence, it is essential to recognize which infants should be given what form of iron, in what dose, and during which period in life to achieve optimal preventive effects with minimal, if any, adverse effects. To reach this goal, a detailed understanding of how iron homeostasis in infants and children is regulated and how regulation changes with age is a prerequisite.
MOLECULAR REGULATION OF IRON ABSORPTION
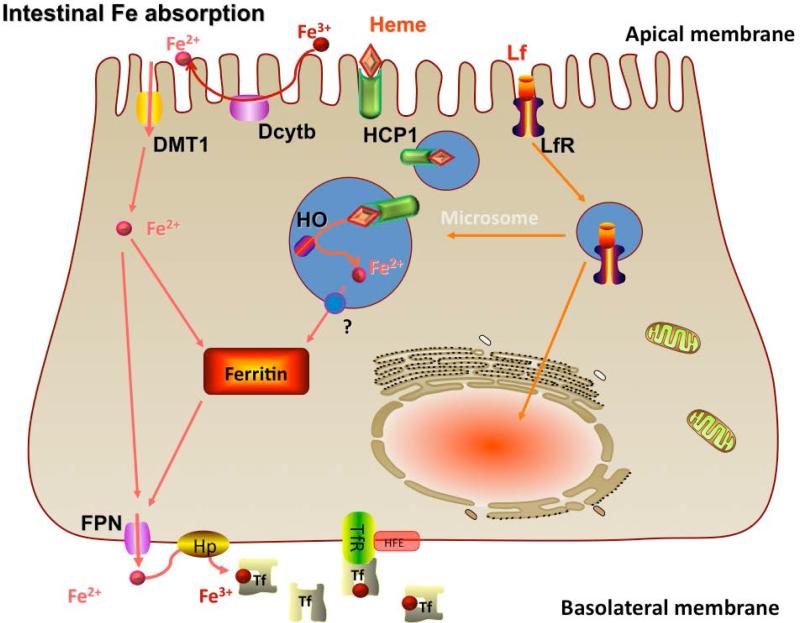
The primary importer of iron across the apical membrane of the intestinal epithelial cell is divalent metal transporter 1 (DMT1, also known as Nramp2) (Fig. 1). DMT1 is essential for iron absorption because mice that lack the gene encoding DMT1 develop severe IDA (5). This transporter is responsible for uptake of ferrous iron and is strongly regulated by iron status (6). While duodenal cytochrome b (Dcytb), a ferric reductase located at the apical membrane, has been shown to be involved in regulation of iron metabolism in rodents (7), studies in knock-out mice do not support this (8). Dcytb is not likely to be involved in iron absorption in humans because humans are known to absorb ferric iron poorly and variations in iron status (hereditary hemochromatosis, ID, controls) are not associated with changes in Dcytb expression (9).
Figure 1.
A schematic model of regulation of intestinal iron absorption including transporters at the apical and basolateral membranes as well as oxido-reduction steps involved in iron transfer. Fe, iron; HCP, heme carrier protein; HFE, human hemochromatosis protein; HO, hydroxyl; Hp, hephaestin; Lf,lactoferrin; LfR, lactoferrin receptor; Tf, transferrin; TfR,transferrin receptor. Reprinted with permission from Lonnerdal and Hernell, 2010.
Intestinal iron absorption. DMT, Divalent metal transporter; Dcytb, Duodenal cytochrome b; HCP, Heme carrier protein; Lf, Lactoferrin; LfR, Lactoferrin receptor; HO, Hydroxyl; FPN, Ferroportin; Fe, Iron; Tf, Transferrin; TfR, Transferrin receptor; Hp, Hephaestin; HFE, Human hemochromatosis protein.
Reprinted with permission from (62)
Once internalized by the enterocyte, iron is transported across the cell, but little is known about intracellular trafficking of iron. Depending on cellular iron status, iron may become bound to ferritin. A recent study suggests that ferritin H may be involved in protection against iron overload (10). Over time, iron bound to ferritin will either be mobilized for further transport or lost by normal sloughing of epithelial cells.
Iron translocated across the cell is exported by ferroportin (FPN) located at the basolateral membrane (Fig.1). FPN is strongly regulated by iron status and is the only known ferrous iron exporter in mammals. FPN mutations in humans lead to iron-loading disorders (11). Ferrous iron is oxidized by hephaestin, a copper-containing membrane-bound ferroxidase that co-localizes with FPN in the basolateral membrane (12). This oxidation of iron is important for iron transfer because hephaestin knock-out mice develop a severe iron deficiency anemia (13). Following export of iron by FPN, iron in ferric form is transported to the liver bound to transferrin and utilized by the reticuloendothelial system for hemoglobin (Hb) synthesis or deposited in iron stores. The communication between the iron stores in the liver and the intestinal epithelial cell was long an enigma but has been shown to be mediated by hepcidin, a peptide synthesized by the liver (14). Hepcidin is an endocrine regulator of iron metabolism that covalently binds to FPN, which causes its internalization and breakdown (15). Iron subsequently accumulates in the intestinal cell and down-regulates the expression of DMT1. As a consequence, iron absorption is effectively down regulated.
IRON METABOLISM DURING THE NEWBORN PERIOD
Although the magnitude of the difference in bioavailability of iron from breast milk and infant formula varies among studies, most investigators agree that iron is absorbed better from breast milk. In part, this may be due to the presence of high concentrations of the iron-binding protein lactoferrin in breast milk and its virtual absence from infant formula (16). A major part of iron in breast milk is associated with lactoferrin. Lactoferrin is relatively resistant against proteolysis and appears in the stool of breastfed infants in intact form (17). Human lactoferrin is absorbed across the apical membrane of the intestinal cell via a specific lactoferrin receptor (Fig.1) and internalized with its bound iron (18). Thus, lactoferrin facilitates a unique mechanism for absorption of iron from breast milk. In contrast, iron in infant formula based on cow milk is largely bound to casein, and phosphopeptides formed during digestion may limit iron absorption (19). Breast milk also contains casein, but in lower concentrations and with different subunits. Relatively more iron is present in low molecular complexes, a form of iron that is more likely to be absorbed. Infant formulas, particularly those for preterm infants, contain higher levels of calcium than breast milk. This has caused some concern because calcium has been shown to decrease iron absorption in adults (20). This inhibitory effect may, however, only occur in the short-term because long-term studies on infants given high levels of calcium fail to show any adverse effect on iron status (21).
Measurements of iron absorption are dependent upon the method used. Studies using radioisotope methodology report substantial differences in iron bioavailability from breast milk and cow milk formula (22). This method has the advantage that absorbed and retained iron is measured by whole body counting. However, iron absorption is also strongly affected by iron status, a factor that was rarely controlled for in early studies. Stable isotopes have been used more commonly in recent studies, and these studies show smaller or no differences in iron absorption from breast milk and infant formula. This may be due to improvements in infant formula composition, but also may be due to methodological limitations. In stable isotope studies, it is assumed that about 80% of absorbed iron is incorporated into red blood cells (23), an assumption based on studies of human adults. In fact, much less iron is incorporated into erythrocytes in infants (24), which may lead to an underestimate of iron absorption. The extent to which this incorporation is affected by age or by iron status is not yet known, nor how it is affected by the form of iron given to the infant. We have shown that iron given as drops or as fortification iron affects iron indicators differently, suggesting different metabolic pathways (25). Whether iron taken up from breast milk (lactoferrin) or from infant formula has different metabolic fates is not yet known.
Using stable isotope methodology, we showed that 6 month old healthy, exclusively breastfed term infants absorbed 16 ± 11 % of iron, with no significant difference between iron-supplemented and unsupplemented infants (24). At 9 months of age, iron absorption from human milk remained at the same level in iron-supplemented infants (16 ± 9 %). Whether there are age-related differences in iron absorption independent from iron status is not known as there have been no developmental studies on iron absorption in iron-replete infants by the same investigators using the same technique. A compilation of studies to date indicates that this is not the case as the results are highly variable but differences in iron status and methodology between studies may obscure such a finding. Homeostatic regulation of iron absorption in infants also needs to be considered. Although no difference was found between iron-supplemented and unsupplemented infants at 6 months of age, unsupplemented infants had considerably higher iron absorption at 9 months of age, i.e. 37 ± 19 % (26). This suggests that homeostatic regulation of iron absorption is absent in young infants but matures and is present at 9 months of age. In further support of this, iron supplementation between 4 and 6 months of age considerably increased Hb concentration regardless of initial iron status. In contrast, continued iron supplementation to 9 months had no effect on Hb concentrations in iron-replete infants (27). When iron homeostasis develops during the period of 6 to 9 months is not yet known, nor whether it is fully developed at 9 months of age. Further studies of various age groups are needed to clarify this.
The molecular reasons for the lack of homeostasis of iron metabolism that we found in young infants are not yet known, but results from rodent models may provide some insights. It has been found that 10-day-old suckling rat pups also lack homeostatic regulation of iron absorption, whereas day 20-day-old weaned pups can regulate iron absorption (28). At day 10, there was no effect of iron status on either DMT1 or FPN expression in intestinal epithelial cells. However, at day 20, iron deficiency strongly up-regulated the expression of these two iron transporters, and iron supplementation strongly down-regulated their expression. Whether the same kind of regulation occurs in human infants is not yet known. It is tempting, though, to speculate that offspring are adapted to absorb as much iron as possible as long as milk, which has a low iron content, is the main food. This is supported by a study in neonatal rats showing that more iron is absorbed in the distal part of the gastrointestinal tract than in adult rats (29). Since DMT1 is primarily located in the proximal part of the small intestine, this suggests that iron may be absorbed via another mechanism in early life. It has also been shown in piglets that DMT1 is largely located intracellularly during the neonatal period and not in the apical membrane where iron is taken up (30). Further, recent work has shown that iron absorption is refractory to hepcidin during the neonatal period, in spite of intact hepcidin signaling during this period (31). Taken together, it is evident that mechanisms for iron absorption and their regulation are different during early life than in adults and that further research in this area is needed.
FACTORS AFFECTING IRON STATUS DURING INFANCY
Since the strongest factor affecting iron absorption in adults is iron status of the individual, it is essential for our understanding of iron metabolism in infants to evaluate their iron status and how it changes with age.
Total body iron in fetuses and newborns is approximately 75 mg/kg (32). At a growth rate of 15-20 g·kg−1·day−1, this translates to an iron accretion rate of 1-1.5 mg·kg−1·day−1, which does not apply to newborn infants because the normal decline in Hb concentration after birth significantly increases iron stores. Therefore, a healthy, term infant is initially independent of external iron and can double its birth weight before iron stores are depleted. Blood Hb concentration is high at birth, about 170 g/L in cord blood at birth in healthy term infants (range 135-210 g/L), and declines with age to its lowest value of 110-120 g/L between 8-18 months of age. This decline is physiologic and is due to breakdown of fetal Hb that is replaced by adult Hb by endogenous erythropoiesis, which typically resumes when Hb has decreased from 170g/L to 120 g/L. Breast milk is low in iron (0.2 to 0.4 mg/L), and even though this iron is well utilized, infants breastfed for longer than 4 to 6 months without receiving iron supplements or iron-fortified complementary foods are at risk of developing IDA.
Iron endowment at birth
Maternal ID does not appear to compromise the iron endowment of their infants, but severe ID, i.e., IDA does. Infants of moderately and severely anemic mothers have lower iron stores and a 3-fold increased risk of low birth weight, placing them at higher risk of ID at an early age. Indeed, the incidence of ID and IDA during the second half-year of life is higher in infants born to mothers with IDA than in infants born to iron-replete mothers (33). The timing of umbilical cord clamping also affects the iron endowment of the newborn. Early cord clamping decreases iron transfer to the infant, whereas delayed cord clamping increases the red cell volume in the infants and, in turn, increases the iron endowment. Delaying umbilical cord clamping for 2 min increases total body iron by about 33% and larger iron stores at 6 months of age (34). Taken together, iron requirements during the first half of infancy depend greatly on the iron endowment of the infant at birth. This is supported by a recent study by Ziegler et al. (35) who showed that infants who were exclusively breast-fed until 4 months of age and allowed to receive complementary foods but not infant formula from 4 to 9 months of age started to show signs of ID from 4 months and, with increasing prevalence, until 9 months of age. By 6 months of age, 5% of the infants had exhausted iron stores (ID). Infants with ID were found to be born with low iron endowment, illustrating the need for adequate iron stores at birth. Maternal iron status accounts for only 6% of the variation in infant iron stores at birth, and the remaining causes of the highly variable size of the birth endowment are not known, but likely include low birth weight, intrauterine growth retardation, prematurity, time of cord clamping, maternal smoking and diabetes in pregnancy (36). Prenatal iron supplementation has no effect on infant iron status at 2 or 5 months (37).
Effect of sex on iron status
Substantial sex differences in iron status have been observed during infancy (38, 39). Hemoglobin, mean corpuscular volume and s-Ft concentrations were found to be lower and TfR and zinc protoporphyrin concentrations to be higher in boys than in girls at 4, 6 and 9 months of age. Moreover, boys at 9 months of age had a higher risk of being classified with IDA than girls (38). Ziegler et al (35) also reported lower iron status of boys than of girls. The sex differences in mean corpuscular volume and zinc protoporphyrin concentrations may reflect normal physiological differences between genders. On the other hand, the differences in Hb and TfR seem to reflect a higher incidence of ID in boys. Sex-specific reference values to define ID may need to be developed for some of the iron status indicators (38).
Provision of various forms of iron
In many countries it is recommended to give iron supplements as iron drops to breastfed infants after the first 4-6 months of age. We compared the effects of initiating iron supplementation at 4 months as compared to 6 months of age in exclusively breastfed infants until 9 months of age in two different settings: Honduras as an example of a developing country with low iron endowment at birth and a high prevalence of IDA in infancy and Sweden as an example of a population with adequate iron stores at birth and low prevalence of IDA in infancy (27). We found no significant benefits of starting iron supplementation at 4 months as compared to 6 months of age in either setting. It was evident, however, that the Honduran infants benefitted from the supplement after 6 months of age as shown by Hb values, several indicators of iron status, and the prevalence of ID at 9 months. In contrast, no effects of the supplements were found in the Swedish infants at any age. This suggests that exclusive breastfeeding until 6 months of age when combined with complementary food of high quality and rich in iron will meet the iron requirement of infants. Unexpectedly, we found that iron supplements given to iron-replete infants resulted in decreased linear growth in both settings (see below).
The form of iron given to infants may affect indicators of iron status differently. We have shown that infants provided iron-fortified cereals between 6 and 9 months of age had significantly higher Hb concentration than infants given the same amount of iron daily in the form of iron drops (25). In contrast, the infants given iron drops had significantly higher serum ferritin concentration than those fed iron-fortified cereals. This suggests that these two forms of iron are metabolized differently, with iron from drops preferentially being deposited in stores, whereas iron in fortified foods is incorporated into erythrocytes. Further studies are needed to elucidate the mechanisms behind these observations.
Iron in infant formula
The iron content of infant formulas has typically been substantially greater than in human milk. As described above, recent studies have shown small differences in iron absorption between human milk and infant formulas. Consistent with these observations we have shown that a considerably lower level of iron fortification of infant formulas results in adequate iron status that is not different from breast-fed infants until 6 months of age (40, 41). Healthy Swedish infants fed a formula providing 1.6 mg of iron/L from 1 month of age were shown to have satisfactory iron status at 6 months of age (41). Infants born with low iron status may need more iron, but it is uncertain whether higher levels of iron fortification of infant formula will result in improved iron status of formula-fed infants up to 6 months of age as iron supplementation of Honduran infants (who have low iron endowments) before 6 months of age failed to improve iron status (27). Most infant formulas contain 4-12 mg of iron/L, which is 10-60 times greater than the level of iron in breast milk. It may be questioned whether infant formula used during the first 6 months of life should contain a vast excess of iron, which provides no benefit in order to meet perceived increased iron requirements during 6-12 months of age. In areas where the same type of infant formula is used during the first 12 months of age, increasing the level of iron fortification in complementary foods may be an alternative possibility, while in areas where different types of milk formulas are used between 0-6 and 6-12 months of age, the follow-on formula may have a higher level of iron fortification (42).
ADVERSE EFFECTS OF INADEQUATE AND EXCESSIVE IRON SUPPLY
An important aspect of the physiology of iron is the hierarchical prioritization of iron distribution when iron supply does not meet iron demand. The various organs in the neonate are not equally likely to become deficient. Thus, the liver, which generally harbors stored iron, becomes deficient before skeletal muscle, heart and brain. The neonatal brain is prioritized above all other non-hematologic organs in the fetus or neonate that is in negative iron balance, but the red cells claim the highest priority. Similar to this inter-organ prioritization, intra-organ prioritization exists as well, with iron-containing proteins dedicated to oxygen delivery (e.g., globins) taking precedence over energy production (i.e., cytochromes) (43). As a result, areas of the brain may become iron deficient even when serum indicators are within normal limits.
The brain is highly metabolic in the newborn and infant, consuming 60% of the total body oxygen consumption rate. As has been shown in rodent studies, this high rate of metabolism is highly iron-dependent, and compromise of iron status leads to significant deficits in regional brain metabolism. The most highly metabolic areas of the brain (e.g., hippocampus) are at greatest risk in the newborn period (44). The brain's electrical efficiency is closely linked to the complexity of its structure (e.g., dendritic arbor, synapse formation), which in turn is dependent on adequate energy metabolism and stimulation. Compromise of the energy status by iron deficiency results in less complex arbors and smaller synaptic heads (45), less electrical potential (46), and less synaptic plasticity (47). The effect is indeed due to the lack of neuronal iron and not due to anemia (47, 48).
Beyond energy and structural effects in rapidly developing brain areas, iron deficiency during late gestation and early neonatal life affects neurotransmitter concentrations and function and myelination. The synthesis of the monoamine neurotransmitters, dopamine, norepinephrine and serotonin are dependent on iron-containing hydroxylases. In early life, iron deficiency results in acute and long-term changes in regional neurotransmitter function (1), which are associated with significant behavioral abnormalities in rats (49). Myelination is also rapidly developing from approximately 32 weeks gestation through the first years postnatally. Iron deficiency compromises oligodendrocyte synthesis of important fatty acids for myelin and also suppresses myelin basic protein and phospholipid 1 and 2 gene expression (50, 51). The slower speed of processing documented in iron-deficient premature infants (52) and 6 month old term infants (53) is thought to be related to this hypomyelinated state.
Iron is unique as there is no natural route for excreting excess iron. Thus, the possibility of overload certainly exists and is well known in adults. However, iron overload from orally provided iron as such has not been described in term human infants, and only implicated in premature infants with a known, or feared, consequence of increased iron-associated oxidative damage. Indications of excessive iron intakes by infants have been observed recently. As mentioned above, we noticed that supplementation of Swedish healthy, term breast-fed infants with iron drops caused decreased linear growth by 9 months of age (54). Since this adverse effect was not noted in Honduran infants we hypothesized that the adverse effect was due to the iron-replete status of the Swedish infants. Indeed, when the Honduran cohort was divided into iron-replete and iron non-replete infants an adverse effect on growth was observed in the iron-replete group. A few other studies have also shown negative effects of iron supplements on growth (55, 56). However, in those studies the effect was noted for reduced weight gain rather than linear growth. It should be noted, though, that the nutritional status of the infants in those studies was compromised overall, which is known to decrease linear growth and cause stunting. Thus, when linear growth is compromised it is possible that the adverse effect of excess iron may be manifested differently and instead affects weight gain. However, a recent study on breastfed US infants given iron drops showed both a significant reduction in length gain and a trend towards reduced weight gain as compared to infants given iron-fortified cereals (57). In the studies cited above, iron drops were given. Since iron fortification is likely to result in less iron being absorbed, the potential risk of excess iron in this form may be lower. However, Lozoff et al (58) found that whereas infants with an initially low Hb (<106 g/L) benefitted from infant formula containing a higher level of iron (12.7 mg/L) and showed better developmental outcomes at 10 years of age than those given formula with less iron (2.3 mg/L) from 6 to 12 months of age, those with an initial Hb above 128 g/L showed worse scores when given formula with the higher level of iron. This suggests that an excess of fortification iron also may result in adverse effects.
The mechanism behind the adverse effect of excess iron is still not known, but may involve the pro-oxidative effects of excess iron or, possibly, an interaction between iron and nutrients involved in growth, such as zinc (59). Another possibility is that excess iron adversely affects the gut microbiota and thereby the cross-talk between the microbiome and the intestinal mucosa. A recent study on Kenyan infants given a micronutrient powder (MNP) with iron daily for 4 months showed increased enterobacteria, particularly Shigella, Clostridium and pathogenic E. coli in their stool as compared to infants given MNP without iron (60). This change in the gut microbiota was accompanied by significantly higher levels of fecal calprotectin, a known marker of inflammation. Further, 27.3% of the infants given MNP with iron required treatment for diarrhea versus 8.3% in the group not given iron. It is possible that the shift in microbiota caused by excess iron affects the gut-brain axis via an as of yet unknown mechanism, possibly explaining the observed adverse effects on cognitive development (58). A shift in gut microbiota has been shown to affect energy utilization and metabolism (61), possibly affecting growth of infants. In affluent societies where the nutritional status of infants is adequate or better, linear growth may be affected, whereas in developing countries where food supply is limited and nutritional status sub-optimal, weight gain may be reduced. Further studies are needed to explore this.
Acknowledgments
Funding: Work in Dr. Georgieff's laboratory is supported by Public Health Service grants P01-HL046925, R01-HD029421, and P01-HD039386
ABBREVIATIONS
- Dcytb
Duodenal cytochrome b
- DMT1
Divalent metal transporter
- FPN
Ferroportin
- Hb
Hemoglobin
- ID
Iron deficiency
- IDA
Iron deficiency anemia
- MNP
Micronutrient powder
- s-Ft
s-ferritin
- s-TfR
s-transferrin receptors
Footnotes
B.L. and O.H. received an honorarium to serve as a member of the Mead Johnson Pediatric Nutrition Iron Expert Panel and write a manuscript. B.L wrote the first draft, and all authors take full responsibility for the manuscript. The sponsor had no involvement in preparing the manuscript. B.L. has received honoraria from Valio, Nestlé, and Humana, serves as a consultant to Arla Foods, Hero Nutritionals, Albion and Biostime, and has received grant support from Mead Johnson Nutrition, Arla Foods and Hero. O.H. has received honoraria from Valio, Nestlé, and HiPP, serves as a consultant to Arla Foods, Hero Nutritionals, and Sobi (Swedish Orphan Biovitrum), and has received grant support from Mead Johnson Nutrition, Arla Foods and Hero. M.G. declares no conflicts of interest.
REFERENCES
- 1.Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64:S34–43. doi: 10.1301/nr.2006.may.S34-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collard KJ. Iron homeostasis in the neonate. Pediatrics. 2009;123:1208–16. doi: 10.1542/peds.2008-1047. [DOI] [PubMed] [Google Scholar]
- 3.Algarin C, Nelson CA, Peirano P, Westerlund A, Reyes S, Lozoff B. Iron-deficiency anemia in infancy and poorer cognitive inhibitory control at age 10 years. Dev Med Child Neurol. 2013;55:453–8. doi: 10.1111/dmcn.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Domellöf M. Iron requirements, absorption and metabolism in infancy and childhood. Curr Opin Clin Nutr Metab Care. 2007;10:329–35. doi: 10.1097/MCO.0b013e3280523aaf. [DOI] [PubMed] [Google Scholar]
- 5.Gunshin H, Fujiwara Y, Custodio AO, Direnzo C, Robine S, Andrews NC. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J Clin Invest. 2005;115:1258–66. doi: 10.1172/JCI24356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharp P, Srai SK. Molecular mechanisms involved in intestinal iron absorption. World J Gastroenterol. 2007;13:4716–24. doi: 10.3748/wjg.v13.i35.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Latunde-Dada GO, Van der Westhuizen J, Vulpe CD, Anderson GJ, Simpson RJ, McKie AT. Molecular and functional roles of duodenal cytochrome B (Dcytb) in iron metabolism. Blood Cells Mol Dis. 2002;29:356–60. doi: 10.1006/bcmd.2002.0574. [DOI] [PubMed] [Google Scholar]
- 8.Gunshin H, Starr CN, Direnzo C, Fleming MD, Jin J, Greer EL, et al. Cybrd1 (duodenal cytochrome b) is not necessary for dietary iron absorption in mice. Blood. 2005;106:2879–83. doi: 10.1182/blood-2005-02-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gleeson F, Ryan E, Barrett S, Russell J, Kelleher B, Crowe J. Duodenal Dcytb and hephaestin mRNA expression are not significantly modulated by variations in body iron homeostasis. Blood Cells Mol Dis. 2005;35:303–8. doi: 10.1016/j.bcmd.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Vanoaica L, Darshan D, Richman L, Schumann K, Kuhn LC. Intestinal ferritin H is required for an accurate control of iron absorption. Cell Metab. 2010;12:273–82. doi: 10.1016/j.cmet.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Moreno-Carralero MI, Munoz-Munoz JA, Cuadrado-Grande N, Lopez-Rodriguez R, Jose Hernandez-Alfaro M, del-Castillo-Rueda A, et al. A novel mutation in the SLC40A1 gene associated with reduced iron export in vitro. Am J Hematol. 2014;89:689–94. doi: 10.1002/ajh.23714. [DOI] [PubMed] [Google Scholar]
- 12.Yeh KY, Yeh M, Glass J. Interactions between ferroportin and hephaestin in rat enterocytes are reduced after iron ingestion. Gastroenterology. 2011;141:292–9. 9, e1. doi: 10.1053/j.gastro.2011.03.059. [DOI] [PubMed] [Google Scholar]
- 13.Fuqua BK, Lu Y, Darshan D, Frazer DM, Wilkins SJ, Wolkow N, et al. The multicopper ferroxidase hephaestin enhances intestinal iron absorption in mice. PLoS One. 2014;9:e98792. doi: 10.1371/journal.pone.0098792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nemeth E, Ganz T. The role of hepcidin in iron metabolism. Acta Haematol. 2009;122:78–86. doi: 10.1159/000243791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–3. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 16.Lönnerdal B, Iyer S. Lactoferrin: molecular structure and biological function. Annu Rev Nutr. 1995;15:93–110. doi: 10.1146/annurev.nu.15.070195.000521. [DOI] [PubMed] [Google Scholar]
- 17.Davidson LA, Lonnerdal B. Persistence of human milk proteins in the breast-fed infant. Acta Paediatr Scand. 1987;76:733–40. doi: 10.1111/j.1651-2227.1987.tb10557.x. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki YA, Shin K, Lonnerdal B. Molecular cloning and functional expression of a human intestinal lactoferrin receptor. Biochemistry. 2001;40:15771–9. doi: 10.1021/bi0155899. [DOI] [PubMed] [Google Scholar]
- 19.Hurrell RF, Lynch SR, Trinidad TP, Dassenko SA, Cook JD. Iron absorption in humans as influenced by bovine milk proteins. Am J Clin Nutr. 1989;49:546–52. doi: 10.1093/ajcn/49.3.546. [DOI] [PubMed] [Google Scholar]
- 20.Hallberg L, Rossander-Hulten L, Brune M, Gleerup A. Bioavailability in man of iron in human milk and cow's milk in relation to their calcium contents. Pediatr Res. 1992;31:524–7. doi: 10.1203/00006450-199205000-00024. [DOI] [PubMed] [Google Scholar]
- 21.Lönnerdal B. Calcium and iron absorption--mechanisms and public health relevance. Int J Vitam Nutr Res. 2010;80:293–9. doi: 10.1024/0300-9831/a000036. [DOI] [PubMed] [Google Scholar]
- 22.Saarinen UM, Siimes MA, Dallman PR. Iron absorption in infants: high bioavailability of breast milk iron as indicated by the extrinsic tag method of iron absorption and by the concentration of serum ferritin. J Pediatr. 1977;91:36–9. doi: 10.1016/s0022-3476(77)80439-3. [DOI] [PubMed] [Google Scholar]
- 23.Fomon SJ, Ziegler EE, Serfass RE, Nelson SE, Frantz JA. Erythrocyte incorporation of iron is similar in infants fed formulas fortified with 12 mg/L or 8 mg/L of iron. J Nutr. 1997;127:83–8. doi: 10.1093/jn/127.1.83. [DOI] [PubMed] [Google Scholar]
- 24.Fomon SJ, Ziegler EE, Nelson SE. Erythrocyte incorporation of ingested 58Fe by 56-day-old breast-fed and formula-fed infants. Pediatr Res. 1993;33:573–6. doi: 10.1203/00006450-199306000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Domellöf M, Lind T, Lönnerdal B, Persson LA, Dewey KG, Hernell O. Effects of mode of oral iron administration on serum ferritin and haemoglobin in infants. Acta Paediatr. 2008;97:1055–60. doi: 10.1111/j.1651-2227.2008.00899.x. [DOI] [PubMed] [Google Scholar]
- 26.Domellöf M, Lönnerdal B, Abrams SA, Hernell O. Iron absorption in breast-fed infants: effects of age, iron status, iron supplements, and complementary foods. Am J Clin Nutr. 2002;76:198–204. doi: 10.1093/ajcn/76.1.198. [DOI] [PubMed] [Google Scholar]
- 27.Domellöf M, Cohen RJ, Dewey KG, Hernell O, Rivera LL, Lönnerdal B. Iron supplementation of breast-fed Honduran and Swedish infants from 4 to 9 months of age. J Pediatr. 2001;138:679–87. doi: 10.1067/mpd.2001.112895. [DOI] [PubMed] [Google Scholar]
- 28.Leong WI, Bowlus CL, Tallkvist J, Lonnerdal B. DMT1 and FPN1 expression during infancy: developmental regulation of iron absorption. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1153–61. doi: 10.1152/ajpgi.00107.2003. [DOI] [PubMed] [Google Scholar]
- 29.Frazer DM, Wilkins SJ, Anderson GJ. Elevated iron absorption in the neonatal rat reflects high expression of iron transport genes in the distal alimentary tract. Am J Physiol Gastrointest Liver Physiol. 2007;293:G525–31. doi: 10.1152/ajpgi.00579.2006. [DOI] [PubMed] [Google Scholar]
- 30.Lopez V, Suzuki YA, Lönnerdal B. Ontogenic changes in lactoferrin receptor and DMT1 in mouse small intestine: implications for iron absorption during early life. Biochem Cell Biol. 2006;84:337–44. doi: 10.1139/o06-059. [DOI] [PubMed] [Google Scholar]
- 31.Darshan D, Wilkins SJ, Frazer DM, Anderson GJ. Reduced expression of ferroportin-1 mediates hyporesponsiveness of suckling rats to stimuli that reduce iron absorption. Gastroenterology. 2011;141:300–9. doi: 10.1053/j.gastro.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 32.Widdowson EM, Spray CM. Chemical development in utero. Arch Dis Child. 1951;26:205–14. doi: 10.1136/adc.26.127.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colomer J, Colomer C, Gutierrez D, Jubert A, Nolasco A, Donat J, et al. Anaemia during pregnancy as a risk factor for infant iron deficiency: report from the Valencia Infant Anaemia Cohort (VIAC) study. Paediatr Perinat Epidemiol. 1990;4:196–204. doi: 10.1111/j.1365-3016.1990.tb00638.x. [DOI] [PubMed] [Google Scholar]
- 34.Chaparro CM, Neufeld LM, Tena Alavez G, Eguia-Liz Cedillo R, Dewey KG. Effect of timing of umbilical cord clamping on iron status in Mexican infants: a randomised controlled trial. Lancet. 2006;367:1997–2004. doi: 10.1016/S0140-6736(06)68889-2. [DOI] [PubMed] [Google Scholar]
- 35.Ziegler EE, Nelson SE, Jeter JM. Iron stores of breastfed infants during the first year of life. Nutrients. 2014;6:2023–34. doi: 10.3390/nu6052023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siddappa AM, Rao R, Long JD, Widness JA, Georgieff MK. The assessment of newborn iron stores at birth: a review of the literature and standards for ferritin concentrations. Neonatology. 2007;92:73–82. doi: 10.1159/000100805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finkelstein JL, O'Brien KO, Abrams SA, Zavaleta N. Infant iron status affects iron absorption in Peruvian breastfed infants at 2 and 5 mo of age. Am J Clin Nutr. 2013;98:1475–84. doi: 10.3945/ajcn.112.056945. [DOI] [PubMed] [Google Scholar]
- 38.Domellöf M, Lönnerdal B, Dewey KG, Cohen RJ, Rivera LL, Hernell O. Sex differences in iron status during infancy. Pediatrics. 2002;110:545–52. doi: 10.1542/peds.110.3.545. [DOI] [PubMed] [Google Scholar]
- 39.Yang Z, Lönnerdal B, Adu-Afarwuah S, Brown KH, Chaparro CM, Cohen RJ, et al. Prevalence and predictors of iron deficiency in fully breastfed infants at 6 mo of age: comparison of data from 6 studies. Am J Clin Nutr. 2009;89:1433–40. doi: 10.3945/ajcn.2008.26964. [DOI] [PubMed] [Google Scholar]
- 40.Lönnerdal B, Hernell O. Iron, zinc, copper and selenium status of breast-fed infants and infants fed trace element fortified milk-based infant formula. Acta Paediatr. 1994;83:367–73. doi: 10.1111/j.1651-2227.1994.tb18121.x. [DOI] [PubMed] [Google Scholar]
- 41.Hernell O, Lönnerdal B. Iron status of infants fed low-iron formula: no effect of added bovine lactoferrin or nucleotides. Am J Clin Nutr. 2002;76:858–64. doi: 10.1093/ajcn/76.4.858. [DOI] [PubMed] [Google Scholar]
- 42.Koletzko B, Baker S, Cleghorn G, Neto UF, Gopalan S, Hernell O, et al. Global standard for the composition of infant formula: recommendations of an ESPGHAN coordinated international expert group. J Pediatr Gastroenterol Nutr. 2005;41:584–99. doi: 10.1097/01.mpg.0000187817.38836.42. [DOI] [PubMed] [Google Scholar]
- 43.Georgieff MK, Schmidt RL, Mills MM, Radmer WJ, Widness JA. Fetal iron and cytochrome c status after intrauterine hypoxemia and erythropoietin administration. Am J Physiol. 1992;262:R485–91. doi: 10.1152/ajpregu.1992.262.3.R485. [DOI] [PubMed] [Google Scholar]
- 44.de Deungria M, Rao R, Wobken JD, Luciana M, Nelson CA, Georgieff MK. Perinatal iron deficiency decreases cytochrome c oxidase (CytOx) activity in selected regions of neonatal rat brain. Pediatr Res. 2000;48:169–76. doi: 10.1203/00006450-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Brunette KE, Tran PV, Wobken JD, Carlson ES, Georgieff MK. Gestational and neonatal iron deficiency alters apical dendrite structure of CA1 pyramidal neurons in adult rat hippocampus. Dev Neurosci. 2010;32:238–48. doi: 10.1159/000314341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pisansky MT, Wickham RJ, Su J, Fretham S, Yuan LL, Sun M, et al. Iron deficiency with or without anemia impairs prepulse inhibition of the startle reflex. Hippocampus. 2013;23:952–62. doi: 10.1002/hipo.22151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fretham SJ, Carlson ES, Wobken J, Tran PV, Petryk A, Georgieff MK. Temporal manipulation of transferrin-receptor-1-dependent iron uptake identifies a sensitive period in mouse hippocampal neurodevelopment. Hippocampus. 2012;22:1691–702. doi: 10.1002/hipo.22004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carlson ES, Tkac I, Magid R, O'Connor MB, Andrews NC, Schallert T, et al. Iron is essential for neuron development and memory function in mouse hippocampus. J Nutr. 2009;139:672–9. doi: 10.3945/jn.108.096354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Unger EL, Hurst AR, Georgieff MK, Schallert T, Rao R, Connor JR, et al. Behavior and monoamine deficits in prenatal and perinatal iron deficiency are not corrected by early postnatal moderate-iron or high-iron diets in rats. J Nutr. 2012;142:2040–9. doi: 10.3945/jn.112.162198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Connor JR, Menzies SL. Relationship of iron to oligodendrocytes and myelination. Glia. 1996;17:83–93. doi: 10.1002/(SICI)1098-1136(199606)17:2<83::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 51.Clardy SL, Wang X, Zhao W, Liu W, Chase GA, Beard JL, et al. Acute and chronic effects of developmental iron deficiency on mRNA expression patterns in the brain. J Neural Transm Suppl. 2006:173–96. doi: 10.1007/978-3-211-33328-0_19. [DOI] [PubMed] [Google Scholar]
- 52.Amin SB, Orlando M, Eddins A, MacDonald M, Monczynski C, Wang H. In utero iron status and auditory neural maturation in premature infants as evaluated by auditory brainstem response. J Pediatr. 2010;156:377–81. doi: 10.1016/j.jpeds.2009.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roncagliolo M, Garrido M, Walter T, Peirano P, Lozoff B. Evidence of altered central nervous system development in infants with iron deficiency anemia at 6 mo: delayed maturation of auditory brainstem responses. Am J Clin Nutr. 1998;68:683–90. doi: 10.1093/ajcn/68.3.683. [DOI] [PubMed] [Google Scholar]
- 54.Dewey KG, Domellöf M, Cohen RJ, Landa Rivera L, Hernell O, Lönnerdal B. Iron supplementation affects growth and morbidity of breast-fed infants: results of a randomized trial in Sweden and Honduras. J Nutr. 2002;132:3249–55. doi: 10.1093/jn/132.11.3249. [DOI] [PubMed] [Google Scholar]
- 55.Idjradinata P, Watkins WE, Pollitt E. Adverse effect of iron supplementation on weight gain of iron-replete young children. Lancet. 1994;343:1252–4. doi: 10.1016/s0140-6736(94)92151-2. [DOI] [PubMed] [Google Scholar]
- 56.Lind T, Seswandhana R, Persson LA, Lönnerdal B. Iron supplementation of iron-replete Indonesian infants is associated with reduced weight-for-age. Acta Paediatr. 2008;97:770–5. doi: 10.1111/j.1651-2227.2008.00773.x. [DOI] [PubMed] [Google Scholar]
- 57.Ziegler EE, Nelson SE, Jeter JM. Iron status of breastfed infants is improved equally by medicinal iron and iron-fortified cereal. Am J Clin Nutr. 2009;90:76–87. doi: 10.3945/ajcn.2008.27350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lozoff B, Castillo M, Clark KM, Smith JB. Iron-fortified vs low-iron infant formula: developmental outcome at 10 years. Arch Pediatr Adolesc Med. 2012;166:208–15. doi: 10.1001/archpediatrics.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lind T, Lönnerdal B, Stenlund H, Ismail D, Seswandhana R, Ekström EC, et al. A community-based randomized controlled trial of iron and zinc supplementation in Indonesian infants: interactions between iron and zinc. Am J Clin Nutr. 2003;77:883–90. doi: 10.1093/ajcn/77.4.883. [DOI] [PubMed] [Google Scholar]
- 60.Jaeggi T, Kortman GA, Moretti D, Chassard C, Holding P, Dostal A, et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut. 2014 doi: 10.1136/gutjnl-2014-307720. doi: 10.1136/gutjnl-2014-307720. [DOI] [PubMed] [Google Scholar]
- 61.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–23. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lönnerdal B, Hernell O. Homeostatic Regulation of Iron and Its Role in Normal and Abnormal Iron Status in Infancy and Childhood. Annales Nestlé. 2010;68:96–104. [Google Scholar]