Abstract
Natural killer (NK) cells, a cytotoxic lymphocyte lineage, are able to kill tumor cells in vitro and in mouse models. However, whether these cells display an anti-tumor activity in cancer patients has not been demonstrated. Here we have addressed this issue in patients with several hematological cancers. We found a population of highly activated CD56dimCD16+ NK cells that have recently degranulated, evidence of killing activity, and it is absent in healthy donors. A high percentage of these cells expressed natural killer cell p46-related protein (NKp46), natural-killer group 2, member D (NKG2D) and killer inhibitory receptors (KIRs) and a low percentage expressed NKG2A and CD94. They are also characterized by a high metabolic activity and active proliferation. Notably, we found that activated NK cells from hematological cancer patients have non-NK tumor cell antigens on their surface, evidence of trogocytosis during tumor cell killing. Finally, we found that these activated NK cells are distinguished by their CD45RA+RO+ phenotype, as opposed to non-activated cells in patients or in healthy donors displaying a CD45RA+RO− phenotype similar to naïve T cells. In summary, we show that CD45RA+RO+ cells, which resemble a unique NK population, have recognized tumor cells and degranulate in patients with hematological neoplasias.
Keywords: CD45, Trogocytosis, NK cell, Cytotoxicity, Hematological cancer
Highlights
-
•
Expression of both CD45 isoforms RA and RO identifies anti-leukemia NK cells.
-
•
Anti-leukemia NK cells proliferate, degranulate and perform trogocytosis in vivo.
-
•
The presence of CD45RARO population identifies hematological cancer patients.
NK cells are thought to have an intrinsic anti-tumor activity. However, the presence of anti-leukemia NK cells in patients is unknown. We present a relatively simple protocol to identify and characterize them. This is based on differential protein expression and on the fact that they gain tumor cell proteins by the process of trogocytosis. These phenotypic differences should be taken into account in analysis to identify different NK cell subpopulations. Hence, NK cells are actively recognizing tumor cells in leukemia patients; but this seems to be insufficient to eradicate disease. Future efforts should enhance the antitumor activity of this population.
1. Introduction
The immune system recognizes and eliminates tumor cells (Dunn et al., 2002), which defend themselves by different mechanisms (Villalba et al., 2013). The lymphocyte lineage natural killer (NK) cell belongs to the innate immune system (Lanier, 2008, Vivier et al., 2008) and show strong anti-leukemia activity when engrafted in allogeneic settings in hematological cancer patients (Velardi, 2008, Ruggeri et al., 2007, Anel et al., 2012). However, the presence of an anti-leukemia NK cell population in patients with hematological malignancies has not been proven.
NK cells are not a homogenous population and different subsets have different physiological activities. Moreover, different stimuli (e.g., cytokines vs targets cells) give rise to different immunophenotypes (Fujisaki et al., 2009, Sanchez-Martinez et al., 2014). In peripheral blood, human NK cells are mostly CD3−CD56dim cells with high cytotoxic activity, while CD3−CD56brigth cells excel in cytokine production (Bryceson et al., 2011). In vitro evidence indicates that CD56bright NK cells are precursors of CD56dim NK cells and this might also be the case in vivo (Domaica et al., 2012). In addition, combined analysis of CD56 and CD16 expression during NK cell development indicates that their profiles change as follows: CD56brigthCD16− → CD56brigthCD16dim → CD56dimCD16dim → CD56dimCD16+. Additional markers can be used to identify specific subsets within these NK cell populations (Moretta, 2010, Freud et al., 2014).
Identification of antileukemic NK cells in vivo is complex. CD69 expression increases after NK cell activation but it is not exclusive of NK cells encountering tumor cells (Fogel et al., 2013, Elpek et al., 2010). Due to the clinical interest of NK cells, it is therefore highly relevant to identify more precisely the NK cell population(s) with antitumor functions.
CD45 is a protein tyrosine phosphatase that is specifically expressed in leucocytes (Kaplan et al., 1990). CD45 regulates receptor signaling by direct interaction with components of the receptor complexes or by dephosphorylating and activating various Src family kinases (SFK) (Rhee and Veillette, 2012). However, it can also hinder cytokine receptor signaling by inhibiting Janus kinases (JAK) (Irie-Sasaki et al., 2001) or by dephosphorylating Src activating residues (Rhee and Veillette, 2012). CD45 activity is critical for efficient immune response, because its deficiency results in severe combined immunodeficiency (SCID) in mice (Kishihara et al., 1993, Byth et al., 1996, Mee et al., 1999) and humans (Kung et al., 2000, Tchilian et al., 2001).
Total CD45 expression increases with T cell maturation (Hermiston et al., 2009), but the CD45 family comprises several isoforms derived from a single complex gene (Hermiston et al., 2009). Naive T lymphocytes usually express the long CD45RA isoform. Activated and memory T cells express CD45RO, the shortest CD45 isoform, generated by activation-induced alternative splicing of CD45 pre-mRNA (Warren and Skipsey, 1991, Roth, 1994, Lynch and Weiss, 2000, Hermiston et al., 2009). It has been proposed that CD45RO expression also identifies memory NK cells (Fu et al., 2011). Little is known about CD45 expression and function in NK cells, although it is commonly accepted that CD45 positively regulates their activation by dephosphorylating the inhibitory site of SFKs, thus leading to cytokine and chemokine production. However, in vitro cytotoxicity is only slightly impaired in NK cells derived from CD45-deficient mice (Huntington et al., 2005, Hesslein et al., 2006, Mason et al., 2006). As most of what is known was obtained in mouse models and cannot be transposed to humans, the role of CD45 in human NK cells is an open issue and could depend on the type and strength of the activation.
Here we show that, in contrast to T cells, NK cells can express both CD45 isoforms: CD45RA and CD45RO. Moreover, these CD45RARO NK cells recognize tumor cells in patients with hematological cancers and, subsequently, degranulate.
1.1. Experimental Procedures
1.1.1. Cell Culture
The K562 cell line (ATCC CCL 243) and the lymphoblastoid EBV cell line PLH (IHW Number: 9047) were maintained in logarithmic growth in RPMI 1640 medium (Gibco® GlutaMAX™ media) with 10% fetal bovine serum (FBS) (Gibco®). Cells were cultured at 37 °C in a humidified chamber with 5% CO2 in air, and passaged 1:10 twice a week.
1.1.2. Peripheral Blood Mononuclear Cell (PBMC) Purification
Bone marrow and peripheral blood samples were obtained from patients with different hematological diseases and from healthy donors after informed consent. Cells were purified by Ficoll-Hypaque (Sigma) density-gradient centrifugation as described earlier (Allende-Vega et al., 2015). Briefly, 3–6 ml of 1:2 diluted blood or 1:3 diluted bone marrow samples in RPMI were added on top of 5 ml of Histopaque. Cells were centrifuged at 1600 rpm and at 20 °C without break for 30 min. Mononuclear cells were collected from the interlayer white ring. After washing in RPMI, cells were suspended in complete RPMI medium supplemented with 10% FBS (Invitrogen).
1.1.3. In Vitro NK Cell Stimulation Protocol
PBMCs, 1.106 cells/ml, were stimulated during 10 or 20 days with a high dose of IL-2 (1000 U/ml, eBiosciences) or with the lymphoblastoid EBV cell line PLH together with IL-2 (100 U/ml) and IL-15 (5 ng/ml, Miltenyi).
1.1.4. Selection of Patients and Healthy Donors
Data and samples from patients with different hematological cancers were collected at the Oncology and Clinical Hematology Department of the CHU Montpellier, France, after patient's informed consent (Allende-Vega et al., 2015). Patients were enrolled in two independent clinical programs approved by the “Comités de Protection des Personnes Sud Méditerranée I”: ref 1324 and ID-RCB: 2011-A00924-37. All samples from cancer patients were collected at diagnosis and included HD, Healthy donor (nbs = 10); MM, multiple myeloma (nbs = 19, nbms = 20); B-CLL, B-cell chronic lymphocytic leukemia (nbs = 15); BCL, B-cell lymphoma (nbs = 14); AML, acute myeloid leukemia (nbs = 14); bs, blood samples; bms, bone marrow samples.
1.1.5. Multicolor Staining of Cell Surface Markers
PBMCs were stained with 7AAD (Beckman) to identify viable cells and with the following anti-CD25-FITC, − CD45RO-FITC, − CD161-FITC, − CD3-PE, − CD19-PE, − CD62L-PE, − CD69-PE, − CD138-PE, − CD314(NKG2D)-PE, − CD3-ECD, − CD19-ECD, − CD38-ECD, − CD56-PECy7, CD3-APC, − CD56-APC, − GzB-AlexaFluor700, − CD19-AlexaFluor700, − CD20-APC-AlexaFluor750, − CD45-APC-AlexaFluor750, − CD45RA-APC-AlexaFluor750, − CD5-PacificBlue, − CD16-PacificBlue, − CD57-PacificBlue, − CD45-KromeOrange, − CD16-KromeOrange (Beckman), − CD158b-FITC, − CD158a-PE, − CD107a-HV500, − Ki-67-V450 (BD Biosciences), − CD45RA-FITC, − CD45RO-PE, − CD159a(NKG2A)-PE, − CD335(NKp46)-PE, − CD94-PE-Vio770, − CD335(NKp46)-PE-Vio770, − CD45RO-APC, − CD14-VioBlue, − CD19-VioBlue, − CD158e-VioBlue (Miltenyi Biotec) and -CD71-APC (ImmunoTools) antibodies against surface markers for cell phenotyping. Briefly, 1x106 cells were incubated with the different antibodies in PBS with 2% FBS at 37 °C for 30 min. Cells were then washed and suspended in 200–250 μl PBS 2% FBS and staining was analyzed using a Gallios flow cytometer (Beckman) and the Kaluza software.
Viable lymphocytes were gated using FSC-SSC and 7AAD staining. B lymphocytes (CD19+), T lymphocytes (CD3+CD56−) and NK cells (CD56+CD3−) were differentiated based on CD19, CD3 or CD56 expression. NK cells were then separated in four distinct populations based on CD45RA and CD45RO expression: CD45RA+RO− (CD45RA), CD45RA+RO+ (CD45RARO), CD45RAdimRO− (CD45RAdim), CD45RAdimRO+ (CD45RAdimRO). These different populations were then analyzed for CD16, CD57, CD62L, CD69, CD71, CD94, CD107a, CD158a, CD158b, CD158e, CD159a (NKG2A), CD161, CD314 (NKG2D), CD335 (NKp46), Ki-67, GzB expression and cell size and granularity (FSC and SSC).
1.1.6. In Vitro CD107a Degranulation Assay
After PBMC purification and NK cell quantification, 3 million cells were incubated at 37 °C for 4 h or overnight with K562 target cells at an Effector (NK cell): Target ratio of 1:10 in a final volume of 500 μl (RPMI Glutamax with 10% FBS and 10U/ml IL2). The medium also contained 1.5 μl anti-CD107a antibody (BD Biosciences, Franklin Lakes, NJ) and 1 μl monensin to prevent CD107a degradation (BD Golgi-Stop BD Biosciences). Then, cells were resuspended in 50 μl of an antibody cocktail containing the anti-CD45RO-FITC, − CD69-PE, − CD19-ECD, − 7AAD, − CD56-PECy7, − CD3-APC, − CD45RA-APCAlexaFluor750, − CD107a-HV500 and − CD16-KO antibodies (BD Biosciences, Beckman). Samples were analyzed on a Beckman Coulter FACS Gallios flow cytometer using the Kaluza software. Events were initially gated on forward and side scatter (SSC) to identify lymphocytes. A bivariate plot of CD56 versus CD3 was used to acquire at least 10,000 NK cells.
1.1.7. Multicolor Staining for Cell Surface and Intracellular Markers
After PBMC purification, 1 million cells were pre-blocked by incubation with 10% normal human serum at RT for 15 min and then stained with 50 μl of the PANEL Ki-67 antibody cocktail against cell surface markers (anti-CD45RO-PE, − CD19-ECD, − CD56-PC7, − CD3-APC, − CD45RA-APCAlexaFluor750 and − CD16-KO antibodies) (BD Biosciences, Beckman). Cells were washed twice with Staining Buffer and resuspended in 250 μl BD Cytofix-Cytoperm solution at 4 °C for 20 min. Cells were washed twice in BD Perm-Wash solution. Next, cells were fixed-permeabilized in 50 μl BD Perm-Wash solution containing an antibody cocktail against intracellular markers (anti-GzB-AlexaFluor700, − Ki-67-V450) as described in the figures at 4 °C for 30 min in the dark. Cells were washed twice in BD Perm-Wash solution and resuspended in Staining Buffer prior to flow cytometric analysis on a Beckman Coulter FACS Gallios flow cytometer using the Kaluza software. Events were initially gated on forward and side scatter (SSC) to identify lymphocytes. A bivariate plot of CD56 versus CD3 was used to acquire at least 10,000 NK cells.
1.1.8. Identification of Pure Single NK Cells
Primary CD56+ NK cells were enriched and purified from PBMCs of B-cell lymphoma patients with the CD56+ NK cell isolation kit (Miltenyi Biotec, Auburn, CA, USA). The purity (% of CD56+CD3−) of CD56+ NK cells, measured by flow cytometry, was > 90%. Purified CD56+ NK cells have been stained with anti-CD335(NKp46)-PE to formally identify NK cells together with anti-CD45RA-FITC, − CD45RO-APC and − CD19-VioBlue (detection of trogocytosis-capable NK cells). Purified and stained NK cells have been analyzed with the DEPArray™ System (Silicon Biosystems, Menarini). This technology allowed us to detect, enumerate and take pictures of single cells.
1.1.9. DEPArrayTM Procedure
Cell sorting experiments were performed as described in the manufacturer's instructions and in (Lianidou et al., 2013). Briefly, DEPArray cartridges were manually loaded with 14 μl of sample and 800 μl of the buffer solution in which purified and stained NK cells had to be recovered. After loading the cartridge into the DEPArray system, ∼ 9.26 μl of sample was automatically injected by the system into a microchamber of the cartridge where the cells were spontaneously organized into a preprogrammed electric field consisting of 16,000 electrical cages in which individual cells are trapped. Image frames covering the entire surface area of the microchamber for each of four fluorescent filter cubes (FITC, PE, APC and DAPI-Hoechst-VioBlue-PacificBlue) and bright field images were captured. Captured images were digitally processed and presented in a software module that enables selection of cells of interest by the operator.
1.1.10. Statistics
All the experiments shown in the figures were performed at least with samples from six patients for each malignancy and the same number of healthy donors (HD). The statistical analysis was performed using the Student t test: *p < 0.01; **p < 0.001; ***p < 0.0001. Average values were expressed as mean plus or minus the standard error (SD).
2. Results
2.1. Expression of Different CD45 Isoforms in Patients With Hematological Malignancies
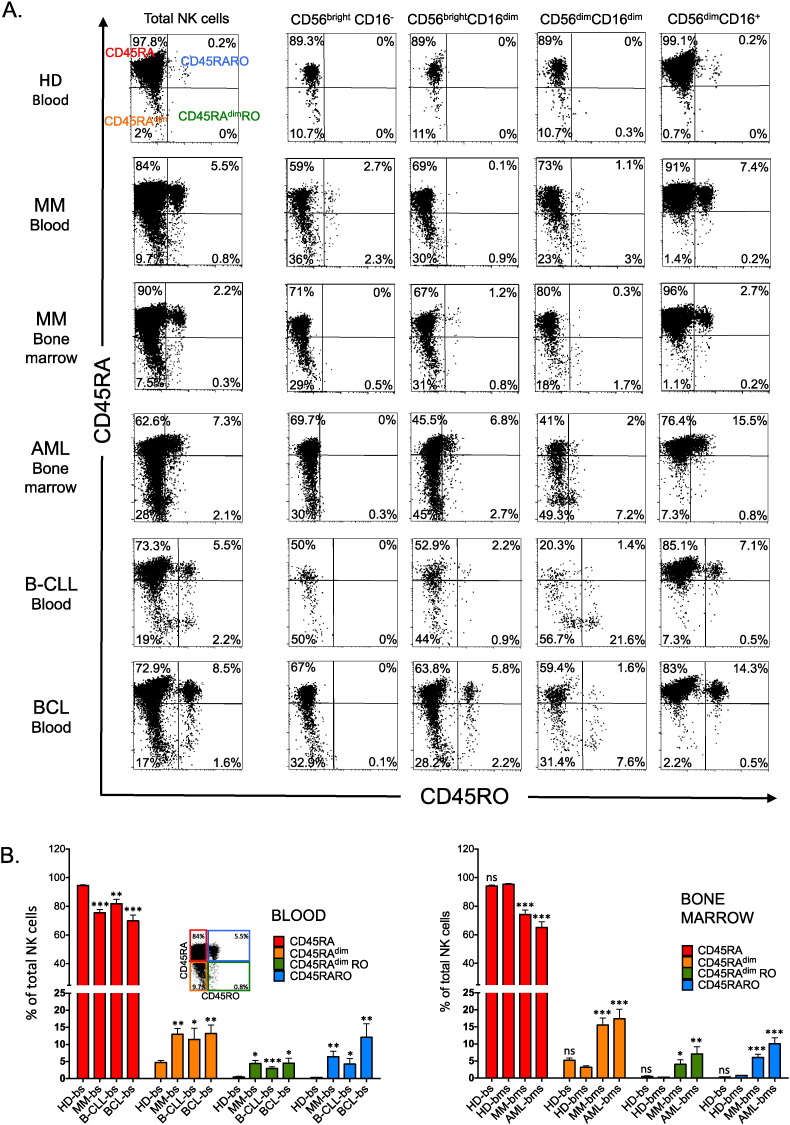
In healthy donors, NK cells were mainly CD45RA cells with few CD45RAdim cells, found particularly in immature NK cell subsets. CD45RARO cells represented between 0 and 0.75% of all NK cells and belonged exclusively to the fully mature CD56+CD16+ subset (Fig. 1A top panels and supplemental Table 1). NK cells derived from healthy donor bone marrows showed equal distribution (Fig. 1B). Blood samples from patients with multiple myeloma (MM) contained four times more CD45RAdim cells and between 1 and 20% of CD45RARO cells (Fig. 1A and supplemental Table 2). As MM is characterized by accumulation of tumor cells in the bone marrow, we also investigated whether bone marrow NK cells, which should be in closer contact with tumor cells, were more activated than circulating NK cells. This was not the case as the percentage of CD45RAdim and CD45RARO cells was similar in blood and bone marrow samples (Fig. 1A and supplemental Table 2).
Fig. 1.
Patients with hematological malignancies and healthy donors have different NK cell subset profiles. A) PBMCs from blood samples (bs) of a healthy donor and of a patient with multiple myeloma (MM) or from bone marrow (bms) of the patient with MM or samples of patients with other hematological diseases were stained for FACS analysis with anti-CD19 (B cells), − CD3 (T cells, CD3+CD56−) and − CD56 (NK cells, CD56+CD3−), to identify the different lymphocyte populations, and also with anti-CD16, to identify NK cell subsets at different stage of maturation, and with − CD45RA, and − CD45RO antibodies. Numbers in the quadrants indicate the percentage of cells. B) Percentage of different NK cell populations based on CD45RA and RO expression in healthy donors and in patients with hematological cancers. The populations correspond to the quadrants in A: upper left (CD45RA), bottom left (CD45RAdim), upper right (CD45RARO) and bottom right (CD45RAdimRO). The bars show the mean ± SD for each medical condition, Student t-test compare to healthy donor blood (left panel) or bone marrow (right panel) samples: *p < 0.01; **p < 0.001; ***p < 0.0001. HD, Healthy donor; MM, multiple myeloma; B-CLL, B-cell chronic lymphocytic leukemia; BCL, B-cell lymphoma; AML, acute myeloid leukemia; bs, blood samples; bms, bone marrow samples.
Similar increases in the CD45RAdim and CD45RO populations were also observed in bone marrow samples from patients with acute myeloid leukemia (AML) or in blood samples of patients with B-cell chronic lymphocyte leukemia (B-CLL) and B-cell lymphoma (BCL) (Fig. 1A and supplemental Table 3). In summary, the C45RARO cell population was statistically increased in all analyzed samples from patients with blood malignancies compared to healthy controls (Fig. 1B and supplemental Fig. 1). The gating strategy to identify CD45RARO cells is described in supplemental Fig. 1B).
2.2. Phenotypic Characterization of CD45RARO Population
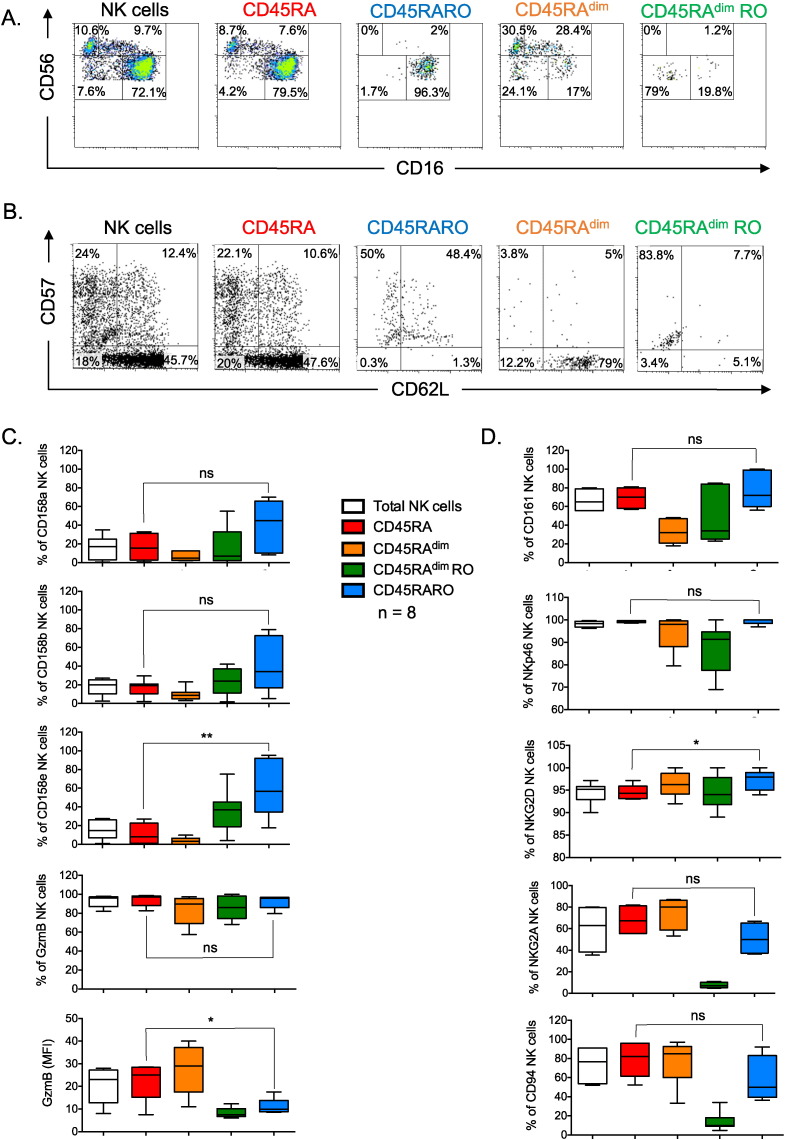
As indicated in Fig. 1, CD45RARO cells belonged to the CD56+CD16+ subset (Fig. 2A) and mostly express the maturation marker CD57 (Fig. 2B) although CD62L was coexpressed by half of them. The CD45RARO population contained higher percentage of cells that expressed KIRs, although it was statistically significant only for CD158e (Fig. 2C and supplemental Fig. 2). The percentage of granzyme B (GzmB)+ cells was similar to other subsets, but the intracellular level of this cytokine was lower (Fig. 2C). This could be due to a deficient production or a recent degranulation that has emptied the intracellular stores. CD45RARO cells also expressed similar levels than CD45RA of another maturation marker the CD161-Killer cell lectin-like receptor subfamily B, member 1 (KLRB1) or the natural cytotoxicity receptor (NCR) NKP46 and slightly higher levels of the activating NKG2D receptor (Fig. 2D and supplemental Fig. 3). However, they showed lower levels of the CD94 glycoprotein and, probably, the inhibitory NK receptor NKG2A (Fig. 2D and supplemental Fig. 3). In summary, CD45RARO cells are fully mature NK cells that mainly express NK receptors of mature cells.
Fig. 2.
The phenotypic characterization of CD45RARO shows that they are fully mature cells. PBMCs from a representative BCL patient were stained as in Fig. 1 to identify the CD45RARO population and the maturation development was revealed by expression of CD56 CD16 (A) or CD57 CD62L (B). Numbers in the quadrant indicate the percentage of cells. C–D) PBMCs from 5 BCL patients were stained as in Fig. 1 to identify the CD45RARO population and the expression of different molecules on the different NK cell subsets was revealed by using antibodies against KIRs 158a, b and e, GzmB, the Lectin Like Transcript-1 (LLT1) receptor CD161, the NCR NKP46, the activating receptor NKG2D, the inhibitory receptor NKG2A (D) and the molecule CD94.
2.3. Expression of Different CD45 Isoforms in Vivo: Patients With Cytomegalovirus (CMV)-Reactivation
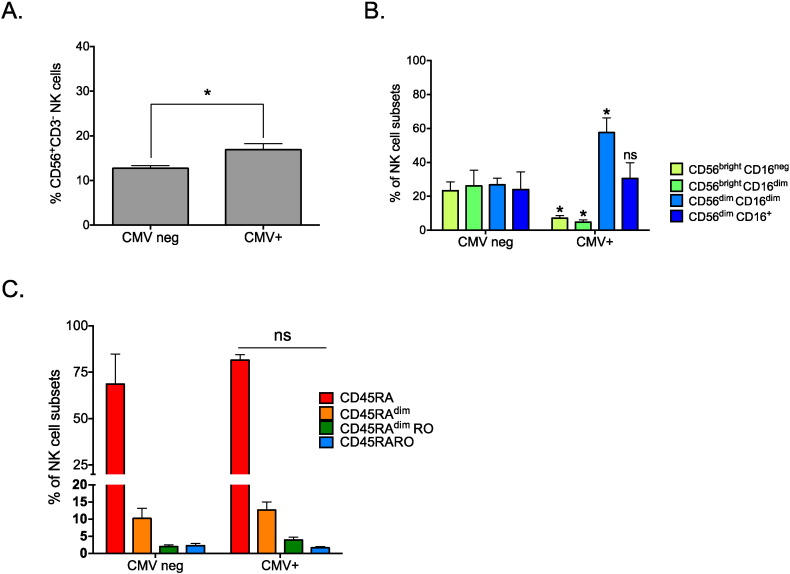
We next asked whether other conditions that lead to NK cell activation, such as viral infections, could give rise to a similar phenotype. We thus analyzed peripheral blood mononuclear cell (PBMC) samples from patients with reactivation (CMV+) or not (CMVneg) of CMV infection following kidney transplantation. CMV reactivation induced an increase in the total number of NK cells (Fig. 3A). In addition, CMV+ patients showed an increase in CD56dimCD16dim cells associated with a reduction of the CD56brigth subsets compared to CMVneg patients (Fig. 3B). The reason of these changes is not clear to us, but could be due to different factors, such as CMV-induced NK cell maturation (Della Chiesa et al., 2013), or an effect on the expression of the different NK cell markers in CMV-infected cells, as previously described for decidual NK cells (Siewiera et al., 2013). These changes were accompanied by minor variations in the expression pattern of CD45 isoforms (Fig. 3C). These results indicate that the expression pattern of CD45 isoforms in NK cells activated by viral infection or hematological cancers is different. Specifically, CD45RARO cells are mainly present in samples from patients with hematological cancers, whereas they represent a minor fraction in virus-infected patients.
Fig. 3.
CMV+ patients and patients with hematological cancer have different NK cell subset profiles. A) PBMCs from patients with reactivation (CMV+) or not (CMVneg) of CMV infection following kidney transplantation were purified as in Fig. 1 and the percentage of NK cells was calculated. B) The abundance (in percentage) of NK cells at different stages of maturation (CD56 CD16) was analyzed in the samples described in (A). C) The percentage of each NK cell subsets (CD45 isoforms) is shown. There were not any differences between the two groups of patients. In (B) and (C) bars represent the mean ± SD of at least four individuals for each medical condition.
2.4. Metabolic Characterization of CD45RARO Population
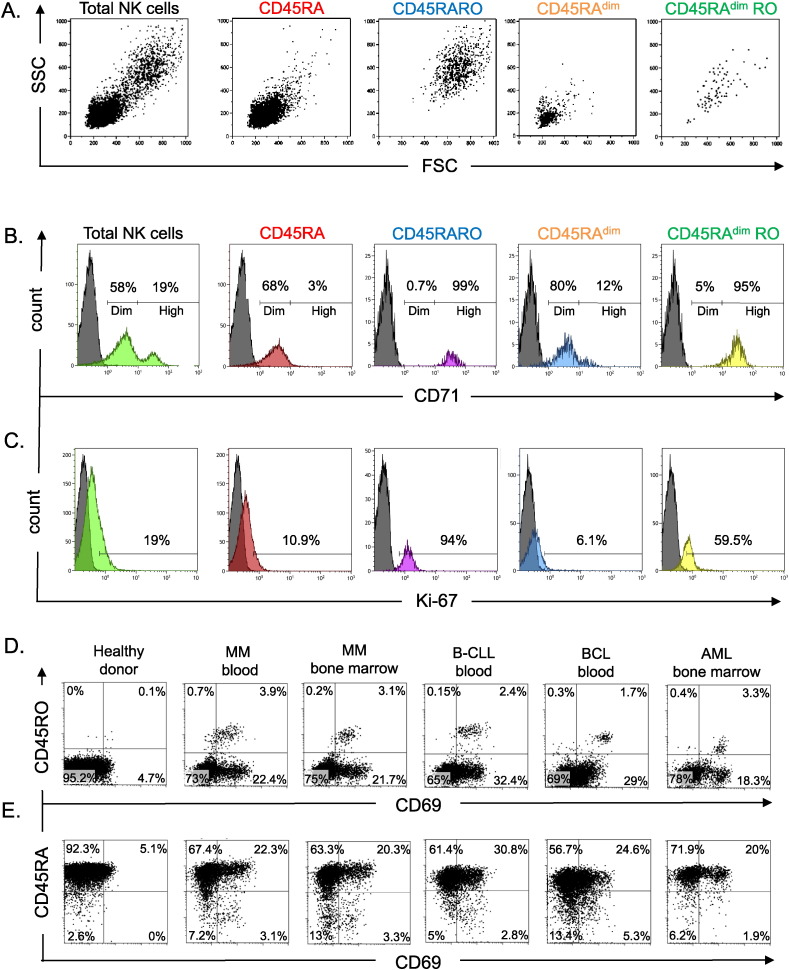
Activated lymphocytes generally increase their size (Zarcone et al., 1987, Skak et al., 2008) and become highly metabolically active cells (Sanchez-Martinez et al., 2014, Sanchez-Martinez et al., 2015). The Ser-Thr kinase mammalian target of rapamycin (mTor) probably links the metabolic shift and the cytoskeletal organization after NK cell activation (Marcais and Walzer, 2014). We observed a subset of NK cells from MM patients that was larger in size (FS) and with high granularity (SS) and corresponded to CD45RARO cells (Fig. 4A and supplemental Fig. 5C). This suggested that they were activated cells that had a higher metabolic activity compared to the other NK subsets. Hence, we activated NK cells in vitro by incubating them with the Epstein Barr Virus (EBV) lymphoblastoid cell line PLH. To support NK cell survival we added low concentrations of two NK cell activating cytokines: IL-2 (100 U/ml) and IL-15 (5 ng/ml) (Anel et al., 2012). We incubated NK cells for up to 20 days to reflect long-term activation. In agreement with previous reports (Zarcone et al., 1987, Skak et al., 2008), 10-day activation induced an increase in size (FCS) and granularity (SSC) that was more relevant at day 20 (supplemental Fig. 4A).
Fig. 4.
Functional characterization of CD45RARO NK cells. A) FS and SS values of the different NK cell subsets (based on the expression of CD45 isoforms) derived from a blood sample of a patient with MM. B–C) Expression of CD71 and Ki67 in the different NK cell subsets in a representative BCL patient. D–E) Representative graphs showing the expression of CD45RO or CD45RA versus CD69 in NK cells from blood (bs) or bone marrow samples (bms) of patients with different blood-borne cancers. Numbers in the quadrant indicate the percentage of cells.
After 3 days of in vitro activation, NK cells started losing CD45RA (supplemental Fig. 4B). However, it was questionable if a real CD45RARO population appeared or cells were losing CD45RA whereas gaining CD45RO. 10 days after initial activation, most cells are CD45RA−CD45RO+. However, at day 20 a CD45RARO population appeared in the culture. This was not exclusive of the presence of accessory cells because long-term activation with cytokines produced a similar pattern (supplemental Fig. 4B). In summary, CD45RARO NK cells also exist in vitro after long activation. Next, we evaluated in vitro activation of patient CD45RARO population. Three days of cytokine-induced activation induced a strong rearrangement on the expression of CD45 isoforms and it was impossible to evaluate the faith of individual populations (Supplemental Fig. 4C). These results additionally suggested that CD45RARO cells could change their CD45 phenotype, at least in vitro.
We then assessed the expression of transferrin receptor protein 1 (TfR1 or CD71), which is required for iron delivery from transferrin to the cells. CD71 expression increases in active metabolic cells because iron is a cofactor for fundamental biochemical activities, such as oxygen transport, energy metabolism and DNA synthesis (Wang and Pantopoulos, 2011). In agreement with the superior metabolic activity suggested by high FS and SS of CD45RARO cells, CD71 expression was higher in CD45RO+ cells in both healthy controls and patients with hematological malignancies (Fig. 4B and supplemental Fig. 5A). Moreover, most of these cells also expressed the proliferation marker Ki-67 (Fig. 4C and supplemental Fig. 5B). In summary, CD45RARO cells represent a NK subset of highly metabolic cells in proliferation.
CD69 expression increases after NK cell stimulation and is considered a bona fide marker of NK cell activation (Elpek et al., 2010), including ex vivo (Vey et al., 2012). Analysis of CD69 expression in the different CD45 populations in patients showed that CD45RO+ cells were mainly CD69+ (Fig. 4D); but not vice versa, as most CD69+ cells were not CD45RO. The very low amount of CD45RO+ cells in healthy donors precluded any meaningful analysis of this population.
In healthy donors, CD45RAdim cells were mainly CD69− (Fig. 4E). CD45RAdim cells were significantly increased in patients and many were also CD69+. However, reduction of CD45RA expression was not always associated with gain of CD69 expression. In fact, patients' samples were enriched particularly in CD45RAdim CD69− and CD45RA CD69+ and, to a lower extent, in CD45RAdim CD69+ cells. This finding suggests that loss of CD45RA and gain of CD69 expression identify two different physiological processes and that these two populations might have different functions.
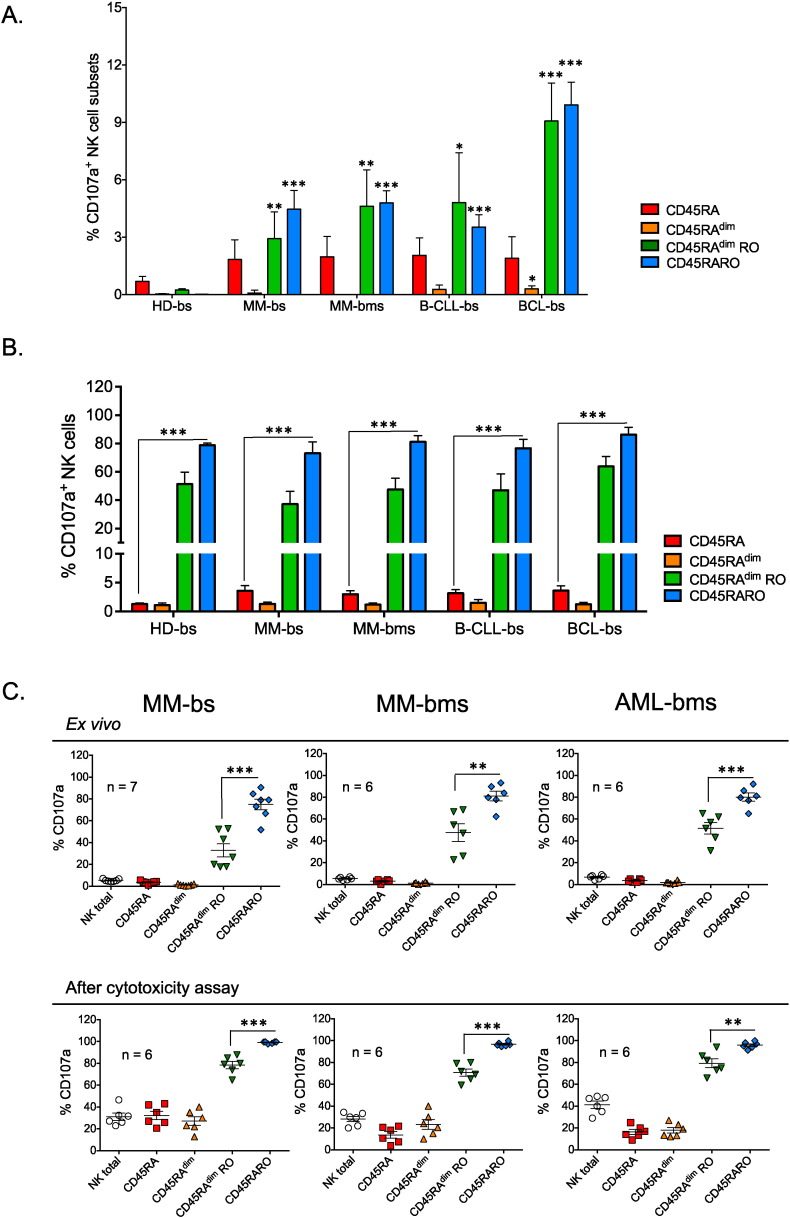
2.5. Functional Characterization of CD45RARO Population
To identify the function of the different NK cell subsets, first we assessed cell degranulation by ex vivo staining of PBMCs with anti-CD107a antibodies (Fig. 5A). In healthy donors, around 1% NK cells were CD107a+. Most of these cells were CD45RA, with a small number of CD45RO+ cells. Remarkably, most CD45RARO and half of CD45RAdimRO cells were CD107a+ (Fig. 5B).
Fig. 5.
CD45RARO identifies degranulating NK cells. PBMCs from healthy donors (HD) and patients with different hematological malignancies were purified as in Fig. 1. A) Number of CD107a+ cells in each NK cell subset (CD45RA RO expression described in Fig. 1A) per million of NK cells. Bars represent the mean ± SD for each medical condition; Student t-test compare to healthy donor samples. B) Percentage of CD107a+ NK cells in the four different subsets. C) Upper panels, Percentage of CD107a+ cells in different NK cell subsets isolated from bone marrow samples (bms) of patients with MM (shown also the percentage in the corresponding blood sample, bs, for comparison) or AML. Bottom panels, Percentage of CD107a+ cells in different NK cell subsets after exposure to target K562 tumor cells (in vitro cytotoxicity assay). PBMCs were incubated for 4 h with target K562 tumor cells at the effector:target ratio of 10:1.
NK cells from patients with hematological cancers showed a large increase in CD107a+ cells (Fig. 5A and supplemental Fig. 6A), particularly among the CD45RO+ subsets, which are specifically increased in these patients (Fig. 1). Reduction of CD45RA expression was not associated with increased degranulation (Fig. 5B). Like in healthy donors, the CD45RARO and, to a lower extent, CD45RAdimRO fractions contained mostly cells that had degranulated (Fig. 5B and supplemental Fig. 6B). The median CD107a-mean fluorescence intensity (MFI) of these two populations was largely increased compared to CD45RO− populations (supplemental Fig. 6A). This was not exclusive of circulating NK cells, because similar results were obtained also for NK cells derived from bone marrow samples of patients with MM and AML (Fig. 5B and C upper panels). In contrast, CD45RARO cells showed low GzmB content (Fig. 2C). Our explanation is that CD45RARO cells had recently degranulated in vivo.
Fig. 6.
CD45RARO cells have performed trogocytosis on tumor cells. PBMCs from patients with BCL (A) or AML (C) were purified as in Fig. 1 and were stained with different antibodies. Numbers in the quadrant indicate the percentage of cells. In this experiment, the NK cell population corresponded to CD56+NKP46+ cells. B) Purified NK cells (CD56+ selection) from a B-CLL patient have been were stained with NKp46, to formally identify NK cells, together with CD45RA, CD45RO and CD19. They were analyzed with the DEPArray™ System.
CD45RARO cells continued to show the higher degranulation rate after an in vitro analysis using K562 as target cells (Fig. 5C bottom panels), although other populations significantly increased degranulation. Interestingly, the different CD45 NK cell subsets did not change after the 4-hour in vitro cytotoxic assay (supplemental Fig. 7). This and the in vitro activation results (supplemental Fig. 4) showed that expression of CD45RA and CD45RO is stable at short times but can change after long lasting activation.
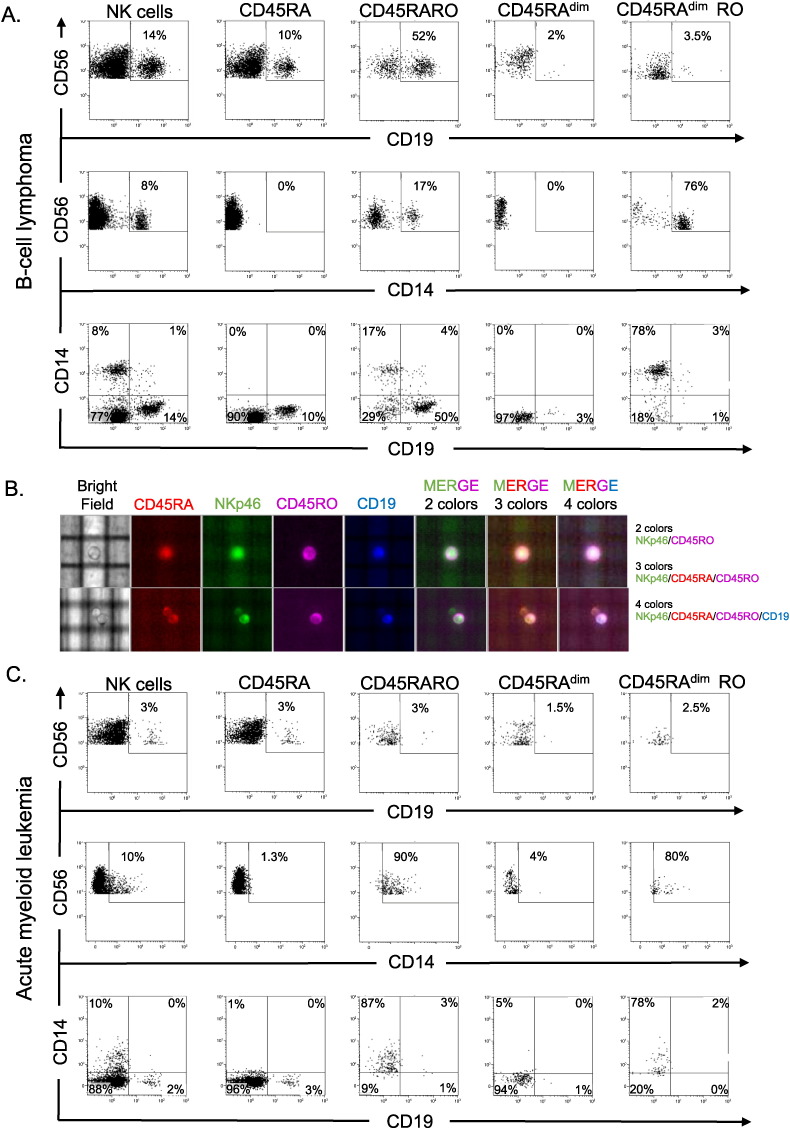
2.6. CD45RARO Have Performed Trogocytosis in Vivo
To investigate if CD45RARO cells were performing antitumor activity in vivo, we investigated if these cells have performed trogocytosis on tumor targets. Trogocytosis is a process whereby lymphocytes, i.e. NK cells (Suzuki et al., 2015, Nakamura et al., 2013), gain surface molecules from interacting cells and express them on their own surface and has been observed in B lymphoblastic leukemia (B ALL) ex vivo (Soma et al., 2015). We observed that long-time activated NK cells (see supplemental Fig. 4) performed trogocytosis in two AML cell lines (supplemental Fig. 8). In fact, NK cells extracted at least two proteins expressed in AML cells, CD14 and CD33, with considerable efficiency. This showed that human NK cell efficiently performed trogocytosis and we investigated if this was the case in vivo. Because in this experiment we studied markers of other cell types, we used a double labeling to identify NK cells and gated on CD56+NKP46+ cells. In a BCL patient, we observed that 14% of the NK cells expressed the BCL marker CD19 in their membrane (Fig. 6A). This value increased to 52% in the CD45RARO population and it was much lower in the other populations. NK cells also gained at lower level expression of the myeloid marker CD14, although the population was predominantly CD45RAdimRO. However, the NK cells that stained positive for both CD19 and CD14 were very rare. This suggested that two different NK cell populations were performing trogocytosis. The CD45RARO cells were doing it on tumor cells. We observed the very similar results in another CD19+ disease: B-CLL (supplemental Fig. 9). Next, we used the purified NK cells (CD56+ selection) from whole blood of a B-CLL patient and analyzed them with the DEPArray™ System, which allowed identifying, visualizing and taking pictures of single cells. We labeled cells with NKp46 to formally identify NK cells together with CD45RA, CD45RO and CD19. Fig. 6B showed that single cells expressed all markers. Thus, we showed a picture of a human NK cell that has just performed in vivo trogocytosis in a tumor cell (see also the graphical abstract).
To exclude that NK cells were not gaining CD19 in all tumors, we investigated NK cells from an AML patient (Fig. 6B). Around 10% of NK cells expressed the AML marker CD14 and only 3% expressed CD19, which was expressed in all NK cell populations. In contrast, 90% of CD45RARO expressed CD14 showing that they have massively performed trogocytosis in a CD14+ population in AML patients.
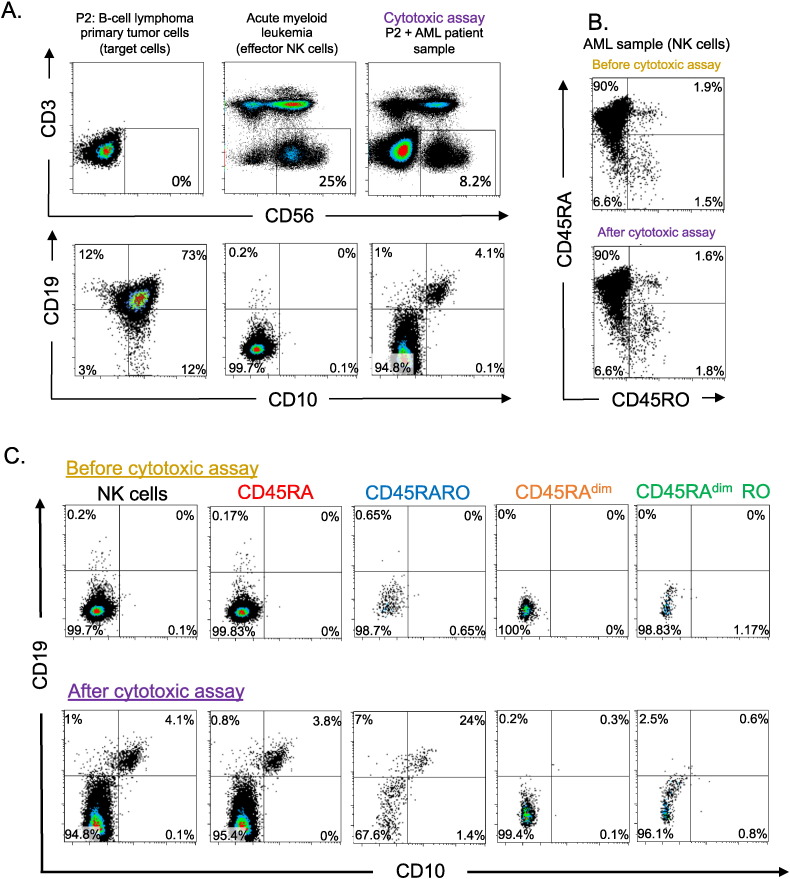
Next, we investigated if CD45RARO cells derived from a AML patient that had performed trogocytosis and gained CD14 expression were able to take CD19 from tumor cells of a BCL patient. Tumor CD19+ cells and NK cells from the AML patient did not express the same membrane markers (Fig. 7A). We distinguished BCL cells by CD19 and CD10 staining and after 16-h cytotoxic assay, we observed that 4% of NK cells form the AML patient gained expression of both CD10 and CD19 (Fig. 7A). The CD45RARO population was mainly stable all through the assay (Fig. 7B). The population that performed trogocytosis mainly was the CD45RARO (Fig. 7C). This showed that the CD45RARO population was prompted to recognize and interact with allogeneic tumor cells. In summary, our data showed that NK cells performed trogocytosis on tumor cells and that the CD45RARO population is mainly responsible of this.
Fig. 7.
CD45RARO cells are prompted to perform trogocytosis on allogeneic tumor cells. PBMCs from an AML patient were incubated with purified tumor cells from a BCL patient (E: (NK cell):T ratio 0.3:1), which are CD10CD19 for 16 h before staining with different antibodies. A) Top panels identified the BCL cells, the NK cells before and after cytotoxic assay. In the bottom panels CD10CD19 expression was analyzed in the NK cells described in the top panels. Numbers in the quadrant indicate the percentage of cells. B) CD45RA RO expression before and after cytotoxic assay was analyzed in NK cells. C) The expression of CD10CD19 was analyzed in different NK cell CD45 subsets. The numbers in graphics represent the percentages of cells in the specific quadrant.
3. Discussion
Identification of human NK cell populations is important for understanding their physiology and for improving their therapeutic use in the clinic. Altogether our results indicate that CD45RARO cells are fully mature NK cells (CD56dimCD16+CD57+KIR+CD161+), which are activated (high size and granularity, CD69+CD71+KI67+NKG2D+) and that have degranulated (CD107a+ and low GzmB content) and performed trogocytosis (CD19+ in BCL and B-CLL and CD14+ in AML). Moreover, they are prompted to perform trogocytosis on different target cells. These findings suggest that they are effector cells with maximal cytotoxic activity against cancer cells. It seems that a population of highly mature NK cells encounters its targets and respond by becoming effector cells. In addition, we observed that a population of NK cells has performed trogocytosis in non-tumor, myeloid, cells at least in BCL and B-CLL patients. It is well known that NK cells kill dendritic cells and macrophages in several contexts, but the role here is unknown. Moreover, the population that has performed it is mainly CD45RAdimRO, a generally minor population.
The large size and granularity of CD45RARO cells could preclude their observation when standard FCS-SSC parameters for the classical lymphocyte populations are used. It is essential to understand that activated lymphocytes increase in size and granularity, which distinguish them for naïve lymphocytes. This is important for future studies of CD45RARO NK cells in solid cancers, which could also induce a similar phenotype because NK cell infiltration is associated with a good prognosis in several cancers (Senovilla et al., 2012, Mamessier et al., 2013, Mamessier et al., 2012). However, our work does not show the irrefutable proof that CD45RARO cells are bona-fide NK cells, although all results point in this direction. Alternative analyses are needed to definitively state the nature of these cells.
Target cell availability is probably maximal for NK cells in blood borne cancers, hence, we believe that these diseases will show the highest CD45RARO NK cell numbers; although these cells are unable to control the disease. Leukemogenesis in mouse is enhanced when the host immune system is impaired (Garaude et al., 2008, Kaminski et al., 2012) and more hematological cancer patients present severe NK cell dysfunctions (Baier et al., 2013). Others and we have shown the requirement of fully functional NK cells to eradicate blood-borne tumors in several mouse models (Karre et al., 1986, Van Den Broek et al., 1995, Pardo et al., 2002, Aguilo et al., 2009, Charni et al., 2009, Charni et al., 2010, Ramírez-Comet et al., 2014). The use of alloreactive NK cells may represent a new cancer treatment, specifically for tumors of hematopoietic origin. Indeed, KIR–KIR ligand incompatibility in the graft-versus-host (GvH) direction, which is mainly based on NK cell alloreactivity, improves the outcome after unrelated cord blood stem cell transplantation (UCBT) in the clinic (Willemze et al., 2009, Stern et al., 2008). Moreover, NK cells: i) are not responsible of GvH disease (GvHD); ii) can be injected as “differentiated” cells and thus do not need to survive within the patient's body for a long time; iii) protect from opportunistic infections (Willemze et al., 2009), probably through their immunoregulatory effects on B and T cells, macrophages and, more importantly, polymorphonuclear cells (Bhatnagar et al., 2010). However, evaluation of NK cell activation in vivo is difficult because we lack effective methods for their analysis. CD69 expression has routinely been used (Elpek et al., 2010, Vey et al., 2012); though, our results show that CD69 expression does not imply degranulation, which is believed to be the most essential component of the NK cell anti-tumor activity (Bryceson et al., 2011), or trogocytosis. Conversely, our work indicates that CD45RO expression identifies degranulating NK cell subsets in patients with hematological malignancies. We believe that efficient antitumor treatments that involve also NK cell activity, such as monoclonal antibodies against tumor antigens, should also increase these NK cell populations. Other options for treatment include new chemicals that can be associated with immunotherapy to boost the immune response (Villalba et al., 2014) and that could improve the NK cell-mediated response (Catalán et al., 2015).
CD45 activity is regulated by dimerization and spontaneous CD45 homodimerization at the plasma membrane inhibits its activity (Xu and Weiss, 2002). The size of CD45 extracellular domain is inversely proportional to the extent of CD45 dimerization and thus self-inhibition (Xu and Weiss, 2002). Larger CD45 isoforms, such as CD45RA, dimerize less efficiently and, accordingly, they should better promote TCR signaling than smaller isoforms, such as CD45RO (Rhee and Veillette, 2012). However, CD45 activity also depends on its plasma membrane localization and thus on its extracellular domain (Mustelin et al., 2005, Rhee and Veillette, 2012). At least in T cells, too high CD45 activity leads to dephosphorylation of the activating residues in Src kinases, whereas too low CD45 activity might leave phosphorylated the inhibitory residues. Therefore, it is important for efficient NK cell activation that CD45 activity remains within a specific window (Hermiston et al., 2009) and the amount of specific CD45 isoforms will regulate the final activity. We found that CD45RARO NK cells show maximal degranulation and trogocytosis, suggesting that expression of both CD45RA and CD45RO isoforms might give to NK cells the appropriate level of CD45 activity for efficient signaling to boost cytotoxicity. CD45 is required for full NK cell cytotoxicity in vivo in mice (Hesslein et al., 2011); however, it is not required in vitro (Mason et al., 2006, Hesslein et al., 2006, Huntington et al., 2005). In agreement, we observed that other NK cell subsets, which express different CD45 isoforms, improved degranulation in vitro. This suggests that NK cells depend less of CD45 expression in vitro than in vivo.
Expression of different CD45 isoforms changes the recognition of CD45 ligands. For example, the abundance and types of O-glycans on the different CD45 isoforms regulate the cell sensitivity to galectin-1 (Earl et al., 2010). Galectin-1, which is abundantly produced by tumor cells, blocks T cell-mediated cytotoxic responses (Ito et al., 2012) and induces apoptosis of thymocytes and T cells (Earl et al., 2010). It is possible that anti-tumor NK cells are selected based on their resistance to galectin-1 or to other ligands through expression of CD45RO. Indeed, as O-glycans bind mainly to CD45 extracellular domain, cells that express short CD45 isoforms, like CD45RO, will have relatively fewer O-glycans and thus will be more resistant to galectin-1.
Ex vivo we found very few NK cells that express CD45RO in peripheral blood samples from healthy donors. This is surprising, especially if CD45RO expression identifies memory NK cells, as it has been proposed (Fu et al., 2011). This finding suggests that the amount of memory NK cells might be extremely low in blood or bone marrow samples, or that CD45RO may not be a marker of memory NK cells. Alternatively, CD45RO expression in NK cells could have been specifically lost during ex vivo sample handling. We think that this is unlikely because NK cells express slightly higher levels of total CD45 than other lymphocyte types (data not shown). In fact, we found that CD45RO is mostly associated with effector NK cells; however, differently from what observed in most T cell populations, CD45RA down-regulation is not required for NK cell activation. This suggests that in NK cells the expression of different CD45 isoforms plays a different role than in T cells.
In summary, we show here that NK cells that recognize tumor cells are present in all examined patients of hematological cancers. Hence, NK cells are actively recognizing tumor cells in leukemia patients; but this seems to be insufficient to eradicate disease. Protocols to enhance such population should improve patient's prognosis. Finally, the presence of this population identifies blood-borne cancer patients.
4. Financial Support and Acknowledgements
All our funders are public or charitable organizations. This work was supported by the program “Chercheur d'avenir” from the Region Languedoc-Roussillon (09-13195) (MV), a scientific program from the “Communauté de Travail des Pyrénées” (CTPP5/12 to MV), the charities CIEL, L'Un pour l'Autre and Ensangble (09/2013) (MV), a grant from FEDER Objectif Competitivite (10-007762) (MV), a grant from the European Community Program SUDOE (CLiNK SOE2/P1/E341 to MV and J-FR), an AOI from the CHU Montpellier (N°221826) (GC and MV) and fellowships from the ARC (DOC20121206007) and La Ligue Contre le Cancer (TDKB13362) (EK) and Ministère de l'Enseignement Supérieur et de la Recherche (MESR) (DNV). FACs analysis was performed at the platform Montpellier Rio Imaging (MRI).
Conflicts of Interest
The authors EK and MV have presented a patent application for the use of CD45RARO cells as a biomarker of hematological cancers (Martin Villalba and Ewelina Krzywinska. Methods for Diagnosing Hematological Cancers. EP14306134.9.).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2015.08.021.
Appendix A. Supplementary Data
Supplementary material.
References
- Aguilo J.I., Garaude J., Pardo J., Villalba M., Anel A. Protein kinase C-theta is required for NK cell activation and in vivo control of tumor progression. J. Immunol. 2009;182:1972–1981. doi: 10.4049/jimmunol.0801820. [DOI] [PubMed] [Google Scholar]
- Allende-Vega N., Krzywinska E., Orecchioni S., Lopez-Royuela N., Reggiani F., Talarico G., Rossi J.F., Rossignol R., Hicheri Y., Cartron G., Bertolini F., Villalba M. The presence of wild type p53 in hematological cancers improves the efficacy of combinational therapy targeting metabolism. Oncotarget. 2015;6:19228–19245. doi: 10.18632/oncotarget.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anel A., Aguilo J.I., Catalan E., Garaude J., Rathore M.G., Pardo J., Villalba M. Protein kinase C-theta (PKC-theta) in natural killer cell function and anti-tumor immunity. Front. Immunol. 2012;3:187. doi: 10.3389/fimmu.2012.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier C., Fino A., Sanchez C., Farnault L., Rihet P., Kahn-Perles B., Costello R.T. Natural killer cells modulation in hematological malignancies. Front. Immunol. 2013;4:459. doi: 10.3389/fimmu.2013.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar N., Hong H.S., Krishnaswamy J.K., Haghikia A., Behrens G.M., Schmidt R.E., Jacobs R. Cytokine-activated NK cells inhibit PMN apoptosis and preserve their functional capacity. Blood. 2010;116:1308–1316. doi: 10.1182/blood-2010-01-264903. [DOI] [PubMed] [Google Scholar]
- Bryceson Y.T., Chiang S.C., Darmanin S., Fauriat C., Schlums H., Theorell J., Wood S.M. Molecular mechanisms of natural killer cell activation. J. Innate Immun. 2011;3:216–226. doi: 10.1159/000325265. [DOI] [PubMed] [Google Scholar]
- Byth K.F., Conroy L.A., Howlett S., Smith A.J., May J., Alexander D.R., Holmes N. CD45-null transgenic mice reveal a positive regulatory role for CD45 in early thymocyte development, in the selection of CD4 + CD8 + thymocytes, and B cell maturation. J. Exp. Med. 1996;183:1707–1718. doi: 10.1084/jem.183.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalán E., Charni S., Aguiló J.-I., Enríquez J.-A., Naval J., Pardo J., Villalba M., Anel A. MHC-I modulation due to metabolic changes regulates tumor sensitivity to CTL and NK cells. Oncoimmunology. 2015;4:e985924. doi: 10.4161/2162402X.2014.985924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charni S., Aguilo J.I., Garaude J., De Bettignies G., Jacquet C., Hipskind R.A., Singer D., Anel A., Villalba M. ERK5 knockdown generates mouse leukemia cells with low MHC class I levels that activate NK cells and block tumorigenesis. J. Immunol. 2009;182:3398–3405. doi: 10.4049/jimmunol.0803006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charni S., De Bettignies G., Rathore M.G., Aguilo J.I., Van Den Elsen P.J., Haouzi D., Hipskind R.A., Enriquez J.A., Sanchez-Beato M., Pardo J., Anel A., Villalba M. Oxidative phosphorylation induces de novo expression of the MHC class I in tumor cells through the ERK5 pathway. J. Immunol. 2010;185:3498–3503. doi: 10.4049/jimmunol.1001250. [DOI] [PubMed] [Google Scholar]
- Della Chiesa M., Muccio L., Moretta A. CMV induces rapid NK cell maturation in HSCT recipients. Immunol. Lett. 2013;155:11–13. doi: 10.1016/j.imlet.2013.09.020. [DOI] [PubMed] [Google Scholar]
- Domaica C.I., Fuertes M.B., Uriarte I., Girart M.V., Sardanons J., Comas D.I., Di Giovanni D., Gaillard M.I., Bezrodnik L., Zwirner N.W. Human natural killer cell maturation defect supports in vivo CD56(bright) to CD56(dim) lineage development. PLoS One. 2012;7:e51677. doi: 10.1371/journal.pone.0051677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn G.P., Bruce A.T., Ikeda H., Old L.J., Schreiber R.D. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- Earl L.A., Bi S., Baum L.G. N- and O-glycans modulate galectin-1 binding, CD45 signaling, and T cell death. J. Biol. Chem. 2010;285:2232–2244. doi: 10.1074/jbc.M109.066191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elpek K.G., Rubinstein M.P., Bellemare-Pelletier A., Goldrath A.W., Turley S.J. Mature natural killer cells with phenotypic and functional alterations accumulate upon sustained stimulation with IL-15/IL-15Ralpha complexes. Proc. Natl. Acad. Sci. U. S. A. 2010;107:21647–21652. doi: 10.1073/pnas.1012128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel L.A., Sun M.M., Geurs T.L., Carayannopoulos L.N., French A.R. Markers of nonselective and specific NK cell activation. J. Immunol. 2013;190:6269–6276. doi: 10.4049/jimmunol.1202533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freud A.G., Yu J., Caligiuri M.A. Human natural killer cell development in secondary lymphoid tissues. Semin. Immunol. 2014;26:132–137. doi: 10.1016/j.smim.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X., Liu Y., Li L., Li Q., Qiao D., Wang H., Lao S., Fan Y., Wu C. Human natural killer cells expressing the memory-associated marker CD45RO from tuberculous pleurisy respond more strongly and rapidly than CD45RO-natural killer cells following stimulation with interleukin-12. Immunology. 2011;134:41–49. doi: 10.1111/j.1365-2567.2011.03464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaki H., Kakuda H., Shimasaki N., Imai C., Ma J., Lockey T., Eldridge P., Leung W.H., Campana D. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69:4010–4017. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaude J., Kaminski S., Charni S., Aguilo J.I., Jacquet C., Plays M., Hernandez J., Rodriguez F., Hipskind R.A., Anel A., Villalba M. Impaired anti-leukemic immune response in PKCtheta-deficient mice. Mol. Immunol. 2008;45:3463–3469. doi: 10.1016/j.molimm.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Hermiston M.L., Zikherman J., Zhu J.W. CD45, CD148, and Lyp/Pep: critical phosphatases regulating Src family kinase signaling networks in immune cells. Immunol. Rev. 2009;228:288–311. doi: 10.1111/j.1600-065X.2008.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesslein D.G., Takaki R., Hermiston M.L., Weiss A., Lanier L.L. Dysregulation of signaling pathways in CD45-deficient NK cells leads to differentially regulated cytotoxicity and cytokine production. Proc. Natl. Acad. Sci. U. S. A. 2006;103:7012–7017. doi: 10.1073/pnas.0601851103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesslein D.G., Palacios E.H., Sun J.C., Beilke J.N., Watson S.R., Weiss A., Lanier L.L. Differential requirements for CD45 in NK-cell function reveal distinct roles for Syk-family kinases. Blood. 2011;117:3087–3095. doi: 10.1182/blood-2010-06-292219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntington N.D., Xu Y., Nutt S.L., Tarlinton D.M. A requirement for CD45 distinguishes Ly49D-mediated cytokine and chemokine production from killing in primary natural killer cells. J. Exp. Med. 2005;201:1421–1433. doi: 10.1084/jem.20042294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie-Sasaki J., Sasaki T., Matsumoto W., Opavsky A., Cheng M., Welstead G., Griffiths E., Krawczyk C., Richardson C.D., Aitken K., Iscove N., Koretzky G., Johnson P., Liu P., Rothstein D.M., Penninger J.M. CD45 is a JAK phosphatase and negatively regulates cytokine receptor signalling. Nature. 2001;409:349–354. doi: 10.1038/35053086. [DOI] [PubMed] [Google Scholar]
- Ito K., Stannard K., Gabutero E., Clark A.M., Neo S.Y., Onturk S., Blanchard H., Ralph S.J. Galectin-1 as a potent target for cancer therapy: role in the tumor microenvironment. Cancer Metastasis Rev. 2012;31:763–778. doi: 10.1007/s10555-012-9388-2. [DOI] [PubMed] [Google Scholar]
- Kaminski S., Adjali O., Jacquet C., Garaude J., Keriel A., Lassaux A., Hipskind R., Sitbon M., Taylor N., Villalba M. The protooncogene Vav1 regulates murine leukemia virus-induced T-cell leukemogenesis. Oncoimmunology. 2012;1:600–608. doi: 10.4161/onci.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan R., Morse B., Huebner K., Croce C., Howk R., Ravera M., Ricca G., Jaye M., Schlessinger J. Cloning of three human tyrosine phosphatases reveals a multigene family of receptor-linked protein-tyrosine-phosphatases expressed in brain. Proc. Natl. Acad. Sci. U. S. A. 1990;87:7000–7004. doi: 10.1073/pnas.87.18.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karre K., Ljunggren H.G., Piontek G., Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- Kishihara K., Penninger J., Wallace V.A., Kundig T.M., Kawai K., Wakeham A., Timms E., Pfeffer K., Ohashi P.S., Thomas M.L. Normal B lymphocyte development but impaired T cell maturation in CD45-exon6 protein tyrosine phosphatase-deficient mice. Cell. 1993;74:143–156. doi: 10.1016/0092-8674(93)90302-7. [DOI] [PubMed] [Google Scholar]
- Kung C., Pingel J.T., Heikinheimo M., Klemola T., Varkila K., Yoo L.I., Vuopala K., Poyhonen M., Uhari M., Rogers M., Speck S.H., Chatila T., Thomas M.L. Mutations in the tyrosine phosphatase CD45 gene in a child with severe combined immunodeficiency disease. Nat. Med. 2000;6:343–345. doi: 10.1038/73208. [DOI] [PubMed] [Google Scholar]
- Lanier L.L. Up on the tightrope: natural killer cell activation and inhibition. Nat. Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lianidou E.S., Mavroudis D., Georgoulias V. Clinical challenges in the molecular characterization of circulating tumour cells in breast cancer. Br. J. Cancer. 2013;108:2426–2432. doi: 10.1038/bjc.2013.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch K.W., Weiss A. A model system for activation-induced alternative splicing of CD45 pre-mRNA in T cells implicates protein kinase C and Ras. Mol. Cell. Biol. 2000;20:70–80. doi: 10.1128/mcb.20.1.70-80.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamessier E., Bertucci F., Sabatier R., Birnbaum D., Olive D. “Stealth” tumors: breast cancer cells shun NK-cells anti-tumor immunity. Oncoimmunology. 2012;1:366–368. doi: 10.4161/onci.18528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamessier E., Pradel L.C., Thibult M.L., Drevet C., Zouine A., Jacquemier J., Houvenaeghel G., Bertucci F., Birnbaum D., Olive D. Peripheral blood NK cells from breast cancer patients are tumor-induced composite subsets. J. Immunol. 2013;190:2424–2436. doi: 10.4049/jimmunol.1200140. [DOI] [PubMed] [Google Scholar]
- Marcais A., Walzer T. mTOR: a gate to NK cell maturation and activation. Cell Cycle. 2014;13:3315–3316. doi: 10.4161/15384101.2014.972919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason L.H., Willette-Brown J., Taylor L.S., Mcvicar D.W. Regulation of Ly49D/DAP12 signal transduction by Src-family kinases and CD45. J. Immunol. 2006;176:6615–6623. doi: 10.4049/jimmunol.176.11.6615. [DOI] [PubMed] [Google Scholar]
- Mee P.J., Turner M., Basson M.A., Costello P.S., Zamoyska R., Tybulewicz V.L. Greatly reduced efficiency of both positive and negative selection of thymocytes in CD45 tyrosine phosphatase-deficient mice. Eur. J. Immunol. 1999;29:2923–2933. doi: 10.1002/(SICI)1521-4141(199909)29:09<2923::AID-IMMU2923>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Moretta L. Dissecting CD56dim human NK cells. Blood. 2010;116:3689–3691. doi: 10.1182/blood-2010-09-303057. [DOI] [PubMed] [Google Scholar]
- Mustelin T., Vang T., Bottini N. Protein tyrosine phosphatases and the immune response. Nat. Rev. Immunol. 2005;5:43–57. doi: 10.1038/nri1530. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Nakayama M., Kawano M., Ishii T., Harigae H., Ogasawara K. NK-cell fratricide: dynamic crosstalk between NK and cancer cells. Oncoimmunology. 2013;2:e26529. doi: 10.4161/onci.26529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo J., Balkow S., Anel A., Simon M.M. Granzymes are essential for natural killer cell-mediated and perf-facilitated tumor control. Eur. J. Immunol. 2002;32:2881–2887. doi: 10.1002/1521-4141(2002010)32:10<2881::AID-IMMU2881>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Ramírez-Comet N., Aguiló J.I., Rathoré M.G., Catalán E., Garaude J., Uze G., Naval J., Pardo J., Anel A., Villalba M. IFN-α signaling through PKC-θ is essential for anti-tumor NK cell function. Oncoimmunology. 2014;3 doi: 10.4161/21624011.2014.948705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee I., Veillette A. Protein tyrosine phosphatases in lymphocyte activation and autoimmunity. Nat. Immunol. 2012;13:439–447. doi: 10.1038/ni.2246. [DOI] [PubMed] [Google Scholar]
- Roth M.D. Interleukin 2 induces the expression of CD45RO and the memory phenotype by CD45RA + peripheral blood lymphocytes. J. Exp. Med. 1994;179:857–864. doi: 10.1084/jem.179.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri L., Mancusi A., Burchielli E., Aversa F., Martelli M.F., Velardi A. Natural killer cell alloreactivity in allogeneic hematopoietic transplantation. Curr. Opin. Oncol. 2007;19:142–147. doi: 10.1097/CCO.0b013e3280148a1a. [DOI] [PubMed] [Google Scholar]
- Sanchez-Martinez D., Krzywinska E., Rathore M.G., Saumet A., Cornillon A., Lopez-Royuela N., Martinez-Lostao L., Ramirez-Labrada A., Lu Z.Y., Rossi J.F., Fernandez-Orth D., Escorza S., Anel A., Lecellier C.H., Pardo J., Villalba M. All-trans retinoic acid (ATRA) induces miR-23a expression, decreases CTSC expression and granzyme B activity leading to impaired NK cell cytotoxicity. Int. J. Biochem. Cell Biol. 2014;49:42–52. doi: 10.1016/j.biocel.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Sanchez-Martinez D., Azaceta G., Muntasell A., Aguilo N., Nunez D., Galvez E.M., Naval J., Anel A., Palomera L., Vilches C., Marzo I., Villalba M., Pardo J. Human NK cells activated by EBV lymphoblastoid cells overcome anti-apoptotic mechanisms of drug resistance in haematological cancer cells. Oncoimmunology. 2015;4:e991613. doi: 10.4161/2162402X.2014.991613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senovilla L., Vacchelli E., Galon J., Adjemian S., Eggermont A., Fridman W.H., Sautes-Fridman C., Ma Y., Tartour E., Zitvogel L., Kroemer G., Galluzzi L. Trial watch: prognostic and predictive value of the immune infiltrate in cancer. Oncoimmunology. 2012;1:1323–1343. doi: 10.4161/onci.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siewiera J., El Costa H., Tabiasco J., Berrebi A., Cartron G., Le Bouteiller P., Jabrane-Ferrat N. Human cytomegalovirus infection elicits new decidual natural killer cell effector functions. PLoS Pathog. 2013;9:e1003257. doi: 10.1371/journal.ppat.1003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skak K., Frederiksen K.S., Lundsgaard D. Interleukin-21 activates human natural killer cells and modulates their surface receptor expression. Immunology. 2008;123:575–583. doi: 10.1111/j.1365-2567.2007.02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma L., Wu D., Chen X., Edlefsen K., Fromm J.R., Wood B. Apparent CD19 expression by natural killer cells: a potential confounder for minimal residual disease detection by flow cytometry in B lymphoblastic leukemia. Cytometry B Clin. Cytom. 2015;88:145–147. doi: 10.1002/cyto.b.21179. [DOI] [PubMed] [Google Scholar]
- Stern M., Ruggeri L., Mancusi A., Bernardo M.E., De Angelis C., Bucher C., Locatelli F., Aversa F., Velardi A. Survival after T cell-depleted haploidentical stem cell transplantation is improved using the mother as donor. Blood. 2008;112:2990–2995. doi: 10.1182/blood-2008-01-135285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki E., Kataoka T.R., Hirata M., Kawaguchi K., Nishie M., Haga H., Toi M. Trogocytosis-mediated expression of HER2 on immune cells may be associated with a pathological complete response to trastuzumab-based primary systemic therapy in HER2-overexpressing breast cancer patients. BMC Cancer. 2015;15:39. doi: 10.1186/s12885-015-1041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchilian E.Z., Wallace D.L., Wells R.S., Flower D.R., Morgan G., Beverley P.C. A deletion in the gene encoding the CD45 antigen in a patient with SCID. J. Immunol. 2001;166:1308–1313. doi: 10.4049/jimmunol.166.2.1308. [DOI] [PubMed] [Google Scholar]
- Van Den Broek M.F., Kagi D., Zinkernagel R.M., Hengartner H. Perforin dependence of natural killer cell-mediated tumor control in vivo. Eur. J. Immunol. 1995;25:3514–3516. doi: 10.1002/eji.1830251246. [DOI] [PubMed] [Google Scholar]
- Velardi A. Role of KIRs and KIR ligands in hematopoietic transplantation. Curr. Opin. Immunol. 2008;20:581–587. doi: 10.1016/j.coi.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Vey N., Bourhis J.H., Boissel N., Bordessoule D., Prebet T., Charbonnier A., Etienne A., Andre P., Romagne F., Benson D., Dombret H., Olive D. A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood. 2012;120:4317–4323. doi: 10.1182/blood-2012-06-437558. [DOI] [PubMed] [Google Scholar]
- Villalba M., Rathore M.G., Lopez-Royuela N., Krzywinska E., Garaude J., Allende-Vega N. From tumor cell metabolism to tumor immune escape. Int. J. Biochem. Cell Biol. 2013;45:106–113. doi: 10.1016/j.biocel.2012.04.024. [DOI] [PubMed] [Google Scholar]
- Villalba M., Lopez-Royuela N., Krzywinska E., Rathore M.G., Hipskind R.A., Haouas H., Allende-Vega N. Chemical metabolic inhibitors for the treatment of blood-borne cancers. Anti Cancer Agents Med. Chem. 2014;14:223–232. doi: 10.2174/18715206113136660374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E., Tomasello E., Baratin M., Walzer T., Ugolini S. Functions of natural killer cells. Nat. Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- Wang J., Pantopoulos K. Regulation of cellular iron metabolism. Biochem. J. 2011;434:365–381. doi: 10.1042/BJ20101825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren H.S., Skipsey L.J. Loss of activation-induced CD45RO with maintenance of CD45RA expression during prolonged culture of T cells and NK cells. Immunology. 1991;74:78–85. [PMC free article] [PubMed] [Google Scholar]
- Willemze R., Rodrigues C.A., Labopin M., Sanz G., Michel G., Socie G., Rio B., Sirvent A., Renaud M., Madero L., Mohty M., Ferra C., Garnier F., Loiseau P., Garcia J., Lecchi L., Kogler G., Beguin Y., Navarrete C., Devos T., Ionescu I., Boudjedir K., Herr A.L., Gluckman E., Rocha V. KIR-ligand incompatibility in the graft-versus-host direction improves outcomes after umbilical cord blood transplantation for acute leukemia. Leukemia. 2009;23:492–500. doi: 10.1038/leu.2008.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Weiss A. Negative regulation of CD45 by differential homodimerization of the alternatively spliced isoforms. Nat. Immunol. 2002;3:764–771. doi: 10.1038/ni822. [DOI] [PubMed] [Google Scholar]
- Zarcone D., Prasthofer E.F., Malavasi F., Pistoia V., Lobuglio A.F., Grossi C.E. Ultrastructural analysis of human natural killer cell activation. Blood. 1987;69:1725–1736. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.