Abstract
Background Rapid tests are now widely available to assist the diagnosis of influenza; implementation may optimise the use of antiviral and antibiotic agents in the clinical management of influenza.
Objective To explore the clinical management of children with influenza‐like illness (ILI) when rapid influenza tests were and were not performed.
Methods Between 15 January 2007 and 30 April 2007, a standardised questionnaire was used to record the clinical features of children aged 1–12 years who presented to office‐based paediatricians in Germany with febrile ILI during periods of local influenza activity. For each paediatric contact, a clinical diagnosis of either ‘influenza positive’, ‘influenza negative’ or ‘suspected ILI’ was made. Where performed, the outcome of a Clearview Exact Influenza A + B rapid test was recorded. Prescriptions for antiviral agents and antibiotic medications were also recorded.
Results A total of 16 907 questionnaires were evaluated. After fever (an entry criteria for all children), cough (84·6%), fatigue/decreased activity (83·0%), rhinorrhoea (73·7%) and headache (67·1%) were the most common symptoms. Influenza was clinically diagnosed in 56·8% (9596/16 907) of cases. The antiviral oseltamivir was prescribed for 24·6% (178/725) of children who were influenza positive by symptom assessment alone and 60·1% (4618/7685) of children who were influenza positive by rapid test. Antibiotics were less commonly prescribed for children who were influenza positive by rapid test [3·5% (271/7685) versus 17·2% (125/725) for symptom assessment alone].
Conclusions In children with ILI, a positive rapid test result for influenza promotes the rational use of antiviral agents and reduces the inappropriate use of antibiotic medications.
Keywords: Antiviral, antibiotic, diagnosis, influenza, rapid test
Introduction
The burden of influenza on children is substantial. Infection rates may exceed 30% in pre‐school and school‐age children, 1 , 2 with day care attendance further increasing the risk of infection. 3 One in four infected children can go on to develop acute otitis media, 4 , 5 while complications involving the upper and lower respiratory tract, such as sinusitis and pneumonia, are also common. 4 , 5 , 6 , 7 Hospitalisation rates for children <2 years are comparable with those for adults >65 years, 8 , 9 , 10 , 11 and clinic and emergency room visits for children <5 years may be up to 250 times more common than hospital admissions. 12 Children are also an important vector for the spread of influenza within the community. 13 , 14 Despite these observations, immunisation against influenza is not uniformly recommended in children. 15
In the absence of cohesive vaccination programmes, antiviral therapy is an alternative intervention for reducing the burden of influenza in children. Currently, the neuraminidase inhibitors oral oseltamivir and inhaled zanamivir are the recommended antivirals for the management of influenza in children. 16 When used as recommended and early after the onset of symptoms, both agents can significantly reduce the severity and duration of symptoms and risk of associated complications. 17 , 18 , 19 , 20 , 21 , 22 Although both medications are indicated for use in children, 23 , 24 oseltamivir can be used in younger children (aged ≥1 year versus aged ≥5 years for zanamivir) and the availability of an oral formulation may make it more suitable for use in some children. For optimal clinical benefit, both antivirals must be initiated within 48 hours of the onset of symptoms. For this reason, a diagnosis of influenza must be established early in the course of the illness.
In clinical practice, symptom assessment is the principal method for diagnosing influenza. In children, however, diagnosis may be complicated or delayed by the presence of both typical (e.g. fever, cough, sore throat, rhinorrhoea) and atypical (e.g. abdominal complaints) symptoms 5 that are shared with other common, and often co‐circulating, respiratory viruses (e.g. respiratory syncytial virus). 25 Children are also less able to clearly describe their symptoms than adults. While surveillance data on local influenza activity can assist the diagnosis, new strategies to improve the early diagnosis of influenza are urgently required.
A number of rapid influenza tests are now available to assist the diagnosis of influenza. 26 , 27 Most are immunoassays, in which nasopharyngeal aspirates or swabs are mixed with substrates that are impregnated with monoclonal antibodies against influenza A and/or B viral antigens. Results are visualised by colour changes that are indicative of the presence or absence of viral antigen and are typically available within 30 minutes. By providing additional support for the initial clinical diagnosis, these assays have been used to promote the rational use of antiviral agents and discourage the inappropriate prescribing of antibiotics. 28 Rapid influenza testing has also been associated with a reduction in the use of other diagnostic assays and the associated costs. 29 , 30 , 31 , 32
Here, we describe the findings of an observational non‐randomised study that explored the clinical management of children with influenza‐like illness (ILI) in Germany. Prescribing habits when rapid influenza tests were and were not performed were assessed.
Methods
A total of 794 office‐based paediatricians in Germany were recruited through the mailing list of Roche Germany to participate in the survey. Of these, 705 paediatricians were given access to and performed free‐of‐charge rapid influenza tests (‘rapid test group’). In order to reflect standard practice in Germany (where physicians rarely have access to rapid tests), a control group of 89 paediatricians who were not given rapid tests was also recruited. Educational material discussing influenza in children and the need for quicker and more accurate diagnosis (but not containing reference to antiviral therapy) was provided to all participating physicians by the sponsor. All participating physicians received monetary compensation for conducting the rapid test and/or documenting clinical signs of infection according to the GOÄ (Gebührenordnung für Ärzte), the applicable remuneration system for all privately applied medical services in Germany. No incentive was offered to prescribe any particular medication.
Between 15 January 2007 and 30 April 2007, a standardised questionnaire was used to record the clinical management of each child aged 1–12 years who presented to the participating physician with febrile (≥38°C) ILI during periods of local influenza activity. This activity was monitored using the RealFlu report that was available to all participating paediatricians via http://www.grippe‐online.de. When completing the questionnaire, the paediatricians in both the rapid test and control groups had to record the following information (Figure 1): (i) their clinical diagnosis (made before the rapid test in the case of the rapid test group and recorded as either influenza positive, influenza negative or suspected ILI); (ii) the child’s characteristics; (iii) the clinical features of the child’s ILI (including time from symptom onset to presentation); (iv) the child’s pre‐visit medications; (v) the outcome of a Clearview Exact Influenza A + B rapid test (Inverness Medical Innovations, Inc., Waltham, MA, USA), if used (rapid test group only); (vi) any prescribed treatments for the child and (vii) any prescribed treatments for the child’s family members.
Figure 1.
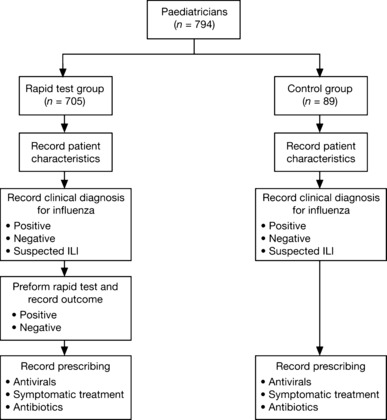
Questionnaire completion process followed by paediatricans participating in the study.
In the analysis, only those questionnaires that involved children aged 1–12 years and included information on both the clinical diagnosis and rapid influenza test were considered. Responses were analysed in the overall survey population, the rapid test group and the control group.
Results
During the survey period, 18 906 questionnaires were completed. Of these, 16 907 were completed correctly (i.e. involved children aged 1–12 years, and included information on both the clinical diagnosis and rapid influenza test) and were included in the survey analysis.
Influenza activity
Over the 15 week study period, influenza A/H3N2 and A/H1N1 viruses were prevalent in Germany (89% and 10%, respectively, of clinical isolates tested, according to the sentinel system at the Robert Koch Institute, Berlin, Germany). Influenza B virus activity was low (1% of clinical isolates). Influenza activity peaked during week 7 of the survey period (week commencing Monday 26 February 2007).
Demographics
The demographics of the 16 907 children whose questionnaires were evaluated are shown in Table 1. The mean age of the overall survey population was 5·9 years, with slightly more boys presenting than girls. Of the 7277 children in the overall analysis population who provided information on their influenza vaccination status, only 903 (12·4%) were vaccinated; of these children, 291 (32·2%) had a rapid test performed and were rapid test positive for influenza. The majority of children in the overall analysis population were healthy (82·8% without any chronic or pre‐existing illness, 13 991/16 907 children), and the most common pre‐visit medications prescribed were antipyretics, analgesics and anti‐inflammatories (63·4%, 10 727/16 907 children). Both the rapid test and control groups of children had a similar mean age and other demographics.
Table 1.
Demographics of the children aged 1–12 years who were evaluated in the analysis (overall, control and rapid test groups)
| Overall (n = 16 907) | Control (n = 1036) | Rapid test (n = 15 871) | |
|---|---|---|---|
| Age (years) | |||
| Mean | 5·9 | 5·9 | 5·9 |
| SD | 3·21 | 3·20 | 3·21 |
| Sex | |||
| n | 16 690 | 1018 | 15 672 |
| Male (n) (%) | 8729 (52·3) | 553 (54·3) | 8176 (52·2) |
| Female (n) (%) | 7961 (47·7) | 465 (45·7) | 7496 (47·8) |
| Vaccinated | |||
| n | 7277 | 509 | 6768 |
| Yes (n) (%) | 903 (12·4) | 74 (14·5) | 829 (12·2) |
| No (n) (%) | 6374 (87·6) | 435 (85·5) | 5939 (87·8) |
| Chronic pre‐existing illness | |||
| Yes (n) (%) | 1904 (11·3) | 142 (13·7) | 1762 (11·1) |
| No (n) (%) | 13 991 (82·8) | 840 (81·1) | 13 151 (82·9) |
| Pre‐visit medication | |||
| Antipyretics, analgesics and anti‐inflammatories (n) (%) | 10 727 (63·4) | 714 (68·9) | 10 013 (63·1) |
| Cough syrups (n) (%) | 3831 (22·7) | 278 (26·8) | 3553 (22·4) |
| Others (n) (%) | 1419 (8·4) | 113 (10·9) | 1306 (8·2) |
| None (n) (%) | 4932 (29·2) | 258 (24·9) | 4674 (29·4) |
Clinical features
The majority (82%) of the children in the overall analysis population presented within 48 hours of symptom onset; 50% presented within 24 hours (Figure 2). The most commonly reported clinical symptoms in addition to fever (an entry criteria for all children) were cough (84·6% of cases), fatigue/decreased activity (83·0% of cases), rhinorrhoea (73·7% of cases) and headache (67·1% of cases) (Table 2). With the exception of rhinorrhoea, clinical symptoms were reported with a similar frequency in the rapid test and control groups (Table 2).
Figure 2.
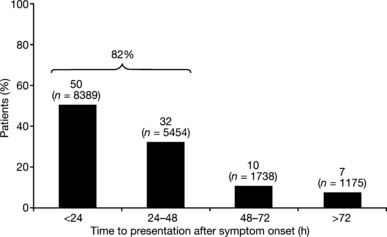
Time to presentation following symptom onset for the children aged 1–12 years who were evaluated in the analysis (overall patient group; n = 16 907).
Table 2.
Clinical symptoms of the children aged 1–12 years who were evaluated in the analysis (overall, control and rapid test groups)
| Overall (n = 16 907) | Control (n = 1036) | Rapid test (n = 15 871) | |
|---|---|---|---|
| Systemic symptoms | |||
| Chills/sweats (n) (%) | 10 034 (59·3) | 589 (56·9) | 9445 (59·5) |
| Myalgia (n) (%) | 7366 (43·6) | 466 (45·0) | 6900 (43·5) |
| Headache (n) (%) | 11 349 (67·1) | 683 (65·9) | 10 666 (67·2) |
| Fatigue/decreased | |||
| activity (n) (%) | 14 036 (83·0) | 859 (82·9) | 13 177 (83·0) |
| Nausea (n) (%) | 5320 (31·5) | 352 (34·0) | 4968 (31·3) |
| Respiratory symptoms | |||
| Cough (n) (%) | 14 305 (84·6) | 884 (85·3) | 13 421 (84·6) |
| Rhinorrhoea (n) (%) | 12 454 (73·7) | 601 (58·0) | 11 853 (74·7) |
| Pharyngitis, (n) (%) | 9308 (55·1) | 620 (59·8) | 8688 (54·7) |
| Others | |||
| Vomiting (n) (%) | 2771 (16·4) | 195 (18·8) | 2576 (16·2) |
| Diarrhoea (n) (%) | 927 (5·5) | 45 (4·3) | 882 (5·6) |
Clinical diagnoses
A clinical diagnosis that was positive for influenza was the most common outcome for the overall analysis population (56·8%, 9596/16 907 children) followed by a clinical diagnosis of suspected ILI (41·4%, 7006/16 907 children). A negative clinical diagnosis was uncommon (1·8%, 305/16 907 children). In the rapid test group, clinicians were less likely to make a positive or negative clinical diagnosis and more likely to make clinical diagnosis of suspected ILI than in the control group (Figure 3).
Figure 3.
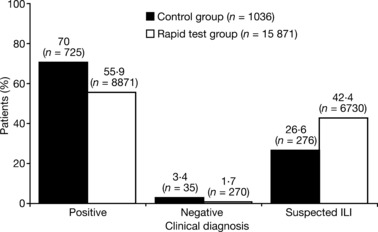
Clinical diagnoses for the children aged 1–12 years who were evaluated in the analysis (control group versus rapid test group; clinical diagnosis made before rapid test in the latter).
Concordance of the rapid test result with the clinical diagnosis
The concordance between the outcome of the clinical diagnosis and the result of the rapid influenza test for the children in the rapid test group is presented in Figure 4. Overall, 62·8% of the children who were clinically diagnosed as influenza positive were confirmed as influenza positive by the rapid test, while 81·9% of those who were clinically diagnosed as influenza negative were also confirmed as negative by the rapid test. Of the children with clinical diagnosis of suspected ILI, 68·9% were influenza negative and 30·6% were influenza positive (although performed, the result of the rapid influenza test was not recorded in 0·4% of cases).
Figure 4.
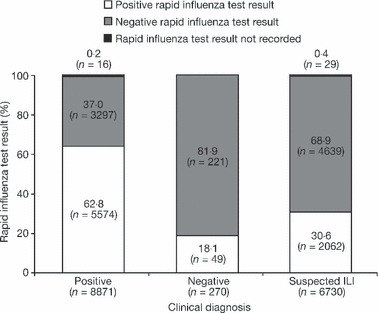
Concordance between the clinical diagnosis and rapid test outcome (rapid test group; clinical diagnosis made before rapid test).
Physicians’ prescribing behaviour
The medications prescribed for the children in the control group (clinical diagnosis only) and rapid test group (clinical diagnosis followed by rapid test) following a positive diagnosis of influenza are shown in Figure 5A. In children where a positive diagnosis of influenza was made on the basis of symptoms alone (control group), symptomatic treatments were the most commonly prescribed interventions (72·4%, 525/725 children). Oseltamivir was the most commonly prescribed antiviral therapy (24·6%, 178/725 children). A combination of oseltamivir and symptomatic treatment was prescribed in 9·7% of cases (70/725 children) where influenza was clinically diagnosed. Amantadine was rarely prescribed (0·1%, 1/725 children) and zanamivir was not prescribed. In the rapid test group, oseltamivir was the most commonly prescribed medication following a positive test result (60·1%, 4618/7685 children). Symptomatic treatments were also prescribed for 47·1% (3623/7685) of children. In 16·3% of children (1251/7685), both oseltamivir and symptomatic treatment were prescribed. Use of amantadine and zanamivir was uncommon [3·0% (234/7658) and 0·1% (7/7658) of children, respectively]. The medications that were prescribed for prophylaxis of the family members of the children who formed the control and rapid test groups are shown in Figure 5B. Oseltamivir was more commonly prescribed for the family members of children in the rapid test group who were influenza positive by the rapid test than for those of the children who were influenza positive by clinical assessment only in the control group (11·5% versus 5·9%, respectively).
Figure 5.
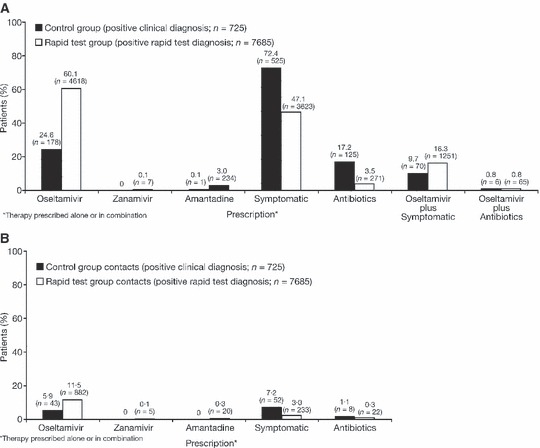
Physicians’ prescribing behaviour for (A) the management of the children aged 1–12 years who were evaluated in the analysis and (B) the prophylaxis of their family members, when rapid influenza tests were and were not performed (control versus rapid test group).
Figure 5 (A) also shows the proportion of children in the control group (symptom assessment only) and children in the rapid test group who were prescribed antibiotics. Antibiotics were less commonly prescribed for the children who were influenza positive by the rapid influenza test [3·5% (271/7658) versus 17·2% (125/725) for positive diagnosis by symptom assessment alone in the control group]. When influenza was diagnosed clinically or by rapid test, antibiotics were rarely prescribed in combination with oseltamivir in the control (0·8%, 6/725 children) or rapid test (0·8%, 65/7685 children) groups. Children in the rapid test group with a negative rapid test result for influenza were most commonly prescribed symptomatic treatment [69·9% (5689/8141)] and antibiotics [13·4% (1092/8141)], either alone or in combination, and rarely received antivirals (data not shown). Those in the control group with a negative or suspected clinical diagnosis for influenza also commonly received symptomatic treatment and/or antibiotics, but were rarely prescribed antivirals (data not shown).
Discussion
The clinical diagnosis of influenza continues to present a challenge for physicians. 33 This assessment examined the influence of rapid influenza testing on the clinical management of children with ILI, and has identified shifts in clinical practice in three key areas.
Firstly, we found that the antiviral medication oseltamivir was more commonly prescribed for children who were diagnosed influenza positive by rapid test (rapid test group) than by clinical assessment alone (control group; 60·1% versus 24·6%, respectively). Bonner et al. (2003) reported a similar finding; in their prospective study, the clinical management of children who presented to an emergency department with acute respiratory disease when the results of rapid influenza tests were and were not made available (‘physician aware’ and ‘physician unaware’ groups, respectively) was evaluated. 32 For the children who were influenza positive (n = 202), the number of prescriptions for antiviral drugs was significantly higher in the physician aware group than in the physician unaware group [18·8% (18/96) versus 9·4% (7/106), respectively; P = 0·02 for comparison]. These results suggest one of two possible findings: (i) that the decision to initiate antiviral therapy is linked with the level of confidence in the diagnosis of influenza and that physicians have more confidence in the outcome of rapid tests coupled with clinical diagnosis than clinical assessment alone, or (ii) that rapid testing is considered by some to be a requirement for the initiation of antiviral therapy. Although rapid testing is the standard practice in Japan, the latter conclusion appears unlikely, as oseltamivir is recommended for use in patients ‘who present with symptoms typical of influenza when influenza virus is circulating in the community’. 23 Whatever the underlying cause, positive rapid influenza tests do appear to increase the use of antivirals. Wider antiviral use may have a significant impact on the disease burden of influenza in children, especially in the absence of widespread vaccination 15 , 34 , 35 and the suboptimal level of protection afforded, 36 as observed in the current survey. For instance, in clinical studies, oseltamivir not only reduces the severity and duration of symptoms in children, but also the risk of influenza‐associated complications, such as otitis media. 18 , 37 These actions may also provide wider benefits, by reducing the impact on carers (e.g. work days lost) and healthcare systems (e.g. hospitalisations). The fact that most children (82%) presented within 48 hours of symptom onset is also an important finding, especially as early presentation was not encouraged as part of the survey and both oseltamivir and zanamivir must be started within this period to provide meaningful benefits. We also found that 50% of children presented within 24 hours of symptoms onset. This is a significant observation, since treatment effects with the neuraminidase inhibitors are more pronounced with earlier initiation. 38 , 39 Zanamivir was less commonly prescribed than oseltamivir, possibly due to its limited indication in children and the need for inhaled rather than oral administration (which children may find difficult). 24 Physicians rarely prescribed oseltamivir when a child tested negative for influenza by rapid test (1·3% versus 60·1% for a positive rapid test), but were more likely to prescribe antibiotics in such cases (13·4% versus 3·5%, respectively). Symptomatic relief was often given regardless of the outcome of the rapid test.
Secondly, children who were influenza positive by the rapid test (rapid test group) were also prescribed fewer antibiotics than children who were diagnosed by symptom assessment alone (control group; 3·5% versus 17·2%, respectively). Again, a similar observation was made by Bonner et al. (2003): significantly fewer influenza‐positive children in the physician aware (or rapid test) group were prescribed antibiotics than those in the physician unaware (or control) group [7·7% (4/52) versus 31·1% (23/74), respectively; P < 0·001 for comparison]. As previously, this finding suggests that the decision to prescribe antibiotics is influenced by the level of confidence in the diagnosis of influenza and that physicians are more willing to withhold antibiotics when the diagnosis of viral illness is supported by a rapid influenza test. This observation may have important implications for clinical practice, since conserving the utility of antibiotics by limiting inappropriate use is a current healthcare priority. Rapid influenza testing could therefore represent a previously untapped sparing strategy. It is also noteworthy that wider antiviral use in influenza may in itself decrease antibiotic usage by reducing the incidence of secondary complications of bacterial origin, such as sinusitis and pneumonia. 28 In addition to antibiotic usage, rapid influenza testing may also promote the cost‐effective use of medications and avoid unnecessary, but often routine, ancillary tests, such as blood examinations, urinalyses and chest radiographs. 31 , 32 , 40
Thirdly, we noted that the physicians who were able to perform rapid influenza tests (rapid test group) were less likely to make firm clinical diagnoses (e.g. influenza positive, influenza negative) and more likely to make a clinical diagnosis of suspected ILI than physicians who were unable to perform rapid influenza tests (control group). This finding represents a further modification of clinical practice. The fact that the clinical symptoms observed in the two groups were generally similar provides further support for this argument. It is also noteworthy that the symptoms identified by this survey are consistent with those reported previously in studies involving children with influenza. 5
In our survey, 62·8% of positive clinical diagnoses and 81·9% of negative clinical diagnoses were supported by the application of a rapid influenza test. The Clearview Exact Influenza A + B assay has a specificity for influenza A and B viruses of 98·5% and 97·4%, respectively, and a sensitivity of 81·7% and 88·6%, respectively. (Clearview Exact Influenza A + B test Product Monograph) As such, while the test results do not provide a definitive assessment of the accuracy of the clinical diagnosis in the survey, they do suggest that approximately two out of every three positive clinical diagnoses and four out of every five negative clinical diagnoses were correct. It is noteworthy, however, that this impressive level of diagnostic accuracy was still associated with suboptimal clinical management of children with ILI. In a prior study, Peltola et al. (2005) found the accuracy of clinical diagnosis to be considerably lower; only 31·9% [88/276] of children ≤13 years who were clinically diagnosed as influenza positive actually had laboratory confirmed influenza. 33 The fact that the latter study was performed throughout the influenza season, not just when influenza was circulating as in the current study, may explain the discrepancy, but further research is warranted.
When interpreting these findings, it should be kept in mind that the raw data used in the above analyses were generated in an observational non‐randomised fashion. While acknowledging that this is not a randomised controlled study, it should be noted that the approach employed enabled the rapid accrual of an extensive body of data and that this would not have been possible if conventional approaches had been used; this is evidenced by the previously reported clinical studies on this topic, which were limited to a single centre and involved small numbers of patients. 31 , 32 , 40 The fact that our findings are consistent with those of these earlier studies provides additional support for the methodology employed. It should also be noted that the number of physicians (and therefore the number of children) in the rapid test group was considerably larger than in the control group. This can be attributed to the fact that interest in participation was much greater when the rapid test was made available.
In conclusion, the findings of this analysis suggest that rapid influenza testing modifies the clinical management of children with ILI by promoting the rational use of antiviral agents and reducing the inappropriate use of antibiotics. These actions may not only improve the clinical management of influenza in children, thereby reducing its burden, but also avoid the emergence of antibiotic resistance.
Conflict of Interest
Lance C. Jennings received honororia and travel assistance from GlaxoSmithKline, F. Hoffman ‐ La Roche Ltd., Sanofi Pasteur, Solvay, Baxter, Wyeth, CSL and Quidel Corporation for participation in advisory and scientific meetings. Heino Skopnik acted as a paid advisor to F. Hoffman ‐ La Roche Ltd and received funding for research carried out in other influenza projects. He also gave paid lectures on paediatric influenza. Isabel Burckhardt is an employee of F. Hoffman ‐ La Roche Ltd. Irene Hribar is an employee of Roche Pharma AG. Klaus A. Deichmann has acted as a paid consultant to Roche Pharmaceuticals, attended 2 symposia on behalf of Roche and received reimbursement for attending a press conference on influenza by the same company.
Acknowledgements
The authors would like to thank: RxMD (Chennai, India) for assisting in the collation of the raw data generated by survey; Paul Smith and Thomas Müller (Statistics for Research, Basel, Switzerland) for supporting the implementation of the survey analysis plan; Scott Malkin (Gardiner‐Caldwell Communications, Macclesfield, UK) for assisting in the development of the manuscript; and F. Hoffmann‐La Roche Ltd (Basel, Switzerland and Grenzach‐Wyhlen, Germany) for providing financial support for the survey and above activities.
References
- 1. Heikkinen T. Influenza in children. Acta Paediatr 2006; 95:778–784. [DOI] [PubMed] [Google Scholar]
- 2. Monto AS, Sullivan KM. Acute respiratory illness in the community. Frequency of illness and the agents involved. Epidemiol Infect 1993; 110:145–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hurwitz ES, Haber M, Chang A et al. Studies of the 1996–1997 inactivated influenza vaccine among children attending day care: immunologic response, protection against infection, and clinical effectiveness. J Infect Dis 2000; 182:1218–1221. [DOI] [PubMed] [Google Scholar]
- 4. Heikkinen T, Silvennoinen H, Peltola V et al. Burden of influenza in children in the community. J Infect Dis 2004; 190:1369–1373. [DOI] [PubMed] [Google Scholar]
- 5. Peltola V, Ziegler T, Ruuskanen O. Influenza A and B virus infections in children. Clin Infect Dis 2003; 36:299–305. [DOI] [PubMed] [Google Scholar]
- 6. Tsolia MN, Logotheti I, Papadopoulos NG et al. Impact of influenza infection in healthy children examined as outpatients and their families. Vaccine 2006; 24:5970–5976. [DOI] [PubMed] [Google Scholar]
- 7. Lahti E, Peltola V, Virkki R, Ruuskanen O. Influenza pneumonia. Pediatr Infect Dis J 2006; 25:160–164. [DOI] [PubMed] [Google Scholar]
- 8. Neuzil KM, Mellen BG, Wright PF, Mitchel EF Jr, Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med 2000; 342:225–231. [DOI] [PubMed] [Google Scholar]
- 9. Izurieta HS, Thompson WW, Kramarz P et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med 2000; 342:232–239. [DOI] [PubMed] [Google Scholar]
- 10. Simonsen L, Fukuda K, Schonberger LB, Cox NJ. The impact of influenza epidemics on hospitalizations. J Infect Dis 2000; 181:831–837. [DOI] [PubMed] [Google Scholar]
- 11. Smith NM, Bresee JS, Shay DK et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55:1–42. [PubMed] [Google Scholar]
- 12. Poehling KA, Edwards KM, Weinberg GA et al. The underrecognized burden of influenza in young children. N Engl J Med 2006; 355:31–40. [DOI] [PubMed] [Google Scholar]
- 13. Monto AS, Davenport FM, Napier JA, Francis T Jr. Modification of an outbreak of influenza in Tecumseh, Michigan by vaccination of schoolchildren. J Infect Dis 1970; 122:16–25. [DOI] [PubMed] [Google Scholar]
- 14. Glezen WP, Couch RB. Interpandemic influenza in the Houston area, 1974–76. N Engl J Med 1978; 298:587–592. [DOI] [PubMed] [Google Scholar]
- 15. Ramet J, Weil‐Olivier C, Sedlak W. Influenza vaccination: the paediatric perspective. Vaccine 2007; 25:780–787. [DOI] [PubMed] [Google Scholar]
- 16. Fiore AE, Shay DK, Haber P et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep 2007; 56:1–54. [PubMed] [Google Scholar]
- 17. Nicholson KG, Aoki FY, Osterhaus AD et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet 2000; 355:1845–1850. [DOI] [PubMed] [Google Scholar]
- 18. Whitley RJ, Hayden FG, Reisinger KS et al. Oral oseltamivir treatment of influenza in children. Pediatr Infect Dis J 2001; 20:127–133. [DOI] [PubMed] [Google Scholar]
- 19. Kaiser L, Wat C, Mills T et al. Impact of oseltamivir treatment on influenza‐related lower respiratory tract complications and hospitalizations. Arch Intern Med 2003; 163:1667–1672. [DOI] [PubMed] [Google Scholar]
- 20. Hayden FG, Osterhaus AD, Treanor JJ et al. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. GG167 Influenza Study Group. N Engl J Med 1997; 337:874–880. [DOI] [PubMed] [Google Scholar]
- 21. Hedrick JA, Barzilai A, Behre U et al. Zanamivir for treatment of symptomatic influenza A and B infection in children five to twelve years of age: a randomized controlled trial. Pediatr Infect Dis J 2000; 19:410–417. [DOI] [PubMed] [Google Scholar]
- 22. The MIST Study Group . Randomised trial of efficacy and safety of inhaled zanamivir in treatment of influenza A and B virus infections. The MIST (Management of Influenza in the Southern Hemisphere Trialists) Study Group. Lancet 1998; 352:1877–1881. [PubMed] [Google Scholar]
- 23. Roche Products Limited . Tamiflu® Summary of Product Characteristics, 2007. Available at: http://emc.medicines.org.uk/default.aspx
- 24. GlaxoSmithKline UK . Relenza® Summary of Product Characteristics, 2008. Available at: http://emc.medicines.org.uk/default.aspx (Accessed 3 March 2009).
- 25. Fleming DM, Pannell RS, Elliot AJ, Cross KW. Respiratory illness associated with influenza and respiratory syncytial virus infection. Arch Dis Child 2005; 90:741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Charles PG, Grayson ML. Point‐of‐care tests for lower respiratory tract infections. Med J Aust 2007; 187:36–39. [DOI] [PubMed] [Google Scholar]
- 27. World Health Organization (WHO) . WHO recommendations on the use of rapid testing for influenza diagnosis, 2005. Available at: http://www.who.int/csr/disease/avian_influenza/guidelines/rapid_testing/en/ (Accessed 3 March 2009).
- 28. Low D. Reducing antibiotic use in influenza: challenges and rewards. Clin Microbiol Infect 2008; 14:298–306. [DOI] [PubMed] [Google Scholar]
- 29. Poehling KA, Zhu Y, Tang YW, Edwards K. Accuracy and impact of a point‐of‐care rapid influenza test in young children with respiratory illnesses. Arch Pediatr Adolesc Med 2006; 160:713–718. [DOI] [PubMed] [Google Scholar]
- 30. Sharma V, Dowd MD, Slaughter AJ, Simon SD. Effect of rapid diagnosis of influenza virus type a on the emergency department management of febrile infants and toddlers. Arch Pediatr Adolesc Med 2002; 156:41–43. [DOI] [PubMed] [Google Scholar]
- 31. Esposito S, Marchisio P, Morelli P, Crovari P, Principi N. Effect of a rapid influenza diagnosis. Arch Dis Child 2003; 88:525–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bonner AB, Monroe KW, Talley LI, Klasner AE, Kimberlin DW. Impact of the rapid diagnosis of influenza on physician decision‐making and patient management in the pediatric emergency department: results of a randomized, prospective, controlled trial. Pediatrics 2003; 112:363–367. [DOI] [PubMed] [Google Scholar]
- 33. Peltola V, Reunanen T, Ziegler T, Silvennoinen H, Heikkinen T. Accuracy of clinical diagnosis of influenza in outpatient children. Clin Infect Dis 2005; 41:1198–1200. [DOI] [PubMed] [Google Scholar]
- 34. Santibanez TA, Santoli JM, Bridges CB, Euler GL. Influenza vaccination coverage of children aged 6 to 23 months: the 2002‐2003 and 2003–2004 influenza seasons. Pediatrics 2006; 118:1167–1175. [DOI] [PubMed] [Google Scholar]
- 35. Centers for Disease Control and Prevention (CDC) . Influenza vaccination coverage among children aged 6–59 months – six immunization information system sentinel sites, United States, 2006–07 influenza season. MMWR Morb Mortal Wkly Rep 2007; 56:963–965. [PubMed] [Google Scholar]
- 36. Centers for Disease Control and Prevention (CDC) . Assessment of the effectiveness of the 2003–04 influenza vaccine among children and adults – Colorado 2003. Morb Mortal Weekly Rep 2004; 53:707–710. [PubMed] [Google Scholar]
- 37. Barr CE, Schulman K, Iacuzio D, Bradley JS. Effect of oseltamivir on the risk of pneumonia and use of health care services in children with clinically diagnosed influenza. Curr Med Res Opin 2007; 23:523–531. [DOI] [PubMed] [Google Scholar]
- 38. Aoki FY, Macleod MD, Paggiaro P et al. Early administration of oral oseltamivir increases the benefits of influenza treatment. J Antimicrob Chemother 2003; 51:123–129. [DOI] [PubMed] [Google Scholar]
- 39. Whitley RJ, Reisinger K, Dutkowski R. Early oseltamivir initiation produces marked reductions in illness duration, symptom severity and secondary complications in children with influenza. Options for the Control of Influenza VI 2007, Toronto, June 17–23 2007, Abstract O98 [Generic].
- 40. Noyola DE, Demmler GJ. Effect of rapid diagnosis on management of influenza A infections. Pediatr Infect Dis J 2000; 19:303–307. [DOI] [PubMed] [Google Scholar]


