Abstract
Purpose
There is considerable evidence for systemic vascular dysfunction in primary open-angle glaucoma (POAG). We performed nailfold capillary video microscopy to observe directly the nature of nonocular microvasculature abnormalities in POAG.
Methods
We enrolled 199 POAG patients and 124 control subjects from four sites. We used JH-1004 capillaroscopes to perform nailfold capillary video microscopy on the fourth and fifth digits of each subject's nondominant hand. Videos were evaluated for hemorrhages, dilated capillary loops > 50 μm, and avascular zones > 100 μm by graders masked to case status. Multivariable odds ratios (ORs) and 95% confidence intervals (CIs) for POAG were obtained by means of logistic regression analyses that were applied to data from all cases and controls. Corresponding estimates of moderate or severe POAG versus mild POAG (based on the Hodapp-Anderson-Parrish scale) were obtained among cases only.
Results
After controlling for demographic factors, family history of glaucoma, systemic diseases, and use of anticoagulation and antiplatelet therapy, for each 100 nailfold capillaries assessed, all types of microvascular abnormalities were significantly associated with POAG. Specifically, the presence of any dilated capillaries (OR = 2.9; 95% CI, 1.6–5.6), avascular zones (OR = 4.4; 95% CI, 1.7–11.3) and hemorrhages (OR = 12.2; 95% CI, 5.9–25.1) were associated with POAG. Among cases, the frequency of microvascular abnormalities was not associated with glaucoma severity (P ≥ 0.43).
Conclusions
These data provided support for nonocular capillary bed abnormalities in POAG. Comparable vascular abnormalities in the optic nerve may render it susceptible to glaucomatous damage.
Keywords: primary open-angle glaucoma, nailfold capillary microscopy, hemorrhages, avascular zones, dilated capillaries
Primary open-angle glaucoma (POAG) is an insidious disease characterized by optic disc excavation with corresponding vision loss due to loss of retinal ganglion cells and their axons. The etiology of this disease is poorly understood. While there are obvious ocular biomarkers for POAG, such as optic nerve head excavation, neuroretinal rim erosion, and elevated intraocular pressure (IOP),1 and recent studies have identified genetic loci that alter the risk of POAG and glaucoma-related traits,2 there are no established systemic biomarkers. The ultimate goal for a better understanding of POAG etiology and improved POAG screening is to identify biomarkers for pathogenic factors that precede IOP elevation and optic neuropathy.
There is evidence for dysfunction of Schlemm's canal endothelium3 and systemic vascular endothelium4–7 in POAG. For example, human Schlemm's canal endothelium generates nitric oxide in response to shear but this response was absent in comparable cells from two glaucomatous specimens.3 In POAG patients, vascular endothelial cell dysfunction can be manifest as impaired vascular autoregulation, which has been documented in the retinal8 and cerebral vasculature.9 Endothelial cell dysfunction in POAG extends beyond the CNS. Henry et al.5 have reported that in untreated POAG patients without a history of elevated IOP, the brachial artery fails to dilate after exposure to acetylcholine. Arteries dissected from gluteal fat biopsies of normal pressure glaucoma patients also demonstrate exaggerated vasoconstriction in response to 5-hydroxytryptamine and endothelin-1.5 Furthermore, POAG patients across the spectrum of IOP show impaired brachial artery flow–mediated vasodilation.6,7
Another marker of systemic vascular abnormalities is findings from nailfold capillary microscopy. Starting in the 1970s, nailfold capillaroscopy has been used to study the vascular component of various connective tissue diseases such as dermatomyositis, systemic lupus erythematosus, and rheumatoid arthritis.10 Nailfold capillaroscopy with its ability to provide high magnification views of hairpin capillary networks offers an alternative window to assess systemic vascular dysfunction in glaucoma. A recent study using nailfold capillary microscopy has demonstrated that the presence of dilated capillaries, avascular zones, and nailfold hemorrhages are common in Korean patients who predominantly have the normal tension subtype of POAG.11 It is not certain whether similar associations may be observed in POAG patients with higher IOP and other ethnicities after controlling for potential confounders such as systemic disease and anticoagulation therapy; furthermore, it is unclear if such abnormalities may be associated with POAG severity. Thus, we conducted a study to assess nailfold microvasculature features in relation to POAG, where POAG is predominately of the high-tension disease subtype, and in relation to POAG disease severity.
Methods
Study Design and Population
We conducted a multisite, clinic-based, cross-sectional case-control nailfold capillary microscopy study from January 2012 to March 2015. The POAG cases and control subjects were recruited from three clinical sites in Chicago, Illinois (21 cases and 50 controls from the private practice of Zaparackas and Knepper Ltd.; 47 cases and 3 controls from Northwestern University, Department of Ophthalmology; and 65 cases and 49 controls from The John H. Stroger Cook County Hospital, Division of Ophthalmology) and one site in Boston, Massachusetts (66 cases and 22 controls from Massachusetts Eye and Ear, Department of Ophthalmology). Respective institutional review boards approved this study and each subject consented in writing after being informed about the nature of research at each site. This study adhered to the tenets of the Declaration of Helsinki.
Eligibility Criteria and Staging of Severity in POAG Cases
All POAG cases and controls were at least 35 years of age. There were no IOP inclusion criteria for POAG cases but high tension cases and normal tension cases were defined by highest known IOP > 21 mm Hg and IOP ≤ 21 mm Hg, respectively. Controls were selected from eye clinics at each respective site and had IOP ≤ 21 mm Hg, normal slit lamp examinations, cup disc ratio (CDR) ≤ 0.6, and CDR asymmetry ≤ 0.1. The POAG cases demonstrated visual field loss on reliable tests consistent with nerve fiber layer loss. These POAG cases also had slit lamp biomicroscopy findings that did not reveal secondary causes for elevated IOP and open angles on gonioscopy. A reliable visual field (VF) from the more severely affected eye closest to the time of nailfold capillary microscopy was used to stage POAG. The POAG severity was graded as mild, moderate, or severe on the basis of the Hodapp-Anderson-Parrish scale.12 Specifically, mean deviation (MD) better than −6 dB was graded as mild; MD between −6 dB and −12 dB as moderate; and MD worse than 12 dB as severe disease. An important exclusion criterion for cases and controls was the presence of any connective tissue disease (see next section for details).
Collection of Covariate Data
We extracted information from the participants' medical record regarding basic demographic features (age, sex, and race/ethnicity), glaucoma-related characteristics (IOP and physician-recorded CDR), and preexisting medical conditions (e.g., hypertension, diabetes mellitus). Special attention was given for connective tissue diseases such as rheumatoid arthritis, dermatomyositis, and systemic sclerosis, since nailfold capillary abnormalities are associated with these conditions.13 Participants with any connective tissue disease, including rheumatoid arthritis, dermatomyositis, and autoimmune diseases (e.g., multiple sclerosis, systemic lupus erythematosus) were excluded. We also extracted information on the use of anticoagulation and antiplatelet therapy, as use of these agents may precipitate nailfold hemorrhage with minimal provocation. Information was extracted from the medical record closest to the performance of nailfold capillary microscopy.
Measurement of Nailfold Capillaroscopic Findings
The nailfold is located just proximal to the lunula and cuticle (Fig. 1). Cedar oil is placed on the nailfold to promote epidermal translucency and facilitate visualization of the underlying vascular networks. As the nailfold microvasculature can vary from finger to finger, we examined the fourth and fifth digits on each subject's nondominant hand.13 This approach allowed for adequate vascular sampling and minimized effects of microvascular changes introduced by nonmedical conditions. We used a JH-1004 microscope (Jiangsu Jiahau Electronic Instrument Co., Jiangsu, China) set at ×280 magnification to image and video the microvasculature (Fig. 2). Figure 3 illustrates a still shot captured from video capillaroscopy on a control subject, demonstrating the open hairpin–looped capillaries with an inverted U-configuration arranged next to the cuticle.
Figure 1.
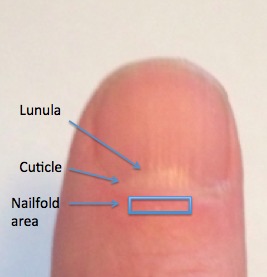
External photograph of a thumb illustrating the lunula, cuticle, and nailfold area (rectangle).
Figure 2.

A subject has placed the fourth digit of the nondominant hand in a slot directly under the microscope while the examiner focuses on the digital microvasculature and inspects the video on the computer screen.
Figure 3.
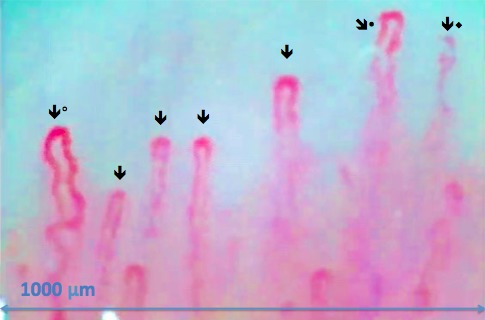
A capillaroscopic image of a 68-year-old white male without glaucoma (×280). The arrows illustrate the open hairpin capillaries in a 1-mm strip. The filled circle (•) illustrates capillaries that are somewhat ectatic and tortuous. During video capillaroscopy, crossed vascular loop (°) and out-of-focus capillaries are commonly encountered (♦).
We recorded 2 to 4 minutes of nailfold capillary video per patient, which was stored on a laptop computer and forwarded to a shared drive where it was analyzed by masked observers. For each 100 capillaries viewed, the readers assessed for dilated capillaries > 50 μm, avascular zones > 100 μm, and hemorrhage count.
Statistical Analysis
For univariate analyses of continuous demographic and clinical features, unpaired t-tests were used. For univariate analyses of categorical variables, χ2 tests were used to compare frequencies between POAG cases and controls. Mantel-Haenszel χ2 tests were used to determine whether ordinal categories of nailfold video microscopy findings showed a relation to glaucoma severity among cases. Severity was categorized as an ordinal variable with two categories (one for mild cases and two for moderate and severe cases).
To assess the association between nailfold microvascular abnormalities and POAG, univariate and two multivariable-adjusted logistic regression analyses were conducted. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for each analysis. In the first multivariable-adjusted model (model 1), we adjusted for age (years), sex, race/ethnicity (African, Asian, and Latino), family history of glaucoma, and clinical site. In the second multivariable-adjusted model (model 2), we additionally adjusted for presence of malignancy, hypertension or diabetes, and use of aspirin/warfarin or other blood thinners. For each nailfold video microscopy finding, we evaluated the association with any nonzero value as well as ordinal categories of values, with the reference group being those without a particular nailfold video microscopy finding. For evaluating multivariable-adjusted tests for trends across categories of nailfold video microscopy findings, we used the significance of the ordinal variable representing each category (e.g., 0 for no hemorrhages per 100 capillaries, 1 for the “>0 and ≤1” category, 2 for the “>1 and ≤2” category and 3 for “>2” category) in multivariable models.
To determine the association between nailfold video microscopy findings and disease severity, we used data among cases only. We used a similar approach adjusting for the same covariates as well as POAG medications to conduct univariate and multivariable-adjusted logistic regression analyses, where the outcome was moderate or severe POAG (n = 117) versus mild POAG (n = 82).
To evaluate whether associations between nailfold video microscopy findings and either glaucoma status or severity of VF loss may differ by age (by median age in controls [64 years]), sex, and family history, we tested the significance of various interaction terms in Wald tests added to each multivariable-adjusted model (model 2).
Odds ratios and 95% CIs were calculated for each analysis. All statistical tests were two sided, and significance level was set at P < 0.05. Statistical analysis was performed by using the SAS 9.4 package (SAS Institute, Inc., Cary, NC, USA).
Results
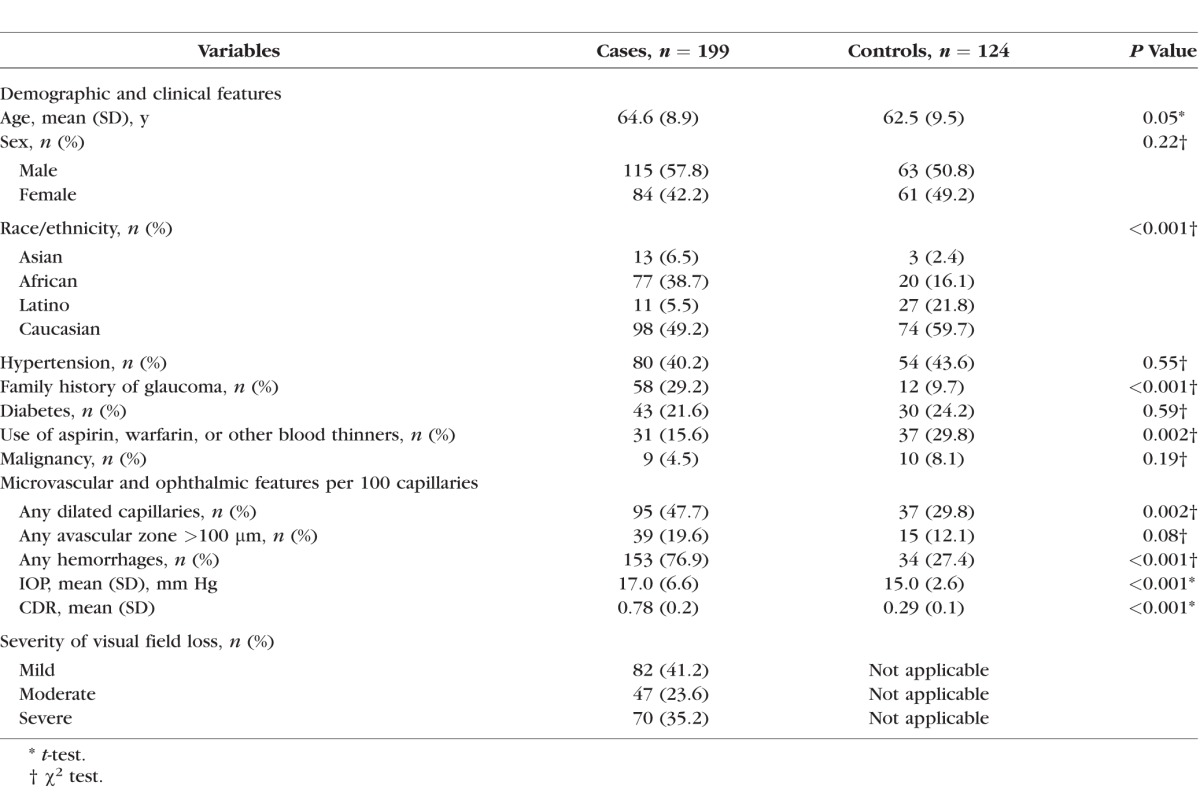
We enrolled 199 POAG patients (82 with mild, 47 with moderate, and 70 with severe VF loss) and 124 controls (Table 1). Of the 199 POAG patients, 24 (12%) had highest known IOP ≤ 21 mm Hg and 175 (88%) had highest known IOP > 21 mm Hg. Compared to control subjects, the POAG patients were more likely to have a family history of glaucoma and be of African or Asian descent. In contrast, controls were somewhat more likely to be of Latino descent and were more likely to take aspirin, warfarin, or other blood thinners. The mean IOP and CDR were significantly higher in cases than in controls. In addition, all types of nailfold microvascular abnormalities were significantly more frequent in cases than in controls per 100 capillaries sampled: 47.7% of cases versus 29.8% of controls had dilated capillaries (P = 0.002); 19.6% of cases versus 12.1% of controls had avascular zones > 100 μm (P = 0.08), and 76.9% of cases versus 27.4% of controls had hemorrhages (P < 0.001). Other characteristics did not differ between the POAG and control groups.
Table 1.
Baseline Demographic and Clinical Features in Glaucoma Cases and Controls
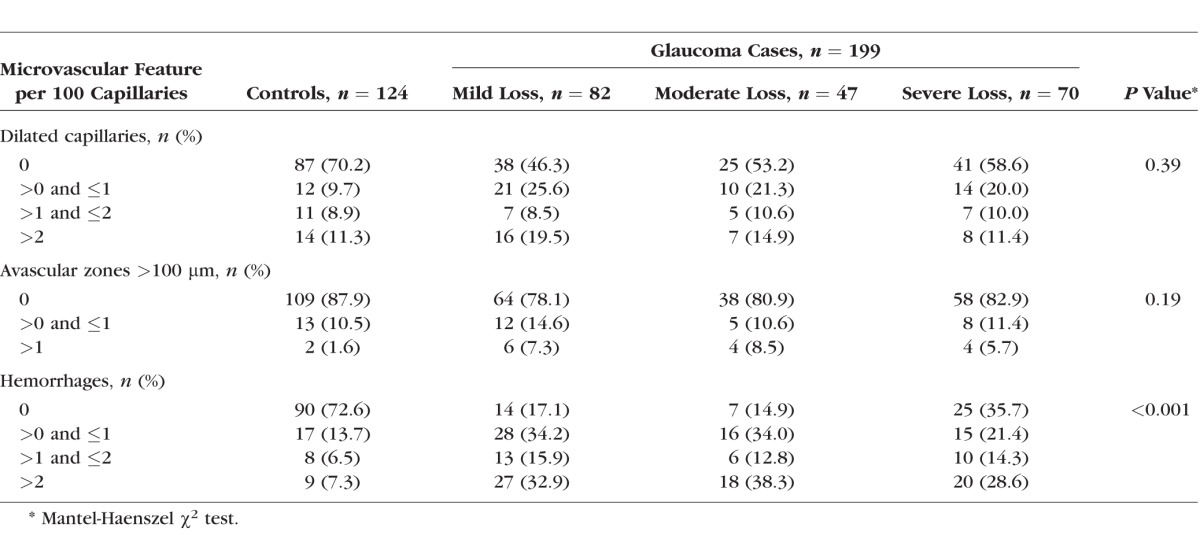
When we evaluated the univariate relationship between the number of dilated capillaries, avascular zones, and hemorrhages and the severity of VF loss, we observed only an increased number of hemorrhages with increasing VF loss severity (P < 0.001) (Table 2).
Table 2.
Descriptive Analysis of Nailfold Video Microscopy Findings by Severity of Glaucoma Visual Field Loss
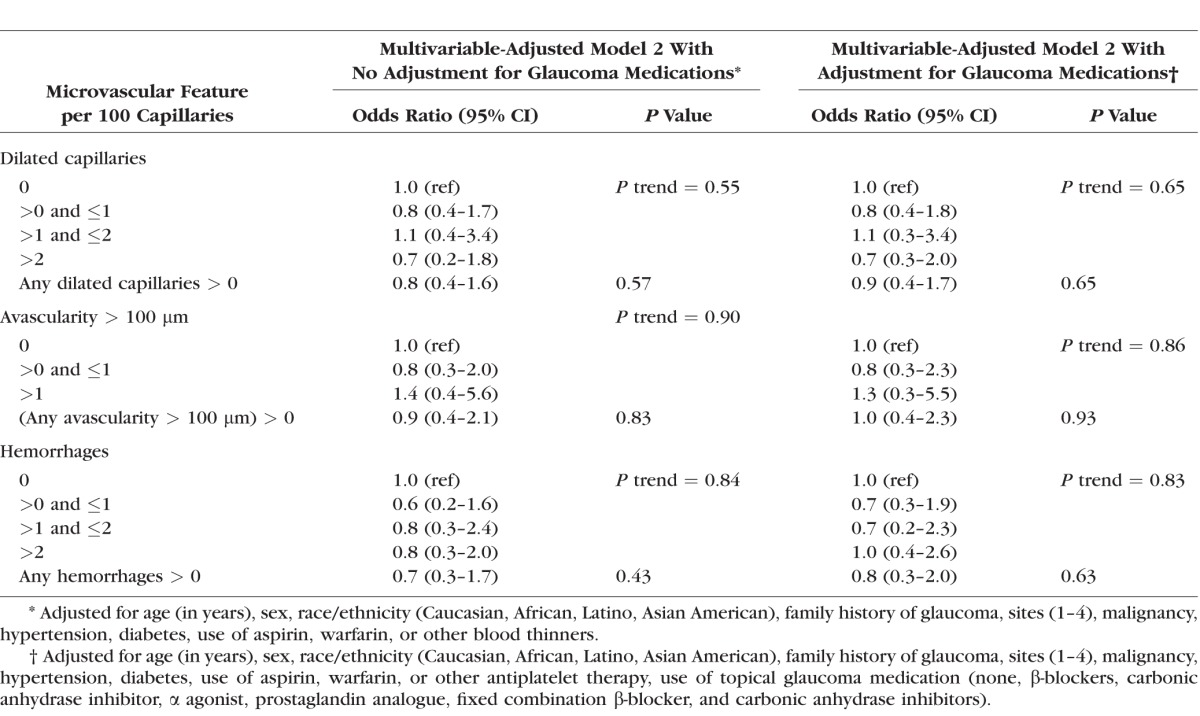
Compared to the univariate analyses, the multivariable-adjusted model results showed stronger relations between nailfold video microscopy findings and POAG (Table 3). All types of nailfold video microscopy findings per 100 capillaries sampled were significantly associated with POAG in expanded multivariable-adjusted models (model 2), that is, dilated capillaries: OR = 2.9 (95% CI, 1.6–5.6); avascular zones > 100 μm: OR = 4.4 (95% CI, 1.7–11.3); and hemorrhages: OR = 12.2 (95% CI, 5.9–25.1). Furthermore, significant trends were observed between greater abnormalities and higher odds of POAG across all types of nailfold microscopy abnormalities (P for trend ≤ 0.003).
Table 3.
Univariate and Multivariable Logistic Regression Analyses of Nailfold Video Microscopy Findings in Relation to Glaucoma
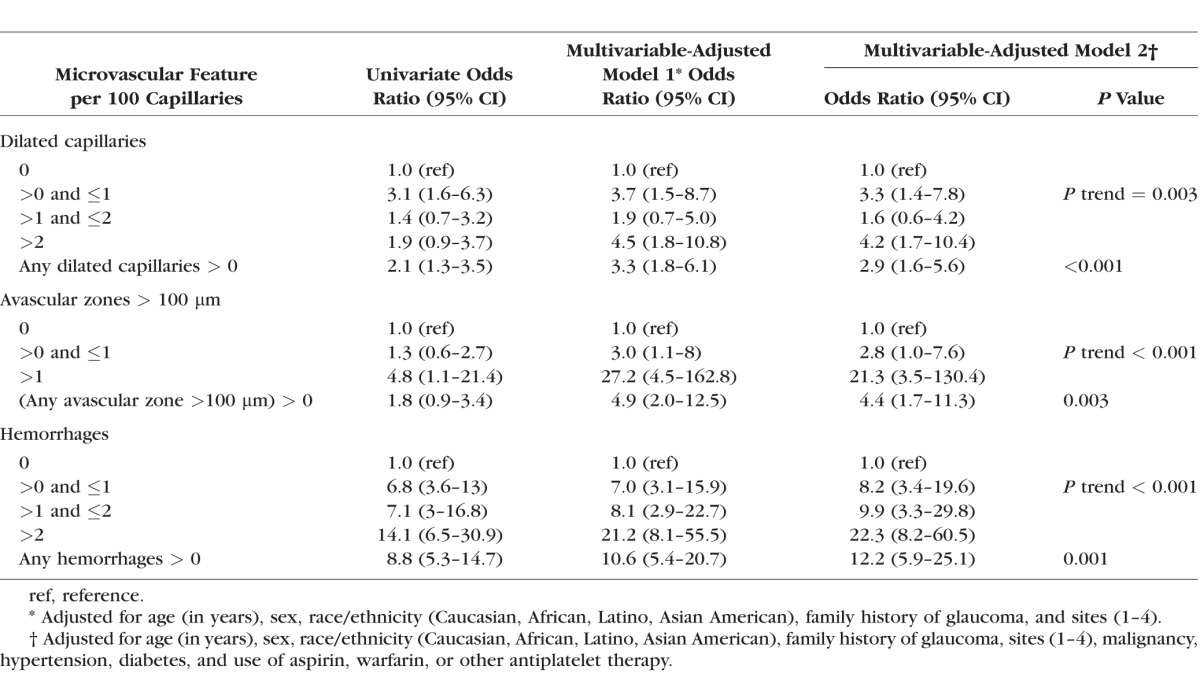
The relationship between nailfold microvascular abnormalities and VF loss severity (moderate or severe VF loss versus mild VF loss) was analyzed among cases only with logistic regression (Table 4), with and without additional control for glaucoma medications. None of the nailfold microvascular abnormalities were significantly associated with severity of VF loss. Therefore, the associations between abnormalities in nailfold video microscopy findings with POAG observed in Table 2 and with POAG in Table 3 were likely driven by the major differences between controls and POAG cases overall and not by POAG groups defined by disease severity. Post hoc power calculations indicated that with our sample of 199 cases, we had 80% power to detect the following ORs between moderate plus severe cases versus mild POAG cases: 2.4 for any dilated capillaries; 2.5 for any avascular zones; and 3.8 for any hemorrhages.
Table 4.
Multivariable Logistic Regression Analyses Among Cases Only (n = 199) of Nailfold Video Microscopy Findings in Relation to the Outcome of Moderate or Severe Visual Field Loss (n = 117) Versus Mild Visual Field Loss (n = 82)
When we evaluated whether the relationship between the presence of any individual nailfold video microscopy finding and either POAG or disease severity differed according to age, sex, and glaucoma family history, we observed no significant interactions in relation to POAG status (data not shown). However, there was a possible interaction between having any avascular zones/100 capillaries > 100 μm and family history of glaucoma, where the association with any avascular zones/100 capillaries > 100 μm and POAG was stronger in those without family history (P for interaction = 0.05).
Figure 4 shows a frame from the nailfold capillary video that captured newly formed hemorrhages and an avascular zone > 100 μm in a 59-year-old patient with moderate-stage POAG included in this study. The patient had six hemorrhages ranging from newly formed to older deposits containing brown hemosiderin (the latter not captured in the frame depicted in Fig. 4). The glaucomatous characteristics of this patient included inferior neuroretinal rim thinning (Fig. 5A), a corresponding superior paracentral scotoma (Fig. 5B) and focal defect in the lamina cribrosa defect on enhanced depth imaging ocular coherence tomography, in the region of a prior disc hemorrhage (Fig. 5C).
Figure 4.
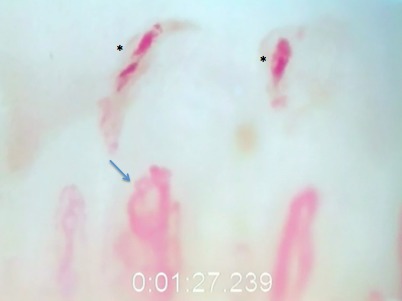
A capillaroscopic image from a 59-year-old white male with POAG (×280). It illustrates fresh epicapillary hemorrhages (*) and an avascular zone > 100 μ from left to right starting at the blue arrow.
Figure 5.
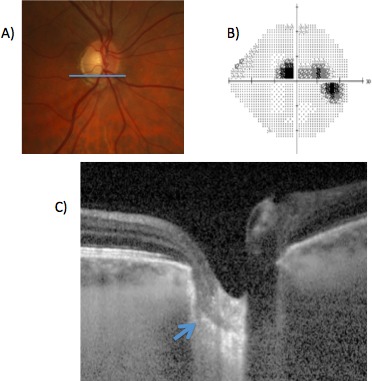
Optic nerve photograph (A), Humphrey 24-2 visual field gray scale (B) and EDI-OCT scan of the right eye in the POAG patient illustrated in Figure 4. The EDI-OCT scan is shown at the level of the inferior pole of the optic nerve illustrated by the blue bar in (A). There is a defect in the lamina cribrosa (blue arrow) in an area corresponding to the paracentral visual field defect illustrated in (B). Of note, there was a history of a disc hemorrhage in this section of the disc, although no optic nerve hemorrhage was noted at the time of nailfold capillaroscopy. EDI-OCT, enhanced depth imaging–ocular coherence tomography.
Discussion
In this US multisite, case-control study, the presence of any dilated capillaries > 50 μm, avascualar zones > 100 μm, and hemorrhages was associated with POAG in multivariable analysis. These findings are consistent with a Korean case-control study of POAG that included predominately normal tension glaucoma (NTG) cases.11 The association between nailfold hemorrhages and POAG was particularly strong. While we did not observe associations between nailfold capillary microvascular abnormalities and POAG disease severity among cases only, this analysis may have been underpowered. Short- and long-term longitudinal observations with larger sample sizes will be needed to determine whether there is a true association between nailfold microvascular abnormalities and glaucoma severity.
Other researchers have used nailfold capillary microscopy to study open-angle glaucoma in different ways. Gasser and Flammer14 have examined 30 patients with NTG, 30 patients with POAG, and 30 controls and noted markedly reduced blood velocities in NTG patients, especially after cold exposure. Bozic et al.15 have studied various morphologic features (but not hemorrhages) in NTG (n = 30) versus high tension glaucoma (n = 30) and found more vascular tortuosity in NTG patients. In a case-only analysis, Lee et al.16 have found significant correlation between dilated capillaries and serum matrix metalloproteinase (MMP)-9 levels as well as between avascular zones and serum MMP-9 levels in 25 NTG patients. No relation between nailfold hemorrhages and MMP-9 levels have been detected.16 The authors postulate that elevated MMP-9 levels cause basement membrane weakening that could alter the nailfold capillary architecture. None of these studies are as large as our study, nor did they perform multivariable analysis to address whether other covariates could confound the relation between glaucoma status and nailfold capillary features.
The mechanism underlying the relationship between nailfold microvascular abnormalities and POAG is unknown. The hairpin capillaries in the nail bed may be particularly susceptible to vascular dysregulation from impaired nitric oxide signaling because of their unique architectural arrangement. There is considerable evidence that intergenic single nucleotide polymorphisms (SNPs) between CAV1 and CAV2 are associated with both POAG17,18 and higher IOP.19,20 Caveolin 1 (CAV1) is a structural protein, which lies next to nitric oxide synthase 3 (NOS3—an enzyme responsible for nitric oxide generation) on inverted lipid rafts situated on endothelial abluminal surfaces called caveolae.21 Along with endothelin, CAV1 reciprocally regulates NOS322 to control nitric oxide generation, which is abluminally secreted from the endothelium to control vascular tone.23 Interestingly, CAV1 SNPs24 and increased endothelin levels25 are also associated with systemic sclerosis, which likewise is associated with nailfold microvascular abnormalities including hemorrhages.26 We hypothesize that dysregulation in the nailfold vasculature alters blood flow and shear force rates in ways that lead to extravasation of blood in POAG patients. Alternatively, the cell stiffness demonstrated in Schlemm's canal endothelium27 may extend to vascular endothelium, rendering nailfold capillary walls more susceptible to rupture.
Another factor that may be important in contributing to the vasculopathy in POAG is a reduced number of circulating endothelial progenitor cells, which has also been associated with impaired brachial artery vasodilation in response to distal limb ischemia.7 Endothelial progenitor cells are CD34+ cells derived from the bone marrow that migrate to damaged endothelium for repair.28 CD34+ cells are also decreased in rheumatoid arthritis,29 systemic lupus erythematosus,30 and systemic sclerosis,31 all diseases with nailfold capillary abnormalities that are comparable to POAG.32 Most notably, in systemic sclerosis, concomitant nailfold microvascular abnormalities (dilated capillaries and avascular zones) and reduced CD34+ cells have been reported.31,33 We hypothesize that a reduced number of these cells could lead to dilated capillaries and capillary dropout in vascular beds relevant to glaucoma.
It remains unknown if nailfold capillary abnormalities in POAG are related to optic nerve hemorrhages.34 In this study, 76.9% of POAG patients had nailfold hemorrhages with one patient having 18 such nailfold hemorrhages. It seems that nailfold hemorrhages are far more common than disc hemorrhages35 and the reason might relate to the vascular architectural differences between the two tissue beds. Also, the nailfold capillary is supported only by pericytes, whereas the optic nerve capillaries are supported by both pericytes and astrocytes to form the blood–brain barrier.36 Finally optic nerve hemorrhages may be less frequent than nailfold hemorrhages because the former occur in the globe, which is a pressurized compartment. Furthermore, the optic nerve is relatively sheltered from external stimuli and less subject to microtrauma than the nail bed.
Study limitations included its cross-sectional nature, which did not allow us to determine if nailfold microvascular pathology occurred before or after optic nerve degeneration in POAG. However, the strong association between nailfold pathology and disease (specifically the 12-fold risk of POAG associated with nailfold hemorrhages) and the higher odds of POAG with greater abnormalities suggest the former is a real possibility. Also this work did not reveal the mechanism of nailfold microvasculopathy in POAG, nor did it reveal whether other forms of glaucoma have similar nailfold microvascular changes. There was an insufficient distribution of normal tension and high tension cases to determine if the nature of nailfold capillary microvascular changes was different in these disease subtypes. Finally, this was a clinic-based study and subjects may not be representative of a population-based sample. In a population-based sample, some cases are untreated, which may alter the spectrum of nailfold microvascular abnormalities discovered.
Our study did have several strengths. It was a fairly large study performed at multiple sites, with ethnically heterogeneous POAG patients having a broad range of disease severity from mild to severe. Graders masked to glaucoma case status interpreted the nailfold capillary videos, reducing the chance of reader bias. We collected data on multiple covariates, including use of blood thinners, and controlled for them in multivariable analysis, minimizing the chance that the association between nailfold microvascular change and POAG was the result of uncontrolled confounding. Also, this study further establishes the feasibility and applicability of studying the nailfold capillary network in POAG noninvasively. The procedure is easy to perform, and the high magnification allows for in vivo visualization of capillaries, something that is not easily accomplished in ophthalmic vascular beds. In addition, since it is doubtful that local ophthalmic treatments alter the nailfold capillary system, the observed abnormalities are likely to be systemic in nature.
In conclusion, nailfold microvascular pathology was independently associated with POAG case status. These data support the notion that POAG is a systemic disease with a vascular component and may provide insight into why IOP lowering treatment slows, but does not halt disease progression.37–39 This study adds to the growing evidence that systemic vascular factors are operative in POAG pathogenesis. Longitudinal observation of nailfold capillary morphology in glaucoma suspects with ocular hypertension or a positive family history is warranted to determine if microvascular alterations are acquired over time.
Acknowledgments
Supported by Grant R01 EY015473 (LRP) from the National Institutes of Health. The Harvard Glaucoma Center of Excellence (LRP and JLW), a Harvard Medical School Distinguished Scholar Award (LRP), the BrightFocus Foundation Grant G2011047 (PAK), the Rosemary O'Meara and Kathleen F. Connelly Memorial Funds (PAK), and the Illinois Society for the Prevention of Blindness (PAK) also supported this work. APT is supported by Research to Prevent Blindness. LRP has been a speaker for Allergan. He also served as a nonpaid consultant to Novartis and a paid consultant to Bausch + Lomb. He has received support to travel to the Exfoliation Glaucoma Think Tank Meeting in New York City by the Glaucoma Foundation. None of the other authors have conflicts of interest, including financial interests, activities, relationships, and affiliations.
Disclosure: L.R. Pasquale, Novartis (C), Bausch + Lomb (C), Allergan (R), Glaucoma Foundation NYC (R, S); A. Hanyuda, None; A. Ren, None; M. Giovingo, None; S.H. Greenstein, None; C. Cousins, None; T. Patrianakos, None; A.P. Tanna, None; C. Wanderling, None; W. Norkett, None; J.L. Wiggs, None; K. Green, None; J.H. Kang, None; P.A. Knepper, None
References
- 1. Kokotas H,, Kroupis C,, Chiras D,, et al. Biomarkers in primary open angle glaucoma. Clin Chem Lab Med. 2012; 50: 2107–2119. [DOI] [PubMed] [Google Scholar]
- 2. Takamoto M,, Araie M. Genetics of primary open angle glaucoma. Jpn J Ophthalmol. 2014; 58: 1–15. [DOI] [PubMed] [Google Scholar]
- 3. Ashpole NE,, Overby DR,, Ethier CR,, Stamer WD. Shear stress-triggered nitric oxide release from Schlemm's canal cells. Invest Ophthalmol Vis Sci. 2014; 55: 8067–8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buckley C,, Hadoke PW,, Henry E,, O'Brien C. Systemic vascular endothelial cell dysfunction in normal pressure glaucoma. Br J Ophthalmol. 2002; 86: 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Henry E,, Newby DE,, Webb DJ,, O'Brien C. Peripheral endothelial dysfunction in normal pressure glaucoma. Invest Ophthalmol Vis Sci. 1999; 40: 1710–1714. [PubMed] [Google Scholar]
- 6. Su WW,, Cheng ST,, Ho WJ,, Tsay PK,, Wu SC,, Chang SH. Glaucoma is associated with peripheral vascular endothelial dysfunction. Ophthalmology. 2008; 115: 1173–1178. e1171. [DOI] [PubMed] [Google Scholar]
- 7. Fadini GP,, Pagano C,, Baesso I,, et al. Reduced endothelial progenitor cells and brachial artery flow-mediated diation as evidence of endothelial dysfunction in ocular hypertension and primary open-angle glaucoma. Acta Ophthalmol. 2010; 88: 135–141. [DOI] [PubMed] [Google Scholar]
- 8. Feke GT,, Pasquale LR. Retinal blood flow response to posture change in glaucoma patients compared with healthy subjects. Ophthalmology. 2008; 115: 246–252. [DOI] [PubMed] [Google Scholar]
- 9. Tutaj M,, Brown CM,, Brys M,, et al. Dynamic cerebral autoregulation is impaired in glaucoma. J Neurol Sci. 2004; 220: 49–54. [DOI] [PubMed] [Google Scholar]
- 10. Mariq HR,, Downey JA,, LeRoy EC. Standstill of nailfold capillary blood flow during cooling in scleroderma and Raynaud's syndrome. Blood Vessels. 1976; 13: 338–349. [DOI] [PubMed] [Google Scholar]
- 11. Park HY,, Park SH,, Oh YS,, Park CK. Nail bed hemorrhage: a clinical marker of optic disc hemorrhage in patients with glaucoma. Arch Ophthalmol. 2011; 129: 1299–1304. [DOI] [PubMed] [Google Scholar]
- 12. Hodapp E,, Parrish RK,, II,, Anderson DR. Clinical Decisions in Glaucoma. St. Louis: CV Mosby; 1993. [Google Scholar]
- 13. Grassi W,, DeAngelis R. Mistakes in capillaroscopy. : Cutolo M, Atlas of Capillaroscopy in Rheumatic Disease. Milan: Elsevier; 2011: 87. [Google Scholar]
- 14. Gasser P,, Flammer J. Blood-cell velocity in the nailfold capillaries of patients with normal-tension and high-tension glaucoma. Am J Ophthalmol. 1991; 111: 585–588. [DOI] [PubMed] [Google Scholar]
- 15. Božic M,, Senćanic PH,, Spahić G,, et al. Is nail fold capillaroscopy useful in normotensive and primary open-angle glaucoma: a pilot study. Curr Eye Res. 2010; 35: 1099–1104. [DOI] [PubMed] [Google Scholar]
- 16. Lee NY,, Park HY,, Park SH,, Park CK. The association of nailfold capillaroscopy with systemic matric metalloproteinase-9 concentration in normal-tension glaucoma. Curr Eye Res. 2015; 40: 1001 –100. [DOI] [PubMed] [Google Scholar]
- 17. Wiggs JL,, Kang JH,, Yaspan BL,, et al. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma in Caucasians from the USA. Hum Mol Genet. 2011; 20: 4707–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thorleifsson G,, Walters GB,, Hewitt AW,, et al. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nat Genet. 2010; 42: 906–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hysi PG,, Cheng CY,, Springelkamp H,, et al. Genome-wide analysis of multi-ancestry cohorts identifies new loci influencing intraocular pressure and susceptibility to glaucoma. 2014; 46: 1126–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen F,, Klein AP,, Klein BE,, et al. Exome array analysis identifies CAV1/CAV2 as a susceptibility locus for intraocular pressure. Invest Ophthalmol Vis Sci. 2015; 56: 544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Razani B,, Woodman SE,, Lisanti MP. Caveolae: from cell biology to animal physiology. Pharmacol Rev. 2002; 54: 431–467. [DOI] [PubMed] [Google Scholar]
- 22. Garcia-Cardena G,, Martasek P,, Masters BS,, et al. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin: functional significance of the nos caveolin binding domain in vivo. J Biol Chem. 1997; 272: 25437–25440. [DOI] [PubMed] [Google Scholar]
- 23. Haefliger IO,, Flammer J,, Luscher TF. Nitric oxide and endothelin-1 are important regulators of human ophthalmic artery. Invest Ophthalmol Vis Sci. 1992; 33: 2340–2343. [PubMed] [Google Scholar]
- 24. Manetti M,, Allanore Y,, Sad M,, et al. Evidence for caveolin-1 as a new susceptibility gene regulating tissue fibrosis in systemic sclerosis. Ann Rheum Dis. 2012; 71: 1034–1041. [DOI] [PubMed] [Google Scholar]
- 25. Vancheeswaran R,, Magoula T,, Efrat G,, et al. Circulating endothelin-1 levels in systemic sclerosis subsets: a marker of fibrosis or vascular dysfunction? J Rheumatol. 1994; 21: 1838–1844. [PubMed] [Google Scholar]
- 26. Sambataro D,, Sambataro G,, Zaccaro E,, et al. Nailfold videocapillary micro-haemorrhage and giant capillary counting: an accurate approach for steady state definition of disease activiity in systemic sclerosis. Arthritis Res Ther. 2014; 16: 462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Overby DR,, Zhou EH,, Vargas-Pinto R,, et al. Altered mechanobiology of Sclemm's canal endothelial cells in glaucoma. Proc Natl Acad Sci U S A. 2014; 111: 13876–138881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ma F,, Morancho A,, Montaner J,, Rosell A. Endothelial progenitor cells and revascularization following stroke. Brain Res. 2015; 1623: 150–159. [DOI] [PubMed] [Google Scholar]
- 29. Grisar J,, Aletaha D,, Steiner CW,, et al. Depletion of endothelial progenitor cells in the peripheral blood of patients with rheumatoid arthritis. Circulation. 2005; 111: 204–211. [DOI] [PubMed] [Google Scholar]
- 30. Denny MF,, Thacker S,, Mehta H,, et al. Interferon-α promotes abnormal vasculogenesis in lupus: a potential pathway for premature atherosclerosis. Blood. 2007; 110: 2907–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Andrigueti FV,, Arismendi MI,, Ebbing PC,, Kayser C. Decreased numbers of endothelial progenitor cells in patients in the early stages of systemic sclerosis. Microvasc Res. 2015; 98: 82–87. [DOI] [PubMed] [Google Scholar]
- 32. Kin KM,, Cheng TT,, Chen CJ. Clinical applications of nailfold capillaroscopy in different rheumatic diseases. J Intern Med. 2009; 20: 238–247. [Google Scholar]
- 33. Avouac J,, Vallucci M,, Smith V,, et al. Low circulating endothelial progenitor cell levels and high VEGF serum levels are associated with the late nailfold capillaroscopic pattern in systemic sclerosis. Arthritis Rheum. 2012; 64: S647–S648. [Google Scholar]
- 34. Patel HY,, Buys YM,, Trope GE. Nailfold capillaroscopy assessment in patients with glaucoma with a current optic disc hemorrhage. Can J Ophthalmol. 2015; 50: 155–158. [DOI] [PubMed] [Google Scholar]
- 35. Budenz DL,, Anderson DL,, Feuer WJ,, et al. Detection and prognostic significance of optic disc hemorrhages during the Ocular Hypertension Treatment Study. Ophthalmology. 2008; 113: 2137–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim JH,, Kim JH,, Park JA,, et al. Blood-neural barrier: intercellular communication at glio-vascualr interface. J Biochem Mol Biol. 2006; 39: 339–345. [DOI] [PubMed] [Google Scholar]
- 37. Heijl A,, Leske MC,, Bengtsson B,, Hyman L,, Bengtsson B,, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002; 120: 1268–1279. [DOI] [PubMed] [Google Scholar]
- 38. The Collaborative Normal Tension Glaucoma Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998; 126: 487–497. [DOI] [PubMed] [Google Scholar]
- 39. Kass MA,, Heuer DK,, Higginbotham EJ,, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma [ discussion in Arch Ophthalmol. 2002; 120: 829–830]. Arch Ophthalmol. 2002; 120: 701–713. [DOI] [PubMed] [Google Scholar]


