Abstract
The photothermal heating and release properties of biocompatible organic nanoparticles, doped with a near-infrared croconaine (Croc) dye, were compared with analogous nanoparticles doped with the common near-infrared dyes ICG and IR780. Separate formulations of lipid-polymer-hybrid nanoparticles and liposomes, each containing Croc dye, absorbed strongly at 808 nm and generated clean laser-induced heating (no production of 1O2 and no photobleaching of the dye). In contrast, laser-induced heating of nanoparticles containing ICG or IR780 produced reactive 1O2 leading to bleaching of the dye and also decomposition of co-encapsulated payload such as the drug Doxorubicin. Croc dye was especially useful as a photothermal agent for laser controlled release of chemically sensitive payload from nanoparticles. Solution state experiments demonstrated repetitive fractional release of water soluble fluorescent dye from the interior of thermosensitive liposomes. Additional experiments used a focused laser beam to control leakage from immobilized liposomes with very high spatial and temporal precision. The results indicate that fractional photothermal leakage from nanoparticles doped with Croc dye is a promising method for a range of controlled release applications.
Keywords: Near infrared dye, lipid polymer nanoparticles, liposomes, photothermal heating, drug release, reactive oxygen species
1. Introduction
Laser induced photothermal heating has many biomedical applications such as cell ablation,1 drug release,2 and gene delivery.3 The laser absorbing chromophore can either be gold nanostructure,4 organic dye,5 carbon nanotube,6 inorganic nanoparticle,7 or polymeric material.8 There is a major technical advantage if the chromophore strongly absorbs in the near-infrared (NIR) window because NIR light penetrates furthest through skin and tissue.9 Gold nanostructures are popular choices for photothermal heating but they have potential performance drawbacks such irreversible morphology changes upon heating, slow rates of diffusion, and slow clearance from the body.10 On a per mass basis, organic dyes have the same photothermal heating capacity as gold nanostructures,11 but most organic NIR dyes are limited by poor stability and photobleaching.12 Typically, the photobleaching is due to a chemical reaction with reactive oxygen species (ROS) such as singlet oxygen (1O2) produced by energy transfer from the dye triplet excited state to nearby molecular oxygen which is known as type II reaction.13 In the Type I reaction, the excited dye reacts directly with the substrate. Photothermal dyes that simultaneously emit heat and generate ROS are attractive for applications that aim to cause rapid cell death.14 However, certain photothermal applications are best conducted with chromophores that do not simultaneously generate ROS. Consider the process of photothermal release of chemically sensitive payloads, such as drugs, biologics, or oligonucleotides, where the concomitant production of ROS has the likely detrimental effect of causing payload decomposition. While many studies have demonstrated laser triggered release of cargo from nanoparticles, very few of them, if any, have evaluated if the released cargo is damaged.15
It is apparent that new classes of NIR organic dyes are needed for biomedical applications that require stable levels of clean photothermal heating over long periods of time or alternatively over repeated heating cycles. Recent reports have demonstrated that organic nanoparticles containing either porphyrin dye,16 gadolinium bis(naphthalocyanine) sandwich complex17 or modified heptamethine indocyanine dye18, 19 enable exclusive photothermal heating without photobleaching. In addition, there is recent evidence for laser-induced leakage from liposomes, containing a porphyrin chromophore, that does not involve bulk heating of the sample.20 As a complement to these ongoing developments, we have discovered that croconaine (Croc) dyes (chemical structure in Figure 1) exhibit many of the desired features for high performance photothermal heating using a cheap 808 nm diode laser.21, 22 In brief, Croc dyes absorb strongly at around 800 nm and they have very short picosecond excited state lifetimes, low fluorescence quantum yields, and very little intersystem crossing to the excited triplet state. They efficiently convert 808 nm laser light into heat with negligible production of 1O2. We have shown that Croc dyes can be encapsulated inside macrocyclic molecules and also within silicated micelles to give water soluble nanoparticles with diameters < 15 nm.22 In this present study, we address the need for larger, biocompatible organic nanoparticles with diameters of 50-200 nm that are commonly used for controlled release applications and have the potential to exploit the enhanced permeability and retention effect for tumor targeting.23 Particles of this size are not likely to be excreted from the body so it is important to use biodegradable building blocks. We describe the construction of Croc doped lipid-polymer hybrid nanoparticles and Croc doped liposomes and show that both systems exhibit superior photothermal properties when compared to identical nanoparticle systems doped with two commonly used commercial NIR dyes, indocyanine green (ICG) and IR780 (structures in Figure 1a).24, 25 We demonstrate how this high performance photothermal system permits new types of nanoparticle release strategies with very high levels of spatial and temporal control.
Figure 1.
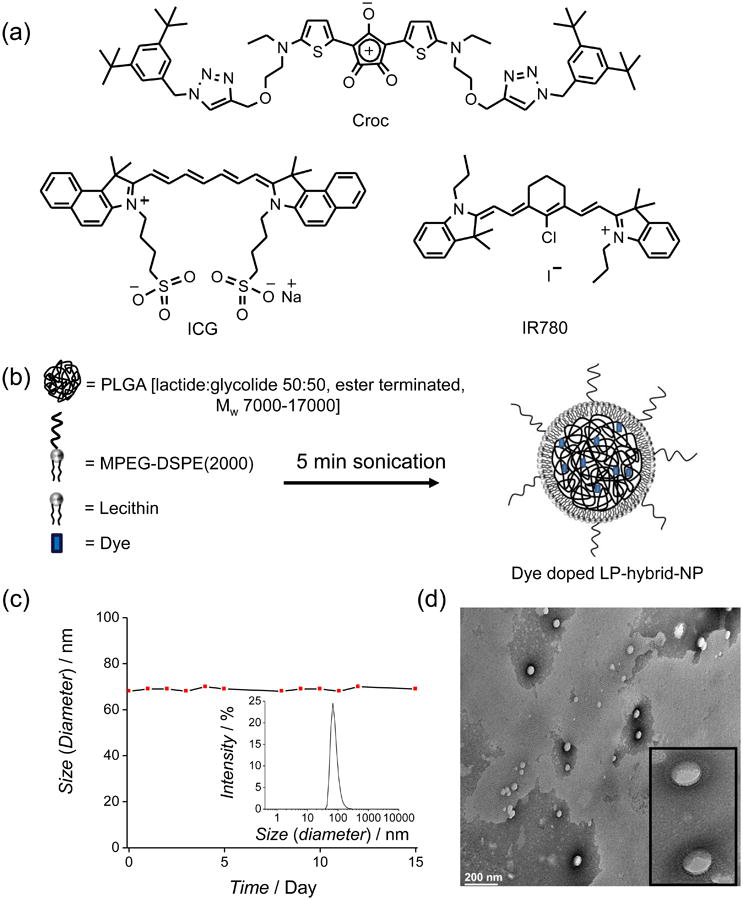
(a) Molecular structures of NIR dyes. (b) Schematic representation of dye doped LP-hybrid-NP fabrication. (c) DLS study of Croc/LP-hybrid-NP in HEPES buffer at pH 7.4. Hydrodynamic diameter 68±1 nm and PDI = 0.109 (see insert) does not change over 15 days. (d) TEM image of Croc/LP-hybrid-NP; inset shows a 5× expansion of one section of the image.
2. Materials and Methods
2.1. Materials
The Croc dye used in this study was prepared using our published method22 and has the following photophysical properties in CHCl3: λabs 795 nm, ε 2.7 × 105 M−1cm−1, λem 810 nm, ΦF 0.06.22 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid (HEPES), PLGA [poly(D, L-lactide-co-glycolide); lactide:glycolide 50:50, ester terminated, Mw 7000-17000], cardiogreen [Indocyanine Green (ICG)], IR780 iodide, DPBF (1,3-diphenyl isobenzofuran), carboxyfluorescein (CF), and phosphotungstic acid were obtained from Sigma Aldrich. Doxorubicin hydrochloride (DOX.HCl) was purchased from Fisher Scientific. 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) were purchased from Avanti Polar Lipids (USA) and stored in a freezer at −20°C. N-(Carbonyl-methoxypolyethyleneglycol-2000)-1,2-distearoyl--sn-glycero-3-phosphoethanolamine, ammonium salt (MPEG-DSPE(2000)) was purchased from Corden Pharma (Switzerland) and stored in a freezer at −20°C. Lecithin (90% Soybean) was purchased from Alfa Aesar and stored in a freezer at −20°C. Singlet Oxygen Sensor Green (SOSG) was purchased from Life technologies (USA). Amicon Ultra (Ultra-15) centrifugal filter units with a molecular weight cutoff of 10kDa were bought from Millipore (USA). Before opening, the chemicals were warmed to room temperature.
2.2. Lipid-Polymer Hybrid Nanoparticles (LP-hybrid-NP)
2.2.1. Nanoparticle Preparation
Stock solutions for LP-hybrid-NP synthesis were MPEGDSPE (2000): 1 mg/mL in 4 wt% ethanol:water solution; lecithin: 1 mg/mL in 4 wt% ethanol:water solution; PLGA: 2.5 mg/mL in acetonitrile. Stock solutions were stored at 4°C and replaced after three weeks, with the exception of PLGA which was always prepared as a fresh solution. To synthesize LP-hybrid-NP, MPEG-DSPE(2000) stock (200 μL) and lecithin stock (12.5 μL) were added into deionized H2O (3.7 mL). Solid organic dye (0.5 mg) was dissolved in the PLGA stock (400 μL) and was added slowly drop-wise. The resulting solution was sonicated using a probe sonicator for 5 min (sonicator frequency 20 kHz and power 130 W), which produced a clear solution. The solution was filtered and washed 3 times using Amicon Ultra-4 centrifugal filter [MWCO 10 kDa] and HEPES buffer at pH 7.4 [4000 × g, 30 min each time] to remove remaining organic solvent and free organic dye. The final weight ratio of components in the assembled nanoparticle was dye:DSPE-PEG(2000):lecithin:PLGA = 0.5:0.2:0.012:1.
2.2.2. Fluorescein and DOX Release from LP-hybrid-NP
DOX and fluorescein release from Croc/DOX/LP-hybrid-NP and Croc/Fluorescein/LP-hybrid-NP was determined by a dialysis method using a dialysis membrane with 8 kDa MWCO (prevents passage of the nanoparticles) and HEPES (pH 7.4) as buffer. The sample in a cuvette was warmed to 37 °C for 2 min prior to irradiation for 15 min with an 808 nm diode laser which increased the sample temperature to 52 °C (control experiments did not laser irradiate). After laser irradiation the samples were incubated in the dark at 37 °C on a rotating incubator set at 200 rpm. At time points, the dialysate was taken out to estimate the amount of released fluorescein or DOX. Fluorescence intensity of DOX was measured at 591 nm when excited at 480 nm. Fluorescence intensity of fluorescein was measured at 512 nm when excited at 480 nm. All experiments were performed in triplicate. To achieve 100% release of fluorescein or DOX, the samples were treated with CH3CN, which dissolved the nanoparticles. The percentage of release F(%) was calculated as F(%) = 100*(Ft-F0)/(Ftotal-F0), where Ft is the fluorescein or DOX intensity at the measured time point, F0 is intensity at t = 0, and Ftotal is the intensity after treatment with CH3CN.
2.3. Liposome Studies
2.3.1. Liposome Preparation
Appropriate ratios of POPC (10 mg/mL), DPPC (10 mg/mL), MPEG-DSPE(2000) (10 mg/mL) and Croc (1 mg/mL) in CHCl3 were combined to give Croc/POPC (3:97) or Croc/MPEG-DSPE(2000)/DPPC (2:5:93). The solvent was removed by N2 stream for 10 min and the residual solvent was removed under high vacuum for 2 hours to produce a Croc/lipid film. The lipid films were rehydrated with an appropriate amount of HEPES buffer (20 mM HEPES, 150 mM NaCl, pH 7.4) to give a final lipid+Croc concentration of 6.67 mM. After 1 h of incubation at T > tm of the lipid, the hydrated films were subjected to five freeze/thaw cycles (consisting of freezing the samples in liquid nitrogen followed by melting in a water bath to T > tm of the lipid). The disperse multilamellar vesicles were incubated at T > tm for 5 min, vortexed, then extruded 21 times through a polycarbonate membrane (200 nm pore size, 19 mm diameter, Whatman Nuclepore Track-Etch Membrane Filtration Products) using a Liposofast extruder (Avestin, Ottawa, Canada) at T > tm to produce a suspension of unilamellar vesicles. The unincorporated Croc dye was removed by size exclusion using a column filled with Sephadex G-25 Superfine gel and cold HEPES buffer as eluent. An analogous procedure was used to make liposomes containing IR780 or DPBF in place of Croc.
2.3.2. Carboxyfluorescein Release Assay
Unilamellar vesicles were prepared with a lipid composition of thermosensitive Croc/MPEG-DSPE(2000)/DPPC: (2:5:93) or nonthermosensitive Croc/POPC (3:97) using the procedure above, except the film was hydrated with carboxyfluorescein (CF) solution (50 mM CF, 20 mM HEPES, 100 mM NaCl at pH 7.4). The unencapsulated CF and Croc were removed by size exclusion using a column filled with Sephadex G-25 Superfine gel and cold HEPES buffer as eluent. CF is self-quenched at high concentrations inside the liposomes. An increase in CF fluorescence indicates release of CF from the vesicles into solution. Addition of 1 % (w/v) Triton X-100 lyses the vesicles and releases the CF, allowing for normalization to 100% leakage. The percentage of CF release was calculated from the fluorescence intensity (Ex: 492 nm, Em: 517 nm) using the equation F(%) = 100*(Ft-F0)/(Ftotal-F0), where Ft is the CF intensity at the measured time point, F0 is intensity at t = 0, and Ftotal is the intensity after treatment with Triton X-100. The laser triggered liposome release experiments in solution used a 808 nm diode laser (6 W/cm2) to heat microwells containing the samples. The non-thermosensitive formulation of Croc:POPC (3:97) is in a liquid crystalline phase before laser heating and so although laser irradiation produces substantial photothermal heating there is no membrane phase transition and no enhanced leakage of CF.
2.4. Photothermal Heating of Immobilized Liposomes in a Gel
Separate 60 × 15 mm petri dishes were loaded with a suspension of gelatin (7 mL, 50 mg/mL) containing CF-loaded Croc/MPEG-DSPE(2000)/DPPC (2:5:93) liposomes (1.2 mL) at 30 °C. The petri dishes containing the gelatin/liposome suspension were allowed to sit for 3 hours at 15 °C, creating a robust gel, and then localized spots (4 mm2) on the gel surface were irradiated with an 808 nm diode laser (15 W/cm2). Four spots on one gel surface were irradiated continuously for 30, 60, 90, or 120 sec, and the temperature at the end of each heating period was measured using a thermal imaging camera (ICI, USA). The four spots on the other gel surface were irradiated for a designated number of heating cycles, where each cycle consisted of laser irradiation for 15 sec (which raised the temp of the spot to 41 °C) followed by a 60 sec break in laser heating. Fluorescence images of the released CF in the irradiated spots were captured using a Lumina IVIS machine (Perkin Elmer, USA) with GFP excitation and emission filter sets and a 1 sec acquisition time. Images were processed using ImageJ and the uniform fluorescence of the nonirradiated gel was subtracted as background. Region of interest (ROI) analyses measured total and mean pixel intensity within each ROI.
3. Results and Discussion
The study compared the photothermal heating properties of lipid-polymer hybrid nanoparticles and liposomes that were doped with the three different NIR dyes in Figure 1a. To help the reader, the two nanoparticle systems are described using a short systematic nomenclature that starts with the name of dye followed by the other composites. For example, Croc doped lipid-polymer hybrid nanoparticles are named Croc/LP-hybrid-NP and Croc doped POPC liposomes are named Croc/POPC. The work is presented in the following sequence. First a comparison of the dye doped lipid-polymer hybrid nanoparticles reveals the superior ability of Croc/LP-hybrid-NP to undergo clean photothermal heating without generating ROS. Then there is a demonstration of laser activated photothermal release of molecular payload from Croc doped lipid-polymer hybrid NP and thermosensitive liposomes.
3.1. Fabrication and Characterization of Dye Doped LP-Hybrid-NP
A rapid and straightforward literature sonication procedure was used to prepare the LP hybrid NP as a core-shell architecture with a hydrophobic core of PLGA [ester terminated poly(lactic-co-glycolic acid)] coated by a monolayer of two polar lipids, lecithin and MPEGDSPE(2000) (Figure 1b, Figure S1 and S2).26 It is worth noting that all three substances are approved for use in humans by the US FDA. The hydrophobic core readily accommodates additional hydrophobic molecules such as drugs or dyes.27, 28, 29 The PEG(2000) chains protruding from the surrounding shell form a protective corona that sterically protects the nanoparticles from self-aggregation and undesired interaction with biological surfaces. All nanoparticle preparations exhibited a narrow size distribution. Figure 1c shows representative DLS data for nanoparticles doped with Croc dye (Croc/LP-hybrid-NP), indicating a high level monodispersity [polydispersity index (PDI) = 0.109] and a particle diameter of 68±1 nm in HEPES buffer at pH 7.4. Stock solutions of the nanoparticles could be stored for more than 15 days with no change in particle size distribution.
Transmission electron microscopy (TEM) was used to visualize the morphology of Croc/LP-hybrid-NP that had been deposited on a carbon coated copper grid (300 mesh) and stained with 2% (w/v) phosphotungstic acid. The TEM images show well-ordered and almost uniform spherical structures with average diameters of 65 nm (Figure 1d). The negative staining indicates higher contrast on the circumference of the polymeric nanoparticles consistent with a polymer core and a surrounding monolayer of polar lipid. Absorption spectra of the Croc/LP-hybrid-NP show two bands at 800 nm and 720 nm (Figure 2b). The peak at 720 nm is due to partial self-aggregation of Croc dye inside the particle. The amount of Croc self-aggregation inside the nanoparticles did not change significantly after sitting for 24 h, even when the solvent was 2 % FBS (w/w) at pH 7.4 (HEPES buffer) (Figure S3a).
Figure 2.
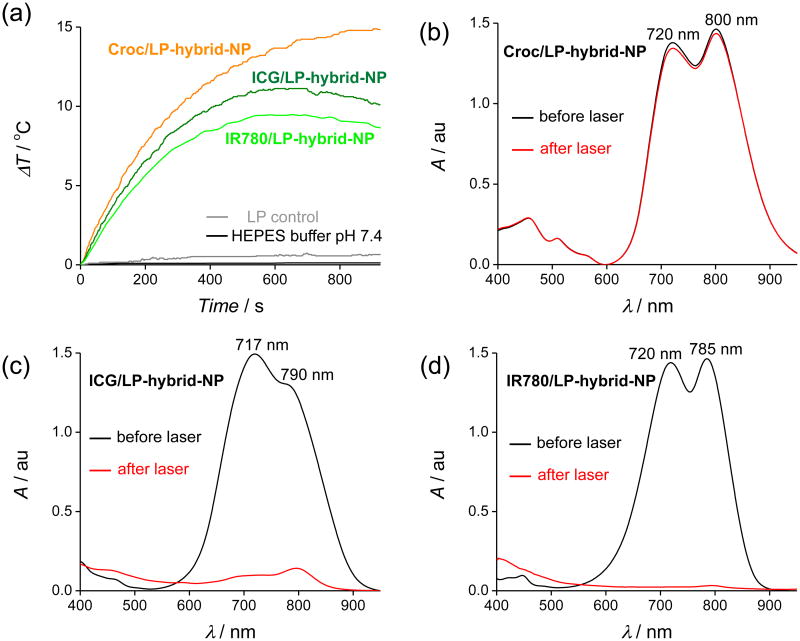
(a) Sample temperature profiles during laser irradiation (808 nm) for Croc/LP-hybrid-NP (orange), ICG/LP-hybrid-NP (deep green), and IR780//LP-hybrid-NP (light green) in HEPES buffer at pH 7.4. Control experiments: LP-hybrid-NP without any dye at pH 7.4 (HEPES buffer) (grey) and HEPES buffer alone at pH 7.4 (black). (b-d) UV/Vis absorption of Croc/LP-hybrid-NP, ICG/LP-hybrid-NP, and IR780//LP-hybrid-NP respectively at pH 7.4 (HEPES buffer) before (black) and after (red) laser irradiation (15 min). Significant loss of ICG and IR780 absorption is observed after laser irradiation.
3.2. Photothermal Heating of Dye Doped LP-Hybrid-NP
For comparison studies, the following dye doped lipid-polymer hybrid NP were prepared using identical methods: Croc/LP-hybrid-NP, ICG/LP-hybrid-NP, and IR780/LP-hybrid-NP (for nanoparticle characterization data, see Figure S4). Separate cuvettes containing samples of each nanoparticle system in pH 7.4 HEPES buffer were irradiated for 15 min with an 808 nm diode laser and the solution temperature was monitored with a thermocouple. In addition, absorption spectra of each sample were acquired before and after laser heating. As shown in Figure 2a, the solution temperature of the Croc/LP-hybrid-NP sample increased to a steady state of 15 °C above ambient, with minimal change in the Croc absorption spectrum. Additional laser irradiation experiments revealed a linear relationship between solution temperature increase and laser power (Figure S5). The remarkable stability of the Croc/LP-hybrid-NP to laser irradiation was further tested by repeating the irradiation experiments in 2 % FBS (w/w) and there was no change in the photothermal behavior (Figure S3d). The results clearly indicate very high photostability and photothermal durability of Croc/LP-hybrid-NP.
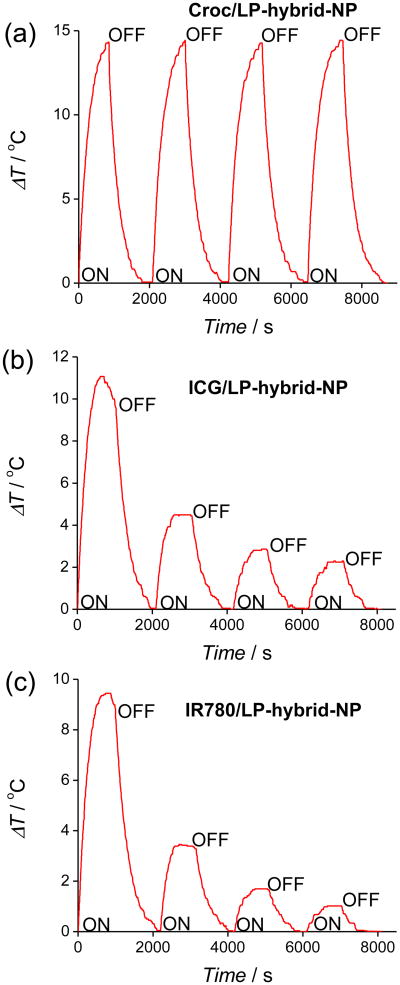
The same laser heating experiments with ICG/LP-hybrid-NP and IR780/LP-hybrid-NP produced different heating results. The absorption spectra in Figure 2c-d and fluorescence spectra in Figure S6 showed that 15 min of laser irradiation almost completely destroyed each dye's ability to absorb the 808 nm laser light. This time-dependent loss in absorption cross section explains why the maximum temperature increase is smaller (∼10 °C for both systems) and also why there is a gradual decrease in sample temperature over the later stages of the laser irradiation (Figure 2a). This difference in dye photostability was readily apparent in cycling experiments that repeatedly irradiated the same sample. As shown in Figure 3, each sample was subjected to four cycles of laser irradiation for 15 min followed by a break without light. The sample of Croc/LP-hybrid-NP showed no change in maximum temperature over the four iterations, whereas, the samples of ICG/LP-hybrid-NP and IR780/LP-hybrid-NP exhibited a large decrease in photothermal heating after each irradiation step in the cycle. However, the size of the LP-hybrid-NPs was unchanged after several cycles of laser irradiation.
Figure 3.
Four heating cycles of (a) Croc/LP-hybrid-NP, (b) ICG/LP-hybrid-NP, and (c) IR780/LP-hybrid-NP at pH 7.4 (HEPES buffer) with laser irradiation.
3.3. Oxygen Photosensitization by Dye Doped LP-Hybrid-NP
The photobleaching of ICG/LP-hybrid-NP and IR780/LP-hybrid-NP is consistent with generation of singlet oxygen (1O2) by dye photosensitization, and this hypothesis was confirmed by conducting two sets of chemical trapping studies. The first set of studies incorporated the lipophilic organic compound diphenylbenzofuran (DPBF) within the core of the nanoparticles. DPBF is well known to react quickly with 1O2 to form a product with a decreased absorption maxima and associated fluorescence (Figure S7). The property makes DPBF an excellent chemical sensor for 1O2 that is produced within the lipophilic core of a bilayer membrane or an organic nanoparticle.30 Three analogous batches of the nanoparticles were prepared with DPBF and one of the three NIR dyes incorporated inside, i.e., ICG/DPBF/LP-hybrid-NP, IR780/DPBF/LP-hybrid-NP, and Croc/DPBF/LP-hybrid-NP (Figure S7). Each nanoparticle sample was irradiated for 15 min with an 808 nm laser and the changes in DPBF fluorescence emission spectra (Figure S8) were recorded. In the case of Croc/DPBF/LP-hybrid-NP there were no spectral changes (Figure S8a), whereas the samples of ICG/DPBF/LP-hybrid-NP and IR780/DPBF/LP-hybrid-NP showed large decreases in the characteristic fluorescence emission peaks for DPBF at 453 and 477 nm (Figure S8b-c). This suggests that irradiation of ICG/DPBF/LP-hybrid-NP and IR780/DPBF/LP-hybrid-NP produced significant amount of reactive 1O2 that reacted with both the NIR dye and the DPBF 1O2 sensor. Control experiments showed no bleaching of the dyes in the absence of laser irradiation. As expected, dye photobleaching led to diminished photothermal heating in the later stages of the irradiation experiments (Figure S9).
Another set of trapping experiments tested for the appearance of 1O2 in the aqueous solution outside the nanoparticles. The 1O2 sensor was the water soluble fluorescent probe Singlet Oxygen Sensor Green (SOSG), which becomes highly fluorescent after reacting with 1O2.31 Separate aqueous solutions of SOSG (pH 7.4) mixed with Croc/LP-hybrid-NP, ICG/LP-hybrid-NP, or IR780/LP-hybrid-NP were laser irradiated for 15 minutes. As expected, irradiation of the Croc/LP-hybrid-NP produced no change in SOSG emission, whereas irradiation of ICG/LP-hybrid-NP or IR780/LP-hybrid-NP produced large increases in SOSG emission indicating considerable 1O2 production (Figure S10).
3.4. Photo-oxidation of Doxorubicin Encapsulated Inside Dye Doped LP-Hybrid-NP
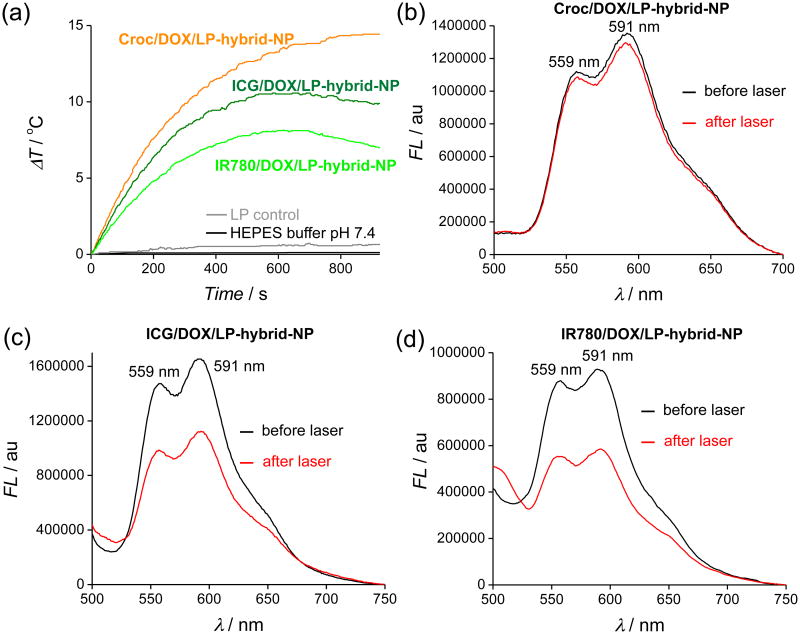
The significant differences in oxygen photosensitization prompted us to evaluate the effect of 808 nm laser irradiation on dye doped LP-hybrid-NP that also contained the anticancer drug doxorubicin (DOX).32 DOX is an effective topoisomerase II inhibitor and a fluorescent compound that is often utilized in clinical nanomedicine and preclinical studies.33 The reactivity of DOX with ROS has not been examined in great detail. However, several studies have found that UV irradiation of solutions containing DOX and photosensitizers leads to destruction of the DOX.34, 35, 36 The degradation is indicated by a loss in the characteristic red fluorescence emission and greatly reduced DOX cytotoxicity. We conducted conceptually similar DOX degradation experiments by first preparing three separate batches of dye doped LP-hybrid-NP that also incorporated the lipophilic free base of DOX, i.e., ICG/DOX/LP-hybrid-NP, IR780/DOX/LP-hybrid-NP, and Croc/DOX/LP-hybrid-NP (Figure S11). Each NP system exhibited the characteristic DOX fluorescence emission bands at 559 and 591 nm when excited at 480 nm (Figure 4). The Croc/DOX/LP-hybrid-NP exhibited long storage stability and excellent photothermal heating performance (Figure S12), including negligible change in the DOX fluorescence intensity after laser irradiation for 15 min (Figure 4a,b). The ICG/DOX/LP-hybrid-NP and IR780/DOX/LP-hybrid-NP had a comparable size and PDI (Figure S13). As expected, the laser heating performance was weaker, with evidence of significant dye photobleaching. Furthermore, there was a 30-35% loss in DOX fluorescence intensity after laser irradiation for 15 minutes (Figure 4c,d) presumably due to reaction with photogenerated 1O2. The structures of the DOX decomposition products were not elucidated but the literature clearly indicates that photo-oxidized DOX has greatly decreased therapeutic efficacy.36
Figure 4.
(a) Temperature change profiles during laser irradiation for Croc/DOX/LP-hybrid-NP (orange), ICG/DOX/LP-hybrid-NP (dark green), and IR780/DOX/LP-hybrid-NP (light green) at pH 7.4 (HEPES buffer). Control experiment: LP-hybrid-NPs without any dye at pH 7.4 (HEPES buffer) (grey) and HEPES buffer alone at pH 7.4 (black). (b-d) DOX fluorescence (λex = 480 nm) in samples of Croc/DOX/LP-hybrid-NP, ICG/DOX/LP-hybrid-NP, and IR780/DOX/LP-hybrid-NP, respectively, before (black) and after (red) laser (15 min) irradiation. The DOX decrease in the three samples was 4%, 30% and 35%, respectively.
3.5. Laser Promoted Release of Payload from LP-Hybrid-NP
The clean photothermal heating of Croc doped nanoparticles (i.e., no generation of destructive 1O2) is very attractive for laser activated drug release applications. This possibility was first tested by measuring the effect of laser irradiation on the leakage of encapsulated molecular payload from Croc doped LP-Hybrid-NP. A dialysis assay compared the rate of DOX escape from Croc/DOX/LP-Hybrid-NP after the sample had been irradiated with 808 nm diode laser for 15 min (which increased the sample temperature to 52 °C) to a control sample that was kept entirely in the dark. As shown in Figure S14a, the half-life for DOX leakage from the nanoparticles in the dark was 18 hours, and this was shortened to 6 hours by 15 min of preliminary laser irradiation. DLS data indicated that the size of the NPs was unchanged after the laser irradiation (Figure S15). The same trend was observed in a separate dialysis experiment that encapsulated the fluorescent dye fluorescein (i.e., Croc/fluorescein/LP-Hybrid-NP, see Figure S16). In this case, the half-life for fluorescein leakage from the nanoparticles in the dark was 8.5 hours, and this was shortened to 4 hours by 15 min of preliminary laser irradiation (Figure S14b). Thus, an initial 15 min period of laser irradiation was able to induce a modest increase in the relatively slow leakage rate of drug or dye payload from Croc/payload/LP-Hybrid-NP. These leakage rates match those observed with other PLGA nanoparticle systems.32, 37 In order to explain the enhanced leakage, we determined the PLGA glass transition temperature (Tg) to be 42-46 °C (Figure S17). We hypothesize that laser induced heating of the Croc/payload/LP- Hybrid-NP induces a temporary thermal phase change in the PLGA core from a rigid, glassy state to a compliant, rubbery state, which facilitates diffusion of encapsulated payload towards the periphery of the nanoparticle. However, complete escape from the nanoparticle is still fairly slow since the payload has to diffuse through the surrounding shell, which is a densely packed monolayer of polar lipids.27
3.6. Laser Promoted Release of Payload from Thermosensitive Liposomes
Liposomes are effective biocompatible nanoparticle systems for drug delivery.38 In the literature, there are different ways of creating thermosensitive liposomes.39 One approach is to use membrane formulations that undergo a thermal phase transition of the saturated acyl chains in the bilayer membrane from gel to fluid phase leading to greatly enhanced leakage of aqueous contents from the liposomes.40 There are only a few reports of thermosensitive liposomes that incorporate NIR dyes as the photothermal agent, in part because of anticipated problems due to dye photobleaching and oxygen photosensitization,41 but also because of the emerging success using gold nanostructures to absorb the NIR light.42 While gold nanoparticles have many attractive features, some structures (such as nanorods) are known to undergo heat driven morphology changes that alter their light absorption and limit their potential for applications that require repetitive photothermal heating.
To demonstrate the potential of Croc doped liposomes for laser activated release of aqueous contents, thermosensitive liposomes were prepared with Croc dye in the lipophilic interior of the membrane and water soluble carboxyfluorescein (CF) trapped in the aqueous interior. The release of CF from the liposomes is easily monitored by its increase in fluorescence intensity as it is diluted into the external solution (Figure 5a). Two liposome systems were studied, a nonthermosensitive formulation comprised of Croc:POPC (3:97) and a thermosensitive formulation comprised of Croc/MPEG-DSPE(2000)/DPPC (2:5:93) with a measured phase transition temperature of 42.2°C (Figure S18). There was substantial leakage from the thermosensitive liposome system (Figure 5b). Furthermore, substantial CF leakage started when the bulk solution temperature passed through the membrane phase transition temperature (Figure 5b).
Figure 5.
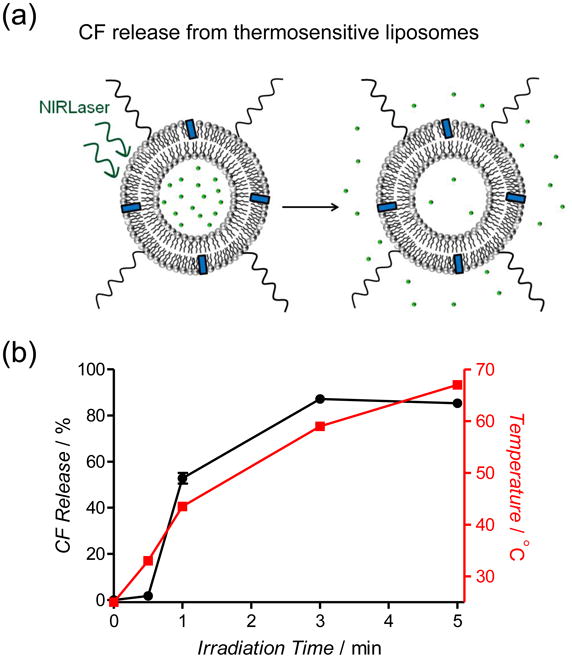
(a) Schematic representation of photothermal CF release from thermosensitive liposomes due to laser irradiation. (b) Photothermal CF release (black line) from Croc/MPEGDPPE(2000)/DPPC (2:5:93) liposomes at pH 7.4 after laser (808 nm, 6 W/cm2) irradiation. The red line is the sample temperature.
An attractive feature of this family of thermosensitive liposomes is the potential for fractionated release. That is, the leakage stops if the sample temperature dips below the membrane phase transition temperature. The unique ability of Croc dye to undergo multiple photothermal heating cycles without loss of heating efficiency makes it attractive for methods that use repetitive doses of laser irradiation to achieve fractionated liposome leakage. Figure 6 shows the results of two sets of CF leakage experiments that demonstrate this concept. Figure 6a shows the controlled incremental CF release from thermosensitive Croc/MPEGDSPE(2000)/DPPC (2:5:93) liposomes in aqueous solution due to repetitive 1 minute doses of 808 nm laser irradiation with 10 minute breaks. In Figure 6b-c is a more complex CF leakage experiment that highlights the spatial as well as the temporal attributes of a laser beam. Two petri dishes were each partially filled with identical layers of a relatively rigid “gel” containing a homogeneous distribution of thermosensitive Croc/MPEG-DSPE(2000)/DPPC (2:5:93) liposomes that were loaded with CF. Preliminary tests showed that a relatively narrow 808 nm laser beam (4 mm2) directed at the gel surface produced a localized photothermal heating spot due to laser absorption by the Croc dye in the liposomes. Furthermore, the heating caused release of CF from the liposomes and the increased CF fluorescence at the heating spot could be observed by fluorescence imaging of the entire petri dish. The first experiment (Figure 6b) used the laser beam to create four separate heating spots (A – D) with continuous laser irradiation times ranging from 30 to 120 seconds. As shown by the associated bar graph in Figure 6d, a longer laser irradiation time produced a commensurate increase in local CF leakage. The second experiment used the same laser beam, but this time the photothermal heating at a single spot was cycled a specified number of times, with each cycle involving 15 seconds of laser and 60 seconds of dark. The four spots in Figure 6c (E – H) correspond to 1 to 4 photothermal heating cycles, respectively. The associated graph in Figure 6e shows that the amount of local CF leakage increased almost linearly with the number of heating cycles. The high photostability of the Croc dye is the key attribute that allowed this fractionated liposome leakage of CF over multiple cycles. This type of repetitive release cannot be achieved with thermosensitive liposomes containing ICG or IR780, because they quickly photobleach.
Figure 6.
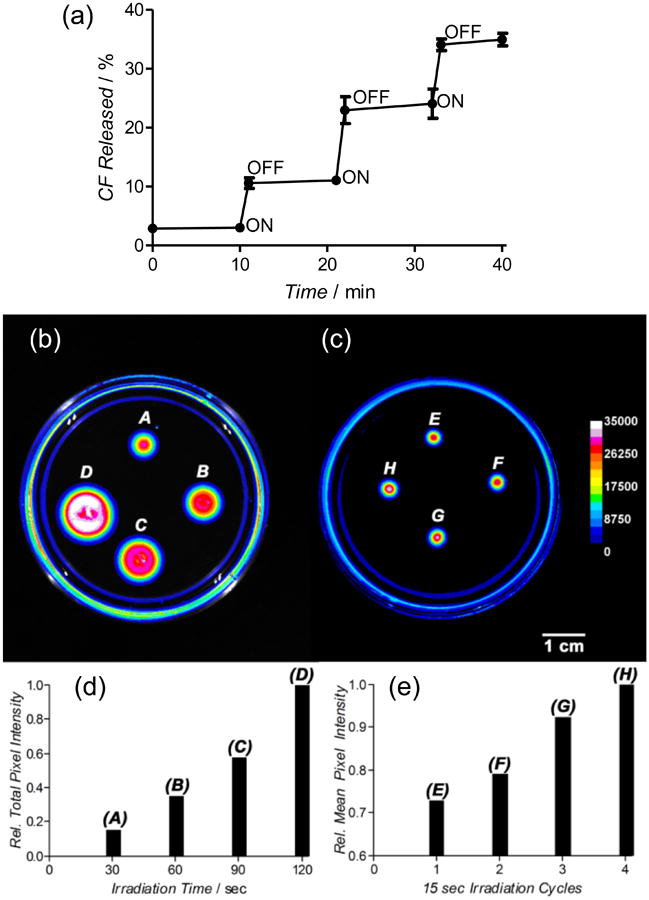
(a) Fractionated laser induced photothermal release of CF from thermosensitive liposomes made of Croc/MPEG-DPPE(2000)/DPPC (2:5:93) in a solution of HEPES buffer (pH 7.4). (b) Fluorescence image of a petri dish holding a gel with an even distribution of immobilized Croc/MPEG-DSPE(2000)/DPPC (2:5:93) liposomes. The four fluorescent spots indicate CF release due to continuous laser (808 nm, 15 W/cm2) irradiation of a 4 mm2 zone for the following time periods and final temperatures (A) 30 sec, 50 °C, (B) 60 sec, 56 °C, (C) 90 sec, 57 °C, (D) 120 sec, 59 °C. (c) Fluorescence image of an identical petri dish holding a gel with an even distribution of immobilized Croc/MPEG-DSPE(2000)/DPPC (2:5:93) liposomes. The four fluorescent spots indicate CF release due to laser (808 nm, 15 W/cm2) irradiation of a 4 mm2 zone for the following cycles of 15 sec irradiation followed by 60 sec break, (E) 1 cycle, (F) 2 cycles, (G) 3 cycles, (H) 4 cycles. Each 15 sec heating period raised the temperature of the spot to 41 °C. (d) Comparison of relative total fluorescence pixel intensity of the four spots in image (b). (e) Comparison of relative mean fluorescence pixel intensity of the four spots in image (c).
Comparison of Figures 6b and 6c shows that fractionated photothermal heating is an effective way to minimize the thermal diffusion of leaked CF from the spot of heating. The effect is nicely illustrated by comparing the wider zone of released CF in spot B (diameter 10 mm, spot irradiated constantly for 60 seconds which produced a maximum temperature of 56 °C) with smaller spot H (diameter 3.6 mm, spot irradiated four times for 15 seconds with one minute breaks, each heating cycle only reached a maximum temperature of 41 °C). Both spots received the same total dose of laser photons but there was less thermal diffusion of the released CF in spot H due to the fractionated irradiation which allowed a cooling period between each irradiation. These simple experiments indicate how clean (i.e., no production of undesired 1O2) and fractionated photothermal release of payload from thermosensitive liposomes containing highly photostable Croc dye can be combined with the precise spatial precision of a laser beam for various types of controlled release applications. For example, we envision possible methods in nanomedicine that immobilize Croc-containing thermosensitive liposomes in superficial in vivo locations (e.g., tattooed under the skin43) and use focused laser beam irradiation to trigger predictable amounts of leaked pharmaceutical over time. This method is attractive for situations that need to maintain dosage levels within an optimal therapeutic window. It may be possible to automate the process by connecting the repetitive laser heating to a diagnostic feedback system that monitors the level of the released payload in the external media.
4. Conclusions
Croc dye has several attractive photothemal properties. It absorbs strongly at 808 nm, a highly effective NIR wavelength for biological optical applications, and generates clean laser-induced heating (no generation of 1O2) without photobleaching of the dye. In contrast, laser-induced heating of nanoparticles that contain the common commercial NIR dyes ICG or IR780 simultaneously produces reactive 1O2, which leads to bleaching of the dye and also decomposition of co-encapsulated payload such as the drug Doxorubicin. Croc doped lipid-polymer hybrid nanoparticles have several favorable features for effective photothermal therapy.44, 45 The nanoparticles are fabricated by a straightforward, rapid, and reproducible sonication method, and all of the nanoparticle components (except the Croc dye) are approved by the US FDA for use in humans. The special ability of Croc doped lipid-polymer nanoparticles to produce consistent photothermal heating over multiple heating cycles should enable future development of alternative strategies in photothermal therapy, such as repetitive fractionated heating of tumors,46 or photothermally enhanced uptake of chemotherapeutics.47 Croc dye is especially useful for repetitive fractional release of water soluble payload from the interior of thermosensitive liposomes. While the shallow penetration of NIR light through skin and tissue is a technical limitation for certain biomedical applications, the precise spatial and temporal control of a laser beam compares favorably with other methods used to induce hyperthermia.48
Supplementary Material
Acknowledgments
We are grateful for funding support from the Walther Cancer Foundation Advancing Basic Cancer Research Grant (2013/14) administered by the Harper Cancer Research Institute (USA), the NSF, and NIH grants R01GM059078 (B.D.S.) and T32GM075762 (S.K.S. and F.M.R.).
Abbreviations
- Croc
Croconaine
- ICG
Indocyanine Green
- IR780 iodide
2-[2-[2-Chloro-3-[(1,3-dihydro-3,3-dimethyl-1-propyl-2H-indol-2-ylidene)ethylidene]-1-cyclohexen-1-yl]ethenyl]-3,3-dimethyl-1-propylindolium iodide
- NIR
Near-infrared
- TEM
Transmission Electron Microscopy
- DLS
Dynamic Light Scattering
- DSC
Differential Scanning Calorimetry
- HEPES
2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid
- PLGA
[poly(D, L-lactide-co-glycolide)]
- DPBF
1,3-diphenyl isobenzofuran
- SOSG
Singlet Oxygen Sensor Green
- CF
carboxyfluorescein
- DOX
Doxorubicin
- POPC
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- DPPC
1,2-dipalmitoyl-sn-glycero-3-phosphocholine
- MPEG-DSPE(2000)
N-(Carbonyl-methoxypolyethyleneglycol-2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine, ammonium salt
- 1O2
singlet oxygen
- PDI
Polydispersity Index
- ROS
Reactive Oxygen Species
Footnotes
Supporting Information. Chemical structures, schematic summaries of experimental methods, and additional data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Lal S, Clare SE, Halas NJ. Nanoshell-Enabled Photothermal Cancer Therapy: Impending Clinical Impact. Acc Chem Res. 2008;41:1842–1851. doi: 10.1021/ar800150g. [DOI] [PubMed] [Google Scholar]
- 2.Leung SJ, Romanowski M. NIR-Activated Content Release from Plasmon Resonant Liposomes for Probing Single-Cell Responses. ACS Nano. 2012;6:9383–9391. doi: 10.1021/nn304434a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huschka R, Barhoumi A, Liu Q, Roth JA, Ji L, Halas NJ. Gene Silencing by Gold Nanoshell-Mediated Delivery and Laser-Triggered Release of Antisense Oligonucleotide and siRNA. ACS Nano. 2012;6:7681–7691. doi: 10.1021/nn301135w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hauck TS, Jennings TL, Yatsenko T, Kumaradas JC, Chan WC. Enhancing the Toxicity of Cancer Chemotherapeutics with Gold Nanorod Hyperthermia. Adv Mater. 2008;20:3832–3838. [Google Scholar]
- 5.Yuan A, Wu J, Tang X, Zhao L, Xu F, Hu Y. Application of Near-Infrared Dyes for Tumor Imaging, Photothermal, and Photodynamic Therapies. J Pharm Sci. 2013;102:6–28. doi: 10.1002/jps.23356. [DOI] [PubMed] [Google Scholar]
- 6.Moon HK, Lee SH, Choi HC. In Vivo Near-Infrared Mediated Tumor Destruction by Photothermal Effect of Carbon Nanotubes. ACS Nano. 2009;3:3707–3713. doi: 10.1021/nn900904h. [DOI] [PubMed] [Google Scholar]
- 7.Jaque D, Maestro LM, del Rosal B, Haro-Gonzalez P, Benayas A, Plaza JL, Rodrigueza EM, Solé JG. Nanoparticles for Photothermal Therapies. Nanoscale. 2014;6:9494–9530. doi: 10.1039/c4nr00708e. [DOI] [PubMed] [Google Scholar]
- 8.Cheng L, Yang K, Chen Q, Liu Z. Organic Stealth Nanoparticles for Highly Effective in Vivo Near-Infrared Photothermal Therapy of Cancer. ACS Nano. 2012;6:5605–5613. doi: 10.1021/nn301539m. [DOI] [PubMed] [Google Scholar]
- 9.Parrish JA. New Concepts in Therapeutic Photomedicine; Photochemistry, Optical Targeting and the Therapeutic Window. J Invest Dermatol. 1981;77:45–50. doi: 10.1111/1523-1747.ep12479235. [DOI] [PubMed] [Google Scholar]
- 10.Khlebtsov N, Dykman L. Biodistribution and Toxicity of Engineered Gold Nanoparticles: A Review of in Vitro and in Vivo Studies. Chem Soc Rev. 2011;40:1647–1671. doi: 10.1039/c0cs00018c. [DOI] [PubMed] [Google Scholar]
- 11.Jiang R, Cheng S, Shao L, Ruan Q, Wang J. Mass-Based Photothermal Comparison Among Gold Nanocrystals, PbS Nanocrystals, Organic Dyes, and Carbon Black. J Phys Chem C. 2013;117:8909–8915. [Google Scholar]
- 12.Engel E, Schraml R, Maisch T, Kobuch K, König B, Szeimies RM, Hillenkamp J, Bäumler W, Vasold R. Light-Induced Decomposition of Indocyanine Green. Invest Ophthalmol Vis Sci. 2008;49:1777–1783. doi: 10.1167/iovs.07-0911. [DOI] [PubMed] [Google Scholar]
- 13.Sheng Z, Hu D, Zheng M, Zhao P, Liu H, Gao D, Gong P, Gao G, Zhang P, Ma Y, Cai L. Smart Human Serum Albumin-Indocyanine Green Nanoparticles Generated by Programmed Assembly for Dual-Modal Imaging-Guided Cancer Synergistic Phototherapy. ACS Nano. 2014;8:12310–12322. doi: 10.1021/nn5062386. [DOI] [PubMed] [Google Scholar]
- 14.Guo M, Mao H, Li Y, Zhu A, He H, Yang H, Wang Y, Tian X, Ge C, Peng Q, Wang X, Yang X, Chen X, Liu G, Chen H. Dual Imaging-guided Photothermal/Photodynamic Therapy Using Micelles. Biomaterials. 2014;35:4656–4666. doi: 10.1016/j.biomaterials.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wan Z, Mao H, Guo M, Li Y, Zhu A, Yang H, He H, Shen J, Zhou L, Jiang Z, Ge C, Chen X, Yang X, Liu G, Chen H. Highly Efficient Hierarchical Micelles Integrating Photothermal Therapy and Singlet Oxygen-Synergized Chemotherapy for Cancer Eradication. Theranostics. 2014;4:399–411. doi: 10.7150/thno.8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacDonald TD, Liu TW, Zheng G. An MRI-Sensitive, Non-Photobleachable Porphysome Photothermal Agent. Angew Chem Int Ed. 2014;53:6956–6959. doi: 10.1002/anie.201400133. [DOI] [PubMed] [Google Scholar]
- 17.Mathew S, Murakami T, Nakatsuji H, Okamoto H, Morone N, Heuser JE, Hashida M, Imahori H. Exclusive Photothermal Heat Generation by a Gadolinium Bis(naphthalocyanine) Complex and Inclusion into Modified High-Density Lipoprotein Nanocarriers for Therapeutic Applications. ACS Nano. 2013;7:8908–8916. doi: 10.1021/nn403384k. [DOI] [PubMed] [Google Scholar]
- 18.Cheng L, He W, Gong H, Wang C, Chen Q, Cheng Z, Liu Z. PEGylated Micelle Nanoparticles Encapsulating a Non-Fluorescent Near-Infrared Organic Dye as a Safe and Highly-Effective Photothermal Agent for In Vivo Cancer Therapy. Adv Funct Mater. 2013;23:5893–5902. [Google Scholar]
- 19.Gong H, Dong Z, Liu Y, Yin S, Cheng L, Xi W, Xiang J, Liu K, Li Y, Liu Z. Engineering of Multifunctional Nano-Micelles for Combined Photothermal and Photodynamic Therapy Under the Guidance of Multimodal Imaging. Adv Funct Mater. 2014;24:6492–6502. [Google Scholar]
- 20.Carter KA, Shao S, Hoopes MI, Luo D, Ahsan B, Grigoryants VM, Song W, Huang H, Zhang G, Pandey RK, Geng J, Pfeifer BA, Scholes CP, Ortega J, Karttunen M, Lovell JF. Porphyrin–Phospholipid Liposomes Permeabilized by Near-Infrared Light. Nat Commun. 2014;5:3546. doi: 10.1038/ncomms4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spence GT, Hartland GV, Smith BD. Activated Photothermal Heating Using Croconaine Dyes. Chem Sci. 2013;4:4240–4244. [Google Scholar]
- 22.Spence GT, Lo SS, Ke C, Destecroix H, Davis AP, Hartland GV, Smith BD. Near-Infrared Croconaine Rotaxanes and Doped Nanoparticles for Enhanced Aqueous Photothermal Heating. Chem Eur J. 2014;20:12628–12635. doi: 10.1002/chem.201403315. [DOI] [PubMed] [Google Scholar]
- 23.Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the Enhanced Permeability and Retention Effect for Tumor Targeting. Drug Disc Today. 2006;11:812–818. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Yu J, Javier D, Yaseen MA, Nitin N, Richards-Kortum R, Anvari B, Wong MS. Self-Assembly Synthesis, Tumor Cell Targeting, and Photothermal Capabilities of Antibody-Coated Indocyanine Green Nanocapsules. J Am Chem Soc. 2010;132:1929–1938. doi: 10.1021/ja908139y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang E, Luo S, Tan X, Shi C. Mechanistic Study of IR-780 Dye as a Potential Tumor Targeting and Drug Delivery Agent. Biomaterials. 2014;35:771–778. doi: 10.1016/j.biomaterials.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 26.Fang RH, Aryal S, Hu CMJ, Zhang L. Quick Synthesis of Lipid-Polymer Hybrid Nanoparticles with Low Polydispersity Using a Single-Step Sonication Method. Langmuir. 2010;26:16958–16962. doi: 10.1021/la103576a. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Chan JM, Gu FX, Rhee JW, Wang AZ, Radovic-Moreno AF, Alexis F, Langer R, Farokhzad OC. Self-Assembled Lipid-Polymer Hybrid Nanoparticles: A Robust Drug Delivery Platform. ACS Nano. 2008;2:1696–1702. doi: 10.1021/nn800275r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan JM, Zhang L, Yuet KP, Liao G, Rhee JW, Langer R, Farokhzad OC. PLGA–Lecithin–PEG Core–Shell Nanoparticles for Controlled Drug Delivery. Biomaterials. 2009;30:1627–1634. doi: 10.1016/j.biomaterials.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 29.Raemdonck K, Braeckmans K, Demeestera J, Smedt SCD. Merging the Best of Both Worlds: Hybrid Lipid-Enveloped Matrix Nanocomposites in Drug Delivery. Chem Soc Rev. 2014;43:444–472. doi: 10.1039/c3cs60299k. [DOI] [PubMed] [Google Scholar]
- 30.Wozniak M, Tanfani F, Bertoli E, Zolese G, Antosiewicz J. A New Fluorescence Method to Detect Singlet Oxygen Inside Phospholipid Model Membranes. Biochim Biophys Acta. 1991;1082:94–100. doi: 10.1016/0005-2760(91)90304-z. [DOI] [PubMed] [Google Scholar]
- 31.Lin H, Shen Y, Chen D, Lin L, Wilson BC, Li B, Xie S. Feasibility Study on Quantitative Measurements of Singlet Oxygen Generation Using Singlet Oxygen Sensor Green. J Fluoresc. 2013;23:41–47. doi: 10.1007/s10895-012-1114-5. [DOI] [PubMed] [Google Scholar]
- 32.Zheng M, Yue C, Ma Y, Gong P, Zhao P, Zheng C, Sheng Z, Zhang P, Wang Z, Cai L. Single-Step Assembly of DOX/ICG Loaded Lipid-Polymer Nanoparticles for Highly Effective Chemo-Photothermal Combination Therapy. ACS Nano. 2013;7:2056–2067. doi: 10.1021/nn400334y. [DOI] [PubMed] [Google Scholar]
- 33.Ang CY, Tan SY, Zhao YL. Recent Advances in Biocompatible Nanocarriers for Delivery of Chemotherapeutic Cargoes Towards Cancer Therapy. Org Biomol Chem. 2014;12:4776–4806. doi: 10.1039/c4ob00164h. [DOI] [PubMed] [Google Scholar]
- 34.Chen M, Hein S, Le DQS, Feng W, Foss M, Kjems J, Besenbacher F, Zou X, Bünger C. Free Radicals Generated by Tantalum Implants Antagonize the Cytotoxic Effect of Doxorubicin. Int J Pharm. 2013;448:214–220. doi: 10.1016/j.ijpharm.2013.03.041. [DOI] [PubMed] [Google Scholar]
- 35.Ramu A, Mehta MM, Leaseburg T, Aleksic A. The Enhancement of Riboflavin-Mediated Photo-oxidation of Doxorubicin by Histidine and Urocanic Acid. Cancer Chemother Pharmacol. 2001;47:338–346. doi: 10.1007/s002800000236. [DOI] [PubMed] [Google Scholar]
- 36.Ramu A, Mehta MM, Liu J, Turyan I, Aleksic A. The Riboflavin-Mediated Photooxidation of Doxorubicin. Cancer Chemother Pharmacol. 2000;46:449–458. doi: 10.1007/s002800000174. [DOI] [PubMed] [Google Scholar]
- 37.Yang J, Lee J, Kang J, Oh SJ, Ko HJ, Son JH, Lee K, Suh JS, Huh YM, Haam S. Smart Drug-Loaded Polymer Gold Nanoshells for Systemic and Localized Therapy of Human Epithelial Cancer. Adv Mater. 2009;21:4339–4342. doi: 10.1002/adma.200900334. [DOI] [PubMed] [Google Scholar]
- 38.Torchilin VP. Recent Advances with Liposomes as Pharmaceutical Carriers. Nat Rev Drug Discov. 2005;4:145–60. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 39.Leung SJ, Romanowsk M. Light-Activated Content Release from Liposomes. Theranostics. 2012;2:1020–1036. doi: 10.7150/thno.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li L, ten Hagen TLM, Schipper D, Wijnberg TM, van Rhoon GC, Eggermont AMM, Lindner LH, Koning GA. Triggered Content Release from Optimized Stealth Thermosensitive Liposomes Using Mild Hyperthermia. J Control Release. 2010;143:274–279. doi: 10.1016/j.jconrel.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Giraudeau C, Moussaron A, Stallivieri A, Mordon S, Frochot C. Indocyanine Green: Photosensitizer or Chromophore? Still a Debate. Curr Med Chem. 2014;21:1871–1897. doi: 10.2174/0929867321666131218095802. [DOI] [PubMed] [Google Scholar]
- 42.You J, Zhang P, Hu F, Du Y, Yuan H, Zhu J, Wang Z, Zhou J, Li C. Near-Infrared Light-Sensitive Liposomes for the Enhanced Photothermal Tumor Treatment by the Combination with Chemotherapy. Pharm Res. 2014;31:554–565. doi: 10.1007/s11095-013-1180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shio MT, Paquet M, Martel C, Bosschaerts T, Stienstra S, Olivier M, Drug AF. Delivery by Tattooing to Treat Cutaneous Leishmaniasis. Sci Rep. 2014;4:4156. doi: 10.1038/srep04156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaque D, Maestro LM, del Rosal B, Haro-Gonzalez P, Benayas A, Plaza JL, Rodríguez EM, Solé JG. Nanoparticles for Photothermal Therapies. Nanoscale. 2014;6:9494–9530. doi: 10.1039/c4nr00708e. [DOI] [PubMed] [Google Scholar]
- 45.Devadas MS, Devkota T, Guha S, Shaw SK, Smith BD, Hartland GV. Spatial Modulation Spectroscopy for Imaging and Quantitative Analysis of Single Dye-doped Organic Nanoparticles Inside Cells. Nanoscale. 2015;7:9779–9785. doi: 10.1039/c5nr01614b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gutwein LG, Singh AK, Hahn MA, Rule MC, Knapik JA, Moudgil BM, Brown SC, Grobmyer SR. Fractionated Photothermal Antitumor Therapy with Multidye Nanoparticles. Int J Nanomed. 2012;7:351–357. doi: 10.2147/IJN.S26468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tian B, Wang C, Zhang S, Feng L, Liu Z. Photothermally Enhanced Photodynamic Therapy Delivered by Nano-Graphene Oxide. ACS Nano. 2011;5:7000–7009. doi: 10.1021/nn201560b. [DOI] [PubMed] [Google Scholar]
- 48.Mura S, Nicolas J, Couvreur P. Stimuli-Responsive Nanocarriers for Drug Delivery. Nat Mater. 2014;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.