Abstract
Objective
Experience on thrombolysis and/or thrombectomy for acute major ischemic strokes in the setting of deep (less than 40,000/mm3) thrombocytopenia is limited.
Methods
Case report and review of the literature.
Results
A 63-year-old female with myelodysplastic syndrome presented with left middle cerebral artery stroke within 2 hours of symptom onset. Severe thrombocytopenia (10.000/mm3) precluded systemic thrombolysis. However, endovascular thrombectomy provided successful recanalization and dramatic clinical recovery with NIHSS score decreasing from 20 to 2 soon after the procedure. Her modified Rankin scale was 1 at the end of the third month.
Conclusion
This exceptional case highlights that neurothrombectomy could be feasible and of justifiable merit even in the setting of critically low thrombocytopenia if a meticulous procedure is followed in subjects with severe acute stroke.
Keywords: acute stoke, platelet counts, thrombectomy, thrombocytopenia, tissue plasminogen activator
Introduction
A platelet count of less than 100,000/mm3 is considered as a contraindication for intravenous (IV) thrombolysis for acute ischemic stroke per the current European Stroke Organization (ESO) and American Heart Association (AHA) guidelines [1, 2]. In contrast to ESO guidelines, AHA clearly recommends that IV tissue plasminogen activator (tPA) can be initiated before the results of clotting tests and blood counts become available in case of possible delays due to laboratory procedures. This recommendation is emerged from the low incidence of thrombocytopenia in subjects without relevant medical history and is not based on a systematic study demonstrating an acceptable level of safety. In patients with thrombocytopenia, interventional thrombectomy without using pharmacological agents is a more safer option. Accordingly, lower thresholds of platelet counts were used as exclusion criteria in the interventional thrombectomy studies, such as 30,000/mm3 in Multi-MERCİ and 40,000/mm3 in MR-CLEAN study [3, 4]. The case presented herein defines a much more lower threshold for attempting recanalization in a safe manner in the setting of acute ischemic stroke.
Case Report
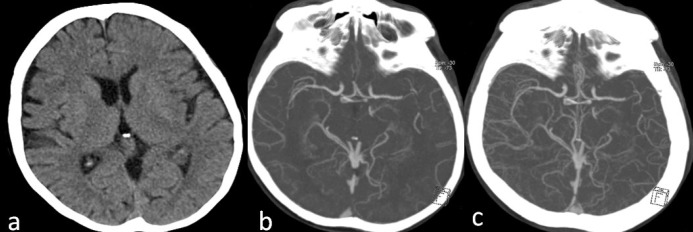
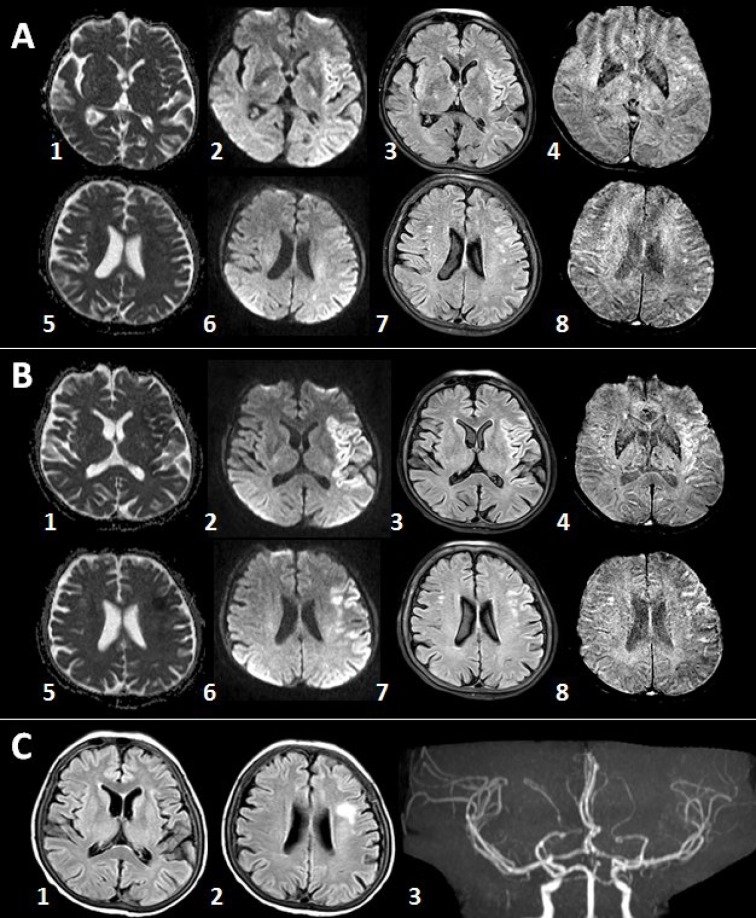
A 63-year-old-female with myelodysplastic syndrome (MDS) presented with acute onset of speech disturbance and right-sided weakness. On admission, her neurological exam revealed global aphasia, confusion, right hemiplegia, right hemianopia and conjugated eye deviation to the left, all pointing to a fully developed left middle cerebral artery (MCA) stem syndrome. Her initial National Institute of Health Stroke Scale (NIHSS) score was 20. Albeit the presence of hypertension and coronary artery disease in her past medical history, she was not using any anticoagulant or antiplatelet agents due to transfusion-refractory thrombocytopenia caused by MDS over the last one-year period. The initial laboratory work-up at the emergency department showed significant thrombocytopenia (10.000/mm3) along with a slightly elevated international normalized ratio (1.69); other laboratory parameters were within normal limits. Noncontrast cranial computed tomography (CT) was unremarkable (Figure 1a). CT angiography revealed occlusion of distal M1 segment of the left middle cerebral artery (Figures 1b and 1c). After meticulous discussions and detailed consenting with the legal representatives, she was taken to the angiography suite for endovascular treatment. Angiography showed abrupt cutoff in the distal M1 segment with poor collateral filling (Figures 2a and 2b). Mechanical thrombectomy was performed using the Catch Mini Plus ® system (Balt, Montmercy, France) deployed through an Excelsior® SL-10 microcatheter (Stryker, Fremont, CA) and a recanalization status of TICI-2b was attained at the end of the procedure (Figures 2c and 2d). The procedure was completed 3.5 hours after symptom onset. Six units of platelets were administered during and after the procedure. Cranial magnetic resonance imaging (MRI) obtained 5 hours after completion of the procedure showed patchy areas of restricted diffusion in left MCA territory (Figure 3A (1–3) and (5–7)) without any hemorrhage detectable on susceptibility weighted images (Figure 3A (4,8)). Her response to treatment was dramatic. The NIHSS score decreased to 2 following the procedure. Another MRI obtained 53 hours after symptom onset showed small parietoinsular and frontal infarcts and again no evidence of hemorrhage (Figure 3B (1-8)). At the end of the third month, her only neurological deficit was slight right facial and arm paresis; she received a score of 1 on the modified Rankin scale. The follow-up brain MRI showed a small frontal infarct and normal cerebral arterial vasculature (Figure 3C (1–3)). Platelet number fluctuated around 20,000/mm3 during this period.
Figure 1. a: Pretreatment cranial CT showed normal findings. b: Arterial phase CT angiography showed an abrupt cutoff in the horizontal portion of the left MCA distally with poor-to-moderate collateral status. c: Venous phase CT angiography confirmed the collateral insufficiency.
Figure 2. Left MCA distal M1 occlusion as depicted in anteroposterior (a) and lateral (b) projections was successfully recanalized except for a small posterior parietal perfusion deficit (c, d, same planes).
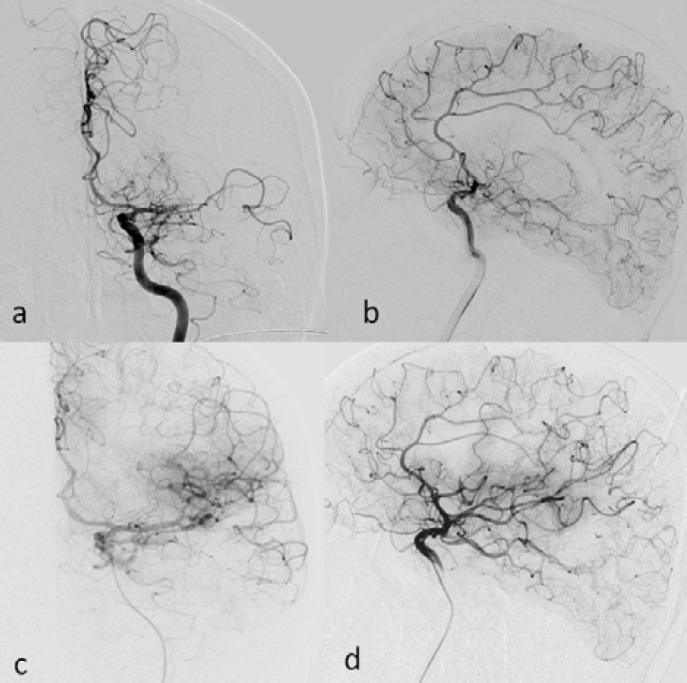
Figure 3. A: MRI obtained 8.5 hours after symptom onset showed patchy areas of restricted diffusion in the left MCA territory along with no hemorrhagic transformation. B: Another MRI obtained 53 hours after symptom onset showed FLAIR hyperintense lesions without any evidence of hemorrhage. C: Day 90 MRI shows only a small frontal infarct along with patent left MCA. Images: Apparent diffusion coefficient (ADC) maps: A1, A5, B1, B5; Diffusion-weighted imaging (DWI): A2, A6, B2, B6; Fluid attenuation inversion recovery (FLAIR): A3, A7;B3, B7, C1, C2; Susceptibility weighted imaging (SWI): A4, A8;B4, B8.
Discussion
Unsuspected thrombocytopenia can be found in up to 0.8% of the acute stroke patients scheduled for thrombolysis [5–7]. Although the exact prevalence is not known, it is not uncommon to encounter low platelet counts in the acute stroke setting, especially considering those cases with a previously established diagnosis. While the potential for hemorrhagic complications comprise the primary concern in such patients, several thrombocytopenic conditions such as sepsis, drug reactions, immune thrombocytopenia (ITP), malignancy-related disseminated intravascular coagulopathy (DIC), heparin-induced thrombocytopenia (HIT), thrombotic thrombocytopenic purpura (TTP), and antiphospholipid syndrome are also paradoxically thrombotic [8–11]. Therefore, an action plan for acute stroke thrombolysis is needed in these patients. It is fortunate that, albeit the presence of limited experience, mechanical thrombectomy without using any pharmacological agents remains as a valid option for this purpose [12].
Local/systemic thrombolysis or mechanical thrombectomy have successfully been used in severe life-threatening thrombotic conditions, such as acute myocardial infarction, massive pulmonary embolism, and massive stroke, in subjects with mild to moderate thrombocytopenia (platelet counts between 40,000 and 100,000/mm3) [13, 14]. According to the quite limited experience with IV tPA in acute stroke patients with unsuspected mild thrombocytopenia (minimum platelet count reported in literature: 59,000/mm3) [5], systemic thrombolytic treatment has not resulted in hemorrhagic complications or death during follow-up. However, platelet counts (even higher than 150.000/mm3) have been labeled as a potential predictor of symptomatic hemorrhagic complications after IV thrombolysis in several studies [15]. Endovascular therapy is usually the preferred therapeutic option for these patients; however, published experience with this modality in patients with low platelet counts is also quite limited. In the MERCI/Multi MERCI cohort, mechanical thrombectomy was used in four patients with moderate thrombocytopenia (>40.000/mm3) [12]. Albeit no significant hemorrhage was seen, none of these patients had a good outcome (Modified Rankin’s score: mRS<4) despite successful recanalization. The authors connected this finding to the poor baseline health status of the patients.
There are very few reports in the literature regarding the treatment of acute thromboembolic events in patients with severe thrombocytopenia (platelet counts less than 40,000/mm3). We found only three such patients with acute stroke treated by intravenous thrombolysis or mechanical thrombectomy [12, 16]. Two of these patients were in the MERCI/Multi MERCI cohort. The first was a 52-year-old female with terminal gestational trophoblastic carcinoma, complicated with thrombocytopenia developing after chemotherapy and sepsis. The apparent life expectancy was less than 3 months. Endovascular treatment was attempted for severe ICA occlusion (NIHSS: 24) within 1.5 hours after symptom onset, but no recanalization was achieved. A massive hemorrhage occurred which eventually led to the death of the patient. The second was a 60-year-old male with a platelet count of 37,000/mm3 who developed severe (NIHSS: 17) MCA territory infarction and was successfully recanalized 4.2 hours after symptom onset. No hemorrhage was observed. His 90-day mRS was 4 [12]. An additional 39-year-old severely thrombocytopenic (27.000 mm3, due to TTP) man with expressive aphasia due to MCA occlusion was successfully treated with IV tPA followed by therapeutic plasma exchange. He fully recovered [16]. The case presented herein is the fourth report in the literature, and mechanical thrombectomy produced an excellent result. Of note, among patients ever been attempted for the treatment of thromboembolic insults, this case had the lowest platelet count. We were not able to find any cases with MDS and thromboembolic stroke in our Pubmed® search [17]. However, we are aware that there are at least 95 cases with an established diagnosis of MDS who had stroke according to FDA database and social media [18]. The stroke risk in MDS increases with male gender, age >60 years, hypertension and Lenalidomide use. Our case is also the first case of stroke in a patient with MDS described in the peer-reviewed literature.
The threshold at which platelet function becomes severely compromised is a critical question. Platelet transfusion guidelines generally recommend platelet transfusion in patients scheduled for major surgery when the platelet count is between 80,000 and 100,000/mm3. Anticoagulation is generally held in patients when the platelet count drops to values below 50,000/mm3. Meanwhile, chemotherapy patients are generally transfused and children with ITP are treated to keep platelet counts above 10,000/mm3. ITP patients generally do not spontaneously bleed beyond the skin even with platelet counts lower than 10,000/mm3. Before the availability of platelet transfusions, spontaneous bleeding, such as intracranial hemorrhage and gastrointestinal bleeding, did not occur until platelets dropped to values less than 5000/mm3 among humans receiving chemotherapy. A certain number of platelets, between 7000 to 10 000/mm3, are needed just to maintain vascular integrity in humans [19]. Despite these announced thresholds, experimental data usually indicate that the most basic level of hemostasis can be maintained with relatively few number of platelets. An experimental data from mice have nicely shown that platelet number less than 2.5% of normal can be enough for maintenance of hemostasis [20]. The presence of functionally more active platelets in the peripheral blood, perhaps due to an unbalanced increase in thrombin concentration, may further help to establish hemostasis. Our single-case experience supports this assumption and draws the attention of stroke experts to the difficulties in decision making in such cases. We think that mechanical thrombectomy may be warranted in cases with significant thrombocytopenia presenting with acute stroke. Unless the procedure is complicated with vascular rupture or dissection, positive clinical results can be expected.
References
- European stroke organisation (ESO) executive committee EWC. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25:457–507. doi: 10.1159/000131083. [DOI] [PubMed] [Google Scholar]
- Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW, Jr, Qureshi AI, Rosenfield K, Scott PA, Summers DR, Wang DZ, Wintermark M, Yonas H. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- Smith WS, Sung G, Saver J, Budzik R, Duckwiler G, Liebeskind DS, Lutsep HL, Rymer MM, Higashida RT, Starkman S, Gobin YP, Frei D, Grobelny T, Hellinger F, Huddle D, Kidwell C, Koroshetz W, Marks M, Nesbit G, Silverman IE. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008;39(4):1205–1212. doi: 10.1161/STROKEAHA.107.497115. Multi MERCI Investigators, [DOI] [PubMed] [Google Scholar]
- Fransen PS, Beumer D, Berkhemer OA, van den Berg LA, Lingsma H, van der Lugt A, van Zwam WH, van Oostenbrugge RJ, Roos YB, Majoie CB, Dippel DW. MR CLEAN, a multicenter randomized clinical trial of endovascular treatment for acute ischemic stroke in the Netherlands: study protocol for a randomized controlled trial. Trials. 2014;15:343. doi: 10.1186/1745-6215-15-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer L, Huttner HB, Kiphuth IC, Huttner HB, Kiphuth IC, Ringwald J, Hilz MJ, Schwab S, Köhrmann M. Waiting for platelet counts causes unsubstantiated delay of thrombolysis therapy. Eur Neurol. 2013;69(5):317–320. doi: 10.1159/000345702. [DOI] [PubMed] [Google Scholar]
- Cucchiara BL, Jackson B, Weiner M, Messe SR. Usefulness of checking platelet count before thrombolysis in acute ischemic stroke. Stroke. 2007;38(5):1639–1640. doi: 10.1161/STROKEAHA.106.480889. [DOI] [PubMed] [Google Scholar]
- Rost NS, Masrur S, Pervez MA, Viswanathan A, Schwamm LH. Unsuspected coagulopathy rarely prevents IV thrombolysis in acute ischemic stroke. Neurology. 2009;73(23):1957–1962. doi: 10.1212/WNL.0b013e3181c5b46d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G, Favaloro EJ, Franchini M. Paradoxical thrombosis, part 2: anticoagulant and antiplatelet therapy. J Thromb Thrombolys. 2012;34(3):367–373. doi: 10.1007/s11239-012-0748-0. [DOI] [PubMed] [Google Scholar]
- Mihalov J, Timarova G. A seeming paradox: ischemic stroke in the context of idiopathic thrombocytopenic purpura. Clin Appl Thromb Hemostat. 2014. [DOI] [PubMed]
- Patterson SL, LaMonte MP, Mikdashi JA, Haines ST, Hursting MJ. Anticoagulation strategies for treatment of ischemic stroke and antiphospholipid syndrome: case report and review of the literature. Pharmacotherapy. 2006;26(10):1518–1525. doi: 10.1592/phco.26.10.1518. [DOI] [PubMed] [Google Scholar]
- Gore JM, Spencer FA, Gurfinkel EP, López-Sendón J, Anderson FA, FitzGerald G, Granger CB. Thrombocytopenia in patients with an acute coronary syndrome (from the Global Registry of Acute Coronary Events [GRACE]) Am J Cardiol. 2009;103(2):175–180. doi: 10.1016/j.amjcard.2008.08.055. [DOI] [PubMed] [Google Scholar]
- Nogueira RG, Smith WS, Merci, Multi MWC. Safety and efficacy of endovascular thrombectomy in patients with abnormal hemostasis: pooled analysis of the MERCI and multi MERCI trials. Stroke. 2009;40(2):516–522. doi: 10.1161/STROKEAHA.108.525089. [DOI] [PubMed] [Google Scholar]
- Collins D, Moloney MA, O’Donnell D, Brophy D, Sheehan SJ. Acute aortic occlusion in a patient with heparin-induced thrombocytopenia treated by thrombectomy. Ir J Med Sci. 2012;181(3):397–400. doi: 10.1007/s11845-010-0531-1. [DOI] [PubMed] [Google Scholar]
- Doll JA, Kelly JP. ST-segment elevation myocardial infarction treated with thrombolytic therapy in a patient with thrombotic thrombocytopenic purpura. J Thromb. 2014;38(1):124–126. doi: 10.1007/s11239-013-1018-5. [DOI] [PubMed] [Google Scholar]
- Tanne D, Kasner SE, Demchuk AM, Koren-Morag N, Hanson S, Grond M, Levine SR. Markers of increased risk of intracerebral hemorrhage after intravenous recombinant tissue plasminogen activator therapy for acute ischemic stroke in clinical practice: the multicenter rt-PA stroke survey. Circulation. 2002;105(14):1679–1685. doi: 10.1161/01.cir.0000012747.53592.6a. [DOI] [PubMed] [Google Scholar]
- Boattini M, Procaccianti G. Stroke due to typical thrombotic thrombocytopenic purpura treated successfully with intravenous thrombolysis and therapeutic plasma exchange. BMJ case reports. 2013:2013. doi: 10.1136/bcr-2012-008426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finelli PF, Harrison RB, Uphoff DF. Myelodysplastic syndrome and sagittal sinus thrombosis. J Stroke. 1998;7(3):211–212. doi: 10.1016/s1052-3057(98)80010-4. [DOI] [PubMed] [Google Scholar]
- eHealthMe. Would you have Cerebrovascular accident (Stroke) when you have Myelodysplastic syndrome? Accessed 22.3.2015 http://www.ehealthme.com/cs/myelodysplastic+syndrome/cerebrovascular+accident.
- Mitchell WB, Bussel JB. How low can you go? Blood. 2013;121(24):4817–4818. doi: 10.1182/blood-2013-04-497610. [DOI] [PubMed] [Google Scholar]
- Morowski M, Vogtle T, Kraft P, Kleinschnitz C, Stoll G, Nieswandt B. Only severe thrombocytopenia results in bleeding and defective thrombus formation in mice. Blood. 2013;121(24):4938–4947. doi: 10.1182/blood-2012-10-461459. [DOI] [PubMed] [Google Scholar]