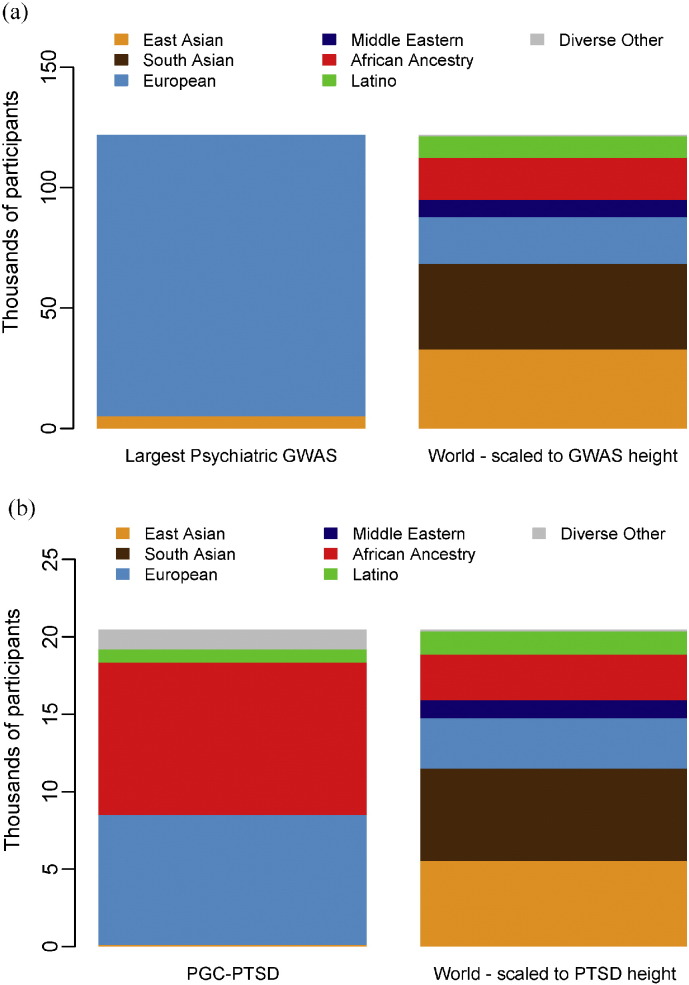
In recent years there have been significant insights into the complex aetiologies of neurodevelopmental brain disorders. For example, neuropsychiatric genetics has achieved success with the identification of 108 loci for schizophrenia (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014). Furthermore, meta-analyses of genome-wide association study (GWAS) results encompassing thousands of samples have been completed for other psychiatric disorders including attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorders, bipolar disorder, and major depressive disorder. However, published results on neuropsychiatric disorders have – thus far – predominantly included samples of European ancestry. In Fig. 1a, we compare world ancestry to the ancestry of individuals in the largest psychiatric GWAS meta-analyses published prior to 2015 (Total N = 121,985). The lack of African samples in the meta-analyses so clearly depicted here, raises concern that Africa will be left behind in terms of neuropsychiatric genetic research and subsequent treatment innovation.
Fig. 1.
Proportion of world population groups in psychiatric GWAS. (a) Proportion investigated in the largest meta-analyses published prior to 2015 for four leading psychiatric disorders (schizophrenia (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014), bipolar disorder (Psychiatric GWAS Consortium Bipolar Disorder Working Group, 2011), major depressive disorder (Ripke et al., 2013), and ADHD (Neale et al., 2010); total N = 121,985). (b) Proportion investigated in PGC-PTSD studies (total N = 20,468).
World population data from http://en.wikipedia.org/w/index.php?title=World_population&oldid=648649676.
There is biological rationale for conducting genetic research in African populations. It has been shown that modern humans originated in Africa and subsequently migrated to other parts of the world (Campbell and Tishkoff, 2008). As the cradle of humanity, Africa and its indigenous populations are a valuable resource when it comes to genetic research. Modern African genomes are characterised by a unique pattern of variation as a result of migration and admixture in earlier generations as well as recombination, natural selection and mutation. With an increase in allelic diversity and shorter segments of linkage disequilibrium, African genomes hold informative alleles which are useful for fine mapping of disease causing alleles (Campbell and Tishkoff, 2008). However, there is limited knowledge on African-specific functional variants highlighting the need to investigate African population groups, particularly for neuropsychiatric disorders.
As genetic findings are translated into intervention, genetic research focused solely on European populations threatens to widen the existing large disparity between Africa and the rest of the world in mental health treatment. The vast majority of work is being conducted in high-income settings, such as the U.S.A. and Denmark, with a large proportion of subjects of Northern European ancestry (Fig. 1a). To date, there have been no large-scale studies on the genetics of neuropsychiatric disorders in African populations. The few studies that have been conducted have been on small samples, typically under a thousand in number (Kolassa et al., 2010). Recent successes in studies of schizophrenia have demonstrated that very large scale meta-analysis is necessary to identify genetic variants associated with neuropsychiatric disorders. Without engaging African scientists and physicians and performing studies of African populations, there is a significant risk that the recent advances in neuropsychiatric genetics will result in a widening of the massive research and treatment gaps between Africa and the rest of the world. Indeed, one of the aims of the movement for global mental health is to decrease inequality in mental health outcomes particularly for low- and middle-income countries (Patel, 2012), typical of much of the African continent.
Researchers in Africa are faced with a number of unique challenges. Research funding is scarce, and African scientists are often not eligible for training mechanisms offered by the National Institutes of Health and other funding agencies. Much of the funding that is available focuses on public mental health issues rather than on neuroscience or the integration of neuroscience with public mental health (Stein et al., 2015). Also, many African countries lack the infrastructure required to conduct large-scale neuropsychiatric genetics research, e.g. refrigeration for blood samples and cloud-based technology for phenotypic data collection. Furthermore, there is a shortage of highly skilled geneticists and clinician-scientists, highlighting the need for training and capacity building in African countries. In particular, clinicians need to develop culturally appropriate tools for the diagnosis and phenotyping of these disorders. There are also several ethical considerations, especially in the context of collaborative global health partnerships between high and low to middle income countries. These include ethical issues about informed consent; poverty, low literacy, language barriers and poor access to healthcare (De Vries et al., 2011). Additionally, fairness in international collaboration needs to be ensured, with researchers having equal access to data, and intellectual property rights adequately addressed. When working with individuals with mental health problems, in countries with a lack or paucity of mental health prioritization, these types of challenges are exacerbated. Lastly, existing microarray panels may not adequately capture common haplotypes in African populations. This highlights the need for genotyping chips which contain tag single nucleotide polymorphisms (SNPs) that are able to encapsulate common variation across African population groups (Gurdasani et al., 2015).
Despite these challenges, a number of emerging studies may hold promise for future neurogenetics research in Africa. For example, the Drakenstein Child Health Study (http://www.paediatrics.uct.ac.za/scah/dclhs) is a multidisciplinary South African birth cohort study investigating genetic and environmental risk factors for common mental disorders. The cohort consists of 1200 mother–child pairs and a subset of these individuals has already been genotyped with a genome-wide panel of markers shown to be relevant to psychiatric disorders. The post-traumatic stress disorder (PTSD) subgroup of the multi-national Psychiatric Genomics Consortium (PGC) (http://www.med.unc.edu/pgc) aims to carry out large-scale GWASs and has included South African samples in their analyses. To date, the PTSD-PGC group has access to approximately 20,000 samples from study sites. As depicted by Fig. 1b, the ancestry of individuals in the PGC-PTSD studies is more diverse than large psychiatric GWAS in general. The Enhancing Neuro Imaging Genetics through Meta-Analysis (ENIGMA) Network (http://enigma.ini.usc.edu/about-2/), a consortium investigating brain structure, function and disease using brain imaging and genomics, has also included samples from South Africa. This consortium comprises 70 institutions world-wide and consists of different disease working groups including those for schizophrenia, bipolar disorder and PTSD, respectively. Lastly, the Human Heredity and Health in Africa (H3Africa) (http://h3africa.org/) initiative seeks to improve health in African populations by investigating genomic and environmental factors contributing to common disease. This initiative includes a study investigating the genetic basis of schizophrenia in the southern African Xhosa-speaking population group. Using exome-sequencing and by investigating genome-wide copy-number variation, this study aims to identify genes associated with schizophrenia. Similarly, an initiative tentatively called the Neuropsychiatric Genetics in African Populations (Neuro-GAP), by the Stanley Center for Psychiatric Research of the Broad Institute of MIT and Harvard University, in collaboration with the University of Cape Town and a number of other African institutions, aims to improve and achieve equity in mental health by expanding the infrastructure and research findings from large-scale psychiatric genetic epidemiology to Africa. This will be achieved by enhancing neuropsychiatric genetic research capacity in Africa through the training of scientists, conducting very large-scale sample collection and analysis through supporting the development of locally led research programmes in neuropsychiatric genetics and leveraging unique opportunities in population genetics.
In conclusion, while there is a clear need for further work in elucidating the genetics of neuropsychiatric disorders in African populations, several challenges will first need to be tackled. An effective local network of neurogenetic researchers needs to be established in order to discover genetic variation predisposing to neuropsychiatric disorders. This research needs to avoid prior pitfalls of “safari research” by engaging and training African scientists and physicians to improve phenotyping and perform studies of African populations to ensure long-term capacity to translate genetic findings in a way that will benefit African peoples.
Conflicts of Interest
Dan Stein has received research grants and/or consultancy honoraria from AMBRF, Biocodex, Cipla, Lundbeck, National Responsible Gambling Foundation, Novartis, Servier, and Sun.
Funding Source
The authors are members of the Neuropsychiatric Genetics in African Populations (Neuro-GAP) consortium. Dan Stein is funded by the Medical Research Council of South Africa.
References
- Campbell M.C., Tishkoff S.A. African genetic diversity: implications for human demographic history, modern human origins, and complex disease mapping. Annu. Rev. Genomics Hum. Genet. 2008;9:403–433. doi: 10.1146/annurev.genom.9.081307.164258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries J., Bull S.J., Doumbo O., Ibrahim M., Mercereau-Puijalon O., Kwiatkowski D. Ethical issues in human genomics research in developing countries. BMC Med. Ethics. 2011;12 doi: 10.1186/1472-6939-12-5. (5–6939-12-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdasani D., Carstensen T., Tekola-Ayele F., Pagani L., Tachmazidou I., Hatzikotoulas K. The African Genome Variation Project shapes medical genetics in Africa. Nature. 2015;517:327–332. doi: 10.1038/nature13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolassa I., Kolassa S., Ertl V., Papassotiropoulos A., Dominique J. The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol-O-methyltransferase Val 158 Met polymorphism. Biol. Psychiatry. 2010;67:304–308. doi: 10.1016/j.biopsych.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Neale B.M., Medland S., Ripke S., Anney R.J., Asherson P., Buitelaar J. Case–control genome-wide association study of attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49:906–920. doi: 10.1016/j.jaac.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V. Global mental health: from science to action. Harv. Rev. Psychiatry. 2012;20:6–12. doi: 10.3109/10673229.2012.649108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychiatric GWAS Consortium Bipolar Disorder Working Group Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat. Genet. 2011;43:977–983. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripke S., Wray N.R., Lewis C.M., Hamilton S.P., Weissman M.M., Breen G. A mega-analysis of genome-wide association studies for major depressive disorder. Mol. Psychiatry. 2013;18:497–511. doi: 10.1038/mp.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D.J., He Y., Phillips A., Sahakian B.J., Williams J., Patel V. Global mental health and neuroscience: potential synergies. 2015;2:178–185. doi: 10.1016/S2215-0366(15)00014-0. [DOI] [PubMed] [Google Scholar]