Abstract
Please cite this paper as: Gildea et al. (2010) A comparative antibody study of the potential susceptibility of Thoroughbred and non‐Thoroughbred horse populations in Ireland to equine influenza virus. Influenza and Other Respiratory Viruses 4(6), 363–372.
Background In Ireland, horses may be protected against equine influenza virus (EIV) as a result of natural exposure or vaccination. Current mandatory vaccination programmes are targeted at highly mobile horses. A correlation between antibody levels as measured by single radial haemolysis (SRH) and protective immunity against EIV has been established.
Objectives The objective of this study was to determine the susceptibility of selected populations of horses by quantifying their antibodies to EIV.
Methods Blood samples were collected from Thoroughbred weanlings, yearlings, racehorses and broodmares, teaser stallions and non‐Thoroughbred horses. Antibodies against EIV H3N8 and H7N7 were measured by SRH.
Results The order of susceptibility to Equine Influenza (EI) in the populations examined in Ireland was as follows: Thoroughbred weanlings > teasers > non‐Thoroughbred horses and ponies > Thoroughbred yearlings > Thoroughbred horses in training > Thoroughbred broodmares. The H3N8 antibody levels of the weanlings, yearlings, broodmares and horses in training were similar to their H7N7 antibody levels, suggesting that their antibodies were primarily vaccinal in origin. The teasers and non‐Thoroughbreds had higher H3N8 antibody levels than H7N7 antibody levels, suggesting that the majority of seropositive horses in these populations had been exposed to H3N8 by natural infection.
Conclusions Weanlings, teasers and non‐Thoroughbred horses were identified as most susceptible to EIV. The results suggest that it would be advisable that weanlings are vaccinated prior to attendance at public sales, that teaser stallions are vaccinated prior to each breeding season and that mandatory vaccination be implemented for participation in non‐Thoroughbred events.
Keywords: Equine, influenza, Ireland, susceptibility
Introduction
Equine influenza virus (EIV) of the H3N8 subtype has long been regarded as the most important respiratory pathogen of horses because of its highly contagious nature and rapid spread among susceptible animals. 1 The introduction of EIV to immunologically naïve populations has resulted in substantial financial losses because of restriction of movement and the cancellation of race meetings and other equestrian events. In South Africa in 1986 2 and in Hong Kong in 1992 3 , racing was suspended for 5 and 1 month respectively, following an incursion of EIV. The introduction of EIV into Australia for the first time in 2007 resulted in the infection of over 76 000 horses on over 10 000 premises in New South Wales and Queensland, costing a reported one billion Australian dollars. 4 At present, the only countries that have never experienced an incursion of EIV are New Zealand and Iceland. The virus is endemic in Europe and America where outbreaks of disease result in financial loss because of disruption of training schedules.
Large outbreaks of equine influenza (EI) are often associated with the congregation of horses at equestrian events and their subsequent dispersal over a wide geographical area. 5 In endemic countries, the economic losses owing to EI can be minimised by targeted vaccination of highly mobile horses. In Ireland, the Turf Club implements a mandatory vaccination programme for racehorses and vaccination is required for yearlings and horses in training, prior to entry to the major Thoroughbred sales. All horses participating in Federation Equestre Internationale (FEI) competitions must be vaccinated, and several horse and pony societies have a mandatory vaccination policy. The overall aim of such vaccination policies is to ensure sufficient herd immunity to protect equestrian events rather than individual horses. Mandatory vaccination of racehorses and competition horses was introduced in Ireland in 1981. Despite sporadic outbreaks and one countrywide epidemic in 1989, this policy has been successful as no major equestrian event has been cancelled because of EI for almost three decades. This is similar to the situation in the United Kingdom. 6
In Ireland, horses may be protected against EI as a result of exposure to virus by natural infection or by vaccination. A definite correlation between antibody levels against the virus haemagglutinin protein as measured by the single radial haemolysis (SRH) test and protective immunity against EI has been established in both experimental challenge studies and in the field. 7 , 8 , 9 , 10 , 11 Published data suggest that horses with antibody levels of 85 mm2 or greater are clinically protected against EIV and that those with antibody levels of 150 mm2 or greater are virologically protected and do not shed virus after challenge. 8 , 10 , 11 Horses with antibody levels of less than 50 mm2 are 15 times more likely to be the index case within a yard and represent a significant risk to other horses. 12
There are five EI vaccines; two inactivated whole virus vaccines, two subunit vaccines and a canary pox recombinant vaccine available in Ireland. The kinetics of the antibody response to these vaccines is similar (Gildea et al., in preparation). The cold‐adapted, modified live EI vaccine that is available in the United States and does not induce antibodies correlating with protection 13 , 14 is not available in Ireland. All five vaccines contain H3N8 viruses from the American and Eurasian lineages. The inactivated and subunit vaccines that are administered to the majority of vaccinated horses in Ireland contain a representative H7N7 virus that is not included in the canary pox vaccine. 15 Although H7N7 viruses cocirculated with H3N8 viruses in horses for many years, these viruses have not been isolated for over two decades and are considered to be extinct. 16 The World Organisation for Animal Health or OIE (Office International des Epizooties) stipulates that there is no requirement for inclusion of an H7N7 virus in equine influenza vaccines. 17 However, antibodies against this virus are a useful aid in the differentiation of vaccinated horses from naturally infected horses.
The objective of this study was to determine the susceptibility of selected populations of horses in Ireland by quantifying their antibodies to EIV H3N8. It was not possible to differentiate between H3N8 antibodies because of natural exposure and vaccination, but the measurement of antibodies against H7N7 provided information on the populations vaccinated with one of the four commercially available vaccines containing a representative of that subtype.
Materials and methods
Samples and horse populations
Whole blood samples (5–10 ml) were collected for analysis by SRH from the populations listed in the following paragraphs. The vaccination history of the horses included in this study was unknown. No diagnosis of EIV was made on any of the selected premises where samples were collected, and there was no overlap between premises or animals in any of the groups listed.
Thoroughbred weanlings
Two hundred blood samples were collected from five weanlings on 40 premises located in over 10 of the 32 counties in Ireland.
Thoroughbred yearlings
Two hundred and fifteen blood samples were collected from five yearlings on 43 premises in 10 counties.
Thoroughbred broodmares
Two hundred and five blood samples were collected from five broodmares on 41 premises in 10 counties. Analysis of 131 broodmares in this population revealed a minimum and maximum age of 4 and 25 years respectively and a mean age of 9·44 ± 0·364 SE years.
Teaser stallions
Blood samples were collected from 70 teaser stallions in 10 counties. These horses were of varying age but all sexually mature (greater than 2 years of age) as teaser stallions are used to determine whether mares are in oestrus.
Thoroughbred racehorses
Blood samples were collected from 233 Flat and National Hunt (Jump) horses in 16 counties. Analysis of 182 horses in this population revealed a minimum and maximum age of 2 and 9 years respectively and a mean age of 3·61 ± 0·111 SE years.
Non‐thoroughbred
Two hundred and thirty‐six samples were collected from non‐Thoroughbred horses and ponies of varying age, gender and discipline in 10 different counties in Ireland.
Serology
Antibodies against A/eq/Newmarket/2/93 (H3N8) a representative of the Eurasian lineage, A/eq/Kildare/92 (H3N8) a representative of the American lineage, A/eq/South Africa/4/03 (H3N8) a representative of the Florida sublineage of the American lineage and A/eq/1/Prague/56, the prototype H7N7 virus, were measured using the SRH test according to standard procedures. 18 In brief, optimised viral antigens were coupled to sheep red blood cells (SRBC) with chromium chloride. Agarose plates (1% wt/vol) were prepared containing the sensitised SRBC’s and guinea pig complement. The test sera were heat inactivated at 56 ± 1°C for 30 minutes, 10 μl aliquoted into wells on the plate and incubated at 34 ± 1°C for 20–24 hours.
Control antisera against A/eq/Newmarket/77 (H7N7), A/eq/Newmarket/2/93 (H3N8), A/eq/Newmarket/1/93 (H3N8) and A/eq/South Africa/4/03 (H3N8) from the European Directorate for the Quality of Medicines and Healthcare (EDQM) were included on each plate as appropriate. The haemolytic zones resulting from the lysis of the antigen‐coated SRBCs by the antibody in the test sera were measured with a viewer and digital recording apparatus (Mitutoya, Aurora, Illinois, USA). The area of haemolysis was calculated and results were expressed in millimetre square. The results were interpreted in accordance with published data; horses with antibody levels of 150 mm2 or greater were classified as virologically protected, horses with antibody levels less than 150 mm2 but greater than 85 mm2 were classified as clinically protected, horses with antibody levels less than 85 mm2 but greater than 50 mm2 were classified as partially protected and horses with antibody levels less than 50 mm2 were classified as potential index cases. 11 , 12
Statistical analysis
spss version 16.0 for Windows (Chicago, Illinois, USA) was used to analyse the data. Mean H3N8 antibody values were calculated from SRH results obtained against the subtype 2 antigens A/eq/Kildare/92, A/eq/Newmarket/2/93 and A/eq/South Africa/4/03. ANOVA, post hoc Bonferroni and independent T tests were carried out where appropriate to compare mean antibody levels in the equine populations included in this study. The correlation between H7N7 and H3N8 antibodies in horses that were seropositive to H7N7 was examined using Pearson’s correlation test. The H3N8 antibody levels of horses that were seropositive for both H3N8 and H7N7 were compared with those of horses that were seronegative for H7N7 by a one sample T test. A significance level of P < 0·05 was used for all statistical tests.
Results
Descriptive statistics of equine influenza H3N8 and H7N7 antibody results obtained from the six populations examined are displayed in Table 1 and the significant difference between the groups is illustrated in 1, 2. The weanlings had similar mean H3N8 and H7N7 antibody levels. This was also true for the yearlings, broodmares and horses in training. The teasers and non‐Thoroughbreds had significantly higher H3N8 antibody levels than H7N7 antibody levels (P < 0·01).
Table 1.
Descriptive statistics of H3N8 and H7N7 antibody results
| H3N8 SRH (mm2) | H7N7 SRH (mm2) | ||
|---|---|---|---|
| Weanling | Mean | 14·3 ± 2·61 SE | 12·8 ± 2·77 SE |
| Median | 0·0 | 0·0 | |
| Std. Deviation | 36·88 | 39·21 | |
| Minimum | 0·0 | 0·0 | |
| Maximum | 218·4 | 182·2 | |
| Yearling | Mean | 94·4 ± 5·34 SE | 97·5 ± 6·19 SE |
| Median | 91·8 | 102·5 | |
| Std. Deviation | 78·31 | 90·80 | |
| Minimum | 0·0 | 0·0 | |
| Maximum | 278·7 | 305·2 | |
| Broodmare | Mean | 143·2 ± 3·64 SE | 176·3 ± 4·27 SE |
| Median | 146·1 | 178·5 | |
| Std. Deviation | 52·13 | 61·14 | |
| Minimum | 0·0 | 0·0 | |
| Maximum | 276·6 | 285·3 | |
| Teaser | Mean | 65·7 ± 6·80 SE | 30·9 ± 7·80 SE |
| Median | 63·7 | 0·0 | |
| Std. Deviation | 56·90 | 65·24 | |
| Minimum | 0·0 | 0·0 | |
| Maximum | 240·2 | 222·1 | |
| Horses in training | Mean | 121·8 ± 3·30 SE | 143·3 ± 4·54 SE |
| Median | 123·5 | 154·9 | |
| Std. Deviation | 49·35 | 67·82 | |
| Minimum | 0·0 | 0·0 | |
| Maximum | 283·3 | 268·6 | |
| Non‐thoroughbred | Mean | 84·3 ± 4·38 SE | 30·2 ± 4·13 SE |
| Median | 87·7 | 0·0 | |
| Std. Deviation | 67·33 | 63·40 | |
| Minimum | 0·0 | 0·0 | |
| Maximum | 249·9 | 246·9 | |
SRH, single radial haemolysis.
Figure 1.
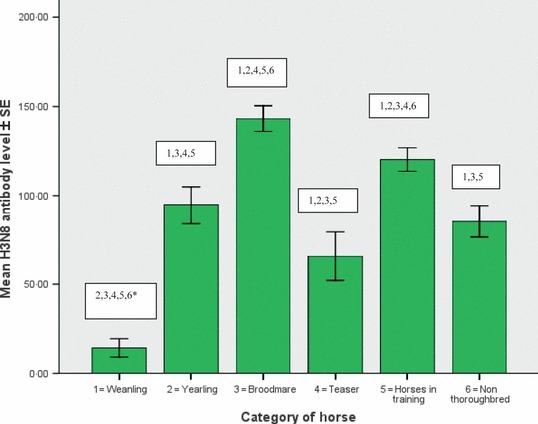
Mean H3N8 antibody level of weanlings, yearlings, broodmares, teasers, horses in training and non‐Thoroughbreds. Comparison of mean antibody levels for example, 2, 3, 4, 5, 6* = mean H3N8 antibody level of weanlings is significantly different from that of the yearlings, broodmares, teasers, horses in training and non‐Thoroughbreds (P < 0·01).
Figure 2.
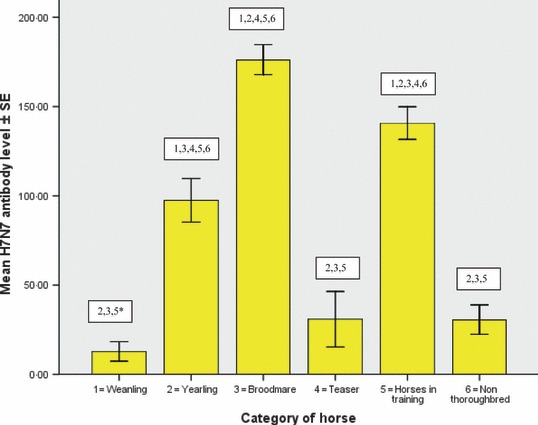
Mean H7N7 antibody level of weanlings, yearlings, broodmares, teasers, horses in training and non‐Thoroughbreds. Comparison of mean antibody levels for example, 2, 3, 5* = mean H7N7 antibody level of weanlings is significantly different from that of the yearlings, broodmares and horses in training (P < 0·01).
There was a significant difference in mean H3N8 antibody levels between all of the populations except in the case of yearlings, non‐Thoroughbreds and teasers. There was a significant difference in mean H7N7 antibody levels between all of the populations except in the case of weanlings, non‐Thoroughbreds and teasers. 2, 3 summarise the number of horses in each population with different levels of antibody (<50, 50–85, 85–150 and >150 mm2) and Table 4 summarise the distribution (number of premises) of weanlings, yearlings and brood mares with different levels of antibodies.
Table 2.
Equine influenza H3N8 single radial haemolysis (SRH) levels in selected populations
| SRH | Weanling | Yearling | Broodmare | Teaser | Horses in training | Non‐TB | Total |
|---|---|---|---|---|---|---|---|
| <50 mm2 | 183 (91·5%) | 74 (34·4%) | 7 (3·4%) | 28 (40·0%) | 18 (8·1%) | 77 (32·6%) | 387 (33·7%) |
| >50 mm2 | 4 | 26 | 20 | 14 | 20 | 39 | 123 |
| <85 mm2 | (2·0%) | (12·1%) | (9·8%) | (20·0%) | (9·0%) | (16·5%) | (10·7%) |
| >85 mm2 | 8 | 58 | 82 | 24 | 123 | 78 | 373 |
| <150 mm2 | (4·0%) | (27·0%) | (40·0%) | (34·3%) | (55·2%) | (33·1%) | (32·5%) |
| >150 mm2 | 5 | 57 | 96 | 4 | 62 | 42 | 266 |
| (2·5%) | (26·5%) | (46·8%) | (5·7%) | (27·8%) | (17·8%) | (23·2%) | |
| Total | 200 | 215 | 205 | 70 | 223 | 236 | 1149 |
Table 3.
Equine influenza H7N7 single radial haemolysis (SRH) levels in selected populations
| SRH | Weanling | Yearling | Broodmare | Teaser | Horses in training | Non‐TB | Total |
|---|---|---|---|---|---|---|---|
| <50 mm2 | 177 (88·5%) | 86 (40·0%) | 8 (3·9%) | 56 (80·0%) | 30 (13·5%) | 186 (78·8%) | 543 (47·3%) |
| >50 mm2 | 11 | 13 | 5 | 1 | 3 | 10 | 43 |
| <85 mm2 | (5·5%) | (6·0%) | (2·4%) | (1·4%) | (1·3%) | (4·2%) | (3·7%) |
| >85 mm2 | 4 | 45 | 55 | 5 | 70 | 18 | 197 |
| <150 mm2 | (2·0%) | (20·9%) | (26·8%) | (7·1%) | (31·4%) | (7·6%) | (17·1%) |
| >150 mm2 | 8 | 71 | 137 | 8 | 120 | 22 | 366 |
| (4·0%) | (33·0%) | (66·8%) | (11·4%) | (53·8%) | (9·3%) | (31·9%) | |
| Total | 200 | 215 | 205 | 70 | 223 | 236 | 1149 |
Table 4.
Equine influenza H3N8 and H7N7 single radial haemolysis (SRH) levels on weanling, yearling and broodmare premises
| SRH | Weanling premises n = 40 | Yearling premises n = 43 | Broodmare premises n = 41 | |||
|---|---|---|---|---|---|---|
| H3N8 | H7N7 | H3N8 | H7N7 | H3N8 | H7N7 | |
| (a) ≥1 of 5 horses tested on each premises | ||||||
| <50 mm2 | 40 (100·0%) | 39 (97·5%) | 29 (67·4%) | 29 (67·4%) | 6 (14·6%) | 7 (17·1%) |
| >50 mm2 | 8 (20·0%) | 14 (35·0%) | 38 (88·4%) | 32 (74·4%) | 41 (100·0%) | 41 (100·0%) |
| >85 mm2 | 7 (17·5%) | 7 (17·5%) | 36 (83·7%) | 31 (72·1%) | 41 (100·0%) | 41 (100·0%) |
| >150 mm2 | 3 (7·5%) | 6 (15·0%) | 24 (55·8%) | 27 (62·8%) | 38 (92·7%) | 41 (100·0%) |
| (b) all horses (5) tested on each premises | ||||||
| <50 mm2 | 31 (77·5%) | 26 (65·0%) | 5 (11·6%) | 11 (25·6%) | 0 (0·0%) | 0 (0·0%) |
| >50 mm2 | 0 (0·0%) | 0 (0·0%) | 14 (32·6%) | 14 (32·6%) | 35 (85·4%) | 34 (82·9%) |
| >85 mm2 | 0 (0·0%) | 0 (0·0%) | 10 (23·3%) | 10 (23·3%) | 23 (56·1%) | 29 (70·7%) |
| >150 mm2 | 0 (0·0%) | 0 (0·0%) | 2 (4·7%) | 3 (7·0%) | 2 (4·9%) | 8 (19·5%) |
The H3N8 antibody levels of horses that were seropositive to both subtypes correlated significantly with their H7N7 antibody levels (P < 0·01). The H3N8 antibody levels of horses that were seropositive for both H3N8 and H7N7 were significantly higher than the H3N8 antibody levels of horses that were seronegative for H7N7 (P < 0·01).
Levels of H3N8 antibodies in the weanling, yearling, broodmare, teaser, horses in training and non‐Thoroughbred populations at the time of sampling are summarised in 2, 4.
TB weanlings
One hundred and eighty‐three of the 200 weanlings had an H3N8 antibody level of <50 mm2. On 31 (77·5%) of the 40 premises, all five weanlings had an H3N8 antibody measurement of <50 mm2; thus, all of the horses tested on these premises were potential index cases. On 15 of the 31 premises, all five weanlings were seronegative for H3N8. On 13 of these premises, the weanlings tested were also seronegative for H7N7.
Only five (2·5%) of the 200 weanlings were virologically protected against H3N8, and these five weanlings were located on four different premises. Eight (4·0%) weanlings were clinically protected and four (2·0%) were partially protected against H3N8. Antibodies to the H7N7 virus were recorded in 23 (11·5%) weanlings on 14 premises. Ten of the 17 weanlings that had H3N8 antibody levels of >50 mm2 also had H7N7 antibody levels of >50 mm2.
TB yearlings
Over one‐third of the yearlings, i.e. 74 (34·4%), had index case potential against H3N8. In five of the 43 premises, all five yearlings had an antibody measurement of <50 mm2, and in two of these premises, all five yearlings were seronegative. Over 50% of the 215 yearlings tested had either virological or clinical protection against H3N8. Antibody levels indicative of virological protection were detected in 57 (26·5%) of the 215 yearlings in 24 (55·8%) of the 43 premises. Fifty‐eight (27·0%) of the yearlings on 29 of the premises were clinically protected. Of these 29 premises, 17 had yearlings that were both virologically and clinically protected. Twenty‐six (12·1%) of the 215 yearlings were partially protected.
Antibodies against the H7N7 virus were recorded in 131 of the 215 yearlings (60·9%) at the time of sampling. In 14 (32·6%) of the 43 premises, all five yearlings had measurable antibody levels to H7N7. In 11 (25·6%) of the 43 premises, four of the five yearlings had measurable antibody levels. In four (9·3%) premises, three of the five animals had measurable antibodies to H7N7, while in two (4·7%) premises two of the five animals sampled had measurable antibodies. In one single (2·3%) premise, only one of the five yearlings had detectable antibodies to H7N7. In 11 (25·6%) of the 43 premises, all five yearlings sampled were seronegative for H7N7. On one of these premises, all five yearlings were either virologically or clinically protected against H3N8.
TB broodmares
Only seven (3·4%) of the 205 broodmares sampled had index case potential, and five of these were seronegative for H3N8. There was no premises sampled during the course of this study where all five broodmares were potential index cases (Table 4b). The majority of the broodmares were either virologically or clinically protected. H3N8 antibody levels indicative of virological protection were detected in 96 (46·8%) of the 205 broodmares in 38 of the 41 premises. Eighty‐two (40·0%) of the 205 broodmares were clinically protected, while 20 (9·8%) of the mares were partially protected against the virus.
Antibodies to the H7N7 virus were recorded in 197 (96·1%) of the 205 broodmares at the time of sampling. Of the eight broodmares that did not have measurable antibodies to H7N7, two had clinical protection against H3N8, while four of the eight were partially protected against the H3N8 virus. The remaining two mares that were seronegative to H7N7 did not have measurable antibodies to EIV H3N8.
Teaser stallion population
Twenty‐eight (40%) of the 70 animals sampled in this category had index case potential. Twenty‐one (30%) of these were seronegative. Only four (5·7%) animals in this group were virologically protected, 24 (34·3%) were clinically protected and 14 (20%) had partial protection.
Fifty‐six (80%) teasers were seronegative for H7N7 of which two horses had virological protection against H3N8. Thirteen of these horses had clinical protection against H3N8, while 13 were partially protected against the virus. Of the remaining 28 horses that were seronegative to H7N7, 21 were also seronegative for H3N8, and the remainder had index case potential.
Thoroughbred horses in training
Eighteen (8·1%) of the 223 horses in training had index case potential, and six of these were seronegative. Sixty‐two horses (27·8%) were virologically protected, 123 (55·2%) were clinically protected and 20 (9·0%) were partially protected.
Antibodies to the H7N7 virus were recorded in 196 (87·9%) of the 223 horses. Of the 27 horses that did not have measurable antibodies to H7N7, one horse had virological protection against H3N8, 11 horses had clinical protection and five horses were partially protected. Of the remaining 10 horses that were seronegative to H7N7, five were also seronegative to H3N8, and the remainder had index case potential.
Non‐thoroughbred population
Seventy‐seven (32·6%) of the 236 horses included in this section had index case potential and 66 of these were seronegative. Forty‐two (17·8%) had virological protection, 78 (33·1%) were clinically protected and 39 (16·5%) were partially protected.
Antibodies to EIV H7N7 were detected in 51 (21·6%) of the 236 horses. Of the 185 (78·4%) horses that did not have measurable antibodies to H7N7, 15 horses had virological protection against H3N8, 57 horses had clinical protection and 37 horses were partially protected. Of the remaining 76 horses that were seronegative to H7N7 67 were seronegative for H3N8 and the remainder had index case potential.
Discussion
The horse industry in Ireland is broadly subdivided into the Thoroughbred or racing, and the non‐Thoroughbred sports and pleasure horse sectors. The precise number of horses is unknown; however in 2008, 12 419 Thoroughbred foals were registered as born in Ireland, and at that time there were approximately the same number of registered Thoroughbred yearlings. 19 There were 20 038 registered Thoroughbred broodmares, and over 12 000 horses returned in training. 19 In addition, it was estimated that there were over 110 000 non‐thoroughbred horses. 20 Horses from each of these categories were included in this study to determine the sectors of the industry that are most at risk from influenza. Ireland has an active EI surveillance programme (funded by the Department of Agriculture) that serves as an early warning system for the entire industry. The highly contagious nature of the virus and the level of susceptibility of the horse population contribute to the dissemination of virus and the spread of disease. It is estimated that one infected horse is capable on average of infecting 10 susceptible, in‐contact horses. 21 The identification of susceptible populations facilitates targeted vaccination to reduce the impact of an influenza outbreak and disruption of equestrian events.
Results obtained in a study carried out by Davies and Grilli 22 in the U.K., which examined the susceptibility of humans to influenza virus, indicated that the infection rate for those with no detectable antibodies against the virus was 80%. In the equine population, horses with SRH antibody levels of <50 mm2 are at greatest risk from influenza virus. 12 Data from experimental challenge studies and observations in the field suggest that horses with SRH antibody levels of >85 mm2 are likely to be protected against clinical disease and that those with levels of 150 mm2 or greater are likely to be protected against infection and virus shedding. 8 , 10 , 11 Thus, in this study, SRH antibodies against H3N8 were considered as a correlate of protection. Antibodies against H7N7 were considered as indicative of vaccination as this subtype is believed to be extinct 16 but is contained in four of the five vaccines on the Irish market. To our knowledge, this is the first study to compare SRH antibody levels of these different equine populations within a country although young Thoroughbred racehorses have been investigated in Newmarket. 23
Results of this study indicate that the order of susceptibility to EI in the populations examined in Ireland was as follows: Thoroughbred weanlings > teasers > non‐Thoroughbred horses and ponies > Thoroughbred yearlings > Thoroughbred horses in training > Thoroughbred broodmares, with the Thoroughbred weanlings being the most susceptible and the Thoroughbred broodmares being the least susceptible to EIV infection. The fact that the H3N8 antibody levels of the weanlings, yearlings, broodmares and horses in training were similar to their H7N7 antibody levels suggests that their antibodies were primarily vaccinal in origin. This is consistent with the mandatory vaccination programme for racehorses implemented by the Turf Club and the vaccination requirements for some Thoroughbred sales. The teasers and non‐Thoroughbreds had significantly higher H3N8 antibody levels than H7N7 antibody levels, suggesting that the majority of the horses that were seropositive in these populations had been exposed to H3N8 by natural infection. The H3N8 antibody levels of horses in the study that were seropositive to both subtypes correlated significantly with their H7N7 antibody levels, i.e. there was no evidence of increased antibody response to H3N8 as a result of natural infection in vaccinated horses.
It is estimated that the weanlings included in this study were 6–10 months of age. Maternal antibodies against EI usually decline within six months. 24 , 25 , 26 Vaccination of foals before the maternal antibodies have waned may be of benefit if using a canary pox recombinant vaccine 27 but is not generally recommended if using conventional inactivated or subunit vaccines. 25 , 26 , 28 In Ireland, most breeders do not commence vaccination until the foals are more than eight months of age. Over 70% and 88% of weanlings in this study were seronegative for H3N8 and H7N7, respectively, and on 13 premises, all of the weanlings tested were seronegative for both subtypes. This suggests that the majority of the weanlings had no detectable maternal antibodies and had not commenced their vaccination programme. It was not possible to determine whether some of the low levels of antibody detected against H3N8 and H7N7 were residual maternal antibodies or a response to first vaccination.
Over 65% of the yearlings in this study had SRH antibody levels against H3N8 of >50 mm2. Their mean SRH antibody level was 94 mm2, which was higher than that recorded by Newton et al. 23 in 222 yearlings entering training in Newmarket (64 mm2). It is recommended that EI vaccination commences after maternal antibodies have waned 26 and the sales companies in Ireland require that yearlings entering the autumn sales have up to date vaccination records and have at least completed the primary course of two vaccinations. The SRH levels against H7N7 suggested that the majority (>60%) of the yearlings had been vaccinated with one of the four vaccines containing this subtype and on one of the 43 premises, the high H3N8 antibody levels and absence of H7N7 antibodies were consistent with vaccination with the canary pox recombinant vaccine or exposure by natural infection.
Although the yearlings in this study had a much lower index case potential than the weanlings (34% compared to 92%), over a third of them had low SRH levels, suggesting a high susceptibility to EI. Vaccination in accordance with Turf Club Rules and the vaccine manufacturer’s recommendations can frequently leave horses with low antibody titres for several months between their second and third vaccination. 10 , 26 Therefore, the time of year that the primary course is administered and the timing and frequency of the subsequent booster vaccination might explain why some vaccinated animals do not have protective antibody levels. The existence of poor responders to vaccination is also well recognised, and these horses play an important role in the amplification of virus and the spread of infection. 11 Vaccination records in the official passports of yearlings included in a study carried out by Newton et al., 23 indicated that 23% of them were not previously vaccinated. In this study, 54 of the 215 yearlings (25%) were seronegative to both H3N8 and H7N7, suggesting that they were either poor responders or had not been vaccinated. This included three premises where all the yearlings tested were seronegative.
The Turf Club requires that racehorses receive two primary vaccinations administered 21–92 days apart, followed by a third vaccination administered 150–215 days after the second dose and annual vaccination thereafter. In this study, the horses in training had higher antibody levels than the weanlings or the yearlings. The mean SRH level of 121 mm2 was similar to the level of 115 mm2 reported for 2 year olds in training in Newmarket. 23 Young horses are particularly susceptible to EI because of mixing at training facilities and racetracks and possibly the impact of training regimes on the immune system. 29 The 8% identified as having index case potential in this study, particularly the five seronegative horses represent a risk to their cohorts. Possible explanations for their poor immune status include that they responded poorly to vaccination or that they had only recently entered the training yard and had not been vaccinated.
It is recommended that to ensure a good supply of colostral antibodies for the foal, brood mares be vaccinated in the latter stages of pregnancy but not later than 2 weeks prior to foaling. 26 However, there are no mandatory requirements for vaccination of this population in Ireland. Broodmares were the best protected population investigated in this study with a mean SRH level of 143 mm2 and an index case potential of only 3·4%. Only two mares that were seronegative for H3N8 and H7N7 were identified in a population of 205. The majority of Thoroughbred broodmares would have been vaccinated for several years while in training and they may also have been exposed to virus by natural infection at some time.
The majority of teasers are pony stallions. Because of the nature of their work, they are exposed to a large number of broodmares during the breeding season. Forty per cent of the teasers included in this study had index case potential and 21 of 70 were seronegative for both H3N8 and H7N7. Only 4 were virologically protected against H3N8. The mean antibody level for H3N8 and H7N7 was 66 and 31 mm2, respectively. The results strongly suggest that the teaser population is inadequately vaccinated and could play a major role in transmission to susceptible broodmares during an outbreak.
The level of susceptibility observed in the non‐Thoroughbred population was similar to that observed in the Thoroughbred yearling population in that approximately half of the population was either virologically or clinically protected, while the other half was only partially protected or had index case potential. Horses competing under the auspices of the FEI require EI vaccination within 6 months +21 days of the competition and many equine associations and clubs require vaccination. Vaccination is recommended for all horses in Ireland, but there is no mandatory vaccination for the majority of pleasure horses. The mean H3N8 and H7N7 antibody levels for the non‐Thoroughbreds tested in this study were 84 and 30 mm2, respectively. Antibodies against H7N7, which are indicative of vaccination, were observed in only 21·6% of this population at the time of sampling. The results suggest many non‐Thoroughbreds have antibodies as a result of natural infection but that the overall immunological status could be significantly improved by regular vaccination.
The circumstances surrounding the induction of antibodies may influence their correlation to protection against EI and the only really satisfactory way to determine whether horses are protected is to conduct virus challenge experiments. Such experiments are not practical with horses retained for competition, breeding and pleasure riding. The nature of the immune response to natural infection differs from that elicited by vaccination and in our study the potential vulnerability of some of the horses in, for example, the non‐Thoroughbred sector may be over estimated. Resistance to re‐infection has been documented to exist for 1 year and to persist even after levels of antibody become barely detectable. 30 Similarly, the level of protective immunity of some of the vaccinated Thoroughbreds in this study could well prove to be over estimated if they were exposed to a virus strain that differed significantly from the vaccine strains. Higher SRH antibody levels are required to protect against a heterologous virus. 31 , 32 These caveats aside, the results obtained from this study provide useful information on the potential susceptibility of different populations to EI.
This study identified weanlings, teasers and non‐Thoroughbred horses as the populations most susceptible to EI on the basis of their SRH antibody levels. There is currently no mandatory EI vaccination for entry to weanling sales in Ireland. Although to date there is little epidemiological evidence to indicate that the weanling population is particularly susceptible to influenza, our results suggest that it would be advisable that weanlings have commenced their vaccination programme prior to attendance at public sales. Unvaccinated teaser stallions played a key role in the spread of EI on a public stud in Ireland in 2008 (Gildea et al., in preparation), and this study has identified the potential for this to occur on other farms. As a minimum, teaser stallions should be vaccinated against EI in accordance with the manufacturers’ instructions and preferably receive a booster before each breeding season. Outbreaks of EI in Ireland are usually diagnosed in the non‐Thoroughbred population and in horses in training (Gildea et al., in preparation). During the 1989 EI epidemic in Ireland, the disease was first identified in the non‐Thoroughbreds and then spilled over into the racehorse population. 5 Currently, vaccination against EI is not required for entry to many of the non‐Thoroughbred sales, fairs and local shows. The introduction of mandatory vaccination for participation in such events would reduce the risk of amplification of virus in this population and dissemination to other populations. Only through a combined approach of vaccination, meticulous surveillance and the evaluation of levels of immunological protection in our equine populations can we minimise disruption to training schedules and equestrian events.
Acknowledgement
All of the experimental work was funded by the Department of Agriculture under the National Development Plan and carried out at the Irish Equine Centre. The results will be submitted as part of a PhD thesis by Sarah Gildea to the University of Limerick.
References
- 1. Timoney PJ. Equine influenza. Comp Immunol Microbiol Infect Dis 1996; 19:205–211. [DOI] [PubMed] [Google Scholar]
- 2. Guthrie AJ, Stevens KB, Boaman PP. The circumstances surrounding the outbreak and spread of equine influenza in South Africa. Rev Sci Tech 1999; 18:179–185. [DOI] [PubMed] [Google Scholar]
- 3. Powell DG, Watkins KL, Li PH, Shortridge KF. Outbreak of equine influenza among horses in Hong Kong during 1992. Vet Rec 1995; 136:531–536. [DOI] [PubMed] [Google Scholar]
- 4. Garner MG, Cowled B, East IJ, Maloney BJ, Kung NY. Evaluating the effectiveness of early vaccination in the control and eradication of equine influenza – A modeling approach. Prev Vet Med 2010; doi:10.1016/j.prevetmed.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 5. Van Maanen C, Cullinane A. Equine influenza virus: an update. Vet Q 2002; 2:79–94. [DOI] [PubMed] [Google Scholar]
- 6. Newton JR, Daly JM, Spencer L, Mumford JA. Description of the outbreak of equine influenza (H3N8) in the United Kingdom in 2003, during which recently vaccinated horses in Newmarket developed respiratory disease. Vet Rec 2006; 158:185–192. [DOI] [PubMed] [Google Scholar]
- 7. Mumford JA, Wood J. Establishing an acceptable threshold for equine influenza vaccines. Develop Biol Standard 1992; 79:137–146. [PubMed] [Google Scholar]
- 8. Mumford JA, Wilson H, Hannant D, Jessett DM. Antigenicity and immunogenicity of equine influenza vaccines containing Carbomer adjuvant. Epidemiol Infect 1994; 112:421–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Townsend HGG, Morley PS, Newton JR, Wood JLN, Haines DM, Mumford JA. Measuring serum antibody as a method of predicting infection and disease in horses during outbreaks of influenza; in: Equine Infectious Diseases VIII: Proceedings of the Eight International Conference on Equine Infectious Diseases 1999; 33–37. [Google Scholar]
- 10. Newton JR, Townsend HGG, Wood JLN, Sinclair R, Hannant D, Mumford JA. Immunity to equine influenza: relationship of vaccine induced antibody in young thoroughbred racehorses to protection against field infection with influenza A/equine‐2 viruses (H3N8). Equine Vet J 2000; 32:65–74. [DOI] [PubMed] [Google Scholar]
- 11. Mumford JA. Biology, epidemiology and vaccinology of equine influenza. Proceeding of the International Symposium, Budapest, 10‐11 December 2001: 7–12.
- 12. Wood JLN. Equine Influenza: A Review of the History and Epidemiology and a Description of Recent Outbreak. MSc Dissertation, London School of Hygiene and Tropical Medicine, University of London, London, 1991. [Google Scholar]
- 13. Townsend HG, Penner SJ, Watts TC et al. Efficacy of a cold‐adapted, intranasal, equine influenza vaccine: challenge trials. Equine Vet J 2001; 33:637–643. [DOI] [PubMed] [Google Scholar]
- 14. Lunn PD, Hussey S, Sebring R et al. Safety, efficacy, and immunogenicity of a modified‐live equine influenza virus vaccine in ponies after induction of exercise‐induced immunosuppression. J Am Vet Med Assoc 2001; 218:900–906. [DOI] [PubMed] [Google Scholar]
- 15. Toulemonde EC, Daly J, Sindle T, Guigal PM, Audonnet JC, Minke JM. Efficacy of a recombinant equine influenza vaccine against challenge with American lineage H3N8 influenza virus responsible for the 2003 outbreak in the United Kingdom. Vet Rec 2005; 156:367–371. [DOI] [PubMed] [Google Scholar]
- 16. Webster RG. Are equine 1 influenza viruses still present in horses? Equine Vet J 1993; 25:537–538. [DOI] [PubMed] [Google Scholar]
- 17. OIE Bulletin . Expert surveillance panel of equine influenza vaccines – conclusions and recommendations; Mill Hill, London (United Kingdom) 18th January, 2008, (2), 42–45.
- 18. OIE 2008. Manual of standards for diagnostic tests and vaccines, equine influenza, Chapter 2.5.7; 875–877.
- 19. HRI , 2008. Horse racing Ireland fact book; Irish thoroughbred racing industry statistics. Year ended 31st December, 2008.
- 20. HSI , 2008. Horse sport Ireland. Profile of the Irish sports horse industry; 30th January 2008: 13.
- 21. Glass K, Wood LJ, Mumford JA, Jesset D, Grenfell BT. Modelling equine influenza 1: a stochastic model of within‐yard epidemics. Epidemiol Infect 2002; 128:491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Davies JR, Grilli EA. Natural or vaccine induced antibody as a predictor of immunity in the face of natural challenge with influenza virus. Epidemiol Infect 1989; 102:325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Newton JR, Lakhani KH, Wood JL, Baker DJ. Risk factors for equine influenza serum antibody titres in young thoroughbred racehorses given an inactivated vaccine. Prev Vet Med 2000; 46:129–141. [DOI] [PubMed] [Google Scholar]
- 24. Van Oirschot JT, Bruin G, Boer‐Luytze E, Smolders G. Maternal antibodies against equine influenza virus in foals and their interference with vaccination. J Vet Med 1991; 38:391–396. [DOI] [PubMed] [Google Scholar]
- 25. Van Maanen C, Bruin G, De Boer‐Luijtze E, Smolders G, De‐Boer GF. Interference of maternal antibodies with the immune response of foals after vaccination against equine influenza. Vet Q 1992; 14:13–17. [DOI] [PubMed] [Google Scholar]
- 26. Cullinane A, Weld J, Osborne M, Nelly M, McBride C. Field studies on equine influenza vaccination regimes in Thoroughbred foals and yearlings. Vet J 2001; 161:174–185. [DOI] [PubMed] [Google Scholar]
- 27. Minke JM, Toulemonde EC, Dinic S, Cozette V, Cullinane A, Audonnet JC. Effective priming of foals born to immune dams against influenza by Canarypox‐Vectored Recombinant Influenza H3N8 Vaccine. J Comp Path 2007; 137:S76–S80. [DOI] [PubMed] [Google Scholar]
- 28. Wilson WD, Mihalyi JE, Hussey S, Lunn DP. Passive transfer of maternal immunoglobulin isotype antibodies against tetanus and influenza and their effect on the response of foals to vaccination. Equine Vet J 2001; 33:644–650. [DOI] [PubMed] [Google Scholar]
- 29. Folsom RW, Littlefield‐Chabaud MA, French DD, Pourciau SS, Mistric L, Horohov DW. Exercise alters the immune response to equine influenza virus and increases susceptibility to infection. Equine Vet J 2001; 33:664–669. [DOI] [PubMed] [Google Scholar]
- 30. Hannant D, Mumford JA, Jesset DM. Duration of circulating antibody and immunity following infection with equine influenza virus. Vet Rec 1988; 122:125–128. [DOI] [PubMed] [Google Scholar]
- 31. Newton JR, Verheyen K, Wood JLN, Yates PJ, Mumford JA. Equine influenza in the United Kingdom in 1998. Vet Rec 1999; 145:449–452. [DOI] [PubMed] [Google Scholar]
- 32. Daly JM, Yates PJ, Newton RJ et al. Evidence supporting the inclusion of strains from each of the two co‐circulating lineages of H3N8 equine influenza viruses in vaccines. Vaccine 2004; 22:4101–4109. [DOI] [PubMed] [Google Scholar]


