Abstract
Background
We sought to determine the prognostic variables associated with overall survival (OS) and recurrence-free probability (RFP) in patients with primary and secondary sternal tumors treated with surgical resection.
Methods
A retrospective analysis of patients who underwent resection of primary or secondary sternal tumors at two cancer institutes between 1995 and 2013 was performed. OS and RFP were estimated using the Kaplan-Meier method, and predictors of OS and RFP were analyzed using the Cox proportional hazards model.
Results
Seventy-eight patients underwent sternal resection with curative (n=67; 86%) or palliative (n=6; 8%) intent. Seventy-three patients (94%) had malignant tumors, of which 28 (36%) were primary and 45 (57%) were secondary malignant. Thirteen patients (17%) underwent complete and 65 (83%) underwent partial sternal resection. There were no perioperative deaths, and grade III/IV complications were noted in 17 patients (22%). The 5-year OS was 80% for patients with primary malignant tumors, 73% for patients with non-breast secondary malignant tumors, and 58% for patients with breast tumors (p=0.85). In the overall cohort, R0 resection was associated with prolonged 5-year OS (84% vs 20%) on both univariate (p=0.004) and multivariate (adjusted HR, 3.37; p=0.029) analysis. On subgroup analysis, R0 resection was associated with improved OS and RFP only for patients with primary malignant tumors.
Conclusions
Sternal resection can achieve favorable OS for patients with primary and secondary sternal tumors. R0 resection is associated with improved 5-year OS and RFP in patients with primary malignant tumors. We did not detect a similar effect in patients with breast or non-breast secondary tumors.
Keywords: sternum, cancer, outcomes
Introduction
Resection of tumors involving the sternum is a challenging procedure, as it puts both hemithoraces at risk of instability and requires complex reconstruction. Therefore, it is imperative to characterize patient and tumor subgroups that will benefit from these extensive resections.
Sternal resections have been performed for primary and secondary sternal tumors, including breast cancer, with both curative and palliative intent. We [1] and others [2] have reported that sternal resections can be performed safely. For patients with primary sternal tumors, 5-year overall survival (OS) of 49% to 92% has been reported, and tumor grade and completeness of resection have been shown to be associated with OS in multiple reports [1-4]. Secondary sternal tumors compose a heterogeneous group that includes metastases as well as locally invasive and recurrent mediastinal, lung, breast, and other tumors.
Breast cancer, the most widely studied secondary sternal tumor, has a 5-year OS of 20% to 60% [5-15]. Breast cancer can involve the sternum through local invasion by the primary tumor, local invasion at the time of recurrence, or hematogenous metastasis. Although any chest wall invasion portends worse survival, highly selected patients—such as those with isolated bone recurrence, lack of nodal involvement, and an extended disease-free interval—can experience long-term survival after resection [5-15].
There is limited data available on sternal resection of nonbreast secondary tumors; 5-year OS of 20% to 50% has been reported [2,16]. A few small studies have reported survival and recurrence outcomes for the various sternal malignancies [2, 16-18].
The purpose of this study is to examine the clinical outcomes and prognostic factors associated with OS and recurrence-free probability (RFP) for patients with primary and secondary sternal tumors and breast cancer treated with sternectomy.
Material and Methods
This study was approved by the institutional review boards at Memorial Sloan Kettering Cancer Center (New York, New York) and the National Cancer Institute (Naples, Italy).
Patients
A retrospective review of prospectively maintained databases was conducted to identify patients who had undergone sternectomy as part of their treatment for primary, nonbreast secondary, and breast cancers between 1995 and 2013. Clinical, pathologic, and follow-up data were obtained. The study included 54 patients from Memorial Sloan Kettering and 24 from the National Cancer Institute of Italy.
Patients with sternal involvement through local invasion by the primary tumor, local invasion at the time of recurrence, or hematogenous metastasis were included. Recurrent breast cancer was defined as a tumor involving the primary site of resection, along with sternal invasion. Sternal metastasis was defined as a tumor involving the sternum, without presence of disease at the original site of resection. Patients with internal mammary lymph node metastases were included in this group. If the sternum was involved in such cases, the bone and the lymph nodes were resected en bloc. In the majority of these patients, the sternum was the only site of disease, and sternal resection was performed with curative intent. It is routine practice at our institutions to discuss such patients and their treatment plans at a multidisciplinary tumor board.
Surgery was considered (1) in cases of a solitary metastasis, where resection was deemed by the multidisciplinary team to be the treatment option most likely to extend life expectancy, or (2) when the sternal disease had caused skin breakdown or incipient breakdown such that resection was considered appropriate to improve quality of life.
Sternal Resection
Sternal resection and reconstruction were performed using a multidisciplinary approach. In the absence of skin involvement, a midline incision was made and skin flaps were raised. Manubrial resection with or without resection of the clavicular head was performed without dividing the sternal body. Peritumoral soft tissue was excised en bloc, and intraoperative frozen section was used to confirm negative soft tissue margins. A 4-cm bone margin was used whenever possible. Larger tumors involving the sternal body were resected by dividing the cartilaginous portions of the anterior ribs.
The choice of material for reconstruction was based on a previously described algorithm [19]. In brief, we covered large anterior and antero-lateral defects with prosthesis, most commonly a marlex mesh-methymethacrylate sandwich [20]. This was augmented by soft tissue coverage. In the presence of skin breakdown or infection, absorbable mesh, titanium struts, or a large vascularized muscle flap was used. Common Terminology Criteria for Adverse Events version 4 was used to grade postoperative complications [21].
Statistical Methods
Clinical characteristics were compared using Fisher exact test (for categorical variables) and Wilcoxon rank-sum test (for continuous variables). Long-term outcomes, overall survival (OS), and recurrence-free probability (RFP) were estimated using the Kaplan-Meier method. Patients were followed until death (in the OS analyses) and cancer recurrence or progression (in the RFP analysis). Patients who did not experience the event of interest by the end of the study were censored at the time of the last available follow-up. The effect of potential risk factors on OS and PFS were investigated in univariate analyses using log-rank test. For RFP, a multivariate Cox proportional hazards models was fit to investigate the independent effect of the clinicopathological factors that were associated with RFP on the univariate analysis at p < 0.1.
Results
Patient and Tumor Characteristics
During the study period, 78 patients underwent sternal resections (Table 1). Thirty-four patients (44%) had sternal pain at the time of presentation. Patients with benign tumors were younger (median age, 38 years; p=0.013). Primary malignant and nonbreast secondary tumors were more common in men. Overall, breast cancer was the most common sternal tumor (n=24; 31%).
Table 1.
Clinical Characteristics of Patients with Sternal Tumors
| Characteristic | Benign (n=5) | Primary Malignant (n=28) | Secondary Malignant (n=21) | Breast (n=24) | P |
|---|---|---|---|---|---|
| Age, years, median (range) | 32 (24-65) | 50 (20-76) | 57 (23-72) | 60 (48-69) | 0. 027 |
| Sex | |||||
| Female | 3 (60) | 11 (39) | 8 (38) | 23 (96) | <0.001 |
| Male | 2 (40) | 17 (61) | 13 (62) | 1 ( 4) | |
| Smoking | |||||
| Never | 3 (60) | 15 (54) | 12 (57) | 19 (79) | 0. 43 |
| Former/current | 2 (40) | 12 (46) | 8 (43) | 4 (21) | |
| Tumor size, cm (range) | 1.5 (0-6.3) | 4.2 (1-12) | 8 (2.1-15) | 4.2 (1.2-7) | 0. 004 |
| Location of the tumor | 0. 014 | ||||
| Manubrium | 0 (0) | 7 (25) | 9 (43) | 1 (4) | |
| Sternal body | 4 (80) | 20 (71) | 10 (48) | 18 (75) | |
| Lower sternum | 1 (13) | 0 (0) | 1 (5) | 1 (4) | |
| Manubrium + sternal body | 0 ( 0) | 1 ( 4) | 1 ( 5) | 4 (17) | |
| Tumor characteristics* | |||||
| Locally invasive | — | 25 (100) | 5 (45) | 1 (6) | — |
| Metastatic | — | — | 5 (45) | 10 (59) | |
| Recurrence with local invasion | — | — | 1 ( 9) | 6 (35) | |
| Neoadjuvant treatment | — | ||||
| Chemotherapy | 0 | 1 (4) | 4 (22) | 3 (13) | |
| Radiation | 0 | 0 | 2 (11) | 1 (4) | |
| Intent of resection | |||||
| Curative | 5 (100) | 28 (100) | 17 (89) | 19 (82) | |
| Palliative | — | 0 | 2 (11) | 4 (18) | |
| Sternectomy | |||||
| Total | 0 | 6 (21) | 4 (19) | 3 (13) | 0. 76 |
| Subtotal | 5 (100) | 22 (79) | 17 (81) | 21 (87) | |
| Pathologic margin status | |||||
| R0 | 4 (80) | 23 (82) | 15 (71) | 15 (61) | 0. 48 |
| R1/2 | 1 (20) | 5 (18) | 6 (29) | 9 (38) | |
| Adjuvant treatment* | |||||
| Chemotherapy | 0 | 1 (7) | 3 (19) | 4 (21) | |
| Radiation | 0 | 0 | 4 (25) | 1 (5) | |
| Grade III/IV complications | 0 | 5 (20) | 5 (24) | 7 (29) | |
| 5-year OS, % | 100% | 80.2% | 72.8 | 58.3 | 0. 69 |
Note. Unless otherwise noted, data are no. (%).
Data available for 54 patients.
Missing data - % calculated based on number of cases where data were available.
Chondrosarcoma (n=16; 21%) was the most common primary malignant sternal tumor (Appendix Table 1). The sternal body, with or without anterior rib involvement, was the most common location, followed by the manubrium. Data on type of tumor invasion were available for only 11 nonbreast secondary and 17 breast tumors. Among patients with nonbreast secondary tumors, 5 had direct sternal invasion, 5 had hematogenous metastases, and 1 had recurrent soft tissue sarcoma that invaded the sternum. Among patients with breast cancer, 1 had primary breast cancer that invaded the sternum, 10 had metastatic lesions, and 6 had recurrent breast cancer that invaded the sternum.
The majority of patients underwent resection with curative intent (95%); palliative resection was performed for 2 patients with secondary malignant tumors and 4 patients with breast tumors.
Treatment Modalities
The use of neoadjuvant and adjuvant chemotherapy and radiation treatment is shown in Table 1. The majority of patients (83%) had subtotal sternal resection to achieve an R0 resection. Among the tumor types, the rate of R0 resection was lowest for breast cancer (61%). Presence of disease in soft tissue margins was the most common reason for incomplete resection. One patient had bony infiltration by the tumor, resulting in a positive bone margin despite wide grossly negative margins. There was no difference in the rate of R0 resection between complete (77%) and partial (72%) sternectomies. We also did not find any difference in R0 resection rates between locally invasive and metastatic tumors (67% vs 73%). Primary tumors that received incomplete resection are as follows: benign- 1 large periosteal myxoma that involved the sternal body and anterior ribs on both sides; primary malignant- 3 leiomyosarcomas and 2 chondrosarcomas. We were unable to identify any factors that would predispose these tumors to an incomplete resection.
The use of skeletal and soft tissue reconstruction is shown in Appendix Table 2. Marlex mesh-methylmethacrylate sandwich was the most common skeletal reconstruction modality (n=42; 54%). Pectoral muscle flap was the most common soft tissue coverage modality (n=19; 25%).
There were no perioperative deaths, and 17 patients (22%) had grade III/IV complications. Among these 17 patients, 2 had postoperative hemorrhage requiring reexploration. Four patients developed postoperative pneumonia, 2 of whom required prolonged intubation secondary to respiratory failure. Other complications included arrhythmia (n=3), acute coronary syndrome (n=1), vocal cord paralysis (n=1), and delirium (n=6). Three patients had postoperative wound infections that were treated with local wound care. Six patients underwent explantation of the skeletal reconstruction (time since surgery, 3 weeks to 4 years); 4 of these patients had breast cancer and had undergone radiation treatment after the initial breast surgery (3 with infected prosthesis and 1 with flap necrosis). One patient had mechanical fracture of a titanium plate, which required re-reconstruction, and 1 had mesh erosion through the soft tissue, which necessitated explantation. Reconstruction after explantation was performed using muscle and omental flaps.
Overall Survival and Recurrence Free Progression
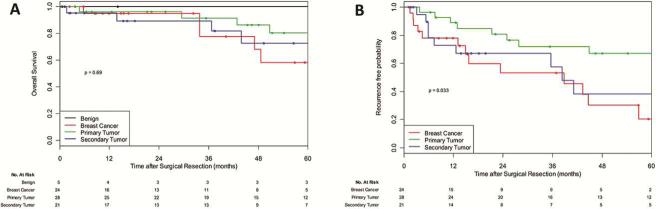
After a median follow-up of 37 months, 16 patients had died and 17 had experienced recurrence or progression. There were no deaths or recurrences in patients with benign tumors. The 5-year OS was 80% for patients with primary malignant tumors, 73% for patients with nonbreast secondary malignant tumors, and 58% for patients with breast cancer (Figure 1A). The 5-year RFP was 67% for patients with primary malignant tumors, 38% for patients with nonbreast secondary malignant tumors, and 20% for patients with breast cancer (Figure 1B).
Figure 1.
Survival outcomes after resection of malignant sternal tumor, according to tumor type.A, Overall survival after resection of sternal tumor (p=0.59). B, Recurrence-free probability after resection of sternal tumor (p=0.06).
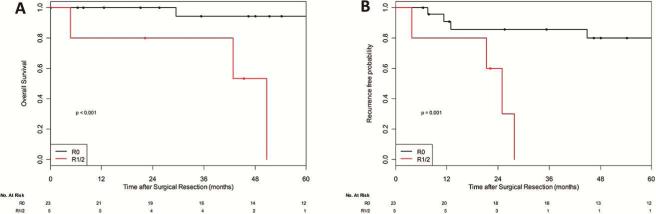
For primary malignant tumors, R0 resection was associated with significantly prolonged 5-year OS (93% vs 0%; p<0.001) and RFP (78% vs 0%; p=0.003) (Figure 2). Among patients with non-breast secondary malignant tumors, there was no significant difference between patients who underwent an R0 versus R1/2 resection, in OS (5-year OS: R0-74% vs R1/2 −50%, p=0.88) as well as in RFP (R0-43% vs R1/2 −0, p=0.61).
Figure 2.
Survival outcomes after resection for primary malignant tumor, as a function of completeness of resection. A, Overall survival after resection of sternal tumor (p<0.001).B, Recurrence-free probability after resection of sternal tumor (p=0.003).
Among breast cancer patients, there was a trend toward prolonged 5-year OS among those who received R0 resection (76% for R0 vs 25% for R1/2; p=0.13), which was not statistically significant. Interestingly, the RFP was similar between breast cancer patients who received R0 resection and those who received R1/2 resection (18% for R0 vs 19% for R1/2; p=0.73).
Univariate analysis of predictors of survival revealed that an R0 resection (84% vs 20%; p=0.004) was associated with prolonged 5-year OS (Table 2). When potential predictors of recurrence were evaluated by univariate analysis, presence of primary malignant tumor, complete sternectomy, and R0 resection were associated with improved RFP (Table 2). On multivariable analysis, only presence of primary malignant tumor (HR, 2.4 [95% CI, 1.1-5.6]; p=0.036) and complete sternectomy (HR, 2.6 [95% CI, 1.1-5.9]; p=0.027) were associated with improved RFP.
Table 2.
Association between Clinicopathologic Factors and 5-year OS and RFP
| Variable | No. (%) | 5-year OS, % | P | 5-year RFP,* % | P |
|---|---|---|---|---|---|
| All patients | 73 | 72.1 | 46.2 | ||
| Age, years | 0.17 | 0.24 | |||
| ≤60 | 41 (59) | 71.1 | 51.5 | ||
| >60 | 29 (41) | 73.2 | 37.7 | ||
| Sex | 0.79 | 0.52 | |||
| Female | 40 (57) | 74.5 | 39.7 | ||
| Male | 30 (43) | 69.2 | 55.9 | ||
| Smoking | 0.11 | 0.68 | |||
| Never | 45 (67) | 80.8 | 43.8 | ||
| Former/current | 22 (33) | 41.5 | 44.3 | ||
| Comorbidity | 0.68 | 0.37 | |||
| Absence | 47 (67) | 73.4 | 41.6 | ||
| Presence | 23 (33) | 69.9 | 56.5 | ||
| Surgery | 0.41 | 0.051 | |||
| Total sternectomy | 13 (19) | 62.5 | 21.9 | ||
| Subtotal sternectomy | 57 (81) | 74.1 | 51.6 | ||
| Malignant disease | 0.49 | 0.012 | |||
| Primary | 25 (36) | 80.2 | 67.2 | ||
| Secondary | 45 (64) | 65.9 | 29.4 | ||
| Pathologic margins | 0.003 | 0.037 | |||
| R0 | 50 (71) | 84.3 | 55.7 | ||
| R1/R2 | 20 (29) | 19.7 | 10.4 |
Note. Unless otherwise noted, data are no. (%) and include patients with malignant tumors only
Data available for 54 patients.
** Missing data - % calculated based on number of cases where data were available.
Comment
We found that sternal resections can be performed with low morbidity and mortality. Primary malignant sternal tumors portend favorable OS and RFP after resection, and their outcomes are significantly improved with R0 resection. Overall survival for breast cancer was poorer than that for primary and other secondary malignant tumors. The recurrence rate after resection of breast tumors was the highest among all tumor types. We were unable to show a benefit of R0 resection, in terms of improving OS or RFP, for secondary malignant and breast tumors.
Chondrosarcomas are the most common primary malignant sternal tumors. A few series have reported outcomes of resection of sternal sarcomas and found 5-year OS of 49% to 92% [1-3, 5-7]. Our findings for primary malignant sternal tumors were similar, with a 5-year OS of 79%. Remarkably, patients with primary sternal tumors that received complete resection had a 5-year OS of 98%, whereas none of the patients with primary sternal tumors that received incomplete resection were alive at 5 years. Lequaglie et al. [16] found improved survival with radical resections (60% vs 30%). In addition to striving for a gross margin of 4 to 5 cm around the tumor, it is now our routine practice to perform intraoperative frozen section on key representative soft tissue margins to ensure complete resection. On multivariate analysis of all malignant sternal tumors (Table 3), R0 resection was associated with improved OS; however, in an analysis of individual tumor types, only patients with primary malignant tumors benefited from R0 resection. Accordingly, our results support complete surgical resection to achieve negative margins when performing primary sternal tumor resection with curative intent. For primary tumors, patients who have an R1 resection can be closely followed with serial imaging. Redo surgery in such patients can be challenging and morbid. It is reasonable to offer patients reresection if there is local recurrence.
Table 3.
Multivariate Analysis of Factors Associated with RFP in Malignant Tumors
| Parameter | Pr > χ2 | HR | 95% CI |
|---|---|---|---|
| RFP | |||
| Total vs subtotal sternectomy | 0.021 | 2.663 | 1.160-6.112 |
| Secondary vs primary malignancy | 0.024 | 2.600 | 1.132-5.973 |
| R1/2 vs R0 | 0.092 | 1.964 | 0.895-4.313 |
In our series, breast cancer patients had a 5-year OS of 58% and an RFP of 20%. Previous series on resection of isolated chest wall breast cancer recurrences have reported 5-year OS ranging from 20% to 60% [4, 6-8, 11-15]. It is important to note that this series consists of a relatively small number of highly selected patients who likely had favorable tumor biology. These survival statistics cannot be generalized to all patients with sternal breast cancer metastases. Although we were unable to find a statistical difference in OS between the patients who received R0 resection and those who received R1/2, 76% of patients who received R0 resection and 25% of those who received R1/2 resection were alive at 5 years. However, the recurrence rates were similar between the two groups. Lequaglie et al. [16] made a similar finding—that is, 40% of their patients with recurrent breast cancer who underwent radical resection were alive at 10 years. Several reports, including our previous institutional series [11] and a recent series by Shen et al. [18], noted the underlying systemic nature of recurrent breast cancer. In these cases, the initial clinical manifestation is often isolated chest wall recurrence, and the patients have an equally poor OS with or without resection. For breast cancer, it is accepted that disease-free interval, presence of other metastasis, and hormone receptor status are better prognostic factors than complete resection of visible disease [22].
Chagpar et al. [23] showed that breast cancer patients with isolated chest wall recurrence had a favorable long-term survival if they were initially node negative and if their recurrence occurred >24 months after the treatment of the primary tumor. These factors point toward the underlying tumor biology, and such patients can potentially be considered for aggressive chest wall or sternal resection. In our group of patients with isolated sternal involvement by breast cancer, long-term survival was reasonable. We did not find a statistical benefit of R0 resection; however, it is recommended that, if sternal resection is to be undertaken, a complete resection should be the goal of the procedure.
Limited data are available for other secondary malignant tumors, with reported 5-year OS ranging from 20% to 50% [2,16,18]. Tumor types vary between studies, and it is difficult to make direct comparisons. We did not find significant differences in OS or RFP between patients who underwent R0 resection and those who underwent R1/2 resection. This could be a function of patient selection or merely a reflection of stage IV disease. However, owing to the lack of a non-surgically treated comparison group, we cannot definitively make that conclusion.
This study addresses a rare problem in a heterogenous population, which makes it difficult to systematically study the treatment and prognostic variables. There were no set criteria for selection of patients with non-breast secondary tumors and breast cancer with isolated sternal involvement. Some patients had their original tumors treated elsewhere and were referred to us only for management of sternal disease. Therefore, we did not have data on their original tumor characteristics or their post–resection oncologic management. Moreover, larger cohorts are needed to draw definitive conclusions regarding the effect of pathologic margin status in this subgroup.
In conclusion, in this large series of sternal tumors, we have shown that sternal resection of benign and primary malignant tumors can yield long-term survival. Complete resection with negative margins is associated with improved outcomes. For nonbreast secondary tumors and breast cancer with isolated sternal involvement, complete resection does not impart any benefit in terms of OS or RFP. However, long-term survival is possible in selected patients. With contemporary perioperative care, sternal resection can be performed with low morbidity and mortality; therefore, it can be offered as a palliative measure.
Discussion
28. Resection for Primary and Metastatic Tumors of the Sternum: An Analysis of Prognostic Variables. Paper presented by Usman Ahmad, MD, New York, NY. uahmad31@gmail.com
Discussion by Traves Crabtree, MD, Missouri crabtreet@wustl.edu Dr. T. Crabtree (St. Louis MO):
You showed us that the R0 resections did better in terms of survival. Could you go back and find out if you could predict who was going to get an R1 resection? Would you be able to see say you wouldn't operate on those patients or that you would change the resection approach or strategy?
28. Resection for Primary and Metastatic Tumors of the Sternum: An Analysis of Prognostic Variables. Response by Usman Ahmad, MD, New York, NY.
DR. AHMAD: I think that's a good question.
Preoperative identification of risk factors that would predict an R0 resection is difficult, and I went back and looked at all the incomplete resections for both primary and secondary tumors and found, as I mentioned briefly, that the cause for incomplete resection most commonly was involvement of mediastinal structures, which supposedly could not have been detected in the preoperative imaging. I'm not sure if I found that intraoperatively if I would change my management.
If I go in to resect a primary sternal tumor where my goal is to do an R0 resection and I find either pleural metastases or ascending aortic invasion from a sternal tumor, I would probably call it a palliative resection and do an R1 or R2 resection.
28. Resection for Primary and Metastatic Tumors of the Sternum: An Analysis of Prognostic Variables. Paper presented by Usman Ahmad, MD, New York, NY. uahmad31@gmail.com
Discussion by Richard K. Freeman, MD, Indiana richard.freeman@stvincent.org Dr. R. Freeman (Indianapolis, IN):
That was a very nice presentation. So if I understand it, breast cancer patients had the lowest rate of R0 resection, the highest rate of recurrence, and the lowest rate of overall survival. So should we really be operating on breast cancer patients with sternal tumors I guess is my first question.
And the second one is, what was your incidence of respiratory insufficiency requiring prolonged intubation and after this series?
28. Resection for Primary and Metastatic Tumors of the Sternum: An Analysis of Prognostic Variables. Response by Usman Ahmad, MD, New York, NY.
DR. AHMAD: Thank you for those questions. I think your first question is right on the point. It is the most common sternal tumor and we have got to figure out whether we are making these patients better or not. And I'm going to delve a little bit into the breast cancer literature. We don't have much thoracic oncology literature on the subject.
But if we look at isolated chest wall recurrences from breast cancer, it is now considered a disease entity in itself, and there are a set of prognostic factors that have been identified, including things like a disease-free interval of more than two years, node negativity at the time of initial tumor presentation, or tumor markers, including the estrogen receptor, progesterone receptor nHER2. Now, I think those are the prognostic factors that really drive the survival in these patients.
I tried hard to look for data in the chest wall recurrence literature to find if there were any reports on patients who were not resected, and the best I could glean was a five-year survival of about 20% in patients who either had bad prognostic factors or could not undergo complete resection of an isolated chest wall metastases.
So, like I mentioned, in the absence of a nonsurgical arm, it's really hard for me to conclude whether surgery is beneficial for them or not in setting up a sternal tumor. However, comparing it to historical data, there does seem to be some improvement.
I'm sorry, your second question was?
DR. FREEMAN: Respiratory procedure rates.
DR. AHMAD: There were four patients who had pneumonias, and two of them required prolonged intubation and ICU stays.
28. Resection for Primary and Metastatic Tumors of the Sternum: An Analysis of Prognostic Variables. Paper presented by Usman Ahmad, MD, New York, NY. uahmad31@gmail.com
Discussion by Daniel L. Miller, MD, Georgia daniel.miller@wellstar.org Dr. D. Miller (Marietta, GA):
I have one comment, when you are resecting metastatic or recurrent breast cancer, you have to achieve an R0 resection as well as removed all the infected and necrotic tissue or you are going to be left with a situation that could be worse than what you started with, especially in regards to quality of life for the patient. Ten percent of your patients had to have their prosthetic patches removed. Sometimes by going in and taking out their tumor, you are not really going to improve their situation with no survival benefit and you set them up for significant complications such as necrotic flaps, recurrent infection, and requiring multiple surgeries. You have to be extremely selective in these patients. How many of these were infected preoperatively?
28. Resection for Primary and Metastatic Tumors of the Sternum: An Analysis of Prognostic Variables. Response by Usman Ahmad, MD, New York, NY.
DR. AHMAD: I don't know the answer to that question, but I think your point is very important. Now, I mentioned that these breast and non-breast secondary tumors were resected with a curative intent, although I think that's a simplistic way of looking at tumor biology. As we know, once they have metastasized, there are going to be other micro metastases that will pop up at some point. However, achieving an R0 resection I think is the key and we should strive to do that. I completely agree. Thank you.
28. Resection for Primary and Metastatic Tumors of the Sternum: An Analysis of Prognostic Variables. Paper presented by Usman Ahmad, MD, New York, NY. uahmad31@gmail.com
Discussion by Stephen C. Yang, MD, Maryland syang@jhmi.edu Dr. S. Yang (Baltimore, MD):
Congratulations on doing an international study. You didn't mention anything about chemo or radiation therapy preoperatively or postoperatively. At least in my practice we get them referred sort of after the fact when they have gotten chemo and radiation and there is a godawful ugly mess there. So what percent of the time were they referred primarily? And then can you comment about whether we should be doing adjuvant or neoadjuvant chemoradiation therapy?
28. Resection for Primary and Metastatic Tumors of the Sternum: An Analysis of Prognostic Variables. Response by Usman Ahmad, MD, New York, NY.
DR. AHMAD: As I mentioned, unfortunately, the majority of these patients were referred only for management of their sternal tumors and we don't know, or at least I don't have the data on how their primary tumors were managed. I can't imagine that a breast cancer patient had a lumpectomy or a mastectomy and did not undergo radiation at the time of their treatment of initial tumor.
However, when they were referred for the sternal tumors, about 5% of them had combined chemoradiation, about 2% of them had chemotherapy, and 3% radiation separately in the neoadjuvant setting and a similar number in the adjuvant setting. So a relatively small number.
If you ask my if I'm seeing a patient and somebody comes to my clinic with de novo presentation of a breast cancer invading into the sternum, I would probably give that patient chemotherapy, radiation, the standard of care based on their tumor markers, and then look at their disease progression over time and see if the patient, based on their prognostic factors, is a candidate for an aggressive resection.
If somebody comes to me with their primary tumor has been treated and now it is an isolated sternal met, I would look at the primary tumor and its prognostic factors, and I think we again have to borrow from the chest wall data in the breast cancer world and see if their disease-free interval is long enough, if they have ER/PR positivity and node negativity, and if they meet those criteria, I would offer them a resection.
DR. YANG: So given that you have to have an R0 resection or if you had an R1 or R2, would you give them adjuvant chemoradiation?
DR. AHMAD: I think the idea is very tempting. I don't know if it's going to change my results. I don't have the data to support that.
28. Resection for Primary and Metastatic Tumors of the Sternum: An Analysis of Prognostic Variables. Paper presented by Usman Ahmad, MD, New York, NY. uahmad31@gmail.com
Discussion by Mitchell Magee, MD, Texas mitchell.magee@hcahealthcare.com Dr. M. Magee (Dallas, TX):
So as a corollary to that, are there certain patient tumor characteristics that you would consider alternatively giving primary radiation therapy as opposed to surgery or neoadjuvant radiation therapy prior to surgery? So if you saw a small tumor, that was their only met, would you just consider palliating them with radiation as opposed to resecting them or giving them neoadjuvant therapy before you resect them?
28. Resection for Primary and Metastatic Tumors of the Sternum: An Analysis of Prognostic Variables. Response by Usman Ahmad, MD, New York, NY.
DR. AHMAD: I think that's a good question and I don't know the data for recurrent breast cancer response to radiation only. If it's a small tumor and it looks resectable, depending on their performance status, et cetera, our routine is to present these patients at tumor boards and get a consensus from all teams involved, and if resection is the choice, then I would probably offer resection up front.
Supplementary Material
Acknowledgments
Financial support: NIH/NCI Cancer Center Support Grant P30 CA008748
Appendix Table 1.
Histologic Profile of Resected Sternum Tumors. N = 78
| Histologic Profile | No. (%) |
|---|---|
| Benign | 5 (6) |
| Schwannoma | 1 |
| Aneurysmal bone cyst | 1 |
| Fibrous dysplasia | 1 |
| Periosteal myxoma | 1 |
| Chondroma | 1 |
| Primary malignant | 28 (36) |
| Chondrosarcoma | 16 |
| Leiomyosarcoma | 3 |
| Desmoid | 3 |
| Ewing sarcoma | 2 |
| Osteosarcoma | 1 |
| Epithelioid sarcoma | 1 |
| Fibrosarcoma | 1 |
| Malignant lymphoma | 1 |
| Clear cell sarcoma | 1 |
| Secondary malignant | 45 (58) |
| Breast carcinoma | 24 |
| Non-small cell lung cancer | 3 |
| Thyroid carcinoma | 2 |
| Melanoma | 2 |
| Chondrosarcoma (metastatic) | 2 |
| Mesothelioma | 2 |
| Hodgkin lymphoma | 1 |
| Squamous cell carcinoma (tongue) | 1 |
| Dermatofibrosarcoma protuberans | 1 |
| Basal cell carcinoma | 1 |
| Uterine leiomyosarcoma | 1 |
| Ovarian serous carcinoma | 1 |
| Osteosarcoma (metastatic) | 1 |
| Giant cell lung carcinoma | 1 |
| Squamous cell carcinoma (mediastinum) | 1 |
| Malignant epithelioid carcinoma | 1 |
Appendix Table 2 Types of Skeletal and Soft-Tissue Reconstruction. N = 78
| Reconstruction | No. (%) |
|---|---|
| Skeletal | |
| None | 5 (6) |
| Mesh (Gore-Tex/Vicryl/Marlex) | 18 (23) |
| Marlex mesh-methylmethacrylate | 42 (54) |
| Titanium plate | 8 (10) |
| Cryopreserved bone allograft | 3 (4) |
| Riblike reconstruction | 2 ( 3) |
| Soft tissue | |
| None | 29 (37) |
| Pectoralis major | 19 (25) |
| Rectus abdominis | 8 (10) |
| Omentum | 7 (9) |
| Latissimus dorsi | 7 (9) |
| Vastus lateralis | 1 (1) |
| Combined | 7 (9) |
| Pectoralis major + omentum | 4 |
| Pectoralis major + rectus abdominis | 2 |
| Pectoralis major + latissimus dorsi | 1 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at: STSA 61st Annual Meeting, Tucson, AZ, November 5-8, 2014
References
- 1.Martini N, Huvos AG, Burt ME, Heelan RT, Bains MS, McCormack PM, Rusch VW, Weber M, Downey RJ, Ginsberg RJ. Predictors of survival in malignant tumors of the sternum. J Thorac Cardiovasc Surg. 1996;111:96–105. doi: 10.1016/S0022-5223(96)70405-1. [DOI] [PubMed] [Google Scholar]
- 2.Incarbone M, Nava M, Lequaglie C, Ravasi G, Pastorino U. Sternal resection for primary or secondary tumors. J Thorac Cardiovasc Surg. 1997;114:93–9. doi: 10.1016/S0022-5223(97)70121-1. [DOI] [PubMed] [Google Scholar]
- 3.Chapelier AR, Missana MC, Couturaud B, Fadel E, Fabre D, Mussot S, Pouillart P, Dartevelle PG. Sternal resection and reconstruction for primary malignant tumors. Ann Thorac Surg. 2004;77:1001–6. doi: 10.1016/j.athoracsur.2003.08.053. [DOI] [PubMed] [Google Scholar]
- 4.Walsh GL, Davis BM, Swisher SG, Vaporciyan AA, Smythe WR, Willis-Merriman K, Roth JA, Putnam JB., Jr A single-institutional, multidisciplinary approach to primary sarcomas involving the chest wall requiring full-thickness resections. J Thorac Cardiovasc Surg. 2001;121:48–60. doi: 10.1067/mtc.2001.111381. [DOI] [PubMed] [Google Scholar]
- 5.Koppert LB, van Geel AN, Lans TE, van der Pol C, van Coevorden F, Wouters MW. Sternal resection for sarcoma, recurrent breast cancer, and radiation-induced necrosis. Ann Thorac Surg. 2010;90:1102–1108. doi: 10.1016/j.athoracsur.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 6.Girotti P, Leo F, Bravi F, Tavecchio L, Spano A, Cortinovis U, Nava M, Pastorino U. The “rib-like” technique for surgical treatment of sternal tumors: lessons learned from 101 consecutive cases. Ann Thorac Surg. 2011;92:1208–15. doi: 10.1016/j.athoracsur.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Soysal O, Walsh GL, Nesbitt JC, McMurtrey MJ, Roth JA, Putnam JB., Jr Resection of sternal tumors: extent, reconstruction, and survival. Ann Thorac Surg. 1995;60:1353–8. doi: 10.1016/0003-4975(95)00641-W. [DOI] [PubMed] [Google Scholar]
- 8.Noble J, Sirohi B, Ashley S, Ladas G, Smith I. Sternal/para-sternal resection for parasternal local recurrence in breast cancer. Breast. 2010;19:350–4. doi: 10.1016/j.breast.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Noguchi S, Miyauchi K, Nishizawa Y, Imaoka S, Koyama H, Iwanaga T. Results of surgical treatment for sternal metastasis of breast cancer. Cancer. 1988;62:1397–401. doi: 10.1002/1097-0142(19881001)62:7<1397::aid-cncr2820620726>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 10.Faneyte IF, Rutgers EJ, Zoetmulder FA. Chest wall resection in the treatment of locally recurrent breast carcinoma: indications and outcome for 44 patients. Cancer. 1997;80:886–91. [PubMed] [Google Scholar]
- 11.Downey RJ, Rusch V, Hsu FI, Leon L, Venkatraman E, Linehan D, Bains M, van Zee K, Korst R, Ginsberg R. Chest wall resection for locally recurrent breast cancer: is it worthwhile? J Thorac Cardiovasc Surg. 2000;119:420–8. doi: 10.1016/s0022-5223(00)70119-x. [DOI] [PubMed] [Google Scholar]
- 12.Warzelhan J, Stoelben E, Imdahl A, Hasse J. Results in surgery for primary and metastatic chest wall tumors. Eur J Cardiothorac Surg. 2001;19:584–8. doi: 10.1016/s1010-7940(01)00638-8. [DOI] [PubMed] [Google Scholar]; Veronesi G, Scanagatta P, Goldhirsch A, Rietjens M, Colleoni M, Pelosi G, Spaggiari L. Results of chest wall resection for recurrent or locally advanced breast malignancies. Breast. 2007;16:297–302. doi: 10.1016/j.breast.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 13.van Geel AN, Wouters MW, van der Pol C, Schmitz PI, Lans T. Chest wall resection for internal mammary lymph node metastases of breast cancer. Breast. 2009;18:94–9. doi: 10.1016/j.breast.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 14.van der Pol CC, van Geel AN, Menke-Pluymers MB, Schmitz PI, Lans TE. Prognostic factors in 77 curative chest wall resections for isolated breast cancer recurrence. Ann Surg Oncol. 2009;16:3414–21. doi: 10.1245/s10434-009-0662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lequaglie C, Massone PB, Giudice G, Conti B. Gold standard for sternectomies and plastic reconstructions after resections for primary or secondary sternal neoplasms. Ann Surg Oncol. 2002;9:472–9. doi: 10.1007/BF02557271. [DOI] [PubMed] [Google Scholar]
- 16.Briccoli A, Manfrini M, Rocca M, Lari S, Giacomini S, Mercuri M. Sternal reconstruction with synthetic mesh and metallic plates for high grade tumours of the chest wall. Eur J Surg. 2002;168:494–9. doi: 10.1080/110241502321116523. [DOI] [PubMed] [Google Scholar]
- 17.Wouters MW, van Geel AN, Nieuwenhuis L, van Tinteren H, Verhoef C, van Coevorden F, Klomp HM. Outcome after surgical resections of recurrent chest wall sarcomas. J Clin Oncol. 2008;26:5113–8. doi: 10.1200/JCO.2008.17.4631. [DOI] [PubMed] [Google Scholar]
- 18.Shen MC, Massarweh NN, Lari SA, Vaporciyan AA, Selber JC, Mittendorf EA, MacGregor MC, Smith BD, Kuerer HM. Clinical course of breast cancer patients with isolated sternal and full-thickness chest wall recurrences treated with and without radical surgery. Ann Surg Oncol. 2013;20:4153–60. doi: 10.1245/s10434-013-3202-4. [DOI] [PubMed] [Google Scholar]
- 19.Weyant MJ, Bains MS, Venkatraman E, Downey RJ, Park BJ, Flores RM, Rizk N, Rusch VW. Results of chest wall resection and reconstruction with and without rigid prosthesis. Ann Thorac Surg. 2006;81:279–85. doi: 10.1016/j.athoracsur.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 20.McCormack P, Bains MS, Beattie EJ, Jr, Martini N. New trends in skeletal reconstruction after resection of chest wall tumors. Ann Thorac Surg. 1981;31:45–52. doi: 10.1016/s0003-4975(10)61315-x. [DOI] [PubMed] [Google Scholar]
- 21.Common Terminology Criteria for Adverse Events, version 4.0. doi: 10.1016/j.jaad.2012.02.010. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-0614_QuickReference_5x7.pdf. Accessed on February 2nd, 2015. [DOI] [PubMed]
- 22.Schmoor C, Sauerbrei W, Bastert G, Schumacher M. Role of isolated locoregional recurrence of breast cancer: results of four prospective studies. J Clin Oncol. 2000;18:1696–708. doi: 10.1200/JCO.2000.18.8.1696. [DOI] [PubMed] [Google Scholar]
- 23.Chagpar A, Meric-Bernstam F, Hunt KK, Ross MI, Cristofanilli M, Singletary SE, Buchholz TA, Ames FC, Marcy S, Babiera GV, Feig BW, Hortobagyi GN, Kuerer HM. Chest wall recurrence after mastectomy does not always portend a dismal outcome. Ann Surg Oncol. 2003 Jul;10(6):628–34. doi: 10.1245/aso.2003.01.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.