Abstract
Background and Objective In this study we investigated the levels of cytokines and chemokines produced locally and systemically after influenza vaccination of patients undergoing tonsillectomy.
Methods Blood and saliva were collected prior to, and 1 or 2 weeks after vaccination at the time of the tonsillectomy. The cytokine and chemokine concentrations were determined in both unstimulated (whole blood, serum and saliva) and in vitro influenza stimulated peripheral blood mononuclear cell (PBMC) and tonsillar lymphocyte (TMC) cultures.
Results We found that influenza vaccination elicited protective levels of serum haemagglutination inhibition antibodies and a significant local antibody response in the saliva. No significant differences were observed in the cytokine or chemokine levels 1 or 2 weeks post‐vaccination in either the serum or saliva. Similarly, no significant differences were found in the gene expression levels in PBMC after vaccination, but interleukin (IL)‐2, IL‐4, γ‐interferon and transforming growth factor‐β were slightly elevated at 1 week post‐vaccination but decreased by 2 weeks post‐vaccination. In contrast, increased concentrations of a mixture of type 1, type 2 and inflammatory cytokines were produced 1 and 2 weeks after influenza vaccination by in vitro‐stimulated PBMC and TMC.
Conclusion We show that cytokine responses can be measured after influenza vaccination in in vitro‐stimulated lymphocytes but not directly in the blood or saliva. These results will provide a useful baseline that can be used for comparison of the immune response in human volunteers involved in clinical trials of novel influenza vaccines.
Keywords: Chemokine, cytokine, human, influenza virus, vaccine
Introduction
Influenza is a major respiratory pathogen causing annual outbreaks, which results in a considerable economic burden because of loss of productivity, hospitalization and cost of treatment of the disease. 1 Annual vaccination using parenterally administered inactivated virus vaccine is the main method of influenza prophylaxis, 2 and is particularly targeted at the high‐risk groups. Antiviral drugs are also available for both prophylaxis and for treatment in the early phase of infection and are effective at reducing morbidity.
After parenteral influenza vaccination, complex immunological processes occur at the site of immunization, systemically (blood and lymph) and in the upper respiratory tract. 2 Influenza‐specific antibody secreting cells (ASC) are found in elevated number in the blood and tonsils 1 week after vaccination, 3 but not in the nasal mucosa. 4 , 5 Subsequently, an increased production of anti‐influenza antibodies is observed in the body fluids such as the blood and saliva. 3 , 6 Several important groups of molecules, e.g. cytokines, chemokines and adhesion molecules, mediate the production of antibodies and are produced by cells like lymphocytes, macrophages and dendritic cells. Cytokines are the mediators of immunoregulation, but many cytokines also contribute to the symptoms and pathology of infection. Proinflammatory cytokines such as α‐interferon (IFN‐α), tumour necrosis factor‐α (TNF‐α) and interleukin (IL)‐1α and IL‐1β are produced at the site of infection by influenza‐infected epithelial cells and by macrophages, and are often associated with clinical signs such as fever, fatigue and anorexia. 7 , 8 , 9 TNF‐α is important in stimulating macrophages and neutrophils and in activation of other cytokines such as IL‐1 and IL‐6. 10 IFN‐α enhances the production of IFN‐γ, 11 which promotes an antiviral response, mediates stimulation of macrophages and natural killer cells, 12 , 13 up‐regulates the expression of major histocompatibility complex molecules and is an effective stimulant of immunoglobulin class switching. 14 Other cytokines have an anti‐inflammatory effect, e.g. transforming growth factor‐β (TGF‐β) and IL‐10 and are important in controlling and reducing inflammation. Furthermore, cytokines are often divided into type 1 (IL‐2, IFN‐γ) and type 2 (IL‐4, IL‐5, IL‐6 and IL‐10) depending on whether they stimulate a cellular or humoral type of response respectively. 15 , 16
Naturally or experimentally induced influenza infection in humans and mice induces a strong type 1 response, particularly IFN‐γ activating cytotoxic T‐cells, 17 , 18 , 19 , 20 and macrophages with an increased expression of Fc receptors. In a murine model, both the formulation and the number of vaccine doses of an influenza vaccine determined the type of cytokine response. 21 , 22 Split virus vaccine, which is the most commonly used vaccine formulation in man, induced a type 2‐biased cytokine response. In contrast, whole inactivated influenza vaccine induced a type 1 response after the first dose of vaccine and a more mixed type 1 and 2 response after the second dose of vaccine. However, there are only limited data on the cytokine response elicited after parenteral vaccination in man. Therefore, in the present study we investigated the cytokine and chemokine responses elicited locally and systemically after parenteral influenza vaccination using split influenza vaccine in adult patients scheduled for tonsillectomy.
Materials and methods
Patients and samples
Two separate studies were used to collect the patient material to analyse the local and systemic cytokine and chemokine responses. These studies were approved by the local ethics committee (REK. VEST NO 79.99 and 170.04) and written informed consent was obtained from all the volunteers before inclusion in the studies. The first study (study I) consisted of 33 subjects (24 women and 9 men, age range 16–56 years, mean 25 years old). These patients were vaccinated in October–November 2002 with the 02/03‐season Fluarix® vaccine (GlaxoSmithKline, Uxbridge, UK) containing A/New Caledonia/20/99 (H1N1), A/Panama/2007/99 (H3N2) and B/Shangong/7/79. The second study (study II) was performed in October–November 2004, and consisted of 25 subjects (13 women and 12 men, age range 18–59 years, mean 27 years), who were vaccinated with the 04/05‐season Fluarix® vaccine (GlaxoSmithKline) containing A/New Caledonia/20/99 (H1N1), A/Wyoming/3/2003 (H3N2) and B/Jiangsu/10/2003.
All volunteers were patients scheduled for tonsillectomy at the Haukeland University Hospital (Bergen, Norway), and were chosen from patients with recurrent tonsillitis and hypertrophic tonsils but who were otherwise healthy. The patients had not experienced any infection during the preceding 3 months, had no history of allergy and did not report having experienced a recent infection with influenza or been vaccinated with influenza vaccine. The patients were randomized into three groups: one control group consisting of non‐vaccinated subjects, and two groups who were immunized with parenteral influenza vaccine 1 or 2 weeks before tonsillectomy. In conjunction with the clinical examination of the patients before the operation, peripheral blood was collected by intravenous puncture, and saliva was collected with an absorbent pad on a stick (Orasure®; Epitope, Beaverton, OR, USA) according to the manufacturer’s instructions. Blood samples were sent to the Microbiology and Immunology Laboratory at Haukeland University Hospital and analysed for antibodies to influenza A virus, influenza B virus, parainfluenza viruses and common allergens, in addition to routine preoperative tests. The subjects were then vaccinated with the current season’s trivalent influenza vaccine. One or two weeks later, bilateral tonsillectomy was performed, and at this time point blood and saliva were collected.
Cell culture supernatants
In study I, lymphocytes were harvested and prepared from peripheral blood and tonsil tissue, as previously described. 21 Lymphocytes were counted and resuspended at a final density of 2 × 106 cells per ml in tissue culture medium (RPMI 1640 medium containing 10% foetal calf serum (FCS), L‐glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 0.25 μg/ml fungizone). The lymphocytes were stimulated in 24‐well tissue culture plates, with 1 × 106 cells per well and were stimulated with either phytohemagglutinin (10 μg/ml final concentration, positive control), or haemagglutinin vaccine antigen (HA, 1 μg/ml final concentration) from the H3N2 vaccine strain, or medium alone (negative control/mock) in a total volume of 1 ml medium. The lymphocytes were cultured for 24 hours; the supernatant was then harvested, aliquoted and immediately stored at −70°C.
Haemagglutination inhibition (HAI) assay
Serum samples from study II were pre‐treated with receptor‐destroying enzyme (Denka Seiken, Tokyo, Japan) overnight at 37°C and then incubated for 1 hour at 56°C to remove nonspecific inhibitors. The HAI test was conducted with 8 HA units of the appropriate virus and 0.7% (v/v) turkey erythrocytes, as described elsewhere. 23 A serum HAI titre of ≥40 has been deemed protective. 24
Enzyme‐linked immunosorbent assay (ELISA)
The concentrations of anti‐influenza virus antibodies in the serum and saliva from study II were measured using ELISA as described in our earlier studies. 3 , 6 Purified surface glycoproteins (generously provided by GlaxoSmithKline) from the vaccine strains were used as antigens. Ninety‐six‐well microtitre plates were coated with 0.1 μg of HA in 100 μl of phosphate‐buffered saline (PBS) per well overnight at 4°C. The wells were blocked with 10% FCS in PBS before serum and saliva samples were applied in duplicate at appropriate dilutions. For detection, we used peroxidase‐conjugated goat anti‐human polyvalent immunoglobulins (A‐8400; Sigma‐Aldrich, St Louis, MO, USA). The reaction was developed with 100 μl/well o‐phenylenediamine (P‐2045; Dako, Glostrup, Denmark) at a concentration of 0.7 mg/ml and stopped with 100 μl of 0.5 m H2SO4 per well. The plates were thoroughly washed between each step with multiple washes of PBS. The absorbance was read with a Mikrotek Emax microplate reader (Molecular Devices, Sunnyvale, CA, USA) and analysed with SoftMax Pro software (Apple Macintosh version). A standard curve was prepared using anti‐human polyvalent immunoglobulins (I‐8758; Sigma‐Aldrich) as the capture antibody (1:100 dilution) and purified human immunoglobulin (I‐4506; Sigma‐Aldrich) as the standard on each microtitre plate analysed.
Multiplexed immune assay
The multiplex bead immune assay was performed on serum and saliva from study II using Cytokine Panel III 25‐plex kit (LHC0009; BioSource, Nivelles, Belgium; analytes: IL‐1β, IL‐1 RA, IL‐2, IL‐2R, IL‐4, IL‐5, IL‐6, IL‐7, IL‐8, IL‐10, IL‐12p40, IL‐13, IL‐15, IL‐17, TNF‐α, IFN‐α, INF‐γ, GM‐CSF, MIP‐1α, MIP‐1β, IP‐10, MIG, Eotaxin, RANTES, MCP‐1) based on the xMAP technology (Luminex Corp., Austin, TX, USA). Lymphocyte culture supernatants from study I were analysed with a 10‐plex cytokine assay (LHC0001; BioSource; analytes: IL‐1β, IL‐2, IL‐4, IL‐5, IL‐6, IL‐8, IL‐10, TNF‐α, INF‐γ, GM‐CSF). The assays were run according to the manufacturer’s instructions in a Luminex100 instrument (Luminex Corp.) and analysed using Star Station 1.0 software (Applied Cytometry Systems, Dinnington, UK).
Cytokine (mRNA) gene expression
Whole blood samples (2.5 ml) were collected in PAXgene tubes (no. 762125; PreAnalytix GmbH, Hombrechtikon, CH) which freezes and preserves the mRNA expression levels. Total RNA was purified using PAXgene Blood RNA kit (no. 762134; PreAnalytix) as recommended by the manufacturer. Complementary DNA was produced using the First strand cDNA synthesis kit (no. K1612; Fermentas, Vilnius, Lithuania) and a poly‐T primer according to the manufacturer’s instructions. Messenger RNA fractions were quantified and qualitatively tested using Bioanalyser 2100 (Agilent Technologies Waldbronn, Germany) and RNA 6000 Pico Assay (no. 5065‐4472; Agilent Technologies). Relative human cytokine gene expression was measured using TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA, USA): Hs00174097_m1 (IL‐1β), Hs00174114_m1 (IL‐2), Hs00174122_m1 (IL‐4), Hs00174200_m1 (IL‐5), Hs00174131_m1 (IL‐6), Hs00174103_m1 (IL‐8), Hs00174086_m1 (IL‐10), Hs00174143_m1 (INF‐γ), Hs00174128_m1 (TNF‐α) and Hs00852894_g1 (TGF‐β) on a 7500 RealTime PCR system (Applied Biosystems), and Sequence Detection Software v.1.2.2 software (Applied Biosystems). The cytokine gene expression levels were normalized to the expression of the endogenous β‐actin housekeeping gene: Hs99999903_m1 (β‐actin). Gene expression was determined as fold increase given by the formula: 2 –ΔΔCT, where ΔCT is the difference in threshold cycles for target and integrated β‐actin RNA endogenous control and ΔΔCT = ΔCT at a given time point after vaccination − ΔCT at day 0 (User Bulletin no. 2, Applied Biosystems).
Results
In the present study we investigated the local and systemic cytokine responses at both the mRNA and protein levels after parenteral influenza vaccination in patients scheduled for tonsillectomy because of recurrent tonsillitis and hypertrophic tonsils. The volunteers did not report recent influenza virus infection or influenza vaccination, and they had no history of allergy, which was confirmed by routine diagnostic analysis of total IgE and specific IgE of the six most common allergens in Norway (data not shown).
Serum haemagglutination inhibition (HAI) antibody titres
Serum samples were collected immediately before vaccination and 1 or 2 weeks after vaccination at the time of tonsillectomy. The serum samples were tested using the HAI assay (Figure 1), and an HAI titre of 40 or greater is considered protective against influenza virus infection. 24 Generally, the HAI titres were low before vaccination and increased significantly to above the protective threshold 1 (P = 0.0451) and 2 weeks (P = 0.0132) after vaccination for the H1N1 strain, and after 2 weeks for the H3N2 (P = 0.0019) and B (P = 0.0343) viruses. (Mann–Whitney test). The data shown here are from vaccine study II, but similar results were also obtained in study I (results not shown) using the relevant (02/03 season) influenza antigen.
Figure 1.
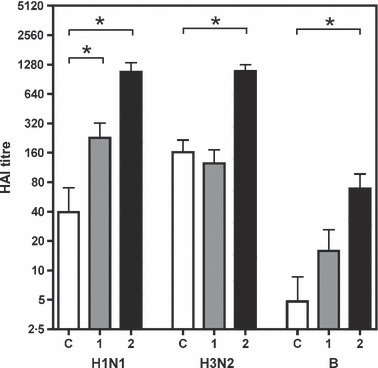
The serum haemagglutination inhibition (HAI) titres (indicated to the left) from study II elicited after vaccination against the vaccine viruses (H1N1, H3N2 and B strains shown below) immediately before operation. The bars show the mean (+standard error of mean; SEM) titres at operation for non‐vaccinated controls (C, open bars), and for patients operated 1 week (1, grey bars) or 2 weeks (2, black bars) after vaccination. *Significant difference between pre‐ and post‐vaccination samples (P < 0.05, by Mann–Whitney test).
Influenza virus‐specific antibody levels in serum and saliva
The concentration of influenza virus‐specific antibodies in the serum and the saliva from study II were measured using ELISA (Figure 2A). Vaccination induced a significant increase in antibody titres at both 1 and 2 weeks to all three vaccine strains. In an earlier study, 6 we found that influenza virus‐specific antibodies in saliva consisted mainly of secretory IgA1. In the present study, we measured the concentration of total influenza virus specific antibodies in the saliva (Figure 2B). The levels of pre‐vaccination influenza virus‐specific antibodies in the saliva were low; however, 1 week after vaccination, the concentrations increased significantly to the B strain. A significant increase in saliva antibodies was observed by 2 weeks post‐vaccination compared to pre‐vaccination levels against all three viruses.
Figure 2.
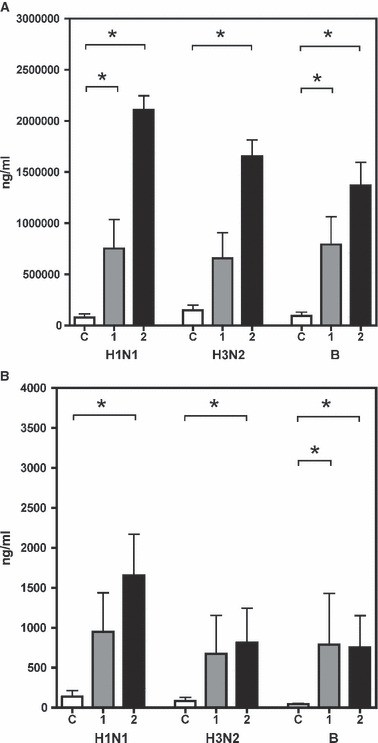
The concentration (ng/ml, indicated on the left) of influenza virus‐specific antibodies to the three vaccine viruses (H1N1, H3N2 and B strains, stated below) from study II (A) in serum and (B) in saliva. The bars show the mean (+SEM) concentrations at operation for controls (C, open bars), and for patients operated 1 week (1, grey bars) or 2 weeks (2, black bars) after vaccination. *Significant difference between pre‐ and post‐vaccination samples (P < 0.05, by Mann–Whitney test).
Cytokine and chemokine levels in serum and saliva
We analysed the concentration of 25 cytokines and chemokines directly in the serum and saliva after vaccination using the multiplex bead assay system (Luminex 100). The cytokine levels in the serum were generally low (results not shown). A number of analytes had a slightly elevated level (above 10–20 pg/ml) in the serum (IL‐1RA, IL‐2R, IL‐7, IL‐12p40, GM‐CSF, Eotaxin, RANTES and MCP), whereas the other analytes were not found above the detection limit. Comparison of the cytokine levels after vaccination in the different groups was complicated by the fact that the individual variation was often higher than the inter‐group variation, giving a high standard deviation for the measured analytes. IL‐12p40 was the only analyte which changed after vaccination and a significant decrease in concentration was observed after vaccination (Wilcoxon matched pairs t‐test). This change was also observed in the non‐vaccinated control subjects, indicating that the decrease in IL‐12p40 is probably due to patient stress prior to tonsillectomy. RANTES was found in the highest concentrations in the serum. However, it has been reported that platelets may spontaneously secrete RANTES upon blood collection in vacutainers and centrifugation. 25
Surprisingly, the cytokine and chemokine levels in the saliva were generally much higher then those of serum (results not shown). The variation in these samples was very high, and comparison of the pre‐ and post‐samples (Wilcoxon matched pairs test) did not reveal any statistical differences in the analyte concentrations. Generally the levels of many analytes decreased after vaccination, with the lowest levels observed after 1 week and slight increases found after 2 weeks.
Cytokine levels in supernatants from in vitro‐stimulated lymphocytes
Peripheral blood and tonsillar lymphocytes (TMC) from study I secreted a number of cytokines after in vitro stimulation with influenza H3 antigen at 1 or 2 weeks post‐vaccination (Figure 3). Peripheral blood lymphocytes produced significantly increased levels of eight of the ten tested cytokines (Figure 3A) belonging to pro‐ and anti‐inflammatory, type 1 and type 2 cytokines. A similar response was observed in the TMC (Figure 3B) where six of the ten tested cytokines increased significantly after in vitro stimulation. A significant increase in cytokine production of IFN‐γ, IL‐10 and TNF‐α was observed in the blood and TMC at both 1 and 2 weeks post‐vaccination. Two weeks after vaccination, a significant increase in GM‐CSF, IL‐2, IL‐5, IL‐6 and IL‐8 occurred in either the blood, the tonsils or in both. Only very low concentrations of IL‐4 were detected in both tonsillar and peripheral blood cultures, which agrees with the previous findings of Guthrie et al. 26
Figure 3.
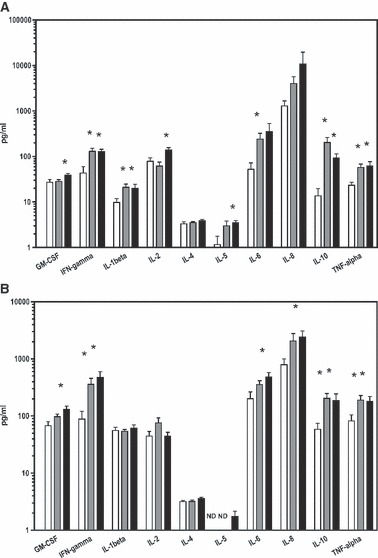
Lymphocytes from (A) peripheral blood (PBMC) and (B) tonsils (TMC) were harvested and stimulated with influenza antigen in vitro. The cytokines secreted into the medium (supernatant) were measured after 24 hours by multiplex bead immuno assay. The bars indicate the mean value with SEM. (A) The open bars are pre‐vaccination levels, the grey bars are the levels of the patients operated 1 week after vaccination and the black bars are the levels of the patients operated 2 weeks after vaccination. (B) The open bars are the levels of the non‐vaccinated group of patients, the grey bars are the levels of the patients operated 1 week after vaccination and the black bars are the levels of the patients operated 2 weeks after vaccination. The cytokine tested are indicated below and the levels on the left (pg/ml) of the graph. *Statistically significant difference between pre‐vaccination (A) non‐vaccinated (B) and post‐vaccination samples (P < 0.05, by Mann–Whitney test).
Quantitative PCR (QPCR) of cytokine gene expression
Gene expressions of cytokines were assayed in cDNA samples by real‐time QPCR from peripheral lymphocytes from patients included in study II (Figure 4). Of the 10 cytokines tested, only two cytokines (IL‐5 and IL‐10) were under the detection limit (data not shown). The expression of cytokines was low both 1 and 2 weeks after vaccination, but the detectable cytokines were generally slightly elevated after vaccination, particularly in the group of patients operated 1 week after vaccination. The QPCR results support the serum and saliva cytokine results indicating that there is no intensive cytokine production and secretion that can be measured without in vitro stimulation at 1 or 2 weeks after vaccination.
Figure 4.
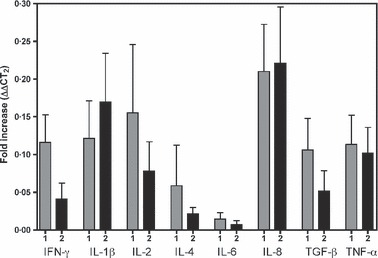
The gene expression levels of cytokines (stated below) in lymphocytes isolated from peripheral blood (PMNC). The bars (+SEM) shows the fold increase (ΔΔCT2) of gene expression in the patient groups operated 1 week (1, grey bars) or 2 weeks (2, black bars) after vaccination relative to pre‐vaccination levels (fold increase).
Discussion
In this study we vaccinated adults with a split influenza virus vaccine, and examined the local and systemic cytokine profiles prior to, and 1 and 2 weeks after vaccination. Cytokines are important molecules facilitating the communication between immune competent cells and the surrounding tissue. This communication is essential in modulation of the directions and intensity of the immune response, promoting activation, proliferation and establishment of a memory pool of lymphocytes. Monitoring the cytokine response after vaccination may provide an important tool which will allow measurement of the efficacy and safety of the vaccine, particularly in human clinical trials of vaccines containing avian subtypes to address the current influenza pandemic threat.
Parenteral influenza vaccination induces a rapid systemic and tonsillar ASC response, which is associated with a rapid and strong systemic response but a short‐lived local antibody response. 3 , 6 Similarly, in this study, we observed that influenza vaccination with the split virus vaccine elicited a particularly good systemic antibody responses with protective antibody titres observed 1 and 2 weeks post‐vaccination. 3 , 18 , 27 , 28
The measurement of cytokine levels in body fluids such as serum and saliva represents a huge challenge. Great care has to be taken when collecting, handling and storing the samples to avoid degradation of the cytokines in the sample. In this study we employed a technique using multiple bead sets singly labelled with different monovalent anti‐cytokine antibodies. The different bead sets are differentially stained with fluorescent markers enabling each bead to be individually identified into a bead set/region by the assay reader. This technique allows the simultaneous detection of multiple analytes in the same sample volume. None of the 25 cytokine and chemokine levels in the serum and the saliva changed significantly after influenza vaccination (results not shown). However, the cytokine levels tended to decrease slightly at 1 week after vaccination and return to pre‐vaccination levels after 2 weeks. This may indicate that there are changes in the cytokine levels after vaccination in the period between vaccination and 1 week later. Therefore, testing in the time frame initially after vaccination (1–3 days post‐vaccination) may be more appropriate to observe changes in the cytokine levels induced by vaccination. The measurement of cytokines in serum and saliva is complicated by the fact that the individual variations are often greater than the responses among the groups. The basal levels of cytokines in saliva were generally higher than in the serum, and this may be related to the fact that the patients were suffering from recurrent tonsillitis and hypertrophic tonsils, which may result in increased local immune activity.
As the soluble cytokine levels in serum and saliva did not change significantly after vaccination, we investigated whether vaccination had an effect on the gene expression levels of cytokines. Quantitative real‐time PCR was used to investigate 10 common cytokines related to inflammation, and type 1 and 2 responses. The gene expression of many of these cytokines (IL‐1β, IL‐2, IL‐4, IL‐8, IFN‐γ, TNF‐α, TGF‐β) was only slightly elevated 1 and 2 weeks after vaccination. However, the gene expression levels were higher at 1 week than at 2 weeks after vaccination for some of the cytokines (IL‐2, IL‐4, IFN‐γ, TGF‐β).
We found that in vitro‐stimulated PBMC and TMC were characterized by increased production of type 1, type 2, pro‐ and anti‐ inflammatory cytokines at 1 and 2 weeks post‐vaccination. In addition, the concentration of cytokines was generally higher in tonsillar than in peripheral blood cultures. Others have observed considerably higher concentrations of IFN‐γ in stimulated tonsillar than in peripheral blood cultures 2 weeks after vaccination. 26 In our work we have shown using a larger panel of cytokines that the concentrations of eight cytokines in the blood and six cytokines in the tonsils increased significantly after vaccination. Furthermore, Guthrie et al. 26 observed that influenza vaccination elicited higher levels of a type 1 (IFN‐γ) and type 2 (IL‐5) cytokine in in vitro‐stimulated PBMC 2 weeks after vaccination. The cytokine profile exhibited a mixed type 1 and type 2 profile, with increases observed in IFN‐γ, IL‐2 and IL‐5, IL‐6 and IL‐10, which is similar to our findings in a murine model where the split virus vaccine also induced a mixed type 1 and type 2 profile. 21 Interestingly in elderly subjects an age‐related decline in type 1 cell responses occurs and is associated with an age‐related decline in the efficacy of the vaccine. 29 Others have found that if there is an increase in aggregates in the vaccine the cytokine balance shifts to a type 2 in the murine model and in humans this may have been associated with adverse reactions (oculorespiratory syndrome). 30 In a murine model we previously found that the formulation and number of doses of a vaccine influence the type of cytokine response and interestingly the whole virus vaccine produced a cytokine response more similar to natural infection than the split virus vaccine.
The lower levels of cytokine levels detected 1 week post‐vaccination in the serum and saliva may indicate that there is an exhaustion of cytokines possibly by binding to up‐regulated levels of cytokine receptors or by cytokine‐producing cells. The slightly higher gene expression levels perhaps indicate that there is still some up‐regulation of these genes 1 week post‐vaccination, which returns to pre‐vaccination levels after 2 weeks and that we have missed the most active phase of cytokine production. In contrast, in vitro stimulation of blood and tonsillar cells resulted in a significant increase in cytokine production generally with higher levels of cytokines observed in the local compartment (tonsils). The split virus vaccine induced a mixed type 1 and 2 cytokine response. With the increasing number of clinical trials of pandemic vaccines, investigation of the cytokine response will further aid in understanding the vaccine induced immunity.
Acknowledgements
We thank the staff at the outpatient clinic and wards of the Department of Otolaryngology, Head and Neck Surgery, Haukeland University Hospital, for most valuable assistance, and GlaxoSmithKline for providing purified influenza virus antigens. We also thank Marianne Eidsheim at the Broegelmann Research Laboratory for technical assistance.
References
- 1. Jefferson T, Demicheli V. Socioeconomics of influenza; in Nicholson KG, Webster RG, Hay AJ. (eds): Textbook of Influenza. Oxford: Blackwell Sciences, 1998. [Google Scholar]
- 2. Cox RJ, Brokstad KA, Ogra P. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand J Immunol 2004;59:1–15. [DOI] [PubMed] [Google Scholar]
- 3. Brokstad KA, Cox RJ, Olofsson J, Jonsson R, Haaheim LR. Parenteral influenza vaccination induces a rapid systemic and local immune response. J Infect Dis, 1995;171:198–203. [DOI] [PubMed] [Google Scholar]
- 4. Brokstad KA, Cox RJ, Eriksson JC, Olofsson J, Jonsson R, Davidsson A. High prevalence of influenza specific antibody secreting cells in nasal mucosa. Scand J Immunol 2001;54:243–247. [DOI] [PubMed] [Google Scholar]
- 5. Brokstad KA, Eriksson JC, Cox RJ et al. Parenteral vaccination against influenza does not induce a local antigen specific immune response in the nasal mucosa. J Infect Dis 2002;185:878–884. [DOI] [PubMed] [Google Scholar]
- 6. Brokstad KA, Cox RJ, Oxford JS, Haaheim LR. IgA, IgA subclasses, and secretory component levels in oral fluid collected from subjects after parental influenza vaccination. J Infect Dis 1995;171:1072–1074. [DOI] [PubMed] [Google Scholar]
- 7. Dinarello CA. Role of interleukin‐1 in infectious diseases. Immunol Rev 1982;127:119–146. [DOI] [PubMed] [Google Scholar]
- 8. Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol 1992;10:411–452. [DOI] [PubMed] [Google Scholar]
- 9. Dinarello CA, Bernheim HA, Duff GW et al. Mechanism of fever induced by recombinant human interferon. J Clin Invest 1884;74:906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang H, Czura CJ, Tracey KJ. Tumor necrosis factor; in Thomson AW, Lotze MT (eds): The Cytokine Handbook. 4th edn San Diego: Academic Press, 2003. [Google Scholar]
- 11. Sareneva T, Matikainen S, Kurimoto M, Julkunen I. Influenza A virus‐induced IFN‐alpha/beta and IL‐18 synergistically enhance IFN‐gamma gene expression in human T cells. J Immunol 1998;160:6032–6038. [PubMed] [Google Scholar]
- 12. Schreiber GH, Schreiber RD. Interferon‐gamma; in Thomson AW, Lotze MT. (eds): The Cytokine Handbook. 4th edn San Diego: Academic Press, 2003. [Google Scholar]
- 13. Lin Y‐X, Leonard WJ. Interleukin‐2; in Thomson AW, Lotze MT. (eds): The Cytokine Handbook. 4th edn San Diego: Academic Press, 2003. [Google Scholar]
- 14. Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon‐gamma. Annu Rev Immunol 1997;15:749–795. [DOI] [PubMed] [Google Scholar]
- 15. Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 1986;136:2348–2357. [PubMed] [Google Scholar]
- 16. Romagnani S. Human TH1 and TH2 subsets: doubt no more. Immunol Today 1991;12:256–257. [DOI] [PubMed] [Google Scholar]
- 17. Johnson PR, Feldman S, Thompson JM, Mahoney JD, Wright PF. Immunity to influenza A virus infection in young children: a comparison of natural infection, live cold‐adapted vaccine, and inactivated vaccine. J Infect Dis 1986;154:121–127. [DOI] [PubMed] [Google Scholar]
- 18. Fazekas G, Rosenwirth B, Dukor P, Gergely J, Rajnavolgyi E. IgG isotype distribution of local and systemic immune responses induced by influenza virus infection. Eur J Immunol 1994;24:3063–3067. [DOI] [PubMed] [Google Scholar]
- 19. Liang S, Mozdzanowska K, Palladino G, Gerhard W. Heterosubtypic immunity to influenza type A virus in mice. Effector mechanisms and their longevity. J Immunol 1994;152:1653–1661. [PubMed] [Google Scholar]
- 20. Brown DM, Roman E, Swain SL. CD4 T cell responses to influenza infection. Semin Immunol 2004;16:171–177. [DOI] [PubMed] [Google Scholar]
- 21. Szyszko E, Brokstad K, Cox RJ, Hovden A‐O, Madhun A, Haaheim LR. The impact of influenza vaccine formulation on serum antibody and cytokine profiles. Scand J Immunol 2006;64:467–475. [DOI] [PubMed] [Google Scholar]
- 22. Hauge S, El‐Madhun AS, Cox RJ, Brokstad KA, Haaheim LR. A comparison of the humoral and cellular immune responses at different immunological sites after split influenza virus vaccination of mice. Scand J Immunol 2007;65:14–21. [DOI] [PubMed] [Google Scholar]
- 23. Cox RJ, Brokstad KA, Zuckerman MA, Wood JM, Haaheim LR, Oxford JS. An early humoral immune response in peripheral blood following parenteral inactivated influenza vaccination. Vaccine 1994;12:993–999. [DOI] [PubMed] [Google Scholar]
- 24. Hobson D, Curry RL, Beare AS, Ward‐Gardner A. The role of serum haemagglutination‐inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972;70:767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boehlen F, Clemetson KJ. Platelet chemokines and their receptors: what is their relevance to platelet storage and transfusion practice? Transfus Med 2001;11:403–417. [DOI] [PubMed] [Google Scholar]
- 26. Guthrie T, Hobbs CG, Davenport V, Horton RE, Heyderman RS, Williams NA. Parenteral influenza vaccination influences mucosal and systemic T cell‐mediated immunity in healthy adults. J Infect Dis 2004;190:1927–1935. [DOI] [PubMed] [Google Scholar]
- 27. Nichol KL, Mallon KP, Mendelman PM. Cost benefit of influenza vaccination in healthy, working adults: an economic analysis based on the results of a clinical trial of trivalentlive attenuated influenza virus vaccine Vaccine 2003;21:2207–2217. [DOI] [PubMed] [Google Scholar]
- 28. Fukuda K, Levandowski RA, Bridges CB, Cox NJ. Inactivated influenza vaccines; in Plotkin SA, Orenstein WA. (eds): Vaccines, Philadelphia, PA: Saunders, 2004. [Google Scholar]
- 29. Deng Y, Jing Y, Campbell AE, Gravenstein S. Age‐related impaired type 1 T cell responses to influenza: reduced activation ex vivo, decreased expansion in CTL culture in vitro, and blunted response to influenza vaccination in vivo in the elderly. J Immunol 2004;172:3437–3446. [DOI] [PubMed] [Google Scholar]
- 30. Babiuk S, Skowronski DM, De Serres G, HayGlass K, Brunham RC, Babiuk L. Aggregate content influences the Th1/Th2 immune response to influenza vaccine: evidence from a mouse model. J Med Virol 2004;72:138–142. [DOI] [PubMed] [Google Scholar]


