Abstract
Purpose
To profile which cytokine genes are differentially expressed (DE) as up- or downregulated by cultured human trabecular meshwork (TMEs) and Schlemm's canal endothelial cells (SCEs) after three experimental treatments consisting of selective laser trabeculoplasty (SLT) irradiation, exposure to media conditioned either by SLT-irradiated TMEs (TME-cm) or by SCEs (SCE-cm). Also, to profile which cytokines are upregulated ex vivo in SLT-irradiated human conventional aqueous outflow pathway (CAOP) tissues.
Methods
After each treatment, Affymetrix microarray assays were used to detect upregulated and downregulated genes for cytokines and their receptors in TMEs and SCEs. ELISA and protein antibody arrays were used to detect upregulated cytokines secreted in SLT-irradiated CAOP tissues ex vivo.
Results
The SLT irradiation upregulated numerous cytokine genes in TMEs, but only a few in SCEs. Exposure to TME- and SCE-cm induced SCEs to upregulate many more cytokine genes than TMEs. Selective laser trabeculoplasty irradiation and exposure to TME-cm downregulated several cytokine genes in TMEs but none in SCEs. Selective laser trabeculoplasty irradiation induced one upregulated and three downregulated cytokine-receptor genes in TMEs but none in SCEs. Exposure to TME-cm induced upregulation of one and downregulation of another receptor gene in TMEs, whereas two unique cytokine-receptor genes were upregulated in SCEs. Cytokine protein expression analysis showed that at least eight cytokines were upregulated in SLT-irradiated human CAOP tissues in situ/ex vivo.
Conclusions
This study has helped us identify a cytokine signaling pathway and to consider newly identified mechanisms regulating aqueous outflow that may lay the foundation for the future development of cytokine-based glaucoma therapies.
Keywords: cytokines, aqueous outflow, glaucoma therapy, in vitro; ex vivo studies
More than 3 decades ago, it was predicted that a laser instrument capable of emitting nanosecond pulses of green light would “activate” irradiated cells due to thermal effects induced by the photothermolysis of melanin granules.1 Only a decade later, the neodymium-doped yttrium aluminum garnet (Nd-YAG) laser instrument became available, as well as the selective laser trabeculoplasty (SLT) procedure.2 Soon thereafter, it was shown that melanin granules in SLT-irradiated TMEs do become depigmented as they undergo “selective” photothermolysis.3,4 Later, it was also learned that SLT-irradiated trabecular meshwork cells (TMEs) and Schlemm's canal endothelial cells (SCEs) undergo the upregulation of numerous genes at the genomic-wide level as they become activated.5–7
On learning that Argon laser-irradiated TMEs secrete IL-1α, IL-1β, and TNF-α,8 we established that SLT-irradiated TMEs and SCEs also secrete the same three cytokines.5,6 Moreover, we also learned that adding selected recombinant cytokines onto SCE monolayers induced marked increases in fluid flow.5,6 Cytokines have also been identified in the aqueous humor of glaucoma patients,9 and certain chemokines are constitutively secreted by TMEs and may maintain a population of resident mononuclear-cells in the conventional aqueous outflow pathway (CAOP).10
Moving forward, using Affymetrix microarrays, we have now identified 28 upregulated and 18 downregulated cytokine genes after exposing TMEs and SCEs to either SLT irradiation or to media conditioned by SLT-irradiated TMEs or SCEs. In addition, we identified six cytokine-receptor genes upregulated or downregulated in TMEs or SCEs after at least one of these treatments. Further, using ELISA and protein antibody arrays, we profiled at least eight upregulated cytokines in SLT-irradiated CAOP explants ex vivo representing an extensive cytokine-driven signaling pathway.
Methods
Specimens
In Vitro.
We established human TMEs or SCEs in primary culture using published methods.11–13 For each experiment, TMEs from one donor and SCEs from another donor were collected (using passage ≤ 5). Confluent monolayers were then treated either by SLT irradiation or by exposure to conditioned media, using our protocols.5,6 The cells were first grown in Dulbecco's modified Eagle's medium (DMEM) containing 15% fetal calf serum (FCS) and then the FCS was replaced to use 10% heat inactivated FCS during the experimental in vitro treatments.
Ex Vivo.
The day of harvesting, corneo-scleral rims were placed in freshly prepared DMEM media and used in ex vivo experiments. We followed tenets of the Declaration of Helsinki and received institutional review board approval from the University of California San Francisco Committee on Human Research approval number 10-04506.
Experiments
In Vitro.
In triplicate experiments, TME or SCE monolayers containing approximately 3.9 million cells per sample received one of the following three treatments: (1) SLT irradiation using the frequency-doubled, Q-switched, Nd:YAG instrument (F-D Nd:YAG) (Lumenis, Inc., San Jose, CA, USA) to deliver a standard number of low-fluency shots (0.8 mJ/pulse or 600 mJ/cm2); (2) exposure to media conditioned for 36 hours after SLT irradiation by either cells of the same type, or (3) by cells of the other type. Twelve hours after each of three treatments, total RNA was extracted from each monolayer for cytokine-gene expression assays. Thirty-six to 48 hours after each of three treatments, media from TMEs or SCEs were collected and assayed for cytokine-protein expression.5,6
Ex Vivo.
Each corneo-scleral ring was maintained in cell-culture media and divided into halves, with one-half receiving 83 SLT laser pulses measuring approximately 0.60 mJ/shot amounting to an average of 49.42 mJ/specimen, and the other half remaining untreated as a control. After SLT treatment, the CAOP tissues were dissected from surrounding tissues and placed in 35-mm culture dishes containing 0.5 mL of culture media and 5% fetal bovine serum. After 48 hours, the media were assayed to measure the total protein concentration using the BCA kit (Pierce Thermo Scientific, Rockford, IL, USA). Subsequently, using cytokine antibody array, ELISA, or both methods, treated and untreated samples were assayed in duplicate loading the same amount of total protein. Antibody array densitometry data were collected for each cytokine spot using ImageJ (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA) and ELISA concentration was determined using VERSAmax Microplate Reader (TECAN; Morrisville, NC, USA).
Growth media were used as a “blank” to ascertain that no extraneous cytokine background was detected. Moreover, antibodies used in such assays were monoclonal antibodies with reactivity against human proteins. Typically very low to zero values were detected in negative controls.
Assays
Affymetrix Microarray.
Gene expression for cytokines and their corresponding cytokine receptors was obtained from the extracted total RNA using the Affymetrix Human U133 plus 2.0 platform and the GeneChip Expression Analysis Software (Affymetrix, Santa Clara, CA, USA).5,6
Antibody Array and ELISA.
For three specific cytokines,5,6 the in vitro secretion of cytokines from the 3.9 million cells taken from each cultured TME and SCE samples was measured. Next, cytokines secreted ex vivo by SLT-irradiated or untreated CAOP tissues were measured using both Human Cytokine Antibody Array methods (Ray Biotech, Inc., Norcross, GA, USA) and/or ELISA kits based on antibody availability (R&D Systems, Inc., Minneapolis, MN, USA; Ray Biotech, Inc.; Antibodies Online, Inc., Atlanta, GA, USA; Abca, Inc., Cambridge, MA, USA; My Bio Source, Inc., San Diego, CA, USA).
Statistical Analyses
Affymetrix Cytokine Gene Expression.
The word “cytokine” is used to refer collectively to all cytokines, chemokines, interleukins, and other proteins purported to have some cytokine function. Previously published Affymetrix measurements were used for the analysis of cytokine gene expression.5,6 Criteria of the Gene Ontology (GO) annotation database (http://www.geneontology.org) were used to select 824 probe sets for 437 candidate cytokine genes for Affymetrix analysis. After filtering out 317 probes expressing an average log2 intensity less than 5, there remained 507 probes for 319 genes for further analysis. Using an empirical Bayes model, moderated t-statistics and their associated P values were calculated for each log2 intensity difference.14 These P values were adjusted for multiple comparisons using the false discovery rate (FDR) method of Benjamini and Hochberg.15 The average log2 intensity difference between each experimental condition and its corresponding control was calculated by raising 2 to the power of the log2 intensity difference for each probe set. All calculations were made using the limma library of the R/Bioconductor software package16 in SAS 9.3 (SAS Institute, Inc., Cary, NC, USA).
Upregulated Cytokine Genes.
The top 50 upregulated probe sets for each of the six experimental comparisons were selected and combined to represent potentially upregulated genes. For genes with multiple probe sets, the particular probe with the largest fold value was selected for further analysis, resulting in 83 upregulated cytokine genes. Only cytokine genes with a calculated fold change ≥ 1.5 and a one-sided FDR-adjusted P value less than 0.1 for at least one experimental condition qualified as upregulated genes. After excluding noncytokine genes (i.e., receptors, enzymes, cluster of differentiation proteins), 28 unique cytokine genes remained identified as upregulated based on the results of the six experimental comparisons.
Downregulated Cytokine Genes.
Using an approach parallel to that used above for detection of upregulated genes, the bottom 50 downregulated probe sets were selected to represent possibly downregulated genes. Only cytokine genes with a calculated fold value ≤ 0.5 and a one-sided FDR-adjusted P value less than 0.1 for at least one experimental condition were considered downregulated genes. After excluding noncytokine genes from 39 candidates, 18 unique cytokine genes remained identified as downregulated.
Affymetrix Cytokine-Receptor Gene Expression.
Upregulated cytokine genes were identified using Affymetrix gene chip analysis, and their 37 corresponding receptor genes were identified using the GO and GeneCards database. Differentially expressed receptor genes, both upregulated and downregulated, were then selected by using the same methods as for identification of cytokine genes described above. Six unique cytokine-receptor genes were identified as differentially expressed (DE) in the six experimental comparisons evaluated.
Cytokine Secretion.
For in vitro experiments, cytokines secreted by cultured TMEs and SCEs were measured and FDR-adjusted Wilcoxon two-sample one-sided P values for all treatment-control comparisons were calculated for each cytokine. Box plots were constructed for comparison between laser-treated and untreated controls. For ex vivo experiments, the difference in the mean log2 intensity measurements between the treated media samples and the untreated media samples was calculated for each specimen and for each cytokine. Depending on the specific cytokine, between 5 and 22 specimens were available for analysis. To test for equality between laser-treated and control observations, the resultant specimen-specific paired means were analyzed using the Wilcoxon signed rank test for paired data, and the FDR-adjusted one-sided Wilcoxon P value was obtained for each cytokine. A cytokine was considered upregulated when its protein expression exhibited a fold change ≥ 1.5 along with a one-sided P value less than 0.1. Only cytokines that were identified as upregulated by gene expression analysis underwent testing for protein expression using specific antibodies. Thus, because TNF-α was not upregulated by this criterion, it was not tested for protein expression using specific antibodies.
Results
Cytokine Gene Expression
Upregulated Cytokine Genes.
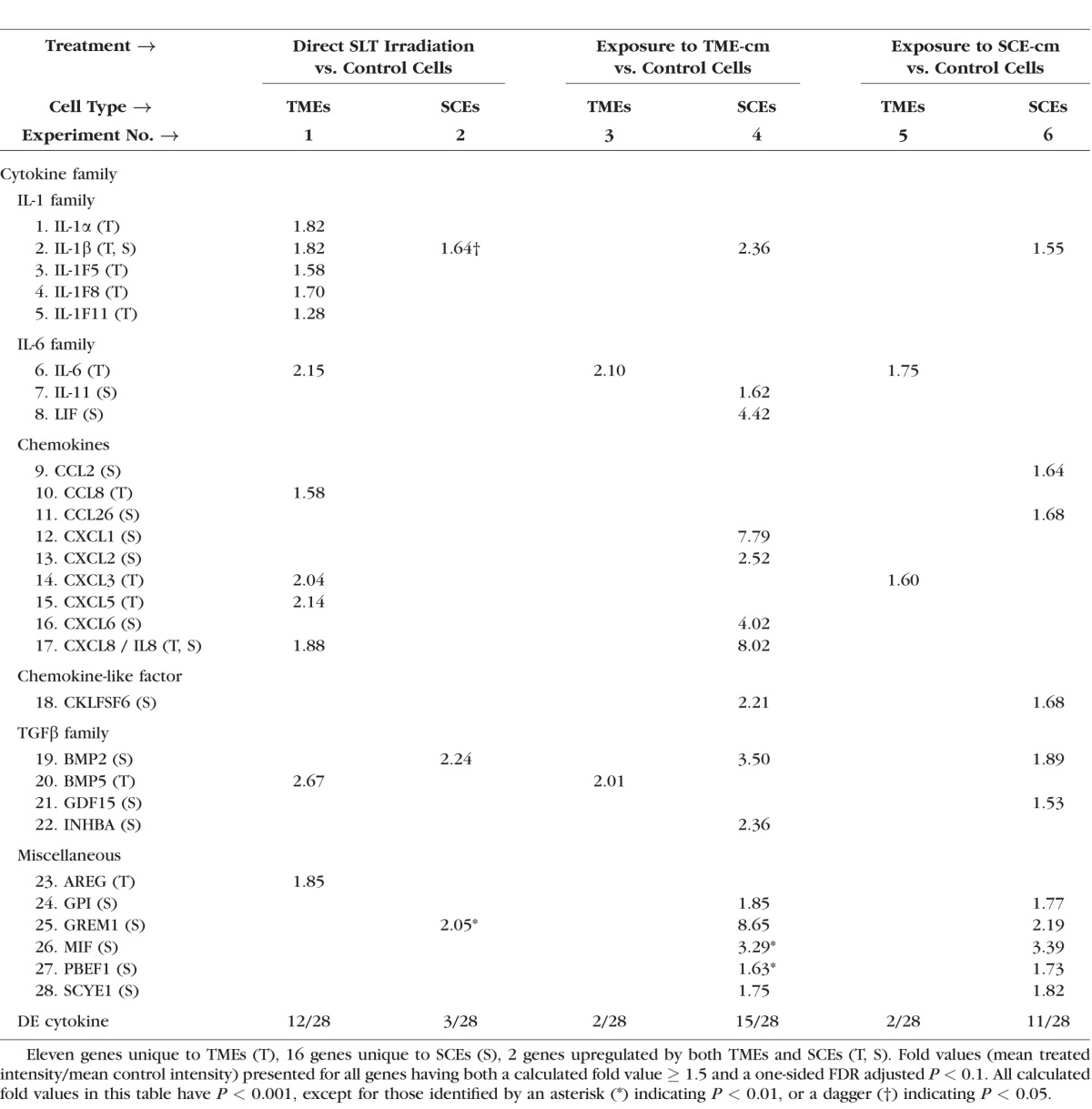
Table 1 shows a consistent asymmetry between TMEs and SCEs in their responses to each of three treatments. In each treatment, one cell type responded much more profoundly than did the other. Among the 28 upregulated cytokine genes, laser irradiation induced upregulation of 12 cytokine genes in TMEs (experiment 1) compared with only 3 genes in SCEs (experiment 2). Exposure to media conditioned SLT-irradiated TMEs (TME-cm) induced upregulation of only 2 cytokine genes in TMEs (autocrine stimulation, experiment 3) compared with 15 cytokine genes in SCEs (paracrine stimulation, experiment 4), the most extensive response detected. Exposure to media conditioned by SLT-irradiated SCEs (SCE-cm) induced upregulation of only 2 cytokine genes in TMEs (paracrine stimulation, experiment 5) compared with 11 cytokine genes in SCEs (autocrine stimulation, experiment 6).
Table 1.
Treated Versus Control Fold Values Calculated for Upregulated Cytokine Genes in Six Experiments Using Affymetrix Gene Expression Data
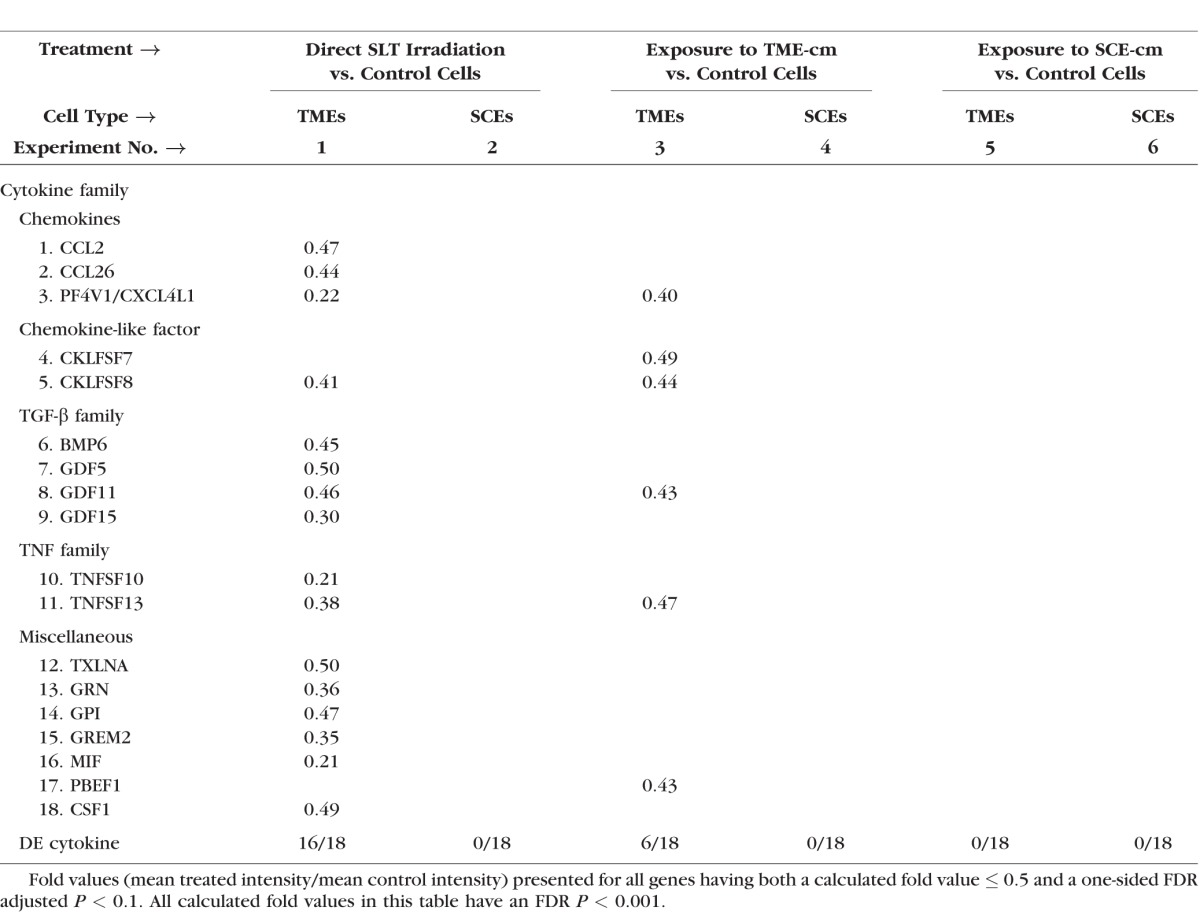
Downregulated Cytokine Genes.
Table 2 shows 18 downregulated cytokine genes in the TMEs with 16 cytokine genes detected after SLT irradiation (experiment 1) and 6 genes detected after TME-cm exposure (experiment 3). In contrast to the TMEs, the SCEs underwent the downregulation of no cytokine genes. This extensive and exclusive downregulation of cytokine genes only in TMEs emphasizes the fact that each cell type yielded distinct responses and should be verified.
Table 2.
Treated Versus Control Fold Values Calculated for Downregulated Cytokine Genes in Six Experiments Using Affymetrix Gene Expression Data
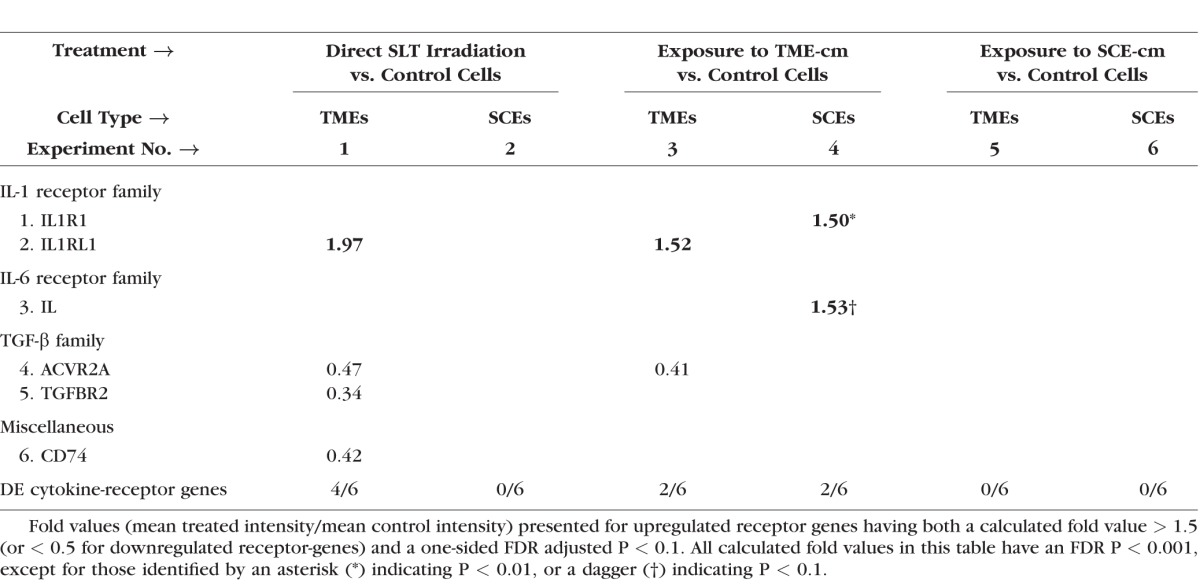
Cytokine-Receptor Gene Expression
Table 3 shows that very asymmetric responses were also detected in the cytokine-receptor gene-expression responses detected in TMEs versus SCEs for each of three treatments. Compared with controls, the SLT irradiation induced downregulation of three cytokine-receptor genes and upregulation of one in TMEs (experiment 1), whereas no receptors were detected DE in SCEs (experiment 2). Exposure to TME-cm induced upregulation of IL1RL1 and downregulation of ACVR2A in TMEs (experiment 3) compared with the unique upregulation of two receptor genes in SCEs (experiment 4). Exposure to SCE-cm induced no DE receptors in both TMEs and SCEs (experiments 5 and 6). The listed cytokine families: IL-1, IL-6, TGF-β, and a “miscellaneous family,” have at least one cytokine-receptor gene detected as DE, but no receptor genes were DE for any chemokines or chemokine-like families. Because no chemokine receptors were found DE in either TMEs or SCEs, we postulate that any chemokines released by these cells might bind instead onto mononuclear cells. Such mononuclear cells are recruited to the CAOP in increased numbers post-SLT.10,17,18
Table 3.
Treated Versus Control Fold Values Calculated for Cytokine-Receptor Genes in Six Experiments Using Affymetrix Gene Expression Data
Cytokine Secretion In Vitro
Figure 1 shows protein expression levels (pg/mL) for IL-1α, IL-1β, and IL-8 (CXCL8) secreted by a standard sample of approximately 3.9 million TME or SCE cells compared with untreated controls using ELISA. On a per-cell basis, untreated TMEs uniformly secreted a higher cytokine level than did untreated SCEs (experiment 1 vs. 3). In addition, after treatment with a standard number of SLT shots, both TMEs and SCEs secreted a larger cytokine concentration than did their respective untreated controls. However, for each of the three cytokines shown in Figure 1, lasered SCEs (SCE L) secreted higher cytokine levels than did their controls (SCE C), but the SCEs did so at much lower levels even when compared with untreated TMEs (experiment 4 vs. 1).
Figure 1.
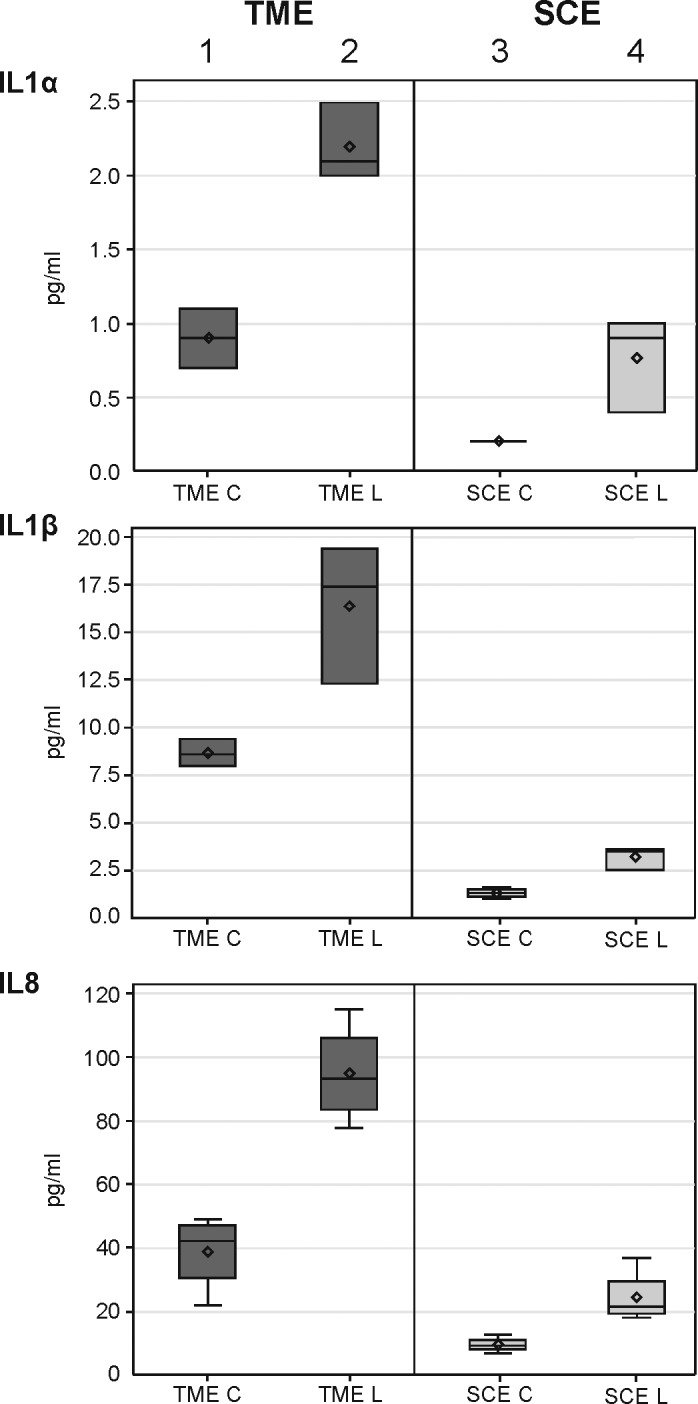
Box plots showing the expression for three cytokines in cultured TMEs and SCEs treated with SLT-irradiation (L) and compared with untreated controls (C). The expression level was determined for IL-1α, IL-1β, and IL-8 (CXCL8) using ELISA with one-sided Wilcoxon P value for each treatment-control comparison measured less than 0.05.
Cytokine Secretion Ex Vivo
Figure 2 presents fold change results for cytokines secreted by SLT-irradiated CAOP tissues ex vivo compared with untreated controls. Among 28 cytokines evaluated, each cytokine expressing a fold change ≥ 1.5 along with a P value less than 0.1 qualified as upregulated. Figure 2 shows that there were at least eight cytokines that were upregulated, which belonged to four major families (IL-1, IL-6, chemokine, and TGF-β) among six families considered.
Figure 2.
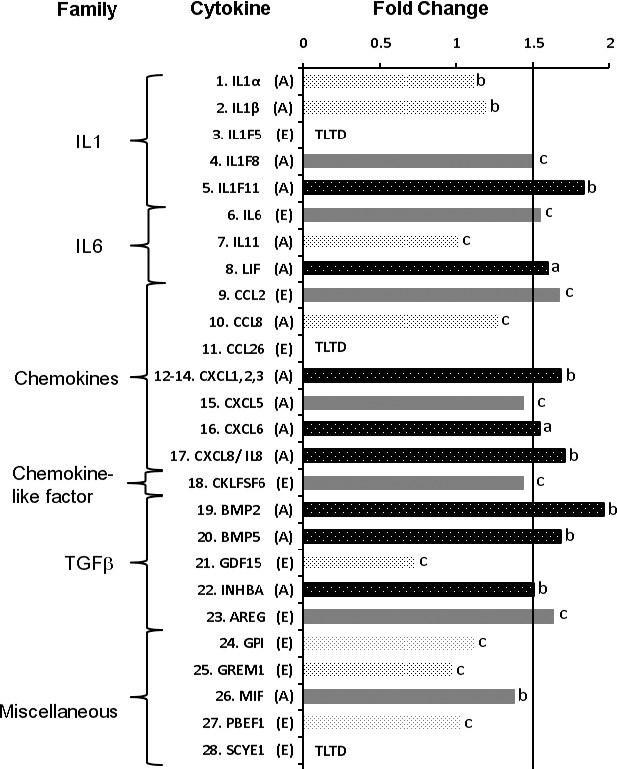
For in situ/ex vivo cytokine expression, the data consisted of intensity measurements taken from between 5 and 22 specimen pairs for each cytokine, depending on the particular assay. Using antibody array assay (A) and ELISA (E), the cytokine expression levels were assayed from an equal amount of supernatants of 48-hour cultured human CAOP tissues after SLT irradiation. Separate antibody array assays could not be carried out for CXCL1, 2, and 3 due to the GRO antibody reacting with CXCL1, CXCL2, and CXCL3. Fold values = mean treated intensity/mean control intensity. A cytokine having both a calculated fold value ≥ 1.5 and a one-sided FDR adjusted Wilcoxon P value less than 0.1 was considered upregulated. Cytokine-specific Wilcoxon FDR P values: (A) < 0.005; (B) < 0.05; and (C): ≥ 0.1. TLTD, too low to detect using ELISA.
Discussion
Selective laser trabeculoplasty irradiation is as an effective modality to lower the elevated IOP in patients with glaucoma,4,19 which “activates” irradiated TMEs and SCEs to upregulate a profile of eight cytokines in CAOP tissues. This cytokine profile appears to maintain aqueous outflow homeostasis post-SLT irradiation via two proposed processes: one involves cytokines maintaining communication between TMEs and SCEs; the other involves setting up a positive “feedback-loop” in the SCEs by cytokine stimuli from both a paracrine and an autocrine pathway. These two processes modulate SCE conductivity via several mechanisms, including intercellular junction disassembly, remodeling of the extracellular matrix (ECM) in juxtacanalicular connective tissues, and other effects.20–24
Paracrine Signaling Pathway
Trabecular meshworks were more responsive to the SLT irradiation than were SCEs, whereas the SCEs were more responsive to exposure to media conditioned by either lasered TMEs or SCEs (Table 1). Further inspection of the cytokine gene expression results for experiments 4 and 5, presented in Table 1, shows SCEs upregulated 15 cytokine genes after the paracrine exposure to TME-cm (experiment 4), but only 2 cytokine genes were upregulated by TMEs after the paracrine exposure to SCE-cm (experiment 5). This difference in response between the two cell types, along with the fact that 13 of these 15 genes were uniquely upregulated by SCEs (see items marked with symbol “S” in Table 1), provides strong support for the existence of the proposed unidirectional paracrine interaction as depicted in Figure 3A, that proceeds from the internally located TMEs toward the externally located SCEs, the same direction as aqueous flows across TMEs then SCEs under physiologic conditions.
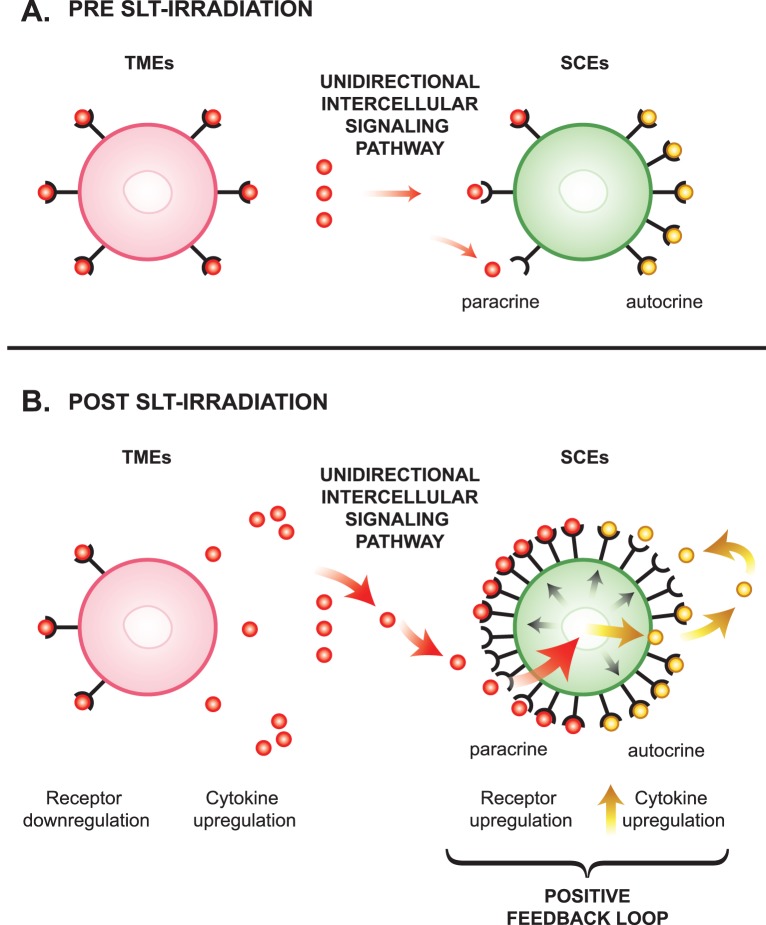
Figure 3.
Proposed unidirectional intercellular signaling pathway and proposed positive feedback loop. (A) Pre-SLT irradiation: cytokine receptor binding prior to laser treatment. (B) Post-SLT irradiation: cytokine receptor binding after laser treatment.
Among six cytokine receptors DE in Table 3, the cytokine receptor for TGFBR2 has been reported to be expressed in both TM tissues and in cultured TMEs.25 Table 3 shows that among 4 cytokine receptors DE by TMEs, three were downregulated in SLT-irradiated TMEs (i.e., becoming relatively less sensitive to further cytokine stimulation), whereas SLT-irradiated SCEs upregulated two receptors detected (i.e., becoming relatively more sensitive to further cytokine stimulation). These relationships support the existence of a predominantly unidirectional paracrine-signaling pathway under normal physiologic conditions that proceeds from TMEs toward SCEs (Fig. 3A).
Taken together, the cytokine expression results in Table 1, the receptor results presented in Table 3, and the cytokine secretion results shown in Figure 1 provide cohesive and compelling evidence that TMEs and SCEs are distinct cell types with unique cytokine-gene expression profiles and interactions that may function to modulate aqueous outflow.
Positive Feedback Loop
Figure 3B depicts a positive feedback loop whose existence is suggested by the findings in experiment 4 (Tables 1 and 3) and experiment 6 (Table 1), showing that SCEs are more responsive than TMEs to both paracrine and autocrine stimulation. Figure 3B depicts the notion that after SLT irradiation, upregulated cytokines derived from TMEs bind onto upregulated SCE receptors through a proposed unidirectional paracrine pathway. This binding initiates a positive loop that begins by activating the SCEs (large red arrow), which respond by upregulating more cytokines (yellow arrows) and cytokine-receptors (black arrows). This loop would dramatically increase the ability of the SCEs to bind and respond to cytokine stimuli derived from TMEs through a paracrine pathway or to cytokine stimuli derived from SCEs through an autocrine pathway as already mentioned.
We further propose this feedback loop may exist in vivo and provide a molecular mechanism to explain the relative chronic IOP lowering effect induced by the SLT irradiation in treated glaucoma patients.26 That is, the SLT irradiation may create an environment in which SCE cells become more responsive to cytokine signaling than before SLT irradiation, even after the TMEs return to produce lower levels of cytokines at baseline conditions.
Figure 1 shows that on a per-cell basis and after SLT irradiation, TMEs secreted approximately twice the concentration of three cytokines examined as were secreted by SCEs. In view of the fact that in vivo TMEs are approximately 10 times more abundant than SCEs,27–30 the TMEs should secrete approximately 20 times greater an amount of cytokines compared with SCEs. These estimates support the concept that TMEs “run the show” by secreting a robust cytokine load that on reaching and binding onto SCEs may function to modulate outflow via cellular and/or ECM effects as mentioned at the outset. Also, based on these estimates, we expected to find fewer cytokines secreted ex vivo by SCEs compared with TMEs. However, at least 50% of eight upregulated cytokines in Figure 2 were secreted uniquely by SCEs (being estimated in gene expression results, Table 1). We offer an explanation for these unexpected findings based on our gene-expression analyses, which demonstrated that SCEs are more responsive than TMEs to the cytokines in TME-cm or in SCE-cm through a paracrine or autocrine pathway (experiments 4 and 6, in Table 1). TME- and SCE-derived cytokines on binding onto upregulated receptors on the SCEs in a paracrine and autocrine manner induced the upregulation and secretion of many more SCE cytokines. Such interactions are basic to the functioning of the positive feedback loop, which over time, enhance the concentration of cytokines uniquely secreted by SCEs in the media ex vivo and thus became detectable (Fig. 2), supporting our proposed model of a positive feedback loop as depicted in Figure 3B. Taken together, we now propose the ex vivo experimental CAOP model represents a promising tool for future studies.
Cytokine Modulation of Aqueous Outflow
At least eight cytokines upregulated in SLT-irradiated CAOP tissues have been previously reported as regulating permeability of endothelial and epithelial barriers throughout the body.6,20,31–36 The cytokines, on binding onto receptors, interact with nuclear factor-κΒ,23 and/or Rho-associated protein kinase (ROCK)/RhoA, converging on Myosin Light-Chain, and induce Acto-myosin contraction37 with the subsequent disassembly of intercellular junctions making up the SCE fluid barriers.38,39
Alternatively, the mechanism whereby cytokines increase aqueous outflow might involve reducing SCE “stiffness” as to promote giant vacuole formation, which would modulate aqueous outflow.22 Wordinger et al.21 emphasized that the cytokines BMP-4 and Gremlin are “antagonists” in term of inducing ECM-production, which also have opposite effects on aqueous outflow in glaucomatous TME cells and tissues.24
However, our results showed that only SCEs upregulated GREM1 and BMP2 genes and only the BMP2 protein was upregulated in ex vivo SLT-irradiated CAOP tissues (Fig. 2). Coupled with the information presented by Wordinger et al.21 ex vivo using CAOP tissues and transformed cell lines, further study is required to establish whether GREM1 and BMP2 interact with each other during the regulation of aqueous outflow after SLT irradiation.
Pitfalls
Interleukin-1α was secreted by TMEs and SCEs in vitro (Fig. 1), whereas it was not DE at the gene expression by SCEs (Table 1). This discrepancy may be related to different sensitivities between Affymetrix and ELISA. Also, the fact that the IL1-α gene was not DE in SCEs using a “fold-change” criterion does not imply this gene was not expressed at any level; possibly, IL-1α is not upregulated at the mRNA level.
Conclusions
We have provided support for the concept that cytokines, on binding onto receptors in the CAOP, increase conductivity and promote fluid flow across cellular barriers.39,40 Our studies have enhanced our understanding of multiple and complex interactions occurring at the molecular and cellular levels, which are involved in aqueous outflow regulation and can be affected by the mechanism of action of SLT irradiation. Moreover, by regulating the rate of aqueous outflow through the CAOP, at least eight newly identified cytokines possess great therapeutic potential for the treatment of glaucoma.
Acknowledgments
We thank Mark A. Terry, MD, David Hwang, MD, Daniel Goodman, MD, Richard Alvarado, MD, and Jyotsom Ganatra, MD, for generously providing us with tissues for our experiments; Suling Wang, our medical illustrator, for providing Figure 3; and Michael Deiner PhD, Janet Chen, MD, and Judith Hellman, MD, for editorial assistance. We also thank the following patients for their contributions: Mary A. Anderson, Mr. and Mrs. Sanford R. Robertson, Earl Skeel, June M. Carros and Doris M. Raffetto, Diane S. Heiman, and Burton L. Wise.
Supported by major grants from the National Eye Institute of the National Institutes of Health (1R01EY021509), the Thomas J. Long Foundation, the McBean Family Foundation, the Joan Leidy Foundation, That Man May See, Inc., the Department of Ophthalmology at the University of California San Francisco, and Research to Prevent Blindness (JAA).
Disclosure: J.A. Alvarado, None; P. Chau, None; J. Wu, None; R. Juster, None; A.S. Shifera, None; M. Geske, None
References
- 1. Anderson R,, Parrish J. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983; 220: 524–527. [DOI] [PubMed] [Google Scholar]
- 2. Latina M,, Sibayan SA,, Shin DH,, Noecker RJ,, Marcellino G. Q-switched 532-nm Nd:YAG laser trabeculoplasty (selective laser trabeculoplasty): a multicenter pilot, clinical study. Ophthalmology. 1998; 105: 2082–2090. [DOI] [PubMed] [Google Scholar]
- 3. Latina M,, Park C. Selective targeting of trabecular meshwork cells: in vitro studies of pulsed and CW laser interactions. Exp Eye Res. 1995; 60: 359–372. [DOI] [PubMed] [Google Scholar]
- 4. Alvarado J,, Shifera A. Progress towards understanding the functioning of the trabecular meshwork based on lessons from studies of laser trabeculoplasty. Br J Ophthalmol. 2010; 94: 1417–1418. [DOI] [PubMed] [Google Scholar]
- 5. Alvarado J,, Yeh RF,, Franse-Carman L,, Marcellino G,, Brownstein MJ. Interactions between endothelia of the trabecular meshwork and of Schlemm's canal: a new insight into the regulation of aqueous outflow in the eye. Trans Am Ophthalmol Soc. 2005; 103: 155–170. [PMC free article] [PubMed] [Google Scholar]
- 6. Alvarado J,, Alvarado RG,, Yeh RF,, Franse-Carman L,, Marcellino GR,, Brownstein MJ. A new insight into the cellular regulation of aqueous outflow: how trabecular meshwork endothelial cells drive a mechanism that regulates the permeability of Schlemm's canal endothelial cells. Br J Ophthalmol. 2005; 89: 1500–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Izzotti A,, Longobardi M,, Cartiglia C,, Rathschuler F,, Saccà SC. Trabecular meshwork gene expression after selective laser trabeculoplasty. PLoS One. 2011; 6: e20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bradley JM,, Anderssohn AM,, Colvis CM,, et al. Mediation of laser trabeculoplasty-induced matrix metalloproteinase expression by IL-1beta and TNFalpha. Invest Ophthalmol Vis Sci. 2000; 41: 422–430. [PubMed] [Google Scholar]
- 9. Chua J,, Vania M,, Cheung CM,, et al. Expression profile of inflammatory cytokines in aqueous from glaucomatous eyes. Mol Vis. 2012; 18: 431–438. [PMC free article] [PubMed] [Google Scholar]
- 10. Shifera A,, Trivedi S,, Chau P,, Bonnemaison LH,, Iguchi R,, Alvarado JA. Constitutive secretion of chemokines by cultured human trabecular meshwork cells. Exp Eye Res. 2010; 91: 42–47. [DOI] [PubMed] [Google Scholar]
- 11. Polansky JR,, Weinreb RN,, Baxter JD,, Alvarado J. Human trabecular cells: I. Establishment in tissue culture and growth characteristics. Invest Ophthalmol Vis Sci. 1979; 18: 1043–1049. [PubMed] [Google Scholar]
- 12. Alvarado J,, Wood I,, Polansky J. Human trabecular cells. II. Growth pattern and ultrastructural characteristics. Invest Ophthalmol Vis Sci. 1982; 23: 464–478. [PubMed] [Google Scholar]
- 13. Alvarado J,, Betanzos A,, Franse-Carman L,, Chen J,, González-Mariscal L. Endothelia of Schlemm's canal and trabecular meshwork: distinct molecular functional, and anatomic features. Am J Physiol Cell Physiol. 2004; 286: C621–C634. [DOI] [PubMed] [Google Scholar]
- 14. Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004; 3: 1–25. [DOI] [PubMed] [Google Scholar]
- 15. Benjamini Y,, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B. 1995; 57: 289–300. [Google Scholar]
- 16. Smyth GK. Limma: linear models for microarray data. : Gentleman R,, Carey V,, Huber W,, Irizarry RA,, Dudoit S, Bioinformatics and Computational Biology Solutions Using R and Bioconductor. New York; Springer; 2005: 397–420. [Google Scholar]
- 17. Alvarado JA,, Katz LJ,, Trivedi S,, Shifera AS. Monocyte modulation of aqueous outflow and recruitment to the trabecular meshwork following selective laser trabeculoplasty. Arch Opthalmol. 2010; 128: 731–737. [DOI] [PubMed] [Google Scholar]
- 18. Alvarado J,, Murphy C. Outflow obstruction in pigmentary and primary open angle glaucoma. Arch Ophthalmol. 1992; 110: 1769–1778. [DOI] [PubMed] [Google Scholar]
- 19. Damji KF,, Bovell AM,, Hodge WG,, et al. Selective laser trabeculoplasty versus argon laser trabeculoplasty: results from a 1-year randomised clinical trial. Br J Ophthalmol. 2006; 90: 1490–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Capaldo CT,, Nusrat A. Cytokine regulation of tight junctions. Biochim Biophys Acta. 2009; 1788: 864–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wordinger RJ,, Fleenor DL,, Hellberg PE,, et al. Effects of TGF-beta2, BMP-4, and gremlin in the trabecular meshwork: implications for glaucoma. Invest Ophthalmol Vis Sci. 2007; 48: 1191–1200. [DOI] [PubMed] [Google Scholar]
- 22. Stamer WD,, Braakman ST,, Zhou EH,, et al. Biomechanics of Schlemm's canal endothelium and intraocular pressure reduction. Prog Retin Eye Res. 2015; 44: 86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang N,, Chintala SK,, Fini ME,, Schuman JS. Activation of a tissue-specific stress response in the aqueous outflow pathway of the eye defines the glaucoma disease phenotype. Nat Med. 2001; 7: 304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Acott TS,, Kelley MJ. Extracellular matrix in the trabecular meshwork. Exp Eye Res. 2008; 86: 543–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sethi A,, Mao W,, Wordinger RJ,, Clark AF. Transforming growth factor-beta induces extracellular matrix protein cross-linking lysyl oxidase (LOX) genes in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011; 52: 5240–5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shazly TA,, Smith J,, Latina MA. Long-term safety and efficacy of selective laser trabeculoplasty as primary therapy for the treatment of pseudoexfoliation glaucoma compared with primary open-angle glaucoma. Clin Ophthalmol. 2010; 5: 5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alvarado J,, Murphy C,, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984; 91: 564–579. [DOI] [PubMed] [Google Scholar]
- 28. Alvarado J,, Murphy C,, Polansky J,, Juster R. Age-related changes in trabecular meshwork cellularity. Invest Ophthalmol Vis Sci. 1981; 21: 714–727. [PubMed] [Google Scholar]
- 29. Grierson I,, Howes RC,, Wang Q. Age-related changes in the canal of Schlemm. Exp Eye Res. 1984; 39: 505–512. [DOI] [PubMed] [Google Scholar]
- 30. Grierson I,, Howes RC. Age-related depletion of the cell population in the human trabecular meshwork. Eye (Lond). 1987; 1: 204–210. [DOI] [PubMed] [Google Scholar]
- 31. Choi YS,, Choi HJ,, Min JK,, et al. Interleukin-33 induces angiogenesis and vascular permeability through ST2/TRAF6-mediated endothelial nitric oxide production. Blood. 2009; 114: 3117–3126. [DOI] [PubMed] [Google Scholar]
- 32. Pan W,, Stone KP,, Hsuchou H,, Manda VK,, Zhang Y,, Kastin AJ. Cytokine signaling modulates blood-brain barrier function. Curr Pharm Des. 2011; 17: 3729–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. DiStasi MR,, Ley K. Opening the flood-gates: how neutrophil-endothelial interactions regulate permeability. Trends Immunol. 2009; 30: 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gavard J,, Hou X,, Qu Y,, et al. A role for a CXCR2/phosphatidylinositol 3-kinase gamma signaling axis in acute and chronic vascular permeability. Mol Cell Biol. 2009; 29: 2469–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Helbing T,, Herold EM,, Hornstein A,, et al. Inhibition of BMP activity protects epithelial barrier function in lung injury. J Pathol. 2013; 231: 105–116. [DOI] [PubMed] [Google Scholar]
- 36. Petreaca ML,, Yao M,, Liu Y,, Defea K,, Martins-Green M. Transactivation of vascular endothelial growth factor receptor-2 by interleukin-8 (IL-8/CXCL8) is required for IL-8/CXCL8-induced endothelial permeability. Mol Biol Cell. 2007; 18: 5014–5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rao PV,, Deng P,, Sasaki Y,, Epstein DL. Regulation of myosin light chain phosphorylation in the trabecular meshwork: role in aqueous humour outflow facility. Exp Eye Res. 2005; 80: 197–206. [DOI] [PubMed] [Google Scholar]
- 38. Alvarado JA,, Iguchi R,, Juster R,, Chen JA,, Shifera AS. From the bedside to the bench and back again: predicting and improving the outcomes of SLT glaucoma therapy. Trans Am Ophthalmol Soc. 2009; 107: 167–181. [PMC free article] [PubMed] [Google Scholar]
- 39. Alvarado J,, Iguchi R,, Martinez J,, Trivedi S,, Shifera AS. Similar effects of selective laser trabeculoplasty and prostaglandin analogs on the permeability of cultured Schlemm canal cells. Am J Ophthalmol. 2010; 150: 254–264. [DOI] [PubMed] [Google Scholar]
- 40. Alvarado J,, Iguchi R,, Juster R,, Chen JA,, Shifera AS. From the bedside to the bench and back again: predicting and improving the outcomes of SLT glaucoma therapy. Trans Am Ophthalmol Soc. 2009; 107: 167–181. [PMC free article] [PubMed] [Google Scholar]