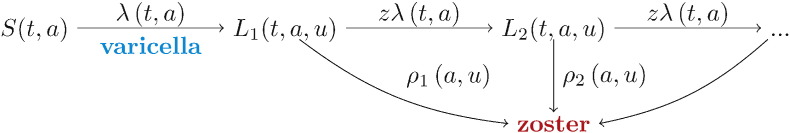Abstract
Introduction
Varicella zoster virus (VZV) is the etiological agent of varicella and herpes zoster (HZ). It has been hypothesised that immune boosting of latently infected persons by contact with varicella reduces the probability of HZ. If true, universal varicella vaccination may increase HZ incidence due to reduced VZV circulation. To inform decision-making, we conduct cost-effectiveness analyses of varicella vaccination, including effects on HZ.
Methods
Effects of varicella vaccination are simulated with a dynamic transmission model, parameterised with Dutch VZV seroprevalence and HZ incidence data, and linked to an economic model. We consider vaccination scenarios that differ by whether or not they include immune boosting, and reactivation of vaccine virus.
Results
Varicella incidence decreases after introduction of vaccination, while HZ incidence may increase or decrease depending on whether or not immune boosting is present. Without immune boosting, vaccination is expected to be cost-effective or even cost-saving. With immune boosting, vaccination at 95% coverage is not expected to be cost-effective, and may even cause net health losses.
Conclusions
Cost-effectiveness of varicella vaccination depends strongly on the impact on HZ and the economic time horizon. Our findings reveal ethical dilemmas as varicella vaccination may result in unequal distribution of health effects between generations.
Keywords: Varicella zoster virus, Vaccination, Transmission model, Cost-effectiveness
Highlights
-
•
Cost-effectiveness of varicella vaccination depends strongly on the impact on herpes zoster and the economic time horizon.
-
•
Varicella vaccination may result in profound trans-generational differences in distribution of health benefits and losses.
-
•
Ethical dilemmas could arise, as unvaccinated groups may be exposed to a substantially increased health hazard.
We study the impact of universal childhood varicella vaccination by dynamic transmission modelling and cost-effectiveness analyses. Scenarios that are considered differ by whether or not immune boosting is included, and whether or not reactivation of vaccine virus is possible. Health effects of varicella vaccination in scenarios with immune boosting are unevenly distributed: cohorts born just before introduction of vaccination and persons who refuse vaccination may pay the price for health gains in vaccinated cohorts by an increased lifetime risk of herpes zoster. These results uncover an ethical dilemma, as varicella vaccination could result in significant trans-generational inequalities of health effects.
1. Introduction
Universal vaccination against acute communicable diseases such as smallpox, poliomyelitis, diphtheria, and measles have been very successful by reducing circulation of the pathogens and associated burden of disease (Ehreth, 2003). A live attenuated vaccine against varicella was developed in the early 1970s, and licensed for universal use in healthy children in the late 1980s and early 1990s. Since then, varicella vaccination has been introduced in an increasing number of countries (Bonanni et al., 2009, European Centre for Disease Prevention and Control (ECDC), 2014). Over the years, the varicella vaccine has proved effective in preventing varicella zoster virus (VZV) infection and varicella disease in clinical trials and after introduction in national immunisation programmes (NIP) (Varicella and Herpes Zoster Vaccines, 2014). Consequently, recommendations for varicella vaccination have been issued by the World Health Organization, the Centers for Disease Control and Prevention, and the European Centre for Disease Prevention and Control (Varicella and Herpes Zoster Vaccines, 2014, Marin et al., 2007, European Centre for Disease Prevention and Control (ECDC), 2015).
Even though the varicella vaccine is safe and effective, not all developed countries have included vaccination in their NIPs (Bonanni et al., 2009). This is mainly due to the low perceived severity of varicella compared to other vaccine-preventable diseases, and uncertainties about the effect of varicella vaccination on the epidemiology of diseases caused by VZV. VZV is the etiological agent of both varicella after primary infection, and of herpes zoster (HZ) after reactivation of the virus. Hope-Simpson hypothesised that HZ incidence increases when circulation of VZV in the population decreases (Hope-Simpson, 1965). This so-called exogenous boosting hypothesis is based on the supposition that lack of exogenous immune boosting in latently infected persons might increase their risk of HZ. The hypothesis is shored by several findings, e.g., the lower incidence of HZ in adults with children than in adults without children (Brisson et al., 2002), and the negative association between HZ incidence and increasing exposure to varicella, social contacts with children or occupational contacts with ill children (Thomas et al., 2002). There are, however, also studies that do not support this hypothesis and the issue is therefore not definitively settled (Ogunjimi et al., 2013).
Cost-effectiveness of varicella vaccination might be affected by several interacting factors. First, reducing VZV circulation by varicella vaccination might result in an increase in HZ incidence (Schuette and Hethcote, 1999, Brisson et al., 2000, Gidding et al., 2005, Karhunen et al., 2010) and an age-shift for HZ to younger ages, resulting in productivity loss. HZ in elderly is more severe than varicella in children, with patients often suffering from severe, long-lasting neurological pain (Oxman, 2000). Second, varicella vaccination will shift the average age at primary infection in unvaccinated individuals to higher ages, as is well-known from epidemiological theory and observations (Anderson and May, 1991). Since varicella is more severe in older than younger persons (Heininger and Seward, 2006), and infection during pregnancy can result in congenital varicella syndrome (Enders and Miller, 2000), these effects also need to be factored in. Third, with populations ageing in many developing countries, a (transient) increase in HZ cases is expected, which may impact on cost-effectiveness analyses. Fourth, the varicella vaccine contains a live attenuated virus, which itself can cause reactivation. However, there is limited quantitative evidence on the frequency of HZ among varicella vaccinees, especially on the long term (Heininger and Seward, 2006). Most cost-effectiveness analyses did not include such effects of varicella vaccination on HZ (Rozenbaum et al., 2008) and may therefore give too optimistic results.
With the aim to inform decision-making regarding introducing varicella vaccination, we provide a comprehensive cost-effectiveness analysis that includes the above factors. The Dutch situation is used as an example because of the good quality data reflecting the pre-vaccination situation in developed countries with a temperate climate in which varicella is in general a childhood disease. Because of the expected age-shift for both varicella and HZ, we pay special attention to generational differences by studying the incidence of both syndromes by birth cohort.
2. Methods
2.1. Data Overview
The analyses are primarily based on two large datasets. First, information on infection status is contained in a population-based serological study of 6251 samples carried out in the Netherlands in 2006–2007 (van Lier et al., 2013). Second, information on age-specific HZ incidence rates is retrieved from 7026 HZ cases reported to general practitioners in 2002–2011 (Stirbu-Wagner et al., 2011). In addition, we have made use of national demographic data of Statistics Netherlands and information on Dutch contact patterns (Mossong et al., 2008). Details are given in the Supplement.
2.2. Model Structure, Statistical Analysis, and Vaccination Scenarios
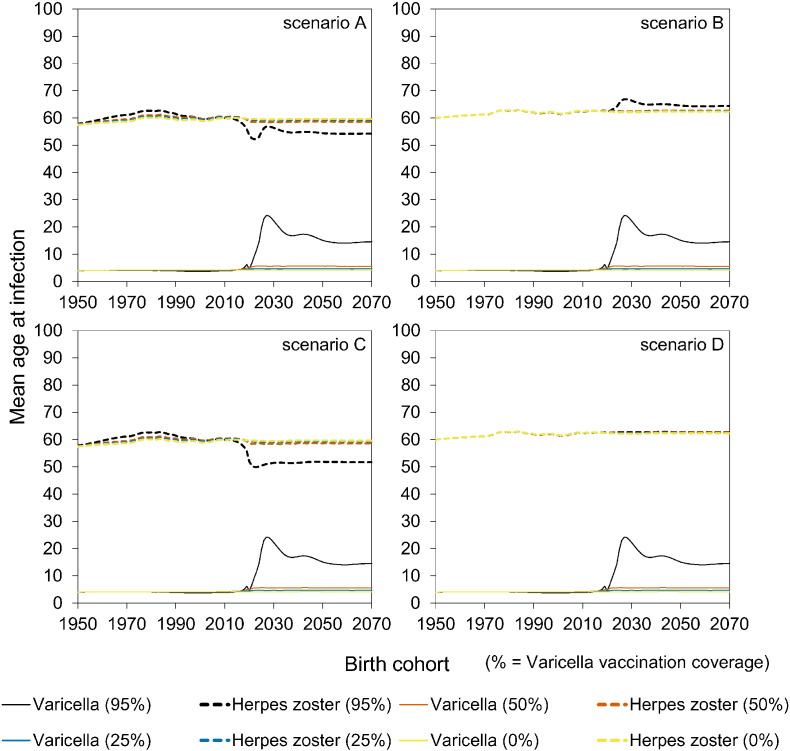
We investigate the effectiveness of universal childhood varicella vaccination using an age-structured transmission model (Guzzetta et al., 2013). First, we use data on varicella and HZ incidence to estimate all relevant transmission parameters in the pre-vaccination era. In a second step, we use the transmission model armed with quantitative parameter estimates to anticipate the impact of varicella vaccination on the age-specific incidences of varicella and HZ. We consider a two-dose varicella vaccination programme with a first dose at 12 months and a second dose at 4 years of age, as this would nicely fit in the existing NIP (Supplement). We simulate a vaccination programme starting (arbitrarily) on January 1, 2020, and analyse four vaccination scenarios, labelled A–D (Table 1). The scenarios differ by whether or not they include immune boosting (scenarios A and C with effect of boosting; B and D without effect of boosting), and whether or not reactivation of vaccine virus is included (scenarios A–B no vaccine virus reactivation; C–D with reactivation). For each scenario, we consider four vaccination coverages: 0%, 25%, 50%, and 95%. Throughout, we assume a vaccine effectiveness of 90% after one dose of varicella vaccine and 95% after two doses, which is reasonable in view of the evidence summarised by WHO (Varicella and Herpes Zoster Vaccines, 2014). We assume that after one vaccine dose there is small probability of breakthrough varicella (10% per infectious contact) that is less infectious (50%) than primary varicella, and we assume that there is no breakthrough varicella after two vaccine doses in persons who respond to vaccination. For simplicity, we do not consider potential waning of vaccine effectiveness. Demographic data of the Netherlands are applied to the modelled incidences of varicella and HZ to obtain the estimated number of cases by year, and by birth cohort. Details on the estimation procedures, model assumptions, and vaccination scenarios are given in the Supplement.
Table 1.
Overview of the four main vaccination scenarios implemented in the dynamic transmission model based on different assumptions about the effects of immune boosting on herpes zoster and vaccine VZV reactivation, and with various vaccination coverages.
| Vaccination scenariosa |
||||
|---|---|---|---|---|
| Assumptions | A | B | C | D |
| Boostingb | Yes | No | Yes | No |
| Vaccine VZV reactivationc | No | No | Yes | Yes |
| Vaccination coverage (%)d | 0;25;50;95 | 0;25;50;95 | 0;25;50;95 | 0;25;50;95 |
-
-Two-dose varicella vaccination programme (first dose: 12 months, second dose: 4 years of age), starting on January 1, 2020;
-
-Vaccine effectiveness of 90% after one dose, 95% after two doses;
-
-Probability breakthrough varicella after one dose: 10% per infectious contact (relative infectiousness after one dose 50%), no breakthrough varicella after two doses.
Yes = exogenous immune boosting has an effect on the probability of VZV reactivation, No = no effects of immune boosting.
Yes = vaccine VZV is able to reactivate with the same rate as wild type VZV, No = no reactivation of vaccine VZV.
0%: baseline without varicella vaccination, 25%: conservative coverage because of expected limited acceptance of varicella vaccination due to the perceived low severity of varicella, 50%: intermediate coverage, 95%: highest coverage based on regular Dutch vaccination coverage data.
2.3. Cost-Effectiveness
We use the output of the transmission modelling with vaccination as input for the cost-effectiveness analyses. All assumptions and parameters regarding treatment costs, vaccination costs, production losses, and QALY (quality-adjusted life-year) losses are described in Data Supplement 3. Because age-shifts play an important role in the analyses, and clinical severity of varicella differs by age, we distinguish health care use and QALY loss of varicella in patients aged < 15 versus ≥ 15 years, following Van Hoek et al. (van Hoek et al., 2012). Similarly, QALY loss of HZ is also age-dependent (van Hoek et al., 2012).
To determine cost-effectiveness of varicella vaccination, taking into account both varicella and HZ, the incremental cost-effectiveness ratio (ICER) is calculated, i.e., the difference in cost between a vaccination programme and no vaccination, divided by the difference in QALYs between a vaccination programme and no vaccination. In accordance with Dutch guidelines for pharmacoeconomic research, costs (4%) and QALYs (1.5%) are discounted from 2020 onwards (Hakkaart-van Roijen et al., 2011). We take a societal costs perspective that includes productivity loss. Costs are expressed in euros (€), at the 2012 price level.
First, the summation from 2020 onwards of the discounted net costs (= costs minus savings), are calculated separately for each year. The discounted net QALYs (= QALYs gained minus QALYs lost) are calculated in the same way. Subsequently, the ICER is calculated as the discounted net costs divided by the discounted net QALYs cumulatively, to get the costs per QALY up to that specific year. The ICER is calculated only when the net QALYs is positive, i.e., when there is health gain. We use a variable time horizon, i.e., we calculate the ICER each year over a total period of 180 years, instead of choosing one single time point as cut-off. Vaccination is considered cost-effective below an ICER threshold of €20,000 per QALY, a limit often used in the Netherlands (Houweling et al., 2010).
2.4. Sensitivity Analyses
In the univariate sensitivity analyses we consider i) a vaccination scenario with two doses given around the age of 1 year, ii) a cost-effectiveness analysis that does not include HZ related costs and QALYs, iii) a cost-effectiveness analysis without discounting of costs and QALYs, iv) a cost-effectiveness analysis with both costs and QALYs discounted at the same rate (4%), v) a cost-effectiveness analysis with a 50% reduced vaccine price, and vi) analyses with alternative cost-effectiveness thresholds (€50,000–€200,000 per QALY).
3. Results
3.1. Effects of Vaccination
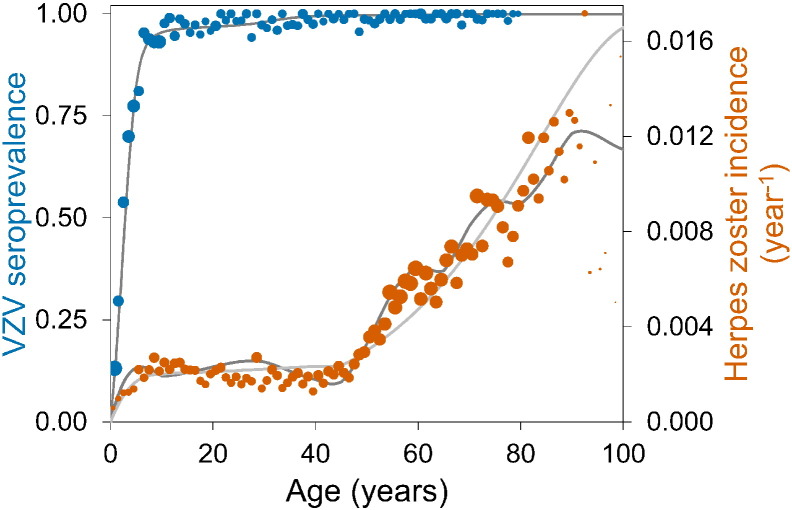
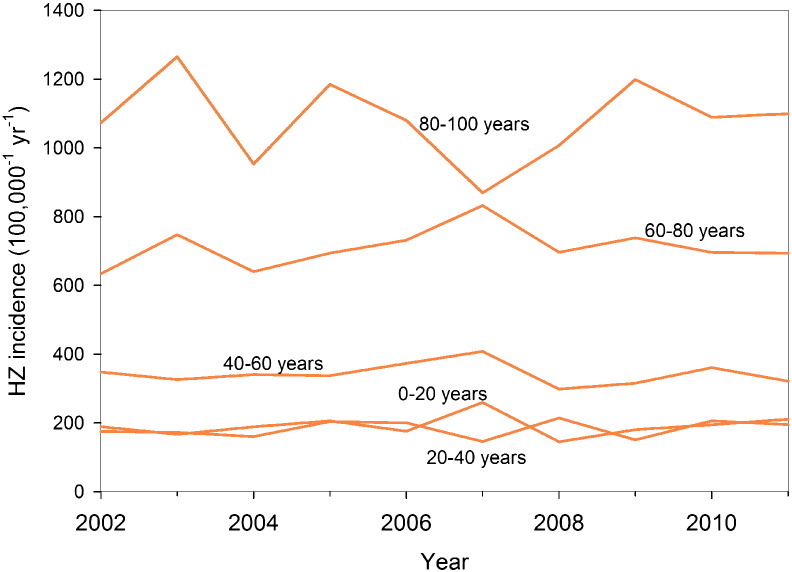
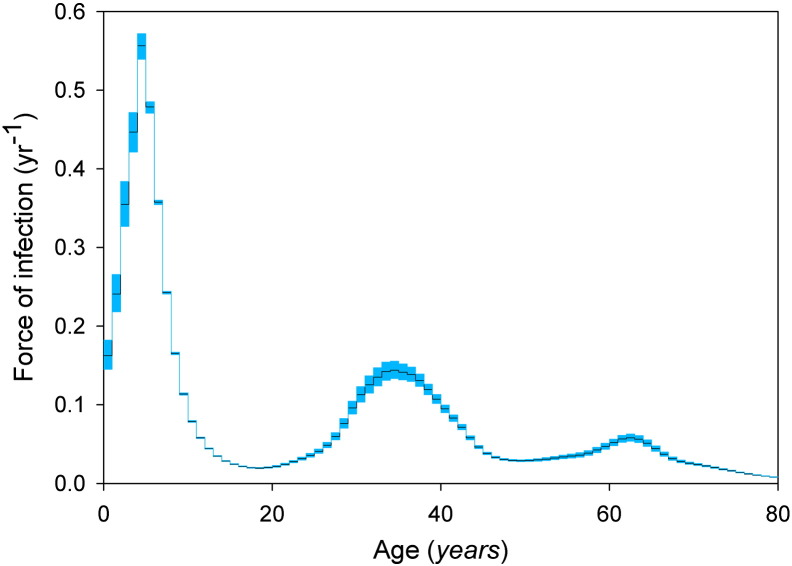
We fit the transmission model to the age-specific VZV serological and HZ incidence data, and obtain quantitative estimates for all epidemiological parameters in the absence of vaccination (Supplement). The results of the two best-fitting model scenarios (with and without immune boosting) are shown with the data in Fig. 1.
Fig. 1.
VZV seroprevalence in 2006–2007 (blue dots) and herpes zoster incidence in the period 2002–2011 (orange dots) in the Netherlands.
The sizes of the dots reflect sample sizes. Total numbers of sera and herpes zoster cases are 6251 and 7026, respectively. Thin grey lines indicate fits of transmission models with and without immune boosting. See the Supplement for details.
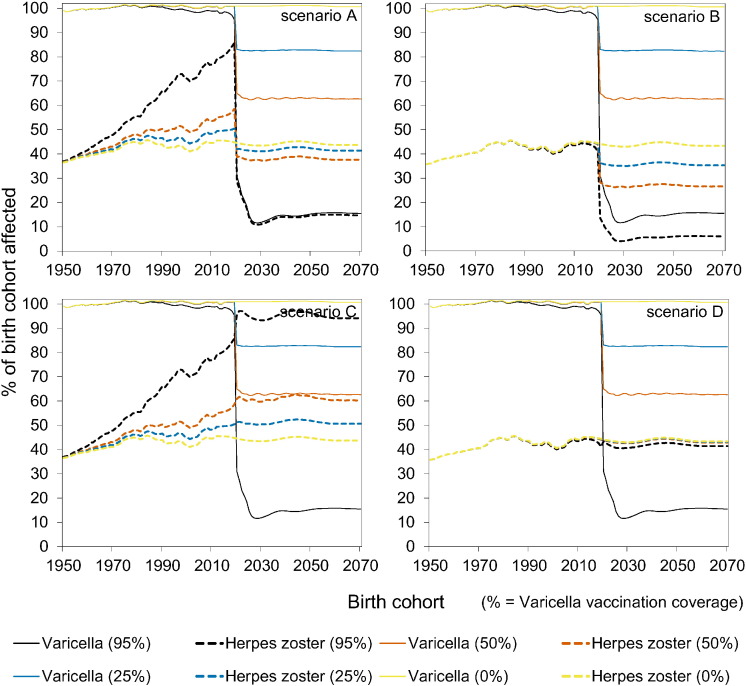
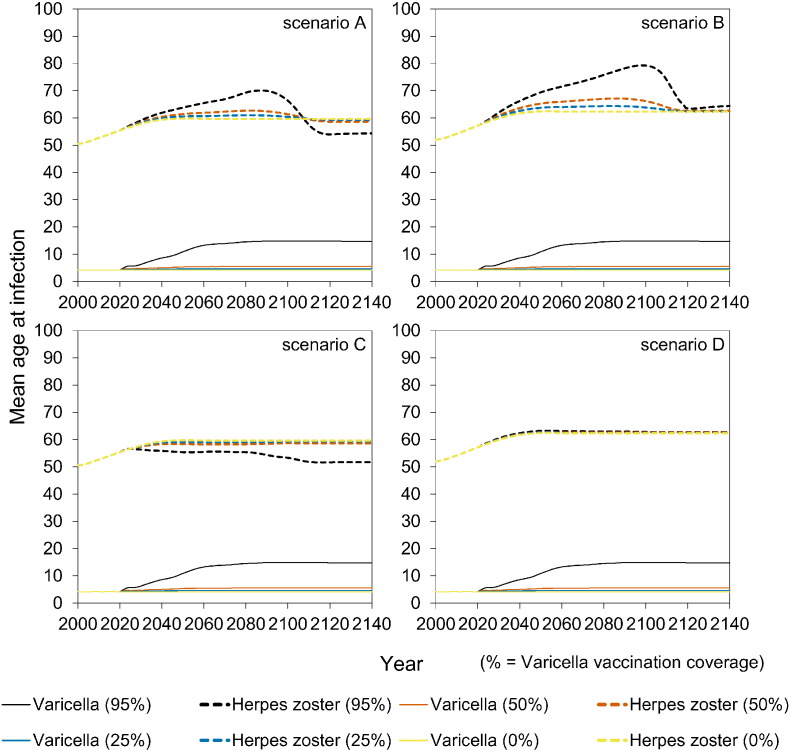
The impact of vaccination on the incidence of varicella and HZ is shown in Fig. 2 by birth cohort. The four scenarios (Table 1) yield identical results for varicella, and differ substantially for HZ. Specifically, in scenario B, both varicella and HZ incidence decrease with increasing vaccination coverage in vaccinated cohorts, and the incidence of varicella and HZ in unvaccinated cohorts is marginally affected. In contrast, in scenario A, the incidence of HZ increases in comparison with scenario B in the vaccinated cohorts, and also in unvaccinated cohorts. This is due to the reduced immune boosting in latently infected persons, which has profound impact especially when vaccination coverage is high. Namely, in scenario A, with high (95%) vaccination coverage the lifetime risk of HZ approaches 100% in unvaccinated persons who are born in the vaccination era, and reaches 80% in cohorts born just before introduction of vaccination (i.e., 2010–2020). In scenario C the situation is even more extreme, as here even all vaccinated cohorts have a high lifetime risk of HZ if vaccination coverage is high. In the scenarios that include boosting (scenarios A and C), the effect of vaccination on varicella and HZ is much smaller in case of low to intermediate vaccination coverage (25% or 50%).
Fig. 2.
Impact of varicella vaccination by birth cohort on the occurrence of varicella and herpes zoster.
The vaccination programme started in 2020. See Table 1 for an overview of scenarios.
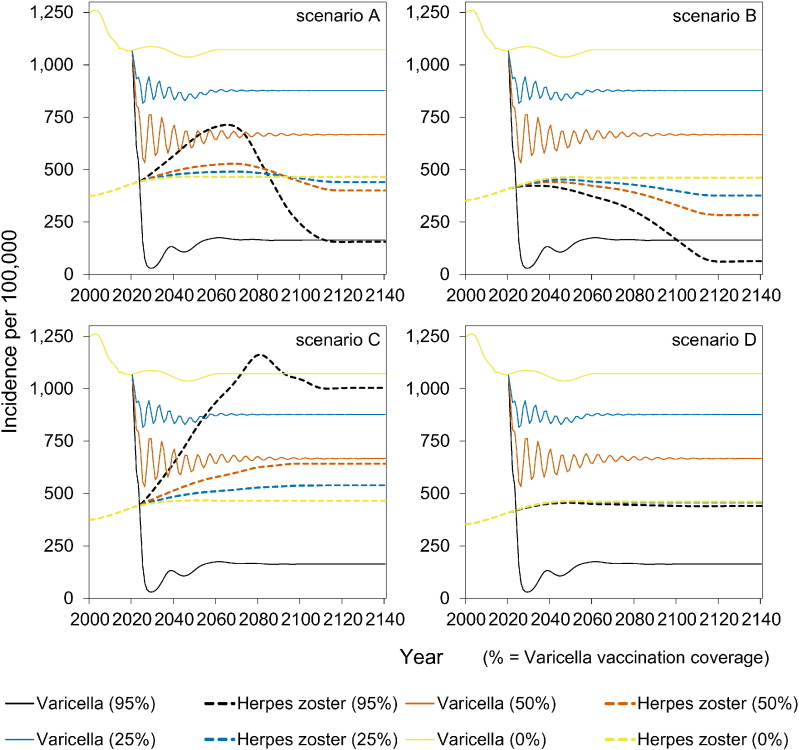
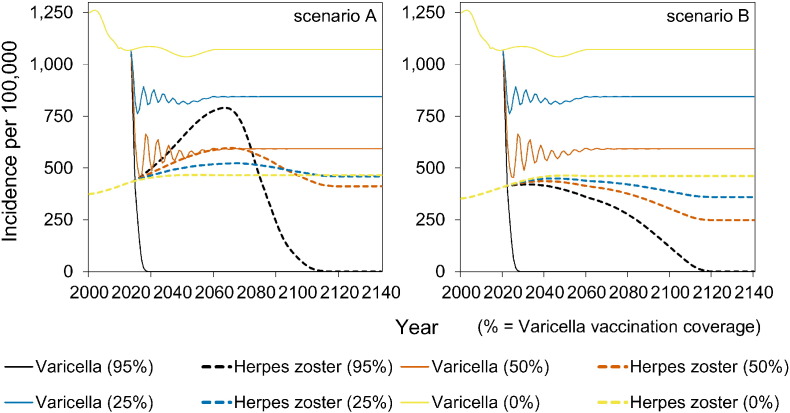
The full impact of vaccination on reducing the incidence of varicella is observed within 5–10 years into the vaccination programme (Fig. 3). In contrast, the potential increase in HZ incidence occurs on a much longer timescale of 20–60 years after start of vaccination (Fig. 3). In the long term, the incidence of HZ decreases to low values in scenarios A and B. In contrast, in scenario C the long-term incidence of HZ increases with increasing vaccination coverage. In scenario D, the HZ incidence is stable.
Fig. 3.
Impact of varicella vaccination over time on the occurrence of varicella and herpes zoster.
The vaccination programme started in 2020. See Table 1 for an overview of scenarios.
At high vaccination coverage (95%), there is a significant increase in the age at varicella from 4 to almost 15 years of age in all scenarios, and even to 24 years of age for some birth cohorts (Figures S7–S8). In scenarios A and B there is a strong transient increase over time in the mean age at HZ to persons in their seventies. In the long run, the mean age at HZ decreases by almost 10 years to persons in their fifties in scenarios with immune boosting (scenarios A and C) (Figure S7–S8).
3.2. Cost-Effectiveness of Vaccination
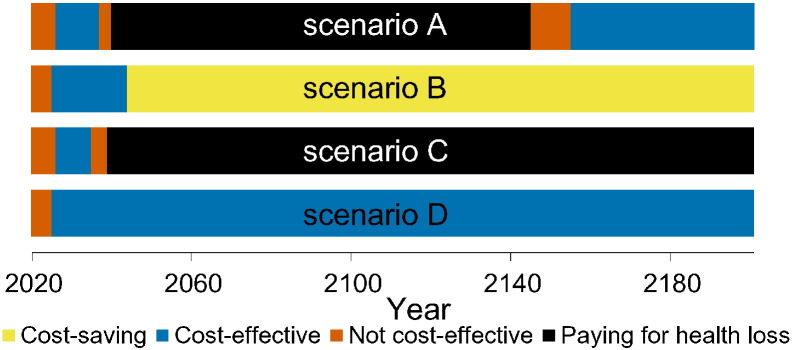
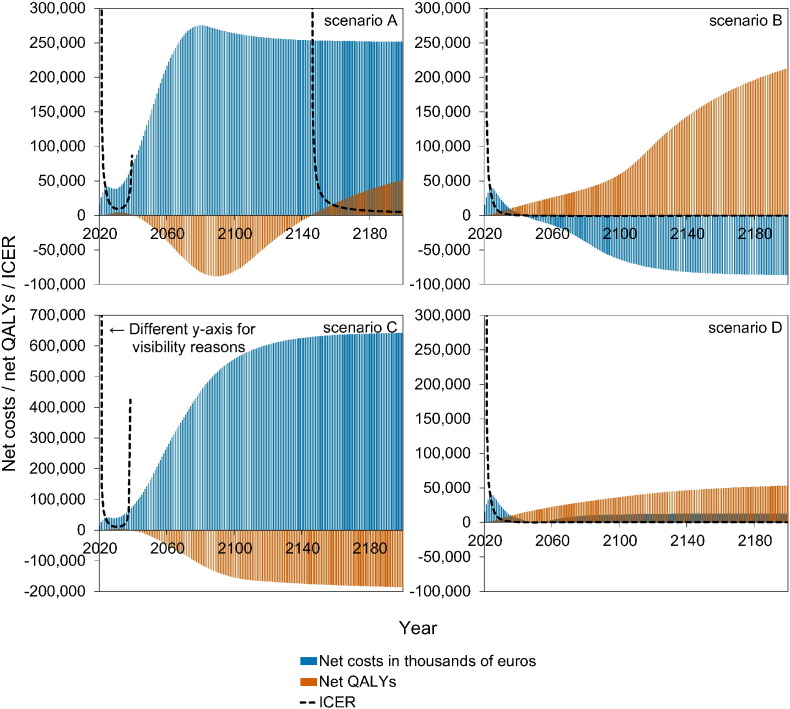
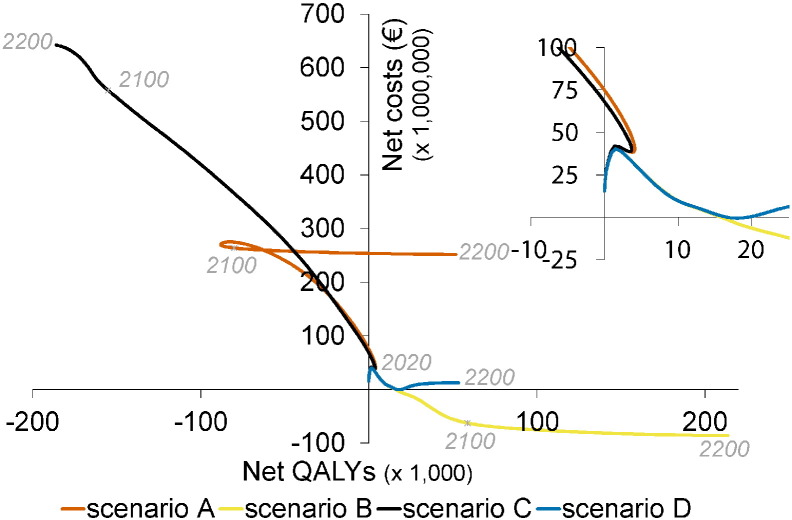
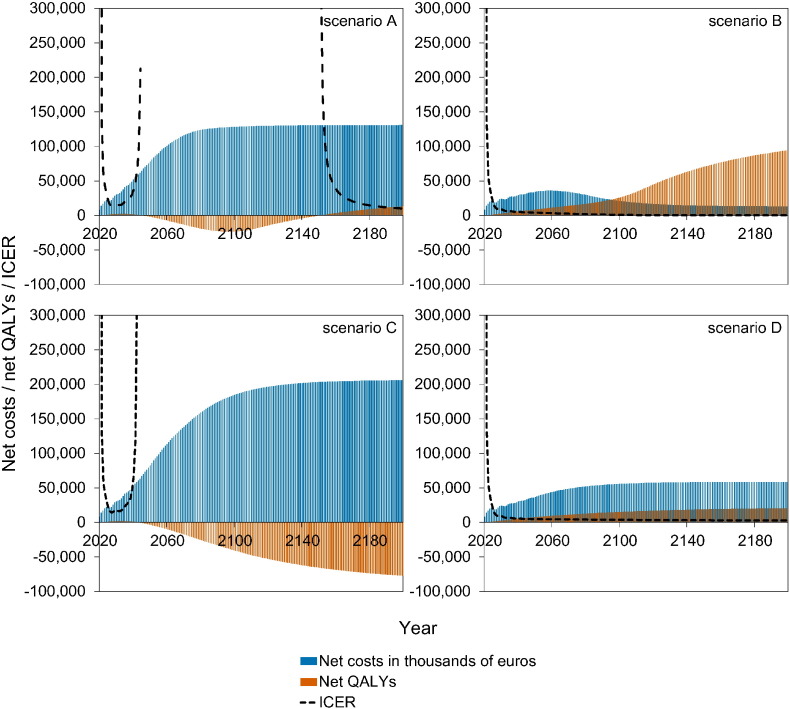
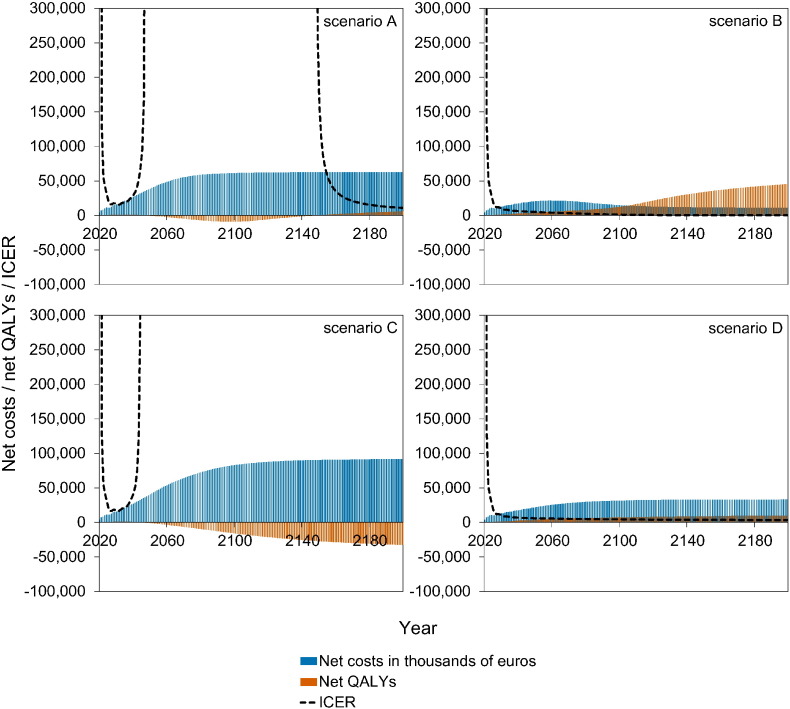
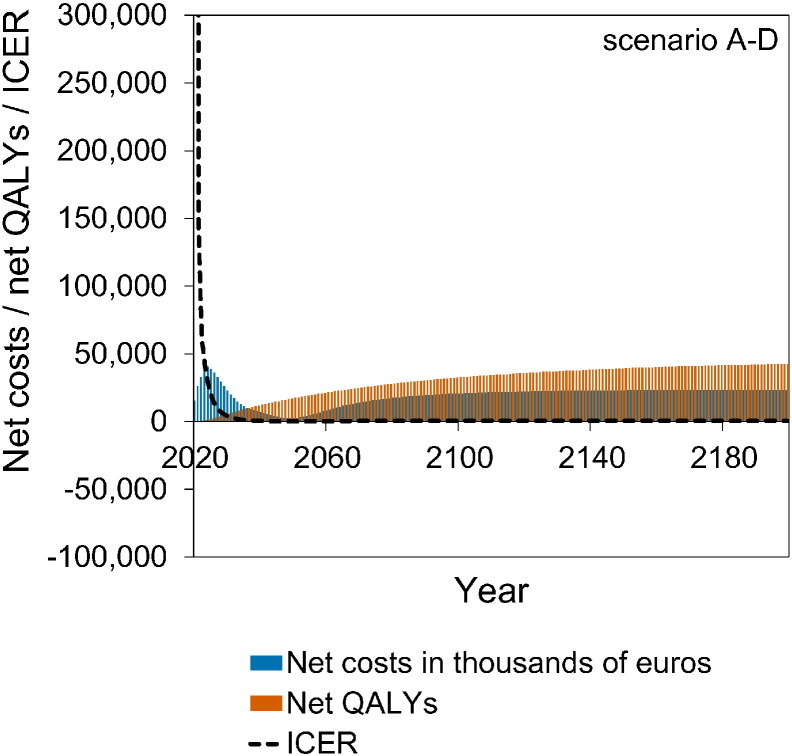
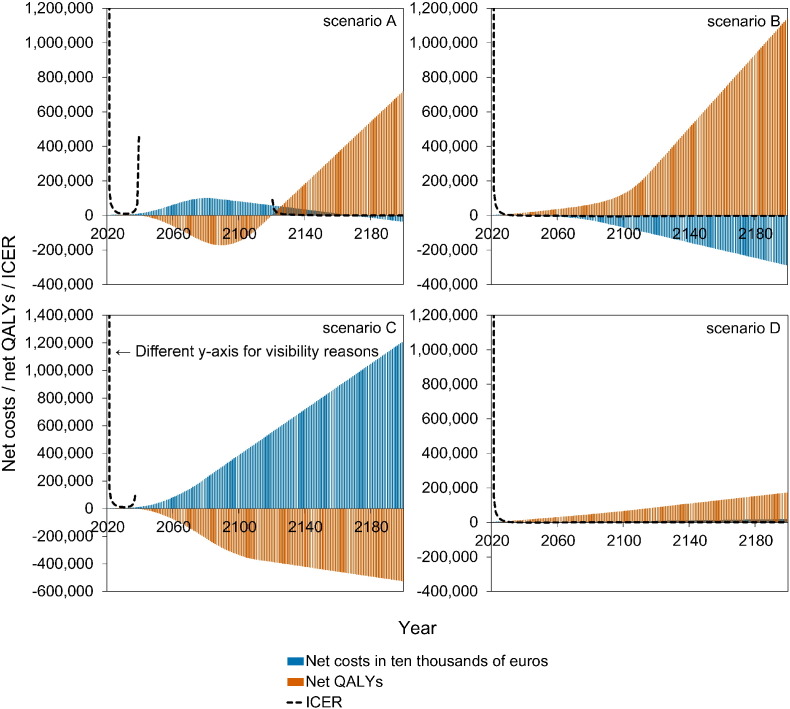
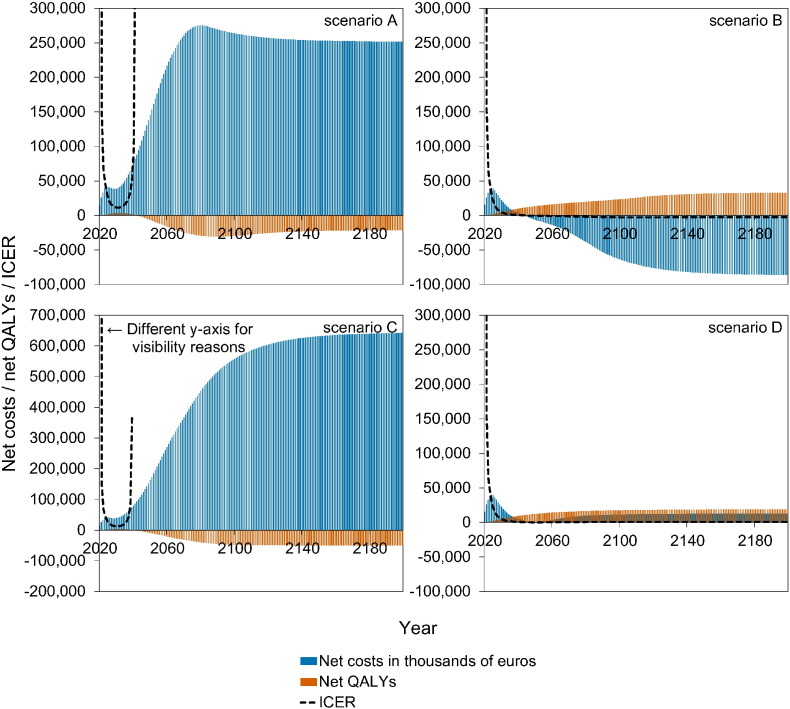
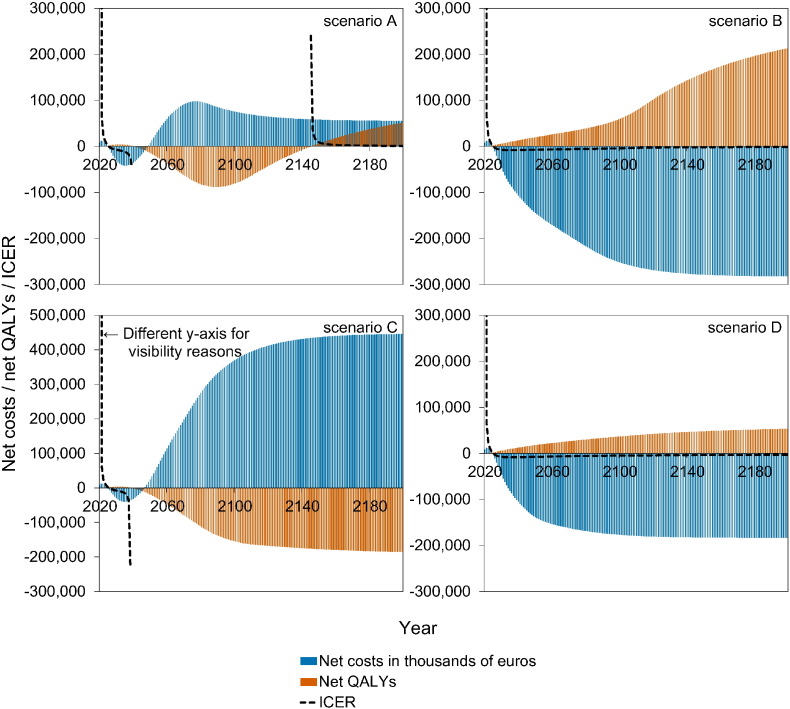
Fig. 4 shows a stylised overview of the cost-effectiveness analyses at high vaccination coverage (95%). Full results are presented in Figures S10–S11. Models without immune boosting (scenarios B and D) are characterised by health gains and limited costs or even savings, while models with immune boosting (scenarios A and C) are characterised by health losses and high costs. In models without immune boosting (scenarios B and D), vaccination at high coverage is expected to be cost-effective (scenario D) or even cost-saving (scenario B). In contrast, in models with boosting (scenarios A and C), vaccination at high coverage is either not cost-effective within 180 years (scenario C) or cost-effective only on the very long term (> 130 years; scenario A), with exception of the first ten years after start of vaccination when varicella incidence is low and HZ incidence not yet increased. In these scenarios, disadvantages for unvaccinated birth cohorts (i.e., QALY loss due to increased HZ) out-weigh health benefits for vaccinated cohorts.
Fig. 4.
Stylised overview of the cost-effectiveness of high-coverage (95%) varicella vaccination programme over time. Incremental cost-effectiveness ratio (ICER) threshold is set at €20,000 per QALY. This figure is based on data contained in Figures S10–S11. See the Supplement for details and sensitivity analyses.
If vaccination coverage is reduced to 50% or 25% (Figure S12–S13), the effect of vaccination on both net costs and net QALYs are smaller. As a result, scenario B ceases to be cost-saving (but remains cost-effective).
3.3. Sensitivity Analyses
The sensitivity analyses (see Supplement) show that the epidemiological impact of vaccination is similar when two vaccine doses are administered around the age of 1 year (Figure S9). If costs and QALYs for HZ are ignored, varicella vaccination is expected to be cost-effective after 5 years of vaccination (Figure S14). Without discounting, scenario A is cost-effective 30 years earlier than in the main analyses, scenario B is cost-saving 10 years earlier, scenario C remains not cost-effective, and scenario D is cost-effective 10 years earlier (Figure S15). When costs and QALYs are discounted at the same rate (4%), scenario A is not cost-effective anymore on the long run, illustrating that the cost-effectiveness hinges on unequal discounting rates in the main analyses (Figure S16). Finally, if the vaccine price is halved, scenario A is cost-effective slightly earlier, scenario B is cost-saving 20 years earlier, scenario C remains not cost-effective, and scenario D is cost-effective 10 years earlier, and even becomes cost-saving (Figure S17). Summarising, in all analyses the ICER decreases strongly in the first years after start of vaccination when upfront costs of the vaccination programme are offset by strong health gains due to reduced VZV circulation. In scenarios with immune boosting (scenario A and C), there is a similarly strong increase in the ICER after approximately 20 years when health benefits caused by reduced VZV circulation are nullified by even stronger negative health effects caused by increasing HZ incidence. Because of abrupt changes in the ICER, using a higher threshold for cost-effectiveness (e.g., €200,000 per QALY) has relatively small impact on the conclusions regarding cost-effectiveness of vaccination.
4. Discussion
Our analyses show that health effects and cost-effectiveness of varicella vaccination depend crucially on the impact on HZ, and the time horizon for economic analysis. In the absence of exogenous immune boosting, vaccination with high coverage is expected to be cost-effective and may even be cost saving, while it is not expected to be cost-effective on reasonable time scales (< 100 years, say) if immune boosting is present. In our analyses, the worst case would be a scenario with immune boosting and reactivation of vaccine virus. In such a scenario, benefits of reduced varicella incidence are offset by a strong increase in HZ incidence. In all our analyses varicella vaccination would be cost-effective on a short time-scale of less than 20 years, when potential negative effects on HZ incidence are not yet felt. Altogether, we conclude that the decision on whether varicella vaccination is cost-effective or not, is dominated by the epidemiological impact of vaccination, and hardly affected by the choices regarding the height of the threshold for cost-effectiveness.
Results by birth cohort further reveal that varicella vaccination may result in inequalities of health effects between generations. Specifically, in scenarios with immune boosting the benefits of vaccination accrue in vaccinated birth cohorts, while the burden and costs are largely due to HZ in unvaccinated persons. Especially cohorts born just before the introduction of vaccination are expected to pay the price for the health gain in vaccinated cohorts. This is true not only in terms of the proportion affected by HZ but also in terms of cost-effectiveness as the age at reactivation is expected to shift to working ages. In addition, in unvaccinated persons the age at infection with VZV increases to older children and young adults, resulting in more severe varicella and potentially an increase in congenital and perinatal varicella. These results reveal an ethical dilemma for policy makers, as groups not included in the vaccination programme may be exposed to a substantially increased health hazard.
Incorporating a catch-up campaign of varicella vaccination does not prevent the health inequalities mentioned above. Although the additional disease burden due to HZ can be partly mitigated by HZ vaccination among older adults (van Hoek et al., 2012), it is debatable whether it would be logical to make additional costs for HZ vaccination to make varicella vaccination, which is possibly not cost-effective on its own, cost-effective.
Earlier studies have shown potential consequences of varicella vaccination on HZ incidence (Schuette and Hethcote, 1999, Brisson et al., 2000, Gidding et al., 2005, Karhunen et al., 2010). However, only a limited number of studies on the cost-effectiveness of varicella vaccination (Rozenbaum et al., 2008, van Hoek et al., 2012, Thiry et al., 2003, Unim et al., 2013, Bilcke et al., 2013) incorporated the potential effects of varicella vaccination on herpes zoster incidence. According to the reviews and additional studies mentioned above, universal childhood varicella vaccination is expected to be cost-effective or even cost-saving from a societal perspective (or cost-effective under the health payer perspective), when effects on herpes zoster are ignored. However, if potential effects on herpes zoster are incorporated, as suggested by the immune boosting hypothesis of Hope-Simpson, vaccination is either not cost-effective or cost-effective on a very long time scale of several decades. Our analyses have revealed profound trans-generational differences in the distribution of health benefits and losses, thereby underscoring the importance to study effects beyond the mean in the population at large. Second, exploration of different scenarios regarding occurrence of immune boosting and reactivation of vaccine virus give justice to the biological uncertainties. Third, ICERs are calculated using a variable time horizon, and our analyses have shown that the time horizon for economic assessment is crucial when health benefits and losses accrue on different time scales.
We acknowledge some study limitations. First, even though different studies seem to be tipping toward the immune boosting hypothesis, there are also negative reports and the issue remains not yet definitely settled (Ogunjimi et al., 2013). In our study we have considered a broad range of biologically plausible scenarios, inevitable leading to a broad range of potential outcomes. Second, due to lack of data on breakthrough infection after two vaccine doses and on possible waning of immunity, the model could not be parameterised including these possible vaccine imperfections. Third, we assume that reactivation of vaccine virus occurs at the same rate as for circulating virus. In practice, reactivation after vaccination is expected to be rarer than after natural infection but the magnitude of this difference remains largely unknown. As a consequence, our scenarios with reactivation of vaccine virus probably represent a worst case scenario with regard to HZ increase after vaccination. Finally, due to lack of information no costs or effects have been included for long-term effects of congenital varicella syndrome.
5. Conclusions
We conclude that cost-effectiveness of varicella vaccination is strongly affected by its impact on HZ, and the time horizon for economic assessment. Although there are reports of increasing HZ incidence in populations with varicella vaccination (Goldman and King, 2013, Kelly et al., 2014), the time since the introduction of vaccination has probably been too short to draw definitive conclusions. Furthermore, evidence on vaccine VZV reactivation on the long-term is still limited (Heininger and Seward, 2006). Therefore, optimal decision-making on varicella vaccination would involve judicious and repeated weighing of the various scenarios as more evidence comes in from countries with vaccination programmes already in place.
Funding
This study was supported by the National Institute for Public Health and the Environment (RIVM), no additional funding has been acquired.
Competing Interests
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; and no other relationships or activities that could appear to have influenced the submitted work.
Dr. Sanders reports receiving grant support from pharmaceutical companies GlaxoSmithKline and Pfizer, and fees paid to the institution (University Medical Center Utrecht) for membership of independent data monitoring board from GSK and Pfizer, outside the submitted work.
Authors' Contributions
HdM and JW initiated this study. MvB designed the transmission model and performed statistical analyses, and AL/AvL designed the cost-effectiveness analyses with input on scenario and parameter choices from HdM, JW, WO, and PJ. AvL, AL, and MvB analysed the data and drafted the manuscript. HdM, JW, WO, PJ, FS, and ES interpreted the analyses and revised the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank Albert Jan van Hoek (Public Health England) for providing the original QALY loss data for herpes zoster by age, Willem Luytjes and Nynke Rots (RIVM) for their input on scenario and parameter choices, Odo Diekmann (Utrecht University) for helpful discussions, and Loes Soetens (RIVM) for her ideas on graphical presentation of the results.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2015.08.017.
Contributor Information
Alies van Lier, Email: alies.van.lier@rivm.nl.
Anna Lugnér, Email: anna.lugner@rivm.nl.
Wim Opstelten, Email: wopstel2@umcutrecht.nl.
Petra Jochemsen, Email: petra.jochemsen@rivm.nl.
Jacco Wallinga, Email: jacco.wallinga@rivm.nl.
François Schellevis, Email: f.schellevis@nivel.nl.
Elisabeth Sanders, Email: lieke.sanders@rivm.nl.
Hester de Melker, Email: hester.de.melker@rivm.nl.
Michiel van Boven, Email: michiel.van.boven@rivm.nl.
Appendix A. Supplementary data
References
- Anderson R.M., May R.M. Oxford University Press; Oxford and New York: 1991. Infectious Diseases of Humans: Dynamics and Control. [Google Scholar]
- Bilcke J., van Hoek A.J., Beutels P. Childhood varicella-zoster virus vaccination in Belgium: cost-effective only in the long run or without exogenous boosting? Hum. Vaccin. Immunother. 2013;9:812–822. doi: 10.4161/hv.23334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanni P., Breuer J., Gershon A., Gershon M., Hryniewicz W., Papaevangelou V. Varicella vaccination in Europe — taking the practical approach. BMC Med. 2009;7:26. doi: 10.1186/1741-7015-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson M., Edmunds W.J., Gay N.J., Law B., De Serres G. Modelling the impact of immunization on the epidemiology of varicella zoster virus. Epidemiol. Infect. 2000;125:651–669. doi: 10.1017/s0950268800004714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson M., Gay N.J., Edmunds W.J., Andrews N.J. Exposure to varicella boosts immunity to herpes-zoster: implications for mass vaccination against chickenpox. Vaccine. 2002;20:2500–2507. doi: 10.1016/s0264-410x(02)00180-9. [DOI] [PubMed] [Google Scholar]
- Ehreth J. The global value of vaccination. Vaccine. 2003;21:596–600. doi: 10.1016/s0264-410x(02)00623-0. [DOI] [PubMed] [Google Scholar]
- Enders G., Miller E. Varicella and Herpes Zoster in Pregnancy and the Newborn. In: Arvin A.M., Gershon A.A., editors. Varicella-Zoster Virus: Virology and Clinical Management. Cambride University Press in association with the VZV Research Foundation; Cambridge: 2000. pp. 317–347. [Google Scholar]
- European Centre for Disease Prevention and Control (ECDC) ECDC; Stockholm: 2015. ECDC Guidance: Varicella Vaccination in the European Union. ( http://ecdc.europa.eu/en/publications/Publications/Varicella-Guidance-2015.pdf) [Google Scholar]
- European Centre for Disease Prevention and Control (ECDC) ECDC; Stockholm: 2014. Immunisation schedules Varicella. (September 26, 2014; Available from: http://vaccine-schedule.ecdc.europa.eu/Pages/Scheduler.aspx) [Google Scholar]
- Gidding H.F., Brisson M., Macintyre C.R., Burgess M.A. Modelling the impact of vaccination on the epidemiology of varicella zoster virus in Australia. Aust. N. Z. J. Public Health. 2005;29:544–551. doi: 10.1111/j.1467-842x.2005.tb00248.x. [DOI] [PubMed] [Google Scholar]
- Goldman G.S., King P.G. Review of the United States universal varicella vaccination program: herpes zoster incidence rates, cost-effectiveness, and vaccine efficacy based primarily on the Antelope Valley Varicella Active Surveillance Project data. Vaccine. 2013;31:1680–1694. doi: 10.1016/j.vaccine.2012.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzzetta G., Poletti P., Del Fava E., Ajelli M., Scalia Tomba G.P., Merler S. Hope-Simpson's progressive immunity hypothesis as a possible explanation for herpes zoster incidence data. Am. J. Epidemiol. 2013;177:1134–1142. doi: 10.1093/aje/kws370. [DOI] [PubMed] [Google Scholar]
- Hakkaart-van Roijen L., Tan S.S., Bouwmans C.A.M. [Guidelines for pharmacoeconomic research] College voor Zorgverzekeringen; Diemen: 2011. Handleiding voor kostenonderzoek — Methoden en standaard kostprijzen voor economische evaluaties in de gezondheidszorg; geactualiseerde versie 2010. (Dutch http://www.zorginstituutnederland.nl/binaries/content/documents/zinl-www/documenten/publicaties/overige-publicaties/1007-handleiding-voor-kostenonderzoek/Handleiding+voor+kostenonderzoek.pdf) [Google Scholar]
- Heininger U., Seward J.F. Varicella. Lancet. 2006;368:1365–1376. doi: 10.1016/S0140-6736(06)69561-5. [DOI] [PubMed] [Google Scholar]
- Hope-Simpson R.E. The nature of herpes zoster: a long-term study and a new hypothesis. Proc. R. Soc. Med. 1965;58:9–20. [PMC free article] [PubMed] [Google Scholar]
- Houweling H., Verweij M., Ruitenberg E.J. on behalf of the National Immunisation Programme Review Committee of the Health Council of the Netherlands. Criteria for inclusion of vaccinations in public programmes. Vaccine. 2010;28:2924–2931. doi: 10.1016/j.vaccine.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Karhunen M., Leino T., Salo H., Davidkin I., Kilpi T., Auranen K. Modelling the impact of varicella vaccination on varicella and zoster. Epidemiol. Infect. 2010;138:469–481. doi: 10.1017/S0950268809990768. [DOI] [PubMed] [Google Scholar]
- Kelly H., Grant K., Gidding H., Carville K. Decreased varicella and increased herpes zoster incidence at a sentinel medical deputising service in a setting of increasing varicella vaccine coverage in Victoria, Australia, 1998 to 2012. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.es2014.19.41.20926. (pii = 20926) [DOI] [PubMed] [Google Scholar]
- Marin M., Guris D., Chaves S.S., Schmid S., Seward J.F. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm. Rep. 2007;56:1–40. [PubMed] [Google Scholar]
- Mossong J., Hens N., Jit M., Beutels P., Auranen K., Mikolajczyk R. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5:e74. doi: 10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunjimi B., Van Damme P., Beutels P. Herpes zoster risk reduction through exposure to chickenpox patients: a systematic multidisciplinary review. PLoS One. 2013;8:e66485. doi: 10.1371/journal.pone.0066485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxman M.N. Clinical Manifestations of Herpes Zoster. In: Arvin A.M., Gershon A.A., editors. Varicella-Zoster Virus: Virology and Clinical Management. Cambridge University Press in association with the VZV Research Foundation; Cambridge: 2000. pp. 246–275. [Google Scholar]
- Rozenbaum M.H., van Hoek A.J., Vegter S., Postma M.J. Cost-effectiveness of varicella vaccination programs: an update of the literature. Expert Rev. Vaccines. 2008;7:753–782. doi: 10.1586/14760584.7.6.753. [DOI] [PubMed] [Google Scholar]
- Schuette M.C., Hethcote H.W. Modeling the effects of varicella vaccination programs on the incidence of chickenpox and shingles. Bull. Math. Biol. 1999;61:1031–1064. doi: 10.1006/bulm.1999.0126. [DOI] [PubMed] [Google Scholar]
- Stirbu-Wagner I., Visscher S., Davids R., Gravestein J.V., Ursum J., Van Althuis T. NIVEL/IQ; Utrecht/Nijmegen: 2011. National Information Network Primary Care (LINH): Facts and Figures on Primary Care in the Netherlands. [Google Scholar]
- Thiry N., Beutels P., Van Damme P., Van Doorslaer E. Economic evaluations of varicella vaccination programmes: a review of the literature. Pharmacoeconomics. 2003;21:13–38. doi: 10.2165/00019053-200321010-00002. [DOI] [PubMed] [Google Scholar]
- Thomas S.L., Wheeler J.G., Hall A.J. Contacts with varicella or with children and protection against herpes zoster in adults: a case–control study. Lancet. 2002;360:678–682. doi: 10.1016/S0140-6736(02)09837-9. [DOI] [PubMed] [Google Scholar]
- Unim B., Saulle R., Boccalini S., Taddei C., Ceccherini V., Boccia A. Economic evaluation of varicella vaccination: results of a systematic review. Hum. Vaccin. Immunother. 2013;9:1932–1942. doi: 10.4161/hv.25228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoek A.J., Melegaro A., Gay N., Bilcke J., Edmunds W.J. The cost-effectiveness of varicella and combined varicella and herpes zoster vaccination programmes in the United Kingdom. Vaccine. 2012;30:1225–1234. doi: 10.1016/j.vaccine.2011.11.026. [DOI] [PubMed] [Google Scholar]
- van Lier A., Smits G., Mollema L., Waaijenborg S., Berbers G., van der Klis F. Varicella zoster virus infection occurs at a relatively young age in The Netherlands. Vaccine. 2013;31:5127–5133. doi: 10.1016/j.vaccine.2013.08.029. [DOI] [PubMed] [Google Scholar]
- Varicella and Herpes Zoster Vaccines: WHO Position Paper, June 2014. Wkly Epidemiol. Rec. 2014;89:265–287. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.