Abstract
Purpose
To evaluate the efficacy of nucleoside reverse transcriptase inhibitors (NRTIs) in the laser-induced mouse model of choroidal neovascularization (CNV).
Methods
We evaluated the NRTIs lamivudine (3TC), zidovudine (AZT), and abacavir (ABC) and the P2X7 antagonist A438079. Choroidal neovascularization was induced by laser injury in C57BL/6J wild-type, Nlrp3−/−, and P2rx7−/− mice, and CNV volume was measured after 7 days by confocal microscopy. Drugs were administered by intravitreous injection immediately after the laser injury. Vascular endothelial growth factor-A in RPE-choroid lysates was measured 3 days after laser injury by ELISA. HEK293 cells expressing human and mouse P2X7 were exposed to the selective P2X7 receptor agonist 2′, 3′-(benzoyl-4-benzoyl)-ATP (Bz-ATP) with or without 3TC, and VEGF-A levels in media were measured by ELISA.
Results
Intravitreous injection of 3TC, AZT, and ABC significantly suppressed laser-induced CNV in C57BL/6J wild-type and Nlrp3−/− mice (P < 0.05) but not in P2rx7−/− mice. Intravitreous injection of A438079 also suppressed the laser-induced CNV (P < 0.05). The NRTIs 3TC, AZT, and ABC blocked VEGF-A levels in the RPE/choroid after laser injury in wild-type (P < 0.05) but not P2rx7−/− mice. Moreover, there was no additive effect of 3TC on CNV inhibition when coadministered with a neutralizing VEGF-A antibody. Stimulation of human and mouse P2X7-expressing HEK293 cells with Bz-ATP increased VEGF secretion (P < 0.001), which was abrogated by 3TC (P < 0.001). Stimulation of primary human RPE cells with Bz-ATP increased VEGFA and IL6 mRNA levels, which were abrogated by 3TC.
Conclusions
Multiple clinically relevant NRTIs suppressed laser-induced CNV and downregulated VEGF-A, via P2X7.
Keywords: NRTI, CNV, choroidal neovascularization, P2X7, VEGF, AMD, infammasome
Nucleoside reverse transcriptase inhibitors block laser-induced CNV in mice in a P2X7-dependent manner. NRTI inhibition of VEGF-A was also P2X7 dependent.
Age-related macular degeneration (AMD) is a leading cause of blindness in industrialized nations.1–3 The majority of severe vision loss in AMD is due to the invasion of choroidal blood vessels into the retina, also known as choroidal neovascularization (CNV). The current standard of care for CNV is anti-VEGF-A therapy, which dramatically reduces blindness due to CNV.4–6 Still, only about 10% of AMD patients develop CNV; there are no effective therapies for the remaining 90% of AMD patients with the “dry” form of disease.
Recently, we reported that multiple nucleoside reverse transcriptase inhibitors (NRTIs), widely used to treat HIV infection, were therapeutic in a mouse model of dry AMD7 by virtue of their intrinsic anti-inflammatory activity that targeted the purinergic receptor P2X7 and the NLRP3 inflammasome pathway. We also found that one NRTI, stavudine (d4T), suppressed laser-induced CNV in mice in a P2X7-dependent fashion.7 Interestingly, the P2X7 receptor is known to regulate VEGF-A expression.8–10 However, it is not clear whether other NRTIs also block laser-induced CNV via P2X7 or whether they affect VEGF-A in CNV. Furthermore, since P2X7 activity is not necessarily synonymous with NLRP3 inflammasome activation, it is also not clear whether NRTIs influence laser-induced CNV via an NLRP3-dependent or -independent mechanism.
In this present study, we found that three clinically relevant NRTIs (lamivudine [3TC], zidovudine [AZT], and abacavir sulfate [ABC]) suppressed laser-induced CNV in mice in a P2X7-dependent, NLRP3-independent fashion. Moreover, the P2X7 antagonist A438079 also inhibited laser-induced CNV, and blockade of VEGF-A by NRTIs required P2X7.
Materials and Methods
Animals
All animal studies were approved by the University of Kentucky Institutional Animal Care and Use Committee and performed according to their guidelines and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. C57BL/6J (wild-type) and P2rx7−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). The Nlrp3−/− mice were obtained from G. Nuñez (University of Michigan).
Laser-Induced Mouse CNV Model
Laser photocoagulation (532 nm, 180 mW, 100 ms, 75 μm) (OcuLight GL; IRIDEX Corp., Mountain View, CA, USA) was performed bilaterally (volume studies: four spots per eye; protein analyses: 20 spots per eye) in 6- to 8-week-old male mice on day 0 to induce CNV in a masked fashion as previously described.11–14
Drug Treatment for CNV
The NRTIs 3TC, AZT, and ABC (SelleckChem, Houston, TX, USA) or the P2X7 antagonist A438079 hydrochloride (Tocris, Minneapolis, MN, USA) were dissolved in PBS. For CNV, each group of mice was injected once with 1 μL of NRTIs (3TC, 125 ng/μL; ABC, 183 ng/μL; AZT, 146 ng/μL), 1 μL of A438079 hydrochloride (3, 30, or 300 ng/μL), or the same volume of vehicle (PBS) into the vitreous humor using a 33-gauge needle (Ito Corp., Tokyo, Japan) immediately after laser injury. Another group of mice was injected with 3TC (125 ng) in combination with an anti-mouse VEGF polyclonal antibody (10 ng; R&D Systems, Minneapolis, MN, USA). Goat whole IgG (10 ng; Jackson ImmunoResearch, West Grove, PA, USA) was used as a biological control for the anti-mouse VEGF antibody.
CNV Volume
At 1 week after the laser injury, eyes were enucleated and fixed with 4% paraformaldehyde for 30 minutes at 4°C. Eyecups were obtained by removing the anterior segments and were washed in PBS, followed by dehydration and rehydration through a methanol series. After blocking in PBS with 1% bovine serum albumin (Sigma-Aldrich Corp., St. Louis, MO, USA) and 0.5% Triton X-100 (Sigma-Aldrich Corp.) for 1 hour at room temperature, eyecups were incubated with 0.7% FITC-isolectin B4 (Vector Laboratories, Burlingame, CA, USA) overnight at 4°C. After washing in PBS with 0.1% Triton X-100, the neurosensory retina was gently detached and severed from the optic nerve. Four relaxing radial incisions were made, and the remaining RPE-choroid-sclera complex was flat mounted in antifade medium (Immu-Mount Vectashield Mounting Medium; Vector Laboratories) and coverslipped. Choroidal neovascularization was visualized using a blue argon laser wavelength (488 nm) and a scanning laser confocal microscope (TCS SP5; Leica, Heidelberg, Germany), and quantified using Image J software (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA) in a masked fashion as previously reported.11–14
Cell Culture
Primary human RPE and HEK293 cells were maintained at 37°C in 5% CO2 and were grown in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin (VWR International, Radnor, PA, USA). HEK293 cells with stable expression of human15 or mouse16 P2X7 receptors (HEK293-hP2X7 or HEK293-mP2X7) were maintained as above and with G418 disulfate salt (0.4 mg/mL; Sigma-Aldrich Corp.). Primary human RPE cells were isolated as described previously.7
VEGF-A ELISA Assay
At 3 days after laser injury, the RPE-choroid complex was sonicated in radioimmunoprecipitation assay (RIPA) buffer (Sigma-Aldrich Corp.) with protease inhibitor (Sigma-Aldrich Corp.) on ice for 15 minutes. The lysate was centrifuged at 20,000g for 15 minutes at 4°C, and the VEGF protein levels in the supernatants were measured by enzyme-linked immunosorbent assay (ELISA) for mouse VEGF-A (Quantikine Immunoassay, R&D Systems) as described by the manufacturer. Total protein of the lysates was quantified by bicinchoninic acid (BCA) assay.
HEK293 or HEK293-hP2X7 cells (5 × 105) were plated into six-well plates in complete medium and allowed to adhere for 24 hours. Cells were then incubated for 24 more hours in the absence or presence of 150 μM 2′,3′-(benzoyl-4-benzoyl)-ATP (Bz-ATP). Cells incubated in the presence of Bz-ATP were also incubated with or without 3TC (100 μM), which was added 30 minutes before Bz-ATP. VEGF release into the supernatants was measured by ELISA for human VEGF-A (Quantikine Immunoassay, R&D Systems) as described by the manufacturer.
Real-Time Quantitative PCR
Primary human RPE cells were plated into 12-well plates in complete medium and allowed to adhere for 24 hours. Cells were then incubated for 2 hours in the absence or presence of Bz-ATP (150 μM). Cells incubated in the presence of Bz-ATP were also incubated with or without 3TC (100 μM), which was added 30 minutes before Bz-ATP. Total RNA was collected in reagent (Trizol; Invitrogen, Carlsbad, CA, USA) and reverse transcribed with a reverse transcription kit (QuantiTect; Qiagen, Valencia, CA, USA). The cDNA was amplified by quantitative real-time PCR (qPCR) (7900 HT Fast Real-Time PCR; Applied Biosystems, Foster City, CA, USA) with Power SYBR Green Master Mix (Invitrogen). Oligonucleotide primers specific for human VEGFA (F 5′ AAGGAGGAGGGCAGAATCAT 3′; R 5′ GCAGTAGCTGCGCTGATAGA 3′) and IL6 (F 5′ CTCCTTCTCCACAAGCGCCTTC 3′; R 5′ GCGCAGAATGAGATGAGTTGTC 3′) mRNA were used and normalized to 18S (F 5′ CGCAGCTAGGAATAATGGAATAGG; R 5′ GCCTCAGTTCCGAAAACCAA 3′). The qPCR cycling conditions were 50°C for 2 minutes, 95°C for 10 minutes, followed by 40 cycles of a two-step amplification program (95°C for 15 seconds and 58°C for 1 minute). Relative expression of target genes was determined by the 2−ΔΔCt method.
Statistical Analysis
All results were expressed as mean plus or minus standard error of the mean (n = number of eyes) with P < 0.05 considered statistically significant as determined by one-way ANOVA with Dunn, Student-Newman-Keuls, or Tukey post hoc tests, as appropriate.
Results
NRTIs Supressed Laser-Induced CNV in a P2X7-Dependent, NLRP3-Independent Fashion
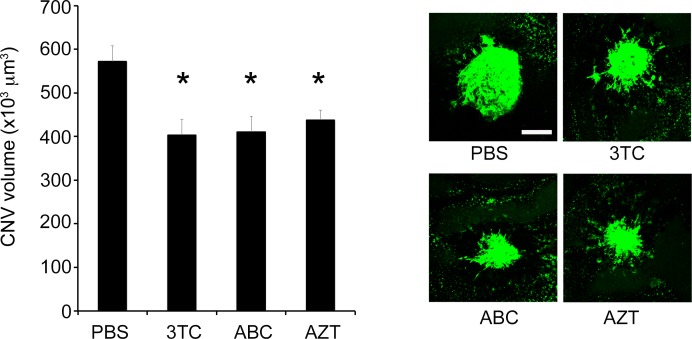
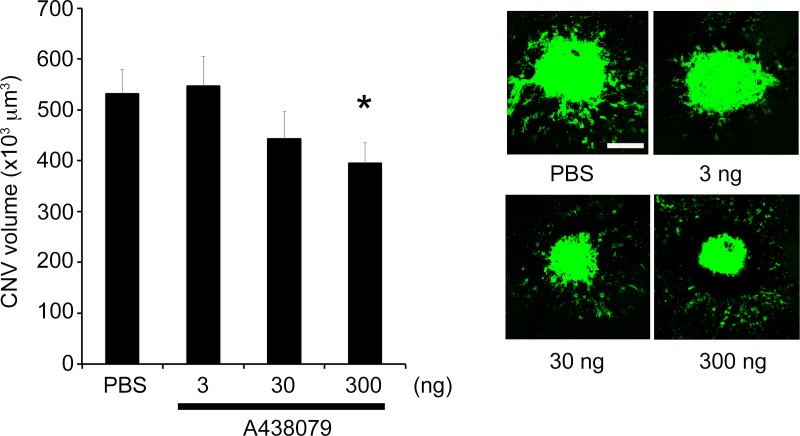
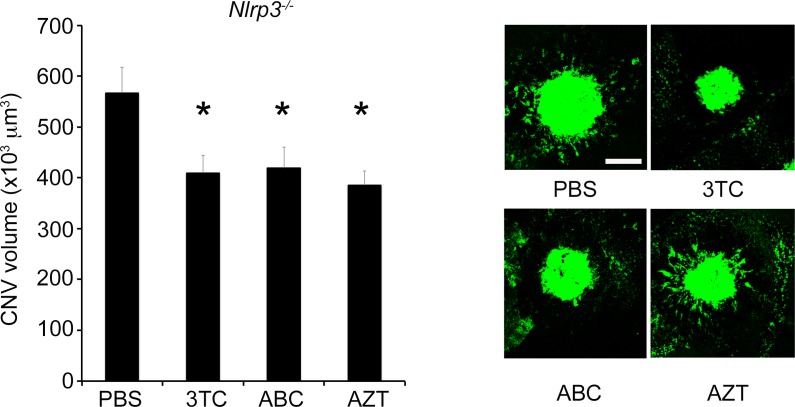
Intravitrous injection of the NRTIs 3TC, AZT, or ABC suppressed the laser-induced CNV in wild-type mice compared to PBS vehicle (Fig. 1). On the other hand, intravitreous injection of 3TC, AZT, or ABC did not suppress the laser-induced CNV in P2rx7−/− mice (Fig. 2). Also supporting the involvement of P2X7 in laser-induced CNV was our finding that the selective P2X7 antagonist A438079 suppressed laser-induced CNV (Fig. 3). Nlrp3 was not required for the antiangiogenic effect of NRTIs, as intravitreous injection of 3TC, AZT, or ABC suppressed laser-induced CNV in Nlrp3−/− mice (Fig. 4).
Figure 1.
NRTIs suppressed laser-induced CNV volume in wild-type mice. Intravitreous administration of 3TC, AZT, and ABC significantly suppressed laser-induced CNV volume in wild-type mice. Data are means ± SEM (n = 7–13 per group). *P < 0.05 by one-way ANOVA and Dunn method. Right: Representative images of FITC-isolectin CNV lesions in RPE-choroid flat mounts. Scale bar: 100 μm.
Figure 2.
NRTIs did not suppress laser-induced CNV in P2rx7−/− mice. Intravitreous injection of 3TC, AZT, and ABC did not suppress the laser-induced CNV in P2rx7−/− mice compared with control (PBS). Data are means ± SEM (n = 4 per group). *P < 0.05 by one-way ANOVA and Dunn method. N.S., not significant. Right: Representative images of FITC-isolectin CNV lesions in RPE-choroid flat mounts. Scale bar: 100 μm.
Figure 3.
A P2X7 antagonist suppressed laser-induced CNV in wild-type mice. The P2X7 antagonist A438079 (300 ng) significantly suppressed laser-induced CNV compared with control (PBS). Data are means ± SEM (n = 5 per group). *P < 0.05 by one-way ANOVA and Dunn method. Right: Representative images of FITC-isolectin CNV lesions in RPE-choroid flat mounts. Scale bar: 100 μm.
Figure 4.
NRTIs suppressed laser-induced CNV in Nlrp3−/− mice. The volume of laser-induced CNV was significantly reduced in Nlrp3−/− mice by intravitreous administration of 3TC, AZT, and ABC compared with control (n = 4–6 per group). *P < 0.05 by one-way ANOVA and Student-Newman-Keuls post hoc test. Right: Representative images of FITC-isolectin CNV lesions in RPE-choroid flat mounts. Scale bar: 100 μm.
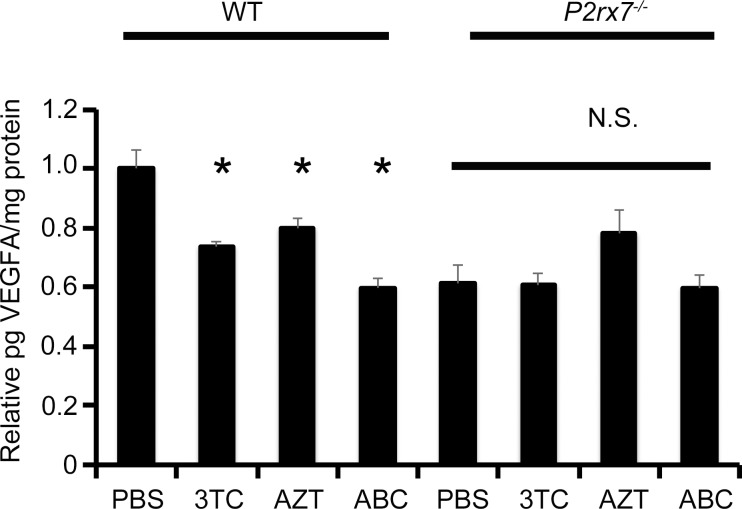
NRTIs Suppressed VEGF-A Levels in a P2X7-Dependent Fashion
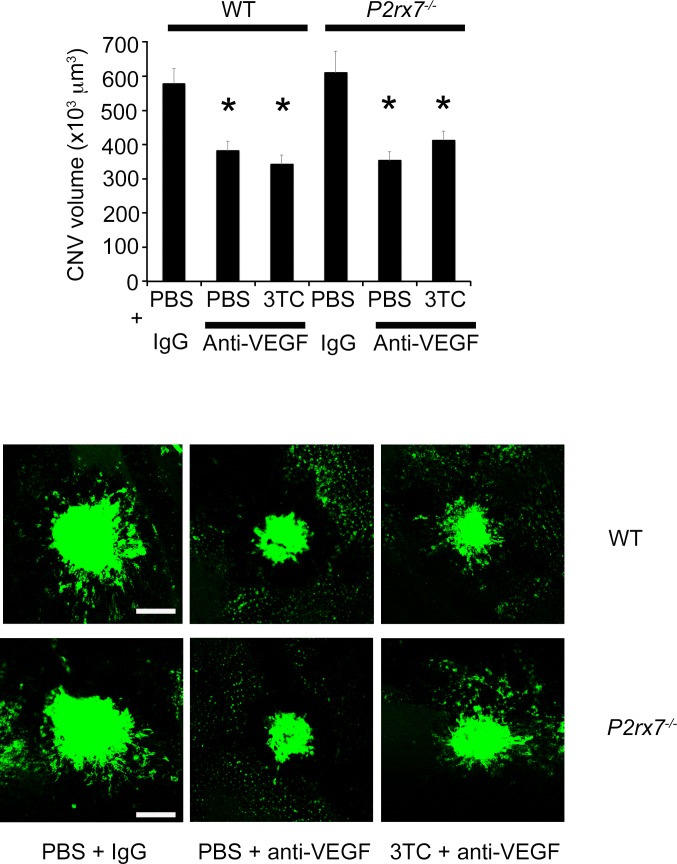
The mean level of VEGF-A in the RPE/choroid, which peaks on day 3 after laser injury,17 was significantly reduced in 3TC-, AZT- and ABC-treated eyes compared with control eyes in wild-type mice, but not in P2rx7−/− mice (Fig. 5). Intravitreous injection of an anti-mouse VEGF-A antibody reduced laser-induced CNV in wild-type mice compared with a control IgG; in support of the idea that the antiangiogenic effect of NRTIs is achieved by VEGF-A inhibition, 3TC did not suppress CNV when coadministered with this VEGF-A neutralizing antibody (Fig. 6).
Figure 5.
NRTIs suppressed VEGF-A levels in the RPE/choroid in laser-induced CNV. VEGF-A levels in the RPE/choroid were significantly reduced in 3TC-, AZT-, or ABC-treated groups compared with control group (PBS). Data are means ± SEM (n = 6–10 per group). Groups treated with 3TC, AZT, or ABC were not significantly different compared with control group in P2rx7−/− mice. *P < 0.05 versus control by one-way ANOVA and Tukey post hoc test. N.S., not significant.
Figure 6.
Coadministration of 3TC with anti-mouse VEGF antibody did not reduce CNV. Intravitreous injection of an anti-mouse VEGF antibody (10 ng) reduced laser-induced CNV volume in wild-type and P2rx7−/− mice compared with an IgG control (10 ng). Intravitreous injection of 3TC in combination with the anti-mouse VEGF antibody suppressed laser-induced CNV to a similar extent in wild-type and P2rx7−/− mice. Data are means ± SEM (n = 4–6 per group). *P < 0.05 versus control by one-way ANOVA and Student-Newman-Keuls post hoc test. Bottom: Representative images of FITC-isolectin CNV lesions in RPE-choroid flat mounts. Scale bar: 100 μm.
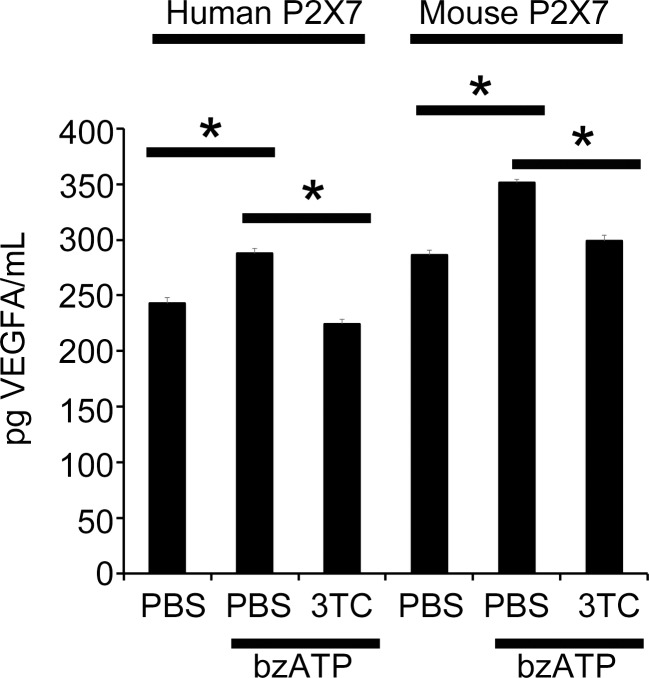
To further test the effect of NRTIs on VEGF-A via P2X7, we utilized HEK293 cells stably expressing the human or mouse P2X7 receptors (HEK293-hP2X7/HEK293-mP2X7). The selective P2X7 agonist Bz-ATP increased VEGF-A secretion into the cell culture media from both HEK293-hP2X7 and HEK293-mP2X7 cells compared to control-treated cells, an effect that was diminished upon 3TC treatment (Fig. 7).
Figure 7.
3TC suppressed VEGF-A upregulation in HEK-P2X7 cells stimulated with Bz-ATP. VEGF-A protein in cell culture media of both HEK293-mP2X7 and HEK293-hP2X7 cells stimulated with Bz-ATP for 24 hours were significantly increased (n = 4 per group). Treatment with 3TC diminished VEGF-A secretion in both Bz-ATP–treated HEK293-hP2X7 and HEK293-mP2X7 cells compared to Bz-ATP alone. *P < 0.001 versus no treatment groups by one-way ANOVA and Student-Newman-Keuls post hoc test. N.S., not significant.
Finally, we explored whether 3TC affected Bz-ATP-induced angiogenic cytokine expression in primary human RPE cells, which are known to produce VEGF-A and IL-6.18,19 We found that 3TC blocked Bz-ATP–induced VEGFA and IL6 mRNA expression (Fig. 8). Like VEGFA, IL6 is proangiogenic, regulated by P2X7,20 and known to be involved in laser CNV.21–23 This result suggests that NRTIs could modulate angiogenesis by affecting the expression of multiple P2X7-dependent cytokines in RPE cells.
Figure 8.
3TC suppressed VEGF-A and IL6 mRNA upregulation in primary human RPE cells stimulated with Bz-ATP. VEGFA and IL6 mRNA levels in primary human RPE cells stimulated with Bz-ATP for 2 hours were significantly increased (n = 4–7 per group). Treatment with 3TC reduced both VEGFA and IL6 mRNA levels in Bz-ATP–treated cells compared to Bz-ATP alone. *P < 0.05 by one-way ANOVA and Student-Newman-Keuls post hoc test.
Discussion
The repurposing of NRTIs is currently being pursued as a therapeutic strategy for the treatment of dry AMD. Since CNV often coexists on a background of dry AMD in humans, it is prudent to also evaluate the effect of NRTIs on CNV. The first distinct role for P2X7 in modulating CNV was recently reported.7 Another study showed that a nonselective P2 receptor antagonist reduced laser CNV in mice, although the role of P2X7 per se was not clear.24 Here we found that multiple clinically relevant NRTIs blocked laser-induced CNV in mice and that P2X7 was required for their antiangiogenic function (Fig. 1, 2). The selective P2X7 antagonist A438079 also inhibited laser-induced CNV in mice (Fig. 3).
There is precedent for the proangiogenic function of P2X7 in experimental cancer systems. P2X7-overexpresing HEK293 and CT26 cells form solid tumors that show an accelerated in vivo growth rate, higher VEGF-A release, and thicker vascular network in mice relative to hypo-expressing P2X7 parental cells. The in vivo growth rate of P2X7-expressing tumors was blunted by VEGF-A neutralization; moreover, P2X7 inhibition blocked tumor growth and reduced VEGF-A levels in this system,9 in line with previous reports indicating that P2X7 regulates VEGF-A expression and secretion in various cell types.8,10,25 Our data are consistent with the idea that the angio-inhibitory effects of NRTIs are mediated by P2X7 inhibition and VEGF-A suppression (Fig. 5–7).
Future work should clarify the identity of the P2X7-expressing cell type(s) that mediate the antiangiogenic effects of NRTIs in laser-induced CNV. Mouse RPE cells in vivo express P2X7, as do mouse RPE primary cell cultures.26 It is also known that RPE cells are a dominant source of VEGF production in the murine retina.27,28 Therefore, it is tempting to speculate that NRTIs modulate angiogenesis by acting directly on RPE cells to control VEGF-A levels. Primary and immortalized (ARPE-19) human RPE cells also express functional P2X7 in cell culture.26 On the other hand, monocytes and macrophages, which express high levels of P2X7,29 are recruited to the choroid after laser injury and drive angiogenesis via VEGF production.17,30,31 Thus, future work will be required to determine whether NRTIs block local VEGF-A release by infiltrating macrophages or by RPE cells. P2X732–34 and VEGF-A35,36 are also involved in regulating immune cell chemotaxis. In contrast to VEGF-A, the proposed chemotactic role of P2X7 in the laser-induced mouse model of CNV is not clear. For these reasons, we speculate that NRTIs could modulate laser-induced CNV by modulating monocyte/macrophage migration.
Nucleoside reverse transcriptase inhibitors could also reduce laser-induced CNV via other P2X7-dependent molecules (Fig. 8). Besides VEGF-A, P2X7 is known to regulate the expression of other proangiogenic cytokines that modulate laser-induced CNV, including IL-621–23 and TNF-α37,38; these cytokines have also been reported in human CNV membranes39 and in the aqueous humor40 of CNV patients. Nucleoside reverse transcriptase inhibitors blocked plasma IL-6 and TNF-α levels in a mouse model of graft-versus-host disease7; future work will be required to determine whether NRTIs also inhibit these cytokines in CNV. P2X7 is also known to regulate the expression of the chemotactic ligand MCP-1/CCL2,22,41 which is known to be involved in neovascularization and immune cell migration.42–44 Interestingly, the CNV lesion size of P2rx7−/− mice is similar to that of wild-type mice (Figs. 1, 2), despite having reduced VEGF-A levels (Fig. 5), which is consistent with the idea that VEGF-A is not the only angiogenic cytokine that modulates laser CNV.
P2X7 is also a critical signaling intermediate in NLRP3 inflammasome activation. Although NRTIs block P2X7-dependent NLRP3 inflammasome activation,7,45 our data indicate that Nlrp3 is not required for NRTI-induced CNV suppression (Fig. 4). NLRP3 expression is increased in histologic sections of human CNV specimens.46 However, the role of the NLRP3 inflammasome in modulating CNV remains contentious. One group has reported that genetic inhibition of NLRP347 reduces laser-induced CNV. On the other hand, a consortium of five research groups could not reproduce the earlier group's findings.48 Regardless of these differences, the data presented here indicate that NRTIs block P2X7-dependent NLRP3-independent laser-induced CNV.
Previous work indicated that d4T blocked laser-induced CNV independent of reverse transcriptase inhibition since its methoxy-modified variant also inhibited P2X7-dependent laser-induced CNV.7 In the future, the creation of methoxy-modified NRTIs for 3TC, ABC, and AZT would support the idea that these NRTIs also reduce laser-induced CNV independent of reverse transcriptase inhibition.
Acknowledgments
The authors thank Nagaraj Kerur, Ana Bastos-Carvalho, Tetsuhiro Yasuma, Charles Wright, and Junichi Fukuhara for discussions. The authors also thank Nicholas Bell, Lindsay Toll, Gregory Botzet, Robinette King, Li Xu, Charles Payne, Gary Pattison, Darrell Robertson, Annette Uittenbogaard, Xiaoqin Zhou, and Kameshwari Ambati for technical assistance. The authors thank Gabriel Nuñez for mice and George R. Dubyak for providing HEK293 P2X7-expressing cells.
This work was supported by NIH Grants DP1GM114862, R01EY018350, R01EY018836, R01EY020672, R01EY022238, and R01EY024068; Doris Duke Distinguished Clinical Scientist Award; Burroughs Wellcome Fund Clinical Scientist Award in Translational Research; Ellison Medical Foundation Senior Scholar in Aging Award; Foundation Fighting Blindness Individual Investigator Research Award; Harrington Discovery Institute Scholar-Innovator Award; Dr. E. Vernon Smith and Eloise C. Smith Macular Degeneration Endowed Chair; and Research to Prevent Blindness departmental unrestricted grant (to JA). The work was also supported by American Heart Association and International Retinal Research Foundation (to BDG) and by Grants NIH T32HL091812 and UL1RR033173 (to BJF).
JA and BJF are named as inventors on patent applications filed by their employer, the University of Kentucky, based on the technology described in this work. JA is a cofounder of iVeena (Holdings, Delivery Systems, Pharma), which has licensed some of this technology. JA has received honoraria and travel support from Allergan unrelated to the work described in this article. All authors of this article, like all investigators, have an intrinsic financial interest in the success of their work.
Disclosure: T. Mizutani, None; B.J. Fowler (P); Y. Kim, None; R. Yasuma, None; L.A. Krueger, None; B.D. Gelfand, None; J. Ambati, iVeena (I, S), Allergan (R, P)
References
- 1. Ambati J,, Ambati BK,, Yoo SH,, Ianchulev S,, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003; 48: 257–293. [DOI] [PubMed] [Google Scholar]
- 2. Ambati J,, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron. 2012; 75: 26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ambati J,, Atkinson JP,, Gelfand BD. Immunology of age-related macular degeneration. Nat Rev Immun. 2013; 13: 438–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gragoudas ES,, Adamis AP,, Cunningham ET,, Jr., Feinsod M,, Guyer DR. Pegaptanib for neovascular age-related macular degeneration. New Engl J Med. 2004; 351: 2805–2816. [DOI] [PubMed] [Google Scholar]
- 5. Brown DM,, Kaiser PK,, Michels M,, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. New Engl J Med. 2006; 355: 1432–1444. [DOI] [PubMed] [Google Scholar]
- 6. Rosenfeld PJ,, Brown DM,, Heier JS,, et al. Ranibizumab for neovascular age-related macular degeneration. New Engl J Med. 2006; 355: 1419–1431. [DOI] [PubMed] [Google Scholar]
- 7. Fowler BJ,, Gelfand BD,, Kim Y,, et al. Nucleoside reverse transcriptase inhibitors possess intrinsic anti-inflammatory activity. Science. 2014; 346: 1000–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hill LM,, Gavala ML,, Lenertz LY,, Bertics PJ. Extracellular ATP may contribute to tissue repair by rapidly stimulating purinergic receptor X7-dependent vascular endothelial growth factor release from primary human monocytes. J Immunol. 2010; 185: 3028–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adinolfi E,, Raffaghello L,, Giuliani AL,, et al. Expression of P2X7 receptor increases in vivo tumor growth. Cancer Res. 2012; 72: 2957–2969. [DOI] [PubMed] [Google Scholar]
- 10. Amoroso F,, Capece M,, Rotondo A,, et al. The P2X7 receptor is a key modulator of the PI3K/GSK3beta/VEGF signaling network: evidence in experimental neuroblastoma. Oncogene. 2015. ;3:e370. [DOI] [PubMed]
- 11. Kleinman ME,, Yamada K,, Takeda A,, et al. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008; 452: 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nozaki M,, Sakurai E,, Raisler BJ,, et al. Loss of SPARC-mediated VEGFR-1 suppression after injury reveals a novel antiangiogenic activity of VEGF-A. J Clin Invest. 2006; 116: 422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nozaki M,, Raisler BJ,, Sakurai E,, et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci U S A. 2006; 103: 2328–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mizutani T,, Ashikari M,, Tokoro M,, Nozaki M,, Ogura Y. Suppression of laser-induced choroidal neovascularization by a CCR3 antagonist. Invest Ophthalmol Vis Sci. 2013; 54: 1564–1572. [DOI] [PubMed] [Google Scholar]
- 15. Humphreys BD,, Virginio C,, Surprenant A,, Rice J,, Dubyak GR. Isoquinolines as antagonists of the P2X7 nucleotide receptor: high selectivity for the human versus rat receptor homologues. Mol Pharmacol. 1998; 54: 22–32. [DOI] [PubMed] [Google Scholar]
- 16. Hong S,, Schwarz N,, Brass A,, et al. Differential regulation of P2X7 receptor activation by extracellular nicotinamide adenine dinucleotide and ecto-ADP-ribosyltransferases in murine macrophages and T cells. J Immunol. 2009; 183: 578–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sakurai E,, Anand A,, Ambati BK,, van Rooijen N,, Ambati J. Macrophage depletion inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003; 44: 3578–3585. [DOI] [PubMed] [Google Scholar]
- 18. Adamis AP,, Shima DT,, Yeo KT,, et al. Synthesis and secretion of vascular permeability factor/vascular endothelial growth factor by human retinal pigment epithelial cells. Biochem Biophy Res Commun. 1993; 193: 631–638. [DOI] [PubMed] [Google Scholar]
- 19. Elner VM,, Scales W,, Elner SG,, Danforth J,, Kunkel SL,, Strieter RM. Interleukin-6 (IL-6) gene expression and secretion by cytokine-stimulated human retinal pigment epithelial cells. Exp Eye Res. 1992; 54: 361–368. [DOI] [PubMed] [Google Scholar]
- 20. Friedle SA,, Brautigam VM,, Nikodemova M,, Wright ML,, Watters JJ. The P2X7-Egr pathway regulates nucleotide-dependent inflammatory gene expression in microglia. Glia. 2011; 59: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Izumi-Nagai K,, Nagai N,, Ozawa Y,, et al. Interleukin-6 receptor-mediated activation of signal transducer and activator of transcription-3 (STAT3) promotes choroidal neovascularization. Am J Pathol. 2007; 170: 2149–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shieh CH,, Heinrich A,, Serchov T,, van Calker D,, Biber K. P2X7-dependent, but differentially regulated release of IL-6, CCL2, and TNF-alpha in cultured mouse microglia. Glia. 2014; 62: 592–607. [DOI] [PubMed] [Google Scholar]
- 23. Solini A,, Chiozzi P,, Morelli A,, Fellin R,, Di Virgilio F. Human primary fibroblasts in vitro express a purinergic P2X7 receptor coupled to ion fluxes microvesicle formation and IL-6 release. J Cell Sci. 1999; 112 (pt 3) ; 297–305. [DOI] [PubMed] [Google Scholar]
- 24. Birke K,, Lipo E,, Birke MT,, Kumar-Singh R. Topical application of PPADS inhibits complement activation and choroidal neovascularization in a model of age-related macular degeneration. PloS One. 2013; 8: e76766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wei W,, Ryu JK,, Choi HB,, McLarnon JG. Expression and function of the P2X(7) receptor in rat C6 glioma cells. Cancer Lett. 2008; 260: 79–87. [DOI] [PubMed] [Google Scholar]
- 26. Guha S,, Baltazar GC,, Coffey EE,, et al. Lysosomal alkalinization, lipid oxidation, and reduced phagosome clearance triggered by activation of the P2X7 receptor. FASEB J. 2013; 27: 4500–4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ford KM,, Saint-Geniez M,, Walshe T,, Zahr A,, D'Amore PA. Expression and role of VEGF in the adult retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2011; 52: 9478–9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saint-Geniez M,, Maldonado AE,, D'Amore PA. VEGF expression and receptor activation in the choroid during development and in the adult. Invest Ophthalmol Vis Sci. 2006; 47: 3135–3142. [DOI] [PubMed] [Google Scholar]
- 29. Burnstock G,, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004; 240: 31–304. [DOI] [PubMed] [Google Scholar]
- 30. Espinosa-Heidmann DG,, Reinoso MA,, Pina Y,, Csaky KG,, Caicedo A,, Cousins SW. Quantitative enumeration of vascular smooth muscle cells and endothelial cells derived from bone marrow precursors in experimental choroidal neovascularization. Exp Eye Res. 2005; 80: 369–378. [DOI] [PubMed] [Google Scholar]
- 31. Murakami Y,, Ikeda Y,, Yonemitsu Y,, et al. Inhibition of choroidal neovascularization via brief subretinal exposure to a newly developed lentiviral vector pseudotyped with Sendai viral envelope proteins. Hum Gene Ther. 2010; 21: 199–209. [DOI] [PubMed] [Google Scholar]
- 32. Kim JE,, Ryu HJ,, Yeo SI,, Kang TC. P2X7 receptor regulates leukocyte infiltrations in rat frontoparietal cortex following status epilepticus. J Neuroinflammation. 2010; 7: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ousingsawat J,, Wanitchakool P,, Kmit A,, et al. Anoctamin 6 mediates effects essential for innate immunity downstream of P2X7 receptors in macrophages. Nat Commun. 2015; 6: 6245. [DOI] [PubMed] [Google Scholar]
- 34. McDonald B,, Pittman K,, Menezes GB,, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010; 330: 362–366. [DOI] [PubMed] [Google Scholar]
- 35. Clauss M,, Weich H,, Breier G,, et al. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Biol Chem. 1996; 271: 17629–17634. [DOI] [PubMed] [Google Scholar]
- 36. Barleon B,, Sozzani S,, Zhou D,, Weich HA,, Mantovani A,, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996; 87: 3336–3343. [PubMed] [Google Scholar]
- 37. Lichtlen P,, Lam TT,, Nork TM,, Streit T,, Urech DM. Relative contribution of VEGF and TNF-alpha in the cynomolgus laser-induced CNV model: comparing the efficacy of bevacizumab adalimumab, and ESBA105. Invest Ophthalmol Vis Sci. 2010; 51: 4738–4745. [DOI] [PubMed] [Google Scholar]
- 38. Shi X,, Semkova I,, Muther PS,, Dell S,, Kociok N,, Joussen AM. Inhibition of TNF-alpha reduces laser-induced choroidal neovascularization. Exp Eye Res. 2006; 83: 1325–1334. [DOI] [PubMed] [Google Scholar]
- 39. Oh H,, Takagi H,, Takagi C,, et al. The potential angiogenic role of macrophages in the formation of choroidal neovascular membranes. Invest Ophthal Vis Sci. 1999; 40: 1891–1898. [PubMed] [Google Scholar]
- 40. Roh MI,, Kim HS,, Song JH,, Lim JB,, Koh HJ,, Kwon OW. Concentration of cytokines in the aqueous humor of patients with naive recurrent and regressed CNV associated with amd after bevacizumab treatment. Retina. 2009; 29: 523–529. [DOI] [PubMed] [Google Scholar]
- 41. Panenka W,, Jijon H,, Herx LM,, et al. P2X7-like receptor activation in astrocytes increases chemokine monocyte chemoattractant protein-1 expression via mitogen-activated protein kinase. J Neurosci. 2001; 21: 7135–7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yamada K,, Sakurai E,, Itaya M,, Yamasaki S,, Ogura Y. Inhibition of laser-induced choroidal neovascularization by atorvastatin by downregulation of monocyte chemotactic protein-1 synthesis in mice. Invest Ophthalmol Vis Sci. 2007; 48: 1839–1843. [DOI] [PubMed] [Google Scholar]
- 43. Ambati J,, Anand A,, Fernandez S,, et al. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med. 2003; 9: 1390–1397. [DOI] [PubMed] [Google Scholar]
- 44. Ambati BK,, Joussen AM,, Kuziel WA,, Adamis AP,, Ambati J. Inhibition of corneal neovascularization by genetic ablation of CCR2. Cornea. 2003; 22: 465–467. [DOI] [PubMed] [Google Scholar]
- 45. Kerur N,, Hirano Y,, Tarallo V,, et al. TLR-independent and P2X7-dependent signaling mediate Alu RNA-induced NLRP3 inflammasome activation in geographic atrophy. Invest Ophthalmol Vis Sci. 2013; 54: 7395–7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tseng WA,, Thein T,, Kinnunen K,, et al. NLRP3 inflammasome activation in retinal pigment epithelial cells by lysosomal destabilization: implications for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013; 54: 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Doyle SL,, Campbell M,, Ozaki E,, et al. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat Med. 2012; 18: 791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hirano Y,, Yasuma T,, Mizutani T,, et al. IL-18 is not therapeutic for neovascular age-related macular degeneration. Nat Med. 2014; 20: 1372–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]