Abstract
Please cite this paper as: Vahlenkamp et al. (2010) Systemic influenza virus H5N1 infection in cats after gastrointestinal exposure. Influenza and Other Respiratory Viruses 4(6), 379–386.
Background Highly pathogenic avian influenza virus (HPAIV) H5N1 infections in felids have been reported in several countries. Feeding on infected birds has been suggested as potential source of infection.
Objectives The study aimed to verify gastrointestinal infection as possible portal of entry for HPAIV H5N1 in cats.
Methods Four cats were infected oculo‐nasopharyngeally with 106 50% egg infectious dose (EID50) of HPAIV H5N1 A/cat/Germany/R606/2006. Two cats were infected intravenously with 106 EID50 and two cats were inoculated orally with 107 EID50 HPAIV embedded in gelatine capsules to mimic gastrointestinal exposure and to avoid virus contact to oropharyngeal or respiratory tissues. Cats were monitored for 6 days by physical examination, virus excretion, and peripheral blood lymphocyte counts. Blood chemical parameters (including AST, ALT, CPK, and TBIL) and viral excretion using pharyngeal and rectal swabs were analyzed.
Results Infected cats showed elevated body temperature up to 41·3°C starting from day 1 or 2 p.i. All infected cats excreted virus in pharyngeal swabs within 2 days p.i. co‐inciding with the development of clinical signs (anorexia, depression, and labored breathing) irrespective of the infection route. Virus dissemination occurred through cell‐free and cell‐associated viremia. Infected cats developed lymphopenia, hepatic necrosis, pneumonia, and significantly elevated levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatine phosphokinase (CPK), and TBIL.
Conclusions The experiments show that the gastrointestinal tract can serve as portal for the entry of HPAIV H5N1 into cats. Infection routes used did not influence viral tissue tropism and course of disease.
Keywords: Cats, gastrointestinal tract, H5N1, influenza virus, oral transmission, pathogenesis
Introduction
Highly pathogenic avian influenza virus (HPAIV) of subtype H5N1 of Asian origin continues to circulate in several Asian and African countries in poultry and wild birds causing considerable concern for veterinary and public health. Concomitant with virus spread among poultry or bird populations felids have been found naturally infected with HPAIV H5N1 in several countries. 1 , 2 Feeding of cats on infected birds has been documented in several cases 3 and is generally considered as the most likely route of infection. A fatal HPAIV H5N1 infection was also documented in dogs in Thailand after scavenging on HPAIV H5N1‐infected chicken carcasses. 4 Oral infection has also been suggested in an outbreak of HPAIV H5N1 in tigers and leopards in a zoo in Thailand. 5 , 6 Under experimental conditions, ingestion of infected uncooked chicken has been shown to cause disease in cats. 7 , 8 The experimental studies further supported the possibility of influenza virus infection through the gastrointestinal tract but could not exclude aerosol infection via the respiratory tract. 7 , 8 Therefore, respiratory and gastrointestinal infection can be suspected in cats. Systemic spread led to virus detection in different organs including liver, heart, brain, kidney, spleen, and pancreas. 8 , 9 , 10 Virus has been isolated from saliva, intestinal contents, urine, and feces of infected cats. 8 , 9 , 11
In humans, limited epidemiological evidence precludes in many instances the identification of the source of HPAIV H5N1 infection. The vast majority of infections probably occurred via the respiratory route. In several cases, however, a gastrointestinal portal of virus entry may have been relevant as documented by diarrhea in infected humans together with the detection of viral RNA in the intestines and of HPAIV in rectal swabs. 12 Thus, infection via the gastrointestinal tract may occur during HPAIV infection in mammals. 13
To analyze gastrointestinal infection with HPAIV H5N1 in a natural mammalian infection model, we inoculated cats orally with HPAIV H5N1 embedded in gelatine capsules and compared this route of infection with the intravenous and oculo‐nasopharyngeal routes.
Material and methods
Cats
Specific pathogen–free animals were obtained from Charles River Laboratories and housed at the high‐containment animal facility (Biosafety Level 3+) at the Friedrich‐Loeffler‐Institut, Germany. Animals were kept individually in separate cages in one room. The animal experiments were approved by the regional ethical committee. All animals were 8–10 months old at the time of infection.
Virus
The influenza virus A/cat/Germany/R606/2006 (H5N1) was used for the infection experiments. It belongs to the clade 2·2 genotype (‘Qinghai‐like’) and is derived from a naturally infected domestic cat found during the HPAI H5N1 virus outbreak among wild birds on the isle of Rügen/Germany in 2006. 14 , 15 The virus was propagated once in embryonated hen eggs. The titer of the inoculated virus was determined by serial dilutions in embryonated hen eggs.
Infection experiments
To verify the gastrointestinal tract as possible route of infection, ten cats were included in the study. Four cats were inoculated with 106 50% egg infectious doses (EID50) via the oculo‐nasopharyngeal route as previously described. 10 Two cats received the virus orally in commercially available gelatine capsules (http://www.capsuleworld.eu; size 14·0 × 5·1 mm). Gelatine capsules were chosen because they dissolve quickly after liquid contact, and therefore, the virus inoculum was likely to be released in the stomach but not in the oropharynx. Capsules were filled with ground dry cat food, and 800 μl of the virus containing 107 EID50 was absorbed to this matrix to prevent dissolution from within. The capsules were closed, applied directly to the base of the cats’ tongue and the mouth of the cat was hold closed. This forced the cat immediately to swallow the capsule without biting or chewing on the capsule, thereby possibly exposing the respiratory tract to the virus. As control for the systemic spread of the infection, two cats were inoculated with 106 EID50 intravenously. Two cats served as uninfected controls.
Clinical monitoring and collection of swab samples
Cats were monitored for 6 days by peripheral blood lymphocyte counts and by physical examination (body temperature, clinical signs incl. depression, anorexia, nasal discharge, conjunctivitis, and respiratory symptoms). Blood chemical parameters (including AST, ALT, CPK, and TBIL) were analyzed on days 0 and 4 p.i. Plasma and peripheral blood mononuclear cell (PBMC) samples were analyzed on day 4 p.i. for viral RNA, and viral excretion was measured on days 0, 2, and 4 p.i. using pharyngeal and rectal swabs. Viral dissemination in the organs was determined by quantitative RT‐PCR.
Real‐time RT‐PCR (rRT‐PCR)
RNA was isolated from swab samples using the QIAampViral RNA Mini kit (Qiagen, Hilden, Germany). One‐step rRT‐PCR specific for an M gene fragment was performed on the Strategene Mx3000 PCR machine using the Superscript III One‐Step RT‐PCR system and Platinum Taq DNA Polymerase (Invitrogen, Darmstadt, Germany) applying 30 minutes at 50°C, 2 minutes at 94°C, and subsequently 42 cycles for 30 seconds each at 94, 56, and 68°C. 10
Blood collection, flow cytometric analysis, and sorting of cells
Whole blood was collected by venipuncture into EDTA vacutainer tubes (Becton‐Dickinson, Heidelberg, Germany). After centrifugation at 300×g for 10 minutes, plasma was removed and the volume replaced with phosphate‐buffered saline (PBS). Blood (200 μl) was removed and 500 μl buffer I (PBS‐, 2 mm EDTQA, 0·1% BSA) added for 10 minutes. After centrifugation at 300×g for 10 minutes and removal of the supernatant, 100 μl of FITC‐conjugated anti‐CD4 (mAb30A) or PE‐conjugated anti‐CD8 (mAb 3·357) antibodies was added for 20 min and incubated on a shaker at room temperature in the dark. After washing with 500 μl buffer I, cells were incubated for 10 min in 1 ml FACS Lysing Solution (Becton‐Dickinson). After centrifugation at room temperature, cells were fixed for 10 min in 200 μl 4% paraformaldehyde. At least 20·000 cells were subsequently analyzed by flow cytometry (FACSCalibur; CellQuest software; BD Biosciences, Heidelberg, Germany).
Enrichment of cell subpopulations was performed by positive selection using anti‐CD4 (mAb30A), anti‐CD8 (mAb 3·357), and anti‐CD14 (TÜK4) antibodies. Cells were subsequently magnetically purified with Dynabeads® Invitrogen, Darmstadt, Germany goat anti‐mouse IgG.
Chemical blood analysis
Enzymatic and chemical blood analysis was performed in the individual cats with the FUJI DRI‐CHEM 3500i Analyzer (Sysmex, Norderstedt, Germany). Serum or plasma samples were investigated before infection and on day 4 p.i. Parameters included gamma‐glutamyl transferase (GGT), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), amyl nitrite (AMYL), blood urea nitrogen (BUN), creatinine (CRE), uric acid (UA), total protein (TP), albumin (ALB), ammonia (NH3), aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatine phosphokinase (CPK), and total bilirubin (TBIL).
Results
Infection experiments
Oculo‐nasopharyngeally infected animals showed elevated body temperature up to 40·3°C from day 2 p.i. onwards (Table 1). The animals also excreted virus continuously from day 2 or day 4 p.i. onward as measured by RT‐PCR in pharyngeal swabs. Virus detection in rectal swabs was inconsistent and detected in one of four animals on day 2 p.i. and two animals on day 4 p.i. The animals showed severe clinical signs of depression, anorexia, and labored breathing from 2 days p.i. onwards.
Table 1.
Route of highly pathogenic avian influenza virus H5N1 infection of cats, body temperature, and virus excretion
| Animal no. | EID50 | Route of infection | Body temperature in °C (dpi) | Pharyngeal swab (dpi) | Rectal swabs (dpi) | Euth (dpi) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 0 | 2 | 4 | 0 | 2 | 4 | ||||
| 081 | 106 | Oc‐na* | 38·8 | 39·9 | 39·3 | 40·0 | 39·0 | − | + | + | − | − | − | 6 |
| 114 | 106 | Oc‐na* | 38·4 | 39·1 | 40·3 | 40·3 | 40·2 | − | − | + | − | − | + | 6 |
| 256 | 106 | Oc‐na* | 38·8 | 38·4 | 39·7 | 39·3 | 39·1 | − | + | + | − | − | ++ | 6 |
| 301 | 106 | Oc‐na* | 38·4 | 39·4 | 39·1 | 40·3 | 39·5 | − | + | + | − | + | − | 6 |
| 262 | 107 | Ga‐in** | 38·5 | 39·5 | 39·7 | 38·9 | nd | − | + | + | − | − | − | 4 |
| 879 | 107 | Ga‐in** | 38·6 | 40·1 | 39·8 | 40·9 | 38·2 | − | + | + | − | + | − | 6 |
| 800 | 106 | i.v.*** | 39·5 | 41·0 | 40·7 | 40·6 | nd | − | + | + | − | + | ++ | 4 |
| 633 | 106 | i.v.*** | 39·2 | 40·6 | 41·3 | 40·8 | nd | − | + | + | − | − | + | 4 |
| 117 | 0 | Control† | 38·7 | 38·9 | 38·9 | 38·5 | 38·7 | − | − | − | − | − | − | 6 |
| 848 | 0 | Control† | 38·6 | 38·8 | 38·7 | 38·7 | 38·4 | − | − | − | − | − | − | 6 |
Elevated body temperatures (above 39°C) are underlined. The rRT‐PCR results are given as: +++ (Ct values of <24), ++ (Ct values of 24 to <31), and + (Ct values of 31 to <38); days post‐infection (dpi); not done (nd).
*Oculo‐nasopharyngeal.
**Gastrointestinal.
***Intravenous.
†Uninfected control.
Cats inoculated via the gastrointestinal route with HPAIV H5N1 in gelatine capsules showed virus excretion and clinical signs, including elevated body temperature, similar to oculo‐nasopharyngeally infected animals. Virus excretion and a rise in body temperature on day 2 p.i. co‐incided with the development of clinical signs.
Cats infected intravenously exhibited elevated body temperatures up to 39·5°C already on day 1 p.i. and developed slightly more severe clinical signs of disease with regard to depression. The two animals and also one of the gastrointestinal inoculated cats were euthanized on day 4 p.i. Body temperatures and results of the RT‐PCR of pharyngeal and rectal swabs from the three groups are given in Table 1. Uninfected control cats remained healthy throughout the observation period.
Virus dissemination
The virus is spread in infected cats irrespective of the route of infection by cell‐free and cell‐associated viremia (Table 2). Using rRT‐PCR, viral RNA was detected on day 4 p.i. in plasma, purified total PBMC as well as CD4+ and CD8+ T cells. Dissemination of the virus occurred to all organs investigated (Table 3). The amount of viral RNA was found to be highest in the lung and liver and lowest in the colon and jejunum. In general, rRT‐PCR Ct values below 24 average in viral titers between 105·0 and 105·2, and Ct values above 24 average in viral titers between 102·3 and 103·3 on MV1Lu cells.
Table 2.
Detection of viral RNA by rRT‐PCR in the peripheral blood on day 4 p.i.
| Animal no. | Route of infection | Plasma | PBMC | CD4+ | CD8+ | CD14+ |
|---|---|---|---|---|---|---|
| 081 | Oc‐na* | + | + | + | + | − |
| 114 | Oc‐na* | + | + | + | + | − |
| 256 | Oc‐na* | ++ | ++ | + | + | − |
| 301 | Oc‐na* | − | + | − | − | − |
| 262 | Ga‐in** | + | + | + | − | − |
| 879 | Ga‐in** | ++ | + | − | − | − |
| 800 | i.v.*** | ++ | ++ | + | − | − |
| 633 | i.v.*** | ++ | ++ | + | − | − |
| 117 | Control† | − | − | − | − | − |
| 848 | Control† | − | − | − | − | − |
The rRT‐PCR results are given as: +++ (Ct values of <24), ++ (Ct values of 24 to <31), and + (Ct values of 31 to <38).
*Oculo‐nasopharyngeal.
**Gastrointestinal.
***Intravenous.
†Uninfected control.
Table 3.
Virus dissemination after highly pathogenic avian influenza virus H5N1 infection of cats by different routes
| Animal no. | Route of infection | Tissue | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Tonsil | Lung | Colon | Jejunum | Liver | Ln mes. | Kidney | CNS | ||
| 081 | Oc‐na* | +++ | ++++ | ++ | + | +++ | ++ | ++ | ++ |
| 114 | Oc‐na* | +++ | ++++ | ++ | + | +++ | ++ | ++ | + |
| 256 | Oc‐na* | ++ | +++ | + | + | ++++ | +++ | +++ | +++ |
| 301 | Oc‐na* | ++++ | ++++ | ++ | ++ | +++ | ++ | ++ | +++ |
| 262 | Ga‐in** | +++ | +++ | ++ | + | +++ | ++ | ++ | + |
| 879 | Ga‐in** | ++ | ++ | ++ | − | +++ | − | ++ | − |
| 800 | i.v.*** | + | +++ | − | − | ++++ | +++ | ++ | ++ |
| 633 | i.v. *** | +++ | ++++ | ++ | ++ | ++++ | +++ | ++ | +++ |
| 117 | Control† | − | − | − | − | − | − | − | − |
| 848 | Control† | − | − | − | − | − | − | − | − |
Animals were euthanized on days 4 and 6 p.i. The rRT‐PCR results are given as: ++++ (Ct values of 10 to <17), +++ (Ct values of 17 to <24), ++ (Ct values of 24 to <31), and + (Ct values of 31 to <38). Average virus isolation titers on MV1Lu cells corresponding to the Ct values clusters +, ++, +++, and ++++ are 102·3, 103·3, 105·0, and 105·2, respectively.
*Oculo‐nasopharyngeal.
**Gastrointestinal.
***Intravenous.
†Uninfected control.
Flow cytometry
CD4+ and CD8+ cell populations (given as percent of total PBMC) were investigated in animals infected oculo‐nasopharyngeally with 106 EID50 HPAIV H5N1 on day 4 p.i. and in uninfected controls. The infected cats developed severe lymphopenia with CD4+ cell numbers of 25·1 ± 5·6% and CD8+ cells numbers of 8·2 ± 4·0%. The CD4+ and CD8+ cell numbers of the uninfected cats were 39·8 ± 5·2% and 11·7 ± 3·4%, respectively. The CD3 cell numbers in the infected (34·4 ± 9·3%) and uninfected animals (56·4 ± 4·9%) also reflect the lymphopenia. The CD4+ and CD8+ cell populations were affected similarly which resulted in comparable CD4+/CD8+ T‐cell ratios in the infected and uninfected group of animals of 3·2 ± 0·9 and 3·5 ± 0·7, respectively.
Blood chemical parameters
HPAIV H5N1 established systemic infection in all inoculated cats (Table 3). Therefore, several blood chemical parameters were investigated which might point to organ damage. Among all parameters analyzed which included GGT, LDH, ALP, AMYL, BUN, CRE, UA, TP, ALB, NH3, AST, ALT, CPK, and TBIL, only AST, ALT, and CPK, as well as TBIL, were significantly elevated within 4 days p.i. in all animals. In Table 4, the values of two animals each infected by the different routes are shown. Overall, the highest values in all four blood parameters were measured in the i.v. infected cats followed by cats infected oculo‐nasopharyngeally and infected via the gastrointestinal tract.
Table 4.
Blood chemical parameters after highly pathogenic avian influenza virus H5N1 infection of cats
| Animal no. | EID50 | Route of infection | Blood parameter* | Units | Day 0 | Day 4 p.i. |
|---|---|---|---|---|---|---|
| 081 | 106 | Oculo‐nasopharyngeal | AST | (U/l) | 42 | 796 |
| ALT | (U/l) | 44 | 642 | |||
| CPK | (U/l) | 250 | >2000 | |||
| TBIL | (mg/dl) | 0·6 | 3·6 | |||
| 256 | 106 | Oculo‐nasopharyngeal | AST | (U/l) | 34 | >1000 |
| ALT | (U/l) | 42 | >1000 | |||
| CPK | (U/l) | 137 | 1691 | |||
| TBIL | (mg/dl) | 0·1 | 2·5 | |||
| 262 | 107 | Gastrointestinal (capsule) | AST | (U/l) | 74 | 223 |
| ALT | (U/l) | 98 | 502 | |||
| CPK | (U/l) | 144 | >2000 | |||
| TBIL | (mg/dl) | 0·7 | 1·9 | |||
| 879 | 107 | Gastrointestinal (capsule) | AST | (U/l) | 18 | 113 |
| ALT | (U/l) | 71 | 118 | |||
| CP | (U/l) | 143 | 338 | |||
| TBIL | (mg/dl) | 0·4 | 3·9 | |||
| 800 | 106 | Intravenous | AST | (U/l) | 23 | 807 |
| ALT | (U/l) | 82 | >1000 | |||
| CPK | (U/l) | 115 | >2000 | |||
| TBIL | (mg/dl) | 0·6 | 2·1 | |||
| 633 | 106 | Intravenous | AST | (U/l) | 69 | >1000 |
| ALT | (U/l) | 96 | >1000 | |||
| CPK | (U/l) | 134 | >2000 | |||
| TBIL | (mg/dl) | 0·6 | 5·3 | |||
| 117 | 0 | Uninfected control | AST | (U/l) | 28 | 51 |
| ALT | (U/l) | 49 | 58 | |||
| CPK | (U/l) | 279 | 196 | |||
| TBIL | (mg/dl) | 0·4 | 0·5 | |||
| 848 | 0 | Uninfected control | AST | (U/l) | 129 | 56 |
| ALT | (U/l) | 42 | 84 | |||
| CPK | (U/l) | 308 | 252 | |||
| TBIL | (mg/dl) | 0·7 | 0·6 |
*Physiological reference values are aspartate aminotransferase (AST) 18–51 U/l; alanine aminotransferase (ALT) 22–84 U/l; creatine phosphokinase (CPK) 87–309 U/l; total bilirubin (TBIL) 0·1–0·5 mg/dl.
Pathology
Pathological investigations of the infected animals revealed lesions in liver and lungs, which were similar in the oculo‐nasopharyngeally infected cats euthanized on day 6 p.i. and the intravenously infected cats euthanized on day 4 p.i. These lesions were also not different in number or extend to the lesions observed in the two gastrointestinal exposed cats euthanized on days 4 and 6 p.i. Within the dark tan liver, there were multiple randomly arranged, sharply demarcated gray to white foci of pinpoint size or up to 3 mm in diameter (hepatocellular necrosis) (Figure 1). In the lung parenchyma, several irregularly sized and shaped, edematous or slightly consolidated, and atelectatic pneumonic, mainly peribronchiolar areas, were present in all infected animals irrespective of the infection route (Figure 2).
Figure 1.
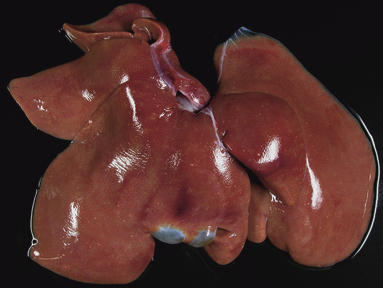
Pathological investigation of the animal inoculated with the gelatine capsules at day 4 p.i. revealed multiple foci of necrosis in the liver.
Figure 2.
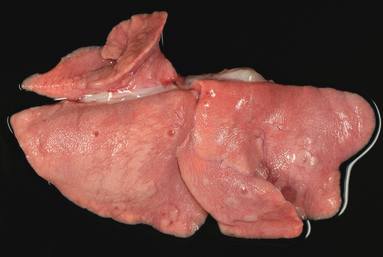
Pathological investigation of the animal inoculated with the gelatine capsules at day 4 p.i. revealed multiple foci of pneumonia herds in the lung.
Histopathology revealed in the liver numerous randomly scattered sharply demarcated areas of coagulative necrosis with the accumulation of karyorrhectic and cellular debris admixed with fibrin and few erythrocytes. In the lungs, bronchointerstitial and focally extensive necrosuppurative bronchopneumonia were the predominant findings. Multifocally, alveoli were filled with homogeneous pale eosinophilic fluid (alveolar edema). The bronchiolar epithelium was partially lost, and lumina were filled with sloughed, necrotic epithelial cells as well as viable and degenerate neutrophils.
Discussion
In this study, we investigated whether HPAIV H5N1 infection can occur via the gastrointestinal tract in cats. We used cats as a mammalian infection model because fatal disease caused by natural HPAIV H5N1 infections has been reported in domestic cats, leopards, tigers, and other different species of wild cats in Asia 5 , 6 , 9 , 11 , 16 and Europe. 14 , 17 Infection experiments revealed that HPAIV H5N1 attaches in the lungs of infected cats predominantly to alpha 2·3 sialoglycan residues present at the surface of type II pneumocytes and alveolar macrophages in the lower respiratory tract, a pattern which most closely resembles that seen in human lung tissue. 18 Cats develop severe clinical signs after infection with HPAIV H5N1 intratracheally, 7 oculo‐nasopharyngeally, 10 and after feeding infected chicken carcasses. 7 The latter studies, however, did not allow to discriminate whether the virus gained entry through the gastrointestinal tract or through respiratory or oropharyngeal epithelia while feeding. Rimmelzwaan and collegues 8 investigated cats infected by feeding on infected carcasses and found virus‐associated ganglioneuritis in the submucosal and myenteric plexus of the small intestine, which might be suggestive of direct infection in these animals from the intestinal lumen. By immunohistochemistry, however, no evidence for virus replication could be found in the epithelium of the digestive tract (esophagus, stomach, duodenum, jejunum, ileum, cecum, and colon), despite the fact that this is an important site of influenza virus replication in birds. 19 The exact mode of infection through the gastrointestinal tract in cats, therefore, still remained to be elucidated.
In this study, HPAIV H5N1 was given orally in commercially available gelatine capsules that dissolve quickly after liquid contact. Capsules were filled with ground dry cat food. After the addition of 107 EID50 HPAIV H5N1, capsules were closed thereby avoiding contact of virus with outer surfaces of the capsules and applied directly to the base of the cats’ tongue to enable immediate swallowing without biting or chewing on the capsule. Capsules dissolved in the stomach, and therefore, the virus was not protected from low pH environment that most closely mimics natural oral exposure. The experiments showed that gastrointestinal exposure is sufficient for infection with HPAIV H5N1. The course of disease was indistinguishable from that seen after infection by either oculo‐nasopharyngeal or intravenous routes. All infected cats developed elevated body temperatures and showed anorexia, depression, and labored breathing within 2 days p.i. Depression seemed to be slightly more severe in the intravenously infected animals. All cats developed systemic infection with the presence of virus as measured by RT‐PCR in respiratory and outer respiratory tissues including colon, jejunum, liver, kidney, lung, and CNS similar to previous studies. 7 , 10
Hemocytological abnormalities described in tigers also include leukopenia and thrombocytopenia. 6 Thus, blood from the oculo‐nasopharyngeally infected cats was analyzed by flow cytometry, and a severe lymphopenia with CD4+ and CD8+ T‐cell depletion was observed.
Previous infection experiments in cats 7 , 10 showed multiple foci of acute necrosis throughout the liver, which was also seen in our experiment irrespective of the infection route. Increased liver enzyme activities have been found in diseased tigers, and preliminary measurements of liver enzyme activities in domestic cats also showed elevated levels. 6 , 14 We focussed also on liver enzymes (AST, ALT) but extended the investigations into a wider range of blood parameters including GGT, LDH, ALP, AMYL, BUN, CRE, UA, TP, ALB, NH3, CPK, and TBIL. Irrespective of the infection route, all inoculated cats developed systemic infection with significantly elevated levels of the enzymes AST, ALT, CPK, and TBIL. CPK is expressed by various tissues and cell types, and elevation in CPK is generally indicative of damage to muscle tissue. The reason for CPK elevation in the cats irrespective of the infection route is not clear. ALT blood tests are used to specifically detect liver damage. The extent of ALT elevation corresponds to the degree of hepatocyte membrane defects. ALT and AST are the two most important markers for liver injury. The significant elevation in TBIL is also indicative of severe liver damage as bilirubin is cleared from the blood by uptake into hepatocytes with subsequent intracellular conjugation before secretion into the bile. In conclusion, HPAIV H5N1 infection in cats causes severe liver damage, and therefore, lung and liver seem to be the main target organs of HPAIV H5N1 in cats.
The gastrointestinal tract can serve as portal of HPAIV H5N1 entry but the feline gastrointestinal tract does not seem to be a site of massive productive virus replication. This concept seems to be supported by the low or negative PCR values as well as the absence of intestinal lesions in the intestines compared to other organs. This might also explain that one gastrointestinal exposed cat (no. 262) did not excrete virus at the indicated times rectally yet got infected and showed virus excretion after the systemic spread in pharyngeal swabs. Similar to our current investigation that detected virus excretion in rectal swabs only inconsistently, also previous studies 8 , 10 demonstrated that virus excretion is predominantly seen in pharyngeal swab samples.
According to previous immunohistochemical investigations, 8 the site of virus uptake or virus replication in the gastrointestinal tract seems difficult to detect. With regard to virus dissemination in the organism, the virus seems to be spread by cell‐free and cell‐associated viremia. Besides positive results in plasma samples, RT‐PCR amplification was obtained also with purified total PBMC as well as CD4+ and CD8+ T cells. We also performed PCR assays that enable the detection of spliced influenza gene segments but did not obtain indications for active influenza virus replication in PBMC or T‐cell subpopulations.
Similar to the disease course in cats, humans infected with HPAIV H5N1 also present with fever, respiratory symptoms, leukopenia, specially lymphopenia, and elevated amino‐transaminases ALT and AST. 20 , 21 , 22 , 23 , 24 There is often not enough epidemiological evidence to identify the source of the infection. The gastrointestinal tract as a portal for HPAIV H5N1 infection cannot be excluded in cases with no record of exposure to sick poultry or sick humans, fatal disease after consumption of raw poultry products or in patients with encephalitis and diarrhea in the absence of respiratory tract disease. 24 , 25 Gastrointestinal manifestations with an elevation in liver enzyme activities and pancytopenia were reported to be unusually prominent in HPAIV H5N1‐infected patients. 20 , 21 , 24 Also, a high proportion of H5N1‐infected patients show severe and watery diarrhea, vomiting and abdominal pain, symptoms that were reported to be diagnosed commonly early in the course of the disease 23 and in some patients before the onset of respiratory signs. 20 Transmission of HPAIV H5N1 by infected feces should be considered in humans. Reports documented diarrhea in infected humans together with the detection of viral RNA in intestines and the presence of virus in rectal swabs. 12 , 25 However, no direct evidence that the gastrointestinal tract can serve as portal of entry or as target organ has been obtained. Despite the lack of direct proof for oral transmission in humans, there is substantial evidence that HPAIV H5N1 infections in mammals can occur through the gastrointestinal tract and that poor preparation and cooking of food cannot be excluded as the cause of infection. 13 Our results in cats support the notion that HPAIV H5N1 can remain infectious in the low pH environment of the stomach and cause systemic infection by the gastrointestinal route of infection.
Acknowledgement
We thank M. Giese for his thorough assay performance and detailed collection of the data, A. Carnitz and K. Wink‐Kruschke for their excellent technical help and all animal caretakers at the Friedrich‐Loeffler‐Institut for their diligent care of the animals.
This study has been supported by the Federal Ministry of Food, Agriculture and Consumer Protection (BMELV), Germany, under the Influenza Research Program FSI and by the EU Network of Excellence, EPIZONE (Contract No FOOD‐CT‐2006‐016236).
References
- 1. Thiry E, Zicola A, Addie D et al. Highly pathogenic avian influenza H5N1 virus in cats and other carnivores. Vet Microbiol 2007; 122:25–31. [DOI] [PubMed] [Google Scholar]
- 2. Vahlenkamp TW, Harder TC. Influenza virus infections in cats and dogs. Vet Immunol Immunopathol 2010; 134:54–60. [DOI] [PubMed] [Google Scholar]
- 3. Kuiken T, Fouchier R, Rimmelzwaan G, Osterhaus A, Roeder P. Feline friend or potential foe? Nature 2006; 440:741–742. [DOI] [PubMed] [Google Scholar]
- 4. Songserm T, Amonsin A, Jam‐on R et al. Fatal avian influenza A H5N1 in a dog. Emerg Infect Dis 2006; 12:1744–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Keawcharoen J, Oraveerakul K, Kuiken T et al. Avian influenza H5N1 in tigers and leopards. Emerg Infect Dis 2004; 10:2189–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thanawongnuwech R, Amonsin A, Tantilertcharoen R et al. Probable tiger‐to‐tiger transmission of avian influenza H5N1. Emerg Infect Dis 2005; 11:699–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kuiken T, Rimmelzwaan G, Van Riel D et al. Avian H5N1 influenza in cats. Science 2004; 306:241. [DOI] [PubMed] [Google Scholar]
- 8. Rimmelzwaan GF, Van Riel D, Baars M et al. Influenza A virus (H5N1) infection in cats causes systemic disease with potential novel routes of virus spread within and between hosts. Am J Pathol 2006; 168:176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Songserm T, Amonsin A, Jam‐on R et al. Avian Influenza H5N1 in Naturally Infected Domestic Cat. Emerg Infect Dis 2006; 12:681–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vahlenkamp TW, Harder TC, Giese M et al. Protection of cats against lethal influenza H5N1 challenge infection. J Gen Virol 2008; 89:968–974. [DOI] [PubMed] [Google Scholar]
- 11. Yingst SL, Saad MD, Felt SA. Qinghai‐like H5N1 from domestic cats, northern Iraq. Emerg Infect Dis 2006; 12:1295–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Buchy P, Mardy S, Vong S et al. Influenza A/H5N1 virus infection in humans in Cambodia. J Clin Virol 2007; 39:164–168. [DOI] [PubMed] [Google Scholar]
- 13. Butler D. Bird‐flu experts question advice on eating poultry. Nature 2006; 440:850–851. [DOI] [PubMed] [Google Scholar]
- 14. Klopfleisch R, Wolf PU, Uhl W et al. Distribution of lesions and antigen of highly pathogenic avian influenza virus (H5N1/Germany/Swan/2006) in domestic cats after natural infection. Vet Pathol 2007; 44:261–268. [DOI] [PubMed] [Google Scholar]
- 15. Weber S, Harder T, Starick E et al. Molecular analysis of highly pathogenic avian influenza virus of subtype H5N1 isolated from wild birds and mammals in northern Germany. J Gen Virol 2007; 88:554–558. [DOI] [PubMed] [Google Scholar]
- 16. Desvaux S, Marx N, Ong S et al. Highly pathogenic avian influenza virus (H5N1) outbreak in captive wild birds and cats, Cambodia. Emerg Infect Dis 2009; 15(3):475–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leschnik M, Weikel J, Möstl K et al. Subclinical Infection with Avian Influenza A (H5N1) Virus in Cats. Emerg Infect Dis 2007; 13:243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Riel D, Munster VJ, De Wit E et al. H5N1 Virus Attachment to Lower Respiratory Tract. Science 2006; 312:399. [DOI] [PubMed] [Google Scholar]
- 19. Alexander DJ. Orthomyxovirus infection; in McFerran JB, McNulty MS. (eds): Virus Infections of Birds. Amsterdam: Elsevier Science Publishers, 1993; 287–316. [Google Scholar]
- 20. Yuen KY, Chan PK, Peiris M et al. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet 1998; 351:467–471. [DOI] [PubMed] [Google Scholar]
- 21. Tran TH, Nguyen TL, Nguyen TD et al. Avian influenza A (H5N1) in 10 patients in Vietnam. N Engl J Med 2004; 350:1179–1188. [DOI] [PubMed] [Google Scholar]
- 22. Chotpitayasunondh T, Ungchusak K, Hanshaoworakul W et al. Human disease from influenza A (H5N1), Thailand, 2004. Emerg Infect Dis 2005; 11:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hui DS. Review of clinical symptoms and spectrum in humans with influenza A/H5N1 infection. Respirology 2008; 13(Suppl 1):S10–S13. [DOI] [PubMed] [Google Scholar]
- 24. Liem NT, Tung CV, Hien ND et al. Clinical features of human influenza A (H5N1) infection in Vietnam: 2004‐2006. Clin Infect Dis 2009; 48:1639–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Jong MD, Bach VC, Phan TQ et al. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N Engl J Med 2005; 352:686–691. [DOI] [PubMed] [Google Scholar]


