Abstract
Background Avian influenza (AI) caused by H7 AI viruses (AIVs) of both low pathogenicity (LP) and high pathogenicity (HP) are notifiable poultry diseases.
Objectives Design and validate two RealTime reverse transcriptase polymerase chain reactions (RRT PCRs) for Eurasian H7 AIV detection and pathotyping.
Methods The H7 RRT PCRs amplified within the (i) HA2 and (ii) cleavage site CS regions of the haemagglutinin gene. Both were validated against 65 H7 AIVs, 57 non‐H7 AIVs and 259 poultry swabs in comparison to M gene (AI generic) RRT PCR and virus isolation (VI). An additional 38 swabs and 20 tissue specimens extended validation against M gene RRT PCR.
Results Both H7 RRT PCRs amplified all 61 Eurasian lineage H7 AIVs and none of 57 non‐H7 AIVs. A total of 297 poultry swabs were used to determine diagnostic sensitivity and specificity relative to M gene RRT PCR, sensitivity was 95·4% and 64·6% for the HA2 and CS RRT PCRs respectively, and specificity 97·9% and 99·6% respectively. The H7 HA2 RRT PCR was more sensitive than VI. This was emphasized by analysis of 37 swabs from turkeys infected experimentally with HPAI H7N1 virus sampled at 24 hours post‐inoculation and LPAI H7N1 chicken infections sampled at 40–64 hours. Although less sensitive, usefulness of the H7 CS RRT PCR was confirmed by the correct molecular pathotyping for all 61 Eurasian lineage H7 AIVs tested.
Conclusions The high sensitivity of H7 HA2 RRT PCR confirms its suitability for use in poultry surveillance and disease diagnosis. H7 CS RRT PCR provides an opportunity for rapid pathotyping of H7 AIVs.
Keywords: H7 avian influenza virus, pathotyping, RRT PCR, validation
Introduction
Among the influenza A viruses (AIVs), those of the haemagglutinin (H) subtypes H5 and H7 pose the greatest threat to farmed poultry. In normal circumstances viruses of these subtypes occur in wild bird reservoirs as low pathogenic avian influenza (LPAI), but upon transmission to poultry these viruses may mutate to highly pathogenic AI (HPAI). 1 This is characterized by a rapid onset of high morbidity and mortality usually within 48 hours, and the risk of highly contagious spread to adjacent uninfected birds. 2
This marked difference in virus virulence appears to be related to the haemagglutinin glycoprotein of AIVs, which is produced as a precursor, HA0, requiring post‐translational cleavage by host proteases before it is functional and virus particles are infectious. 3 In H5 and H7 LPAI viruses the HA0 precursor proteins of have a single arginine at the cleavage site (CS) and another basic amino acid at position ‐4 or ‐3, respectively, from the CS. These viruses are limited to cleavage by extracellular host proteases such as trypsin‐like enzymes and thus restricted to replication at sites in the host where such enzymes are found, i.e. the respiratory and intestinal tracts. Highly pathogenic avian influenza viruses possess multiple basic amino acids (arginine and lysine) at their HA0 cleavage sites 4 , 5 , 6 and are cleavable by an intracellular ubiquitous protease(s), probably one or more proprotein‐processing subtilisin‐related endoproteases of which furin is the leading candidate. 7 Highly pathogenic avian influenza viruses are able to replicate throughout the bird, damaging vital organs and tissues, causing extensive infection and death.
In the last decade there have been HPAI outbreaks in poultry caused by AIVs of H7 subtype. These include Eurasian outbreaks in Italy (H7N1, 1999–2000), The Netherlands (H7N7, 2003), Pakistan (H7N3, 2003), North Korea (H7N7, 2005) and the United Kingdom (UK; H7N7, 2008). 8 , 9 , 10 , 11 , 12 , 13 Outbreaks caused by H7 HPAI in the Americas have occurred in Chile (H7N3, 2002) and Canada (H7N3, British Columbia 2004 and Saskatchewan 2007). 14 , 15 , 16 European H7 LPAI poultry outbreaks have occurred in Italy and England (H7N3, 2002–03, 2006 and 2007), Italy and the UK (H7N2, 2007) and Denmark (H7N1, 2008). 8 , 9 , 17 , 18 , 19 , 20 , 21 American H7 LPAI poultry outbreaks have also occurred, such as in Virginia (H7N2, 2002) 14 , 22 and more recently in Arkansas (H7N3, 2008). 23 Infections of poultry due to both LPAI and HPAI viruses of H5 H7 subtypes are now considered as notifiable diseases by the World Organisation for Animal Health (OIE) 24 and within the European Union (EU). 25 Wild bird surveys have also identified H7 LPAI viruses in species of migratory waterfowl. 26 , 27
The ‘gold standard’ diagnostic approach to identifying AIV outbreaks or isolates from individual suspect cases is virus isolation (VI) in embryonated fowls’ eggs (EFEs), 28 , 29 followed by haemagglutination inhibition tests to identify the H‐subtype of the isolated virus. Following detection of H7 AIV, especially in poultry, there is a need to assess the virulence of the virus by deducing the amino acid sequence of the HA0 CS or by in vivo tests which may require up to several days. 1 While this approach remains the ‘gold standard’, recent years have seen the emergence of validated RealTime reverse transcriptase polymerase chain reaction (RRT PCR) protocols for the sensitive, specific and rapid detection of AIV RNA extracted directly from clinical specimens, where a superior sensitivity relative to AIV VI is regularly observed. 26 , 27 , 30 , 31 , 32 , 33 In this study we describe two RRT PCR assays for the detection of Eurasian H7 AIV isolates, and present accompanying validation data that includes the testing of clinical specimens. One H7 RRT PCR is optimized for the sensitive detection of Eurasian H7 isolates by amplification within the HA2 region of the H7 gene, while the H7 CS RRT PCR provides additional opportunities to include pathotyping through sequencing of this amplicon that encompasses the region of the H7 gene coding for the HA0 CS.
Materials and methods
Viruses
Avian influenza viral isolates were grown in 9‐ to 10‐day‐old specific‐pathogen free (SPF) EFEs and typed using standard protocols 28 , 29 In total 65 H7 and 57 non‐H7 AIV isolates of diverse geographic origin were used in this study, the latter including 24 H5 AIVs (1, 2). These EFE grown AIVs were diluted 100–1000‐fold prior to RNA extraction to give levels of virus that approximate to those present in clinical specimens. 31 , 34 Avian influenza virus titres were expressed as median egg infectious dose (EID50) determined by standard methods. 35 Non‐AI avian pathogens included laboratory isolates of Newcastle Disease virus (NDV), avian paramyxoviruses types 2 and 3 (APMV‐2 and APMV‐3), infectious bronchitis virus (IBV), infectious laryngotracheitis virus (ILTV), avian metapneumovirus (AMPV), avian reovirus and Salmonella senftenberg.
Table 1.
H7 AIVs (n = 65) used to validate the two H7 RRT PCRs
| H7 AIVs | H7 RRT PCR results | ||
|---|---|---|---|
| AI Subtype (LP/HP) | AI virus | HA2 | CS |
| H7N7 LP | A/turkey/England/647/77 | positive | positive |
| H7N1 LP | A/African starling/England‐Q/983/79 | positive | positive |
| H7N3 LP | A/turkey/England/262/79 | positive | positive |
| H7N7 HP | A/chicken/Australia (Bendigo)/85 | negative | positive |
| H7N1 LP | A/ostrich/South Africa/1609/91 | positive | positive |
| H7N1 LP | A/ostrich/South Africa (Oudtshoorn)/5352/92 | positive | positive |
| H7N2 LP | A/psittacine/Italy/1/91 | positive | positive |
| H7N1 LP | A/conure/England/1234/94 | positive | positive |
| H7N1 LP | A/parrot/England/1174/94 | positive | positive |
| H7N1 LP | A/fairy bluebird/Singapore/F92/94 | positive | positive |
| H7N1 LP | A/common iora/Singapore/F89/95 | positive | positive |
| H7N3 HP | A/chicken/Pakistan/CR2/95 | positive | positive |
| H7N7 LP | A/turkey/Ireland/PV74/1995 | positive | positive |
| H7N2 LP | A/turkey/Poland/85/95 | positive | positive |
| H7N4 LP | A/turkey/Poland/95/95 | positive | positive |
| H7N7 LP | A/England/268/96 | positive | positive |
| H7N1 LP | A/ostrich/Zimbabwe/222/96 | positive | positive |
| H7N7 LP | A/turkey/N Ireland/VF‐98‐1545/98 | positive | positive |
| H7N1 LP | A/teal/Taiwan/19·2‐37‐2/98 | positive | positive |
| H7N1 LP | A/duck/Taiwan/27. 2‐65‐9/98 | positive | positive |
| H7N1 LP | A/turkey/Italy/4042/99 | positive | positive |
| H7N1 LP | A/turkey/Italy/4294/99 | positive | positive |
| H7N1 LP | A/turkey/Italy/4829/V99 | positive | positive |
| H7N1 LP | A/chicken/Italy/1081/99 | positive | positive |
| H7N1 HP | A/chicken/Italy/4746/99 | positive | positive |
| H7N1 LP | A/turkey/Italy/977/99 | positive | positive |
| H7N1 HP | A/turkey/Italy/4640/99 | positive | positive |
| H7N3 HP | A/Peregrine falcon/UAE/188/99 | positive | positive |
| H7N1 HP | A/ostrich/Italy/984/00 | positive | positive |
| H7N1 LP | A/chicken/Italy/4/00 | positive | positive |
| H7N1 HP | A/falcon/Italy/2985/00 | positive | positive |
| H7N1 LP | A/turkey/Italy/117/00 | positive | positive |
| H7N1 HP | A/turkey/Italy/3/00 | positive | positive |
| H7N3 HP | A/chicken/Chile/02 | negative | negative |
| H7N3 LP | A/turkey/Italy/02 | positive | positive |
| H7N3 LP | A/turkey/Netherlands/03006814/03 | positive | positive |
| H7N7 HP | A/chicken/Netherlands/3227‐8/03 | positive | positive |
| H7N3 HP | A/chicken/Pakistan/66‐04/40/03 | positive | positive |
| H7N3 HP | A/chicken/British Columbia/514/04 | negative | negative* |
| H7N4 LP | A/mallard/Italy/4810‐79/04 | positive | positive |
| H7N1 LP | A/houbara bustard/Dubai/04 | positive | positive |
| H7N3 LP | A/chicken/England/4054/06 | positive | positive |
| H7N7 LP | A/swan/Germany/R557/06 | positive | positive |
| H7N4 LP | A/swan/Germany/R736/06 | positive | positive |
| H7N7 LP | A/gooseGermany/R752/06 | positive | positive |
| H7N7 LP | A/pochard/Germany/R916/06 | positive | positive |
| H7N1 LP | A/mute swan/Germany/R901/06 | positive | positive |
| H7N1 LP | A/duck/Turkey/55/49/06 | positive | positive |
| H7N2 LP | A/chicken/Wales/1306/07 | positive | positive |
| H7N7 LP | A/mute swan/Hungary/5973‐1/07 | positive | positive |
| H7N3 LP | A/mallard/Italy/1336/07 | positive | positive |
| H7N3 LP | A/chicken/Italy/2837‐54/07 | positive | positive |
| H7N3 LP | A/chicken/Italy3981‐90/07 | positive | positive |
| H7N1 LP | A/mallard/Italy/6103‐5/07 | positive | positive |
| H7N3 LP | A/mallard/Italy/6103‐12/07 | positive | positive |
| H7N3 LP | A/turkey/Italy/4527/07 | positive | positive |
| H7N3 HP | A/chicken/Saskatchewan/HR‐00010/07 | negative | negative* |
| H7N7 LP | A/whooper swan/Norway/07 | positive | positive |
| H7N7 LP | A/mallard/Sweden/123455/08 | positive | positive |
| H7N7 LP | A/mallard/Sweden/100993/08 | positive | positive |
| H7N7 LP | A/swan/Netherlands/12665/08 | positive | positive |
| H7N1 LP | A/duck/Denmark/53‐147‐8/08 | positive | positive |
| H7N1 LP | A/tern/Belgium/09745/08 | positive | positive |
| H7N7 LP | A/mallard/Bulgaria/3/08 | positive | positive |
| H7N7 HP | A/chicken/England/011406/08 | positive | positive |
Positive results by CS H7 RRT PCR include correct pathotype obtained by amplicon sequencing. Twelve of these are isolates from the Italian H7N1 LP and HPAI outbreak(s) in 1999–2000. 10 , 36
*indicates a CS H7 RRT PCR negative which contained a product which was directly purified and sequenced correctly as HPAI.
Table 2.
Non‐H7 AIVs (n = 57) used to validate the two H7 RRT PCRs. All were negative by both methods
| Non‐H7 AIVs | H7 RRT PCR results | ||
|---|---|---|---|
| AI Subtype (LP/HP) | AI virus | HA2 | CS |
| H1N1 | A/wild duck/Taiwan/26·2‐55‐1/98 | negative | negative |
| H1N1 | A/Duck/China (Yangzhou)//229/03 | negative | negative |
| H2N3 | A/duck/Germany/1215/73 | negative | negative |
| H2N3 | A/avian/Netherlands/03008927/03 | negative | negative |
| H2N5 | A/mallard/Italy/5709‐6/07 | negative | negative |
| H3N2 | A/duck/Malaysia/F11107/02 | negative | negative |
| H3N1 | A/duck/China/213/03 | negative | negative |
| H4N6 | A/duck/Czechoslovakia/56 | negative | negative |
| H4N6 | A/wild duck/Taiwan/16·2‐32‐2/98 | negative | negative |
| H4N6 | A/duck/Denmark/74‐66167‐1/02 | negative | negative |
| H5N1 HP | A/chicken/Scotland/59 | negative | negative |
| H5N3 HP | A/tern/South Africa/61 | negative | negative |
| H5N9 HP | A/turkey/Ontario/7732/66 | negative | negative |
| H5N8 HP | A/turkey/Ireland/83 | negative | negative |
| H5N2 LP | A/chicken/Mexico/94 | negative | negative |
| H5N2 LP | A/ostrich/Denmark/72420/96 | negative | negative |
| H5N2 HP | A/chicken/Italy/330/97 | negative | negative |
| H5N2 LP | A/duck/New Zealand/2/97 | negative | negative |
| H5N2 LP | A/chicken/Belgium/150/99 | negative | negative |
| H5N7 LP | A/mallard duck/Denmark/75‐64650/03 | negative | negative |
| H5N1 HP | A/Vietnam/1204/04 | negative | negative |
| H5N3 LP | A/duck/Italy/775/04 | negative | negative |
| H5N2 LP | A/duck/Singapore/F118/04 | negative | negative |
| H5N2 LP | A/ostrich/South Africa/N22704 | negative | negative |
| H5N2 LP | A/wild duck/Denmark/G13/04 | negative | negative |
| H5N1 HP | A/duck/Hunung/04 | negative | negative |
| H5N1 HP | A/chicken/Indonesia/04 | negative | negative |
| H5N1 HP | A/Q‐mesia/England/05 | negative | negative |
| H5N1 HP | A/turkey/Turkey/1/05 | negative | negative |
| H5N3 LP | A/turkey/Italy/05 | negative | negative |
| H5N3 LP | A/teal/England/EL94966/06 | negative | negative |
| H5N3 LP | A/mallard/Germany/R2557/06 | negative | negative |
| H5N3 LP | A/ostrich/Germany/R5/06 | negative | negative |
| H5N1 HP | A/turkey/England/2614/07 | negative | negative |
| H6N2 | A/chicken/South Africa/4796/02 | negative | negative |
| H6N2 | A/waterfowl/Bulgaria/A/05 | negative | negative |
| H6N1 | A/teal/England/EL94955/06 | negative | negative |
| H8N4 | A/turkey/Ontario/6118/66 | negative | negative |
| H8N4 | A/teal/England/7486/06 | negative | negative |
| H9N2 | A/turkey/Wisconsin/1/66 | negative | negative |
| H9N2 | A/chicken/Iran/98 | negative | negative |
| H9N2 | A/chicken/Pakistan/99 | negative | negative |
| H9N2 | A/turkey/Hungary/11/461/2001 | negative | negative |
| H9N9 | A/knot/England/SV497/02 | negative | negative |
| H10N7 | A/duck/Taiwan/15. 2‐31‐3/98 | negative | negative |
| H10N5 | A/mallard/Ireland/PV03‐002852/03 | negative | negative |
| H10N7 | A/mallard/Ireland/04 | negative | negative |
| H10N7 | A/mallard/England/GN63662/06 | negative | negative |
| H11N9 | A/white‐fronted goose/England/106/2001 | negative | negative |
| H11N3 | A/duck broiler/Singapore/F107/05/02 | negative | negative |
| H12N5 | A/duck/Alberta/60/76 | negative | negative |
| H12N2 | A/duck/Belgium/10157/07 | negative | negative |
| H13N6 | A/gull/Maryland/704/77 | negative | negative |
| H13N6 | A/herring gull/Finland/Li9875/05 | negative | negative |
| H14N5 | A/gull/Gurjev/263/82 | negative | negative |
| H15N6 | A/shearwater/Western Australia/79 | negative | negative |
| H16N3 | A/gull/Denmark/68110/02 | negative | negative |
Virus isolation from clinical specimens
Viral isolation was done by inoculating 9‐ to 10‐day‐old EFEs following standard procedures and AIV growth was confirmed by a positive haemagglutination assay (HA) result. 28 , 29
UK field specimens from wild birds and poultry
Four hundred swabs (oro‐buccal and tracheal) were collected from 200 wild birds in the UK during 2006 and originally tested by M gene RRT PCR. One hundred and eighty swabs (oro‐buccal and tracheal) were collected from 90 domestic fowl that had been used in an IBV vaccine study.
Clinical specimens from poultry infected experimentally
Poultry were infected experimentally with HPAI and LPAI viruses of H7N1 subtype, namely A/ostrich/Italy/984/00 (HPAI) and its LPAI progenitor A/chicken/Italy/1081/99. 10 , 36 Fifty‐one birds (33 SPF chickens and 18 turkeys) were directly inoculated in a variety of experiments via the ocular–nasal route with varying doses of H7N1 virus (1 × 101–106 EID50) given in 0·1 ml. Available specimens included 117 swabs (76 buccal, 41 cloacal) which were collected in 1 ml of brain–heart infusion broth (BHIB) containing antibiotics (Table 3). Ten organ specimens were obtained from one of the H7N1 HPAI infected turkeys and from an additional H7N1 HPAI infected chicken not included in Table 3. These were roughly homogenized and similarly stored in 1 ml BHIB (ca 10% w/v). Organs included brain, trachea, lung, liver, spleen, intestine, caecum, breast muscle, thigh muscle and non‐calcified feather calamus, obtained at post‐mortem after humane killing at 48 hours pi due to severe HPAI clinical signs. Swab fluids and the 20 organs were stored frozen at −70°C until required for testing by both H7 RRT PCRs, M gene RRT PCR and VI (Table 3).
Table 3.
Description of 117 swabs from H7N1 experimentally infected poultry used for validation of the two H7 RRT PCRs
| Group ID | Species | Pathogenicity of H7N1 inoculum | Numbers of swabs (buccal ‘B’, cloacal ‘C’) sampled at various times post‐infection (hours) | Total number of swabs per group | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 hours | 24 hours | 32 hours | 40 hours | 48 hours | 64 hours | 72 hours | 120 hours | ||||
| A | Chicken | LP | 1B*, 1C* | 3B | 3B | 6B, 6C, 4B | 10B, 1C | (15B, 15C) | 4B, 5C | 74 | |
| B | Turkey | LP | 3B, 5B | 8 | |||||||
| C | Turkey | HP | 7B, 4C, (5B, 3C) | 1B, 1C | 9B, 5C | 35 | |||||
| 117 total swabs from infection studies | |||||||||||
All swabs were collected from poultry that were directly inoculated with H7N1, except 26 swabs collected from sentinel birds indicated by italics
Parentheses indicate the additional 38 swabs from directly inoculated birds that were used for validation of both H7 RRT PCRs in comparison to M gene RRT PCR.
*indicates swabbing immediately before direct inoculation.
H7 sequence analysis and design of H7 RRT PCR primers and probes
The H7 AIV gene sequences were acquired from influenza virus databases. 37 , 38 These were aligned using the Clustal W algorithm in the Megalign software from the Lasergene bioinformatics package (DNAstar, Madison, WI, USA). This served to identify conserved regions of H7 sequences from Eurasian isolates, and to exclude potentially similar regions from non‐H7 subtypes. PrimerSelect (DNAstar, Madison, WI, USA) software from the Lasergene package was used to guide RRT PCR primer and probe design. Two sets of primers and hydrolysis probes were designed to amplify within the HA2 region and a region of the HA gene containing the CS sequence. Hydrolysis probes were labelled at the 5′ and 3′ ends with the 6‐carboxyfluorescein (FAM) and black hole quencher 1 (BHQ1) respectively (Sigma Genosys, Haverhill, UK), and all primer and probe sequence positions are numbered as the H7 haemagglutinin gene from isolate A/chicken/England/4266/06 (H7N3) (Accesion number: EF467825):
H7 HA2 RRT‐PCR
Primer LH6H7: 5′‐GGC CAG TAT TAG AAA CAA CAC CTA TGA‐3′ (1474–1500, sense)
Primer RH4H7: 5′‐GCC CCG AAG CTA AAC CAA AGT AT‐3′ (1583–1605, antisense)
Probe H7pro11: 5′‐FAM‐CCG CTG CTT AGT TTG ACT GGG TCA ATC T‐BHQ1–3′ (1542–1569, antisense)
H7 CS RRT‐PCR
Primer H7F: 5′‐CGT GCA AGT TTT CTG AGA GG‐3′ (803–822, sense)
Primer H7R: 5′‐GAC CTT CCC ATC CAT TTT CA‐3′ (1057–1076, antisense)
Probe H7‐TM1: 5′‐FAM‐AAC CCG CTA TGG CAC CAA ATA GGC CTC‐BHQ1–3′ (1026–1052, antisense)
RNA extraction
The Mini Viral RNA kit (Qiagen, Crawley, UK) was used to extract RNA manually from egg fluids, BHIB swab fluids and supernatants from organ homogenates (1, 2, 3) as described. 32 , 39 Robotic RNA extraction from the 580 swab specimens from the field was effected by the same kit chemistry adapted to a Universal Biorobot (Qiagen, Crawley, UK).
H7 RRT‐PCRs
Twenty‐three microlitre H7 RRT PCR volumes were prepared using the OneStep RT‐PCR kit (Qiagen) with the final magnesium chloride concentration supplemented to 3·75 mM and RNasin (Promega, Southampton, UK) included at 4 units per reaction. 30 , 32 ROX reference dye (Stratagene, Amsterdam, The Netherlands) was included as recommended by the manufacturer, and for both H7 RRT‐PCRs the final concentration of each primer and probe were 0·40 μM and 0·15 μM respectively. Two microlitres of extracted RNA was added to each reaction mix, and cycling conditions for both H7 RRT‐PCRs were identical to those used previously: 32 30 minute at 50°C, 15 minute at 95°C; then ×40 cycles: 10 second at 95°C, 30 second at 54°C and 10 second at 72°C using Mx3000 RealTime instruments (Stratagene). Fluorescence data was gathered at the end of each 54°C step. The H7 HA2 RRT PCR produces a 132 bp amplicon from all Eurasian H7 AIV isolates which were used to guide primer/probe design. The H7 CS RRT PCR produced a 274 bp amplicon from the A/chicken/England/06 (H7N3) LPAI template and other H7 LPAI viruses were predicted to be of very similar size. The H7 HPAI viruses would yield a larger product by the H7 CS RRT PCR depending on the precise length of the CS sequence. 1
M gene RRT‐PCR
This effects generic detection of all AIV H‐types essentially as described. 30 , 31 It utilized the OneStep RT‐PCR kit (Qiagen) with the final magnesium chloride concentration supplemented to 3·75 mM, with RNasin at 4 units per reaction and ROX included as for the H7 RRT PCRs. Cycling was conducted on a Mx3000 thermocycler: 30 minute at 50°C, 15 minute at 95°C; then ×40 cycles: 10 second at 95°C, 20 second at 60°C.
Hemi‐nested conventional H7 RT‐PCR
Two regions of the H7 gene were selected for conventional (i.e. gel detection) nested RT‐PCR of selected clinical specimens. Amplification was within the (i) HA1 portion and (ii) across the H7 CS region. For (i), primers were designed using an alignment of recent European H7 isolates followed by the PrimerSelect software to produce amplicons that could be sequenced:
First round RT‐PCR primers
Forward primer: 5′‐CAG TCC TTT GTA CCG AGT CCA‐3′ (668–688, sense)
Reverse primer: 5′‐TGA AGG CCC CAT TGA AAC‐3′ (774–791, sense)
Second round PCR primers
Forward primer: 5′‐TCC AGG AGC GAG GCC AC‐3′ (685–700, sense)
Reverse primer: As above for first round.
For (ii), amplification across the CS was achieved by conducting first round PCR with H7 primer pair GK 7·3/7·4 39 and second round PCR with the H7 primers described by Fouchier et al. (2004) 40 . For the first round conventional PCRs, 5 μl RNA was added to give a 50 μl volume containing the respective primers at 1 μM each in a OneStep RT‐PCR mix, which included RNasin as above. Upon completion of the first round, 0·5 μl product was transferred to a 50 μl nested reaction mix, which contained the respective second round primers at 1 μM each. First round cycling was: 30 minute at 50°C, 15 minute at 94°C; then ×35 cycles: 45 second at 94°C, 45 second at 52°C, 45 second at 72°C: with a final extension for 4 minute at 72°C. The nested (second) round used a similar cycle, but with the initial RT 30 minute step at 50°C excluded.
Sequencing of H7 amplicons
The H7 CS RRT PCR product was purified directly from the reaction mix by using the Gel Extraction Kit (Qiagen) following the instructions for non‐electrophoresed amplicons. Cycle sequencing was conducted using the BigDye v3.1 kit (Applied Biosystems (AB), Warrington, UK) with primers H7F and H7R and sequencing products were analysed on an ABI 310 instrument (AB). Hemi‐nested amplicons were purified on gels and sequenced using the respective amplification primers for sequencing. Sequencing data was assembled and inspected with Lasergene software.
Analytical Sensitivity of the H7 RRT PCRs
Ten‐fold dilution series of RNA were constructed from (i) egg‐quantified H7N1 preparations (A/ostrich/Italy/984/00 (HPAI) and A/chicken/Italy/1081/99 (LPAI)) and (ii) from in vitro RNA transcription products corresponding to sequences in these viruses amplified by the H7 HA2 and CS RRT PCRs. In order to produce in vitro RNA transcripts, both H7N1 isolates were first amplified conventionally using the H7 HA2 and H7 CS RRT PCR primer pairs where the 5′ end of the antisense primers included the T7 promoter. 41 Amplification conditions were as for the H7 RRT PCRs (above) except that the final magnesium chloride concentration was 2·5 mM, with ROX and the probes excluded. Amplicons were purified after gel electrophoresis in 3% agarose, and approximately 10 ng of each was added to a T7 in vitro RNA transcription reaction (T7 RiboMax Large Scale RNA Production System; Promega). DNase I digestion was according to the manufacturers’ protocols. T7 RNA transcripts were purified by spun columns (Rneasy Mini Kit; Qiagen) according to the manufacturer’s standard protocol for RNA purification for the H7 CS transcript (>200 nucleotides) and the outlined modified protocol for the smaller H7 HA2 transcript (<200 nucleotides). RNA concentration was determined spectrophotometrically with a NanoDrop instrument (Nanodrop ND1000 spectrophotometer; Thermo Scientific Loughborough, UK). Both H7 RRT PCRs and the respective non‐RT H7 RealTime PCRs (Quantitect Multiplex PCR “No Rox” kit, Qiagen) amplified the in vitro transcribed RNA to check for the relative level of any undigested DNA template. This was determined by a comparison of RT and non‐RT Ct values, where any residual DNA template could be diluted‐out to extinction, i.e. to a theoretical Ct value of >40. Ten‐fold dilutions of both H7 viral RNA and the H7 in vitro transcripts were tested by both H7 HA2 and CS RRT PCRs, where a correlation between Ct value and RNA quantity (both in terms of corresponding EID50 and transcript copy number) was determined as originally described. 31
Results
Specificity of H7 RRT PCR for detection of Eurasian H7 AIVs, other AIV H‐types and other avian pathogens
Both H7 RRT‐PCRs were used to test 65 H7 AIV isolates of diverse geographic origin (Table 1). These EFE grown AIVs were diluted 100–1000‐fold prior to RNA extraction to give titres approximate to those present in clinical specimens. 31 , 34 Sixty‐one of 65 H7 AIV isolates were successfully detected by the H7 HA2 RRT‐PCR, and 62/65 H7 AIVs were detected by the H7 CS RRT‐PCR. The four H7 AIVs not detected by the H7 HA2 RRT PCR were A/chicken/Australia/Bendigo/85 (H7N7), A/chicken/Chile/02 (H7N3) and both Canadian H7N3 isolates, A/chicken/British Columbia/04 (H7N3) and A/chicken/Saskatchewan/07 (H7N3) (Table 1). The H7 CS RRT PCR failed to detect A/chicken/Chile/02 (H7N3) and both Canadian H7N3 isolates. Sequencing of the H7 CS RRT PCR product yielded the correct pathotype for all 61 Eurasian lineage H7 isolates plus the Australian isolate, including successful discrimination of the 12 isolates from the Italian 1999–2000 H7N1 outbreak as LPAI or HPAI (Table 1). Although both Canadian H7N3 isolates failed to generate a fluorescent Real Time product with the H7 CS RRT PCR, amplicon purification followed by sequencing also gave an accurate HPAI pathotyping result (Table 1). Neither H7 RRT PCR detected any of the 57 non‐H7 AIVs, which included 24 H5 AIVs (Table 2), nor any of the non‐AI avian pathogens, NDV, PMV‐2 and ‐3, IBV, ILTV, AMPV, avian reovirus and Salmonella senftenberg.
Efficiency and analytical sensitivity of H7 Eurasian RRT PCRs
H7N1 isolates A/ostrich/Italy/984/00 (HPAI) and A/chicken/Italy/1081/99 (LPAI) were grown in EFEs and EID50 titres determined. 35 RNA was extracted from the H7N1 preparations and used to construct 10‐fold dilution series in duplicate, and these were tested by both H7 HA2 and H7 CS RRT‐PCRs. The Stratagene Mx 3000 instrumentation software plotted a standard curve of Ct values against the logarithmic dilutions: An R 2 value of >0·98 indicated a straight line, where the slope corresponded to efficiency in the range 90–110%. This indicated an optimized RRT PCR protocol. 42 Reproducibility was ensured by storage of H7N1 RNA standards in aliquots at −70°C. The sensitivity limit, measured in terms of infectious virus in the sample prior to RNA extraction, was 101 and 102 EID50/ml for the H7 HA2 and H7 CS RRT PCRs respectively, occurring within Ct values of about 35–37. Amplification of T7 in vitro transcribed RNA from both these H7N1 isolates showed that this analytical sensitivity corresponded to approximately 20 and 200 copies per reaction for the H7 HA2 and H7 CS RRT PCRs respectively.
Assessment of Eurasian H7 RRT PCRs with clinical specimens
Wild bird swabs
Both H7 RRT PCRs and M gene RRT PCR gave negative results with the 399 swab specimens collected from wild birds. These experiments included H7 robot extraction controls that gave a Ct value similar to that obtained by manual RNA extraction (data not shown). One cloacal swab that was positive by M gene RRT PCR and yielded an H9N2 isolate by VI (A/mallard/England/06) was negative by both H7 RRT PCRs.
Poultry swabs
The two H7 RRT‐PCRs, VI and M gene RRT PCR were used to test 180 swabs from 90 domestic fowl and 79 swabs (56 buccal, 23 cloacal) obtained from 51 SPF chickens and turkeys used in H7N1 infection experiments (Table 3). The 180 swabs from the 90 farmed chickens were all negative by both H7 RRT PCRs, M gene RRT PCR and VI. For the 79 swabs from experimentally infected poultry, 56 swabs gave concordant results by H7 HA2 RRT PCR and VI (34 positive and 22 negative), while 23 were H7 HA2 RRT PCR positive but VI negative (Table 4a). By combining data from these poultry swabs (n = 259), the relative sensitivity and specificity for the H7 HA2 RRT PCR compared to VI in EFEs was 100% and 89·8% respectively. It must be emphasized that this specificity calculation is based on the assumption that VI is more sensitive than H7 HA2 RRT PCR, where the 23 VI negatives that amplify by H7 HA2 RRT PCR (Ct range: 27·55–37·91; mean 33·68; median 34·19) are implied as false positives (Table 4a). However, no H7 false positives were identified by H7 HA2 RRT PCR during testing of H7‐negative swabs from wild birds (400) and 180 AI‐negative swabs from domestic fowl. Validation of the H7 HA2 RRT PCR in comparison to the M gene RRT PCR was extended to an additional 38 swabs (Table 3). Among this total of 297 swabs, 289 gave concordant results by both H7 HA2 and M gene RRT PCR methods. The 62 swabs that were positive by both M gene and H7 HA2 RRT PCRs demonstrated a very close association for the Ct value ranges, including mean and median values, obtained by both tests (Table 4b). The eight discordant results included three that were M gene RRT PCR positive but H7 HA2 RRT PCR negative, and five that were M gene RRT PCR negative but H7 HA2 RRT PCR positive. These eight Ct values (Table 4b) revealed all these specimens with apparent discrepant results to be weak positives in the respective AI RRT PCRs. The relative sensitivity and specificity for the H7 HA2 RRT PCR compared to M gene RRT PCR were 95·4% and 97·9% respectively.
Table 4.
Sensitivity and specificity determination for the H7 HA2 RRT PCR in comparison to (a) VI and (b) M gene RRT PCR, using 259 and 297 poultry swabs respectively
| (a) Sensitivity [34/(34 + 0) = 100%], specificity [202/(23 + 202) = 89·8%] | |||
|---|---|---|---|
| n = 259 | Virus isolation | Total | |
| + | − | ||
| H7 HA2+ | 34* | 23** | 57 |
| RRT PCR− | 0 | 22 + 180 = 202 | 202 |
| Total | 34 | 225 | 259 |
| (b) Sensitivity [62/(62 + 3) = 95·4%], specificity [227/(5 + 227) = 97.9%] | |||
| n = 297 | M gene RRT‐PCR | Total | |
| + | − | ||
| H7 HA2+ | 62*** | 5† | 67 |
| RRT PCR− | 3†† | 47 + 180 = 227 | 230 |
| Total | 65 | 232 | 297 |
Normal type numbers indicate specimens from experimentally infected (H7N1) poultry and italic numbers indicate specimens from farmed chickens.
*Ct range: 23·32–35·29; Mean 29·33; Median 29·36.
**Ct range: 27·55–37·91; Mean 33·68; Median 34·19.
***Ct range: M gene RRT PCR: 21·70–38·65; Mean 31·24; Median 31·38. H7 HA2 RRT PCR: 23·32–38·31; Mean 31·12; Median 30·78.
†Ct values: 34·86, 35·01, 37·02, 37·15, 37·18.
††Ct values: 35·48, 37·07, 37·21.
The same sensitivity and specificity determination was conducted for the H7 CS RRT PCR. The 259 swabs that were used in the comparison with VI revealed 243 concordant results by both methods (27 positive and 216 negative; Table 5a). Sixteen discordant results were obtained, seven of these were VI positive but H7 CS RRT PCR negative, and nine were VI negative but H7 CS RRT PCR positive (Ct range: 29·30–36·01; mean 33·71; median 34·18) (Table 5a). The relative sensitivity and specificity for the H7 CS RRT PCR relative to VI in EFEs were 79·4% and 96% respectively. The 38 additional swabs (Table 3) were then included to allow a larger comparison (n = 297) between the H7 CS RRT PCR and the M gene RRT PCR (Table 5b). This revealed 273 concordant results, namely 42 positives plus 231 negatives. Twenty‐four discordant results included 23 low titre M gene RRT PCR positives that were H7 CS RRT PCR negative (Table 5b), plus a single specimen that was M gene RRT PCR negative but H7 CS RRT PCR positive (Ct value 34·65) (Table 5b). The relative sensitivity and specificity for the H7 CS RRT PCR compared to M gene RRT PCR were 64·6% and 99·6% respectively.
Table 5.
Sensitivity and specificity determination for the H7 CS RRT PCR in comparison to (a) VI and (b) M gene RRT PCR, using 259 and 297 poultry swabs respectively
| (a) Sensitivity [27/(27 + 7) = 79·4%] specificity [216/(9 + 216) = 96·0%] | |||
|---|---|---|---|
| n = 259 | Virus isolation | Total | |
| + | − | ||
| H7 CS+ | 27* | 9** | 36 |
| RRT PCR− | 7 | 36 + 180 = 216 | 223 |
| Total | 34 | 225 | 259 |
| (b) Sensitivity [42/(42 + 23) = 64·6%] specificity [231/(1 + 231) = 99·6%] | |||
| n = 297 | M gene RRT PCR | Total | |
| + | − | ||
| H7 CS+ | 42*** | 1† | 43 |
| RRT PCR− | 23†† | 51 + 180 = 231 | 254 |
| Total | 65 | 232 | 297 |
Normal type numbers indicate specimens from experimentally infected (H7N1) poultry and italic numbers indicate specimens from farmed chickens.
*Ct range: 26·61–38·50; Mean 32·02; Median 32·09.
**Ct range: 29·30–36·01; Mean 33·71; Median 34·18.
***Ct range: M gene RRT PCR: 21·70–36·80; Mean 29·36; Median 29·08. H7 CS RRT PCR: 26·71–38·63; Mean 32·50; Median 32·57.
†Ct value: 34·65.
††Ct range: 29·57–38·65; Mean 35·35; Median 35·79.
Poultry organs
Ten organs or tissues from a turkey and a chicken infected experimentally with H7N1 HPAI were collected at 48 hours post‐infection. Total RNA extracts from these materials were tested by the two H7 RRT PCRs and the M gene RRT PCR (Table 6).
Table 6.
Ct values obtained by M gene and both H7 RRT PCRs from organs (48 hours post‐infection) from two H7N1 HPAI infected birds
| Organ | Turkey | Chicken | ||||
|---|---|---|---|---|---|---|
| M gene | H7 HA2 | H7 CS | M gene | H7 HA2 | H7 CS | |
| Brain | 13·81 | 13·37 | 14·72 | 16·01 | 15·26 | 16·10 |
| Trachea | 20·30 | 20·94 | 23·58 | 19·03 | 17·05 | 20·40 |
| Lung | 20·32 | 21·48 | 24·39 | 22·46 | 21·25 | 23·17 |
| Liver | 20·49 | 20·24 | 21·06 | 22·36 | 21·77 | 23·18 |
| Spleen | 16·98 | 16·8 | 18·73 | 21·76 | 21·39 | 24·01 |
| Intestine | 22·36 | 21·09 | 22·83 | 24·28 | 23·47 | 24·51 |
| Caecum | 22·33 | 21·96 | 24·10 | 22·91 | 22·33 | 24·88 |
| Breast | 20·53 | 20·42 | 21·81 | 22·66 | 22·88 | 24·63 |
| Thigh | 24·02 | 23·24 | 25·35 | 24·52 | 24·83 | 25·93 |
| Feathers | 22·90 | 23·71 | 25·78 | 26·44 | 25·98 | 28·37 |
Molecular pathotyping by sequencing of H7 CS RRT PCR products derived from clinical specimens
Sequencing the H7 CS RRT PCR product from the chicken brain (Ct: 16·1) and lung (Ct: 23·17) obtained post‐mortem at 48 hours pi correctly identified the H7N1 isolate as HPAI (Tables 6). Four turkey swabs collected at 24 hours pi (Group C, Table 3) yielded positive H7 CS RRT PCR results (Ct range: 28·2–33·09), and amplicon sequencing gave the correct HPAI result for all four. Seven swabs collected from H7N1 LPAI virus infected chickens at 64 hours pi (Group A, Table 3) yielded positive H7 CS RRT PCR results (Ct range: 27·70–35·77), and amplicon sequencing gave the correct LPAI result for all seven.
Detection of early HPAI viral shedding using H7 RRT PCRs
Eleven swabs were collected from turkeys at 24 hours after direct experimental infection with H7N1 HPAI virus (Group C, Table 3). At that time the birds appeared clinically normal. Five swabs were VI positive, all five of these were positive by H7 HA2 RRT PCR and one was positive by CS RRT PCR (Figure 1). Among the VI negative swabs collected at this time, four were positive by H7 HA2 RRT PCR and one by CS RRT PCR (Figure 1). One of the VI negative swabs gave a relatively higher value, i.e. Ct 30·43 (Figure 1), which corresponded to a theoretical infectivity titre of 4·8 × 102 EID50/ml that suggested a non‐viable and non‐infectious specimen. The other four VI negatives gave H7 HA2 RRT PCR Ct values in the range 37·35–37·84 which corresponded to a lower theoretical infectivity titre range of 0·63–0·85 × 101 EID50/ml (Figure 1). Two of the 24 hours pi swabs were negative by both the H7 RRT PCRs, M gene RRT PCR and VI. These were obtained from two turkeys, which had been inoculated with very low doses (1 × 101 and 1 × 102 EID50/ml) that did not result in productive infection (data not shown).
Figure 1.
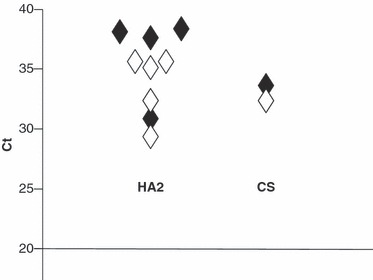
Distribution of H7 RRT PCR Ct values for swabs collected 24 hours pi from H7N1 HPAI directly infected turkeys (Group C, Table 3). Ct values are shown for both the H7 HA2 (n = 9) and CS (n = 2) RRT PCRs where open and filled symbol indicate VI positive and VI negative swabs respectively. Swab results from this group which were negative by H7 HA2 (two, both VI negative) and H7 CS (nine, i.e. five VI negative and four VI positive) RRT‐PCRs are not shown.
H7 RRT PCR detection of early LPAI viral shedding at 40–64 hours pi
Twenty‐six swabs were collected at 40–64 hours pi (Group A, Table 3) from clinically normal chickens that had been inoculated with H7N1 LPAI virus. Five swabs were VI positive, of which five were positive by H7 HA2 RRT PCR, and four positive by CS RRT PCR (Figure 2). Eighteen swabs sampled during this period were VI negative, but positive by H7 HA2 RRT PCR and seven by CS RRT PCR (Figure 2). In addition, three swabs were negative by both H7 RRT PCRs, M gene RRT PCR and VI.
Figure 2.
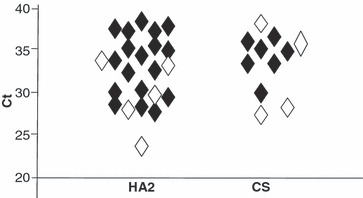
Distribution of H7 RRT PCR Ct values for swabs collected 40–64 hours pi from H7N1 LPAI directly infected chickens (Group A, Table 3). Ct values are shown for both the H7 HA2 (n = 23) and CS (n = 11) RRT PCRs where open and filled symbol indicate VI positive and VI negative swabs respectively. Swab results from this group which were negative by H7 HA2 (three, all VI negative) and H7 CS (15, i.e. 14 VI negative and one VI positive) RRT PCRs are not shown.
Further investigation of ‘weak’ H7 RRT PCR positive clinical specimens
Among the VI negative swabs collected from chickens infected with H7N1 LPAI, eight were selected for amplification by the two H7 hemi‐nested conventional PCRs. These were all H7 HA2 RRT PCR weak positives (i.e. Ct>33, Figure 2) that were H7 CS RRT PCR negative. These included five swabs collected 48 hours pi (Ct range: 37·02–38·21) and three collected 64 hours pi (Ct: 33·75, 34·19 and 35·13). Hemi‐nested conventional PCR in the H7 HA1 region gave positive results for five of these eight swabs and yielded clear confirmatory H7 sequences. These swabs produced H7 HA2 RRT PCR Ct values of 37·15, 38·21 (both 48 hours pi), 33·75, 34·19 and 35·13 (all three 64 hours pi). The first of these was negative by M gene RRT PCR, but this gave similar Ct values for the last four that corresponded to infectivity titres of 0·8, 5·8, 4·4 and 2·5 × 101 EID50/ml. The H7 HA2 RRT PCR Ct 34·19 swab was also successfully amplified with the H7 hemi‐nested conventional PCR across the CS. This provided clear confirmatory sequences that showed the correct pathotype had been identified.
Discussion
Eastern and western hemisphere H7 AIVs are known to be phylogenetically distinguishable from each other, 43 hence this study focused on two RRT PCRs designed for the detection of Eurasian H7 AIVs. Testing a large panel of geographically diverse AIV isolates verified their ability to specifically amplify Eurasian H7 isolates (1, 2). These included 29 Eurasian H7 isolates of diverse geographical origin and N‐types obtained during 2002–2008 (Table 1) that may be considered highly contemporary. Failure to detect four non‐Eurasian H7 isolates by H7 HA2 RRT PCR (Table 1) was unsurprising due to sequence mismatches in the primer/probe binding sequences (data not shown).
Twelve H7N1 isolates were included from the Italian 1999–2000 poultry outbreaks during which both LPAI and HPAI strains were collected. Sequencing of the H7 CS RRT PCR amplicon successfully identified the correct pathotype for each of the twelve Italian H7N1 viruses, as well as for 49 other Eurasian lineage H7 isolates that gave positive fluorescence by H7 CS RRT PCR (Table 1). This affirmed the value of this test in providing an opportunity for more rapid pathotyping. Another RRT PCR pathotyping approach used a CS‐region specific hydrolysis probe for direct discrimination of Asian‐origin ‘Qinghai’ lineage H5N1 HPAI viruses from other H5 AIVs. 44 This has an advantage of providing direct pathotyping during the RRT PCR without recourse to sequencing, and a similar approach for pathotyping H7 AIVs remains attractive. However, variability within the CS sequence of H5 HPAI viruses did not allow this HP‐specific probe to recognize viruses from any of the other H5 HPAI outbreaks that emerged from different H5 lineages. 44 The current study demonstrated H7 CS amplicon sequencing as a more widely applicable and robust means of pathotyping a variety of H7 viruses, which included H7 HPAI viruses from eight geographically distinct poultry outbreaks during the period 1985–2008 (Table 1).
Two recent Canadian H7N3 poultry isolates failed to give clear fluorescence by H7 CS RRT PCR, but gel electrophoresis revealed amplicons of the predicted size and sequencing correctly identified these as HPAI viruses. Sequence analysis of these isolates revealed significant diversity in the probe binding region for the H7 CS RRT PCR, while the primer binding region was relatively conserved (data not shown). However, more extensive mismatches in the primer/probe binding region accounted for H7 CS RRT PCR detection failure for A/chicken/Chile/02 (H7N3 HPAI) that was unsurprising, although sufficient conservation appeared to account for H7 CS RRT PCR detection of A/chicken/Australia (Bendigo)/85 (H7N7 HPAI) (Table 1).
Validation of both H7 RRT PCRs included comparison with VI by testing 259 poultry swabs, which included 79 swabs from poultry infected experimentally with H7N1 LPAI and HPAI viruses. In the case of the H7 HA2 RRT PCR, a relatively poor specificity of 89·8% compared to VI was due to 23 swabs that were RRT PCR positive but VI negative (Table 4a). However, specificity testing with 579 AIV negative field swabs revealed that false positives did not occur. For this reason, validation of the H7 HA2 RRT PCR was extended to comparison with M gene RRT PCR, a method known to be generally more sensitive than VI. 26 , 30 , 31 This was considered a more appropriate comparison as both RRT PCRs amplify their targets from the same analyte, namely extracted RNA, and neither is dependent on the presence of viable infectious virus. Hence validation of the H7 HA2 RRT PCR in comparison to M gene RRT PCR with 297 poultry swabs revealed a higher specificity of 97·9% (Table 4b). This reflected the greater overall sensitivity of M gene RRT PCR in comparison to VI. Clearly, the HA2 RRT PCR can detect a number of H7 positive clinical specimens that are VI negative, as shown by testing poultry swabs collected from birds at the early stages of experimental infections with H7N1 HPAI and LPAI viruses (1, 2). Genuine H7 positive results were confirmed by comparison to M gene RRT PCR results. H7 conventional nested PCRs amplified five available specimens which were ‘weak positive’ by H7 HA2 RRT PCR. The H7 conventional nested PCRs targeted other regions of the H7 gene, whereby amplicon sequencing provided independent evidence for presence of H7 AIV.
Analytical sensitivity determination utilised (i) H7 viral RNA extracted from serial dilutions of two H7N1 isolates that had been titrated in EFEs and (ii) copy numbers of the in vitro transcribed H7 RNA from the same viruses. In the H7 HA2 RRT PCR the detection limit was determined as 101 EID50/ml or 20 molecules, typically at Ct values of 35–37. However, additional investigations of low viral titre and VI negative clinical specimens revealed that H7 HA2 RRT PCR Ct values of 38 (<101 EID50/ml) could be confirmed as very weak positives by considering the results of additional H7 nested PCR investigations which included confirmatory sequencing, as discussed above. This validation study included examples of converting H7 HA2 RRT PCR Ct values into infectivity titres (EID50/ml), 31 particularly for such ‘weak positive’ H7 clinical specimens obtained at early time points during experimental infection. While the Ct values obtained from any sufficiently efficient RRT PCR may be interpreted at least semi‐quantitatively in a research setting, 31 , 42 the routine application for both these H7 RRT PCRs is to provide a qualitative diagnosis.
It must be emphasized that while the H7 nested conventional PCRs provided useful confirmatory data for five H7 HA2 RRT PCR weak positives, the nested approach is not recommended as a routine diagnostic method because of the risk of amplifying carry‐over contamination. The H7 CS nested conventional PCR has also shown its research value in amplifying this region of the likely H7N7 LPAI progenitor of the UK 2008 H7N7 HPAI poultry outbreak from a deep litter specimen from an affected chicken shed. 11 This environmental sample yielded a Ct value of 35·89 by H7 HA2 RRT PCR (unpublished data).
In contrast the H7 CS RRT PCR was characterized by a lower analytical sensitivity, and in comparison with VI and M gene RRT PCR revealed diagnostic sensitivities of 79·4% and 64·6% respectively (Table 5). H7 CS RRT PCR failed to detect seven swabs positive by VI and 23 swabs that were detected by M gene RRT PCR (Table 5). Its failure to detect five (VI) positive swabs collected at the early stages of H7N1 HPAI and LPAI infections (1, 2) underlined the reduced sensitivity of the H7 CS RRT PCR (Table 6). In considering all the 297 tested poultry swabs, H7 HA2 RRT PCR gave positive results for 24 swabs that were ‘No Ct’ by H7 CS RRT PCR, and there were no H7 HA2 RRT PCR ‘No Ct’ results among the 43 swabs positive by H7 CS RRT PCR (data not shown).
Although the H7 CS RRT PCR has a lower sensitivity than the H7 HA2 RRT PCR, it does have applications in investigating ongoing poultry outbreaks. The collection of a statistically valid number of clinical specimens from an epidemiological group of infected poultry gives the opportunity to link rapidly to pathotyping. For this reason the H7 CS RRT PCR fulfils requirements in identifying poultry H7 specimens as either HPAI or LPAI. 28 , 29
The greater sensitivity of the H7 HA2 RRT PCR compared to the H7 CS RRT PCR indicated that the former would be the preferred method for AI scenarios where low titres of H7 may be encountered in swabs, for example in screening programmes such as post H7 poultry outbreak surveillance, tracing or wild bird surveys. Data presented in this study has shown that H7 HA2 RRT PCR is sufficiently sensitive to detect H7 shedding from LPAI and HPAI experimentally‐infected poultry that are not displaying clinical signs. It is also applicable to H7 AI disease diagnosis in a European poultry outbreak investigation in which a statistically valid sample size should include oro‐pharyngeal and cloacal swabs from at least 20 birds in the same epidemiological unit. 28 The slightly reduced diagnostic sensitivity of 95·4% for the H7 HA2 RRT PCR relative to the M gene RRT PCR can be compensated by swabbing twenty birds per epidemiological unit, since flock sensitivity would be sufficient to identify a H7 infected premises (data not shown). Both H7 RRT PCRs have already proven their value during the UK H7 LPAI poultry outbreaks in 2006 and 2007. 17 , 18 , 21 The latter 2007 H7N2 outbreak was originally discovered at a backyard poultry farm, where subsequent surveillance included AI RRT PCR testing of 20 317 samples with extensive use of H7 HA2 RRT PCR during the four week outbreak period. This necessitated testing of poultry farms which were epidemiologically linked to this LPAI outbreak (unpublished data). This surveillance identified a second H7N2 LPAI infected poultry smallholding 18 by H7 HA2 RRT PCR. The H7 HA2 RRT PCR was used to confirm detection of H7N1 LPAI from an outbreak in Denmark in 2008 by testing swabs from farmed domestic ducks that did not display clinical signs (Jørgensen and Handberg, Personal Communication). 20
Both the H7 RRT PCRs confirmed infection with H7N7 HPAI at a large chicken farm in England in 2008. 11 This included testing of tissues, swabs and faeces from affected chickens together with mallards housed on the same premises. The H7 HA2 RRT PCR detected H7 virus in chicken specimens (including faeces), but no active H7 virus infection in the mallards. 11
This study describes the extensive validation of H7 HA2 and H7 CS RRT PCRs, and both are included among the EU recommended AI RRT PCR protocols. 28 Annual AI PCR proficiency testing has been organised by the EU since 2006, and EU and other participating laboratories have been very successful in H7 detection by implementing these H7 RRT PCR protocols. 45 While current international concerns remain largely directed towards H5N1 HPAI outbreaks, the continuing occurrence of H7 LPAI and HPAI outbreaks in poultry underlines the importance of thoroughly validated H7 RRT PCR protocols. Both the H7 RRT PCRs described in this study have already demonstrated their value during LPAI and HPAI H7 poultry outbreaks, both in disease diagnosis and accompanying active surveillance.
Acknowledgements
The authors wish to thank all laboratories that supplied avian influenza virus isolates for use in this study. Manuscript comments were appreciated from both the anonymous reviewers, as was the helpful contribution of Dr Dennis Alexander. Funding from the UK Department of the Environment, Food and Rural Affairs (Defra) is acknowledged for grant SV3029.
References
- 1. Senne DA, Pedersen JC, Suarez DL, Panigrahy B. Rapid diagnosis of avian influenza (AI) and assessment of pathogenicity of avian H5 and H7 subtypes by molecular methods. Dev Biol 2006; 126:171–177. [PubMed] [Google Scholar]
- 2. Swayne DE, Suarez DL. Highly pathogenic avian influenza. Rev Sci Tech 2000; 19:463–482. [DOI] [PubMed] [Google Scholar]
- 3. Rott R. The pathogenic determinant of influenza virus. Vet Microbiol 1992:33: 303–310. Erratum. Vet Microbiol 1993; 34: 398. [DOI] [PubMed] [Google Scholar]
- 4. Vey M, Orlich M, Adler S, Klenk HD, Rott R, Garten W. Hemagglutinin activation of pathogenic avian influenza viruses of serotype H7 requires the protease recognition motif R‐X‐K/R‐R. Virol 1992; 188:408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wood GW, McCauley JW, Bashiruddin JB, Alexander DJ. Deduced amino acid sequences at the haemagglutinin cleavage site of avian influenza A viruses of H5 and H7 subtypes. Arch Virol 1993; 130:209–217. [DOI] [PubMed] [Google Scholar]
- 6. Senne DA, Panigrahy B, Kawaoka Y et al. Survey of the hemagglutinin (HA) cleavage site sequence of H5 and H7 avian influenza viruses: amino acid sequence at the HA cleavage site as a marker of pathogenicity potential. Avian Dis 1996; 40:425–437. [PubMed] [Google Scholar]
- 7. Stieneke‐Gröber A, Vey M, Angliker H et al. Influenza virus hemagglutinin with multibasic cleavage site is activated by furin, a subtilisin‐like endoprotease. EMBO J 1992; 11:2407–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alexander DJ. An overview of the epidemiology of avian influenza. Vaccine 2007; 25:5637–5644. [DOI] [PubMed] [Google Scholar]
- 9. Alexander DJ. Summary of avian influenza activity in Europe, Asia, Africa, and Australasia, 2002–2006. Avian Dis 2007; 51:61–166. [DOI] [PubMed] [Google Scholar]
- 10. Capua I, Marangon S, Dalla Pozza M, To C, Cattoli G. Avian influenza in Italy 1997−2001. Avian Dis 2003; 47:839–843. [DOI] [PubMed] [Google Scholar]
- 11. Defra . Highly Pathogenic Avian Influenza – H7N7 in Egg Laying Poultry in Oxfordshire. Smith Square, London, UK: Nobel House; Epidemiology Report, situation at 2 July 2008. Version 1, Released July 2008. Available at: http://defraweb/animalh/diseases/notifiable/ai/pdf/epireport‐080711.pdf 2008 (accessed 18 May 2009). [Google Scholar]
- 12. Normile D. North Korea collaborates to fight bird flu. Science 2005; 308:175. [DOI] [PubMed] [Google Scholar]
- 13. Stegeman A, Bouma A, Elbers ARW et al. Avian influenza virus (H7N7) epidemic in the Netherlands in 2003: Course of the epidemic and effectiveness of control measures. J Inf Dis 2004; 190:2088–2095. [DOI] [PubMed] [Google Scholar]
- 14. Senne DA. Avian influenza in North and South America, 2002–2005. Avian Dis 2007; 51:167–173. [DOI] [PubMed] [Google Scholar]
- 15. Pasick J, Robinson J, Hooper‐McGrevy K et al. The roles of national and provincial diagnostic laboratories in the eradication of highly pathogenic H7N3 avian influenza virus from the Fraser Valley of British Columbia, Canada. Avian Dis 2007; 51:309–312. [DOI] [PubMed] [Google Scholar]
- 16. Canadian Food Inspection Agency . Avian Influenza – Saskatchewan. Available at: http://www.inspection.gc.ca/english/anima/heasan/disemala/avflu/2007sask/saske.shtml 2007 (accessed 18 May 2009). [Google Scholar]
- 17. Anonymous . Low pathogenic H7N2 avian influenza virus confirmed in Wales. Vet Rec 2007; 160:746. [Google Scholar]
- 18. Anonymous . Second case of H7 avian influenza. Vet Rec 2007; 160:814. [Google Scholar]
- 19. EU . Low pathogenic avian influenza in Italy. Epidemiological situation. Available at: http://ec.europa.eu/food/committees/regulatory/scfcah/animal_health/ai_it05062007.pdf 2007 (accessed 18 May 2009). [Google Scholar]
- 20. EU . Low Pathogenic Avian Influenza H7N1 in Denmark. Available at: http://ec.europa.eu/food/committees/regulatory/scfcah/animal_health/presentations/ai_78052008_dk.pdf 2008 (accessed 18 May 2009).
- 21. Manvell RJ, Londt BZ, Ceeraz V et al. Low pathogenic avian influenza in domestic fowl in Norfolk, England, March and April, 2006. Vet Rec 2008; 162:278–280. [DOI] [PubMed] [Google Scholar]
- 22. Akey BL. Low‐pathogenicity H7N2 avian influenza outbreak in Virginia during 2002. Avian Dis 2003; 47:1099–1103. [DOI] [PubMed] [Google Scholar]
- 23. Low pathogenic avian influenza (poultry), United States of America . Final report 15 July 2008. Available at: http://www.oie.int/wahis/public.php?page=single_report&pop=1&reportid=7176 2008 [accessed 18 May 2009].
- 24. OIE (World Organisation for Animal Health) . World Health Organization for Animal Health, Terrestrial Animal Health Code, chapter 10.4 “Avian influenza”. Paris: OIE; Available at: http://www.oie.int/eng/normes/mcode/en_chapitre_1.10.4.pdf 2008 (accessed 1 December 2008) [Google Scholar]
- 25. CEC . Council Directive 2005/94/EC of 20 December 2005 on Community measures for the control of avian influenza and repealing 92/40/EEC. Official J Eur Commission; L10:16–65. Available at: http://eur‐lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:010:0016:0065:EN:PDF 2006 (accessed 18 May 2009). [Google Scholar]
- 26. Terregino C, De Nardi R, Guberti V et al. Active surveillance for avian influenza viruses in wild birds and backyard flocks in Northern Italy during 2004–2006. Avian Pathol 2007; 36:337–344. [DOI] [PubMed] [Google Scholar]
- 27. Wallensten A, Munster VJ, Latorre‐Margalef N et al. Surveillance of influenza A virus in migratory waterfowl in northern Europe. Emerg Infect Dis 2007; 13:404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. EU . Diagnostic manual for avian influenza. Available at: http://eur‐lex.europa.eu/LexUriServ/site/en/oj/2006/l_237/l_23720060831en00010027.pdf 2006 (accessed 18 May 2009). [Google Scholar]
- 29. OIE (World Organisation for Animal Health) . Avian influenza: Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (2008), chapter 2.3.4. Paris: OIE; Available at: http://www.oie.int/eng/normes/mmanual/2008/pdf/2.03.04_AI.pdf 2008 (accessed 18 May 2009). [Google Scholar]
- 30. Spackman E, Senne DA, Myers TJ et al. Development of a real‐time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 haemagglutinin subtypes. J Clin Microbiol 2002; 40:3256–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee C‐W, Suarez DL. Application of real‐time RT‐PCR for the quantitation and competitive replication study of H5 and H7 subtype avian influenza virus. J Virol Methods 2004; 119:151–158. [DOI] [PubMed] [Google Scholar]
- 32. Slomka MJ, Pavlidis T, Banks J et al. Validated H5 Eurasian real‐time reverse transcriptase–polymerase chain reaction and its application in H5N1 outbreaks in 2005–2006. Avian Dis 2007; 51:373–377. [DOI] [PubMed] [Google Scholar]
- 33. Monne I, Ormelli S, Salviato A et al. Development and Validation of a one‐step RealTime PCR Assay for simultaneous detection of subtype H5, H7, and H9 avian influenza viruses. J Clin Microbiol 2008; 46:1769–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wood GW, Parsons G, Alexander DJ. Replication of influenza A viruses of high and low pathogenicity for chickens at different sites in chickens and ducks following intranasal inoculation. Avian Pathol 1995; 24:545–551. [DOI] [PubMed] [Google Scholar]
- 35. Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg 1938; 27:493–497. [Google Scholar]
- 36. Banks J, Speidel ES, Moore E et al. Changes in the haemagglutinin and the neuraminidase genes prior to the emergence of highly pathogenic H7N1 avian influenza viruses in Italy. Arch Virol 2001; 146:963–973. [DOI] [PubMed] [Google Scholar]
- 37. Bao Y, Bolotov P, Dernovoy D et al. The influenza virus resource at the National Center for Biotechnology Information. J Virol 2008; 82: 596–601. Available at: http://www.ncbi.nlm.nih.gov/genomes/FLU/Database/select.cgi (accessed 18 May 2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Macken C, Lu H, Goodman J, Boykin L. The value of a database in surveillance and vaccine selection; in Osterhaus ADME, Cox N, Hampson AW. (eds): Options for the Control of Influenza IV. Amsterdam: Elsevier Science, 2001. Available at: http://www.flu.lanl.gov/ (accessed 18 May 2009) [Google Scholar]
- 39. Slomka MJ, Coward VJ, Banks J et al. Identification of sensitive and specific avian influenza PCR methods through blind ring trials in the European Union. Avian Dis 2007; 51:227–234. [DOI] [PubMed] [Google Scholar]
- 40. Fouchier RAM, Schneeberger PM, Rozendaal FW et al. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc Natl Acad Sci USA 2004; 101:1356–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fronhoffs S, Totzke G, Stier S et al. A method for the rapid construction of cRNA standard curves in quantitative real‐time reverse transcription polymerase chain reaction. Mol Cell Probes 2002; 16:99–110. [DOI] [PubMed] [Google Scholar]
- 42. McPherson MJ, Møller SG. Real‐time RT‐PCR, chapter 9, 209–231; in: PCR, 2nd edn Taylor and Francis, 2006. [Google Scholar]
- 43. Rohm C, Horimoto T, Kawaoka Y, Suss J, Webster RG. Do hemagglutinin genes of highly pathogenic avian influenza viruses constitute unique phylogenetic lineages? Virol 1995; 209:664–670. [DOI] [PubMed] [Google Scholar]
- 44. Hoffmann B, Harder T, Starick E et al. Rapid and highly sensitive pathotyping of avian influenza A H5N1 virus by using real‐time reverse transcription‐PCR. J Clin Microbiol 2007; 45:600–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Slomka M. The third pan‐European AI PCR Proficiency Panel; in Joint Fourteenth Annual Meetings of the National Laboratories for Avian Influenza and Newcastle Disease of European Union Member States 2008. Available at: http://ec.europa.eu/food/animal/diseases/controlmeasures/avian/docs/programme14th_2008.pdf 2008 (accessed 18 May 2009). [Google Scholar]


