Abstract
Cholera remains an important global cause of morbidity and mortality, which is capable of causing periodic epidemic disease. A number of mathematical models have been developed to help in understanding the dynamics of cholera outbreaks and for use as a tool in planning interventions, including vaccination campaigns. We have explored the utility of models in assessing the spread of cholera in the recent epidemics in Zimbabwe and Haiti. In both instances, a mathematical model was formulated and fitted to cumulative cholera cases to estimate the basic reproductive number ℜ0, and the partial reproductive numbers reflecting potential differences in environmental-to-human versus human-to-human transmission were quantified. In Zimbabwe, estimated ℜ0 for the epidemic using aggregated data at the national level was 1.15; in Haiti, it was 1.55. However, when calculated at a provincial/departmental level, estimated basic reproductive numbers were highly heterogeneous, with a range of 1.11 to 2.72 in Zimbabwe and 1.06 to 2.63 in Haiti. Our models suggest that the underlying patterns of cholera transmission varied widely from region to region, with a corresponding variation in the amenability of outbreaks to control measures such as immunization. These data underscore the heterogeneity of transmission dynamics, potentially linked to differences in environment, socio-economic conditions, and cultural practices. They also highlight the potential utility of these types of models in guiding development of public health intervention strategies.
INTRODUCTION
Historical reports of cholera-like illness can be traced back almost a millennium in South Asia (1). Global, pandemic spread of cholera from its ancestral home in Bengal was first documented in 1817, with the beginning of what has been designated as the first pandemic. We are currently in the throes of the seventh pandemic (caused by toxigenic Vibrio cholerae of the El Tor bio-type), which originated almost 50 years ago in the Celebes. In contrast to the earlier six pandemics, at no time in these past 50 years has cholera retreated to its south Asian home. It has instead established endemicity at multiple sites around the globe and continues to trigger major localized epidemics, including the epidemics in Zimbabwe during 2008 to 2009 (2, 3) and the ongoing epidemics in Haiti that began in 2010 (4). These recent epidemics, which were characterized by increased severity and duration, have demonstrated the need for ongoing work to optimize public health responses and develop prevention strategies for this disease.
To better understand the underlying dynamics of these epidemics, we have developed a series of mathematical models of cholera transmission (5, 6). A key element of these models has been the quantifying of the basic reproductive number of disease transmission (ℜ0) at a regional level, to assess possible geographic differences in transmission dynamics, and to permit better targeting of interventions based on these differences. These models also take into account potential differences in transmission related to what have been characterized as “direct” transmission of the microorganism (direct transmission from human-to-human during the short period of time when the microorganism is “hyper-infectious” after passage in stool) versus “indirect,” or transmission from environment-to-human (7, 8, 9, 10).
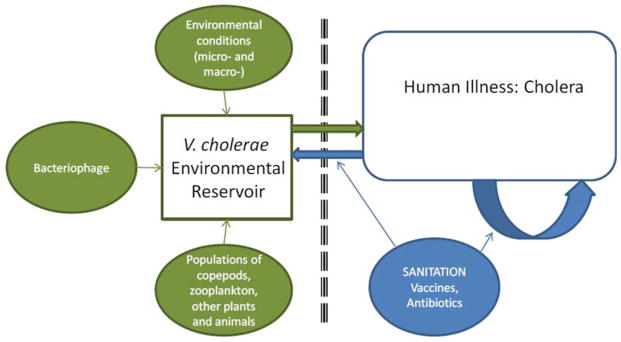
This separation of direct versus indirect transmission pathways is reflected in the conceptual framework shown in Fig. 1 [from Morris (10)]. In this framework, the aquatic, environmental reservoir is critical to long-term maintenance of epidemic V. cholerae. These reservoirs constitute complex biologic systems, with modulation of V. cholerae populations by environmental conditions [the local microenvironment (11, 12)], as well as global macroenvironmental factors, such as the El Niño/Southern Oscillation (ENSO) (13), by predatory bacteriophage populations (14), and by fluctuations in populations of copepods and zooplankton (which may, in turn, be driven by predation by fish) (15), binding to chironomid egg masses, water hyacinth, carriage by birds and mammals, and a host of other variables. However, once the microorganism moves from these sources into human populations, we hypothesize that transmission accelerates, driven by the potential for rapid transmission of hyperinfectious microorganisms to susceptible individuals, with, possibly, a short intervening step in the immediate household environment of the patient (10).
FIGURE 1.
Conceptual framework for cholera transmission. From Morris (10). doi:10.1128 /microbiolspec.VE-0011-2014.f1
Here, we review and compare our modeling studies on cholera for Zimbabwe and Haiti (5, 6) and bring to the fore their implication to the understanding of cholera dynamics and control.
METHODS
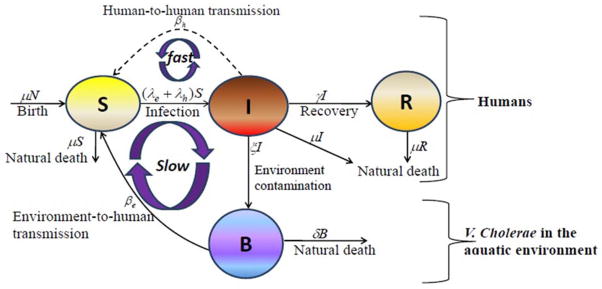
Details regarding the development and application of our models to the epidemics in Zimbabwe and Haiti are reported in Mukandavire et al. (5, 6). In brief, the cholera model compartmentalizes the human population (Fig. 2), of population size N, into susceptibles S, infected I, and recovered R individuals. The concentration of vibrios in contaminated water is denoted by B. Susceptible individuals acquire cholera infection either by ingesting environmental V. cholerae from contaminated aquatic reservoirs (“indirect” transmission, higher infectious dose) or through close contact with infected humans (“direct” transmission, lower infectious dose) at daily per-capita rates and λh= βhI, respectively, with the subscripts e and h denoting environment-to-human and human-to-human transmission routes. The constant k is a shape parameter that determines the human infectious dose: when B equals k, the probability of ingestion resulting in human disease is 0.5. βe and βh are rates of exposure to V. cholerae from the contaminated environment and through human-to-human interaction, respectively. Infected individuals recover from infection at a rate γ. Infected individuals contribute V. cholerae in the aquatic environment at a daily rate χ and vibrios have a net death rate δ in the environment. The resulting model as a system of coupled stochastic differential equations (6) is as follows.
FIGURE 2.
Model flow diagram. S, susceptibles; I, infected; R, recovered individuals; B, concentration of vibrios in contaminated water. From Mukandavire et al. (6). doi:10.1128 /microbiolspec.VE-0011-2014.f2
| (1) |
Where, αi for i = 1,2 are real constants and ξ (t)=(ξ1(t), ξ2(t)) is the Gaussian white noise process to model environmental stochasticity.
For the Zimbabwe epidemic (5), we fitted the deterministic version of the cholera model system (Eq. 1) to weekly data on numbers of cholera cases reported to the Zimbabwe Ministry of Health and Child Welfare (MoHCW) for the period from November 13, 2008, to July 31, 2009. This is the period with a complete cholera data set for all of the provinces in Zimbabwe and marked the onset of the national epidemic. We obtained this data set from the Epidemiology and Disease Control department in the Ministry of Health and Child Welfare. For Haiti, we used daily data on numbers of hospitalized cases published on the Ministry of Public Health and Population (MSPP) website (16). These data may well be underestimates due to weaknesses in the health systems in Zimbabwe and Haiti. However, despite quality issues surrounding the data sets, they presented the best available platform for quantifying the magnitude of the epidemics.
RESULTS
The basic reproductive number (ℜ0) is defined as a measure of the average number of secondary cases generated by a primary case. Understanding its magnitude and variation can help to identify cholera “hot spots” and in designing targeted surveillance and intervention programs. In our models, the two transmission routes of cholera are quantitatively described by partial reproductive numbers, ℜh and ℜe that describe new cases that arise from either the direct human-to-human or the indirect environment-to-human transmission routes, respectively. Estimates for ℜe, ℜh, and ℜ0 for the 10 provinces and the whole country of Zimbabwe are given in Table 1. Similar data for Haiti, by department, are shown in Table 2. The plots for the data fitting can be found in Mukandavire et al. (5, 6).
TABLE 1.
Estimates of ℜe, ℜh, ℜ0, and minimum vaccination coverages for Zimbabwe
| Provinces | Population Size/1000 | ℜe | 95% CI | % ℜ0 | ℜh | 95 % CI | % ℜ0 | ℜ0 | 95 % CI | Vaccination Coverage Resulting in ℜ0 <1 |
|---|---|---|---|---|---|---|---|---|---|---|
| Harare | 2012.78 | 0.9 | (0.57–1.24) | 59.4 | 0.62 | (0.57–0.72) | 40.6 | 1.52 | (1.14–1.96) | 44 |
| Bulawayo | 718.28 | 0.14 | (0.071–0.22) | 10.6 | 1.22 | (1.05–1.39) | 89.4 | 1.36 | (1.12–1.61) | 34 |
| Mashonaland Central | 1056.67 | 0.2 | (0.11–0.29) | 14.3 | 1.18 | (1.11–1.25) | 85.7 | 1.38 | (1.21–1.54) | 35 |
| Mashonaland East | 1196.77 | 0.45 | (0.31–0.58) | 40.4 | 0.66 | (0.58–0.74) | 59.6 | 1.11 | (0.90–1.32) | 13 |
| Mashonaland West | 1300.01 | 0.32 | (0.01–0.62) | 16.9 | 1.54 | (1.33–1.76) | 83.1 | 1.87 | (1.34–2.38) | 59 |
| Midlands | 1554.06 | 0.077 | (0.012–0.14) | 5.5 | 1.31 | (1.21–1.41) | 94.5 | 1.39 | (1.23–1.56) | 36 |
| Manicaland | 1665.45 | 0.2 | (0.099–0.29) | 9.5 | 1.87 | (1.68–2.05) | 90.5 | 2.06 | (1.78–2.34) | 66 |
| Matebeleland South | 693.23 | 1.44 | (0.68–2.2) | 52.9 | 1.28 | (0.52–2.04) | 47.1 | 2.72 | (1.19–4.24) | 81 |
| Matebeleland North | 748.32 | 0.14 | (0.036–0.24) | 8 | 1.58 | (1.4–1.76) | 92 | 1.72 | (1.44–1.99) | 53 |
| Masvingo | 1401.67 | 0.25 | (0.047–0.46) | 15.8 | 1.36 | (1.15–1.56) | 84.2 | 1.61 | (1.20–2.03) | 49 |
| Zimbabwe [Country] | 12347.24 | 0.2 | (0.15–0.25) | 17.3 | 0.95 | (0.93–0.98) | 82.7 | 1.15 | (1.08–1.23) | 17 |
CI, confidence interval.
TABLE 2.
Estimates of ℛe, ℛh, ℛ0, and minimum vaccination coverages for Haiti
| Department | Population size/1000 | ℜe | SE | % ℜ 0 | ℜh | SE | % ℜ 0 | ℜ 0 | SE | Vaccination coverage resulting in ℜ0 <1 |
|---|---|---|---|---|---|---|---|---|---|---|
| Haiti (Country) | 9923.24 | 0.84 | 7.00 × 10−1 | 54.01 | 0.71 | 0.29 | 45.35 | 1.55 | 0.41 | 45.4 |
| Artibonite | 1571.02 | 2.54 | 2.32 × 10−1 | 96.70 | 0.09 | 0.06 | 3.30 | 2.63 | 0.18 | 79.5 |
| Centre | 678.63 | 0.58 | 4.82 × 10−1 | 42.12 | 0.79 | 0.24 | 57.88 | 1.37 | 0.24 | 34.3 |
| Grande Anse | 425.88 | 0.59 | 4.31 × 10−1 | 46.31 | 0.68 | 0.09 | 53.70 | 1.27 | 0.35 | 27.2 |
| Nippes | 311.5 | 0.09 | 2.84 × 10−4 | 8.86 | 0.96 | 0.21 | 91.15 | 1.06 | 0.21 | 6.9 |
| Nord | 970.5 | 0.16 | 7.79 × 10−2 | 10.24 | 1.37 | 0.24 | 89.76 | 1.53 | 0.23 | 44.4 |
| Nord Ouest | 662.78 | 0.20 | 2.56 × 10−4 | 14.26 | 1.20 | 0.11 | 85.74 | 1.40 | 0.11 | 36.4 |
| Nord Est | 358.28 | 0.22 | 1.43 × 10−3 | 14.99 | 1.22 | 0.21 | 85.01 | 1.44 | 0.21 | 38.9 |
| Ouest1 (Ouest) | 1187.83 | 0.45 | 2.04 × 10−4 | 37.67 | 0.74 | 0.06 | 62.33 | 1.18 | 0.06 | 19.9 |
| Port-au-Prince (Ouest) | 2476.79 | 0.74 | 3.68 × 10−4 | 39.27 | 1.15 | 0.13 | 60.73 | 1.89 | 0.13 | 60.5 |
| Sud | 704.76 | 0.21 | 1.32 × 10−3 | 14.65 | 1.23 | 0.09 | 85.36 | 1.44 | 0.09 | 39.5 |
| Sud Est | 575.29 | 0.13 | 2.31 × 10−4 | 11.11 | 1.04 | 0.14 | 88.89 | 1.17 | 0.14 | 18.3 |
| Ouest | 3664.62 | 0.43 | 1.78 × 10−3 | 25.41 | 1.30 | 0.30 | 74.75 | 1.73 | 0.30 | 54.2 |
Ouest department includes Port-au-Prince and Ouest1 hospitalized cases. The population estimates were extracted from http://www.citypopulation.de/Haiti.html.
The basic reproduction numbers provide useful guidelines for the prevention and control of cholera. For example, a vaccination program has to achieve a minimum coverage of
contain a cholera epidemic, where r is the fraction of the vaccinated population who are completely immunized (i.e., with zero susceptibility), and s is the proportional reduction of the susceptibility for those partially immunized. For r = 0 and s = 78% (17, 18), regional vaccination coverages that would have been required to contain the Zimbabwe and Haitian epidemics (Tables 1 and 2) range from about 7% to 82%.
DISCUSSION
The term ℜ0 plays a critical conceptual role in our understanding of the epidemiology of cholera and other infectious diseases. While acknowledging differences in R0 that may arise when comparing different models and model assumptions (19), our data underscore the heterogeneity that may be seen in ℜ0 within a single model as one moves from province to province (or department to department) in the midst of an epidemic. Nonetheless, while there is variability, the ranges from the Zimbabwe and Haiti studies are reasonably close, and, with the exception of the work by Chunara et al. (20), these results are in the same general range as the estimates from other mathematical models of the initial Haitian epidemic (21, 22, 23).
Such heterogeneity may be driven by a number of factors, including environmental factors, socio-economic conditions, and cultural practices. In the absence of more detailed epidemiologic data from these epidemics, it is difficult to identify the specific factors that are operational in each instance. However, at the environmental level, it is interesting to note that in the Zimbabwe model, “indirect” transmission through the environment contributes only a small component (17%) of the total value of ℜ0 using aggregated country level data. In the Haiti model, in contrast, indirect transmission through the environment was estimated to contribute 54% of the value of ℜ0 at a national level. When calculated as the average of values across provinces/departments, differences are still apparent, with an average value of 25% for the contribution of the indirect environmental route to ℜ0 for Zimbabwe and 38% for Haiti. These differences are not unexpected, given that Zimbabwe is land-locked, without the coastline and estuarine areas (present in Haiti) that are known to represent ideal environmental reservoirs for V. cholerae. Interestingly for Haiti, the model also identified Artibonite as a potential disease “hot spot” with ℜ0 values for outbreaks for surrounding departments slightly higher than those further away.
This regional variability in ℜ0 has a potential impact on interventions to limit or prevent further cholera transmission. Our models suggest that, even with limited vaccine uptake, there are certain regions where vaccination campaigns could have a greater impact on disease transmission: that is, the model supports regional targeting of vaccination programs. Regions with a greater concentration of cases linked with “indirect” acquisition of V. cholerae from the environment may benefit most from implementation of strategies to improve water sources and community sanitation; in contrast, for those with high levels of direct, person-to-person transmission, a strong emphasis on household hygiene may be important.
While acknowledging the limitations inherent in these and similar models, mathematical models of disease transmission provide a valuable means of assessing the complex set of variables that come into play in cholera epidemiology. They also have the potential for improving our ability to select and target interventions, particularly at a regional level, so that limited resources that may be available in an outbreak situation can be best allocated to prevent spread of this potentially devastating disease.
Acknowledgments
Work supported in part by NIH grant RO1AI097405 (J. Glenn Morris). Z. Mukandavire was also supported by the UF Science for Life Program, an interdisciplinary program with support from the Howard Hughes Medical Institute.
Footnotes
Conflicts of interest: We declare no conflicts.
References
- 1.Pollitzer R. Cholera. World Health Organization; Geneva, Switzerland: 1959. [Google Scholar]
- 2.WHO. Outbreak news. Cholera, Zimbabwe. Wkly Epidemiol Rec. 2008;83:449–450. [PubMed] [Google Scholar]
- 3.Koenig R. Public health, international groups battle cholera in Zimbabwe. Science. 2009;323:860–861. doi: 10.1126/science.323.5916.860. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. [Accessed February 10, 2011];Haiti cholera outbreak: cholera confirmed in Haiti . 2011 October 21, 2010. http://www.cdc.gov/haiticholera/situation/
- 5.Mukandavire Z, Liao S, Wang J, Gaff H, Smith DL, Morris JG., Jr Estimating the reproductive numbers for the 2008–2009 cholera outbreaks in Zimbabwe. Proc Natl Acad Sci USA. 2011;108:8767–8772. doi: 10.1073/pnas.1019712108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukandavire Z, Smith DL, Morris JG., Jr Cholera in Haiti: reproductive numbers and vaccination coverage estimates. Sci Rep. 2013;3:997. doi: 10.1038/srep00997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merrell DS, Butler SM, Qadri F, Dolganov NA, Alam A, Cohen MB, Calderwood SB, Schoolnik GK, Camilli A. Host-induced epidemic spread of the cholera bacterium. Nature. 2002;417:642–645. doi: 10.1038/nature00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartley DM, Morris JG, Jr, Smith DL. Hyperinfectivity: A critical element in the ability of V. cholerae to cause epidemics? PLoS Med. 2006;3:e7. doi: 10.1371/journal.pmed.0030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson EJ, Harris JB, Morris JG, Jr, Calderwood SB, Camilli A. Cholera transmission: the host, pathogen, and bacteriophage dynamic. Nat Rev Microbiol. 2009;7:693–702. doi: 10.1038/nrmicro2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris JG., Jr Cholera: modern pandemic disease of ancient lineage. Emerg Infect Dis. 2011;17:2099–2104. doi: 10.3201/eid1711.111109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franco AA, Fix AD, Prada A, Paredes E, Palomino JC, Wright AC, Johnson JA, McCarter R, Guerra H, Morris JG., Jr Cholera in Lima, Peru, correlates with prior isolation of Vibrio cholerae from the environment. Am J Epidemiol. 1997;146:1067–1075. doi: 10.1093/oxfordjournals.aje.a009235. [DOI] [PubMed] [Google Scholar]
- 12.Huq A, Sack RB, Nizam A, Longini IM, Nair GB, Ali A, Morris JG, Jr, Khan MN, Siddique AK, Yunus M, Albert MJ, Sack DA, Colwell RR. Critical factors influencing the occurrence of Vibrio cholerae in the environment in Bangladesh. Appl Environ Microbiol. 2005;71:4645–4654. doi: 10.1128/AEM.71.8.4645-4654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodo X, Pascual M, Fuchs G, Faruque AS. ENSO and cholera: a nonstationary link related to climate change? Proc Natl Acad Sci USA. 2002;99:12901–12906. doi: 10.1073/pnas.182203999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson EJ, Chowdhury A, Flynn J, Schild S, Bourassa L, Shao Y, LaRocque RC, Calderwood SB, Qadri F, Camilli A. Transmission of Vibrio cholerae is antagonized by lytic phage and entry into the aquatic environment. PLoS Pathog. 2008;4:e1000187. doi: 10.1371/journal.ppat.1000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinelli Filho JE, Lopes RM, Rivera ING, Colwell RR. Vibrio cholerae O1 detection in estuarine and coatal zooplankton. J Plankton Res. 2011;33:51–62. [Google Scholar]
- 16.Ministry of Public Health and Population. [Accessed February 2, 2011];MSPP website. 2011 http://mspp.gouv.ht/site/index.php?option=com_content&view=article&id=57&Itemid=1.
- 17.Lucas M, Deen JL, von Seidlein L, Wang XY, Ampuero J, Puri M, Ali M, Ansaruzzaman M, Amos J, Cavailler P, Guerin P, Mahoudeau C, Kahozi P, Chaignat CL, Barreto A, Songane FF, Clemens JD. Effectiveness of mass oral cholera vaccination in Beira, Mozambique. N Engl J Med. 2005;352:757–767. doi: 10.1056/NEJMoa043323. [DOI] [PubMed] [Google Scholar]
- 18.Gangarosa EJ, Mosley WH. Epidemiology and surveillance of cholera. In: Barua D, Burrows W, editors. Cholera. WB Saunders; Philadelphia, PA: 1974. pp. 381–403. [Google Scholar]
- 19.Li J, Blakeley D, Smith RJ. The failure of R0. Comput Math Methods Med. 2011;2011:527610. doi: 10.1155/2011/527610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chunara R, Andrews JR, Brownstein JS. Social and news media enable estimation of epidemiological patterns early in the 2010 Haitian cholera outbreak. Am J Trop Med Hyg. 2012;86:39–45. doi: 10.4269/ajtmh.2012.11-0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bertuzzo E, Mari L, Righetto L, Gatto M, Casagrandi R, Blokesch M, Rodriguez-Iturbe I, Rinaldo A. Prediction of the spatial evolution and effects of control measures for the unfolding Haiti cholera outbreak. Geophys Res Lett. 2011;38:L06403. [Google Scholar]
- 22.Chao DL, Halloran ME, Longini IM. Vaccination strategies for epidemic cholera in Haiti with implications for the developing world. Proc Natl Acad Sci USA. 2011;108:7081–7085. doi: 10.1073/pnas.1102149108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuite AR, Tien J, Eisenberg M, Earn DJD, Ma J, Fisman DN. Cholera epidemic in Haiti, 2010: using a transmission model to explain spatial spread of disease and identify optimal control interventions. Ann Intern Med. 2011;154:293–302. doi: 10.7326/0003-4819-154-9-201105030-00334. [DOI] [PubMed] [Google Scholar]