Abstract
Purpose
To investigate visual function in patients with CEP290 Leber congenital amaurosis (LCA-CEP290), using three full-field tests that can be performed by patients with poor fixation.
Methods
Six patients (age range, 9–39 years) with LCA-CEP290 participated in the study. Stimuli for all three tests (full-field stimulus test [FST], pupillometry, and light discomfort threshold [LDT] testing) were generated by the Diagnosys ColorDome ganzfeld, by using achromatic stimuli as well as long- and short-wavelength stimuli to target rod and cone photoreceptors with all three tests and, in the latter two tests, melanopsin photoreceptors.
Results
Dark-adapted FST thresholds in LCA-CEP290 patients were cone mediated and elevated between 4.8 and 6.2 log units above the normal achromatic threshold. The FST threshold was not measurable in one patient. The rod-mediated transient pupillary light reflex (PLR) was absent in all but the youngest patient, where unreliable responses precluded PLR quantification. Cone-mediated transient PLRs were subnormal in five patients, and absent in another. Sustained melanopsin-mediated PLRs were measurable in all patients. Full-field LDT thresholds were elevated compared to normal controls, and were lower for short-wavelengh than for long-wavelength stimuli.
Conclusions
The FST thresholds and transient PLRs were cone mediated in our cohort LCA-CEP290 patients. Rod-mediated PLRs were undetectable, whereas melanopsin-mediated sustained responses were detected in all patients, suggesting a relative preservation of inner-retina function. The LDT elevations for the patients are somewhat paradoxical, given their subjective perception of photoaversion. Relative aversion to short-wavelength light suggests influence from melanopsin on LDTs in these patients.
Keywords: CEP290, NPHP6, Leber congenital amaurosis, pupillometry
Leber congenital amaurosis (LCA) refers to a group of severe inherited retinal disorders characterized by profound congenital vision impairment, nystagmus, and most often autosomal recessive inheritance. Mutations in the gene CEP290 (also known as NPHP6)1 have been found in at least 21% of LCA patients descended from various parts of Europe,1–3 making it the most common cause of nonsyndromic LCA in those populations. The CEP290 protein localizes to the base of the connecting cilium of photoreceptors,4 and loss of function of this protein in the human retina leads to an early loss of rod photoreceptor function and structure, as well as poor cone-mediated central vision.
Both photopic and scotopic full-field electroretinograms (ERGs) tend to be nondetectable, even at an early age.1,3,5 Visual acuity in patients with CEP290 mutations is typically poor, with most falling in the range of hand motion to no light perception.6–8 In spite of poor central vision, optical coherence tomography has shown that foveal cone structure can be relatively preserved decades longer than rod structure,5,9,10 a finding that suggests the potential for therapeutic intervention. Owing to very poor visual acuity and mild photoaversion, LCA-CEP290 has been referred to as a “cone–rod dystrophy.”1–3 On the other hand, LCA-CEP290 has more recently been referred to as a “rod–cone dystrophy”7 owing to relative sparing of the macula and early loss of rod structure.5
Given the poor fixation and large visual sensitivity losses in LCA-CEP290 patients, typical clinical measures of visual function are not ideally suited either to assess functional losses or to track changes in visual function over time. This is important because substantial progress has been made in the development of gene therapies for application to patients with LCA-CEP290, but techniques for assessing therapeutic efficacy in these patients are relatively limited. The primary purpose of this study was to address this limitation by investigating the use of three full-field tests of visual function in patients with nonsyndromic LCA-CEP290: (1) pupillary light reflexes (PLRs); (2) luminance thresholds; and (3) light discomfort (photoaversion). A secondary purpose of this study was to investigate the disagreement in classification of LCA-CEP290 as either a “cone–rod” or “rod–cone” dystrophy through the use of chromatic stimuli to study the relative contributions of different photoreceptor types (rods versus cones) to the PLR and luminance thresholds, as well as through the inclusion of light discomfort threshold (LDT) testing.
For the PLR, we followed a published method that uses 1-second chromatic stimuli at various luminance levels to target rod, cone and melanopsin responses.11 For the luminance thresholds, we tested patients with the full-field stimulus threshold test (FST),12,13 also using chromatic stimuli to determine rod- versus cone-mediation of dark adapted thresholds. As a measure of photoaversion, we adapted a published measure of LDT14,15 by using a full-field stimulus, and we added chromatic stimuli to evaluate the effect of wavelength on LDTs in LCA-CEP290 patients.
Methods
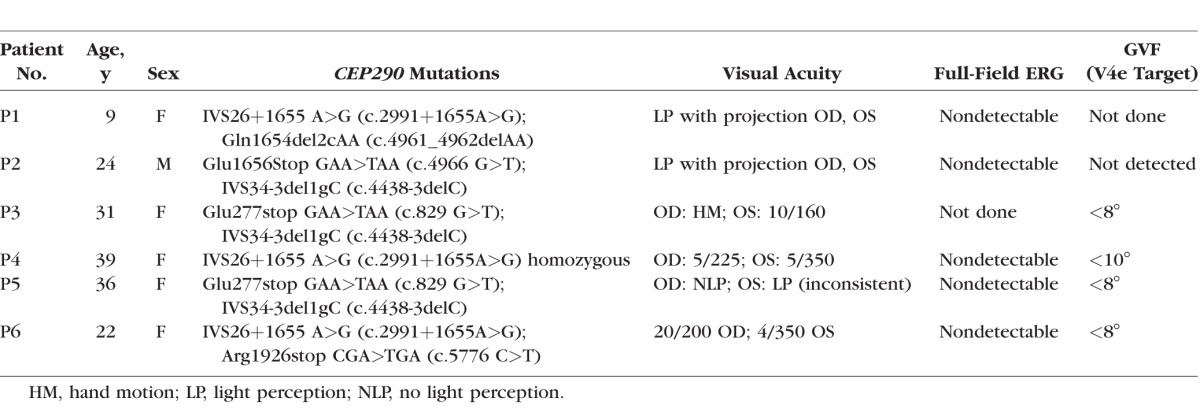
Six patients with LCA-CEP290 participated in the study (P1–P6; ages 9, 24, 31, 39, 36, and 22 years). All six were diagnosed with LCA by a specialist in hereditary retinal diseases (GAF), and were either homozygous or compound heterozygous for disease-associated variants of the CEP290 gene. Genetic testing was performed as described previously.10 In five of the six patients, previously performed clinical full-field ERG had shown nondetectable responses. Patient 4 (sister of patient 3) had no record of having had an ERG. Also, in five of the six patients, Goldmann visual field (GVF) testing had shown less than 20° of visual field to the V4e target (Table 1), and patient 1 had no record of having had a GVF. Visual acuity data from all six of these patients have been published previously,8 although visual acuities given in the present report are updated from the most recent clinical visit (Table 1). P4 had keratoconus, and P6 had optic disc drusen. None of the patients had optic atrophy or retinal coloboma, and none had any known nonocular medical conditions suggestive of a CEP290-associated multisystem ciliopathy syndrome (Joubert syndrome, Senior-Løken syndrome, Meckel-Gruber syndrome, or Bardet-Biedl syndrome).2 Although subtle nonocular findings, such as abnormalities of respiratory cilia, have been associated with LCA-CEP290,16,17 here we refer to LCA-CEP290 in our six patients as “nonsyndromic” to differentiate it from the four syndromes listed above.
Table 1.
LCA-CEP290 Demographics, Genotypes, Visual Acuities, and Historical (Done Years Before Current Study) ERG and Visual Field Data
Pupillometry
The apparatus and stimuli used to elicit and record the PLR are described elsewhere.11,18 In brief, full-field stimuli were generated by the Diagnosys ColorDome ganzfeld associated with the Espion E3 system (Diagnosys, LLC, Lowell, MA, USA), with narrow-band LEDs of 465 nm (blue), and 642 nm (red). Pupil responses were measured with a plastic frame-mounted infrared camera system (Arrington Research, Scottsdale, AZ, USA).19 The PLR was measured in the better-seeing eye of each patient by using 1-second long luminance pulses under conditions designed to target the rod, cone, and melanopsin pathways.11 The rod response was elicited by a pulse of −3 log cd/m2 blue light after a period of 10 minutes dark adaptation. The melanopsin response was elicited by a pulse of 2.6 log cd/m2 blue light without a background light. The cone response was elicited by a 1 log cd/m2 red light on a blue background of 0.78 log cd/m2. The rod- and cone-mediated PLRs were defined as the maximum constriction relative to baseline pupil size (before light onset), per convention,11 and the melanopsin response was measured as the median pupil size between 5 and 7 seconds after the stimulus offset, relative to baseline.11 All three conditions were tested twice, and threshold was calculated as an average of the two trials for each condition. Nine similarly aged, visually normal subjects (mean age 28.4 years; SD 6.2) were tested with the same pupillometry protocol.
Full-Field Stimulus Test
The FST is a measure of luminance threshold using full-field stimuli. The FST stimuli were generated by the narrow-band LEDs of the Espion E3 ColorDome. Stimuli below 0.01 cd-s/m2 (“dim” LEDs) had peak wavelengths of 468 nm (blue) and 632 nm (red); stimuli above 0.01 cd-s/m2 (“bright” LEDs) were 444 nm (blue) and 632 nm (red). A button box was used by the subject to indicate whether or not the brief, full-field stimulus was perceived (see Klein and Birch13 for more information regarding setup and statistical threshold determination with the Diagnosys FST). P1 was only tested in one eye (OD) due to her age, and P5 was unable to reliably perform the FST in either eye. The subjects' pupils were dilated (1.0% tropicamide and 2.5% phenylephrine), and the FST was performed in the dark after 30 minutes of dark adaptation, first testing with a 6500-K white stimulus,13 followed by red and blue stimuli. All three stimuli were tested three times, and the threshold was calculated as an average of the three trials for each stimulus. The right eye was tested before the left eye. Dark-adapted thresholds were assumed to be rod mediated if the threshold was at least 2.2 log units lower for the blue stimulus than for the red stimulus, based on our own dark-adapted normative data, as well as other studies involving the Diagnosys ColorDome.11,12 Thresholds were assumed to be cone mediated if red stimulus thresholds were equal to blue stimulus thresholds, as measured in photopic units. Thresholds were assumed to be a complex mix of rod and cone mediation if blue stimulus thresholds were between 0.1 and 2.1 log units less than red stimulus thresholds.
Rod- and cone-mediated FST thresholds from 10 normally sighted subjects were obtained as part of a previously published study.20 In short, for the control subjects, rod-mediated thresholds were obtained after 30 minutes of dark adaptation (as described above), whereas cone-mediated thresholds were obtained during the cone-plateau phase of dark adaptation in the normal subjects. Dark-adapted white and red stimulus thresholds were not reported in the previous article,20 but are now shown here.
Light Discomfort Threshold Testing
The five older patients (P2–P6) participated in the LDT. For LDT testing, the Espion E3 system was used with “bright” LEDs only, with the addition of a 528 nm (green) stimulus. The button box was used by the subject to start and stop the LDT test. The LDT procedure was partly based on a published method that was designed for testing “photophobia” in migraine patients.14,15 The main difference between the previously published method and our method is the visual angle covered by the light stimulus. In contrast to the small-field stimuli used previously,14,15 the LDT test was performed with full-field stimuli, which minimizes the effect of fixation instability, allowing us to test patients with LCA who have poor fixation and nystagmus.
Before testing, both pupils were dilated (1.0% tropicamide and 2.5% phenylephrine) and one eye was adapted to a 30 cd/m2 6500-K white background light for 5 minutes, then dark adapted for 5 minutes. The fellow eye was patched for measurements made with the 6500-K white stimulus. The initial exposure to a 30 cd/m2 6500-K white light was intended to equate the prior light exposure level among the subjects. Testing was performed monocularly with the 6500-K white stimulus, which was tested three times, with 3 minutes between each test.
The luminance of the light source was increased step-wise, with a 2-second stimulus and a 2-second interstimulus interval of darkness. For the white stimulus, the first stimulus was −0.6 log cd/m2 (0.25 cd/m2), and subsequent stimuli were increased by 0.3 log units (approximately a doubling of the luminance with each step) until the luminance reached the maximum of 3.6 log cd/m2 (3981 cd/m2). The subject was asked to indicate, by button press, when the stimulus was perceived to be “uncomfortable,” which ended the test.14,15 Seven visually normal subjects were also tested with this protocol.
Chromatic testing was performed binocularly in the LCA-CEP290 patients following the test with the white stimulus. Monocular thresholds too often exceeded the highest luminance stimulus available (the test was not stopped at the highest luminance level), therefore the subjects were tested binocularly14 in an attempt to lower thresholds into a measureable range. Red, blue, and green stimuli were each tested once binocularly after at least 18 minutes of dark adaptation. Tests for red, green, and blue stimuli began at 64 cd/m2 and doubled in each luminance step, as in the paradigm used for the white stimuli. The purpose of testing with chromatic stimuli was to evaluate whether there was a short-wavelength bias in eyes without rod function.
Color Naming
A simple method of color naming was performed during the full-field tests. With pupillometry and light discomfort testing, the stimuli were often suprathreshold, and the narrow-band LEDs of the ColorDome provided stimuli that were easily named “red,” “green,” and “blue” by normally sighted individuals. Patients were asked whether they perceived color during chromatic testing with all three full-field tests, and they were asked to name the color (although not necessarily in a forced-choice manner). Their responses aided in interpretation of the results of the three full-field tests.
Procedures adhered to the Declaration of Helsinki and were approved by the institutional review boards (IRBs) of the University of Illinois at Chicago and the Western IRB.
Results
Pupillometry
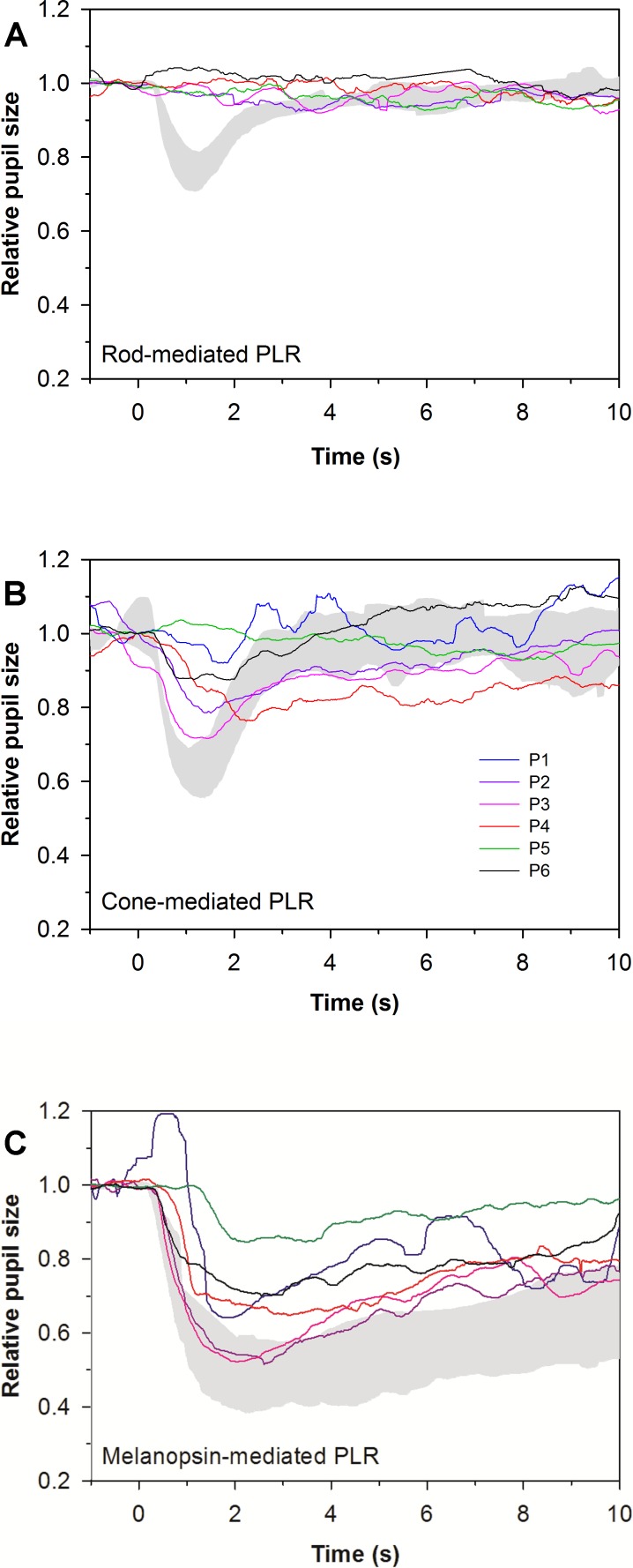
Figure 1 shows the PLRs for the patients, obtained under rod- (Fig. 1A), cone- (Fig. 1B), and melanopsin-mediated (Fig. 1C) conditions. Table 2 lists the PLR amplitudes for all three conditions. The gray region in each panel represents the range of normal. The PLRs are displayed in relative amplitude as a proportion of baseline pupil diameter. The PLR data under the rod condition (Fig. 1A) are only shown for five of the six patients because the results were too noisy to confidently assess in the case of P1, although she appeared to have a detectable rod-mediated response. The rod-mediated PLR was absent in patients 2 to 6. The cone-mediated PLRs (Fig. 1B) were subnormal in patients 2 to 4 and P6, and absent in P5. The mean cone-mediated PLR for the patients was significantly smaller than that of the controls (t = 4.62, P < 0.001). The melanopsin-mediated response (Fig. 1C) was measurable, but reduced in all patients (t = 6.67, P < 0.001). While all patients produced measurable melanopsin-mediated PLRs, the response of one patient (P5; Fig. 1C, green line) showed significantly smaller amplitude and slower onset to constriction. This may reflect the absence of a transient component of the PLR, which should be mediated by rod and/or cone pathway, and the sole dominance of melanopsin in this patient's PLR. As noted in the Methods section, the PLRs were measured twice under each condition and the data shown in Figure 1 and Table 2 are based on the mean of the two responses. The mean PLR amplitude difference between pairs of measurements was 5% for the LCA-CEP290 patients, and the amplitude difference between the two responses for all conditions was less than 12% for all subjects.
Figure 1.
Chromatic full-field pupillometry results, measured in the better-seeing eye in all six patients. Gray areas represent the normal range. (A) Pupillary light reflex responses to rod-targeting stimuli. P1 is excluded from the rod condition owing to noisy recording conditions. The traces for P2 to P6 all show a lack of rod-mediated PLR. (B) Pupillary light reflex responses to cone-targeting stimuli. Cone-mediated PLR amplitudes varied from near-normal to nondetectable. (C) Sustained PLR responses to melanopsin-targeting stimuli. The smallest responses in all three subfigures are from P5 (green trace), who had no response under the rod or cone conditions, but had a modest, delayed response to the bright blue light under the melanopsin condition.
Table 2.
LCA-CEP290 Present Study Monocular Data
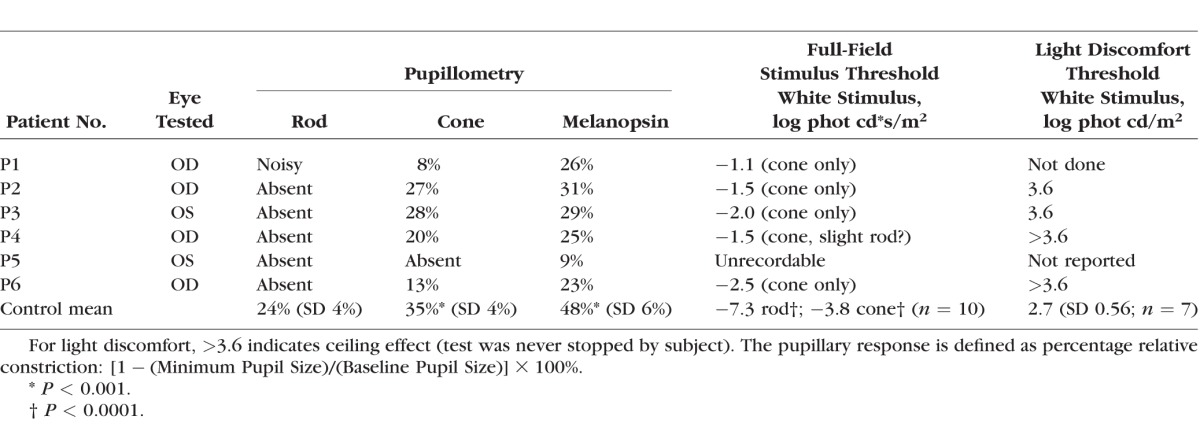
Full-Field Stimulus Thresholds
Reliable FST thresholds were obtained from five of the six patients. P5, who was the exception, was unable to reliably perform the FST, and she also had inconsistent responses to informal clinical testing of light perception. Dark-adapted achromatic FST thresholds in the remaining five patients were elevated between 48 and 62 dB from the normal dark-adapted 6500-K white threshold (individual thresholds for the achromatic stimulus are listed in Table 2). The mean dark-adapted achromatic FST threshold for the five patients was significantly greater than that of the controls (t = 28.5, P < 0.001). Achromatic FST thresholds were measured three times from each of the five patients, and the data shown in Figure 2 and Table 2 are based on the mean of the three responses. The results of the three measurements were highly consistent: the largest variation between any pair of trials was 3.0 dB (in the 9-year-old patient).
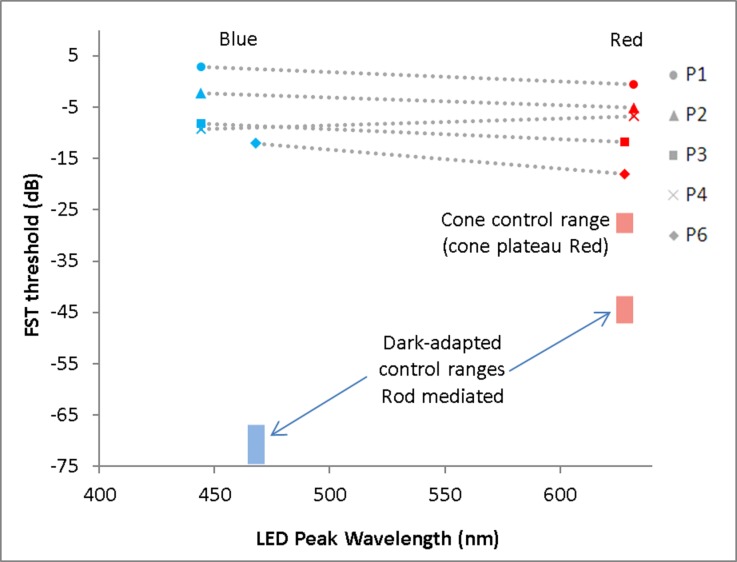
Figure 2.
Dark-adapted FST thresholds for blue and red stimuli, measured in the better-seeing eye of five LCA-CEP290 patients. All but one subject (P4) demonstrated thresholds to short-wavelength stimuli that were at or above the threshold to long-wavelength stimuli, consistent with cone mediation of the responses. In P4 (age 39 years), the short-wavelength thresholds were less than 3 dB lower than the long-wavelength thresholds, suggesting a possible small contribution from rods in her thresholds. Fellow-eye results were very similar, with the exception of P1, who was not tested in the fellow eye. Thresholds for P5, who had inconsistent bare light perception, were not measurable. Control subject responses are shown as blue and red bands at 468 and 628 nm, respectively, where the vertical dimension of each band represents the control range for that stimulus. The blue band (lower left) represents the control range of dark-adapted, rod-mediated thresholds with the blue stimulus. Two red bands are displayed on the right side: the lower red band represents dark-adapted (rod-mediated) thresholds with the red stimulus; the upper red band represents the light-adapted (cone-mediated) thresholds with the same red stimulus, obtained during the cone-plateau phase of dark adaptation. Thresholds are measured in decibels, where zero dB = 0.1 cd-s/m2.
Figure 2 shows the chromatic FST thresholds for the five patients. Data are shown for measurements made with the blue (Fig. 2, left) and red (Fig. 2, right) stimuli (each symbol represents a different patient, given in the key). The colored boxes represent the range of normal for the blue and red stimuli. For the red stimuli, two separate red boxes are shown, one for dark-adapted control thresholds (rod mediated, lower) and one for light-adapted control thresholds (cone mediated, upper). The control thresholds for the dark-adapted blue and red stimuli differed by approximately 25 dB, suggesting that the rod pathway mediated performance. In contrast, dark-adapted thresholds for LCA-CEP290 patients were similar for red and blue stimuli, suggesting that the cone pathway mediated the thresholds. However, when compared to normal cone-mediated thresholds, LCA-CEP290 dark-adapted FST thresholds were elevated between 7 and 25 dB (shown in Fig. 2; t = 8.94, P < 0.0001). In all cases, the cone threshold elevation was at least four times as large as the variation between trials. For P4, the blue stimulus thresholds were slightly lower than the red stimulus thresholds in each eye; therefore, we were unable to entirely rule out a small rod contribution to P4's FST threshold for the blue stimulus.
No significant correlation was found between cone-mediated transient PLR amplitudes and FST red (presumably cone-mediated) thresholds for the five LCA-CEP290 patients who performed both tests (Pearson r = 0.17; P = 0.77).
Light Discomfort Threshold Testing
Four of the tested LCA-CEP290 patients (P2–P4 and P6) were found to have monocular LDTs that were higher than the mean of seven normally sighted subjects (2.7 log cd/m2). Thresholds for the achromatic stimulus are listed in Table 2. Two of the patients (P4 and P6) did not stop the test even at the highest luminance (3.6 log cd/m2), which was not the case for the visually normal controls. From these data we can unambiguously conclude that this cohort of LCA-CEP290 patients was not subnormal (more photoaverse than normal) in its full-field LDTs. The monocular achromatic LDTs were tested three times in a row. The seven normal subjects, as a group, had an average absolute difference of 0.23 log cd/m2 between pairs of trials. Analysis of intravisit variability in the patients is difficult because all but one of the LCA-CEP290 patients had at least one trial in which they did not stop the test. As such, meaningful quantification of the variation in achromatic LDT is not possible. For the same reason, statistical comparison of the LDT values for the patients and controls was not performed.
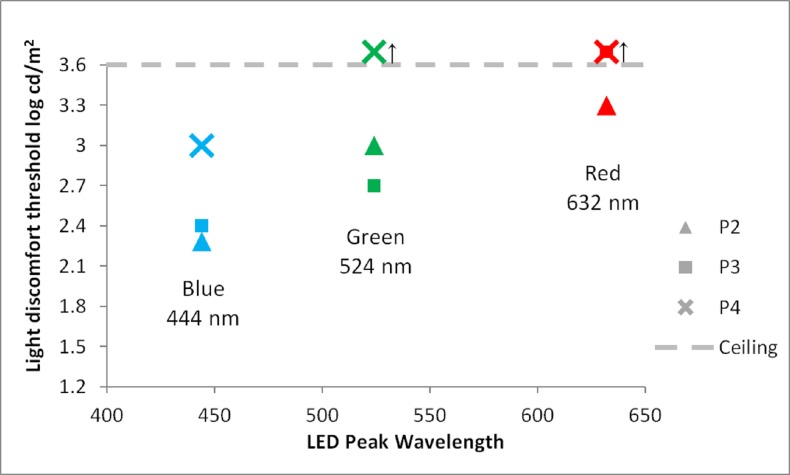
During binocular testing with chromatic stimuli, patients 2 to 4 all were found to have LDTs that were lower for shorter-wavelength stimuli than longer-wavelength stimuli (Fig. 3). P6 failed to stop the test at the highest luminances tested for all chromatic stimuli and is therefore not shown in Figure 3. As with her FST responses, P5's LDT thresholds were inconsistent and are not reported. P1 was not tested with the LDT.
Figure 3.
Full-field binocular LDTs (OU) for patients 2, 3, and 4 (P2, P3, P4). Upward arrow (↑) indicates the subject surpassed the highest luminance stimulus (ceiling effect). The results of all three LCA-CEP290 patients, plotted here, demonstrate greater aversion to the shorter-wavelength stimuli than to longer-wavelength stimuli, suggesting the influence of melanopsin on their LDTs. P6 is not shown because she failed to stop the test at the highest luminance for all three chromatic stimuli.
Color Naming
P1, P2, and P5 denied any subjective perception of color. P3 and P4 claimed only modest perception of color, and P6 claimed she was able to distinguish basic colors. When queried, only one of the six patients (P6) was able to consistently name the colors of the narrow-band stimuli used in the full-field tests. This is an interesting finding in light of FST thresholds and transient PLRs that were cone mediated in this cohort.
Discussion
The purpose of the present study was to investigate the use of three full-field tests of visual function in patients with nonsyndromic LCA-CEP290. We also investigated the relative residual function of rod, cone, and melanopsin photoreceptors in LCA-CEP290 with chromatic stimuli. In general, the six patients in our sample were able to perform the tests well enough to assess rod, cone, and melanopsin function. The data indicated the general absence of rod responses and measurable melanopsin responses. Pupillary light reflex and FST can provide complementary information regarding retinal function and may have their own roles as outcome measures in future clinical trials involving LCA-CEP290 patients.
Pupillometry
Pupillometry using multiple methods has been reported in patients with LCA who do not have an identified mutation,11,21 as well as in a few specific genotypes.22–24 The PLR has also been used to demonstrate efficacy in the various RPE65 gene therapy treatment trials.25–28 The specific PLR paradigm used in this study has been previously demonstrated in three patients with LCA, where the genotype was not specified.11 Chromatic full-field pupillometry has been performed on Abyssinian cats with CEP290 mutations, showing reduction of pupil responses with advancing disease stage.29 However, to our knowledge, the PLR in human LCA-CEP290 has not been described previously.
Pupillometry testing revealed measurable cone responses in five of six LCA-CEP290 patients. From this result alone, full-field pupillometry may have a role as an objective measure of retinal function in LCA-CEP290 patients, as compared with standard full-field ERG, where responses are generally nondetectable.1,3,5 On the other hand, rod-mediated PLRs were not detected in five of six patients, with one patient, the youngest at 9 years of age, being indeterminate owing to noisy responses. Findings of a rod-mediated transient PLR in a 9-year-old would not be inconsistent with the spectral-domain optical coherence tomography and short-wavelength autofluorescence findings of Cideciyan et al.,5 who has concluded that some rod structure is present early in life, even though it is largely lost within the first decade in humans with LCA-CEP290.
Pupillometry revealed measurable melanopsin-mediated sustained responses in all six LCA-CEP290 patients. Most importantly, melanopsin-mediated responses were detected in the patient who had no appreciable rod or cone PLRs. A very similar, long latency melanopsin-mediated PLR has been previously reported in an LCA patient (gene mutation not specified) who also lacked rod- and cone-mediated responses.11 The melanopsin-mediated pupillometry test can demonstrate measurable melanopsin-containing retinal ganglion cells and optic nerve function, even in patients with no light perception,11 and therefore may play a role in patient selection for future treatments.
Full-Field Stimulus Thresholds
The FST was designed specifically with LCA and LCA clinical trials in mind,12 and has been another valuable tool in demonstrating efficacy in RPE65 clinical trials.25,30 Cideciyan et al.5 have reported two-color dark-adapted FST results in eight LCA-CEP290 patients. Six of their LCA-CEP290 patients had cone-mediated responses only, similar to those of our patients. Two of their patients (ages 11 and 17 years) had at least some rod contribution to the dark-adapted FST. We did not find significant rod-mediated responses, even in our 9-year-old LCA-CEP290 patient (P1), with the exception of a possible slight rod contribution to blue stimulus thresholds in one patient (P4).
Cideciyan et al.5 have proposed that cones (as opposed to rods) should be targeted in LCA-CEP290 treatments. We found that LCA-CEP290 FST thresholds were elevated well above both rod and cone normal thresholds, and that intravisit variability was quite small relative to threshold elevations. Therefore, dark-adapted two-color FST should be able to detect sensitivity improvement regardless of which photoreceptor type is targeted, precluding the need for either a bleach recovery20 or a background light24 to isolate cones.
With only five subjects performing both PLR and FST, no significant correlation was found between PLR amplitudes and FST thresholds. Even with larger numbers of subjects, it is possible that a strong correlation between these two tests may not be obtained in LCA-CEP290. Although the stimuli were similar between these two tests in this study, suprathreshold cone-mediated PLRs likely depend on the summed response across several degrees of visual angle,18 whereas the FST could theoretically be mediated by only a small patch of relatively sensitive photoreceptors.12
Light Discomfort Thresholds
From their cohort of 47 patients, Perrault et al.2 have described slight photoaversion as a characteristic feature of LCA-CEP290. Photoaversion has been documented in other studies of LCA-CEP290 as well.3,7 Photoaversion in a retinal dystrophy such as LCA-CEP290, where rod function appears to be impaired to a greater degree than cone function, is somewhat paradoxical. We chose to investigate this complaint because five of our six LCA-CEP290 patients noted at least mild photoaversion, with two reporting it as “mild to moderate.”
The subjective responses used in this form of LDT testing lead to variation in the data, and a more objective method of measurement, such as electromyography31 or lid-fissure measurement,32 may be preferable in future studies. Although the variability inherent in such a subjective test precludes detailed analysis, a couple of general conclusions can be made: thresholds for the patients in our sample were in the normal range or above and thresholds were lower for short-wavelength stimuli than for long-wavelength stimuli. It is interesting that full-field LDT thresholds in the four LCA-CEP290 patients were all above the normal control mean (i.e., they were less sensitive), despite their subjective photoaversion. Therefore, this form of LDT testing would not likely be of great value in monitoring for improvement in any treatment of LCA-CEP290, because of a lack of room for improvement in patient thresholds that are already at least normal.
A possible explanation for the normal-to-elevated LDT thresholds is that the full-field stimulus used in this experiment did not test the particular aspect of their vision on which they base the claim of hypersensitivity to bright light. For example, it is possible that what LCA-CEP290 patients interpret as excessive sensitivity to bright light is a phenomenon related to pattern vision, such as a loss of contrast in bright light situations. The diffusing sphere used in this study does not contain patterns or details that the patients might use to recognize such an effect on their pattern vision.
In a study of “photophobia,” Stringham et al.31 have found that normal subjects are more averse to shorter-wavelength than longer-wavelength stimuli. However, their methods involve more focal stimuli as well as radiometric units, making direct comparison to the present study difficult. It is interesting that our patients, who have very poor vision, were also more averse to the potentially more harmful short-wavelength light. The lack of rod mediation of either PLRs or FST thresholds in our LCA-CEP290 patients essentially rules out rods as a source of the relative discomfort with short-wavelength light. S-cones are a potential source of this short-wavelength bias, but this seems unlikely because the patients did not have higher sensitivity to short-wavelength light in the FDT or PLR. An argument might be made that a subjective (cortical) color bias could affect the LDTs; however, this is also unlikely because only one of the six patients was able to consistently name the colors of the narrow-band chromatic stimuli. It seems most likely that the relatively lower discomfort thresholds for short-wavelength stimuli can be attributed to mediation by melanopsin-containing retinal ganglion cells, given that all patients had measurable melanopsin-mediated PLRs and the peak spectral sensitivity of melanopsin is near that of our short-wavelength LED.
Conclusions
Our primary purpose in this study was to assess tests that could be valuable in patients with the low levels of vision associated with LCA-CEP290. Between pupillometry and FST, responses could be measured in all patients. Both FST and pupillometry appear to have an important role in assessing future treatments of LCA-CEP290. Our five LCA-CEP290 adult patients showed a lack of rod function, and all but one showed some preservation of cone function, as demonstrated by FST and pupillometry. The results from our cohort most support labeling LCA-CEP290 as a rod–cone dystrophy, in spite of reduced visual acuity, poor color perception, and subjective photoaversion. Melanopsin appears to influence not only the sustained response to high-luminance PLR stimuli, but also LDTs to short-wavelength stimuli.
Acknowledgments
Supported by The Grousbeck Family Foundation, Boston, Massachusetts (EMS and GAF); The Pangere Family Foundation, Gary, Indiana (GAF); National Institutes of Health Research Grants EY019510 (JJM) and EY001792 (University of Illinois at Chicago core grant) and an unrestricted departmental grant from Research to Prevent Blindness. The authors alone are responsible for the content and writing of the paper.
Disclosure: F.T. Collison, None; J.C. Park, None; G.A. Fishman, None; J.J. McAnany, None; E.M. Stone, None
References
- 1. den Hollander AI,, Koenekoop RK,, Yzer S,, et al. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am J Hum Genet. 2006; 79: 556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perrault I,, Delphin N,, Hanein S,, et al. Spectrum of NPHP6/CEP290 mutations in Leber congenital amaurosis and delineation of the associated phenotype. Hum Mutat. 2007; 28: 416. [DOI] [PubMed] [Google Scholar]
- 3. Coppieters F,, Casteels I,, Meire F,, et al. Genetic screening of LCA in Belgium: predominance of CEP290 and identification of potential modifier alleles in AHI1 of CEP290-related phenotypes. Hum Mutat. 2010; 31: E1709–E1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chang B,, Khanna H,, Hawes N,, et al. In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum Mol Genet. 2006; 15: 1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cideciyan AV,, Rachel RA,, Aleman TS,, et al. Cone photoreceptors are the main targets for gene therapy of NPHP5 (IQCB1) or NPHP6 (CEP290) blindness: generation of an all-cone Nphp6 hypomorph mouse that mimics the human retinal ciliopathy. Hum Mol Genet. 2011; 20: 1411–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walia S,, Fishman GA,, Jacobson SG,, et al. Visual acuity in patients with Leber's congenital amaurosis and early childhood-onset retinitis pigmentosa. Ophthalmology. 2010; 117: 1190–1198. [DOI] [PubMed] [Google Scholar]
- 7. Yzer S,, Hollander AI,, Lopez I,, et al. Ocular and extra-ocular features of patients with Leber congenital amaurosis and mutations in CEP290. Mol Vis. 2012; 18: 412–425. [PMC free article] [PubMed] [Google Scholar]
- 8. McAnany JJ,, Genead MA,, Walia S,, et al. Visual acuity changes in patients with leber congenital amaurosis and mutations in CEP290. JAMA Ophthalmol. 2013; 131: 178–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cideciyan AV,, Aleman TS,, Jacobson SG,, et al. Centrosomal-ciliary gene CEP290/NPHP6 mutations result in blindness with unexpected sparing of photoreceptors and visual brain: implications for therapy of Leber congenital amaurosis. Hum Mutat. 2007; 28: 1074–1083. [DOI] [PubMed] [Google Scholar]
- 10. Pasadhika S,, Fishman GA,, Stone EM,, et al. Differential macular morphology in patients with RPE65-, CEP290-, GUCY2D-, and AIPL1-related Leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2010; 51: 2608–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Park JC,, Moura AL,, Raza AS,, Rhee DW,, Kardon RH,, Hood DC. Toward a clinical protocol for assessing rod cone, and melanopsin contributions to the human pupil response. Invest Ophthalmol Vis Sci. 2011; 52: 6624–6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roman AJ,, Schwartz SB,, Aleman TS,, et al. Quantifying rod photoreceptor-mediated vision in retinal degenerations: dark-adapted thresholds as outcome measures. Exp Eye Res. 2005; 80: 259–272. [DOI] [PubMed] [Google Scholar]
- 13. Klein M,, Birch DG. Psychophysical assessment of low visual function in patients with retinal degenerative diseases (RDDs) with the Diagnosys full-field stimulus threshold (D-FST). Doc Ophthalmol. 2009; 119: 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vanagaite J,, Pareja JA,, Støren O,, White LR,, Sand T,, Stovner LJ. Light-induced discomfort and pain in migraine. Cephalalgia. 1997; 17: 733–741. [DOI] [PubMed] [Google Scholar]
- 15. Adams WH,, Digre KB,, Patel BC,, Anderson RL,, Warner JE,, Katz BJ. The evaluation of light sensitivity in benign essential blepharospasm. Am J Ophthalmol. 2006; 142: 82–87. [DOI] [PubMed] [Google Scholar]
- 16. Papon JF,, Perrault I,, Coste A,, et al. Abnormal respiratory cilia in non-syndromicLeber congenital amaurosis with CEP290 mutations. J Med Genet. 2010; 47: 829–834. [DOI] [PubMed] [Google Scholar]
- 17. McEwen DP,, Koenekoop RK,, Khanna H,, et al. Hypomorphic CEP290/NPHP6 mutations result in anosmia caused by the selective loss of G proteins in cilia of olfactory sensory neurons. Proc Natl Acad Sci U S A. 2007; 104: 15917–15922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park JC,, McAnany JJ. Effect of stimulus size and luminance on the rod-, cone-, and melanopsin-mediated pupillary light reflex. J Vis. 2015; 15 (3): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kawasaki A,, Kardon RH. Intrinsically photosensitive retinal ganglion cells. J Neuroophthalmol. 2007; 27: 195–204. [DOI] [PubMed] [Google Scholar]
- 20. Collison FT,, Fishman GA,, McAnany JJ,, Zernant J,, Allikmets R. Psychophysical measurement of rod and cone thresholds in Stargardt disease with full-field stimuli. Retina. 2014; 34: 1888–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aleman TS,, Jacobson SG,, Chico JD,, et al. Impairment of the transient pupillary light reflex in Rpe65(-/-) mice and humans with leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2004; 45: 1259–1271. [DOI] [PubMed] [Google Scholar]
- 22. Jacobson SG,, Aleman TS,, Cideciyan AV,, et al. Leber congenital amaurosis caused by Lebercilin (LCA5) mutation: retained photoreceptors adjacent to retinal disorganization. Mol Vis. 2009; 15: 1098–1106. [PMC free article] [PubMed] [Google Scholar]
- 23. Jacobson SG,, Cideciyan AV,, Aleman TS,, et al. Human retinal disease from AIPL1 gene mutations: foveal cone loss with minimal macular photoreceptors and rod function remaining. Invest Ophthalmol Vis Sci. 2011; 52: 70–79. [DOI] [PubMed] [Google Scholar]
- 24. Jacobson SG,, Cideciyan AV,, Peshenko IV,, et al. Determining consequences of retinal membrane guanylyl cyclase (RetGC1) deficiency in human Leber congenital amaurosis en route to therapy: residual cone-photoreceptor vision correlates with biochemical properties of the mutants. Hum Mol Genet. 2013; 22: 168–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jacobson SG,, Cideciyan AV,, Ratnakaram R,, et al. Gene therapy for leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Arch Ophthalmol. 2012; 130: 9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Melillo P,, Pecchia L,, Testa F,, Rossi S,, Bennett J,, Simonelli F. Pupillometric analysis for assessment of gene therapy in Leber Congenital Amaurosis patients. Biomed Eng Online. 2012; 11: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maguire AM,, Simonelli F,, Pierce EA,, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008; 358: 2240–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maguire AM,, High KA,, Auricchio A,, et al. Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009; 374: 1597–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thompson S,, Whiting RE,, Kardon RH,, Stone EM,, Narfström K. Effects of hereditary retinal degeneration due to a CEP290 mutation on the feline pupillary light reflex. Vet Ophthalmol. 2010; 13: 151–157. [DOI] [PubMed] [Google Scholar]
- 30. Hauswirth WW,, Aleman TS,, Kaushal S,, et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008; 19: 979–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stringham JM,, Fuld K,, Wenzel AJ. Action spectrum for photophobia. J Opt Soc Am A Opt Image Sci Vis. 2003; 20: 1852–1858. [DOI] [PubMed] [Google Scholar]
- 32. Zelinger L,, Cideciyan AV,, Kohl S,, et al. Genetics and disease expression in the CNGA3 form of achromatopsia: steps on the path to gene therapy. Ophthalmology. 2015; 122: 997–1007. [DOI] [PubMed] [Google Scholar]