Abstract
Influenza is a highly contagious and debilitating disease that imposes an excess burden of complications and mortality. Antiviral therapy is the primary intervention for treatment and post‐exposure prophylaxis (PEP) of influenza. Amantadine and rimantadine are members of the M2 class of antiviral agents and are moderately effective in influenza management. However, their utility is compromised by high levels of resistance, tolerability concerns and a lack of efficacy against influenza B. An alternative class of agents, the neuraminidase inhibitors (NIs), represent the most advanced form of antiviral therapy available, and act by specifically inhibiting the neuraminidase enzymes that are present on all influenza subtypes. Two NIs, oseltamivir and zanamivir, are currently available for clinical use. Oseltamivir, the most widely used NI, is administered orally as a prodrug (oseltamivir carboxylate) and systemically distributed to all potential infection sites. Zanamivir, a second NI, is administered by inhalation via a disk inhaler and deposited primarily in the respiratory tract. When administered within 48 hours of symptom onset, both agents significantly reduce illness duration and symptom severity, and decrease the rate of influenza‐associated complications. With oseltamivir, greater benefits are detected with earlier treatment initiation (<12 hours). In PEP, both NIs effectively protect the close contacts of index cases from symptomatic influenza. Oseltamivir and zanamivir are generally well tolerated and associated with a low level of resistance. Emerging evidence supports the activity of both NIs against the H5N1avian influenza infection, which is a pandemic candidate. However, the WHO currently recommends the use of oseltamivir for the management of suspected cases, given the systemic nature of the H5N1 challenge. Ongoing studies are exploring the effectiveness of oseltamivir, zanamivir and other NIs for pandemic management.
Keywords: Epidemic, influenza, neuraminidase inhibitors, oseltamivir, pandemic, zanamivir
Introduction
Influenza is a highly contagious viral disease that imposes a sizeable burden on society. Complications are common, and often result in an excess of hospitalizations and mortality. 1 Immunization is the cornerstone of current prevention strategies, given its efficacious nature and good tolerability, and is widely recommended for patients at high risk for complications (e.g. elderly patients or those with comorbid conditions). However, vaccination offers only limited protection for some patient groups (e.g. immunocompromised individuals) and circumstances (e.g. where the antigenic constituents are poorly matched with circulating strains), and is not a viable intervention following infection. 2 , 3
Antiviral therapy is the primary treatment option for influenza. Chemoprophylaxis can also be a useful complement to vaccination in populations where immunization is likely to offer limited protection, such as the frail elderly and immunocompromised. Both concepts were first evaluated with the M2 blockers, amantadine and rimantidine, some four decades ago. At this time, there was general scepticism that an antiviral could provide meaningful benefits in patients showing the symptoms of influenza. This belief was rapidly dispelled by the positive findings of early studies that demonstrated the therapeutic usefulness of the class. Subsequent investigations established the concept of post‐exposure prophylaxis and clearly demonstrated that judicious use could limit the spread of infection within family environments. Despite these positive early findings, their attractiveness for use in influenza management was compromised by the rapid emergence of resistance, together with a relatively low therapeutic index and lack of effect against the influenza B virus. 4
Today, an alternative class of the antiviral agents, the neuraminidase inhibitors, represent the most advanced form of antiviral therapy available. They act by potently and specifically inhibiting the neuraminidase enzymes present on all influenza subtypes, which are essential for the release of viral particles from host cells and the propagation of infection. When administered within 48 h of symptom onset, neuraminidase inhibitors significantly reduce illness duration and symptom severity, and also decrease the incidence of influenza‐associated complications, such as pneumonia, bronchitis and otitis media. When used for prophylaxis, they are also highly effective in limiting the spread of infection, especially in close proximity environments, such as households and care facilities. In both settings, neuraminidase inhibitors are generally well tolerated, without imposing a significant burden of adverse events. 5 , 6
The imminent threat of a new influenza pandemic has fostered renewed interest in antiviral therapy. Oseltamivir is currently the most widely used neuraminidase inhibitor, and is indicated for the treatment and prophylaxis of influenza infections in children (aged ≥1 year) and adults. Alongside zanamivir and the older M2 blockers, it is expected to play a key role in the defence against pandemic influenza, both in the management of index cases and the containment of localized outbreaks. Indeed, the World Health Organization (WHO) recommend early treatment with oseltamivir in cases of confirmed or strongly suspected H5N1 infection. 7 Confidence in the neuraminidase inhibitor class of antiviral agents is based on the robust and comprehensive development programmes conducted for the first two drugs of the series (zanamivir and oseltamivir). Here, experience from the oseltamivir programme is presented as a model system for the development of an effective antiviral for ubiquitous use in epidemic (seasonal) and pandemic influenza. Insights on zanamivir, the first neuraminidase inhibitor to be discovered, and peramivir, the third member of the neuraminidase inhibitor class, are also presented.
Chemical structure and mode of action
Oseltamivir [(3R, 4R, 5S)‐4‐acetylamino‐5‐amino‐3‐(1‐ethylpropoxyl)1‐cyclohexene‐1‐carboxylic acid, ethyl ester, phosphate (1:1)] is an ethyl ester prodrug that is rapidly metabolized by hepatic esterases to its active metabolite, oseltamivir carboxylate, following oral administration. As for zanamivir and peramivir, it was discovered through a process of rational drug design and was specifically designed to bind and inhibit the active site of the influenza virus neuraminidase enzyme (Figure 1). In this way, the neuraminidase inhibitors halt the spread of infection within the host, while allowing an immune response to be raised against the virus, preventing future infection.
Figure 1.
The binding of oseltamivir carboxylate to the active site of neuraminidase (Hoffmann La Roche, 5 data on file).
Preclinical evaluation
As with all new agents, the therapeutic potential of the neuraminidase inhibitors was first explored in the laboratory. In vitro, oseltamivir carboxylate was found to be a potent and selective inhibitor of the viral neuraminidases of various influenza subtypes, with IC50s ≤ 2.0 nm and inhibitory constants (K i) ≤ 1.2 nm. 8 In ferrets infected with influenza A, oseltamivir (25 mg/kg/day initiated 2 h post‐infection and continued for 3 days thereafter) reduced viral titres in nasal washes, decreased the severity and duration of fever and reduced the likelihood of various symptoms of influenza, including lethargy and sneezing, and increased nasal discharge. 8 In mice exposed to various strains of influenza, oseltamivir treatment (1 or 10 mg/kg/day initiated 4 h post‐infection and continued for 5 days) significantly increased the rate of survival compared with placebo‐treated animals (Table 1). 9 A reduced viral titre in lung homogenates and improved survival (80–100% vs. 11–31% in placebo‐treated mice; P < 0.01) were also observed with oseltamivir (10 mg/kg/day b.i.d. for 5 days) in mice infected with influenza A (H1N1 and H3N2) or influenza B. 8 Oseltamivir carboxylate was also effective against the avian influenza subtypes H5N1 and H9N2 in mice. 10 Zanamivir is also an effective inhibitor of various influenza A and B strains. Compared with oseltamivir carboxylate, zanamivir has similar or slightly lower potency against the same influenza strains (IC50s < 5 nm). 8 , 11
Table 1.
Effect of oseltamivir on survival in mice infected with influenza A or B
| Virus | Treatment dose (mg/kg) | Survival (n, %) | P‐value (vs. control) |
|---|---|---|---|
| A/Victoria/3/79 (H3N2) | 10 | 8/8 (100) | <0.01 |
| 0 | 5/16 (31) | ||
| A/Shangdong/09/93 (H3N2) | 10 | 9/10 (90) | <0.01 |
| 1 | 3/10 (30) | <0.05 | |
| 0 | 0/20 (0) | ||
| B/Hong Kong/5/72 | 10 | 8/9 (89) | <0.01 |
| 3.2 | 9/10 (90) | <0.01 | |
| 0 | 2/18 (11) |
Adapted from Sidwell et al. 9
Clinical evaluation
Clinical pharmacokinetics
To be effective in vivo, however, the neuraminidase inhibitors must be sufficiently absorbed to ensure clinically effective concentrations at the sites of influenza infection. Oral absorption has proved to be problematic for some of the neuraminidase inhibitors. Zanamivir must be administered intranasally because of its poor oral absorption (<2%). 12 Similarly, oral peramivir has only showed efficacy when given at high doses (up to 800 mg) due to low oral bioavailability (<10%) and, consequently is not licensed for use in humans. To overcome this issue, peramivir is currently in development as an intravenous (i.v.) formulation. Oseltamivir has avoided these limitations by its administration as an oral prodrug. After oral administration, oseltamivir was found to be readily absorbed from the gastrointestinal tract (80% bioavailability) and rapidly metabolized to the active metabolite, oseltamivir carboxylate. Oseltamivir carboxylate was detected in plasma approximately 30 min after oral dosing, and peak plasma concentrations were reached within 4 h. 13 Steady‐state conditions were achieved in 3 days. Oseltamivir carboxylate was well distributed throughout the body [volume of distribution (V d) 23–26 l), and present at all major infection sites (middle ear, sinuses and lung) at concentrations sufficient to inhibit viral replication. 13 , 14 The active metabolite remained in plasma for some time (t 1/2 6–10 h) and was excreted renally. 13
In children aged 1–12 years, oseltamivir was efficiently metabolized and excreted. However, due to the speed of its clearance, exposure to the active metabolite was reduced compared with older children and adults. A unit‐based dosing regimen was therefore initiated to ensure adequate exposure. 15 , 16 In elderly patients (aged >65 years), a higher exposure to oseltamivir carboxylate was detected when compared with younger subjects (aged 18–55 years). However, as no tolerability concerns were identified, no dose adjustment was required. 17 Dose adjustment was also unnecessary in patients with moderate hepatic impairment 18 and for patients with mild‐to‐moderate renal impairment. 13 Dose adjustment was required only in patients with severe renal impairment (glomerular filtration rate < 30 ml/min). 5 , 13 It has also recently been shown that oseltamivir is safe in patients undergoing dialysis, and provides concentrations of oseltamivir carboxylate that would be expected to be clinically effective. 19
Zanamivir exhibits some, but not all the pharmacokinetic characteristics of oseltamivir. Zanamivir is rapidly absorbed (t max 1–2 h) and is excreted unchanged in the urine (t 1/2 4–5 h). However, because of its intranasal administration via a Diskhaler, zanamivir is deposited primarily in the respiratory tract with no significant systemic exposure. 6 , 12 Due to its metabolic stability and poor systemic distribution, dosage adjustments of zanamivir are not necessary in elderly patients or those with hepatic or renal impairment. 12
Efficacy in experimental influenza
Prior to embarking on studies in patients with naturally acquired influenza, the neuraminidase inhibitors proved their efficacy in experimentally induced influenza. In a randomized, double‐blind, placebo‐controlled study, oseltamivir (20, 100 or 200 mg b.i.d.) or placebo was given to healthy adult volunteers who had been infected with influenza A (A/Texas/36/91; H1N1). 20 Treatment was initiated 28 h after inoculation and continued for 5 days. Compared with placebo, oseltamivir treatment significantly reduced median nasal viral titres (80 vs. 273 log10TCID50h/ml for placebo; P = 0.02) and the duration of viral shedding (58 vs. 107 h for placebo; P = 0.003). Oseltamivir also reduced the severity and duration of influenza symptoms. Initiation of oseltamivir therapy (100 mg daily or 100 mg b.i.d.) 26 h before inoculation with influenza A was sufficient to reduce the incidence of infection as indicated by seroconversion (38% vs. 68% for placebo) in previously healthy individuals, and to also prevent viral shedding (100% vs. 50% in placebo; P < 0.001). 20 In a separate study in adults inoculated with influenza B, oseltamivir significantly reduced the viral titre (by 83%; P = 0.0023), median duration of viral shedding (from 96 to 24 h; P = 0.0005) and symptom score. 21
In a collection of four randomized, double‐blind, placebo‐controlled trials, zanamivir was given prophylactically or as a treatment (4 h before or 1–2 days after inoculation respectively) to 166 susceptible adults inoculated with A/Texas/91 (H1N1). Given prophylactically, zanamivir prevented influenza infection and febrile illness by 82% and 95% respectively. Early treatment intervention significantly reduced the peak viral titres, the median duration of illness and the frequency of febrile illness (P < 0.05 vs. placebo). 22 Similar positive effects were observed in a separate series of studies with intranasal zanamivir, although no prophylactic benefit was observed if zanamivir was given 48 h before viral inoculation. 23 When given intravenously to 14 A/Texas/36/91‐infected individuals, zanamivir (600 mg i.v. twice daily for 5 days beginning 4 h before inoculation) significantly reduced viral shedding to 0% and seroconversion to 14% (vs. 100% for placebo; P < 0.005 for both) and viral titres (0 vs. 11.6 log10TCID50day/ml for placebo; P < 0.005). Fever, upper respiratory tract illness and symptom scores were also significantly reduced (P < 0.005 vs. placebo). 24
Although not licensed, peramivir demonstrated efficacy against experimentally induced influenza in four randomized, double‐blind, placebo‐controlled trials. A total of 288 healthy volunteers (aged 18–45 years) were inoculated with A/Texas/36/91/H1N1 or B/Yamagata/16/88 virus 25 and then received peramivir (100–800 mg/day) for 5 days, starting 24 h after inoculation. Peramivir treatment reduced influenza A viral titres, but only at relatively high doses (400 mg daily and 200 mg twice daily). The same study also evaluated post‐exposure prophylaxis with peramivir. Dosing of 50–800 mg/day was initiated 24 h before inoculation and continued for 4 days. At these doses and with either strain of influenza, peramivir did not significantly reduce viral shedding vs. placebo. This could be explained by the relatively low blood concentrations of peramivir, which suggests a need for an alternative dosing strategy and supports the development of an i.v. regimen for peramivir. 25
Efficacy in naturally acquired influenza: treatment
Following initial demonstrations of efficacy under laboratory conditions, the neuraminidase inhibitors were required to show the efficacy and safety against naturally occurring influenza infection in a ‘real‐world’ setting. The effects of oseltamivir treatment on naturally acquired influenza A and B infections in previously healthy, non‐immunized adults (aged 18–65 years) were assessed in two clinical studies. 26 , 27 All participants presented with fever, one respiratory and one systemic symptom, and were randomized to receive oseltamivir (75 mg or 150 mg b.i.d.) or placebo for 5 days. In all cases, treatment was initiated within 36 h of symptom onset. In these studies, oseltamivir significantly reduced the median duration of illness by 25–35% compared with placebo [71.5 vs. 103.3 h (P < 0.01) 27 and 81.8 and 87.4 vs. 116.5 h (P < 0.05) 26 and 69.9 in the 75 mg and 150 mg groups vs. placebo respectively]. Symptom severity was also significantly reduced 26 , 27 and patients had a more rapid return to everyday activities and normal health. 27
In the study described by Nicholson et al., 26 early treatment initiation (within 24 h) was associated with a more marked reduction in illness duration [74.5 (P = 0.02) and 70.7 (P = 0.01) vs. 117.5 h in the 75 and 150 mg groups vs. placebo respectively]. In the IMPACT study, 28 patients (n = 1426) received 75 mg oseltamivir b.i.d. for 5 days within 6–48 h of symptom onset, and the effects of treatment were assessed at regular intervals for 21 days. As previously described, early treatment initiation increased the effectiveness of oseltamivir therapy. For example, when treatment was initiated within 12 h of symptom onset, illness duration was nearly 4 days shorter than that obtained when treatment was initiated at 48 h (Figure 2). Timely treatment initiation was also associated with reduced symptom severity and a reduced duration of impaired activity, impaired health and fever. 28
Figure 2.
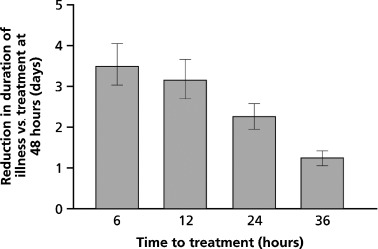
Progressive reductions in the duration of influenza illness (days) in patients receiving early treatment with oseltamivir. Taken from Aoki et al., 28 by permission of Oxford University Press.
Oseltamivir treatment was also shown to reduce the incidence of secondary complications, hospitalizations and the need for antibiotic intervention. 26 , 27 , 29 In a meta‐analysis of 10 clinical studies, involving 3564 patients, 29 oseltamivir treatment (75 mg) significantly reduced the overall number of patients requiring antibiotic intervention (27%; P < 0.001). The incidence of lower respiratory tract infections requiring antibiotic intervention was substantially decreased (55%; P < 0.001), with the frequency of bronchitis reduced by 48% and pneumonia by 39%. A 42% reduction in hospitalization was also observed (P < 0.05). 29
In the largest, randomized, double‐blind, placebo‐controlled trial to investigate the efficacy of oseltamivir in children (aged 1–12 years; n = 695), oseltamivir (2 mg/kg b.i.d. oral suspension for 5 days) reduced the median duration of illness, fever and cough by 36, 25 and 32 h, respectively, compared with placebo (P < 0.0001 for all comparisons). When considered collectively, median symptom duration was significantly reduced by 36 h vs. placebo (P < 0.0001). Viral titres and secondary complications requiring antibiotics were also decreased. 30 In smaller studies, oseltamivir successfully reduced the incidence of fever in children infected with influenza A or B 31 and improved pulmonary function in children with asthma. 32 In elderly patients, the incidence of lower respiratory tract infections requiring antibiotics was significantly reduced following treatment with oseltamivir (12% vs. 19% for placebo; P < 0.02). These data support the licensed indication of oseltamivir for the treatment of adults and children (>1 year old) for influenza A and B infection; treatment should be initiated within 2 days of symptom onset. 5
As with oseltamivir, the efficacy of zanamivir in influenza management is well established. In a pooled analysis of treatment efficacy across six studies involving otherwise healthy adults (n = 1572), zanamivir effectively reduced the duration of illness (vs. no treatment) when initiated within 36–48 h of symptom onset. Participants were classified as either rapid (temperature <37.8°C within 36 h of initiation) or slow (febrile >36 h after dosing) resolvers. In the influenza‐positive population, significantly more zanamivir‐treated patients were shown to be rapid resolvers than placebo‐treated patients [807 (72%) vs. 765 (64%); P < 0.001]. 33 Zanamivir was also effective in reducing fever and symptom severity. In comparison with placebo, more patients with a baseline temperature of ≥37.8°C [630 (68%) vs. 595 (57%); P < 0.001] or ≥38.3°C [382 (67%) vs. 365 (52%); P < 0.001] or severe symptoms at the start of therapy [252 (70%) vs. 222 (63%); P = 0.02] were considered rapid resolvers when treated with zanamivir. In a further analysis of treatment efficacy across eight clinical studies involving a more diverse array of patient types, 34 the median reduction in time to symptom alleviation vs. placebo in the influenza positive population was 1.0 days in children (aged <12 years), 0.8 days in otherwise healthy adults (aged ≥12–65 years) and 0.9 days in elderly (aged >65 years) and at‐risk adults. 34 In a study involving 471 children (aged 5–12 years), 35 a median reduction in illness duration of 1.0 days was recorded with zanamivir (given 36–48 h after symptom onset) vs. no treatment. As a result of this efficacy, zanamivir is indicated for the treatment of influenza in adults and children (aged >5 years); as with oseltamivir, treatment should be initiated within 2 days of symptom onset. 6
Efficacy in naturally acquired influenza: post‐exposure prophylaxis
In addition to their efficacy for the treatment of influenza, it is as important that the neuraminidase inhibitors prevent the further spread of infection. Extensive evaluation of the efficacy of oseltamivir in preventing the onset and spread of infection in close proximity environments, such as households and care facilities, means that oseltamivir is indicated for post‐exposure prophylaxis in adults and children (aged >1 year). 5 In households, a randomized, double‐blind study was conducted in 955 uninfected individuals who received post‐exposure prophylactic treatment with oseltamivir (75 mg once daily) or placebo for 7 days. In this study, the 377 index cases (initially infected) did not receive treatment. 36 Oseltamivir provided a protective efficacy of 89% and 86% for individual and household contacts of index cases respectively. When only those individuals and households with an influenza‐positive index case were considered, protective efficacy remained consistent (89% and 84% respectively).
In a second study, index cases received oseltamivir (75 mg b.i.d.) within 2 days of becoming symptomatic and continued with treatment for 5 days. 37 Uninfected individuals in each household received either prophylactic oseltamivir (75 mg daily) initiated with index case treatment or oseltamivir treatment (75 mg b.i.d.) if symptoms developed. Of those households where the index case had confirmed influenza, further infection occurred in 11% of households where individuals received oseltamivir prophylactically and in 26% of households where individuals were treated at symptom onset (58.5% protective efficacy; P = 0.0114). A similar protective effect (68%; P = 0.0017) was observed in individuals. The protective effect was more pronounced when influenza‐positive contacts at baseline were excluded from the analysis (78.8% and 84.5% for households and individuals respectively). In a subgroup analysis, more children (aged 1–12 years) taking oseltamivir prophylactically avoided infection compared with those treated for initial symptoms only (11% vs. 24% respectively).
Similar benefits have been observed in care facilities. 38 , 39 Bowles et al. described the experience of 10 Canadian long‐term care facilities for older people and their residents who used oseltamivir for treatment or prophylaxis during the 1999/2000 influenza outbreaks. Generally, and irrespective of whether oseltamivir was used for treatment or post‐exposure prophylaxis, influenza outbreaks were brought under control within 48 h of oseltamivir initiation. The incidence of influenza‐associated complications, antibiotic use, hospitalization and death were also significantly reduced. In another study, 548 frail older adults from 31 residential homes across Europe and the USA were randomized to receive 6 weeks prophylaxis with 75 mg oseltamivir once daily or placebo. 39 Oseltamivir administration resulted in a 92% reduction in the incidence of laboratory‐confirmed influenza (P = 0.02), and this protective effect was similar in those elderly adults who had been vaccinated (91%, P = 0.03). Oseltamivir use was also associated with significant reductions in secondary complication rates (85%, P = 0.037).
In healthy adults, zanamivir has also been shown to prevent influenza effectively when used prophylactically and is indicated for post‐exposure prophylaxis of influenza in adults and children aged >5 years. The meta‐analysis by Cooper et al., 34 who considered the efficacy of zanamivir in post‐exposure prophylaxis within households, recorded an 81% reduction in the incidence of influenza and influenza‐like illness. In addition, a 69% reduction in the incidence of laboratory‐confirmed influenza was observed with zanamivir in a community‐based seasonal prophylaxis study involving 1107 patients. 40 However, there is a lack of data to show prophylactic efficacy in young and elderly patients.
Safety and tolerability
Influenza is an indiscriminate disease that infects all individuals regardless of age and physical condition. Consequently, agents to combat influenza infection must be well tolerated, not only in otherwise healthy individuals, but in those with underlying conditions. Safety and tolerability information was collected in all clinical studies of oseltamivir. In all studies, adverse events were generally mild in intensity, with no clinically relevant changes in vital signs, laboratory or cardiac (electrocardiogram) measurements. In a pooled analysis of six studies with oseltamivir in adults, the only adverse events reported more frequently with oseltamivir than placebo were nausea and vomiting, which usually occurred within 2 days of treatment initiation. These symptoms were transient, 90% of patients experienced them only once and ≤1% of patients withdrew from the studies. Notably, the incidence of nausea could be significantly reduced by taking oseltamivir with food. 17 , 41 In a further combined analysis, involving nearly 3000 patients who had received oseltamivir prophylactically, the most common adverse events were headache, upper respiratory tract infection, nausea and fatigue, which occurred to a similar extent in patients who received oseltamivir or placebo. 41 In over 1000 children (aged 1–12 years; 300 with asthma), oseltamivir was also well tolerated.
In general, zanamivir is well tolerated and produces a similar incidence of transient upper respiratory tract and gastrointestinal symptoms to placebo. 42 Although in controlled conditions, inhaled zanamivir did not adversely affect pulmonary function in patients with respiratory disorders, 42 incidences of cough, bronchospasm and reversible decreases in pulmonary function have been noted. 43 For this reason, patients with pulmonary dysfunction who receive zanamivir should have a fast‐acting bronchodilator available and discontinue treatment if respiratory difficulty develops. 42 Although currently there is limited experience with peramivir, this agent appears to be well tolerated, with nausea and headache being the most common side effects. 25
Utility of neuraminidase inhibitors during a pandemic
The likely delay in the generation of a suitable pandemic vaccine means that antiviral agents will be vital in reducing the medical, social and economic impact of an influenza pandemic. 44 Oseltamivir is effective against all avian and human influenza strains, including the highly pathogenic H5N1 strain, in vitro and in vivo. 10 , 11 , 45 Although evidence for the effectiveness of oseltamivir in H5N1‐infected patients is limited, case reports indicate that standard doses of oseltamivir can improve therapeutic outcomes and survival when initiated early in the infection cycle. 45 , 46 , 47 , 48 , 49 For example, six of eight patients with H5N1 infection survived following treatment with oseltamivir given within 48 h of symptom onset. 47 Later intervention is not as successful and it has been suggested that higher and prolonged doses of oseltamivir may be required to manage H5N1 infection. 50 However, at present, WHO guidelines recommend that in cases of confirmed or strongly suspected H5N1 infection, the standard treatment dose of oseltamivir (75 mg b.i.d. for 5 days) should be administered as soon as possible. 7 In addition, the oral dosing form and systemic absorption of oseltamivir make it an ideal treatment for avian/pandemic influenza, being easily and quickly distributed to infection hot spots, and providing systemic protection against a virus that does not seem to be limited to the respiratory tract. 47
Zanamivir has also shown efficacy against the H5N1 viruses that are currently being monitored for pandemic potential. 51 The inclusion of zanamivir in pandemic planning strategies may be important, given the potential for oseltamivir resistance. However, unlike oseltamivir, the systemic absorption of zanamivir is poor (4–17%) 6 and the majority of the dose is deposited in the oropharynx (77.6%) with 13.2% found in the bronchi and lungs. 12 This raises questions as to its suitability for the treatment of a systemic influenza infection, such as H5N1. Currently, there is no clinical evidence for the efficacy of peramivir against the H5N1 avian influenza strain.
Resistance
The structure of the neuraminidase inhibitors is such that they fit specifically and selectively into the active site of viral neuraminidase. For example, oseltamivir carboxylate has a cohexane ring structure and a hydrophobic alkyl group that was specifically designed to fit into the highly conserved active site of the influenza neuraminidase enzyme. As with all antimicrobials, replication of virus in the presence of drug increases the selection pressure for mutations within the target. In the case of oseltamivir, mutations occur within the active site of the neuraminidase enzyme, mutations which alter the ability of oseltamivir carboxylate to bind to the enzyme thus promoting viral resistance. Influenza virus mutations are subtype specific and include R292K and E119V in N2 viruses and H274Y in N1 viruses. 52 Such mutations have resulted in reduced sensitivity of influenza viruses to oseltamivir, in vitro, of up to 100 000‐fold. 30 , 47 , 48 , 53 However, these mutated viruses also have a reduced ability to replicate both in vitro and in vivo, their infectivity is reduced by up to 1000‐fold, and their pathogenicity is also diminished compared with wild‐type viruses. 30 , 54 , 55 , 56 Hence, the consequences of oseltamivir resistance may not be clinically relevant.
In general, the overall incidence of resistance to oseltamivir is low (0.33% in adults and 4% in children 45 ). The apparent greater resistance observed in children may be a consequence of an immature immune system, longer duration of illness, longer periods of viral shedding and higher viral titres. A higher level of resistance has been observed in one study involving Japanese children (18%). 53 However, it is likely that these children were underdosed (using the weight‐based regimen) allowing the influenza virus to replicate, which increased the likelihood of resistance developing. 53 Therefore, reducing the frequency of underdosing may effectively prevent the development of resistant strains of influenza. Few cases of reduced oseltamivir sensitivity have been reported for the H5N1 virus, 47 , 48 all of which were a result of the H274Y mutation.
The resistance profile of zanamivir is good and there have been no reports of patients on acute therapy shedding drug‐resistant virus. 57 However, there is currently insufficient data to conclude that resistance will not be an issue in the future. Resistant variants have been identified in vitro, but all have exhibited diminished viability. 57 At this time, no peramivir‐resistant influenza variants have been detected.
Summary and conclusions
Neuraminidase inhibitors are the primary treatment option for influenza, and can also be used to limit the spread of infection in close proximity environments. Oseltamivir is the leading drug within this class and is reviewed here as a gold‐standard model drug. However, there are no fundamental reasons to prevent zanamivir and peramivir from occupying important positions alongside oseltamivir. In all cases, development was via rational drug design, and for oseltamivir and zanamivir there is a large body of pre‐clinical and clinical data. All three drugs also show: a high level of efficacy, irrespective of influenza subtype, or the age or risk level of the patient; good tolerability; and a low potential for the development of meaningful resistance. These characteristics support the planned pivotal role of the neuraminidase inhibitors in the defence of many countries against pandemic influenza.
Acknowledgements
The author would like to thank Scott Malkin for his assistance in the development of this manuscript and acknowledges honoraria for lectures from GlaxoSmithKline, Roche and Johnson and Johnson, and a grant from Virgil (EU) Network of Excellence for Antiviral Testing.
Conflict of Interests JSO has received grants for scientific research, speaker honoraria and travel to international meetings from GSK, Roche and Johnson and Johnson, who manufacture NA inhibitors.
References
- 1. Menec VH, Black C, MacWilliam L, Aoki FY. The impact of influenza‐associated respiratory illnesses on hospitalizations, physician visits, emergency room visits, and mortality. Can J Public Health 2003;94:59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oxford J. Oseltamivir in the management of influenza. Expert Opin Pharmacother 2005;6:2493–2500. [DOI] [PubMed] [Google Scholar]
- 3. Stephenson I, Nicholson KG. Influenza: vaccination and treatment. Eur Respir J 2001;17:1282–1293. [DOI] [PubMed] [Google Scholar]
- 4. Oxford JS, Galbraith A. Antiviral activity of amantadine: a review of laboratory and clinical data. Pharmacol Ther 1980;11:181–262. [DOI] [PubMed] [Google Scholar]
- 5. Hoffman‐La Roche F. UK. Tamiflu Summary of Product Characteristics (EU/1/02/222/001; revised 22 February 2006). Available at http://www.rocheuk.com/productDB/Documents/rx/spc/Tamiflu_capsules_SPC.pdf (accessed 19 December 2006). [Google Scholar]
- 6. GlaxoSmithKline UK . Relenza Summary of Product Characteristics (PL 10949/0327; revised 23 August 2006). Available at http://emc.medicines.org.uk/emc/assets/c/html/displaydoc.aps?documentid=2608 (accessed 19 December 2006). [Google Scholar]
- 7. World Health Organisation . WHO Rapid Advice Guidelines on Pharmacological Management of Humans Infected with Avian Influenza A (H5N1) Virus. Available at http://www.who.int/medicines/publications/WHO_PSM_PAR_2006.6.pdf (accessed August 2006), 2006. [Google Scholar]
- 8. Mendel DB, Tai CY, Escarpe PA et al. Oral administration of a prodrug of the influenza virus neuraminidase inhibitor GS 4071 protects mice and ferrets against influenza infection. Antimicrob Agents Chemother 1998;42:640–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sidwell RW, Huffman JH, Barnard DL et al. Inhibition of influenza virus infections in mice by GS4104, an orally effective influenza virus neuraminidase inhibitor. Antiviral Res 1998;37:107–120. [DOI] [PubMed] [Google Scholar]
- 10. Leneva IA, Roberts N, Govorkova EA, Goloubeva OG, Webster RG. The neuraminidase inhibitor GS4104 (oseltamivir phosphate) is efficacious against A/Hong Kong/156/97 (H5N1) and A/Hong Kong/1074/99 (H9N2) influenza viruses. Antiviral Res 2000;48:101–115. [DOI] [PubMed] [Google Scholar]
- 11. Govorkova EA, Leneva IA, Goloubeva OG, Bush K, Webster RG. Comparison of efficacies of RWJ‐270201, zanamivir, and oseltamivir against H5N1, H9N2, and other avian influenza viruses. Antimicrob Agents Chemother 2001;45:2723–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dunn CJ, Goa KL. Zanamivir: a review of its use in influenza. Drugs 1999;58:761–784. [DOI] [PubMed] [Google Scholar]
- 13. He G, Massarella J, Ward P. Clinical pharmacokinetics of the prodrug oseltamivir and its active metabolite Ro 64‐0802. Clin Pharmacokinet 1999;37:471–484. [DOI] [PubMed] [Google Scholar]
- 14. Kurowski M, Oo C, Wiltshire H, Barret J. Oseltamivir distributes to influenza virus replication sites in the middle ear and sinuses. Clin Drug Invest 2004;24:49–53. [DOI] [PubMed] [Google Scholar]
- 15. Oo C, Barrett J, Hill G et al. Pharmacokinetics and dosage recommendations for an oseltamivir oral suspension for the treatment of influenza in children. Paediatr Drugs 2001;3:229–236. [DOI] [PubMed] [Google Scholar]
- 16. Oo C, Hill G, Dorr A et al. Pharmacokinetics of anti‐influenza prodrug oseltamivir in children aged 1–5 years. Eur J Clin Pharmacol 2003;59:411–415. [DOI] [PubMed] [Google Scholar]
- 17. Massarella JW, He GZ, Dorr A et al. The pharmacokinetics and tolerability of the oral neuraminidase inhibitor oseltamivir (Ro 64‐0796/GS4104) in healthy adult and elderly volunteers. J Clin Pharmacol 2000;40:836–843. [DOI] [PubMed] [Google Scholar]
- 18. Snell P, Dave N, Wilson K et al. Lack of effect of moderate hepatic impairment on the pharmacokinetics of oral oseltamivir and its metabolite oseltamivir carboxylate. Br J Clin Pharmacol 2005;59:598–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Robson R, Buttimore A, Lynn K, Brewster M, Ward P. The pharmacokinetics and tolerability of oseltamivir suspension in patients on haemodialysis and continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant 2006;21:2556–2562. [DOI] [PubMed] [Google Scholar]
- 20. Hayden FG, Treanor JJ, Fritz RS et al. Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized controlled trials for prevention and treatment. J Am Med Assoc 1999;282:1240–1246. [DOI] [PubMed] [Google Scholar]
- 21. Hayden FG, Robson R, Jennings L et al. Efficacy of oral oseltamivir in experimental human influenza B virus infection [abstract]. Clin Infect Dis 1999;29:1079 (abstract). [Google Scholar]
- 22. Hayden FG, Treanor JJ, Betts RF et al. Safety and efficacy of the neuraminidase inhibitor GG167 in experimental human influenza. J Am Med Assoc 1996;275:295–299. [PubMed] [Google Scholar]
- 23. Calfee DP, Peng AW, Hussey EK, Lobo M, Hayden FG. Safety and efficacy of once daily intranasal zanamivir in preventing experimental human influenza A infection. Antivir Ther 1999;4:143–149. [PubMed] [Google Scholar]
- 24. Calfee DP, Peng AW, Cass LM, Lobo M, Hayden FG. Safety and efficacy of intravenous zanamivir in preventing experimental human influenza A virus infection. Antimicrob Agents Chemother 1999;43:1616–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barroso L, Treanor J, Gubareva L, Hayden FG. Efficacy and tolerability of the oral neuraminidase inhibitor peramivir in experimental human influenza: randomized, controlled trials for prophylaxis and treatment. Antivir Ther 2005;10:901–910. [PubMed] [Google Scholar]
- 26. Nicholson KG, Aoki FY, Osterhaus AD et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet 2000;355:1845–1850. [DOI] [PubMed] [Google Scholar]
- 27. Treanor JJ, Hayden FG, Vrooman PS et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. J Am Med Assoc 2000;283:1016–1024. [DOI] [PubMed] [Google Scholar]
- 28. Aoki FY, Macleod MD, Paggiaro P et al. Early administration of oral oseltamivir increases the benefits of influenza treatment. J Antimicrob Chemother 2003;51:123–129. [DOI] [PubMed] [Google Scholar]
- 29. Kaiser L, Wat C, Mills T et al. Impact of oseltamivir treatment on influenza‐related lower respiratory tract complications and hospitalizations. Arch Intern Med 2003;163:1667–1672. [DOI] [PubMed] [Google Scholar]
- 30. Whitley RJ, Hayden FG, Reisinger KS et al. Oral oseltamivir treatment of influenza in children. Pediatr Infect Dis J 2001;20:127–133. [DOI] [PubMed] [Google Scholar]
- 31. Mitamura K, Sugaya N, Nirasawa M, Shinjoh M, Takeuchi Y. Effectiveness of oseltamivir treatment against influenza type A and type B infection in children. Kansenshogaku Zasshi 2002;76:946–952. [DOI] [PubMed] [Google Scholar]
- 32. Johnston SL, Ferrero F, Garcia ML, Dutkowski R. Oral oseltamivir improves pulmonary function and reduces exacerbation frequency for influenza‐infected children with asthma. Pediatr Infect Dis J 2005;24:225–232. [DOI] [PubMed] [Google Scholar]
- 33. Monto AS, Moult AB, Sharp SJ. Effect of zanamivir on duration and resolution of influenza symptoms. Clin Ther 2000;22:1294–1305. [DOI] [PubMed] [Google Scholar]
- 34. Cooper NJ, Sutton AJ, Abrams KR et al. Effectiveness of neuraminidase inhibitors in treatment and prevention of influenza A and B: systematic review and meta‐analyses of randomised controlled trials. Br Med J 2003;326:1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hedrick JA, Barzilai A, Behre U et al. Zanamivir for treatment of symptomatic influenza A and B infection in children five to twelve years of age: a randomized controlled trial. Pediatr Infect Dis J 2000;19:410–417. [DOI] [PubMed] [Google Scholar]
- 36. Welliver R, Monto AS, Carewicz O et al. Effectiveness of oseltamivir in preventing influenza in household contacts: a randomized controlled trial. J Am Med Assoc 2001;285:748–754. [DOI] [PubMed] [Google Scholar]
- 37. Hayden FG, Belshe R, Villanueva C et al. Management of influenza in households: a prospective, randomized comparison of oseltamivir treatment with or without postexposure prophylaxis. J Infect Dis 2004;189:440–449. [DOI] [PubMed] [Google Scholar]
- 38. Bowles SK, Lee W, Simor AE et al. Use of oseltamivir during influenza outbreaks in Ontario nursing homes, 1999–2000. J Am Geriatr Soc 2002;50:608–616. [DOI] [PubMed] [Google Scholar]
- 39. Peters PH Jr, Gravenstein S, Norwood P et al. Long‐term use of oseltamivir for the prophylaxis of influenza in a vaccinated frail older population. J Am Geriatr Soc 2001;49:1025–1031. [DOI] [PubMed] [Google Scholar]
- 40. Monto AS, Robinson DP, Herlocher ML et al. Zanamivir in the prevention of influenza among healthy adults: a randomized controlled trial. J Am Med Assoc 1999;282:31–35. [DOI] [PubMed] [Google Scholar]
- 41. Dutkowski R, Thakrar B, Froehlich E et al. Safety and pharmacology of oseltamivir in clinical use. Drug Saf 2003;26:787–801. [DOI] [PubMed] [Google Scholar]
- 42. Moscona A. Neuraminidase inhibitors for influenza. N Engl J Med 2005;353:1363–1373. [DOI] [PubMed] [Google Scholar]
- 43. Freund B, Gravenstein S, Elliott M, Miller I. Zanamivir: a review of clinical safety. Drug Saf 1999;21:267–281. [DOI] [PubMed] [Google Scholar]
- 44. Ferguson NM, Cummings DA, Fraser C et al. Strategies for mitigating an influenza pandemic. Nature 2006;442:448–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ward P, Small I, Smith J, Suter P, Dutkowski R. Oseltamivir (Tamiflu) and its potential for use in the event of an influenza pandemic. J Antimicrob Chemother 2005;55(Suppl. 1): i5–i21. [DOI] [PubMed] [Google Scholar]
- 46. Chotpitayasunondh T, Ungchusak K, Hanshaoworakul W et al. Human disease from influenza A (H5N1), Thailand, 2004. Emerg Infect Dis 2005;11:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. De Jong MD, Tran TT, Truong HK et al. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med 2005;353:2667–2672. [DOI] [PubMed] [Google Scholar]
- 48. Le QM, Kiso M, Someya K et al. Avian flu: isolation of drug‐resistant H5N1 virus. Nature 2005;437:1108. [DOI] [PubMed] [Google Scholar]
- 49. Tran TH, Nguyen TL, Nguyen TD et al. Avian influenza A (H5N1) in 10 patients in Vietnam. N Engl J Med 2004;350:1179–1188. [DOI] [PubMed] [Google Scholar]
- 50. Democratis J, Pareek M, Stephenson I. Use of neuraminidase inhibitors to combat pandemic influenza. J Antimicrob Chemother 2006;58:911–915. [DOI] [PubMed] [Google Scholar]
- 51. Trampuz A, Prabhu RM, Smith TF, Baddour LM. Avian influenza: a new pandemic threat? Mayo Clin Proc 2004;79:523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McKimm‐Breschkin JL. Management of influenza virus infections with neuraminidase inhibitors: detection, incidence, and implications of drug resistance. Treat Respir Med 2005;4:107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kiso M, Mitamura K, Sakai‐Tagawa Y et al. Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet 2004;364:759–765. [DOI] [PubMed] [Google Scholar]
- 54. Carr J, Ives J, Kelly L et al. Influenza virus carrying neuraminidase with reduced sensitivity to oseltamivir carboxylate has altered properties in vitro and is compromised for infectivity and replicative ability in vivo . Antiviral Res 2002;54:79–88. [DOI] [PubMed] [Google Scholar]
- 55. Herlocher ML, Truscon R, Elias S et al. Influenza viruses resistant to the antiviral drug oseltamivir: transmission studies in ferrets. J Infect Dis 2004;190:1627–1630. [DOI] [PubMed] [Google Scholar]
- 56. Ives JA, Carr JA, Mendel DB et al. The H274Y mutation in the influenza A/H1N1 neuraminidase active site following oseltamivir phosphate treatment leave virus severely compromised both in vitro and in vivo . Antiviral Res 2002;55:307–317. [DOI] [PubMed] [Google Scholar]
- 57. Colman PM. Zanamivir: an influenza virus neuraminidase inhibitor. Expert Rev Anti Infect Ther 2005;3:191–199. [DOI] [PubMed] [Google Scholar]


