Abstract
Background The best form of protection against influenza is high‐titred virus‐neutralizing antibody specific for the challenge strain. However, this is not always possible to achieve by vaccination due to the need for predicting the emerging virus, whether it be a drift variant of existing human endemic influenza type A subtypes or the next pandemic virus, for incorporation into the vaccine. By activating additional arms of the immune system to provide heterosubtypic immunity, that is immunity active against all viruses of type A influenza regardless of subtype or strain, it should be possible to provide significant benefit in situations where appropriate antibody responses are not achieved. Although current inactivated vaccines are unable to induce heterosubtypic CD8+ T cell immunity, we have shown that lipopeptides are particularly efficient in this regard.
Objectives To examine the role of vaccine‐induced CD8+ T cells in altering the course of disease due to highly virulent H1N1 influenza virus in the mouse model.
Methods The induction of influenza‐specific CD8+ T cells following intranasal inoculation with lipopeptide vaccine was assessed by intracellular cytokine staining (ICS) and the capacity of these cells to reduce viral loads in the lungs and to protect against death after viral challenge was determined.
Results and conclusions We show that CD8+ T cells are induced by a single intranasal vaccination with lipopeptide, they remain at substantial levels in the lungs and are efficiently boosted upon challenge with virulent virus to provide late control of pulmonary viral loads. Vaccinated mice are not only protected from death but remain active, indicative of less severe disease despite significant weight loss.
Keywords: CD8+ T cells, heterosubtypic immunity, lipopeptide, vaccine, virulent influenza virus
Introduction
As countries gear up to face a potential pandemic onslaught by avian H5N1 influenza virus with small stocks of specific vaccine being tested for immunogenicity, and protocols and approvals in place for mass production and use of these vaccines, we find that it is not this virus that has efficiently hopped the species barrier but another, an H1N1 virus thought to originate from classic and Eurasian swine influenza, that is the focus of concern at present. Our inability to contain this virus in its apparent source country and our lack of preparedness with specific vaccine support the view that it is time to think differently about how we vaccinate against influenza.
As proponents of vaccines that will induce cross‐protective as well as strain‐specific responses, we have been working on strategies to induce the main effector of heterosubtypic immunity, the broadly crossreactive CD8+ cytotoxic T lymphocyte (CTL). These studies seek to provide a better understanding of the contribution of CTL to amelioration of disease and the requirements for induction of these cells through vaccination. A strategy we have found to be extremely successful for CTL induction is the use of lipopeptides incorporating short synthetic peptides representing CD8+ and CD4+ T‐cell epitopes. Lipopeptides have been used as vaccines in a number of contexts and have proven safe and immunogenic in animal and human studies (reviewed in 1, 2]). Although different lipids can be incorporated into these vaccines, we have previously shown that, for the induction of anti‐influenza CTL responses, the lipid moiety S‐[2,3‐bis(palmitoyloxy)propyl]cysteine (Pam2Cys) derived from the MALP‐2 lipoprotein of Mycoplasma fermentans, 3 is superior in this regard. 4 , 5 The lipid serves as an adjuvant by targeting Toll‐like receptor (TLR) 2 on the surface of dendritic cells (DC), triggering DC maturation. 4 , 5 As TLR 2 is an endocytic receptor, 6 antigen loading of the DC is facilitated.
Using this minimal epitope approach it is possible to selectively induce influenza‐specific T cells, allowing direct evaluation of their effectiveness in the absence of confounding antibody responses. Although both classes of T cell are induced by the lipopeptide vaccines, we have shown by depletion of the CD4+ subset prior to challenge 7 that these cells have only a minor role as effectors in viral clearance. Instead, co‐induced CD4+ T cells are likely to provide help for substantial memory CD8+ T‐cell development. 8 , 9 , 10 Vaccines of this type are not capable of preventing infection because the primed CD8+ T cells are only reactivated by infected cells. Instead, these vaccines can allow faster resolution of infection, potentially leading to less severe disease and prevention against death from overwhelming infection. In this respect CTL‐inducing vaccines can be thought of as providing a similar outcome to anti‐viral drugs, without the need for timely implementation following infection and constant administration.
In this study we extend our previous observations on the role of lipopeptides in control of influenza of moderate severity 4 , 5 , 11 to examine this in the context of severe infection. Although the ferret model is preferred for the study of protection against influenza, 12 the model is not sufficiently developed for the assessment of T‐cell responses. In contrast, influenza viruses of different virulence that are adapted to use the alpha 2–3 Gal‐linked sialic receptor found in the murine respiratory tract 13 are available and the epitopes recognized by CD4+ and CD8+ T cells in this species are well defined. Here we examine the ability of lipopeptides based on broadly cross‐subtype CTL‐inducing epitopes to alter the course of disease following infection of naïve animals with highly virulent H1N1 influenza virus.
Materials and methods
Influenza viruses
The viruses used were A/Puerto Rico/8/34 (H1N1) influenza virus (PR8, Mount Sinai), and the reassortant virus A/Memphis/1/71(H3N2) x A/Bellamy/42 (H1N1) virus (Mem71, H3N1). Virus stocks were grown for 2 days in the allantoic cavity of 10‐day old embryonated hen’s eggs and infectious allantoic fluid stored at −70C.
Influenza virus infection of mice
Inbred 6‐ to 10‐week‐old specific pathogen free BALB/c mice (H‐2d) were bred and housed at the University of Melbourne, Parkville, Victoria, Australia. Mice were anaesthetized with Penthrane (Abbot Laboratories, North Chicago, IL, USA) and infected intranasally (i.n.) with virus in 50 μl phosphate‐buffered saline (PBS). At 5 or 7 days post‐infection, mice were killed by cervical dislocation and lungs removed and homogenized as previously described. 7 Infectious virus in the lung homogenates was quantified by plaque assay using confluent monolayers of Madin‐Darby canine kidney (MDCK) cells. 14 Vaccinated and control mice were also infected with a lethal dose of PR8 virus and monitored daily. In accordance with University of Melbourne Animal Ethics Committee approved procedures, infected mice were culled at the humane endpoint determined by a combination of weight loss and clinical signs.
Lipopeptide immunization
The lipopeptide vaccine was synthesized as described previously 4 , 15 and incorporated a peptide corresponding to the CD4+ T‐cell epitope GALNNRFQIKGVELKS from the HA2 chain of Mem71 haemagglutinin 16 and a peptide corresponding to the CD8+ T‐cell epitope TYQRTRALV 17 from the nucleoprotein of PR8 influenza virus (NP147–155) that is conserved in all influenza type A viruses. The peptides are linked via a lysine residue and Pam2Cys is attached, with two intervening serine residues, to this lysine to form a branched structure. Mice were immunized i.n. with 20 nmol of lipopeptide dissolved in 50 μl of PBS under light Penthrane anaesthesia.
Flow cytometric analysis of lymphocytes
Lungs, spleen and mediastinal lymph nodes (MLN) were processed through a metal sieve. Red blood cells were removed from washed cell suspensions by lysis in Tris‐buffered ammonium chloride solution. Cells (2 × 107cells/ml) were added to each well of a 96‐well microplate and incubated with or without peptide (TYQRTRALV) for 6 hours at 37°C in 5% CO2. Cells were then stained with Fluorescein isothiocyanate (FITC)‐conjugated rat anti‐mouse CD8 antibody for 30 minutes (clone 53–6·7; Pharmingen, San Diego, CA, USA) then fixed with cytoperm/cytofix (BD Biosciences Pharmingen) for 20 minutes. Cells were washed and stained with PE‐conjugated rat anti‐mouse IFNγ antibody for 30 minutes (Pharmingen). All staining procedures were done at 4°C. CD8+ T cells were enumerated by flow cytometry using a FACScan (Becton Dickinson, San Jose, CA, USA). Data was analysed using CellQuest Software version 5.1.1 (BD Biosciences).
Statistical analysis
Statistical analyses was performed using the non‐parametric Mann–Whitney U‐test and Prism 4 software (GraphPad Software Inc., San Diego, CA, USA).
Results and Discussion
Lipopeptide vaccination of mice resulted in the appearance of CD8+ T cells specific for the TYQRTRALV epitope with the kinetics shown in Figure 1A. Few antigen‐specific CD8+ T cells could be detected in organs prior to 5 days after vaccination but numbers rapidly increased and peaked on day 7 in all three anatomical sites. Specific cells in the spleen and MLN subsequently declined rapidly to almost undetectable levels by day 10, yet CD8+ T cell numbers in the lungs declined at a much slower rate and remained at substantial levels until at least day 20 post‐vaccination. This is compatible with our earlier observation that lipopeptide‐induced long‐lived memory CD8+ T cells could be detected in the lungs several months after vaccination, where they acted as early mediators against subsequent influenza infection. 11
Figure 1.
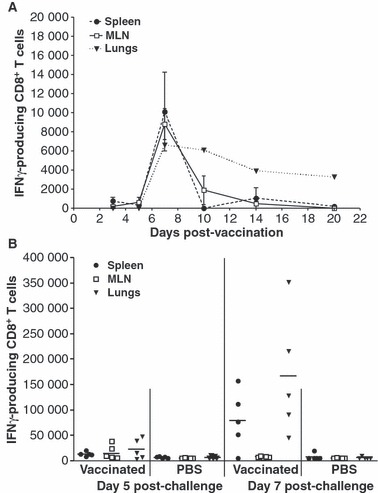
Lipopeptide vaccines induce potent IFN‐γ producing CD8+ T cells. Groups of BALB/c mice (n = 3 in A, n = 5 in B) were immunized i.n. with 20 nmol of lipopeptide in 50 μl of PBS under Penthrane anaesthesia. (A) Kinetics of lipopeptide‐induced CD8+ T cells. At each indicated time point, single cell suspensions of the spleens, MLN and lungs were prepared and stained with anti‐CD8 and anti‐IFN‐γ antibodies then analysed by flow cytometry. Data is presented as the total number of CD8+ T cells producing IFN‐γ in response to the CTL epitope peptide minus the number detected in the absence of peptide stimulation. Mean values and standard deviation are shown. (B) Recall of specific vaccine‐induced CD8+ T‐cells responses by virus. Four weeks post lipopeptide vaccination or inoculation with PBS, mice were challenged with 500 pfu of PR8 virus i.n. Single cell suspensions from organs analysed by flow cytometry as in (A). Each individual symbol represents an individual mouse with the lines indicating the mean value.
Having established a memory pool of CD8+ T cells we then investigated whether these could be boosted in response to viral challenge. Mice were immunized with lipopeptide and 28 days later were infected with 500 pfu of PR8 virus. At 5 and 7 days post‐infection, influenza‐specific CD8+ T cells were enumerated in the spleen, MLN and lungs (Figure 1B). Vaccinated mice had greater numbers of antigen‐specific CD8+ T cells, especially in the spleen (P = 0·0079) and lungs (P = 0·0317), than did unvaccinated PBS control mice 5 days post‐challenge. In the lungs, this represented a greater than sixfold boost on average above the resident effector memory cells induced by lipopeptide immunization prior to challenge. By day 7 post‐infection, the influenza‐specific CD8+ T cells in the spleen and lungs reached even greater levels, increasing a further eightfold either by expansion or recruitment, possibly from the MLN which showed a decrease in cell numbers relative to day 5.
We then determined whether the lipopeptide vaccine‐induced responses could impact upon the course of infection with viruses of different virulence. Mem71 virus, which is a virus of moderate virulence, does not kill naïve BALB/c mice even at very high doses and, although the virus can grow to considerable titres in the lungs, the developing immune response of the infected mouse can clear this virus by 7 days post‐infection. In contrast, PR8 virus is highly lethal to mice at doses <100 pfu; at lower doses, the virus can be cleared but this takes at least three more days than for Mem71 virus, and at lethal doses, lung viral titres peak at higher levels and drop only slightly prior to culling of the animal at the humane endpoint (data not shown).
As shown in Figure 2, lipopeptide vaccination provided substantial viral clearance in the lungs against the moderately virulent Mem71 virus infection, reducing viral loads by 92·1% on day 5 post‐infection compared to those of control mice. A reduction in viral load was also seen in lipopeptide‐vaccinated mice challenged with a sublethal dose of PR8 but this was of lesser magnitude (68·4%). In contrast, no difference was observed in the high viral loads present on day 5 in vaccinated and control mice challenged with a lethal dose of PR8 virus. Nevertheless, since we had shown that boosting of lipopeptide‐induced CD8+ T cell responses by this virulent virus resulted in even higher levels of specific cells on day 7 compared to day 5 post‐infection (Figure 1B), we also examined viral loads on day 7 post lethal PR8 challenge (Figure 2). At this time point, suppression of viral replication in lipopeptide‐primed mice was observed. On average there was 100‐fold less virus in vaccinated mice, with one mouse having undetectable pulmonary virus.
Figure 2.
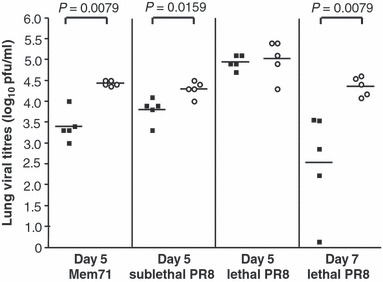
Lung viral clearance after lipopeptide vaccination. Groups of 5 BALB/c mice were immunized i.n. with 20 nmol of lipopeptide (closed squares) or PBS (open circles). Four weeks later, mice were challenged with 104·5 pfu of Mem71 virus or either 50 pfu (sublethal) or 500 pfu (lethal) of PR8 virus in 50 μl of PBS and lungs were collected 5 days after challenge. Another group of mice were challenged with 500 pfu of PR8 virus and lungs removed on day 7 post‐infection. Viral titres were determined by plaque assay on confluent MDCK monolayers. Each individual symbol represents an individual mouse with the lines showing the geometric mean titres. Statistically significant differences between vaccinated and control mice are indicated.
This drop in viral load on day 7 in the course of lethal infection was highly significant but whether it came too late to impact on overwhelming disease remained to be examined. Mice were inoculated with a single dose of lipopeptide and then challenged with a lethal dose of PR8 and monitored daily for weight loss and the development of clinical signs. In accordance with ethical guidelines, mice were culled at the pre‐determined humane endpoint indicative of an inability to overcome the infection. By days 7–12, unvaccinated mice needed to be progressively culled as they began to show severe signs of illness, became inactive and had lost substantial bodyweight (Figure 3). The lipopeptide‐vaccinated group also lost significant weight but they showed only mild signs of infection, such as ruffled fur, and remained active throughout. Consequently these mice recovered and eventually returned to their starting weight.
Figure 3.
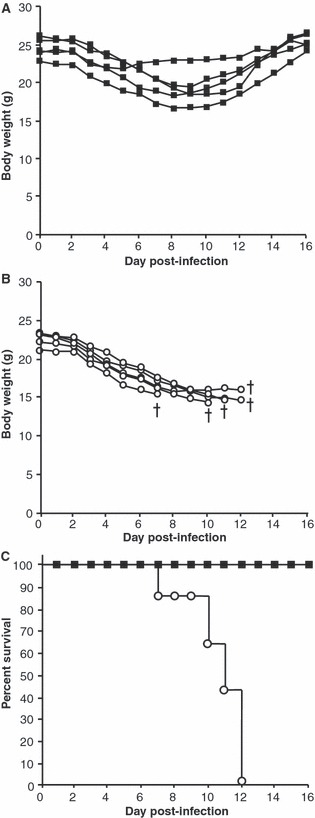
Lipopeptide‐vaccinated mice survived a lethal virus infection. Groups of 5 BALB/c mice were immunized i.n. with 20 nmol of lipopeptide (closed squares, A) or PBS (open circles, B). Four weeks later, mice were infected i.n. with 500 pfu of PR8 virus. Mice were weighed daily and monitored for clinical signs. Individual mouse bodyweight is shown in grams (A and B). Kaplan–Meier plot showing the percentage survival of mice (C). †denotes mice that were culled.
CD8+ T cells induced by prior virus infection are potent mediators in lessening morbidity and mortality caused by a virulent virus 18 but to our knowledge this is the first time that lipopeptide vaccine‐induced CD8+ T cells have been shown to function in this regard. It has previously been shown that the level of memory T cells maintained in the lung dictates the level of viral clearance provided during a secondary influenza challenge 19 (reviewed in 20). It is possible that this is the key to the success of this type of vaccine which appears to be particularly good at priming pulmonary effector memory responses 11 , 21
Importantly, the single lipopeptide, by virtue of its highly conserved CD8+ T‐cell epitope can induce immunity that is effective against different subtypes of virus and impacts on disease progression in the absence of specific antibody. The challenge for any epitope‐based vaccine strategy designed for use in humans is to identify a panel of such broadly cross‐protective CD8+ T‐cell epitopes appropriate for this species and to construct a vaccine with sufficient epitopes to encompass the diversity of highly polymorphic human MHC molecules. Our knowledge of such epitopes has been greatly enhanced by two recent studies by Assarsson et al. 22 and Lee et al. 23 These studies not only provide the necessary information for creating tools to evaluate CD8+ T cell‐inducing vaccine efficacy in humans but also for the construction of epitope‐based vaccines. Peptides that cross‐bind to each of the HLA class I supertypes, i.e., sets of HLA class I molecules with largely overlapping peptide‐binding repertoires, 24 , 25 have the potential to be recognized by a high proportion of the population. The combined coverage of the six supertypes of the major ethnicities (HLA‐A1, A2, A3, A24, B7 and B44) is >98%, 25 overcoming a significant barrier to the application of epitope‐based vaccines.
With the definition of numerous epitopes that have remained invariant throughout the entire evolution of influenza virus it appears that functional constraints on these regions pose unsustainable fitness costs to the virus when mutated, doing much to assuage concerns that CD8+ T‐cell vaccines might impose selective pressure on CTL epitopes. Although mutations that allow escape from recognition by certain influenza virus‐specific CTL have been reported, 26 because influenza is an acute infection that can be ultimately cleared by the developing antibody response, the impact of CTL‐escape variants in an individual would be minimal.
The goal of vaccination for the induction CD8+ T cells, or boosting of existing responses, would be to maintain the memory population at protective levels. These cells are thought to have a very short half‐life after virus infection, estimated at 2–3 years in man. 27 One could envisage that optimal levels of memory CD8+ T‐cell responses might be critical in the elderly in whom antibody‐inducing vaccine efficacy is relatively low. Co‐priming of a strong CD8+ T‐cell response might also reduce the levels of non‐responsiveness in situations of conventional vaccine mismatch, 28 , 29 or in the elderly experiencing ‘original antigenic sin’. 30 , 31 Results of the human studies of McMichael et al. 32 where high levels of CTL reduced the amount and period of viral shedding would also imply a population benefit by reducing transmission opportunity.
Seasonal use of vaccines that significantly boost heterosubtypic CD8+ T‐cell responses would potentially provide the general population with some reduction in morbidity and mortality at the start of a pandemic when specific vaccines to the emerging virus are scarce. In our current situation this is especially important because the global capacity to manufacture specific antibody‐inducing vaccine falls far short of that required to vaccinate the world’s population and it is unlikely that anti‐viral drugs will fill this gap, 33 particularly if resistance becomes an issue. Hopefully the lessons learnt from studies such as our own will help support the argument for exploiting additional arms of the immune response for the control of influenza.
Acknowledgement
This work was supported by the National Health and Medical Research Council of Australia.
References
- 1. BenMohamed L, Wechsler SL, Nesburn AB. Lipopeptide vaccines – yesterday, today, and tomorrow. Lancet Infect Dis 2002; 2:425–431. [DOI] [PubMed] [Google Scholar]
- 2. Brown LE, Jackson DC. Lipid‐based self‐adjuvanting vaccines. Curr Drug Deliv 2005; 2:383–393. [DOI] [PubMed] [Google Scholar]
- 3. Muhlradt PF, Kiess M, Meyer H, Sussmuth R, Jung G. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. J Exp Med 1997; 185:1951–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jackson DC, Lau YF, Le T et al. A totally synthetic vaccine of generic structure that targets Toll‐like receptor 2 on dendritic cells and promotes antibody or cytotoxic T cell responses. Proc Natl Acad Sci USA 2004; 101:15440–15445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lau YF, Deliyannis G, Zeng W, Mansell A, Jackson DC, Brown LE. Lipid‐containing mimetics of natural triggers of innate immunity as CTL‐inducing influenza vaccines. Int Immunol 2006; 18:1801–1813. [DOI] [PubMed] [Google Scholar]
- 6. Schjetne KW, Thompson KM, Nilsen N et al. Cutting Edge: Link between innate and adaptive immunity: Toll‐like receptor 2 internalizes antigen for presentation to CD4+ T cells and could be an efficient vaccine target. J Immunol 2003; 171:32–36. [DOI] [PubMed] [Google Scholar]
- 7. Deliyannis G, Jackson DC, Ede NJ et al. Induction of long‐term memory CD8(+) T cells for recall of viral clearing responses against influenza virus. J Virol 2002; 76:4212–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Belz GT, Wodarz D, Diaz G, Nowak MA, Doherty PC. Compromised influenza virus‐specific CD8(+)‐T‐cell memory in CD4(+)‐T‐cell‐deficient mice. J Virol 2002; 76:12388–12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 2003; 300:337–339. [DOI] [PubMed] [Google Scholar]
- 10. Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 2003; 300:339–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deliyannis G, Kedzierska K, Lau YF et al. Intranasal lipopeptide primes lung‐resident memory CD8+ T cells for long‐term pulmonary protection against influenza. Eur J Immunol 2006; 36:770–778. [DOI] [PubMed] [Google Scholar]
- 12. Maher JA, DeStefano J. The ferret: an animal model to study influenza virus. Lab Anim (NY) 2004; 33:50–53. [DOI] [PubMed] [Google Scholar]
- 13. Ibricevic A, Pekosz A, Walter MJ et al. Influenza virus receptor specificity and cell tropism in mouse and human airway epithelial cells. J Virol 2006; 80:7469–7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tannock GA, Paul JA, Barry RD. Relative immunogenicity of the cold‐adapted influenza virus A/Ann Arbor/6/60 (A/AA/6/60‐ca), recombinants of A/AA/6/60‐ca, and parental strains with similar surface antigens. Infect Immun 1984; 43:457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zeng W, Ghosh S, Lau YF, Brown LE, Jackson DC. Highly immunogenic and totally synthetic lipopeptides as self‐adjuvanting immunocontraceptive vaccines. J Immunol 2002; 169:4905–4912. [DOI] [PubMed] [Google Scholar]
- 16. Jackson DC, Drummer HE, Brown LE. Conserved determinants for CD4+ T cells within the light chain of the H3 hemagglutinin molecule of influenza virus. Virol 1994; 198:613–623. [DOI] [PubMed] [Google Scholar]
- 17. Bodmer HC, Pemberton RM, Rothbard JB, Askonas BA. Enhanced recognition of a modified peptide antigen by cytotoxic T cells specific for influenza nucleoprotein. Cell 1988; 52:253–258. [DOI] [PubMed] [Google Scholar]
- 18. Christensen JP, Doherty PC, Branum KC, Riberdy JM. Profound protection against respiratory challenge with a lethal H7N7 influenza A virus by increasing the magnitude of CD8(+) T‐cell memory. J Virol 2000; 74:11690–11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liang S, Mozdzanowska K, Palladino G, Gerhard W. Heterosubtypic immunity to influenza type A virus in mice. Effector mechanisms and their longevity. J Immunol 1994; 152:1653–1661. [PubMed] [Google Scholar]
- 20. Woodland DL, Kohlmeier JE. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat Rev Immunol 2009; 9:153–161. [DOI] [PubMed] [Google Scholar]
- 21. Day EB, Zeng W, Doherty PC, Jackson DC, Kedzierska K, Turner SJ. The context of epitope presentation can influence functional quality of recalled influenza A virus‐specific memory CD8+ T cells. J Immunol 2007; 179:2187–2194. [DOI] [PubMed] [Google Scholar]
- 22. Assarsson E, Bui HH, Sidney J et al. Immunomic analysis of the repertoire of T‐cell specificities for influenza A virus in humans. J Virol 2008; 82:12241–12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee LY, Ha do LA, Simmons C et al. Memory T cells established by seasonal human influenza A infection cross‐react with avian influenza A (H5N1) in healthy individuals. J Clin Invest 2008; 118:3478–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sette A, Sidney J. HLA supertypes and supermotifs: a functional perspective on HLA polymorphism. Curr Opin Immunol 1998; 10:478–482. [DOI] [PubMed] [Google Scholar]
- 25. Sidney J, Peters B, Frahm N, Brander C, Sette A. HLA class I supertypes: a revised and updated classification. BMC Immunol 2008; 9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Voeten JT, Bestebroer TM, Nieuwkoop NJ, Fouchier RA, Osterhaus AD, Rimmelzwaan GF. Antigenic drift in the influenza A virus (H3N2) nucleoprotein and escape from recognition by cytotoxic T lymphocytes. J Virol 2000; 74:6800–6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McMichael AJ, Gotch FM, Dongworth DW, Clark A, Potter CW. Declining T‐cell immunity to influenza, 1977–82. Lancet 1983; 2:762–764. [DOI] [PubMed] [Google Scholar]
- 28. De Jong JC, Beyer WE, Palache AM, Rimmelzwaan GF, Osterhaus AD. Mismatch between the 1997/1998 influenza vaccine and the major epidemic A(H3N2) virus strain as the cause of an inadequate vaccine‐induced antibody response to this strain in the elderly. J Med Virol 2000; 61:94–99. [PubMed] [Google Scholar]
- 29. Skowronski DM, Masaro C, Kwindt TL et al. Estimating vaccine effectiveness against laboratory‐confirmed influenza using a sentinel physician network: results from the 2005–2006 season of dual A and B vaccine mismatch in Canada. Vaccine 2007; 25:2842–2851. [DOI] [PubMed] [Google Scholar]
- 30. Fazekas de St G, Webster RG. Disquisitions of original antigenic sin. I. Evidence in man. J Exp Med 1966; 124:331–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yarchoan R, Nelson DL. Specificity of in vitro anti‐influenza virus antibody production by human lymphocytes: analysis of original antigenic sin by limiting dilution cultures. J Immunol 1984; 132:928–935. [PubMed] [Google Scholar]
- 32. McMichael AJ, Gotch FM, Noble GR, Beare PA. Cytotoxic T‐cell immunity to influenza. N Engl J Med 1983; 309:13–17. [DOI] [PubMed] [Google Scholar]
- 33. Kieny MP, Costa A, Hombach J et al. A global pandemic influenza vaccine action plan. Vaccine 2006; 24:6367–6370. [DOI] [PubMed] [Google Scholar]


