Abstract
We report a high-risk cancer family with multiple mesotheliomas, cutaneous melanomas, basal cell carcinomas, and meningiomas segregating with a germline nonsense mutation in BAP1 (c.1938T>A; p.Y646X). Notably, most (four of five) mesotheliomas were peritoneal rather than the usually more common pleural form of the disease, and all five mesothelioma patients also developed second or third primary cancers, including two with meningiomas. Another family member developed both cutaneous melanoma and breast cancer. Two family members had basal cell carcinomas, and six others had melanocytic tumors, including four cutaneous melanomas, one uveal melanoma, and multiple benign melanocytic tumors. The family resides in a subtropical area, and several members had suspected exposure to asbestos either occupationally or in the home. We hypothesize that the concurrence of a genetic predisposing factor and environmental exposure to asbestos and UV irradiation contributed to the high incidence of multiple cancers seen in this family, specifically mesothelioma and various skin tumors, respectively.
Keywords: malignant mesothelioma, asbestos, familial cancer, cancer predisposition, BAP1
1. Introduction
Malignant mesothelioma (MM) is an aggressive cancer typically caused by asbestos exposure and accounts for approximately 3,200 deaths per year in the U.S. [1]. Prognosis is poor in MM patients due to chemo- and radio-resistance as well as the ineffectiveness of surgical intervention [2]. Cutaneous melanoma (CM) and uveal melanoma (UM) are also aggressive cancers involving melanocytes of the skin and eye, respectively [3]. UMs account for less than 4% of all melanoma cases and are more common among Caucasians, with an estimated incidence of 6 per million (~1,800 cases) in the U.S. [4]. About half of UM patients have metastatic disease, with poor survival rate when metastasis to the liver is involved [4]. Notably, inactivating somatic mutations of the tumor suppressor gene encoding the BRCA1-associated protein 1 (BAP1) have been reported in nearly 85% of metastasizing UMs [5]. Dissimilar to CMs, ultraviolet (UV) light does not have a clear role in the cause of UMs [4], at least in the absence of a predisposing genetic alteration.
Some individuals develop MM when exposed to small amounts of asbestos, whereas others do not develop MM despite large asbestos exposures [6]. Also, uveal melanomas are not strongly associated with sun exposure, unlike in CM [4]. Thus, these observations suggest the possibility of a role for genetic involvement in families where there is increased susceptibility to MM and UM. The cloning and identification of somatic mutations of the BAP1 tumor suppressor gene in lung and breast cancer cells were first reported by the Rauscher laboratory [7]. Subsequently, BAP1 somatic mutations were discovered in MM [8, 9] and metastasizing UMs [5]. We reported the discovery of germline mutations of BAP1 in two families with multiple MMs, one of which had two family members with UMs (one also having MM), as well as in two additional cases having both MM and UM [9]. Simultaneously, germline BAP1 mutations were reported in two families with atypical melanocytic tumors, CM, and UM [10]. Subsequent work by several groups have confirmed these findings and/or further extended the phenotype to other tumor types [11–15]. Here we describe a new BAP1 family with an unusually high incidence of MMs, CMs, basal cell carcinomas and meningiomas, with five MM patients also developing second or third primary cancers.
2. Materials and Methods
2.1 Patients
All study participants gave informed consent for their participation, and the research protocol was approved by the Institutional Review Board of Fox Chase Cancer Center. Disease diagnoses were based on pathology reports obtained from various hospital laboratories. We also performed a personal interview of the proband, who is a physician, regarding family medical history as well as sun and asbestos exposures. Blood samples from 10 family members were collected and sent to Fox Chase for DNA sequence analysis of BAP1.
2.2 Sequence Analysis
PCR products encompassing all BAP1 coding exons and adjacent intron sequences were amplified for sequencing. The primers and PCR conditions were previously described [16]. The Human Genome Variation Society (HGVS, http://www.hgvs.org/mutnomen) standardized mutation nomenclature was used to describe the BAP1 mutation using cDNA accession # NM_004656 and protein accession # NP_004647 as references.
2.3 Chromosome Microarray Analysis (CMA)
The CMA was performed with Affymetrix Oncoscan arrays. Total genomic DNA was digested with NspI restriction enzyme and ligated to adapters that recognize cohesive 4-basepair (bp) overhangs. A generic primer that recognizes the adapter sequence was used to amplify the adapter-ligated DNA fragments. Amplification products were purified using magnetic beads, fragmented, biotin-labeled, and hybridized to the arrays according to the manufacturer’s recommendations. Next, the hybridized array was washed and scanned with a GeneChip Scanner 3000 7G. The intensities of probe hybridization signals were analyzed by using Affymetrix’s GeneChip Command Console, and copy number analyses were performed using Affymetrix Chromosome Analysis Suite software with default settings.
2.4 Immunohistochemistry
Detection of BAP1 in tumor tissues was performed using a BAP1 antibody (C4, from Santa Cruz Biotechnology), as reported elsewhere [9].
3. Results
This extended family (pedigree shown in Fig. 1) first came to our attention due to the large number of individuals with MMs and various melanocytic tumors. Genomic DNA was isolated from blood of all 10 participants for mutation screening of BAP1. We identified a nonsense mutation in exon 15 (c.1938T>A), which is predicted to result in nonsense-mediated decay of the BAP1 mRNA or truncation of the translated BAP1 protein (p.Tyr646X), with loss of the C-terminal nuclear localization signal (Fig. 2). Individuals who tested positive for this mutation and the types of cancers are shown in the family pedigree (Fig. 1).
Figure 1.
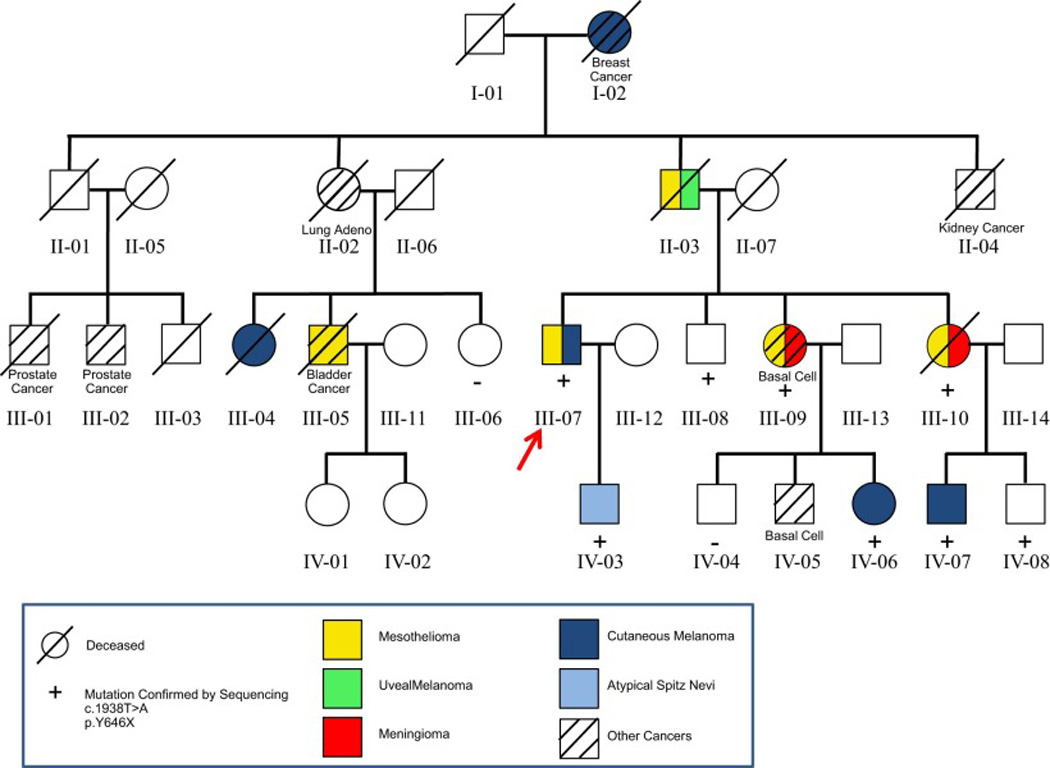
Pedigree of family reported here, whose germline DNA contained a nonsense mutation in BAP1 exon 15 (c.1938T>A). The family of the proband (III-07, arrow) shows numerous malignancies, including six family members (I-02, II-03, III-05, III-07, III-09, and III-10) with two or more primary cancers. Predominant tumors include malignant mesothelioma and cutaneous melanoma, each observed in five family members.
Figure 2.
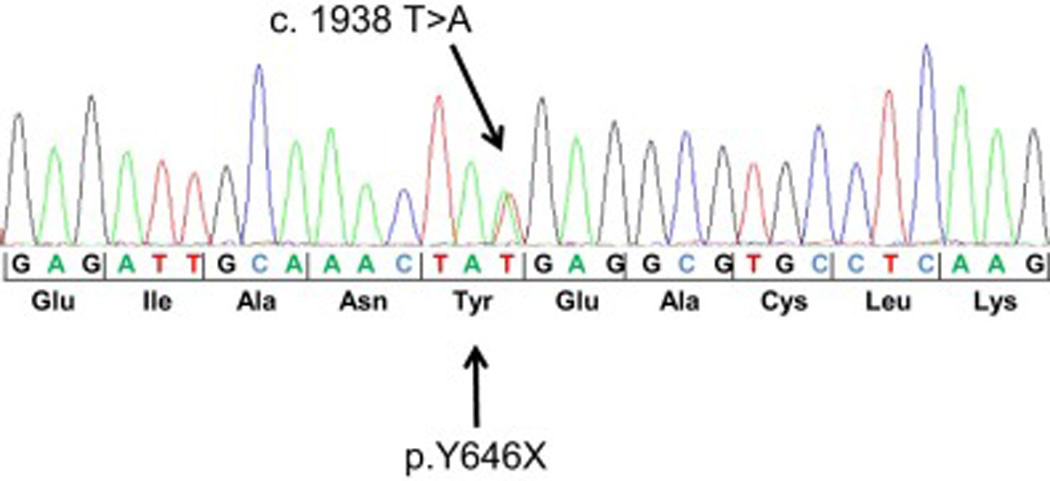
Electropherogram of BAP1 mutation of the index case. The nonsense mutation c.1938T>A is predicted to lead to a BAP1 protein truncation (p.Y646X) or nonsense-mediated decay of the BAP1 mRNA.
The proband, III-07, developed epithelioid peritoneal MM and a superficial CM. His son (IV-03) was diagnosed with a single atypical Spitz nevus, also called juvenile melanoma, as well as several other atypical nevi, at 17 years of age. The index case’s two sisters (III-09 and III-10) both developed meningioma as well as MM (thoracic and peritoneal, respectively); III-09 also developed a third primary tumor: a basal cell carcinoma. Meningioma and MM tissues that were available for case III-10 revealed loss of nuclear BAP1 staining in both tumor tissues but not in adjacent normal brain parenchyma or stroma, respectively (Fig. 3), suggesting biallelic inactivation of the BAP1 gene. To confirm this possibility at the chromosomal and DNA level, meningioma tumor tissue was macrodissected from formalin-fixed, paraffin-embedded sections for DNA isolation. Affymetrix SNP-based copy number analysis of the meningioma DNA revealed only a single copy of chromosome 3, where the BAP1 gene is located (Fig. 4A). Sequencing of the BAP1 gene in the same tumor DNA showed that the vast majority of the remaining BAP1 allele was the mutant copy (Fig. 4B). The copy number analysis also revealed several other genomic imbalances in the meningioma, including losses of one copy each of entire autosomes 1, 4, 8, 9, 13, 14, and 16 in about 50% of cells (Supplemental Fig. 1). Notably, monosomy 3 appears to be the primary (driver) somatic genetic change in this tumor, given that this copy number loss was observed in nearly 100% of tumor cells, with the most clear evidence for loss of heterozygosity (LOH) among all the chromosomal losses observed. One child from each of these two sisters with MM and meningioma developed CM (IV-06 and IV-07). Even though DNA was not available for testing of the proband’s father (II-03), it is likely that he was a BAP1 mutation carrier as he developed both peritoneal MM and UM. Interestingly, this individual survived his initial tumor, UM, but died 30 years later due to MM at 74 years of age. The cousins of the proband developed CM (III-04) or peritoneal MM and bladder cancer (III-05).
Figure 3.
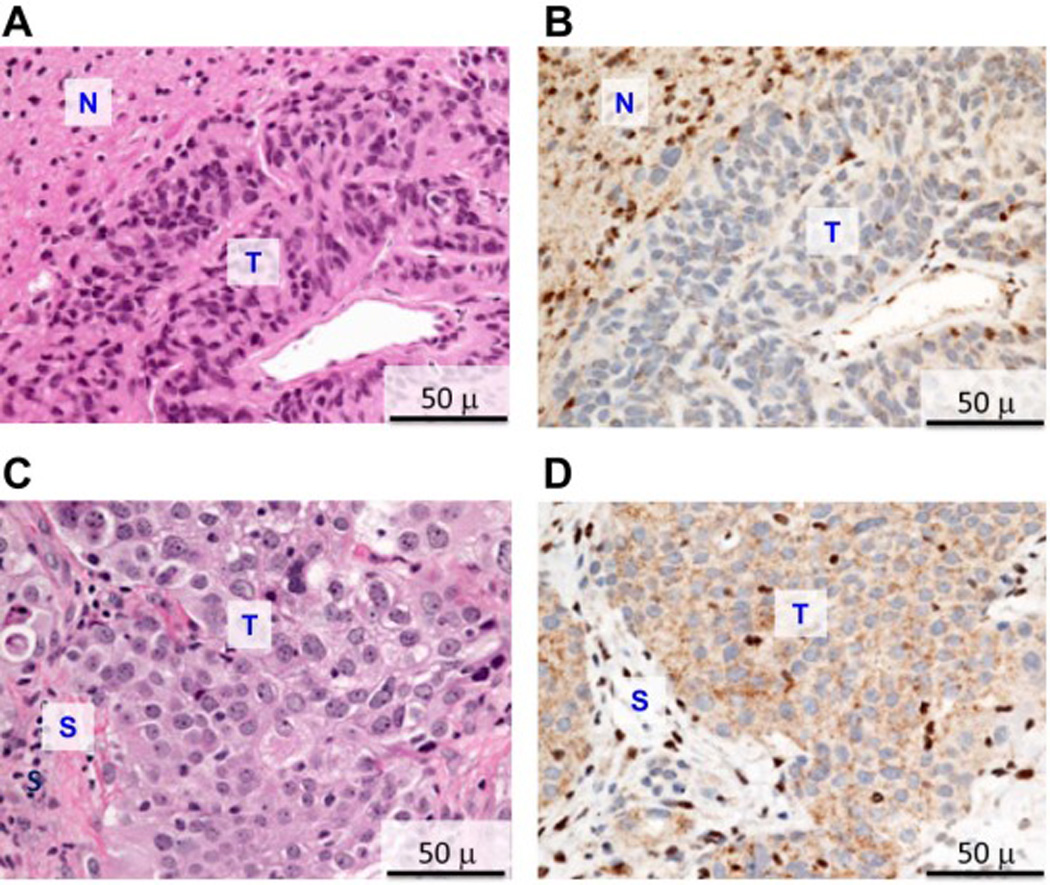
H&E and immunohistochemical staining of meningioma and mesothelioma tissues from patient III-10. A) H&E staining of meningioma. B) BAP1 immunohistochemistry of meningioma, showing absence of nuclear BAP1 staining in tumor (T) and both cytoplasmic and strong nuclear staining in adjacent normal brain parenchyma (N). C) H&E staining of peritoneal mesothelioma. D) BAP1 immunohistochemistry of same mesothelioma, showing absence of nuclear BAP1 staining in tumor (T) and both cytoplasmic and strong nuclear staining in normal stroma (S).
Figure 4.
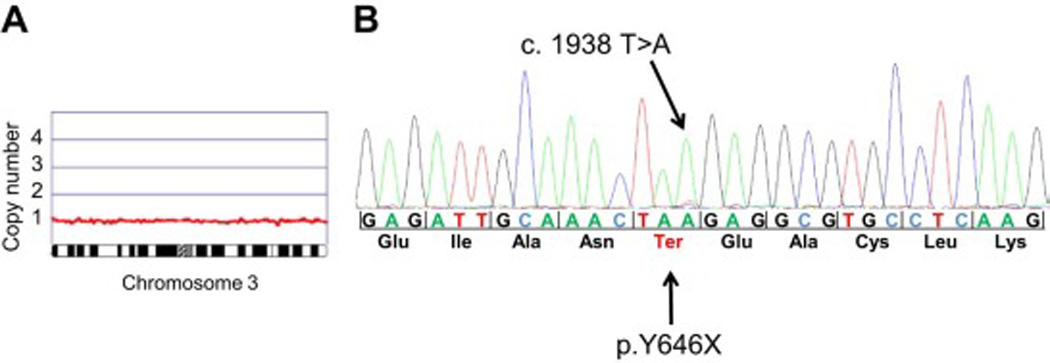
DNA sequencing and chromosome microarray analysis of DNA from FFPE meningioma tissue of patient III-10. A) Oncoscan DNA copy number and allele analysis revealing loss of one copy of chromosome 3. B) Sequence analysis of BAP1 analysis of tumor sample showing loss predominantly of the wild-type BAP1 allele and retention of the mutated BAP1 allele.
4. Discussion
Peritoneal MM accounts for about 20% of all MMs [17]. Epidemiological and animal studies have shown that asbestos exposure is a major cause of peritoneal as well as pleural MM [17, 18]. Three members of the family (I-01, II-03, and II-04) were involved in a construction business in the 1930’s, one of whom (II-03) developed MM. Individual II-03 helped his father build homes while in high school and worked with framing, siding, and concrete/plaster mixing materials as well as with sheet rock. Individual I-01 constructed an addition to his house in the early 1960s, which lasted about 3 months. Construction materials used at the time included asbestos roofing shingles. Since asbestos was widely used before the health problems associated with these fibers were clearly delineated [19], it is likely that asbestos was also present in other materials used in the family’s construction business. Another family member who developed MM (III-09: pleural MM) lived in a home during the time of removal of asbestos shingles from the roof and potentially also was exposed.
MM is generally regarded as a cancer of the 60s and 70s, with a median of about 64 years of age [20]. The age of diagnosis in the five MM cases in this family was 55–74, with a median of 61 years of age.
All nine family members with CM, UM, basal carcinoma or atypical Spitz tumor grew up in Florida, a subtropical area of the U.S. characterized by year-round high levels of sun exposure due to the locale’s proximity to the equator [21], thus possibly contributing to the high incidence of these tumors in this family. Additionally, although UV light does not have a clear role in the cause of UM in the general population [4], BAP1 mutation carriers may be more prone to the carcinogenic effects of sunlight, similar to the way that asbestos-exposed mice carrying a Bap1 mutation show an increased incidence of MM compared to that observed in asbestos-exposed wild type littermates [22].
In addition to the high incidence of MM and various skin and ocular tumors, this family showed another notable tumor type: meningioma. Two sisters (III-09 and III-10) were diagnosed with meningioma as well as MM, and both were found to carry a BAP1 mutation. Similarly, Abdel-Rahman et al. reported a BAP1 family with two meningiomas, one of which was confirmed to be a mutation carrier [11]. Thus, two independent studies indicate that meningioma should be included in the spectrum of neoplasms connected with the BAP1 tumor predisposition syndrome.
It is also noteworthy that family member IV-03, another BAP1 mutation carrier, had an atypical Spitz nevus, and well-documented studies have shown that such benign melanocytic tumors can be the predominant type of tumor in some families with germline mutation of BAP1 [10, 12]. Interviews of III-07, a physician, revealed that individual IV-03 and other younger members of the family who developed CM all had had significant sun exposure.
Other family members had lung, breast or kidney cancers, each of which has been previously reported in some BAP1 families. However, germline DNA was not available for analysis in these individuals. While BAP1 is now recognized as a renal cell carcinoma-predisposition gene [23, 24], additional studies are required to determine whether lung and breast carcinoma belong in the BAP1 syndrome or, instead, represent background consistent with the high incidence of these cancers generally [25].
We previously reported in a mouse model that germline mutation of Bap1 predisposes to the tumorigenic effects of asbestos and that high penetrance of MM requires such environmental exposure [22]. The high incidence of MMs and various skin tumors and UM in the family reported here suggests a devastating perfect storm caused by a potent concurrence of factors. Specifically, the data provide evidence for a gene-environment interaction involving BAP1 and environmental exposure to asbestos and UV irradiation. The findings in this highly unusual family provide strong evidence for a BAP1 cancer syndrome characterized by susceptibility to a growing list of tumor types, including MM, CM, UM, basal cell carcinoma, atypical Spitz nevi, RCC, meningioma, and potentially other neoplasms in association with germline mutation of BAP1. MM and UM are the two cancer types most frequently reported in BAP1 mutation carriers, with a recent review uncovering 39 of 174 (22%) reported BAP1 mutation carriers having MM and 54 of 174 (31%) having UM [26]. Moreover, somatic mutations and deletions of BAP1 have been reported in 60–65% of sporadic MMs [27, 28] and in ~85% of metastasizing UMs [5], lending further support for a strong connection between BAP1 inactivation and these two malignancies. Why somatic BAP1 mutations are so common in these diseases that involve tissues from different germ layers is currently unknown. Notably, a similar situation occurs with regard to germline mutations of TP53 in Li-Fraumeni syndrome, in which the most common types of cancer observed, i.e., osteosarcoma, soft-tissue sarcoma, acute leukemia, breast cancer, brain cancer and adrenal cortical tumors, are derived from a variety of embryonic tissues from the ectoderm and mesoderm [25, 29]. Moreover, as with BAP1, somatic mutations of TP53 are frequent in cancers originating in tissues from various germ layers.
Importantly, many members of this family are cancer survivors, including three who have MM and one or more additional primary cancers. In addition, two mutation carriers (III-08 and IV-08) have not developed malignancies to date. The fact that multiple family members have had more than one primary cancer suggests widespread BAP1-related tumor susceptibility targeting tissues of multiple lineages [16]. Intriguingly, these findings are reminiscent of the multiple cancer phenotype observed in Li-Fraumeni syndrome [29]. Thus, cancer survivors, individuals with benign melanocytic tumors, and mutation carriers who have not yet developed cancer should be monitored closely throughout their lifetime, e.g., through regular dermatologic, ophthalmologic and pulmonary examinations, with the goal of early detection and intervention.
Supplementary Material
Highlights.
Six members of a BAP1 syndrome family developed two or more primary tumors.
Mesotheliomas, melanomas, basal cell carcinomas and meningiomas predominated.
A gene/environment interaction is proposed to play a role in this high-risk family.
Acknowledgments
This work was supported in part by NCI grants R01 CA175691 and P30 CA06927, NIEHS grant P42 ES023720 (UPenn Superfund Research and Training Program Center), a grant from the Mesothelioma Applied Research Foundation (The Anderson Family Grant), an appropriation from the Commonwealth of Pennsylvania, and a gift from the Local #14 Mesothelioma Fund of the International Association of Heat and Frost Insulators & Allied Workers.
Conflict of Interest: JRT and his Fox Chase colleagues are supported by grants from the National Cancer Institute, NIEHS, and Mesothelioma Applied Research Foundation as described elsewhere in this report. JRT, MC, and JP have a pending patent application on BAP1 mutation testing. JAO has served as a consultant and expert witness for both the plaintiff and defense related to her Wake Forest University Occupational Medicine work. JRT has provided consultation regarding genetic aspects of mesothelioma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Henley SJ, Larson TC, Wu M, Antao VC, Lewis M, Pinheiro GA, Eheman C. Mesothelioma incidence in 50 states and the District of Columbia, United States, 2003–2008. Int. J. Occup. Environ. Health. 2013;19:1–10. doi: 10.1179/2049396712Y.0000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flores RM, Pass HI, Seshan VE, Dycoco J, Zakowski M, Carbone M, Bains MS, Rusch VW. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J. Thorac. Cardiovasc. Surg. 2008;135:620–626. doi: 10.1016/j.jtcvs.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 3.Woodman SE. Metastatic uveal melanoma: biology and emerging treatments. Cancer J. 2012;18:148–152. doi: 10.1097/PPO.0b013e31824bd256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jovanovic P, Mihajlovic M, Djordjevic-Jocic J, Vlajkovic S, Cekic S, Stefanovic V. Ocular melanoma: an overview of the current status. Int. J. Clin. Exp. Pathol. 2013;6:1230–1244. [PMC free article] [PubMed] [Google Scholar]
- 5.Harbour JW, Onken MD, Roberson ED, Duan S, Cao L, Worley LA, Council ML, Matatall KA, Helms C, Bowcock AM. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330:1410–1413. doi: 10.1126/science.1194472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goswami E, Craven V, Dahlstrom DL, Alexander D, Mowat F. Domestic asbestos exposure: a review of epidemiologic and exposure data. Int. J. Environ. Res. Public Health. 2013;10:5629–5670. doi: 10.3390/ijerph10115629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen DE, Proctor M, Marquis ST, Gardner HP, Ha SI, Chodosh LA, Ishov AM, Tommerup N, Vissing H, Sekido Y, Minna J, Borodovsky A, Schultz DC, Wilkinson KD, Maul GG, Barlev N, Berger SL, Prendergast GC, Rauscher FJ., 3rd BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene. 1998;16:1097–1112. doi: 10.1038/sj.onc.1201861. [DOI] [PubMed] [Google Scholar]
- 8.Bott M, Brevet M, Taylor BS, Shimizu S, Ito T, Wang L, Creaney J, Lake RA, Zakowski MF, Reva B, Sander C, Delsite R, Powell S, Zhou Q, Shen R, Olshen A, Rusch V, Ladanyi M. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat. Genet. 2011;43:668–672. doi: 10.1038/ng.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Testa JR, Cheung M, Pei J, Below JE, Tan Y, Sementino E, Cox NJ, Dogan AU, Pass HI, Trusa S, Hesdorffer M, Nasu M, Powers A, Rivera Z, Comertpay S, Tanji M, Gaudino G, Yang H, Carbone M. Germline BAP1 mutations predispose to malignant mesothelioma. Nat. Genet. 2011;43:1022–1025. doi: 10.1038/ng.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiesner T, Obenauf AC, Murali R, Fried I, Griewank KG, Ulz P, Windpassinger C, Wackernagel W, Loy S, Wolf I, Viale A, Lash AE, Pirun M, Socci ND, Rutten A, Palmedo G, Abramson D, Offit K, Ott A, Becker JC, Cerroni L, Kutzner H, Bastian BC, Speicher MR. Germline mutations in BAP1 predispose to melanocytic tumors. Nat. Genet. 2011;43:1018–1021. doi: 10.1038/ng.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdel-Rahman MH, Pilarski R, Cebulla CM, Massengill JB, Christopher BN, Boru G, Hovland P, Davidorf FH. Germline BAP1 mutation predisposes to uveal melanoma, lung adenocarcinoma, meningioma, and other cancers. J. Med. Genet. 2011;48:856–859. doi: 10.1136/jmedgenet-2011-100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Njauw CN, Kim I, Piris A, Gabree M, Taylor M, Lane AM, DeAngelis MM, Gragoudas E, Duncan LM, Tsao H. Germline BAP1 inactivation is preferentially associated with metastatic ocular melanoma and cutaneous-ocular melanoma families. PloS One. 2012;7:e35295. doi: 10.1371/journal.pone.0035295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wadt K, Choi J, Chung JY, Kiilgaard J, Heegaard S, Drzewiecki KT, Trent JM, Hewitt SM, Hayward NK, Gerdes AM, Brown KM. A cryptic BAP1 splice mutation in a family with uveal and cutaneous melanoma, and paraganglioma. Pigment Cell Melanoma Res. 2012;25:815–818. doi: 10.1111/pcmr.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wadt KA, Aoude LG, Johansson P, Solinas A, Pritchard A, Crainic O, Andersen MT, Kiilgaard JF, Heegaard S, Sunde L, Federspiel B, Madore J, Thompson JF, McCarthy SW, Goodwin A, Tsao H, Jonsson G, Busam K, Gupta R, Trent JM, Gerdes AM, Brown KM, Scolyer RA, Hayward NK. A recurrent germline BAP1 mutation and extension of the BAP1 tumor predisposition spectrum to include basal cell carcinoma. Clin. Genet. 2014 Sep 15; doi: 10.1111/cge.12501. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Aoude LG, Wadt K, Bojesen A, Cruger D, Borg A, Trent JM, Brown KM, Gerdes AM, Jonsson G, Hayward NK. A BAP1 mutation in a Danish family predisposes to uveal melanoma and other cancers. PloS One. 2013;8:e72144. doi: 10.1371/journal.pone.0072144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheung M, Talarchek J, Schindeler K, Saraiva E, Penney LS, Ludman M, Testa JR. Further evidence for germline BAP1 mutations predisposing to melanoma and malignant mesothelioma. Cancer Genet. 2013;206:206–210. doi: 10.1016/j.cancergen.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 17.Faig J, Howard S, Levine EA, Casselman G, Hesdorffer M, Ohar JA. Changing pattern in malignant mesothelioma survival. Transl. Oncol. 2015;8:35–39. doi: 10.1016/j.tranon.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Z, Armstrong BK, Blundson BJ, Rogers JM, Musk AW, Shilkin KB. Trends in mortality from malignant mesothelioma of the pleura, and production and use of asbestos in Australia. Med. J. Aust. 1985;143:185–187. [PubMed] [Google Scholar]
- 19.Tweedale G. Asbestos and its lethal legacy. Nat. Rev. Cancer. 2002;2:311–315. doi: 10.1038/nrc774. [DOI] [PubMed] [Google Scholar]
- 20.Ohar JA, Ampleford EJ, Howard SE, Sterling DA. Identification of a mesothelioma phenotype. Respir. Med. 2007;101:503–509. doi: 10.1016/j.rmed.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 21.Boscoe FP, Schymura MJ. Solar ultraviolet-B exposure and cancer incidence and mortality in the United States, 1993–2002. BMC Cancer. 2006;6:264. doi: 10.1186/1471-2407-6-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu J, Kadariya Y, Cheung M, Pei J, Talarchek J, Sementino E, Tan Y, Menges CW, Cai KQ, Litwin S, Peng H, Karar J, Rauscher FJ, Testa JR. Germline mutation of Bap1 accelerates development of asbestos-induced malignant mesothelioma. Cancer Res. 2014;74:4388–4397. doi: 10.1158/0008-5472.CAN-14-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Popova T, Hebert L, Jacquemin V, Gad S, Caux-Moncoutier V, Dubois-d'Enghien C, Richaudeau B, Renaudin X, Sellers J, Nicolas A, Sastre-Garau X, Desjardins L, Gyapay G, Raynal V, Sinilnikova OM, Andrieu N, Manie E, de Pauw A, Gesta P, Bonadona V, Maugard CM, Penet C, Avril MF, Barillot E, Cabaret O, Delattre O, Richard S, Caron O, Benfodda M, Hu HH, Soufir N, Bressac-de Paillerets B, Stoppa-Lyonnet D, Stern MH. Germline BAP1 mutations predispose to renal cell carcinomas. Am. J. Hum. Genet. 2013;92:974–980. doi: 10.1016/j.ajhg.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farley MN, Schmidt LS, Mester JL, Pena-Llopis S, Pavia-Jimenez A, Christie A, Vocke CD, Ricketts CJ, Peterson J, Middelton L, Kinch L, Grishin N, Merino MJ, Metwalli AR, Xing C, Xie XJ, Dahia PL, Eng C, Linehan WM, Brugarolas J. A novel germline mutation in BAP1 predisposes to familial clear-cell renal cell carcinoma. Mol. Cancer Res. 2013;11:1061–1071. doi: 10.1158/1541-7786.MCR-13-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Testa JR, Malkin D, Schiffman JD. Connecting molecular pathways to hereditary cancer risk syndromes. Am. Soc. Clin. Oncol. Educ. Book. 2013:81–90. doi: 10.1200/EdBook_AM.2013.33.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rai K, Pilarski R, Cebulla CM, Abdel-Rahman MH. Comprehensive review of BAP1 tumor predisposition syndrome with report of two new cases. Clinical genetics. 2015 Jun 22; doi: 10.1111/cge.12630. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshikawa Y, Sato A, Tsujimura T, Emi M, Morinaga T, Fukuoka K, Yamada S, Murakami A, Kondo N, Matsumoto S, Okumura Y, Tanaka F, Hasegawa S, Nakano T, Hashimoto-Tamaoki T. Frequent inactivation of the BAP1 gene in epithelioid-type malignant mesothelioma. Cancer science. 2012;103:868–874. doi: 10.1111/j.1349-7006.2012.02223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nasu M, Emi M, Pastorino S, Tanji M, Powers A, Luk H, Baumann F, Zhang YA, Gazdar A, Kanodia S, Tiirikainen M, Flores E, Gaudino G, Becich MJ, Pass HI, Yang H, Carbone M. High incidence of somatic BAP1 alterations in sporadic malignant mesothelioma. Journal of Thoracic Oncology. 2015;10:565–576. doi: 10.1097/JTO.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hisada M, Garber JE, Fung CY, Fraumeni JF, Jr, Li FP. Multiple primary cancers in families with Li-Fraumeni syndrome. J. Natl. Cancer Inst. 1998;90:606–611. doi: 10.1093/jnci/90.8.606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


