Abstract
Mixing in microfluidic devices presents a challenge due to laminar flows in microchannels, which result from low Reynolds numbers determined by the channel’s hydraulic diameter, flow velocity, and solution’s kinetic viscosity. To address this challenge, novel methods of mixing enhancement within microfluidic devices have been explored for a variety of applications. Passive mixing methods have been created, including those using ridges or slanted wells within the microchannels, as well as their variations with improved performance by varying geometry and patterns, by changing the properties of channel surfaces, and by optimization via simulations. In addition, active mixing methods including microstirrers, acoustic mixers, and flow pulsation have been investigated and integrated into microfluidic devices to enhance mixing in a more controllable manner. In general, passive mixers are easy to integrate, but difficult to control externally by users after fabrication. Active mixers usually take efforts to integrate within a device and they require external components (e.g. power sources) to operate. However, they can be controlled by users to a certain degree for tuned mixing. In this article, we provide a general overview of a number of passive and active mixers, discuss their advantages and disadvantages, and make suggestions on choosing a mixing method for a specific need as well as advocate possible integration of key elements of passive and active mixers to harness the advantages of both types.
Keywords: mixing, microfluidics, micromixers, flow controls, review
1. Introduction
Microfluidic devices have a wide variety of chemical and biological applications, including medical diagnostics, DNA and protein analysis, and drug development. They can be miniaturized, allowing for quick analysis using portable instrumentation. They also use minimal amounts of samples and consume little reagents, reducing the waste generated and leading to overall low-cost operations.
However, microfluidic devices also have limitations. One of the challenges is mixing, which is often required for sample dilution, reagent homogenization, and chemical or biological reactions. The difficulty in achieving sufficient mixing in a microfluidic device results from laminar flows that can be explained by low Reynolds number. The characteristic length (L) of a microflow is typically on the order of 100 µm. The hydraulic diameter of the channel is used as the characteristic length, and is defined as 4A/Pw where A is the cross sectional area and Pw is the wetted perimeter of the channel at the cross section. The low L values commonly found in microfluidic devices suggest a high surface-area-to-volume ratio, yielding enhanced heat and mass transfer within these devices [1]. However, this low hydraulic diameter, combined with typically small (~1 mm s−1) flow velocities (V) due to extremely high backpressures and with typical kinematic viscosities (υ) of on the order of 10−6 m2 s−1, leads to very small Reynolds numbers for flow in microchannels (~0.1). As a result, flows in microfluidic devices are almost always laminar in nature. The low Reynolds number implies that viscous forces are dominating over inertial forces within the flow, dampening out any flow irregularities that might aid in fluid mixing. Since the Reynolds number for flow is so small within microfluidic devices, they are unable to harness the advantages of turbulent mixing that can be found in macro-scale systems. As a result, microfluidic devices must rely solely on diffusive mixing, which is an inherently slower process and requires a long channel to achieve sufficient mixing [2]. Hessel et al give an in-depth explanation of the principles of mixing, as well as methods to overcome the low Reynolds number in microchannels, and common approaches to determine the mixing efficiency [3]. These approaches for characterizing the mixing efficiency are explained in great detail by Aubin et al [4]. In addition, Kockmann gives an overview of convective micromixers that typically operate under higher Reynolds number flows in larger microchannels (100–1000 µm) [5]. Convective mixers are useful in industrial processes where high throughput and the avoidance of fouling are necessary.
Since microfluidic devices are forced to rely on slow diffusive-mixing process, there is a call in the field to discover new ways in which mixing efficiency can be enhanced. Surprisingly, many laminar flows have the potential to achieve chaotic mixing, and microfluidic devices are currently being fabricated to take advantage of this phenomenon [6, 7]. Enhanced laminar mixing will allow for shorter channels to be used within the devices, increasing the throughput of the devices and ultimately allowing for the realization of more efficient lab-on-a-chip systems [2]. Kumar et al show a rapidly increasing trend in the amount of publications on micromixers from 1999 to 2009, reinforcing the fact that this area of research is thriving in the scientific community [8].
Mixing within microfluidics can be separated into two major categories: active and passive mixing [2, 9–13]. Passive mixing is achieved by altering the structure or configuration of fluid channels. This type of mixing is incorporated into the system during fabrication and is not externally controlled by users. The key benefit of passive mixing is that there are no moving parts within the mixer, resulting in easier fabrication and operation [12]. However, the extent of mixing is determined by the device configuration and can only be adjusted by the users through the imposed flow rates after fabrication. This results in difficulties to obtain the optimal mixing from a given device, and also does not allow users to ‘turn off’ the mixing enhancement if needed once a device is fabricated. Nguyen and Wu reviewed these types of micromixers with a focus on the operation points based on characteristic dimensionless numbers [11].
Active mixers are able to be activated on demand by a user, and controllable mixing may be carried out using pressure gradients, electrical voltages across the fluid, or integrated mixing elements like stirring bars [10, 12, 14, 15]. They typically enhance mixing by stirring the fluid mechanically, magnetically, electrically or acoustically. Although active mixers can be useful in enhancing mixing, particularly for chamber mixing, they tend to be harder to fabricate than passive mixers due to movable parts and often require an external power source [9, 10]. Campbell and Grzybowski reviewed various approaches to active micromixers, proposing a self-assembly method that effectively creates a stirring bar effect [16].
Research in both types of mixing enhancements has led to many novel types of mixers that can be incorporated into microfluidic devices. When utilizing these mixers, multiple system parameters such as flow velocity, energy input, and geometry can be adjusted to obtain ideal mixing, as discussed by Mansur et al [17]. Meijer et al advocate for the use of the mapping method to gain insight into the optimal parameters for commonly used passive mixers [18]. Lee et al reviewed both active and passive mixing enhancement in microfluidic devices, and discussed the advantages and disadvantages of both types of enhancement [2]. Capretto et al provided an in-depth look into active and passive mixers focused on the microscale behavior of the fluid due to the mixing enhancement [13]. Chang and Yang examined all electrokinetic mixers [19] while Jeong et al reviewed the broad applications of micromixing, including chemical and biological detection/ analysis that generally drive the research in the field of microfluidics [20].
Ideal mixing is especially important in reactive systems, where non-idealities lead to inefficiencies in the device. Mixing efficiency can be predicted via simulations using CFD codes, but it is crucial that experimenters be able to accurately measure the mixing efficiency of a particular device experimentally to determine optimal parameters and to understand the nature of the enhancements. Hessel’s review provides a section on mixing characterization that summarizes the nature of these experimental measurements [3].
Mixing evaluation can be undertaken in a variety of ways, which typically rely on flow visualization methods. The most common way to visualize mixing and study mixing efficiency is through mixing a dyed liquid with a clear liquid and examining the color of the product stream over time using a microscope and high-speed camera. For example, the mixing of a blue stream and a yellow stream creates a purely green stream when fully mixed [14]. Fluorescent particles or fluids are also used in conjunction with a microscope to quantitatively characterize the mixing [21, 22]. In a similar manner, 3D flow patterns can be constructed using confocal-fluorescence microscopy [23]. Furthermore, experimenters can mix reactive components and track the reaction along the channel length, which can be correlated to the efficiency of mixing enhancements [24]. These methods can help researchers to qualitatively and quantitatively evaluate the efficiency of designed enhancements, allowing for the optimization of mixing in the device.
In this review, we first discuss the fundamental diffusive mixing present within microchannels. We then examine the common types of passive micromixers (e.g. in geometry or surface properties) with improved performance over pure diffusive mixing. Emphasis is placed on enhancement methods for mixing liquid flows, with a section devoted to multiphase flows as well. We then present active mixing methods and their integration into microfluidic devices, and advocate possible integration of passive and active mixing elements together to take the advantages of both types of mixers. Unsolved problems and promising research directions are then presented, along with recommendations for the implementation of the previously described mixing enhancements for certain processes.
2. Enhanced mixing by passive elements
Passive mixing elements are commonly incorporated into microfluidic devices during fabrication. As the name suggests, these types of elements are passive, and they do not require additional controls by users. They are often achieved by utilizing clever alterations to the channel walls, geometry, or surface properties. Passive mixing elements are usually easy to incorporate, and can provide great enhancement to mixing in microfluidic devices. However, these elements offer no additional mixing control after fabrication; users cannot easily manipulate the level of mixing enhancement. Passive mixing enhancement is recommended for researchers looking for an easy way to incorporate mixing enhancement in processes where the level of mixing control is not crucially important.
2.1. Diffusion and stream splitting/combination
Two different fluids, when put in contact with one another, will eventually mix through molecular diffusion. This fundamental idea was used to develop one of the most primitive methods of mixing within microfluidic devices. Basic microfluidic devices rely on bringing together two split streams into a single larger channel in order to enhance mixing. The mixing generally occurs as a two-step process and is known as stream splitting [25].
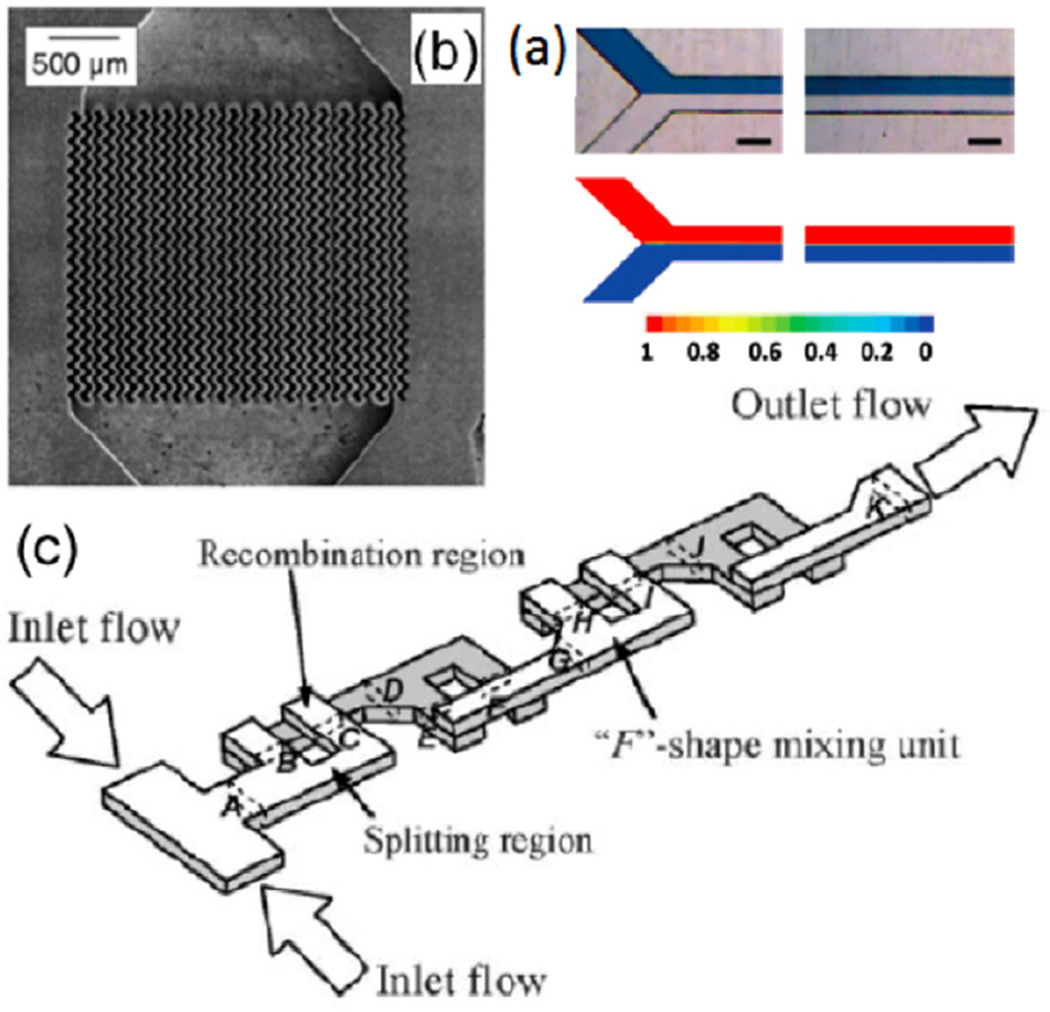
During the first step, the two streams are brought together within a single channel. Next, the diffusion between the two fluids across the interface leads to a uniform mixture at the molecular level. The transport of fluid in this case is a result of Brownian motion along the concentration gradient between the two fluids. Since the interface between the two fluids is long, the diffusion occurs at an accelerated rate across the interface. However, within microfluidic devices, this process is still typically very slow due to a large Peclet number. Bessoth et al utilized long channels with multiple points of combination and a focus on creating a system with a low dead volume in order to effectively mix [26]. Figure 1(a) shows a representation of the process of mixing by combining two channels into one channel in a microfluidic device. Mixing in such a device requires a long channel in order for full mixing to take place [27].
Figure 1.
(a) Images and simulated predictions for concentration contour at the entry (left) and exit (right) of a microfluidic channel at a flow rate of 2 ml min−1. The degree of mixing is minimal because the mixing is relying on diffusion alone (adapted from [27] with permission). (b) Scanning electron micrograph (SEM) of a mixer consisting of 2 × 15 interdigital channels with corrugated walls (adapted from [28] with kind permission from Springer Science and Business Media). (c) Diagram of serpentine laminating micromixer (adapted from [29] with permission from The Royal Society of Chemistry).
This traditional T-shaped or Y-shaped mixer can be enhanced by using complex geometries or by twisting the inlet streams to reduce the mixing path. Once streams are combined, they can also be split and combined again in series to enhance mixing further. Multiple streams running in parallel can be combined in a T-shape manner in order to mix them [5], or 3D flows can even be generated within microfluidic devices if designed correctly, such as in the interdigital micromixers produced by Haverkamp et al and those described by Hessel et al [28, 30] (figure 1(b)). Ahn’s research group utilized a serpentine channel with F-shaped mixing units in order to realize the benefit of stream splitting and recombination to mixing efficiency [29] (figure 1(c)). Many other researchers have also varied the traditional T-shaped mixers in order to enhance mixing [31–33]. Kim et al fabricated a 3D manifold micro-mixer embedded in a microchannel to achieve fast mixing in a short channel length [34]. These variations, however, require a more difficult procedure for the fabrication of channels, which increases the device cost. Dreher et al used CFD simulations to characterize the various flow regimes of a traditional T-mixer, showing different mixing regimes depending on the Reynolds number for the flow [35]. Li et al were able to achieve uniform mixing between two fluid streams in 5.5 µs using a stream combination method combined with a uniquely shaped mixing channel [36].
Though splitting and recombining streams are more effective than relying on diffusion between two streams in contact alone, the mixing still occurs slowly and long channels are often required. A variety of active and passive mixing enhancements can be designed to decrease the necessary mixing channel lengths, enhancing mixing to a much greater extent when combined with stream splitting.
2.2. Slanted wells, ridges or grooves
Slanted wells are a type of passive mixers that are relatively easy to fabricate within a microfluidic device that can provide additional mixing within the system without increasing the overall channel length. These types of mixers came about as an expansion on the traditional method of stream splitting for mixing enhancement within microfluidics. Researchers attempted to increase the mixing rate beyond what would be obtained by diffusion alone by increasing the amount of lateral transport within the channel, in theory decreasing the mixing distance [37].
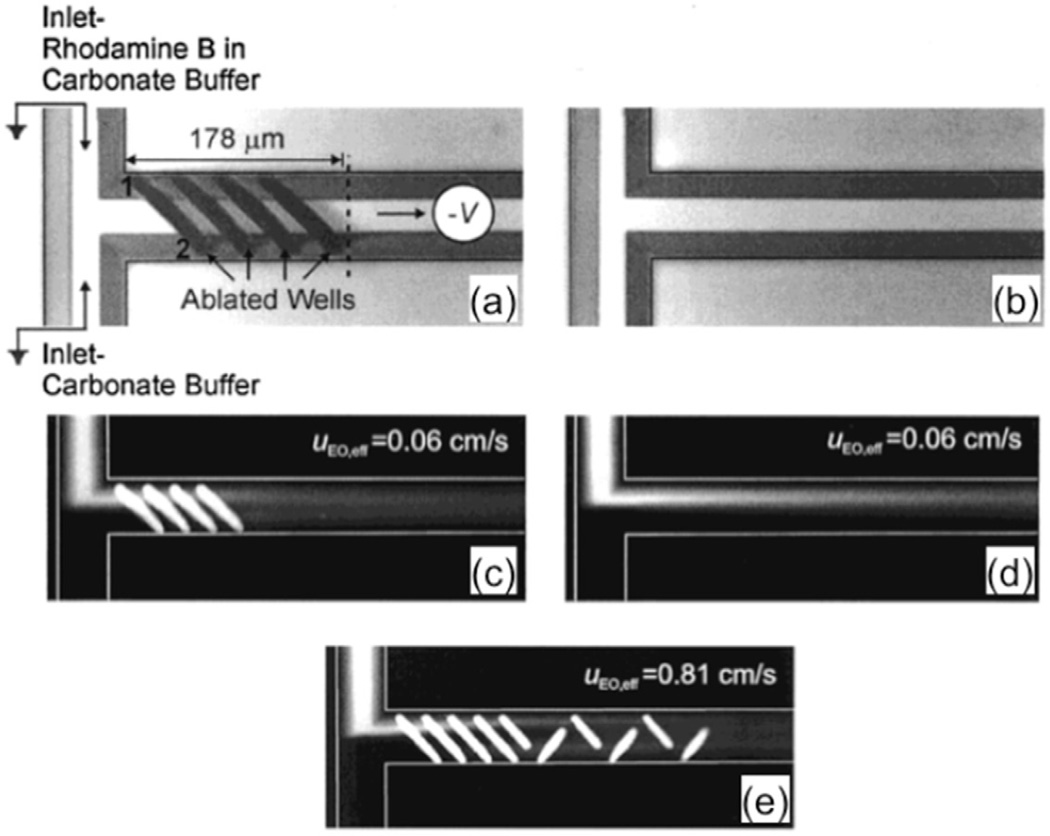
Johnson et al developed this type of passive mixer by using an excimer laser system to create slanted wells along a prefabricated polycarbonate microchannel [37]. Figures 2(a) and (b) show a comparison between a microchannel with slanted wells and a smooth channel. Electroosmotic flow was generated by applying a voltage across the channel, and the resultant mixing was compared between two types of microchannels.
Figure 2.
(a) Configuration of the experimental setup and image of a channel with a series of slanted wells. (b) Image of a smooth channel. (c), (d) Fluorescence image of electroosmotic flow of the corresponding channels in (a) and (b). (e) Fluorescence image for electroosmotic flow past an optimized mixer at a high flow rate (0.81 cm s−1). These figures are adapted from [37] with permission.
Results show that fluid enters and follows the contours of the wells, which induces lateral transport across the channel to aid in mixing. Johnson et al found that for electroosmotic flow, the four well design enhanced mixing over the traditional design, and that the percentage mixing was dependent on the number of wells. Using the four-welled structure, the group achieved 74.7% mixing at a distance of 183 µm past the T-junction when using a flow rate of 0.06 cm s−1. Figure 2(c) shows an experimental graphic representation of the mixing occurring as a result of the slanted walls within the system.
Further experiments were conducted using different numbers of wells and well orientations, and a more optimal configuration for slanted wells was found for higher flow rates. Figure 2(e) shows a more advanced configuration used for higher flow rates up to 0.83 cm s−1. The average depths of the additional partial wells were found to be 100 µm below the channel depth of 31 µm for the majority of the well. These partial wells redirected a portion of the flow back toward the center of the channel, further enhancing the mixing. By utilizing this more advanced structure, Johnson et al achieved 80.5% mixing at a flow rate of 0.81 cm s−1 after only 443 µm, where the four well design only achieved 63.8% mixing and diffusion alone only achieved 21.8% mixing for the same distance [37].
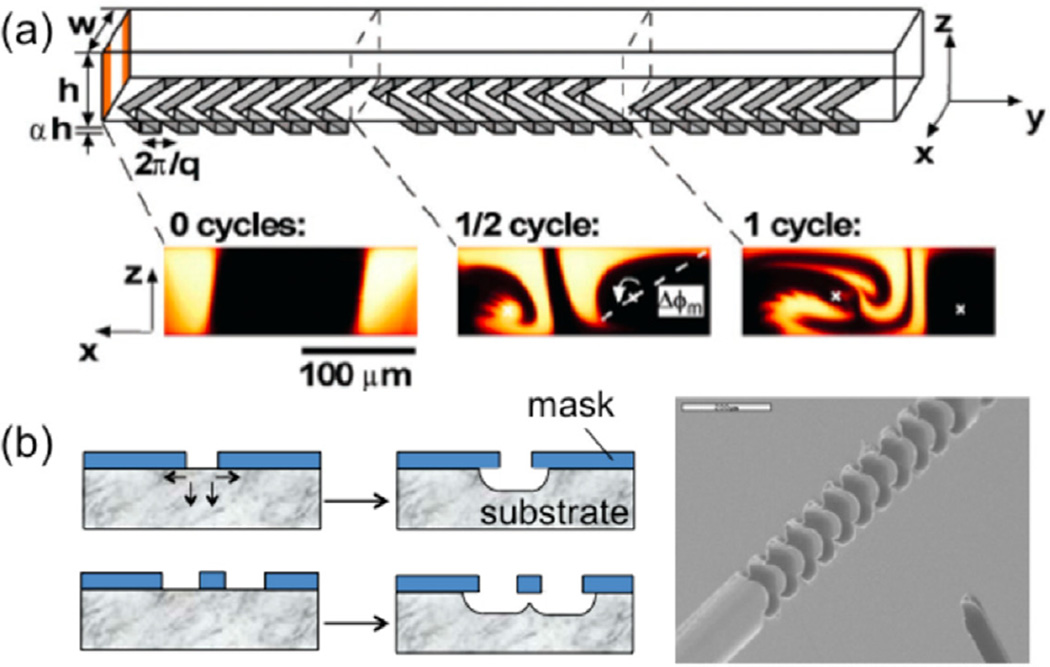
In a similar fashion, Stroock et al developed a passive mixer for microfluidic devices by using oblique ridges [7]. They hypothesized that ‘stirring flows’, or flows that have transverse components that fold fluid elements over the channel cross-section cause chaotic advection [38] within the flow, which in turn enhances the mixing. In short, the traverse flows generated cause the fluid streams to stir into one another [39]. Stroock et al generated this chaotic flow by creating a repeated sequence of rotational and extensional flows. This passive mixer incorporates patterned grooves on the floor of a channel. These patterned grooves follow a ‘staggered herringbone’ structure, which allows for even greater mixing enhancement than the slanted wells. The structure of the staggered herringbone mixer (SHM) contains groove shapes that vary based upon the axial position in the channel. According to Stroock et al, the change in orientation of the ridges causes the centers of rotation to exchange and therefore enhances mixing. Figure 3(a) shows a schematic of the ridges that are used in the SHM and the flow patterns that are generated when a fluid flows through a cycle of these ridges [7].
Figure 3.
(a) Schematic diagram of one-and-a-half cycles of the SHM. A mixing cycle is composed of two sequential regions of ridges; the direction of asymmetry of the herringbones switches with respect to the centerline of the channel from one region to the next. At the bottom are confocal micrographs of vertical cross sections of a channel (from [7]. Reprinted with permission from AAAS). (b) (left) Illustration of isotropic etching and ridges obtained from judicious designs and isotropic etching; (right) SEM picture of a ridged channel in a microfluidic device made from a cyclic olefin copolymer. The scaling bar is 200 µm (adapted with permission from [42]. Copyright 2007 American Chemical Society).
Chaotic flows can be generated within devices that create alternating flow patterns for the fluids. These alternating patterns cause a periodic velocity that ultimately results in chaotic trajectories of the fluid elements. The SHM accomplishes this task by periodically switching the orientation of the ridges. However, there are also many other ways in which to generate this type of flow within microfluidics. For example, Sugioka numerically studied a chaotic active mixer that works by alternating between a pressure-driven directional flow and a vortex flow generated by induced-charge electroosmosis [40]. The group concluded that this type of mixer is effective for producing chaotic mixing within a device, even at large Peclet numbers. Many other researchers have also harnessed chaotic advection as a method for enhancing microfluidic mixing [41].
Xia et al developed another patterned structure to enhance mixing by creating microridges within a channel [42]. These microridges were created as a result of undercutting during the chemical etching of a glass plate, which was then used as a master to create plastic devices. The microridge structure created in this manner mimics that of the herringbone structure created by Stroock et al. These ridges were even easier to fabricate than the slanted well or herringbone structures, due to the fact that their fabrication only requires one step instead of two. The resultant flow patterns were similar to those observed when using slanted wells. Figure 3(b) shows the undercutting created by isotropic etching as well as the final microridge structures created. Mei et al demonstrated a related microfluidic device for biological reactions and luciferase detection [43]. Recently, Marschewski et al created a passive mixer inspired by the herringbone mixer that allows for the mixing of individual reactants in a co-laminar flow while simultaneously avoiding mixing between the individual streams [44].
Passive mixers with patterned grooves allow for enhanced mixing within a microfluidic channel with relatively easy microfabrication methods (due to no moving parts required) and no need for an external power source. If grooves are deep enough, the channel length required for complete mixing drops dramatically when using patterned grooves. However, these types of grooves do have a drawback, as they create dead volume within the channel [9]. Further research in reducing or eliminating this dead volume should be conducted in order to further the advancements in mixing enhancement obtained by using this type of groove.
Many more different types of patterned grooves similar to those studied by Johnson et al and Stroock et al have been researched extensively as passive methods to enhance mixing in microfluidic devices [45–47]. Computational Fluid Dynamics (CFD) models have been used to simulate the flow generated by patterned grooves in order to aid in the development of this type of device. Wang modeled flows over patterned ridges using CFD code to good accuracy, exhibiting that the code can accurately describe the flows occurring [9]. Forbes et al further simulated herringbone style mixing enhancements, offering guidelines for the depth, geometry, and frequency of ridges for optimized performance [48]. Lynn et al also used computer simulations to model flow over a similar type of ridge in order to enhance mass transfer to a capture spot for DNA detection [49]. Many other computer simulations for this type of mixer are also present in the literature [50, 51]. These types of codes can provide design guidelines that will allow researchers in the future to develop new ridge patterns for the channels in order to achieve the best possible mixing, increasing the mixing efficiency of microfluidic devices. Different groove depths, orientations, and spacing can be studied inexpensively using similar codes and analytical descriptions of the mixer before undertaking a research project to develop a better-patterned micromixer [9, 52, 53].
2.3. Multiphase micromixing
In addition to passive mixing enhancements associated with miscible liquids, mixing could also be enhanced in a device that allows for multiphase flows. For example, some researchers have created devices that produce droplets of reagents suspended in an immiscible carrier fluid (e.g. oil). Confining reagents that are to be mixed into a droplet or plug localizes their potential dispersion. In other words, the reagents are unable to penetrate into the surrounding fluid due to immiscibility, and thus they remain concentrated within their droplet. This localization, when combined with other naturally occurring enhancements such as chaotic advection or recirculation, can greatly enhance the mixing within the droplets.
Song et al experimentally studied mixing enhancement due to chaotic advection within droplets in microchannels [54]. The group generated fluid plugs containing three species within a stream of immiscible fluids. These plugs flowed through a winding channel, which in turn caused chaotic advection to occur within the plugs due to periodic recirculation. This recirculation was generated as a result of the shearing interaction of the fluid with the walls [54]. Tice et al used a similar approach to generate plugs within a device, and show that recirculation occurs inside the plugs even without the use of a winding channel [55]. The group found that the recirculation obtained within the plugs was highly sensitive to the initial distribution of the reagents inside the plugs. However, an optimal mixing of the reagents could be achieved by simply altering the relative flow rates of the reagents and immiscible carrier fluid to an experimentally determined value.
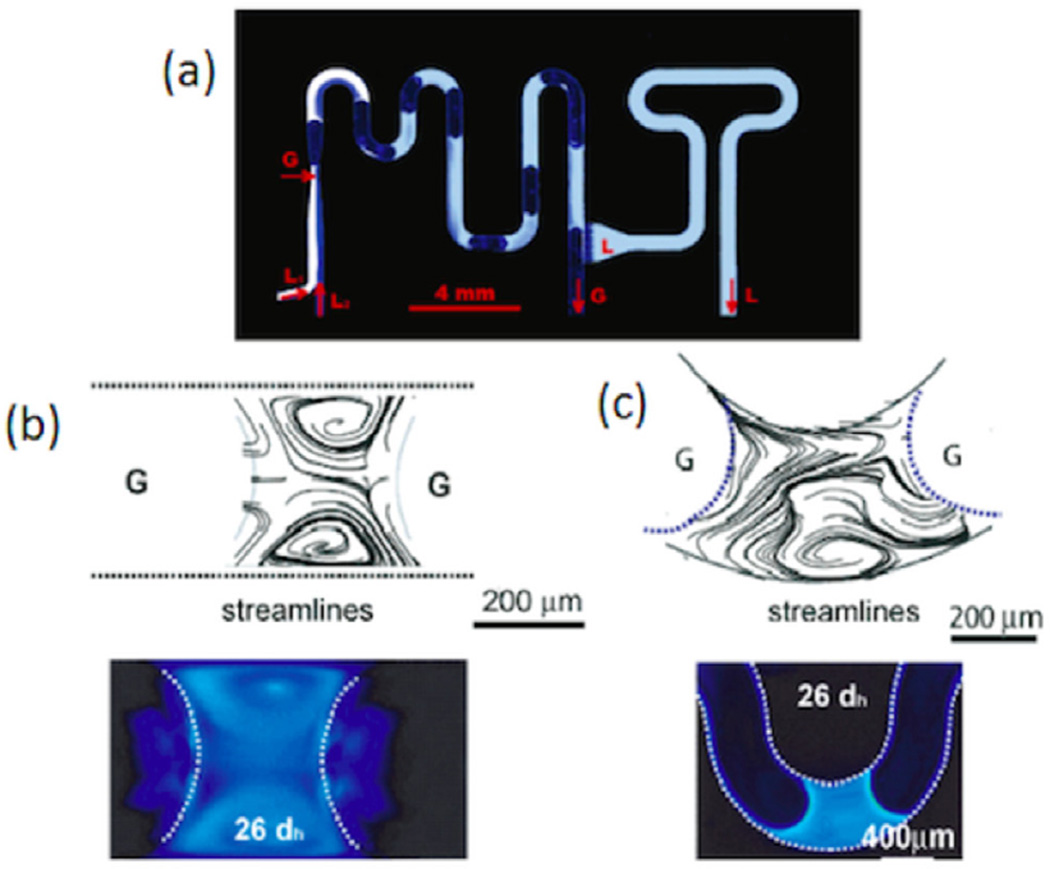
Gunther et al created a device that operates in a similar manner to enhance the mixing within liquid droplets [56]. However, instead of utilizing an immiscible liquid as the carrier, this group segmented the flow into plugs by using a gas. After the mixing occurred in each droplet, the liquid was removed downstream using a capillary separator, and thus the final product from the device is a single phase. The device they used is shown in figure 4(a). This mixer has a slight advantage over the previous mixer utilizing an immiscible liquid, because it can be successfully operated over a much higher range of reagent flow rates. The mixing in this device, in the same way as described above, relies on the recirculation that comes about due to the multiphase aspect of the flow to enhance the mixing. Figures 4(b) and (c) show the streamlines for the recirculation in a straight channel and a winding channel respectively. Garstecki et al were also able to utilize a similar type of mixing enhancement generated by utilizing only a hand-operated source of vacuum to enhance mixing within portable, rugged microfluidic devices [57]. Tangen et al created a droplet-on-demand device capable of injecting nanoliter sized droplets from up to 9 separate injection points that can be integrated into microfluidic devices for mixing purposes [58].
Figure 4.
(a) Visualization of mixing enhancement within liquid plugs and downstream separation of the liquid and gas phases. (b) Streamlines and visualization of recirculation within liquid plugs in straight channels. (c) Streamlines and visualization of recirculation within liquid plugs at the bends of winding channels. These figures were adapted with permission from [56]. Copyright 2005 American Chemical Society.
Dogan et al also examined the mixing enhancement in segmented flows [59]. They computationally modeled the mixing enhancement in winding channels similar to those tested experimentally by Song et al. The group used a finite volume/ front tracking method to conduct 2D numerical simulations. They confirmed numerically that chaotic mixing occurs within the droplet as a result of recirculation. In addition, they found that chaotic advection would be achieved within the droplets as long as segmentation was achieved. Key system parameters that governed the extent of mixing included a weak dependence on the Reynolds number, the corrugation wavelength of the mixing section, and the relative initial distance between bubbles [59]. Che et al also numerically examined this enhancement in 2D winding channels using a particle tracking method that is computationally easy and inexpensive. They found that the optimum mixing occurs in plugs with large curvature, a low Peclet number, a moderate viscosity ratio, and a moderate plug length [60].
2.4. Hydrophobic surfaces and other passive mixing enhancements
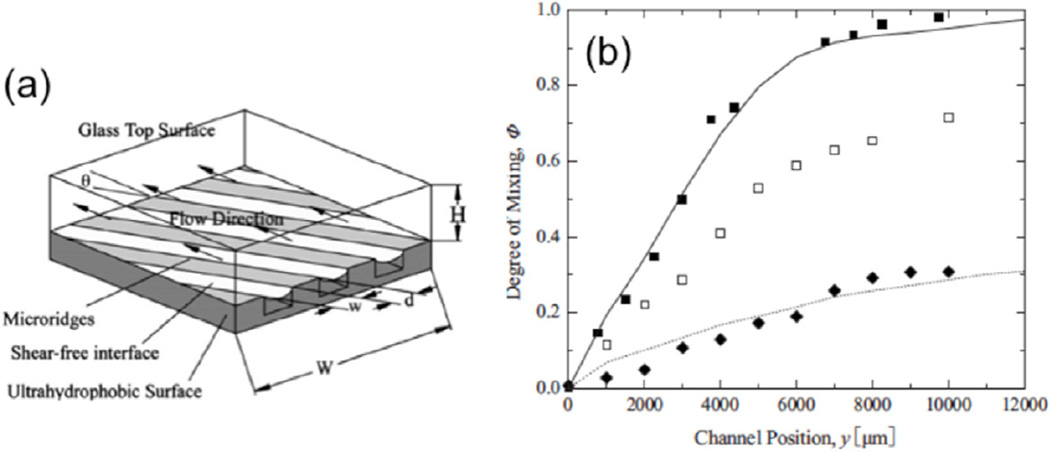
Ou et al studied the use of hydrophobic surfaces with microridges for mixing enhancement within microfluidic systems [61]. This type of enhancement works on a similar principle to those described in the previous section, but the hydrophobic surface creates a shear-free air–liquid interface due to the increased contact angle between the fluid and the channel walls, resulting in a reduction in drag forces on the fluid by more than 40% [62].
Figure 5(a) shows the schematic of the hydrophobic surface with microridges in the microchannels. The microridges were 5 µm deep with spacing of between 30 and 90 µm, oriented at angles between 30 and 90° to the flow direction. The mixing is enhanced due to the generation of a secondary flow similar to those found in normal microchannels with ridges or wells. However, aligning the air–water interface generated by the hydrophobicity with the flow at a transverse angle generates additional enhancement of the mixing (figure 5(b)) by increasing the amount of stretching and folding. This phenomenon is due to the lower resistance to the downward flow into the ridge generated by the shear-free air–liquid interface found between the structures [61]. Another interesting implication of the hydrophobic surfaces is that they allow an air–liquid interface to form within a closed system. This could allow for unique applications within the field of microfluidics that are unavailable without the generation of this interface [61]. Through use of experiments and simulations, Ou et al were able to optimize their design for a passive micromixer, and reduce the mixing length in relation to a smooth channel by over an order of magnitude [61].
Figure 5.
(a) Schematic diagram combining surface hydrophobicity and microridges, allowing water to stand away from the solid surface. (b) Improvement in the degree of mixing by hydrophobic microridges (solid squares) in comparison with hydrophilic microridges (open squares) and smooth surface (solid diamonds). Reprinted with permission from [61]. Copyright 2007 American Physical Society.
The use of ‘wavy walls’ within microfluidic devices also creates mixing enhancement due to the increased interfacial contact area between the fluids. Chen and Cho numerically investigated mixing enhancement in microfluidic devices with wavy walls of varying amplitudes and lengths, and have also studied the effect of adding a charged surface to the walls, which is an additional active enhancement [63]. Results showed that these additions to the channel were able to effectively enhance the mixing. The waves were introduced into the system with amplitudes varying from 20% to 80% of the channel depth, and varying lengths of the wavy region were tested. Wang et al also numerically examined the use of surface modification for mixing enhancement in microfluidic devices [64]. Staggered hydrophobic and hydrophilic regions were simulated through the use of an alternating boundary condition on the channel walls. The competition between these regions generated an oscillatory flow pattern within the channel in order to enhance the mixing.
In addition, Kamio et al achieved mixing enhancement through the use of concentric tubes to create a mixing zone within a microfluidic device [65]. This simple mixing enhancement is reminiscent of stream recombination discussed above. Kockmann and Roberge also used a device with winding channels with repeating contracting and diverging geometries to enhance mixing within a device [66]. The group later experimentally characterized the two-phase mixing properties of this type of device by utilizing a test reaction for several different geometries [24].
3. Enhanced mixing by active forces
Active mixing enhancement, when compared to passive mixing enhancement, offers superior control over the level of mixing obtained. This control, however, comes at the cost of more complex and expensive fabrication due to the moving parts required. Active mixing enhancements can also require an external power source or field for operation. There are a wide variety of active mixers to choose from, allowing for a suitable fit to almost any microfluidic device that requires more control over mixing than the passive mixers mentioned above.
3.1. Microstirrers
Similar to a magnetic stirring bar in a macroscale mixing operation, microstirrers can be fabricated for use in microfluidic devices. This type of active mixing enhancement uses a rotating magnetic field, which causes a microbar to rotate within a fluid system, enhancing the mixing in the vicinity of the bar. Multiple bars can be used simultaneously to enhance the system mixing. This type of mixing bar is literally a miniaturized version of those found in chemistry laboratories, operating on the same general principles [10].
The stirring bars within the microsystem generate circulation loops, which lessen the time needed for mixing. Lu et al created a fabrication process for microstirrers, and studied their effects on the mixing characteristics of fluid systems [14]. The group found that rapid mixing could be obtained within a channel or a large reaction chamber using the stirrers. Also, since the stirrer motion is driven by magnetic actuation, there is no need for electronic wires to intrude into the system to power the mixer. The dimensions of the stirrer, as well as the speed at which it rotates are parameters that can be varied to achieve the desired mixing level within the system. Between speeds of 100 and 600 rpm, Lu et al found the degree of mixing to increase with increasing rotor speed [14].
The main problem associated with using microstirrers is that the mixing enhancement effect does not extend very far from the region defined by the diameter of the stirrer. This can be remedied by designing the stirrer such that its diameter is near that of the channel, and by using multiple stirrers in series or parallel to ensure mixing throughout the length of the channel. However, using an array of stirring bars increases the difficulty of device fabrication. Simulations have been conducted using commercially available software to aid in the design of efficient microstirrers [14].
Mensing et al also explored the use of magnetic microstirrers for mixing in microfluidic devices [12]. Both Mensing and Lu’s mixers were designed to be controlled solely by a magnetic stirring plate as opposed to a strong external field that would require special equipment. The commercial availability of these magnetic stirring plates makes these microstirrers practical for use in laboratories not equipped to generate stronger magnetic fields. Mensing’s microstirrer was able to mix two streams flowing between 0.02 and 10 ml min−1, though the most efficient mixing occurred at or below 4 ml min−1. This wide range of flow rates would allow experimenters to mix streams in various devices to a great extent using a microstirrer [12].
Tierno et al also utilized a magnetic field to create a microstirrer, but used colloidal paramagnetic particles as the ‘stirring bar’ [67]. Lee et al used ferromagnetic particles that could be manipulated with a rotating magnetic field in order to enhance mixing [68]. These particles aligned similar to stir rods under low flow conditions, or as aggregates under high flow conditions, increasing their mixing enhancement potential. Figures 6(a) – (c) show the enhancement obtained in Lee’s device when using this type of particle. Along the same lines, though technically a passive enhancement, Wei and Lee numerically examined a micromixer that could be used to mix magnetic fluids, utilizing stationary, tapered magnets that enhance mixing magnetically without the use of an external magnetic field, therefore cutting down power consumption [69]. Recently, Chen et al studied a device that enhances mixing by actuating artificial cilia with embedded magnetic particles within microchannels both numerically and experimentally for multiple trajectories, as shown in figure 6(d) [70]. Zhou et al developed a similar type of micromixer that does not require the same amount of extensive hardware as the device created by Chen et al [71].
Figure 6.
(a) Fluorescence micrograph of mixing chamber without magnetic particles. No mixing enhancement occurs and the mixing relies solely on diffusion. (b) Fluorescence micrograph of mixing chamber with non-rotating magnetic particles. Mixing is enhanced but not complete using this configuration. (c) Fluorescence micrograph of mixing chamber with rotating magnetic particles. Efficient mixing is observed using this configuration. These figures are adapted from [68] with permission from the Royal Society of Chemistry. Copyright 2009 Royal Society of Chemistry. (d) Mixing enhancements observed over time when using magnetically actuated artificial cilia for three separate cilia trajectories. Adapted from [70] with permission from the Royal Society of Chemistry. Copyright 2013 Royal Society of Chemistry.
Research on microstirrers extends to fabricating micromotors to power a stirrer [15]. The main problem associated with this type of mixer is that the miniaturized motor is more difficult to fabricate than a stirrer alone operating by an external magnetic field. However, such a motor allows for easy variation of rotation speed using a computer, allowing the user to achieve precise levels of mixing within the device. For experimenters who require very precise control over the level of mixing or the shear generated by the stirrer (e.g. when the fluids are shear thinning) this type of stirrer would prove to be the best to use.
3.2. Acoustic mixing
Another unique way to promote mixing in microfluidic devices is by the use of acoustic waves. Frommelt et. al studied the use of surface acoustic waves (SAWs) to generate time-dependent flow patterns which promote mixing in microfluidic devices [72]. These SAWs are a type of elastic energy along the surface of the fluid, which can induce acoustic streaming through the fluid when excited. The produced streamlines have been shown to cause efficient mixing within systems of fluids and movement of liquid droplets even at low Reynolds numbers.
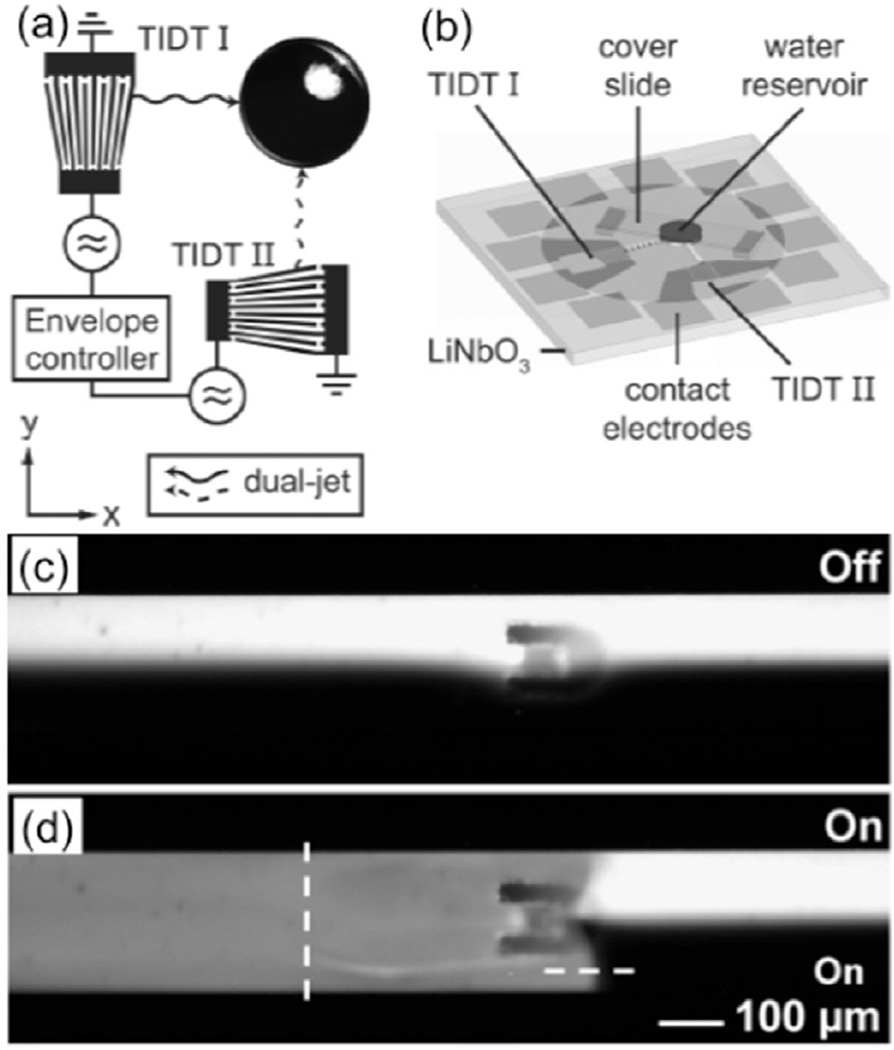
Generating SAWs across a solid surface in contact with a fluid is easily accomplished using interdigital transducers (IDTs) as shown in figure 7(a). Using this type of technology, Frommelt et al attempted to demonstrate a mixing enhancement within liquids by using SAWs at varying frequencies (figure 7(b)). The results showed that there is an optimal frequency of 0.17 Hz to be used in order to obtain the best mixing when utilizing a duel jet setup, creating a uniform distribution of tracer particles within the testing fluid. For these experiments, a droplet of water with a volume of 35 µl was used as the testing fluid. This droplet was held between two hydrophilic planes and was laterally confined by a hydrophobic sample surface. In addition to experimental results, the group was able to verify flow patterns using theoretical calculations. Acoustic-based mixing methods are a versatile technique that can easily be implemented into microfluidic devices to mix fluids within a channel or in open geometries [72].
Figure 7.
(a) Two tapered IDT (TIDT) exciting SAWs in x and y directions. An envelope controller runs predefined programs modulating the amplitude of the SAW arbitrarily. When operating in dual-jet mode, TIDT I works at constant power (solid line), and the power of TIDT II is modulated (dashed line). (b) Diagram of a SAW mixer. Reprinted figures with permission from [72]. Copyright 2008 American Physical Society. (c) A bubble is trapped inside the horseshoe structure. No mixing effect was observed in the absence of acoustic waves. (d) Homogenized mixing of water and fluorescent dye in presence of acoustic waves. The flow is from the right to the left (adapted from [73] with permission from The Royal Society of Chemistry). Copyright 2009 Royal Society of Chemistry.
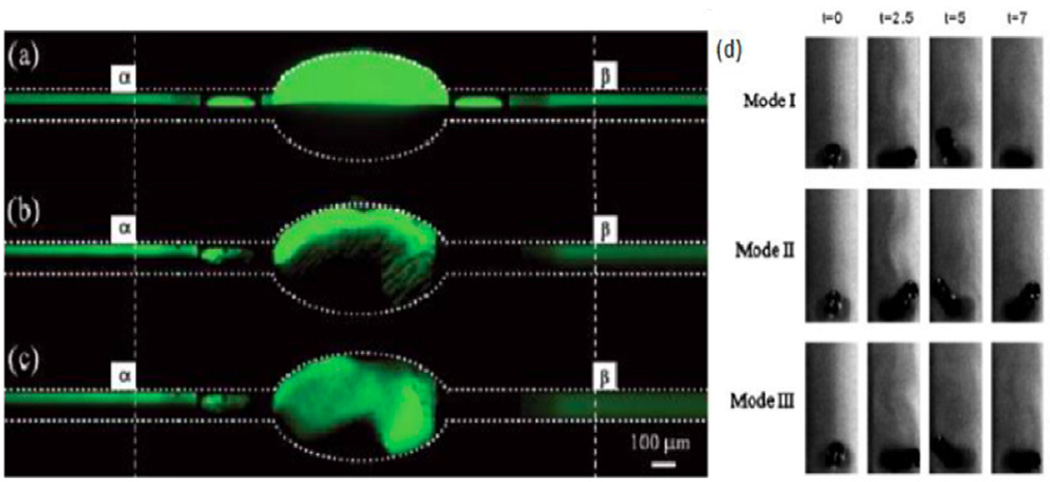
Ahmed et al combined acoustic waves with a microfeature to create a fast bubble mixer within a microchannel [73]. The first step in creating this type of mixer was to design a horseshoe shape within a channel between two fluids, opening in the same direction as the flow direction (figure 7(c)). When the fluids flowed past the horseshoe geometry, an air bubble was caught inside the horseshoe due to the streamlines of the fluids. Once this bubble was trapped, a piezoelectric transducer was used to excite the bubble to its resonance frequency using acoustic waves. This caused the bubble to oscillate and send acoustic streaming through the surrounding fluid in a similar fashion to the SAWs.
The acoustic bubble mixer operates extremely quickly, causing near homogenous mixing past the horseshoe almost instantaneously when operated at the correct frequency (figure 7(d)). Since the horseshoe geometry is relatively easy to fabricate into channels, this type of mixer could easily be used for various microfluidic devices in order to achieve mixing of an entire channel in the near millisecond regime [73]. This bubble mixer was claimed to be ‘the micromixer champion’ by Folch in his book [74]. This research group advanced the acoustic micromixer via bubble inception and cavitation for mixing high-viscosity liquids (Re ~ 0.01) by mixing high-viscosity fluids in the range of 21.2–95.9 mPa s with water [75].
The enhancement designed by Ahmed et al works very well for the enhancement of micromixing, but requires a bubble to be trapped in the device in a pre-designed and microfabricated horseshoe. To counteract this drawback, Wang et al developed a similar mixing enhancement, which introduced the bubble inside the microfluidic device by piezoelectric transducer excitations instead of manually adding the bubble [76]. This enhancement proved to be effective for high-viscosity fluids and relatively high flow rates. Petkovic-Duran et al also successfully used acoustic waves produced by low-cost and easy-to-obtain audio equipment to enhance mixing in microfluidic devices [77]. They simplified the system of Wang et al and Ahmed et al by eliminating the need for a bubble by ensuring that their system has a liquid–air interface due to an open well. This decreased the mixing time by more than an order of magnitude when compared to diffusion alone. Recently, Bezagu et al utilized ultrasonic waves to selectively vaporize a perfluorocarbon phase strategically placed between two fluids, greatly enhancing the mixing of the fluids in roughly 100 ms and allowing for an easy separation of the perfluorocarbon phase after the completion of the enhancement process [78].
3.3. Periodic fluid pulsation
Periodic fluid pulsation or periodic forcing is another method to induce mixing of two flowing fluids within a microsystem. Instead of the traditional approach of bringing two channels together with continuous flow in each channel, the flow in one or both channels is ‘pulsed’ in order to enhance mixing at the interface between the two streams. This pulse is easily achieved by varying the voltage applied across the channels in a time-dependent manner when electroosmotic pumping is used. Since the flow is not constant, streamlines are constantly changing and there are induced changes in the flow patterns of the fluids within the system [79].
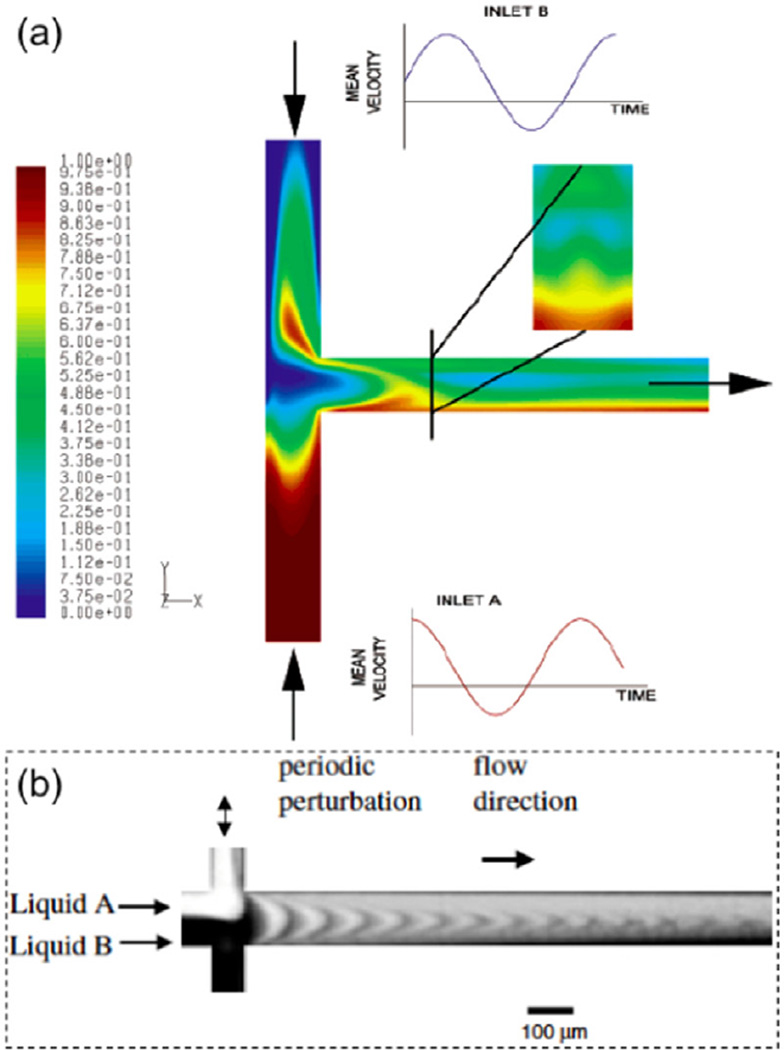
Glasgow and Aubry found that pulsing both streams is the most efficient way to utilize periodic forcing within a simple mixing channel [79]. By utilizing pulsation in both fluid channels, they achieved a degree of mixing 5 times greater than that without any pulsing. Flow pulsation from only one channel also increased the degree of mixing, but to a lesser extent. In-phase and out-of-phase pulsing were both tested, with 90° phase difference providing the best mixing. Figure 8(a) shows the numerically simulated flow patterns that are observed at a 90° phase difference between the pulsing of both streams [80].
Figure 8.
(a) Two inlets indicated by inward arrows have pulsation flows while one outlet is marked by an outward arrow. Pulsation is indicated by the graph next to each inlet, showing the mean velocity as a function of time. The phase difference is 90° between flows at two inlets. Contour plots show concentration of the liquid at half the depth of the channel and at 0.5 mm downstream of the confluence (adapted with permission from [80]. Copyright 2004 American Chemical Society). (b) Experimental results of mixing by perturbed flows generated using one pair of side channels (adapted from [81] with permission. © 2003 IOP Publishing.
This type of periodic forcing is very easy to implement within microfluidic systems, as there is no additional equipment or change in fabrication required to employ it. It works well even at very low Reynolds numbers, and it is easy to manipulate its effects on the mixing within the system by simply changing the forcing parameters. CFD code is also available for simulations in order to determine the optimal wavelengths and amplitudes of the forcing for a given system [79].
Niu and Lee also used the idea of periodic forcing to create active mixing enhancement within microchannels [81]. They created a micromixer utilizing multiple pumps for side channels perpendicular to the direction of the fluid flow. The pumps were then connected to a computer, allowing experimenters to oscillate the pressure drop over the side channel. The manipulations create a sort of ‘source–sink’ mechanism in which the fluid can be easily perturbed in a periodic fashion.
The induced perturbations within the system cause the fluids to flow oblique to the natural flow direction. Similar to the patterned ridge and slanted well methods, this flow induces chaotic mixing within the fluid when the control parameters are manipulated correctly. Chaotic mixing presents a great improvement in mixing time over traditional diffusive mixing, and thus this type of periodic forcing can be used to enhance mixing. Figure 8(b) shows the experimental results obtained using this type of mixer with one pair of side channels. As is shown, the mixing occurs rapidly once the transverse perturbations give rise to an interfacial distortion [81].
Niu and Lee used pumps to generate the perturbations in their analysis, and they also stated that the same chaotic mixing patterns could be generated using an electric field. This would potentially ease the fabrication of devices, as additional external micropumps would not need to be fabricated. In addition, this would transform the apparatus into one very similar to that studied by Glasgow and Aubry. Balasuriya studied the optimal frequency of oscillation for a similar type of enhancement, utilizing both electromagnetic and fluid pumping strategies [82].
Abolhasani et al also utilized oscillations within microfluidic devices, and observed possibilities for mixing enhancements [83]. However, they oscillated the direction of the flow back and forth in order to accomplish this enhancement. A gas was used in addition to the liquid within the channel in order to segment the fluid, and oscillatory flow was generated with electric fields. This type of mixing enhancement presents an interesting and easy-to-implement active mixer within microchannels.
3.4. Thermal mixing enhancement
Diffusion coefficients typically increase with increasing temperature. Mao et al demonstrated that linear temperature gradients could be generated within a microfluidic system across multiple parallel streams simultaneously [84]. These gradients themselves may not enhance mixing in microfluidic devices, but being able to precisely control the temperature of a fluid could help users to control the diffusion rate obtained within the streams. Though these experiments were not conducted to study mixing within the channels, the approach used to induce the temperature gradients could potentially be utilized for developing a novel active mixing enhancement method based on temperature [11].
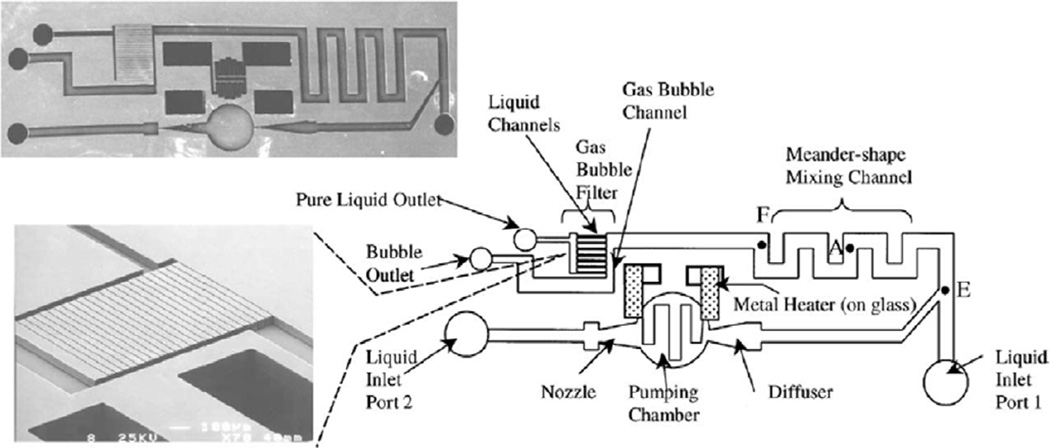
Tsai and Lin developed a device that generates wavy interfaces between the two fluids after a Y-mixer instersection by using a ‘thermal bubble actuated nozzle-diffuser micropump’, as shown in figure 9 [85]. A thermally excitable bubble is first introduced into the system between the two streams at the junction. The bubble is then excited, causing it to oscillate and enhance mixing between the two streams.
Figure 9.
Pictures of a device with schematic drawing representing components of the device. During operation, blue food dye from inlet port 1 and isopropyl alcohol from inlet port 2 mix at point E. The nozzle-diffuser-based bubble pump generates oscillatory flows intrinsically, inducing mixing effects (adapted from [85] with permission). Copyright 2002 Elsevier.
Utilizing the same property changes with temperature, Kim et al also developed a simple active mixing enhancement by utilizing temperature control [86]. Two separate heaters at two distinct locations were used in an alternating fashion within a single mixing chamber. The natural convection flow patterns that arose within the system provided a great mixing enhancement without the need for pumps. Kim et al were careful to prevent unwanted evaporation during the experiment by coating their cartridges with parylene. However, the production of vapor bubbles near a heated element would add extra agitation to the system, enhancing mixing so long as the device was structured to accommodate this phase change.
Though the use of temperature as a mixing parameter within microfluidic devices seems intuitive, this area is still largely unexplored by microfluidics community. This could be partially due to the fact that temperature gradients can damage samples or change their properties. When it is not a concern, temperature gradients could be utilized in the future as effective means of mixing enhancement alongside other methods discussed above.
3.5. Electrokinetic mixing enhancement
Electroosmosis is a commonly used pumping method for microfluidic devices. This type of pumping utilizes electrodes within the channels to create an electrical double layer (EDL). Once the EDL is formed, an external electric field applied would cause a motion of the uncharged liquid in the direction of the electric field. This type of operation can be achieved using both dc and ac currents. Electroosmotic pumping holds multiple advantages over other micropumps, such as eliminating the need for moving parts and the creation of constant flows. They are also easy to integrate into microfluidic devices, making them ideal candidates for lab-on-a-chip applications [87].
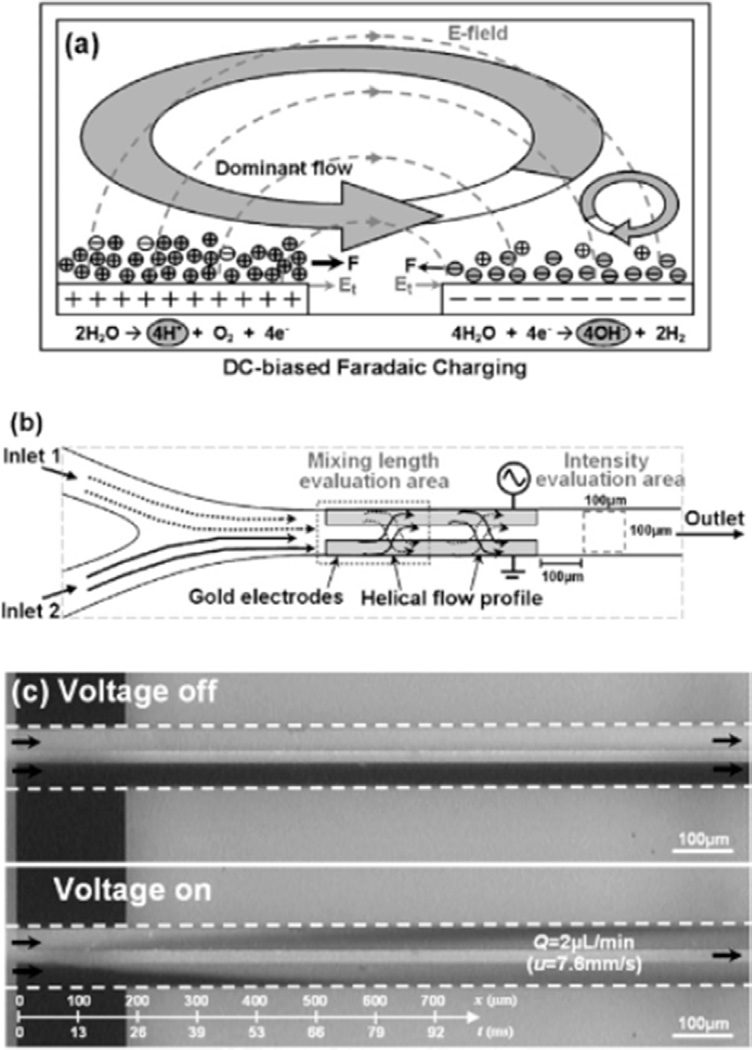
Since this type of pumping is common, it comes as no surprise that the concepts have been exploited as an active mixing enhancement for microfluidic devices. Ng et al developed an ac electrokinetic mixing approach that was able to produce a 92% mixing efficiency over a mixing length of 600 µm in less than 80 ms [88]. The mixer was constructed by placing two coplanar electrodes in a microchannel, oriented parallel to one another. An ac voltage with a dc offset was applied to the electrodes, resulting in an induced flow transverse to the flow direction. This induced flow simulated stirring in the device, and was able to quickly and efficiently mix two fluid streams. Figure 10(a) shows a pictorial representation of the induced flow, and figure 10(b) shows a general schematic for the device. The group tested the effects of varying the dc bias, the polarity, the ac frequency, and the flow rates on the mixing efficiency [88]. These adjustable parameters allow for a high level of fine-tuning when utilizing this type of enhancement. Figure 10(c) shows an image of a typical two fluid laminar flow in a microfluidic channel with and without the ac and dc voltage applied.
Figure 10.
(a) Flow profile generated by the ac electrokinetic mixer and the charge accumulation on the surface of the electrodes. (b) Schematic of the micromixer device and flow profile generation. (c) Laminar flow of two fluids in a microfluidic channel with (bottom) and without (top) the ac and dc voltage components applied. A clear enhancement of the stream mixing can be seen over small distances when the voltages are applied. These figures were adapted from [88] with permission. Copyright 2009 Royal Society of Chemistry.
In a similar manner, Sugioka’s numerical generation of chaotic advection described in section 2.2 utilized induced-charge electroosmosis in conjunction with strategically placed vertical posts to enhance mixing [40]. Both Ng and Sugioka were able to use very low voltages (~1−30 V) to provide adequate mixing enhancement. Tang et al were also able to numerically examine some common mixing enhancements that can be used in conjunction with electroosmotic flow, including wall blocks in the microchannel similar to Sugioka’s posts, as well as an active modification of the zeta potential of the channel surfaces similar to the approach taken by Chen and Cho [40, 63, 89]. Recently, Choi et al effectively enhanced mixing using electrokinetic methods to generate vortexes near an assembled nanoparticle interface [90].
Since electrodes are commonly integrated into microfluidic devices, electrokinetic mixing enhancements are easy to implement, and often do not require large voltages to operate effectively. Moreover, the common use of electroosmotic pumping means that many microfluidic devices already implement the tools necessary for this type of mixing enhancement. However, there are drawbacks to using this type of enhancement, such as portability and the potential to create bubbles within the device when utilizing a dc voltage.
3.6. Other types of active mixing enhancement
In addition to those discussed above, many other forms of active mixing enhancement can be used within microfluidic devices. Aeinehvand et al utilized microballoons inside a centrifugal microfluidic device to enhance mixing [91]. This enhancement was obtained through oscillatory expansion and contraction of the balloon in order to alter the fluid flow. Bynum and Gordon utilized a dual-axis centrifuge to produce a constantly changing meniscus within a microfluidic device, which enhances the mixing properties even for a very thin channel [92]. Berenguel-Alonso et al also successfully created a simple magnetic bead actuator that can be used for microfluidic devices to enhance mixing [93], and Zhu and Nguyen have studied mixing enhancement using ferrofluids and uniform magnetic fields [94]. Overall, the possibilities for active microfluidic mixing enhancement are endless, and the field is still an area of active research.
4. Conclusions
Microfluidic devices have a variety of applications in the chemical and biological fields. They offer a portable and low-cost alternative to experiments/operations typically conducted in a fully equipped scientific laboratory. However, microfluidic devices suffer from inefficient mixing when required for certain applications. This review discusses numerous passive and active techniques to enhance mixing to address this challenge. Table 1 summarizes the enhancement techniques discussed, their advantages and disadvantages, and provides recommendations for their incorporation into microfluidic devices. Enhanced mixing will allow for shorter channels to be used within a device and for the realization of more efficient lab-on-a-chip systems.
Table 1.
Summary of mixing enhancements presented in the review.
| Methods | Pros | Cons | Suggestions/Comments |
|---|---|---|---|
| Passive micromixers | |||
| Stream splitting and recombination |
Basic, but more effective than pure diffusive mixing |
May require long channels for complex micromixer network |
Pay careful attention to the method of recombination; adequate mixing may result without additional elements. |
| Slanted wells or patterned grooves |
Easy to fabricate (no moving parts), CFD code available for optimization |
Requires additional fabrication to adjust the level of mixing |
Useful for mixing enhancement when precise control is not required. |
| Hydrophobicity or surface modification |
Additional mixing enhancement over other passive mixers |
May be complicated to define surface modification zones |
Combining with other passive mixers to maximize mixing potential |
| Multiphase mixing enhancement |
Low fabrication cost, good mixing with little sample dilution, and large mixing property change by adjusting flow rates |
Requires additional purification step as immiscible carrier liquids or gas must be introduced into the device |
Consider for use if introducing an additional fluid into a device is possible without hindering device operation. |
| Active micromixers | |||
| Microstirrers | Versatile; excellent mixing with precise control |
Difficult to fabricate (moving parts); may require multiple stirrers |
Useful for applications requiring precise control over the level of mixing; magnetic actuation easier than mechanical actuation. Utilizing magnetic particles may offset some of the fabrication difficulties. |
| Acoustic waves | Nearly instantaneous mixing; easy to operate |
Need a bubble or air interface | Consider acoustic mixers if quick mixing is needed |
| Flow pulsation | Easy to implement using micropumps or electric fields |
Requires fine-tuning to achieve optimal mixing enhancement |
Convenient when pumps are integrated or do not compromise operation |
| Thermal enhancement | Simple to integrate into a device | Requires heaters | Consider thermal micromixers where heat does not affect samples/reagents |
| Electrokinetic mixing enhancement |
Can be utilized effectively at relatively low voltages. Can be very effective over short mixing lengths. |
Requires integrated electrodes | Consider when electrodes are integrated into the device. Utilize ac voltages to avoid bubble formation. |
Using the methods discussed above, one can greatly enhance the degree of mixing that is limited by the diffusive mixing impairment inherently found in microchannels. The method of mixing enhancement that should be chosen depends heavily on the application. There is no one mixer that will be the best choice regardless of the scenario. For some applications, these current mixing enhancements could be enough for effective utilization of the microfluidic devices. However, more complex systems may require mixing on a smaller timescale or in a more specific matter, and therefore there is still an interest in the field for developing more efficient mixing enhancement methods.
Simple yet effective mixing methods like periodic forcing or thermal mixing enhancement could potentially lead to efficient methodologies when combined with other enhancement methods. The draw of this type of mixing enhancement is that the necessary instrumentation is inherently included in many microfluidic devices, as it is required for the device’s operation. Future research may include efforts on developing novel enhancements using components that are already commonly found in microfluidic devices. This type of enhancement cuts down fabrication costs and simplifies device operation, as no additional fabrication steps or moving parts are necessary.
Creating chaotic advection within the channels of a device is a reliable way to enhance mixing. Passively, there is room for improvement in this area through the development of new structures or surface modifications that will allow for the onset of this phenomenon without creating large amounts of dead volume in the channel. Ideally, scientists would be able to fabricate these structures at low extra cost, adding an effective mixing enhancement to devices quickly and inexpensively. Enhancements that incorporate both passive and active elements could also lead to discoveries of novel micromixers [95]. The field of microfluidics is broad and the applications are vast and continually growing, hence micromixer development will remain an important area of research within the scientific community. With development of new miniaturized devices, the application-specific needs for mixing enhancement will continually evolve, and an understanding of the underlying physics of the microflows will become crucial to the field’s expansion.
Acknowledgments
KLW acknowledges financial support from the National Science Foundation PIRE Grant (OISE-0968313), and the University of Florida (UF) Graduate Research Assistantship.
ZHF acknowledges financial support from the National Institute of Health (R21GM103535 and K25CA149080), and the National Science Foundation (OISE-0968313 and DBI-1353423), and thanks the University of Florida (UF) for a Research Foundation Professorship and Preparatory Grant Program Award, and UF Health Cancer Center for a Pilot Project Award.
References
- 1.Lo R. Application of microfluidics in chemical engineering. Chem. Eng. Process. Tech. 2013;1:1002. [Google Scholar]
- 2.Lee C, Chang C, Wang Y, Fu L. Microfluidic mixing: a review. Int. J. Mol. Sci. 2011;12:3263. doi: 10.3390/ijms12053263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hessel V, Löwe H, Schönfield F. Micromixers—a review on passive and active mixing principles. Chem. Eng. Sci. 2005;60:2479. [Google Scholar]
- 4.Aubin J, Ferrando M, Jiricny V. Current methods for characterising mixing and flow in microchannels. Chem. Eng. Sci. 2010;65:2065. [Google Scholar]
- 5.Kockmann N. Convective micromixers—design and industrial applications. J. Mech. Eng. Sci. 2008;222:807. [Google Scholar]
- 6.Stone H, Kim S. Microfluidics: basic issues, applications, and challenges. AIChE J. 2001;47:1250. [Google Scholar]
- 7.Stroock A, Dertinger SKW, Ajdari A, Mezic I, Stone H, Whitesides G. Chaotic mixer for microchannels. Science. 2002;295:647. doi: 10.1126/science.1066238. [DOI] [PubMed] [Google Scholar]
- 8.Kumar V, Paraschivoiu M, Nigam KDP. Single-phase fluid flow and mixing in microchannels. Chem. Eng. Sci. 2011;66:1329. [Google Scholar]
- 9.Wang H, Iovenitti P, Harvey E, Masood S. Numerical investigation of mixing in microchannels with patterned grooves. J. Micromech. Microeng. 2003;13:801. [Google Scholar]
- 10.Lu L, Ryu K, Liu C. A novel microstirrer and arrays for microfluidic mixing. In: Ramsey JM, van den Berg A, editors. Micro Total Analysis Systems. Berlin: Springer; 2001. pp. 28–30. [Google Scholar]
- 11.Nguyen N, Wu Z. Micromixers—a review. J. Micromech. Microeng. 2005;15:R1. [Google Scholar]
- 12.Mensing G, Pearce T, Graham M, Beebe D. An externally driven magnetic microstirrer. Phil. Trans. A. 2004;362:1059. doi: 10.1098/rsta.2003.1362. [DOI] [PubMed] [Google Scholar]
- 13.Capretto L, Cheng W, Hill M, Zhang X. Micromixing within microfluidic devices. Top. Curr. Chem. 2011;304:27. doi: 10.1007/128_2011_150. [DOI] [PubMed] [Google Scholar]
- 14.Lu L-H, Ryu K, Liu C. A magnetic microstirrer and array for microfluidic mixing. J. Microelectromech. Syst. 2002;11:462. [Google Scholar]
- 15.Barbic M, Mock J, Gray A, Schultz S. Electromagnetic micromotor for microfluidics applications. Appl. Phys. Lett. 2001;79:1399. [Google Scholar]
- 16.Campbell C, Grzybowski B. Microfluidic mixers: from microfabricated to self-assembling devices. Phil. Trans. R. Soc. Lond. A. 2004;362:1069. doi: 10.1098/rsta.2003.1363. [DOI] [PubMed] [Google Scholar]
- 17.Mansur E, Mingxing Y, Yundong W, Youyuan DAI. A state-of-the-art review of mixing in microfluidic mixers. Chin. J. Chem. Eng. 2008;16:503. [Google Scholar]
- 18.Meijer H, Singh M, Kang T, den Toonder J, Anderson P. Passive and active mixing in microfluidic devices. Macromol. Symp. 2009;279:201. [Google Scholar]
- 19.Chang C, Yang R. Electrokinetic mixing in microfluidic systems. Microfluid. Nanofluidics. 2007;3:501. [Google Scholar]
- 20.Jeong G, Chung S, Kim C, Lee S. Applications of micromixing technology. Analyst. 2010;135:460. doi: 10.1039/b921430e. [DOI] [PubMed] [Google Scholar]
- 21.Chen L, Wang G, Lim C, Hun Seong G, Choo J, Lee E, Kang S, Song J. Evaluation of passive mixing behaviors in a pillar obstruction poly(dimethylsiloxane) microfluidic mixer using fluorescence microscopy. Microfluid. Nanofluidics. 2009;7:267. [Google Scholar]
- 22.Yasui T, Omoto Y, Osato K, Kaji N, Suzuki N, Naito T, Okamoto Y, Tokeshi M, Shamoto E, Baba Y. Confocal microscopic evaluation of mixing performance for 3D microfluidic mixer. Anal. Sci. 2012;28:57. doi: 10.2116/analsci.28.57. [DOI] [PubMed] [Google Scholar]
- 23.Fang W, Hsu M, Chen YT, Yang J. Characterization of microfluidic mixing and reaction in microchannels via analysis of cross-sectional patterns. Biomicrofluidics. 2011;5:014111. doi: 10.1063/1.3571495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kockmann N, Karlen S, Girard C, Roberge D. Liquid–liquid test reactions to characterize two-phase mixing in microchannels. Heat Transfer Eng. 2013;34:169. [Google Scholar]
- 25.Schwesinger N, Frank T, Wurmus H. A modular microfluid system with an integrated micromixer. J. Micromech. Microeng. 1996;6:99. [Google Scholar]
- 26.Bessoth F, deMello A, Manz A. Microstructure for efficient continuous flow mixing. Anal. Commun. 1999;36:213. [Google Scholar]
- 27.You J, Kang K, Tran T, Park H, Hwang W, Kim J, I SG. PDMS-based turbulent microfluidic mixer. Lab Chip. 2015;15:1727. doi: 10.1039/c5lc00070j. [DOI] [PubMed] [Google Scholar]
- 28.Haverkamp V, et al. The potential of micromixers for contacting of disperse liquid phases. Fresenius J. Anal. Chem. 1999;364:617. [Google Scholar]
- 29.Kim D, Lee S, Kwon T, Ahn C. A serpentine laminating micromixer combining splitting/recombination and advection. Lab Chip. 2005;5:739. doi: 10.1039/b418314b. [DOI] [PubMed] [Google Scholar]
- 30.Hessel V, Hardt S, Löwe H, Schönfield F. Laminar mixing in different interdigital micromixers: I. Experimental characterization. AIChE J. 2003;49:566. [Google Scholar]
- 31.Lim C, Lam Y, Yang C. Mixing enhancement in microfluidic channel with a constriction under periodic electro-osmotic flow. Biomicrofluidics. 2010;4:014101. doi: 10.1063/1.3279790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin Y, Chung Y, Wu C. Mixing enhancement of the passive microfluidic mixer with J-shaped baffles in the tee channel. Biomed. Microdevices. 2007;9:215. doi: 10.1007/s10544-006-9023-5. [DOI] [PubMed] [Google Scholar]
- 33.Su Y, Chen G, Kenig E. An experimental study on the numbering-up of microchannels for liquid mixing. Lab Chip. 2015;15:179. doi: 10.1039/c4lc00987h. [DOI] [PubMed] [Google Scholar]
- 34.Lim T, et al. Three-dimensionally crossing manifold micro-mixer for fast mixing in a short channel length. Lab Chip. 2011;11:100. doi: 10.1039/c005325m. [DOI] [PubMed] [Google Scholar]
- 35.Dreher S, Kockmann N, Woias P. Characterization of laminar transient flow regimes and mixing in T-shaped micromixers. Heat Transfer Eng. 2009;30:91. [Google Scholar]
- 36.Li Y, Liu C, Feng X, Xu Y, Liu B. Ultrafast microfluidic mixer for tracking the early folding kinetics of human telomere G-quadruplex. Anal. Chem. 2014;86:4333. doi: 10.1021/ac500112d. [DOI] [PubMed] [Google Scholar]
- 37.Johnson T, Ross D, Locascio L. Rapid microfluidic mixing. Anal. Chem. 2002;74:45. doi: 10.1021/ac010895d. [DOI] [PubMed] [Google Scholar]
- 38.Aref H. Stirring by chaotic advection. J. Fluid Mech. 1984;143:1. [Google Scholar]
- 39.Stroock A, Whitesides G. Controlling flows in microchannels with patterned surface charge and topography. Acc. Chem. Res. 2003;36:597. doi: 10.1021/ar0202870. [DOI] [PubMed] [Google Scholar]
- 40.Sugioka H. Chaotic mixer using electro-osmosis at finite péclet number. Phys. Rev. E. 2010;81:036306. doi: 10.1103/PhysRevE.81.036306. [DOI] [PubMed] [Google Scholar]
- 41.Stremler M, Haselton F, Aref H. Designing for chaos: applications of chaotic advection at the microscale. Phil. Trans. R. Soc. Lond. A. 2004;362:1019. doi: 10.1098/rsta.2003.1360. [DOI] [PubMed] [Google Scholar]
- 42.Xia Z, Cattafesta L, Fan ZH. Deconvolution microscopy for flow visualization in microchannels. Anal. Chem. 2007;79:2576. doi: 10.1021/ac062265n. [DOI] [PubMed] [Google Scholar]
- 43.Mei Q, Xia Z, Xu F, Soper S, Fan ZH. Fabrication of microfluidic reactors and mixing studies for luciferase detection. Anal. Chem. 2008;80:6045. doi: 10.1021/ac800843v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marschewski J, et al. Mixing with herringbone-inspired microstructures: Overcoming the diffusion limit in co-laminar microfluidic devices. Lab Chip. 2015;15:1923. doi: 10.1039/c5lc00045a. [DOI] [PubMed] [Google Scholar]
- 45.Howell J, et al. A microfluidic mixer with grooves placed on the top and bottom of the channel. Lab Chip. 2005;5:524. doi: 10.1039/b418243j. [DOI] [PubMed] [Google Scholar]
- 46.Yang Y-J, et al. Novel designs of herringbone chaotic mixers; 1st IEEE Int. Conf. on Nano/Micro Engineered and Molecular Systems, 2006 NEMS, ‘06; 2006. pp. 1307–1310. [Google Scholar]
- 47.Lin D, et al. 3D staggered herringbone mixer fabricated by femtosecond laser direct writing. J. Opt. 2013;15:025601. [Google Scholar]
- 48.Forbes T, Kralj J. Engineering and analysis of surface interactions in a microfluidic herringbone micromixer. Lab Chip. 2012;12:2634. doi: 10.1039/c2lc40356k. [DOI] [PubMed] [Google Scholar]
- 49.Lynn N, Jr, et al. Biosensing enhancement using passive mixing structures for microarray-based sensors. Biosensors Bioelectron. 2014;54:506. doi: 10.1016/j.bios.2013.11.027. [DOI] [PubMed] [Google Scholar]
- 50.Lynn N, Dandy D. Geometrical optimization of helical flow in grooved micromixers. Lab Chip. 2007;7:580. doi: 10.1039/b700811b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Z, Yim C, Lin M, Cao X. Quantitative characterization of micromixing simulation. Biomicrofluidics. 2008;2:034104. doi: 10.1063/1.2966454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams M, Longmuir K, Yager P. A practical guide to the staggered herringbone mixer. Lab Chip. 2008;8:1121. doi: 10.1039/b802562b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xia Z, Mei R, Sheplak M, Fan ZH. Electroosmotically driven creeping flows in a wavy microchannel. Microfluid. Nanofluidics. 2009;6:37. [Google Scholar]
- 54.Song H, Bringer M, Tice J, Gerdts C, Ismagilov R. Experimental test of scaling of mixing by chaotic advection in droplets moving through microfluidic channels. Appl. Phys. Lett. 2003;83:4664. doi: 10.1063/1.1630378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tice J, Song H, Lyon A, Ismagilov R. Formation of droplets and mixing in multiphase microfluidics at low values of the Reynolds and the capillary numbers. Langmuir. 2003;19:9127. [Google Scholar]
- 56.Gunther A, Jhunjhunwala M, Thalmann M, Schmidt M, Jensen K. Micromixing of miscible liquids in segmented gas–liquid flow. Langmuir. 2005;21:1547. doi: 10.1021/la0482406. [DOI] [PubMed] [Google Scholar]
- 57.Garstecki P, Fuerstman M, Fischbach M, Sia S, Whitesides G. Mixing with bubbles: a practical technology for use with portable microfluidic devices. Lab Chip. 2006;6:207. doi: 10.1039/b510843h. [DOI] [PubMed] [Google Scholar]
- 58.Tangen U, Sharma A, Wagler P, McCaskill J. On demand nanoliter-scale microfluidic droplet generation, injection, and mixing using a passive microfluidic device. Biomicrofluidics. 2015;9:014119. doi: 10.1063/1.4907895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dogan H, Nas S, Muradoglu M. Mixing of miscible liquids in gas-segmented serpentine channels. Int. J. Multiphase Flow. 2009;35:1149. [Google Scholar]
- 60.Che Z, Nguyen N, Wong T. Analysis of chaotic mixing in plugs moving in meandering microchannels. Phys. Rev. E. 2011;84:066309. doi: 10.1103/PhysRevE.84.066309. [DOI] [PubMed] [Google Scholar]
- 61.Ou J, Moss G, Rothstein J. Enhanced mixing in laminar flows using ultrahydrophobic surfaces. Phys. Rev. E. 2007;76:016304. doi: 10.1103/PhysRevE.76.016304. [DOI] [PubMed] [Google Scholar]
- 62.Ou J, Perot B, Rothstein J. Laminar drag reduction in microchannels using ultrahydrophobic surfaces. Phys. Fluids. 2004;16:4635. [Google Scholar]
- 63.Chen C, Cho C. Electrokinetically-driven flow mixing in microchannels with wavy surface. J. Colloid Interface Sci. 2007;312:470. doi: 10.1016/j.jcis.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 64.Wang J, Liu Y, Xu Y. The golden-mean surface pattern to enhance flow mixing in micro-channel. Biomed. Microdevices. 2009;11:351. doi: 10.1007/s10544-008-9241-0. [DOI] [PubMed] [Google Scholar]
- 65.Kamio E, Ono T, Yoshizawa H. Design of a new static micromixer having simple structure and excellent mixing performance. Lab Chip. 2009;9:1809. doi: 10.1039/b817536e. [DOI] [PubMed] [Google Scholar]
- 66.Kockmann N, Robergeb D. Scale-up concept for modular microstructured reactors based on mixing, heat transfer, and reactor safety. Chem. Eng. Process.: Process Intensification. 2011;50:1017. [Google Scholar]
- 67.Tierno P, Johansen T, Fischer T. Magnetically driven colloidal microstirrer. J. Phys. Chem. B. 2007;111:3077. doi: 10.1021/jp070579o. [DOI] [PubMed] [Google Scholar]
- 68.Lee S, van Noort D, Lee J, Zhang B, Park T. Effective mixing in a microfluidic chip using magnetic particles. Lab Chip. 2009;9:479. doi: 10.1039/b814371d. [DOI] [PubMed] [Google Scholar]
- 69.Wei Z, Lee C. Magnetic fluid micromixer with tapered magnets. J. Appl. Phys. 2009;105:07B523. [Google Scholar]
- 70.Chen C, Chen C, Lina C, Hua Y. Magnetically actuated artificial cilia for optimum mixing performance in microfluidics. Lab Chip. 2013;13:2834. doi: 10.1039/c3lc50407g. [DOI] [PubMed] [Google Scholar]
- 71.Zhou B, et al. Design and fabrication of magnetically functionalized flexible micropillar arrays for rapid and controllable microfluidic mixing. Lab Chip. 2015;15:2125. doi: 10.1039/c5lc00173k. [DOI] [PubMed] [Google Scholar]
- 72.Frommelt T, Kostur M, Wenzel-Schafer M, Talkner P, Hanggi P, Wixforth A. Microfluidic mixing via acoustically driven chaotic advection. Phys. Rev. Lett. 2008;100:034502. doi: 10.1103/PhysRevLett.100.034502. [DOI] [PubMed] [Google Scholar]
- 73.Ahmed D, Mao X, Shi J, Juluri B, Huang T. A millisecond micromixer via single-bubble-based acoustic streaming. Lab Chip. 2009;9:2738. doi: 10.1039/b903687c. [DOI] [PubMed] [Google Scholar]
- 74.Folch A. Introduction to BioMEMS. 1st edn. Boca Raton, FL: CRC Press; 2012. [Google Scholar]
- 75.Ozcelik A, et al. An acoustofluidic micromixer via bubble inception and cavitation from microchannel sidewalls. Anal. Chem. 2014;86:5083. doi: 10.1021/ac5007798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang S, Huang X, Yang C. Mixing enhancement for high viscous fluids in a microfluidic chamber. Lab Chip. 2011;11:2081. doi: 10.1039/c0lc00695e. [DOI] [PubMed] [Google Scholar]
- 77.Petkovic-Duran K, Manasseh R, Zhu Y, Ooi A. Chaotic micromixing in open wells using audio-frequency acoustic microstreaming. Biotechniques. 2009;47:827. doi: 10.2144/000113242. [DOI] [PubMed] [Google Scholar]
- 78.Bezagu M, et al. A fast and switchable microfluidic mixer based on ultrasound-induced vaporization of perfluorocarbon. Lab Chip. 2015;15:2025. doi: 10.1039/c5lc00247h. [DOI] [PubMed] [Google Scholar]
- 79.Glasgow I, Aubry N. Enhancement of microfluidic mixing using time pulsing. Lab Chip. 2003;3:114. doi: 10.1039/b302569a. [DOI] [PubMed] [Google Scholar]
- 80.Glasgow I, Lieber S, Aubry N. Parameters influencing pulsed flow mixing in microchannels. Anal. Chem. 2004;76:4825. doi: 10.1021/ac049813m. [DOI] [PubMed] [Google Scholar]
- 81.Niu X, Lee Y. Efficient spatial-temporal chaotic mixing in microchannels. J. Micromech. Microeng. 2003;13:454. [Google Scholar]
- 82.Balasuriya S. Optimal frequency for microfluidic mixing across a fluid interface. Phys. Rev. Lett. 2010;105:064501. doi: 10.1103/PhysRevLett.105.064501. [DOI] [PubMed] [Google Scholar]
- 83.Abolhasani M, Oskooei A, Klinkova A, Kumacheva E, Gunther A. Shaken, and stirred: oscillatory segmented flow for controlled size-evolution of colloidal nanomaterials. Lab Chip. 2014;14:2309. doi: 10.1039/c4lc00131a. [DOI] [PubMed] [Google Scholar]
- 84.Mao H, Yang T, Cremer P. A microfluidic device with a linear temperature gradient for parallel and combinatorial measurements. J. Am. Chem. Soc. 2002;124:4432. doi: 10.1021/ja017625x. [DOI] [PubMed] [Google Scholar]
- 85.Tsai J, Lin L. Active microfluidic mixer and gas bubble filter driven by thermal bubble micropump. Sensors Actuators A. 2002;97–98:665. [Google Scholar]
- 86.Kim S, Wang F, Burns M, Kurabayashi K. Temperature-programmed natural convection for micromixing and biochemical reaction in a single microfluidic chamber. Anal. Chem. 2009;81:4510. doi: 10.1021/ac900512x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang X, Cheng C, Wang S, Liu S. Electroosmotic pumps and their applications in microfluidic systems. Microfluid. Nanofluidics. 2009;6:145. doi: 10.1007/s10404-008-0399-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ng W, Goh S, Lam Y, Yang C, Rodríguez I. DC-biased ac-electroosmotic and ac-electrothermal flow mixing in microchannels. Lab Chip. 2009;9:802. doi: 10.1039/b813639d. [DOI] [PubMed] [Google Scholar]
- 89.Tang G, He Y, Tao W. Numerical analysis of mixing enhancement for micro-electroosmotic flow. J. Appl. Phys. 2010;107:104906. [Google Scholar]
- 90.Choi E, Kwon K, Lee S, Kim D, Park J. Non-equilibrium electrokinetic micromixer with nanochannel networks. Lab Chip. 2015;15:1794. doi: 10.1039/c4lc01435a. [DOI] [PubMed] [Google Scholar]
- 91.Aeinehvand MM, et al. Biosensing enhancement of dengue virus using microballoon mixers on centrifugal microfluidic platforms. Biosensors Bioelectron. 2015;67:424. doi: 10.1016/j.bios.2014.08.076. [DOI] [PubMed] [Google Scholar]
- 92.Bynum M, Gordon G. Hybridization enhancement using microfluidic planetary centrifugal mixing. Anal. Chem. 2004;76:7039. doi: 10.1021/ac048840+. [DOI] [PubMed] [Google Scholar]
- 93.Berenguel-Alonso M, Granados X, Faraudo J, Alonso-Chamarro J, Puyol M. Magnetic actuator for the control and mixing of magnetic bead-based reactions on-chip. Anal. Bioanal. Chem. 2014;406:6607. doi: 10.1007/s00216-014-8100-5. [DOI] [PubMed] [Google Scholar]
- 94.Zhu G, Nguyen N. Rapid magnetofluidic mixing in a uniform magnetic field. Lab Chip. 2012;12:4772. doi: 10.1039/c2lc40818j. [DOI] [PubMed] [Google Scholar]
- 95.Yan D, Yang C, Miao J, Lam Y, Huang X. Enhancement of electrokinetically driven microfluidic T-mixer using frequency modulated electric field and channel geometry effects. Electrophoresis. 2009;30:3144. doi: 10.1002/elps.200900162. [DOI] [PubMed] [Google Scholar]