Abstract
Background
Cysteamine has recently been shown to have in vitro properties potentially therapeutically beneficial in cystic fibrosis (CF). In this study we investigated the antimicrobial and mucolytic activity of cysteamine against the complex biologic matrix of CF sputum.
Methods
Sputum samples were obtained from 23 CF adults. Sputum polymicrobial content after in vitro exposure to cysteamine and standard CF antibiotics was assessed after a single exposure and after 14 days low-dose exposure. The effect of cysteamine on sputum spinnbarkeit was assessed.
Findings
Cysteamine reduced sputum polymicrobial burden by 3 · 18 (95% CI 2 · 30–4 · 07, p < 0.001) log10 units after 24 h incubation. Combined cysteamine and tobramycin reduced polymicrobial burden by a further 3 · 75 (95% CI 2 · 63–5 · 07, p < 0 · 001) log10 units above that seen with tobramycin. Repeated low dosing with cysteamine reduced sputum polymicrobial load from day 10 onwards (p = 0.032). Cysteamine reduced CF sputum viscoelasticity, sputum spinnbarkeit cysteamine 11.1 mm/s (95% CI 3.95–18.2) vs DNAse 1.69 mm/s (95% CI 0.73–2.65), p = 0.016. Cysteamine was active against Mycobacterium abscessus as a monotherapy and also potentiated the effects of amikacin and azithromycin.
Conclusion
Further investigation is required into the therapeutic potential of cysteamine in CF to treat emerging as well as established microbial pathogens and as a mucolytic agent.
Keywords: Cysteamine, Cystic fibrosis, Mycobacterium abscessus, Pseudomonas aeruginosa, Sputum
Highlights
-
•
Cysteamine may have a role in treating cystic fibrosis.
-
•
Cysteamine was added to sputum samples from 23 patients with cystic fibrosis.
-
•
Cysteamine reduced microbial load and increased the effectiveness of tobramycin.
-
•
Cysteamine greatly reduced the viscoelasticity of sputum.
-
•
Cysteamine had activity against the emerging pathogen Mycobacterium abscessus.
Cystic fibrosis (CF) is a genetic disease that damages the lungs because the thick sticky mucus produced in CF airways becomes infected. There is a need to develop new treatments for CF lung infections. In this study we have taken sputum samples from 23 people with CF and shown that an investigational drug cysteamine reduces the number of bacteria in the sputum and also makes an antibiotic work better. Cysteamine also reduced sputum stickiness. These results suggest that cysteamine may have a role treating CF lung infections and further research is required to fully assess this.
1. Introduction
Cystic Fibrosis (CF) is an autosomal recessively inherited disease most prevalent in Caucasian populations of European origin (World Health Organization, 2004). In the UK about 10,000 people have CF, globally about 70–100,000 people are affected (World Health Organization, 2004). Although CF is a multisystem condition the majority of CF associated morbidity and mortality is a consequence of chronic suppurative lung disease and ultimately respiratory failure (UK CF Registry, 2014, Goss and Quittner, 2007), currently median (95% CI) age of death in the UK is 29 (27–31) (UK CF Registry, 2014). Infection of the lower airways by Pseudomonas (Emerson et al., 2002), and Burkholderia species (Muhdi et al., 1996, Jones et al., 2004) adversely affects morbidity, quality of life and survival, and there are increasing concerns about the consequences of infection with emergent (Lipuma, 2010) pathogen species such as Mycobacterium abscessus (Esther et al., 2010, Hansen et al., 2006). In CF, Pseudomonas (Emerson et al., 2002) and Burkholderia (Muhdi et al., 1996, Jones et al., 2004) species grow in biofilms and as such are much more resistant to antibiotics compared with planktonic-growing cells of the same isolate (Stewart and Costerton, 2001). The aggressive use of antibiotics to suppress chronic infection and to treat acute exacerbations has contributed to the increased survival of people with CF. However, increasing antibiotic resistance, multiple antibiotic resistance and drug intolerance are emerging as major clinical problems. These issues have led to calls for research into new antimicrobial agents and new antibiotic strategies to target the biofilm and to increase the effectiveness of currently available antibiotics (Bals et al., 2011).
We have previously described the in vitro antimicrobial, anti-biofilm and mucoactive properties of cysteamine as a monotherapy and in combination with CF guideline recommended antibiotics (Charrier et al., 2014). Cysteamine has been routinely used to treat cystinosis for more than 20 years and so a significant body of clinical data already exists as to its characteristics within this patient group. The antimicrobial and mucoactive attributes of cysteamine described to date are potentially therapeutically beneficial in CF, not only in the treatment of acute exacerbations but also chronic management (Charrier et al., 2014). An inherent limitation of the data derived thus far is that they were generated from monocultures of bacteria most commonly isolated from people with CF and against individual components of mucus. To investigate the potential of cysteamine in CF under more physiologically robust conditions, we investigated its mucolytic and antimicrobial activity against sputum from CF patients and report here for the first time on its rheological properties and antimicrobial action against the polymicrobial content of CF sputum. We also report on the activity of cysteamine against M. abscessus isolates from CF participants in this study.
2. Methods and Materials
2.1. Subjects
Patients attending the adult CF clinic at Aberdeen Royal Infirmary, Aberdeen, UK were invited to participate in a cross-sectional study investigating the antimicrobial properties of cysteamine in sputum from people with CF. The inclusion criteria were: diagnosed CF associated lung disease and able to spontaneously expectorate sputum; 57 patients fulfilled the eligibility criteria and were invited to participate. Participants provided a sample of spontaneously expectorated sputum. The clinical data collected included: age, sex, height, weight, ventilatory function (FEV1) and CF genotype. Also recorded were the bacterial species infecting the sputum (Lee et al., 2003) and whether the participant was exacerbating, had a recent exacerbation (completed treatment < 4 weeks previously) or was clinically stable (exacerbation > 4 weeks previously). Antimicrobial therapy at the time of sampling was recorded e.g. azithromycin, inhaled therapies. The study received ethical approval (13/NS/0001) from the North of Scotland Research Ethics Service and all participants provided written informed consent.
2.2. Chemicals and Growth Media
Tobramycin was purchased from Discovery Fine Chemicals (UK). All other chemicals, growth media and antibiotics were obtained from Sigma-Aldrich (UK).
2.3. Effect of Cysteamine and Antibiotics on CF Sputum Microbial burden: Single Exposure
Sputum samples were processed for antimicrobial activity within 4 h of collection. To assess the antimicrobial impact of cysteamine alone and cysteamine in conjunction with antibiotics commonly used to treat infective exacerbations of CF lung disease, 0 · 2 ml of each sputum sample sputum was diluted tenfold in sterile phosphate buffered saline (PBS) and vortexed. Aliquots (0 · 2 ml) of the homogenised diluted sample were exposed to cysteamine only (1 mg/ml) (Charrier et al., 2014), antibiotic (tobramycin [0 · 1 mg/ml] or ciprofloxacin, [0 · 1 mg/ml] only), cysteamine plus antibiotic or vehicle (PBS) only, for 4 h and 24 h at 37 °C. Sputum samples were then serially diluted tenfold (1 × 10− 1 to 1 × 10− 8) and spread-plated on non-selective nutrient agar plates. Cultures were incubated at 37 °C and colony forming units per ml (cfu/ml) of bacteria quantified at 48 h.
2.4. Determination of CF Sputum Microbial Burden: Multiple Exposures
To assess any impact of cysteamine on sputum microbial burden in CF at pharmacological concentrations reported in vivo, multiple dosing experiments were conducted. In these assays, 0 · 2 ml of sputum were exposed daily (first dosing within 4 h of collection as above) to 2 μg/ml of cysteamine, or PBS as a control, for 14 days. 2 μg/ml is a level typically reported when dosing with cysteamine in patients with cystinosis (Langman et al., 2012). On alternate days, 10 μl of each sample was recovered and serially diluted tenfold (1 × 10− 1 to 1 × 10− 8) and spread-plated on non-selective nutrient agar plates. Cultures were incubated at 37 °C and cfu/ml of bacteria quantified at 48 h.
2.5. Quantification of CF Sputum Macrorheological Properties (spinnbarkeit)
Macrorheological analysis was conducted within 4 h of collection of sputum samples. Aliquots (0 · 2 ml) of sputum were incubated for 1 h at 37 °C after the addition of cysteamine (1 mg/ml), PBS or DNAse (500 U/ml). The treated sputum samples were was then transferred to the open end of a 2 ml graduated pipette (Greiner, UK) which was secured vertically. Each sputum sample was allowed to descend inside the pipette under gravity. This process was timed and filmed, and the velocity of the sputum was calculated as distance travelled over time taken in mm/s.
2.6. Antimicrobial Susceptibility of Mycobacteria Abscessus Sputum Isolates in vitro
M. abscessus complex (MAC) was isolated from three participating patients. The susceptibility of these isolates, plus the MAC type strain M. abscessus DSMZ44196 to the antimicrobial effects of cysteamine alone and to cysteamine combined with antibiotics employed in MAC eradication strategies (amikacin, azithromycin and meropenem) was assessed by CLSI broth microdilution procedure (Clinical and Laboratory Clinical Institute, 2012) and checkerboard assay respectively.
2.7. Statistical Considerations
FEV1 was expressed as a percentage of predicted using GLI 2012 (Quanjer et al., 2012) reference equations. Sputum microbial load expressed as colony forming units approximated to a log-normal distribution and was therefore expressed as mean log10 (95% confidence interval). The microbial load of CF sputum samples after incubation with cysteamine, tobramycin, ciprofloxacin or combinations thereof after 4 h and 24 h was modelled using two way repeated measures ANOVA with post hoc testing using Bonferroni adjustment. Analyses were performed using IBM SPSS Statistics for Windows, v22 · 0 (Armonk, NY).
2.8. Role of the Funding Source
This study was funded by Scottish Enterprise. The funder did not contribute to study design, data collection, analysis, this report or the decision to publish.
3. Results
3.1. Patient Population
Twenty three of the eligible 57 patients participated in the study, each provided a sputum sample, their clinical characteristics are outlined in Table 1. All of the 15 patients infected with Pseudomonas aeruginosa were infected by at least one mucoid strain.
Table 1.
Clinical characteristics of participating patients.
| All subjects (n = 23) | Ciprofloxacin subgroup (n = 9) | Low dosing subgroup (n = 13) | Rheology subgroup (n = 6) | |
|---|---|---|---|---|
| Age (yrs) (median, IQR) | 28 (19–36) | 24 (19–37) | 29 (19–36) | 24 (19–45) |
| Female (n,%) | 13 (57%) | 6 (67%) | 6 (46%) | |
| DF508homozygous (n,%) | 16 (70%) | 8 (89%) | 9 (69%) | 5 (83%) |
| DF508heterozygous | 6 (26%) | 1 (11%) | 3 (23%) | 1 (17%) |
| DF508 negative | 1 (4%) | 0 | 1 (8%) | 0 |
| BMI (mean 95% CI) | 22.1 | 22.0 | 22.4 | 22.3 |
| (20.9–23.3) | (19.1–24.9) | (20.5–24.4) | (17.6–27.0) | |
| FEV1% predicted (mean 95% CI) | 62% (49–74) | 54% (37–70) | 69 (52–85) | 53% (29–77) |
| Last exacerbation (n,%) | ||||
| Acute | 9 (39%) | 5 (56%) | 5 (39%) | 3 (50%) |
| < 4 weeks | 8 (35%) | 3 (33%) | 6 (46%) | 2 (33%) |
| > 4 weeks | 6 (26%) | 1 (11%) | 2 (15%) | 1 (17%) |
| Concomitant medication | ||||
| Azithromycin (n,%) | 20 (87%) | 9 (100%) | 11 (85%) | 6 (100%) |
| Inhaled antibiotic (n,%) | 20 (87%) | 8 (89%) | 12 (92%) | 5 (83%) |
| Ivacaftor (n,%) | 2 (9%) | 0 | 0 | 0 |
| Sputum culture (n,%) | ||||
| Staphylococcus aureus | 4 (17%) | 1 (11%) | 3 (23%) | 1 (17%) |
| Pseudomonas aeruginosa | 15 (65%) | 6 (67%) | 8 (62%) | 5 (83%) |
| Burkholderia species | 4 (17%) | 2 (22%) | 2 (15%) | 0 |
| Stenotrophomonas maltophilia | 2 (9%) | 1 (11%) | 2 (15%) | 1 (17%) |
| Mycobacterium abscessus | 3 (13%) | 0 | 0 | 0 |
3.2. Antimicrobial Activity of Cysteamine Against Polymicrobial Burden in CF Sputum
The antimicrobial activity of tobramycin, cysteamine and combined tobramycin/cysteamine were tested in all 23 samples and are presented in Fig. 1. Cysteamine reduced sputum polymicrobial load after 4 h in sputum samples from 20 patients and from 21 patients after 24 h. When compared with untreated samples tobramycin, cysteamine and combined cysteamine/tobramycin reduced polymicrobial load at 4 h and 24 h (all p < 0.001). Overall, tobramycin, cysteamine and combined cysteamine/tobramycin significantly (p < 0 · 001) reduced polymicrobial load by 1 · 42 (95% CI 0 · 92–1 · 92), 3 · 18 (95% CI 2 · 30–4 · 07), and 3 · 86 (95% CI 3 · 11–4 · 61) log10 units respectively. When compared with tobramycin, cysteamine further reduced polymicrobial load by 1 · 76 (95% CI 0 · 89–2 · 63, p < 0 · 001) log10 units. When compared with tobramycin, at 4 h combined cysteamine/tobramycin reduced polymicrobial load by 1.03 (95% CI − 0 · 50–1 · 56, p = 0 · 001) log10 units above that observed with tobramycin. After 24 h of exposure cysteamine/tobramycin further reduced polymicrobial load by 3 · 75 (95% CI 2 · 63–5 · 07, p < 0 · 001) log10 units above that seen with tobramycin (5.65, 95% CI 4.46–6.80).
Fig. 1.
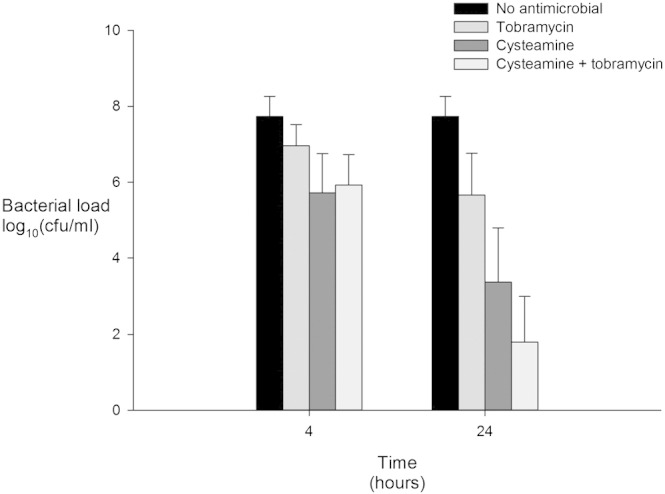
The antimicrobial activity of tobramycin, cysteamine and combined tobramycin and cysteamine on polymicrobial load after 4 and 24 h exposure. Bacterial load expressed as mean (95% confidence interval).
There was sufficient sputum to test antimicrobial activity of ciprofloxacin, cysteamine and combined ciprofloxacin/cysteamine in nine samples, the clinical characteristics of this subgroup are presented in Table 1 and the results are presented in Fig. 2. When compared with untreated samples cysteamine and combined cysteamine/ciprofloxacin reduced polymicrobial load at 4 h and 24 h (all p < 0.005), ciprofloxacin reduced polymicrobial load at 24 h (p = 0.001) but not at 4 h (p = 0.234). Overall ciprofloxacin, cysteamine and combined cysteamine/ciprofloxacin significantly reduced polymicrobial load by 0 · 84 (95% CI 0 · 29–1 · 39, p = 0 · 008), 2 · 76 (95% CI 1 · 32–4 · 20, p = 0 · 002), and 2 · 86 (95% CI 1 · 68–3 · 98, p < 0 · 001) log10 units respectively. When compared with ciprofloxacin, cysteamine further reduced polymicrobial load by 1 · 92 (95% CI 0 · 85–3 · 00, p = 0 · 003) log10 units. Overall when compared with ciprofloxacin combined cysteamine/ciprofloxacin further reduced polymicrobial load by 1 · 99 (95% CI 1 · 02–2 · 96, p < 0 · 001) log10 units, however combined cysteamine/ciprofloxacin did not reduce polymicrobial load over and above that achieved by cysteamine alone.
Fig. 2.
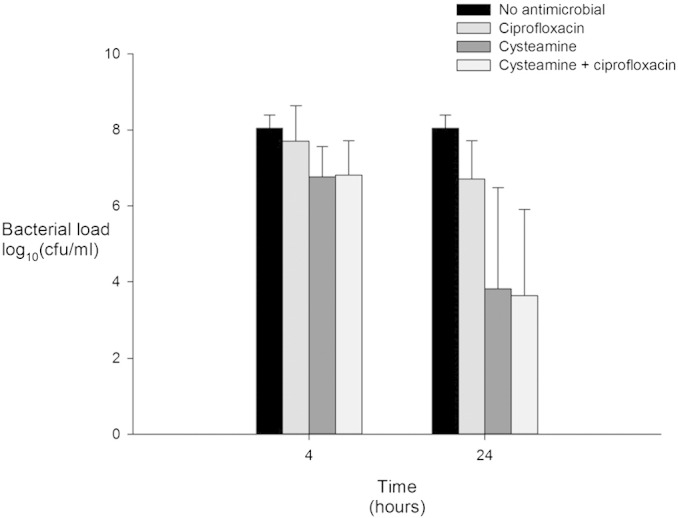
The antimicrobial activity of ciprofloxacin, cysteamine and combined ciprofloxacin and cysteamine on polymicrobial load after 4 and 24 h exposure. Bacterial load expressed as mean (95% confidence interval).
Inclusion of clinical factors in the repeated measures ANOVA analysis demonstrated that sex, CF genotype, FEV1, exacerbation status, or concomitant use of azithromycin, inhaled antibiotics or ivacaftor did not modify the antimicrobial effects reported above.
3.3. Antimicrobial Activity of Cysteamine at a Pharmacological Concentration in CF Sputum
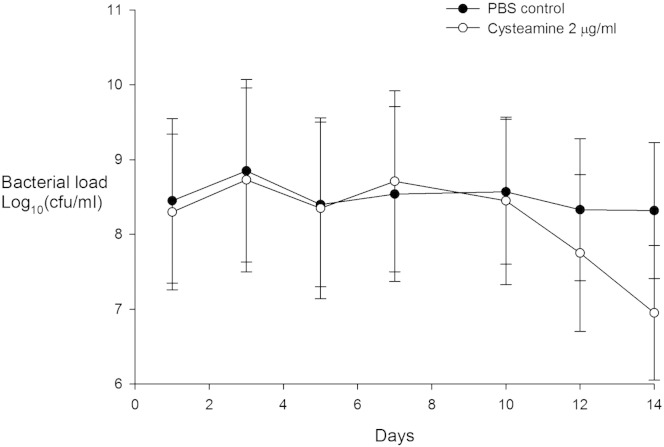
The effects of in vitro daily dosing of sputum from 13 CF patients with 2 μg/ml cysteamine and PBS over a two week period are presented in Fig. 4, the clinical characteristics of this subgroup are presented in Table 1. This dose of cysteamine is typical of the plasma levels observed in patients with cystinosis after dosing with cysteamine (Clinical and Laboratory Clinical Institute, 2012). Cysteamine significantly reduced the polymicrobial burden in CF sputum (Greenhouse–Geisser corrected p = 0 · 032) from about day 10 onwards. Sputum microbial load after 10 days of treatment was lower with cysteamine but this was only significant for day 10 (p = 0 · 044) but not day 14 (p = 0 · 088).
Fig. 4.
The antimicrobial activity of cysteamine (2 μg/ml) administered daily on sputum polymicrobial load over two weeks. Bacterial load expressed as mean (95% confidence interval).
3.4. Sputum Macrorheology/spinnbarkeit
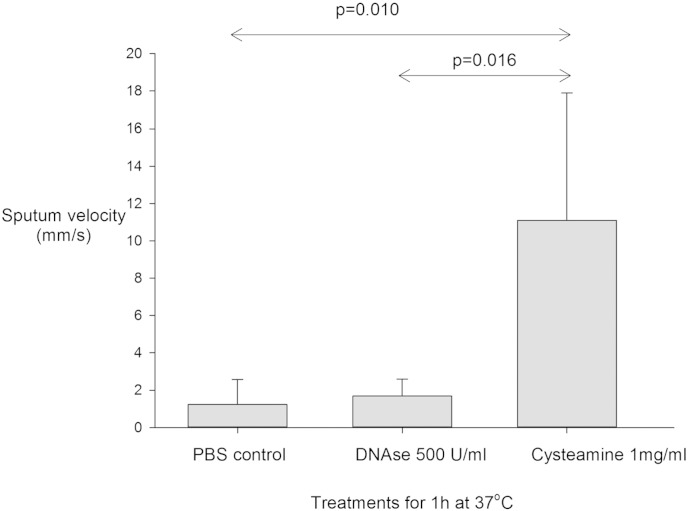
The effect of cysteamine on sputum spinnbarkeit was tested in samples from six participants where the volume of sputum provided permitted analysis, the clinical characteristics of this subgroup are presented in Table 1. Cysteamine was a potent mucolytic in all CF sputum samples tested, outperforming human recombinant DNAse I (p = 0 · 016), a widely used mucolytic in the CF-field (Bilton and Stanford, 2014), in its reduction of sputum viscoelasticity after 1 h treatment at 37 °C (Fig. 3). The effect on sputum spinnbarkeit after 1 h is demonstrated in the Video clip Fig. 5, but improvements in sputum viscosity treated with cysteamine were rapid and noticeable even after 5 min (data not shown).
Fig. 3.
Effect of cysteamine on CF sputum viscoelasticity compared with control and DNAse I treated sputum taken from 6 patients. Sputum velocity expressed as mean (95% confidence interval).
3.5. Antimicrobial Susceptibility of Mycobacterium Abscessus Sputum Isolates in vitro
The M. abscessus isolated from three of the study subjects (in whose sputum samples a significant reduction in polymicrobial burden was brought about with exposure to cysteamine and cysteamine plus tobramycin or ciprofloxacin) were all sensitive to the antimicrobial effects of cysteamine (MIC range 62 · 5–250 μg/ml) when tested in vitro, as was the type strain DSMZ44196 (Table 2). Furthermore, cysteamine potentiated the impact of amikacin in both clinical and type strains, and azithromycin in all but one clinical strain, with fractional inhibitory concentration indices (FICI) (Vanhoof et al., 1978, Choi et al., 2012) demonstrating the more than additive or synergistic potential of combined therapy. Cysteamine had concentration-dependent effects on meropenem sensitivity in the clinical isolates and type strain of M. abscessus tested, showing some concentration-specific antagonism.
Table 2.
Cysteamine activity against a panel of M. abscessus strains, synergy with the clinically relevant antibiotics, amikacin and azithromycin is demonstrated by chequerboard experiments and calculation of fractional inhibitory concentration index (FICI).
| MIC100 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Strain | Cysteamine [μg/ml] | Meropenem [μg/ml] | Amikacin [μg/ml] | Azithromycin [μg/ml] | Meropenem/cysteamine (FICI) | Amikacin/cysteamine (FICI) | Azithromycin/cysteamine (FICI) | |||
| DSM44196 (type) | 62 · 5–125 | 64 | 4–8 | 1–2 | 32–64/62 · 5 (1 · 5–2) | Neutral | 1–4/15 · 625–62 · 5 (0 · 5–0 · 75) | Additive | 0 · 25/15 · 625 (0 · 25–0 · 5) | Synergy |
| MR313292 (clinical) | 125–250 | 16–64 | 4 | 8 | 32–64/250 (2–2 · 5) | Neutral-antagonistic | < 1/< 15 · 125–31 · 25 (0 · 375) | Synergy | 4–8/250 (1 · 25–2) | Neutral |
| MR27419N (clinical) | 125 | 64 | 16 | 1 | 64/125 (2) | Neutral | 4/62 · 5 (0 · 75) | Additive | 0 · 25/< 15 · 625 (0 · 375) | Synergy |
| MR313367D (clinical) | 62 · 5–125 | 16–64 | 16 | 1 | 64/125 (2–5) | Neutral-antagonistic | 4/15 · 625–31 · 25 (0 · 5) | Synergy | 0 · 25/15 · 625 (0 · 375–0 · 5) | Synergy |
All results are presented represent the mean of triplicate samples from experiments conducted three times.
Values shown in the table are MIC100. Where antibiotics have been used in combination, the unhighlighted figures show the MIC100 range for each antibiotic, and the figures highlighted in bold are the fractional inhibitory concentration index (FICI) range. Values ≤ 0.5 are interpreted as synergy.
4. Discussion
Cysteamine is an aminothiol (HSCH2CH2NH2) endogenously present at very low levels as a consequence of coenzyme A metabolism (Besouw et al., 2013, Orloff et al., 1981). In the US and EU, cysteamine has been licensed for the treatment of nephropathic cystinosis for over 20 years. In this observational study the in vitro antimicrobial and mucoactive properties of cysteamine were tested in sputum samples from adults with CF infected with bacteria typical of those reported in the UK CF population (Staphylococcus aureus, P. aeruginosa, Burkholderia species and Stenotrophomonas maltophilia). Cysteamine reduced sputum polymicrobial load ex vivo and at the doses tested this antimicrobial effect was greater than that observed for tobramycin and ciprofloxacin. The combination of cysteamine with tobramycin appeared to be more than additive after 24 h incubation, being greater than the individual effects of cysteamine or tobramycin. The combination of cysteamine/ciprofloxacin appeared to be no more antimicrobial than cysteamine alone, although we have previously demonstrated a synergistic interaction for these two compounds in vitro (Charrier et al., 2014). Whilst the lack of synergy between cysteamine and ciprofloxacin may merely be a consequence of the small number of sputum samples analysed (n = 9), it may be that in the matrix of sputum the combination of cysteamine/ciprofloxacin is unable to exert the additive effect present when cultured under standard media conditions, further work is required to investigate whether in vivo there is synergy between cysteamine and ciprofloxacin. In our present study, we also demonstrated that M. abscessus is sensitive to cysteamine; more so than for other CF bacteria when tested in vitro as a monotherapy. Cysteamine also potentiates the activity of other antimicrobial agents. In addition to cysteamine's antimicrobial effects, in vitro, cysteamine had mucolytic effects, reducing sputum viscosity 8–9 fold, notably cysteamine had more potent (6–7 fold) mucolytic effect than recombinant DNAse. We demonstrated that the antimicrobial effects of cysteamine were not modified by clinical factors e.g. sex, lung function, exacerbation status nor by concurrent medication e.g. azithromycin, inhaled antibiotics or ivacaftor. However, these analyses were of small numbers of patients and lacked statistical power, further work is required to determine whether any antimicrobial effects of cysteamine are modified by concurrent medications, or other factors e.g. disease severity, sex, age.
Previous in vitro studies using type strains and monocultures of bacterial pathogens have shown cysteamine to have direct antimicrobial activity with MIC100 at concentrations in the range of 250–500 μg/ml (in nutrient rich media) against P. aeruginosa and other CF pathogens including Burkholderia cepacia complex, S. aureus and also emerging pathogens including Achromobacter xylosoxidans, and Stenotrophomonas species (Charrier et al., 2014). The current study is a more clinically relevant investigation because cysteamine was exposed to polymicrobial populations of CF-associated pathogens in their natural clinical matrix i.e. sputum from patients with CF. The data derived are a progression from, and concur with previous observed in vitro antimicrobial and mucolytic effects.
The present study demonstrates that cysteamine is antimicrobial and mucoactive within CF sputum. It has also been shown that cysteamine rescues the functional expression of the F508del CF transmembrane conductance regulator (CFTR) in CFTR F508del homozygous mice. In a study of 10 F508del homozygous patients with CF aged 8–25 years the sequential administration of oral cysteamine and the green tea flavonoid epigallocatechin gallate (EGCG) improved CFTR function, decreased sweat chloride concentrations and reduced sputum TNF and CXCL8 protein levels (De Stefano et al., 2014). In the present study, the effects of cysteamine are independent of CFTR genotype, suggesting that future trials of cysteamine as an antimicrobial-mucolytic should not be limited to F508del homozygous patients with CF. Thiols such as cysteamine and N-acetylcysteine have many biological properties, the mucolytic effects of cysteamine are likely to be a consequence of the thiol moiety reducing disulphide bonds in mucus proteins, disrupting their ligand bonding and structure (Samuni et al., 1830). N-acetylcysteine has also been shown to have antimicrobial and antibiofilm activity and synergy with ciprofloxacin against P. aeruginosa has been inconsistently reported (Zhao and Liu, 2010). The antibiofilm activity of N-acetylcysteine has been attributed to thiol disruption of disulphide bonds in enzymes involved in biofilm synthesis, it is possible that similar effects on proteins involved in gene expression, signalling and cell cycling underlie the antimicrobial effects of cysteamine.
The doses of cysteamine applied for the single exposure experiments in the present study were four times the median MIC100 previously established for cysteamine against CF associated pathogens in nutrient-rich media (Charrier et al., 2014). Presently we do not know what, if any, local levels of cysteamine may be achievable in the sputum of CF patients after oral or aerosol delivery. It is known however, that in patients with cystinosis, after a single standard dose of cysteamine at steady state, peak plasma concentration is 2–3 μg/ml (Langman et al., 2012). In an attempt to evaluate ex vivo the effects of cysteamine at these much lower concentrations, sputum samples were exposed daily to 2 μg/ml cysteamine for 14 days. The data from these experiments suggest that significant reductions in total microbial sputum burden are likely to be achieved if cysteamine were to be used as an adjunct to conventional antimicrobial therapy for the typical 14 day course used to treat acute infective exacerbations of CF lung disease. Consideration of the physical properties of cysteamine predict that in vivo the antimicrobial effect of 14 days oral cysteamine is likely to be greater than that observed in the current study. With an acid dissociation constant (pKa) of 10.4, and a partition coefficient (logP) of 0.01 (Drugbank), cysteamine is likely to pass from the systemic circulation into the acidic bronchial secretions (pH 6.0–6.9) (Palmer et al., 2007) such that the concentration of cysteamine will be higher in the bronchial secretions than in the systemic circulation.
A key consideration when developing any potential new antimicrobial strategy, particularly one based on a broad-spectrum agent, is its impact on resistance and whether modifying the existing microbiome creates niches for additional, emerging pathogens. It will be very difficult to establish until introduction into clinical practice whether this is the case for cysteamine, but there is now in vitro and ex vivo evidence that cysteamine is active against emerging pathogens. We have already reported antimicrobial activity against species such as Achromobacter species and Stenotrophomonas species (Charrier et al., 2014) and in the current study we have furthered this work by investigating whether cysteamine has antimicrobial activity against the emerging pathogen M. abscessus. The prevalence of M abscessus infection in patients with CF is increasing and there is great clinical concern since this microorganism is multidrug resistant and patient to patient transmission has been recently demonstrated (Bryant et al., 2013). Three of the participants in this study were infected with M. abscessus and their isolates were all clearly sensitive to cysteamine when tested in vitro, not only as a monotherapy but particularly in combination with amikacin and azithromycin. Although the strains tested were already sensitive to amikacin as defined by CLSI breakpoints, this sensitivity increased markedly in each of the strains tested. The same was true for the M. abscessus type strain. Indeed the MIC100 for cysteamine against M. abscessus was lower than we previously reported for Pseudomonas species and all other CF pathogens. The identification of an orally active agent against M. abscessus could be potentially useful, the synergism between cysteamine and amikacin and azithromycin could be advantageous in the protracted continuation phase of M. abscessus therapy, however the dose-dependent antagonism of meropenem may preclude the use of cysteamine in the initial intensive phase of treatment. Although this study has identified a potentially useful drug for the treatment of patients with CF infected with M. abscessus, only four isolates were tested in an extracellular in vitro context so further work is clearly required.
The study we report here further supports continued development of cysteamine as a novel “re-purposed” candidate CF treatment by confirming activity and therapeutic potential in the biological matrix (CF sputum) and raises the prospect of cysteamine being used to treat M. abscessus. It remains to be confirmed however, the extent to which oral cysteamine is absorbed and enters the bronchial secretions of patients with CF. The tolerability of cysteamine when used in patients with CF also needs to be ascertained because chronic oral cysteamine therapy (in cystinosis) is associated with side-effects that could be detrimental to the overall long term health of patients with CF, e.g. anorexia, nausea, breath odour, lethargy, osteopenia and skin striae. These side effects may not preclude the use of oral cysteamine as an adjunct to conventional antimicrobial treatment of acute infective exacerbations of CF lung disease. They could however, be problematic long term, but overcome by the development of an inhaled form of cysteamine for chronic dosing.
The following is the supplementary data related to this article.
Comparative effects of DNAse I and cysteamine treatment (for 1 h at 37 °C) on the spinnbarkeit of sputum taken from a volunteer with cystic fibrosis.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2015.08.018.
Author Contributions
GD as Chief Clinical Investigator contributed to study inception, obtained funding, contributed to the recruitment of patients, collection of samples, data analysis and manuscript preparation.
DFP, JR, ED, DM conducted the microbiological aspects of the study, and contributed to data analysis and manuscript preparation.
DO'N contributed to study inception, obtained funding, and contributed to data analysis and manuscript preparation.
Declaration of Interests
GD reports grants from Scottish Enterprise during the conduct of the study.
DFP reports grants from Scottish Enterprise, during the conduct of the study; personal fees from NovaBiotics Ltd, outside the submitted work; DFP has a patent Treatment of M. abscessus infections GB14832P pending.
JR reports grants from Scottish Enterprise, during the conduct of the study; personal fees from NovaBiotics Ltd, outside the submitted work.
ED reports grants from Scottish Enterprise, during the conduct of the study; grants from NovaBiotics Ltd, outside the submitted work.
DM reports grants from Scottish Enterprise, during the conduct of the study; personal fees from NovaBiotics Ltd, outside the submitted work.
DO'N reports grants from Scottish Enterprise, during the conduct of the study; personal fees and other from NovaBiotics Ltd, from null, outside the submitted work; DO'N has a patent Biofilms PCT/GB2010/000631 issued, a patent Cysteamine and antibiotics PCT/GB2011/001721 issued, a patent Cysteamine for treating yeasts and moulds B1416727.4, US62/053,523 pending, and a patent Treatment of M. abscessus infections GB14832P pending.
Acknowledgement
This study was funded by Scottish Enterprise Encompass Kick Start Award KSB001.
References
- Bals R., Hubert D., Tümmler B. Antibiotic treatment of CF lung disease: from bench to bedside. J. Cyst. Fibros. 2011;10(Suppl. 2):S146–S151. doi: 10.1016/S1569-1993(11)60019-2. [DOI] [PubMed] [Google Scholar]
- Besouw M., Masereeuw R., van den Heuvel L., Levtchenko E. Cysteamine: an old drug with new potential. Drug Discov. Today. 2013;18(15–16):785–792. doi: 10.1016/j.drudis.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Bilton D., Stanford G. The expanding armamentarium of drugs to aid sputum clearance: how should they be used to optimize care? Curr. Opin. Pulm. Med. 2014;20:601–606. doi: 10.1097/MCP.0000000000000104. [DOI] [PubMed] [Google Scholar]
- Bryant J.M., Grogono D.M., Greaves D., Foweraker J., Roddick I., Inns T., Reacher M., Haworth C.S., Curran M.D., Harris S.R., Peacock S.J., Parkhill J., Floto R.A. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: a retrospective cohort study. Lancet. 2013;381(9877):1551–1560. doi: 10.1016/S0140-6736(13)60632-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier C., Rodger C., Robertson J., Kowalczuk A., Shand N., Fraser-Pitt D., Mercer D., O'Neil D. Cysteamine (Lynovex®), a novel mucoactive antimicrobial & antibiofilm agent for the treatment of cystic fibrosis. Orphanet J. Rare Dis. 2014;9:189. doi: 10.1186/s13023-014-0189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi G.E., Min K.E., Won C.J., Jeon K., Shin S.J., Koh W.J. Activities of moxifloxacin in combination with macrolides against clinical isolates of Mycobacterium abscessus and Mycobacterium massiliense. Antimicrob. Agents Chemother. 2012;56:3549–3555. doi: 10.1128/AAC.00685-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical and Laboratory Clinical Institute . Ninth Edition: Approved Standard M07-A9. CLSI; Wayne, PA, USA: 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. [Google Scholar]
- De Stefano D., Villella V.R., Esposito S., Tosco A., Sepe A., De Gregorio F., Salvadori L., Grassia R., Leone C.A., De Rosa G., Maiuri M.C., Pettoello-Mantovani M., Guido S., Bossi A., Zolin A., Venerando A., Pinna L.A., Mehta A., Bona G., Kroemer G., Maiuri L., Raia V. Restoration of CFTR function in patients with cystic fibrosis carrying the F508del-CFTR mutation. Autophagy. 2014;10:2053–2074. doi: 10.4161/15548627.2014.973737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drugbank Cysteamine. http://www.drugbank.ca/drugs/DB00847 (accessed July 2015)
- Emerson J., Rosenfeld M., McNamara S., Ramsey B., Gibson R.L. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr. Pulmonol. 2002;34:91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- Esther C.R., Jr., Esserman D.A., Gilligan P., Kerr A., Noone P.G. Chronic Mycobacterium abscessus infection and lung function decline in cystic fibrosis. J. Cyst. Fibros. 2010;9:117–123. doi: 10.1016/j.jcf.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss C.H., Quittner A.L. Patient-reported outcomes in cystic fibrosis. Proc. Am. Thorac. Soc. 2007;4:378–386. doi: 10.1513/pats.200703-039BR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C.R., Pressler T., Høiby N., Gormsen M. Chronic infection with Achromobacter xylosoxidans in cystic fibrosis patients; a retrospective case control study. J. Cyst. Fibros. 2006;5:245–251. doi: 10.1016/j.jcf.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Jones A.M., Dodd M.E., Govan J.R., Barcus V., Doherty C.J., Morris J., Webb A.K. Burkholderia cenocepacia and Burkholderia multivorans: influence on survival in cystic fibrosis. Thorax. 2004;59:948–951. doi: 10.1136/thx.2003.017210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langman C.B., Greenbaum L.A., Sarwal M., Grimm P., Niaudet P., Deschênes G., Cornelissen E., Morin D., Cochat P., Matossian D., Gaillard S., Bagger M.J., Rioux P. A randomized controlled crossover trial with delayed-release cysteamine bitartrate in nephropathic cystinosis: effectiveness on white blood cell cystine levels and comparison of safety. Clin. J. Am. Soc. Nephrol. 2012;7:1112–1120. doi: 10.2215/CJN.12321211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.W., Brownlee K.G., Conway S.P., Denton M., Littlewood J.M. Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. J. Cyst. Fibros. 2003;2:29–34. doi: 10.1016/S1569-1993(02)00141-8. [DOI] [PubMed] [Google Scholar]
- Lipuma J.J. The changing microbial epidemiology in cystic fibrosis. Clin. Microbiol. Rev. 2010;23:299–323. doi: 10.1128/CMR.00068-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhdi K., Edenborough F.P., Gumery L., O'Hickey S., Smith E.G., Smith D.L., Stableforth D.E. Outcome for patients colonised with Burkholderia cepacia in a Birmingham adult cystic fibrosis clinic and the end of an epidemic. Thorax. 1996;51:374–377. doi: 10.1136/thx.51.4.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orloff S., Butler J.D., Towne D., Mukherjee A.B., Schulman J.D. Pantetheinase activity and cysteamine content in cystinotic and normal fibroblasts and leukocytes. Pediatr. Res. 1981;15:1063–1067. doi: 10.1203/00006450-198107000-00018. [DOI] [PubMed] [Google Scholar]
- Palmer K.L., Aye L.M., Whiteley M. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J. Bacteriol. 2007;189:8079–8087. doi: 10.1128/JB.01138-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quanjer P.H., Stanojevic S., Cole T.J., Baur X., Hall G.L., Culver B.H., Enright P.L., Hankinson J.L., Ip M.S., Zheng J., Stocks J., E.R.S. Global Lung Function Initiative Multi-ethnic reference values for spirometry for the 3-95-years age range: the global lung function 2012 equations. Eur. Respir. J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuni Y., Goldstein S., Dean O.M., Berk M. The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta. 1830;2013:4117–4129. doi: 10.1016/j.bbagen.2013.04.016. [DOI] [PubMed] [Google Scholar]
- Stewart P.S., Costerton J.W. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358(9276):135–138. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- UK CF Registry Annual data report 2013. 2014. http://www.cysticfibrosis.org.uk/media/598466/annual-data-report-2013-jul14.pdf (accessed June 2015)
- Vanhoof R., Vanderlinden M.P., Hubrechts J.M., Butzler J.P., Yourassowski E. In-vitro activity of antimicrobial agents against Neisseria gonorrhoeae in Brussels. Br. J. Vener. Dis. 1978;54:309–315. doi: 10.1136/sti.54.5.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Report of a joint meeting of WHO/ECFTN/ICF(M)A/ECFS. WHO/HGN/CF/WG/04.02. WHO/HGN/CF/WG; Geneva: 2004. The molecular genetic epidemiology of cystic fibrosis. [Google Scholar]
- Zhao T., Liu Y. N-acetylcysteine inhibit biofilms produced by Pseudomonas aeruginosa. BMC Microbiol. 2010;10:140. doi: 10.1186/1471-2180-10-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparative effects of DNAse I and cysteamine treatment (for 1 h at 37 °C) on the spinnbarkeit of sputum taken from a volunteer with cystic fibrosis.