Abstract
Abstract The recent outbreaks of influenza A/H5N1 and ‘swine influenza’ A/H1N1 have caused global concern over the potential for a new influenza pandemic. Although it is impossible to predict when the next pandemic will occur, appropriate planning is still needed to maximize efficient use of resources and to minimize loss of life and productivity. Many tools now exist to assist countries in evaluating their plans but there is little to aid in writing of the plans. This study discusses the process of drafting a pandemic influenza preparedness plan for developing countries that conforms to the International Health Regulations of 2005 and recommendations of the World Health Organization.
Stakeholders from many sectors should be involved in drafting a comprehensive pandemic influenza plan that addresses all levels of preparedness.
Keywords: Influenza, pandemic, plan, preparedness
Introduction
The 1918 influenza A/H1N1 pandemic resulted in 50–100 million deaths, overwhelmed health services, and disrupted infrastructure worldwide. 1 While all countries were affected, lower income countries may have suffered the highest mortality. 2 Subsequent pandemics (e.g. 1957and 1968) also caused significant morbidity and mortality. 3 The outbreak of highly influenza A/H5N1 virus in humans and the recent outbreak of ‘swine influenza’ A/H1N1 raised concerns of a potential pandemic. 4 , 5 While it remains uncertain if swine influenza will become the next pandemic strain, it is estimated that a new influenza virus could sicken up to 30% of the population in one season. 6 A pandemic caused by a virulent strain would result in millions of deaths. Even a pandemic with low mortality could cause great morbidity and enormous economic losses.
Early response to a novel influenza virus might abort the onset of a pandemic. Non‐pharmaceutical interventions and healthcare surge capacity could slow the progression of a pandemic until a vaccine becomes available. 7 , 8 During 2005, the World Health Organization (WHO) recommended nations implement the International Health Regulations (IHR) and draft national pandemic influenza preparedness plans. 9 Preparedness is expected to improve public health capacity and mitigate effects of such an emergency. Preparing for a pandemic in developing countries is challenging because it requires the use of limited resources intended to control ongoing public health issues which cause the majority of disease (e.g. childhood diarrhea). 10 Many developing countries have not fully adopted IHR or planned for a pandemic. Investments in pandemic preparedness, however, can improve public health infrastructure (e.g. surveillance, laboratory, and outbreak response) and enable authorities in developing countries to better cope with all hazards. We discuss the process of drafting and revising a pandemic influenza preparedness plan for developing countries.
Initial steps
Effective pandemic preparedness and response should involve all sectors of government and civil society because a pandemic is likely to affect entire communities. In less affluent countries, human and material resources are often scarce and other sectors of government may be called upon to maintain essential services. The first step in developing a preparedness plan is engagement of decision makers and technical staff from a spectrum of offices including Ministries of Health, Agriculture, Defense, Internal Affairs, Finance, Labor, Transportation, Communications, and Education. 11 The initial act is to appoint a pandemic planning committee comprised of stakeholders from both private and public sectors, with oversight of ongoing preparedness. The committee’s role includes assigning technical members to draft specific plan segments, setting timelines, reviewing policy, and periodically meeting to verify progress. 12 The roles of the technical staff are to become familiar with the influenza research and adapt international standards to local realities.
Estimating the national impact of a pandemic
Before drafting a plan, staff should project the surge demands a pandemic might place on health care and other infrastructure. Several modeling programs (e.g. FluAid, FluSurge, and FluWorkLoss free‐ware) are available to assist with making projections. 13 It may be necessary to survey national and local infrastructure to determine capacity. Planning committees should be aware that the basic assumptions built into the models are derived from seasonal influenza transmission in a limited number of countries. Health‐seeking behaviors are likely different in developing countries and the models are meant to provide only reference points. 14 Once surge requirements have been estimated, policy decisions may be needed 15 to enhance critical infrastructure. Plans for all WHO pandemic phases should conform to international ethical, legal, and scientific standards. 16 Once a pandemic preparedness plan is drafted, the planning committee must ensure micro‐plans are written and operational at sub‐national levels.
Overview of the sections of a pandemic plan
A comprehensive pandemic plan includes: objectives and principles; incident management structure; surveillance; communication; mitigation measures; maintenance of essential services; agenda to address gaps in knowledge; and review, testing and revision of plans (Table 1). Plans within each section of the plan should state how activities will change in relation to the pandemic phase, and what information will trigger the change (e.g. severity of a pandemic). 9
Table 1.
Overview of the sections of a pandemic plan
| Section | Content |
|---|---|
| 1. Objectives and principles | Preparedness goals General principles of planning Pandemic influenza risk assessment (national and sub‐national) |
| 2. Incident management structure | Members and contact information Lines of authority Legal framework Decision making process |
| 3. Surveillance | Assessment of existing surveillance Recommended improvements Pre‐pandemic surveillance Surveillance during the pandemic |
| 4. Communication | Assessment of existing communications Recommended improvements Frequency, mechanism, and format of messages during each pandemic phase for each audience Thresholds for transition between messages |
| 5. Case management | Assessment of current case management Recommended improvementsTreatment and management guidelines Control of spread among contacts Infection control guidelines |
| 6. Community mitigation | Assessment of current community mitigation strategies Recommended improvements Criteria for school closures Criteria for event cancellation Social distancing measures |
| 7. Pharmaceutical interventions (acquisition, storage, distribution, and use, including safety and effectiveness) | Antivirals Antibiotics Vaccines Recommended improvements |
| 8. Maintenance of essential services | Assessment of health service capacity Recommended improvements Surge capacity of beds, personnel and equipment Mass triage protocols Plans for vulnerable and special populations Plans to support health care staff |
| 9. Agenda to address gaps in knowledge | Assessment of existing key gaps Studies during interpandemic phases Protocols for pandemic phase |
| 10. Review, testing and revision of plans | External review Tabletops and drills Field exercises Use of exercise results to revise plans |
Objectives and principles
The plan should identify the preparedness goals during each pandemic phase and provide a formal risk assessment. 17 Preparedness goals may include slowing the spread of the virus, decreasing morbidity and mortality, and maintaining essential services during the pandemic phase. General principles of preparedness, such as equity, transparency and citing of scientific underpinning, should be discussed. When drafting protocols, it is important to engage individuals involved in implementing the preparedness, and response activities. 18 The objectives and principles section should present the number of ill persons, hospital and critical care admissions, and deaths expected during an unmitigated pandemic. 15 , 16 Estimates of sub‐national morbidity and mortality will help leaders plan surge capacity during periods of high absenteeism.
Incident management structure
Defined lines of authority are essential to coordinate a multi‐sector response. This section should describe the duties of all members of the incident management structure and their leader. Laws cited should address the authorization of the formation of a planning committee and members’ authority to declare an emergency, restrict travel and mass gatherings, close schools and businesses, isolate or quarantine. 19 Triggers prompting a transition of incident management structure activities during each pandemic phase should be specified in this section. Triggers may differ depending on whether a pandemic begins inside or outside national borders. 20 Using terminology consistent with WHO standards will decrease confusion during times of crises. Communication guidelines for the incident management structure are essential. The appendices should include templates of situational reports for the incident command structure and decision‐making during planning. 21
Surveillance
Surveillance is critical for early detection of a pandemic and to monitor its progress. National influenza surveillance, community‐based surveillance, event‐based surveillance and rumor reporting including reporting, laboratory testing, and case definitions, should be described 22 (Figure 1). Plans should detail necessary improvements to build integrated epidemiology and laboratory capacity. The plan should include the process for informing healthcare providers about changes in reporting requirements, strategies for involvement of the public and media, and mechanisms for data dissemination. Specific events that trigger an investigation, such as clusters of severe acute respiratory infections (SARI), should be detailed. Protocols for outbreak investigations, staff responsible, response activities, and ongoing training should be included. 23 The plan should include methods for reporting data from local epidemiology units to the ministry of health, its partners, and WHO in compliance with the IHR (2005).
Figure 1.
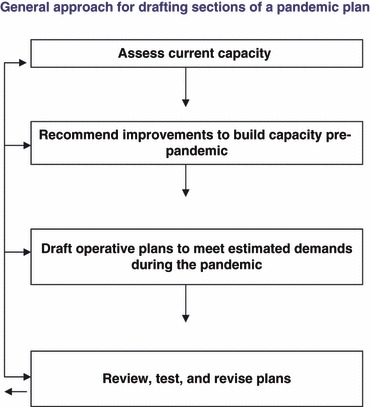
General approach for drafting sections of a pandemic plan.
Laboratory capacity, either nationally or through an agreement with partner nations and the WHO laboratory network, is essential for early detection of novel influenza. Plans should describe laboratory capacity for influenza typing, sub‐typing, and detection of antiviral resistance. 24 The plan should describe the nation’s ability to transport representative specimens; apply standardized laboratory protocols; practice biosafety and biosecurity; and link laboratory with epidemiology data. If laboratory capacity for evaluating novel strains is not available in‐country, the arrangements for transferring specimens to an external reference laboratory should be described. Procedures for the rapid, systematic, and timely sharing of influenza viruses with WHO Collaborating Centers should also be described.
Plans should detail triggers for shifting from routine surveillance to pandemic phase surveillance. For example, the primary pre‐pandemic surveillance objective is early detection of novel influenza strains. Event‐based pre‐pandemic surveillance systems (e.g. surveillance of SARI in healthcare workers) are directed toward early warning and response. Assessments of novel viruses with detailed case‐based investigation are necessary to guide clinical management, containment, and vaccine development. Establishing routine respiratory disease surveillance will help countries determine the epidemiology of seasonal influenza and aid countries in establishing systems for monitoring a pandemic. 25
As a pandemic begins, surveillance objectives include monitoring the geographic spread of the disease, impact on infrastructure (e.g. hospital bed occupancy), and morbidity and mortality in distinct populations. This information is needed to assess the impact of interventions and when these need to be discontinued. Surveillance can monitor subsequent pandemic waves and guide efforts to re‐institute interventions. During this phase, individual case reporting will be impractical; however, established event‐based and routine influenza surveillance can monitor the pandemic progression. Monitoring emergency department visits, pharmacy reports and death reporting may be useful. Reference laboratories should draft protocols to prioritize testing. Laboratory confirmation of individual cases will not be necessary during a pandemic but a laboratory may continue to test for changes in the virus (e.g. antiviral resistance). Pandemic surveillance may be similar to routine surveillance but, should address high absenteeism and a compromised national infrastructure. 1
Risk communication
Communication goals, strategies, and messages will evolve during a pandemic. This section should discuss communicating disease prevention measures to the public; case reporting and treatment guidelines to providers; surveillance findings to decision makers; and the information on new strains to international agencies during different pandemic phases. Thresholds for officially declaring the presence of a pandemic virus are needed. Planning committees should assess and recommend improvements to existing communications mechanisms (e.g. pandemic web pages, public service announcements, and hotlines) between stakeholders and the public. 26
Plans should anticipate the frequency, mechanism, and format of risk communications with media. Health educational messages, in each language spoken within the nation, should be drafted in consultation with risk behavior change communication specialists and included in the appendices. The committee will also have to identify and train spokespersons to deliver these messages. Different messages will be communicated during each pandemic phase. During the pandemic, messages would include cough and hand hygiene, guidance on seeking medical care, social distancing recommendations, and availability and priority for receiving antivirals, vaccines. Guidance for minimizing transmission in special settings (e.g. schools, residential institutions, workplaces, and public transportation) and messages for families caring for sick persons are needed. Strategies for communicating developments and updating clinical guidelines (e.g. new testing requirements for people potentially infected with novel influenza) using various mediums should be detailed. Guidelines include clinical management of suspect cases and how to prioritize use of ventilator support. There should also be regular updates to material in national pandemic websites for the public and healthcare staff.
Mitigation measures
Implementation of mitigation measures during each phase of a pandemic is critical. These include management of individual cases during clusters of novel influenza, potential containment operations, and mitigating disease during the pandemic. 27
Case management
This subsection should include recommendations for management of suspected novel influenza cases and contacts including infection control measures, isolation, and treatment of cases. 28 , 29 Guidelines for the duration and method of monitoring contacts, indications for quarantine, and antiviral prophylaxis are needed. 30 Plans should discuss the role of voluntary or involuntary isolation of cases and quarantine of contacts, implementation thresholds, and of enforcement agency responsible. Plans should describe settings for isolation and quarantine and how persons will be monitored for illness, cared for (e.g. provision of food, water, and other essential needs and a support network). A description of the role of limited antiviral prophylaxis for isolated persons and how to monitor the relative efficacy and duration of quarantine should be included. 31 Detailed infection control measures to encourage respiratory and hand hygiene and whether to recommend and provide masks to the general public should be considered. This subsection should also include guidance for infection control in non‐medical settings with a greater risk of person‐to‐person transmission (e.g. dormitories, military barracks, prisons, and psychiatric residential facilities). The plans should be sufficiently detailed to address the estimated morbidity and mortality and allow continuity of response in case of significant absenteeism or turnover in staff. 32
Community mitigation activities
This subsection is critical for countries in resource‐poor settings. For plans that address school closures/dismissals, limited public gatherings, changes to public transportation to reduce crowding, and other strategies for increasing social distancing, the following components should be included: (i) specifications of the agency responsible; the role law enforcement and the military; and which threshold criteria will be used (e.g. when >10% of children at school have influenza symptoms); 33 (ii) methods for communicating decisions to responsible agencies and the public; (iii) measures to minimize unintended consequences (e.g. impact on workforce if parents stay home to care for children); and, (iv) monitoring the effectiveness and duration of mitigation measures (e.g. 14 day school closures). 34
Pharmaceutical interventions
Plans should reflect the results of assessments on antiviral and antibiotic manufacturing and purchasing. A determination should be made as to whether stockpiles will be established and of their size and maintenance. 35 , 36 If a stockpile will not be established, the plans should describe how antiviral, antibiotic and vaccines may be mobilized during a pandemic. Plans should detail the use of limited pharmaceuticals and which groups will be prioritized for treatment or prophylaxis. 28 , 37 , 38 Plans should cite the justification for the prioritization scheme and describe how pharmaceuticals will be securely stored, distributed (e.g. cold chain), administered, and tracked. 30 Plans should describe how mass influenza and pneumococcal vaccination campaigns will be conducted, including the recording of who has completed the series.
Maintenance of essential services
A severe pandemic may disrupt essential services including power, water, sanitation, commerce, and health care. This section of the plan should discuss mechanisms for ensuring the continuity of key sectors of the economy as a pandemic evolves. Sector‐specific micro‐plans should identify critical activities; which can be reduced, which can be delayed/postponed; which can be moved/transferred (consider regulatory issues/industry standards); which staff/functions are essential and how to cross‐train back‐up personnel; the reliance on backup systems and how they are tested; and how organizations are ensuring partners and subcontractors have developed effective plans. Health plans should include guidance for hospitals, clinics, and other facilities on how to enhance bed capacity. 39 Plans should describe current hospital ward and intensive care bed capacity, the number of ventilators and staff trained to operate them, and who should receive intensive care when demand soon exceeds capacity. Plans should include a registry of volunteers and address any licensure and liability issues. Guidance to local health officials on how to identify, establish, and staff overflow treatment facilities should be provided. Plans should address the role of law enforcement in providing security for hospitals and alternate care facilities. Assessments of the current mortuary and crematory systems and plans to enhance capacity to handle, store, and track corpses are important.
Protocols for mass triage of emergency department patients with fever and respiratory illness should be included in the plans. Triage plans should help determine who needs hospital or outpatient care 40 and describe mechanisms to triage patients to hospital versus home care (e.g. public hotlines). Guidelines for outpatient management and home care for people with less severe cases of influenza should be included in the appendices. Health sector plans should provide guidance to hospitals and clinics about how to monitor and manage suspected influenza among providers and their families, and 41 how to support providers’ families and personal needs. Any plans for creating national or local stockpiles of essential medical supplies and equipment should be described. Storage, distribution, and tracking of personal protective equipment (PPE) should be described; and how supplies will be replenished.
Each sector’s plan should address workforce protection measures, including the provision of PPE; use of employee self‐evaluations/staff screening before worksite entry; promotion of good personal and environmental hygiene in the workplace; and procedures for handling potentially ill staff, flexible leave policies, and travel and return‐to‐work policies. Plans should identify strategies for social distancing in the workplace, such as limiting on‐site visitors and staff, reconfiguring work spaces, splitting teams/shifts, staggering breaks, extending working hours, and alternate worksites. As with other aspects of planning, it is important to address moral and ethical issues, such as the privacy of information, provision of antivirals, vaccines, and non‐medical supplies to select staff; staff’s duty to work; leave entitlement/payment of salary, and benefits; distinctions made between contractors and staff; and, the provision of support for ill/affected staff.
Agenda to address gaps in knowledge
Little information is available to guide pandemic planning. Most data used in projections for surge requirements are based on information obtained from Europe and North America. Countries should include a section in their pandemic plans to describe studies to address gaps in knowledge. During the interpandemic phases, studies could generate data risk assessment. 42 Relevant areas for study may include health seeking during outbreaks, the utility of case definitions, public response to interventions, and the impact of non‐pharmaceutical on transmission. Study protocols developed for implementation during the pandemic could determine antiviral and vaccine efficacy, antiviral resistance, and survival in hospitalized patients.
Review, testing and revision of plans
Pandemic plans should be externally reviewed, tested, and revised according to feedback. Often, when plans are externally reviewed, a number of problems are prevalent. 43 Some plans focus on recognition and containment of H5N1 without considering other sources of novel strains (e.g. swine influenza). Many plans lack calculations of demand during the pandemic, protocols to meet surge capacity, details of non‐pharmaceutical interventions, or operational detail. Site visits often demonstrate lack of staff’s familiarity with plans and poor micro‐planning in sectors outside of public health (e.g. private health sector). To avoid gaps in planning, committees can use tools like the United States Health and Human Services Inventory for Pandemic Preparedness or the WHO Checklist to assess their preparedness. 9 , 44 National committees should test preparedness using tabletop exercises, drills, and simulations to identify gaps. 45 Plans should specify the timeframe and responsible parties tasked to regularly revise the plan to address gaps or changing circumstances.
Summary
In most disasters, preventable suffering occurs as much from improper management of resources as from lack of resources. Resource management and allocation can be improved through planning. Preparedness efforts will facilitate compliance with the IHR and may slow the spread of pandemic influenza and reduce the strain on essential services. A good plan will address gaps between predicted surge requirements and available resources. Successful planning requires active involvement of all levels and sectors of government and thorough, periodic, and independent plan review, adoption of the latest paradigm in pandemic preparedness (e.g. new WHO recommendations).
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry.
Acknowledgements
The Centers for Disease Control & Prevention would like to acknowledge the Spanish Society of Vaccinology and the Hospital Clínic of Barcelona for the support of Raquel G. Alvarez during her time at the Influenza Division.
First Author Biography
The author is the Influenza Division, Centers for Disease Control and Prevention, assignee to the International Centre for Diarrhoeal Disease Research, Bangladesh tasked with assisting the local government with pandemic preparedness. He completed his MPH at the Harvard School of Public Health in 2003 and the Epidemic Intelligence Service in 2005.
Suggested appendices to pandemic plan
-
1
Activity matrix with deadlines, responsible parties, outcome measures (e.g. http://www.pandemicflu.gov/plan/federal/pandemic‐influenza‐implementation.pdf)
-
2
Organizational diagram of national public health command structure (e.g. http://www.bccdc.org/downloads/pdf/epid/reports/BCPIPPpercnt20Aug15‐FINALpercnt20Appendixpercnt20C.pdf)
-
3
Protocols for collection, packaging and shipment of influenza specimens to reference laboratories (e.g. http://www.moh.govt.nz/moh.nsf/pagesmh/4845/$File/national‐laboratory‐guidelines‐pandemic‐influenza.doc).
-
4
Summary description of existing surveillance systems, and plans for implementing enhanced surveillance when appropriate (e.g. http://www.amro.who.int/english/ad/dpc/cd/flu‐snl‐gpis.pdf).
-
5
Rapid response and containment guidelines for clusters of novel influenza cases (http://www.who.int/csr/disease/avian_influenza/guidelines/protocolfinal30_05_06a.pdf).
-
6
Case management guidelines (isolation, infection control measures, treatment)
-
a
During pandemic alert – for suspect or confirmed novel influenza cases
-
b
During pandemic – guidelines for both hospital and home care (e.g. http://www.hhs.gov/pandemicflu/plan/sup5.html#meetcrit)
-
a
-
7
Hospital and clinic infection control guidelines (e.g. http://www.hhs.gov/pandemicflu/plan/sup3.html and http://www.wpro.who.int/NR/rdonlyres/EA6D9DF3‐688D‐4316‐91DF‐5553E7B1DBCD/0/InfectionControlAIinhumansWHOInterimGuidelinesfor.pdf)
-
8
Antiviral and vaccine prioritization list and distribution plans (e.g. http://www.pandemicflu.gov/vaccine/allocationguidance.pdf).
-
9
Guidelines (checklist) for local health departments to develop their own jurisdictional operational plans (47) (e.g. http://www.epi.state.nc.us/epi/gcdc/pandemic/LHDChecklist2007.pdf)
-
10
Guidelines for businesses to prepare to continue essential services and minimize workplace transmission during a pandemic (e.g. http://www.pandemicflu.gov/plan/businesschecklist.html)
-
11
Guidance for hospitals and clinics on steps to take to enhance clinical care capacity during a pandemic (e.g. http://www.vch.ca/pandemic/docs/va03_capacity.pdf).
-
12
Guidance for hospitals and clinics on implementing mass triage plans for patients presenting with fever/respiratory symptoms to identify those in need of hospitalization versus those eligible for home care (e.g. http://www.fchn.org/docs/pandemicfluplan/admission.pdf).
-
13
Training materials for hospitals and clinics to use (Internet trainings, printed materials, and videos) to educate staff on infection control measures/appropriate use of PPE (e.g. http://www.hhs.gov/pandemicflu/plan/sup4.html).
-
14
Guidance for healthcare staff to develop personal and family emergency plans so that they will be able to come to work during the emergencies (e.g. http://www.pandemicflu.gov/plan/pdf/individuals.pdf).
References
- 1. Johnson NP, Mueller J. Updating the accounts: global mortality of the 1918–1920 “Spanish” influenza pandemic. Bull Hist Med 2002; 76:105–115. [DOI] [PubMed] [Google Scholar]
- 2. Murray CJL, Lopez AD, Chin B, Feehan D, Hill KH. Estimation of potential global pandemic influenza mortality on the basis of vital registry data from the 1918–20 pandemic: a quantitative analysis. Lancet 2006; 368:2211–2218. [DOI] [PubMed] [Google Scholar]
- 3. Simonsen L. The global impact of influenza on morbidity and mortality. Vaccine 1999; 17:S3–S10. [DOI] [PubMed] [Google Scholar]
- 4. Mounts AW, Kwong H, Izurieta HS et al. 1999. Case–control study of risk factors for avian influenza A (H5N1) disease, Hong Kong. J Infect Dis 1997; 180:505–508. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization . Swine influenza. 2009. Available at: http://www.who.int/mediacentre/news/statements/2009/h1n1_20090427/en/index.html (Accessed on 30 April 2009).
- 6. Nguyen Van‐Tam JS, Hampson AW. The epidemiology and clinical impact of pandemic influenza. Vaccine 2003; 21:1762–1768. [DOI] [PubMed] [Google Scholar]
- 7. World Health Organization Writing Group . Nonpharmaceutical interventions for pandemic influenza, national and community measures. Emerg Infect Dis 2006; 12:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fedson DS. Preparing for pandemic vaccination: an international policy agenda for vaccine development. J Public Health Policy 2005; 26:4–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization . Checklist for influenza epidemic preparedness. World Health Organization, 2005. Available at: http://www.who.int/csr/resources/publications/influenza/FluCheck6web.pdf (Accessed on 28 July 2008). [Google Scholar]
- 10. Oshitani H, Kamigaki T, Suzuki A. Major issues and challenges of influenza pandemic preparedness in developing countries. EID 2008; 14:875–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. U.S. Department of Homeland Security . Pandemic influenza. Best practices and model protocols. 2007. Available at: http://www.usfa.dhs.gov/downloads/pdf/PI_Best_Practices_Model.pdf (Accessed on 28 July 2008).
- 12. World Health Organization . WHO global influenza preparedness plan: the role of WHO and recommendations for national measures before and during pandemics. 2005. Available at: http://www.who.int/csr/resources/publications/influenza/WHO_CDS_CSR_GIP_2005_5.pdf (Accessed on 28 July 2008).
- 13. U.S. Centers for Disease Control and Prevention . FluSurge 2.0. 2006. Available at: http://www.cdc.gov/flu/tools/flusurge/ (Accessed on 28 July 2008).
- 14. Viboud C, Alonso WJ, Simonsen L. Influenza in tropical regions. PLoS Med 2006; 3:e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. U.S. Department of Health and Human Services . Planning & response activities. Available at: http://www.pandemicflu.gov/plan/index.html (Accessed on January 2008).
- 16. Kinlaw K, Levine R. U.S. Centers for Disease Control. Planning & Response Activities. Ethical guidelines in Pandemic Influenza – Recommendations of the Ethics Subcommittee of the Advisory Committee to the Director, Centers for Disease Control and Prevention. 2007. Available at: http://www.pandemicflu.utah.gov/docs/20070515‐PanFluEthicGuides.pdf (Accessed on 8 June 2009). [DOI] [PubMed]
- 17. World Health Organization . Current WHO phase of pandemic alert. Available at: http://www.who.int/csr/disease/avian_influenza/phase/en/index.html (Accessed on 30 April 2009).
- 18. World Health Organization . WHO pandemic influenza draft protocol for rapid response and containment. 2006. Available at: http://www.who.int/csr/disease/avian_influenza/guidelines/protocolfinal30_05_06a.pdf (Accessed on 28 July 2007).
- 19. World Health Organization . Global consultation on addressing ethical issues in pandemic influenza planning. WHO/CDS/EPR/GIP/2007.1 2006. Available at: http://www.who.int/csr/resources/publications/influenza/WHO_CDS_EPR_GIP_2007_1/en/ (Accessed on 8 June 2009).
- 20. World Health Organization . WHO global influenza preparedness plan. WHO/CDS/CSR/GIP/2005.5. 2005. Available at: http://www.who.int/csr/resources/publications/influenza/WHO_CDS_CSR_GIP_2005_5/en/ (Accessed on 8 June 2009).
- 21. U.S. Department of Health and Human Services . Communicating in a crisis: risk communication guidelines for public officials. 2002. Available at: http://www.riskcommunication.samhsa.gov/RiskComm.pdf (Accessed on 28 July 2008).
- 22. Grein TW, Kamara KBO, Rodier G et al. Rumors of disease in the global village: outbreak verification. Emerg Infect Dis 2000; 6:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. West Virginia Department of Health and Human Resources . General outbreak investigation/notification protocol. January 2007. Available at: http://www.wvdhhr.org/idep/pdfs/idep/Outbreaks/Outbreak_Investigation_Protocol.pdf (Accessed on 28 July 2008).
- 24. Hayden FG. Antiviral resistance in influenza viruses – implications for management and pandemic response. N Engl J Med 2006; 354:785–788. [DOI] [PubMed] [Google Scholar]
- 25. U.S. Centers for Disease Control and Prevention, Pan American Health Organization . Generic protocol for influenza surveillance. 2006. Available at: http://www.ops‐oms.org/English/AD/DPC/CD/flu‐snl‐gpis.pdf (Accessed on 28 July 2008).
- 26. World Health Organization . WHO outbreak communication guidelines. WHO/CDS/2005.28.2005. Available at: http://www.who.int/csr/resources/publications/WHO_CDS_2005_28en.pdf (Accessed on 28 July 2008).
- 27. U.S. Centers for Disease Control and Prevention . Interim pre‐pandemic guidance: community strategy for pandemic influenza mitigation in the United States‐ Early, targeted, layered use of nonpharmaceutical interventions. February 2007. Available at: http://www.pandemicflu.gov/plan/community/community_mitigation.pdf (Accessed on 28 July 2008).
- 28. World Health Organization . WHO rapid advice guidelines on pharmacological management of humans infected with avian influenza A (H5N1) virus. WHO/PSM/PAR/2006.6. 2006. Available at: http://www.who.int/medicines/publications/WHO_PSM_PAR_2006.6.pdf (Accessed on 28 July 2008).
- 29. Glezen WP. Influenza control. N Engl J Med 2006; 355:79–81. [DOI] [PubMed] [Google Scholar]
- 30. World Health Organization . WHO Guidelines on the use of vaccines and antivirals during influenza pandemics. WHO/CDS/CSR/RMD/ 2004.8. 2004. Available at: http://www.who.int/csr/resources/publications/influenza/11_29_01_A.pdf (Accessed on 28 July 2008).
- 31. Rothstein MA, Talbott MK. Encouraging compliance with quarantine: a proposal to provide job security and income replacement. Am J Public Health 2007; 97:S49–S56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. U.S. Centers for Disease Control and Prevention . Interim in public health guidance for the use of facemasks and respirators in non‐occupational community settings during an influenza pandemic. 2007. Available at: http://www.pandemicflu.gov/plan/community/commaskguidance.pdf (Accessed on 28 July 2008).
- 33. Haber MJ, Shay DK, Davis XM et al. Effectiveness of interventions to reduce contact rates during a simulated influenza pandemic. Emerg Infect Dis 2007; 13:581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kahn LH. Pandemic influenza school closure policies. Emerg Infect Dis 2007; 13:344–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ferguson NM, Cummings DA, Fraser C, Cajka CJ, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature 2006; 442:448–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fedson DS. Confronting an influenza pandemic with inexpensive generic agents: can it be done? Lancet Infect Dis 2008; 8:571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Longini IM, Halloran ME, Nizam A, Yang Y. Containing pandemic influenza with antiviral agents. Am J Epidemiol 2004; 159:623–633. [DOI] [PubMed] [Google Scholar]
- 38. U.S. Department of Health and Human Services . Guidance on antiviral drug use during an influenza pandemic. 2008. Available at: http://www.pandemicflu.gov/vaccine/antiviral_use.pdf (Accessed on 2 January 2008).
- 39. Burkle FM. Population‐based triage management in response to surge‐capacity requirements during a large‐scale bioevent disaster. Acad Emerg Med 2006; 13:1118–1129. [DOI] [PubMed] [Google Scholar]
- 40. Christian MD, Hawryluck L, Wax RS et al. Development of a triage protocol for critical care during an influenza pandemic. CMAJ 2006; 175:1377–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. U.S. Centers for Disease Control and Prevention . Interim recommendations for infection control in health‐care facilities caring for patients with known or suspected avian influenza. 2004. Available at: http://www.cdc.gov/flu/avian/professional/pdf/infectcontrol.pdf (Accessed on 28 July 2008).
- 42. Carrat F, Luong J, Lao H, Salle AV, Lajaunie C, Wackernagel H. A ‘small‐worldlike’ model for comparing interventions aimed at preventing and controlling influenza pandemics. BMC Med 2006; 4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Coker RJ, Mounier‐Jack S. Pandemic influenza preparedness in the Asia‐Pacific region. Lancet 2006; 368:886–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Secretariat of the Pacific Community . Pacific island HPAI and pandemic influenza preparedness checklist. April 2007. [cited 2009 April 8]. Available at http://wwwx.spc.int/phs/pphsn/Meetings/PRIPPP/PI_HPAI_Pandemic_Influenza_Preparedness_Checklist_20070404.pdf (Accessed on 28 July 2008).
- 45. European Centre for Disease Prevention and Control . Assessment tool for influenza preparedness in European countries – with a main focus on pandemic preparedness. January 2007. Available at: http://ecdc.europa.eu/pdf/Assessmentpercent20tool.pdf (Accessed on 8 June2009).


