Abstract
Background
Obesity is not a homogeneous condition across individuals since about 25–40% of obese individuals can maintain healthy status with no apparent signs of metabolic complications. The simple anthropometric measure of body mass index does not always reflect the biological effects of excessive body fat on health, thus additional molecular characterizations of obese phenotypes are needed to assess the risk of developing subsequent metabolic conditions at an individual level.
Methods
To better understand the associations of free fatty acids (FFAs) with metabolic phenotypes of obesity, we applied a targeted metabolomics approach to measure 40 serum FFAs from 452 individuals who participated in four independent studies, using an ultra-performance liquid chromatograph coupled to a Xevo G2 quadruple time-of-flight mass spectrometer.
Findings
FFA levels were significantly elevated in overweight/obese subjects with diabetes compared to their healthy counterparts. We identified a group of unsaturated fatty acids (UFAs) that are closely correlated with metabolic status in two groups of obese individuals who underwent weight loss intervention and can predict the recurrence of diabetes at two years after metabolic surgery. Two UFAs, dihomo-gamma-linolenic acid and palmitoleic acid, were also able to predict the future development of metabolic syndrome (MS) in a group of obese subjects.
Interpretation
These findings underscore the potential role of UFAs in the MS pathogenesis and also as important markers in predicting the risk of developing diabetes in obese individuals or diabetes remission after a metabolic surgery.
Abbreviations: T2D, type 2 diabetes; NAFLD, nonalcoholic fatty liver disease; CVD, cardiovascular disease; MS, metabolic syndrome; FFA, free fatty acids; NW, normal weight; HO, metabolically healthy obese; UO, metabolically unhealthy obese; SFA, saturated fatty acid; UFA, unsaturated fatty acid; MUFA, monounsaturated acid; PUFA, polyunsaturated fatty acid; BMI, body mass index; SHOS, the Shanghai Obesity Study; SHDS, the Shanghai Diabetes Study; VLCD, very low carbohydrate diet; OGTT, oral glucose tolerance test; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; TG, triglycerides; HDL, high-density lipoprotein; LDL, low-density lipoprotein; RSD, relative standard deviation; OPLS-DA, orthogonal partial least square discriminant analysis; HbA1c, glycated hemoglobin; DGLA, dihomo-gamma-linolenic acid; GLA, γ-linolenic acid; HA, heptadecanoic acid; PA, palmitoleic acid; AA, arachidonic acid; LA, linoleic acid; DNL, de novo lipogenesis; FATPs, fatty acid transport proteins; SCD, stearoyl-CoA desaturase; DAG, diacylglycerol
Keywords: Free fatty acids, Metabolic syndrome, Obesity, Unsaturated fatty acids, Type 2 diabetes, Insulin resistance
Highlights
-
•
Four independent studies were applied to examine the association of free fatty acids with metabolic status of obesity.
-
•
Our data supported an important role for unsaturated fatty acids in the pathogenesis of metabolic syndrome.
-
•
Two unsaturated fatty acids were predictive of future diabetes risk and diabetes remission after metabolic surgery.
About 25–40% of obese individuals, defined by the body mass index, are metabolically healthy. Because obesity is a risk factor for developing type 2 diabetes, it is important to monitor obese individuals for changes in metabolic status. Simpler means of assessing the efficacy of surgical or dietary interventions are also desirable. We examined blood fatty acid levels in patients to locate potential biomarkers that would signify either greater risk of diabetes acquisition or effectiveness of diabetes treatment. Two unsaturated fatty acids, dihomo-gamma-linolenic acid and palmitoleic acid, were shown to predict acquisition of diabetes and also evaluate diabetes remission post-metabolic surgery.
1. Introduction
Obesity is closely associated with the risk of developing type 2 diabetes (T2D), nonalcoholic fatty liver disease (NAFLD), and cardiovascular disease (CVD). When the nutrient intake exceeds expenditure, tissues such as the adipose, liver, and skeletal become saturated with lipids and result in an increase in lipid export leading to elevated plasma free fatty acids (FFAs) (Kahn et al., 2006, Fabbrini et al., 2010). Previous results from epidemiologic studies have suggested that individuals with higher plasma concentrations of FFAs were at increased risk for T2D (Pankow et al., 2004a, Pankow et al., 2004b, Charles et al., 1997, Paolisso et al., 1995). Higher levels of FFAs have also been linked to peripheral (muscle) insulin resistance through inhibition of insulin-stimulated glucose uptake and glycogen synthesis (Boden, 2003). Plasma FFA levels are chronically elevated in obese individuals (Boden and Shulman, 2002) and therefore, it was hypothesized that increased FFA levels is an important feature of obesity-associated metabolic syndrome (MS) and CVD (Boden, 2011). Normalizing plasma FFA levels has been proposed as a novel therapeutic approach for obesity and metabolic diseases (Boden and Shulman, 2002, Kusunoki et al., 2006).
The metabolic abnormalities found in T2D, NAFLD, and CVD such as glucose intolerance, hypertension, dyslipidemia, and insulin resistance are not found in all obese or overweight individuals, and may also be found in normal-weight individuals (St-Onge et al., 2004). Several recent epidemiological studies reported that a subset of obese subjects (Stefan et al., 2013) can maintain healthy metabolic phenotypes. These metabolically healthy obese individuals were not found at increased risk of cardiovascular diseases (CVD) or all-cause mortality over seven years (Hamer and Stamatakis, 2012). These and other studies have led to a restructuring of the classification of obese individuals as either metabolically healthy (HO) or unhealthy (UO). However, the criteria for delineating obese populations into these two metabolic categories is controversial (Kramer et al., 2013). Because obese individuals are not homogeneous in health, simple anthropometric measure of body mass index (BMI) does not always translate excessive body fat into its biological effects on health (Karelis et al., 2004). Additional types of clinical and biochemical parameters including plasma FFA profiles of obese phenotypes may be useful in assessing future risk of subsequent medical problems. Although previous research has focused on some of the important roles of FFAs in obesity, strong evidence linking a single or a particular group of FFAs with the increased risk of MS is lacking (Boden, 2011).
The overall objective of this study was to assess the association of circulating fatty acid profiles with the metabolic status of obese individuals. We applied a targeted metabolomics approach to quantitatively measure blood concentrations of 40 different FFAs in four different groups of subjects. For each subject, 17 saturated FFAs (SFAs), 10 monounsaturated FFAs (MUFAs), and 13 polyunsaturated FFAs (PUFAs), including n − 3 (omega − 3) and n − 6 (omega − 6) PUFAs, were measured (Supplementary Table S1). Such a targeted metabolomics study was designed to address three specific questions related to FFAs and MS. First, are there significant differences in the FFA profiles among three groups, each with a different metabolic status, normal weight (NW), overweight/obese metabolically healthy (HO), and overweight/obese diabetic (UO) individuals? To answer this question, a cross-sectional study measured differences in FFA profiles and related them to BMI and other metabolic markers. Second, are any FFA profiles predictive for HO over a ten year evolution of health changes for their progression to UO? A longitudinal study was applied to compare baseline FFA profiles between individuals who remained healthy and those who developed MS ten years later. The last two studies involved therapeutic intervention and were used to address another question: do specific FFA patterns reflect significant changes in other metabolic markers related to therapeutic intervention over time? Serum FFA profiles were characterized in obese T2D patients before and after gastric bypass surgery and in obese patients before and after an 8 week dietary intervention utilizing a very low carbohydrate diet. The key result obtained from all four studies was a panel of UFAs, dihomo-gamma-linolenic acid (DGLA) and palmitoleic acid (PA) in particular, were predictive of the risk of developing MS or diabetes remission after metabolic surgery in a group of obese subjects, and were also potential markers for the inflammatory status of the subject.
2. Materials and Methods
2.1. Study Design and Population
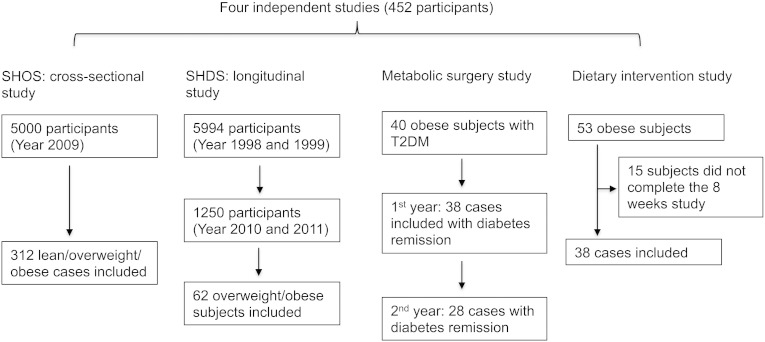
Four independent studies were initiated by the Shanghai Jiao Tong University Affiliated Sixth People's Hospital (Fig. 1).
-
(1)
A total of 312 subjects were selected from the Shanghai Obesity Study (SHOS) and enrolled in a cross-sectional study (Bao et al., 2013). The SHOS was a prospective study designed to investigate the occurrence and development of MS and its related diseases. Beginning in 2009, the SHOS recruited 5000 participants from four communities in Shanghai, China, which included a baseline study as well as, 1.5-, 3-, and 5-year follow-up studies. Of the 312 subjects in the cohort, 132 healthy subjects were normal weight, 107 subjects were either overweight or obese, and 73 subjects had been diagnosed with T2D complicated with hypertension, high cholesterol or hypertriglyceridemia.
-
(2)
10-year longitudinal study: 62 subjects were selected from the Shanghai Diabetes Study (SHDS) (Jia et al., 2007). The SHDS cohort was a multi-stage stratified epidemiological study designed to assess the prevalence of diabetes and associated metabolic disorders. It was initiated in 1998, when 5994 individuals were enrolled from two urban communities, Huayang and Caoyang Districts in Shanghai, China, and 1250 of them completed the follow-up examination in Huayang District between 2010 and 2011. Among 1250 eligible participants, we selected 62 subjects who were overweight/obese and metabolically healthy at baseline, of which, 50 became unhealthy overweight/obese and 12 remained healthy overweight/obese after ten years.
-
(3)
Metabolic surgery intervention study: 40 obese patients with T2D were selected from the Department of Endocrinology and Metabolism outpatient clinic. They received Roux-en-Y gastric bypass surgery, a commonly performed operation for treating obesity-related T2D patients. They were required to complete 1, 3, 6, and 12-month follow-up examinations. Any patient with a history of open abdominal surgery, a serious disease (e.g., heart or lung insufficiency) that was incompatible with surgery, acute T2D complication, severe alcohol or drug dependency, mental disorder, an unstable psychiatric illness, or who was at relatively high surgical risk (e.g., with an active ulcer) was excluded. A second year outcome evaluation was also performed for these patients. The fasting serum specimens of these 40 subjects were collected for FFA analysis at five time points, including baseline before the metabolic surgery, and at month 1, 3, 6, and 12 post-surgery.
-
(4)
Dietary intervention study: the eight-week very low carbohydrate diet intervention study (VLCD) was conducted by initially recruiting 53 obese metabolically healthy volunteers from the Department of Endocrinology and Metabolism outpatient clinic. The details of participant recruitment and dietary intervention (e.g., food composition and supplementation) have been described previously (Gu et al., 2013a, Gu et al., 2013b). Their clinical characteristics and metabolic markers were examined during the dietary intervention. Based on these records, 38 of the 53 subjects who completed the eight-week intervention study were selected because only their serum samples at baseline and eight weeks after intervention were available for FFA analysis.
Fig. 1.
Four independent studies used in this analysis.
All the studies were conducted with ethical approval from the Shanghai Jiao Tong University Affiliated Sixth People's Hospital. Written informed consent was obtained from participants prior to inclusion in the study. A cutoff point of BMI 28 kg/m2 was used to define obesity (≥ 28 kg/m2), a BMI of 24 kg/m2 was used to define overweight (≥ 24 kg/m2) and normal weight was defined as (< 24 kg/m2) based on the recommendation of overweight and obesity for Chinese population (Bei-Fan, 2002). Clinical characteristics and metabolic markers associated with MS were examined for all the participants in four independent studies, including fasting glucose, oral glucose tolerance test (2h-glucose or OGTT), insulin level, systolic and diastolic blood pressures (SBP and DBP), total cholesterol (TC) and triglycerides (TG), and high-density lipoprotein and low-density lipoprotein (HDL and LDL). “Metabolically healthy” was defined as having all of the following: FPG ≤ 6.1 mmol/L, OGTT ≤ 7.8 mmol/L and no previous history of diabetes; SBP/DBP < 140/90 mmHg and no previous history of high blood pressure; fasting plasma TG < 1.7 mmol/L and fasting plasma HDL ≥ 0.9 mmol/L (men) or ≥ 1.0 mmol/L (women), and no previous history of high cholesterol (TC < 5.18 mmol/L); no cardiovascular or endocrine disease history. Failure to meet all of the criteria was classified as “metabolically unhealthy”.
2.2. Sample Preparations and FFA Analysis
All the serum specimens were stored at − 80 °C until analyzed. There were a total of 650 specimens available for FFA analysis: 200 from five time points of metabolic surgery, 76 from two time points of diet intervention, 312 from the cross-sectional study, and 62 from the longitudinal study. Reference standards of these FFAs, an internal standard C19:0-d37, and LC-MS-grade methanol, acetonitrile, water, ammonium acetate, and formic acid were purchased from Sigma-Aldrich (Fisher Scientific, Fair Lawn, NJ). In addition to the internal standard, a mixture of all the reference standards at an appropriate concentration was prepared and run after every ten serum samples for quality control. For all samples, we applied the same protocols to identify and quantify the 40 FFAs using a UPLC-QTOF-MS. The quality control data were as follows: the relative standard deviation (RSD) values of the internal standard were 14.9%, 16.1%, and 6.62%, and the average RSD values of the 40 FFAs in quality control samples were 12.6%, 14.9%, and 14.35%, during the analysis of samples for the metabolic surgery, the dietary intervention and cross-sectional study combined analysis, and in the longitudinal study, respectively.
Serum samples from the four studies were processed and analyzed using the same protocol (Trufelli et al., 2011, Puttmann et al., 1993). Specifically, each sample aliquot of 40 μL was mixed with 10 μL of isotope labeled internal standard (5 μg/mL C19:0-d37), and 500 μL of isopropyl/hexane (v/v = 80/20) with 2% phosphate (2 M). The mixture was extracted with 400 μL of hexane and 300 μL of water. After centrifugation, an aliquot of 400 μL of supernatant was transferred to an Eppendorf microcentrifuge tube and the remaining mixture was further extracted with additional 400 μL of hexane. After centrifugation, an aliquot of 500 μL of supernatant was combined with the first supernatant and dried under vacuum. The residue was reconstituted with 80 μL of methanol, filtered with 0.22-μm membrane (EMD Millipore, Billerica, MA) and then analyzed using UPLC-QTOF-MS.
The set-up parameters for the UPLC-QTOF-MS analysis were as follows. A BEH C18 (2.1 mm × 100 mm, 1.7 μm) chromatographic column was used for separation with column temperature set at 40 °C. The elution solvents were water (A) and acetonitrile/isopropyl (v/v = 80/20, B) with a flow rate of 400 μL/min. The initial gradient was 70% B and kept for 2 min; increased to 75% B in 3 min; increased to 80% in 5 min; increased to 90% in 3 min; increased to 99% in 3 min and kept at 99% for 5 min before switching back to initial condition. The MS was operated at a negative electrospray ionization mode with a capillary voltage of 2.5 kV. The sample cone and the extraction cone were set at 55 V and 4 V, respectively. The source temperature was set to 150 °C, and the desolvation temperature was set to 450 °C with a desolvation gas flow rate of 650 L nitrogen per hour.
2.3. Statistical Analysis
The raw data produced by UPLC-QTOF-MS were initially processed using TargetLynx applications manager version 4.1 (Waters Corp., Milford, MA) to detect peak signals, obtain calibration equations, and calculate the concentration of each FFA. Manual examination and correction were needed to ensure data quality. All statistical computing and graphics were carried out using R version 3.2.1 and SIMCA 13.0.1 software (Umetrics, Sweden).
Prior to the statistical analysis, we examined the distribution of each continuous variable (i.e., clinical characteristics, metabolic markers and FFAs) using the Shapiro–Wilk test, and found that 90% of the variables deviated from normality, thus non-parametric tests were used for this study. We used the Mann–Whitney U test to compare each metabolic marker or FFA between two sample sets, such as HO and UO groups in the cross-sectional study. In the metabolic surgery study, the Kruskal–Wallis test was used to compare the metabolic changes of obese subjects at five different time points. Variables with p-values < 0.05 were considered statistically significant in our study. We calculated the Spearman rank correlation coefficients to measure the relationships between the circulating levels of FFAs, as well as between FFAs and metabolic variables. Their correlations were further visualized using heat map and analyzed using hierarchical clustering. Multivariate logistic regression models were used to estimate the relative risk of having diabetes recurrence after metabolic surgery or developing MS at different FFA levels, adjusting for age, sex, BMI and other confounding factors. Also, p values < 0.05 were considered significant from logistic regression analysis. To evaluate the similarities/differences of FFA profiles between HO and UO groups in the cross-sectional study, a supervised multivariate model called orthogonal partial least square discriminant analysis (OPLS-DA) was built based on their overall metabolic profiles. We further calculated the ROC curve areas of FFAs to evaluate their performance in discriminating HO and UO groups in the cross-sectional study.
2.4. Funding
This work was supported by the International Science & Technology Cooperation Program of China (81170760), the Major State Basic Research Development Program (2011CB504001), the National Natural Science Foundation of China (2014DFA31870), and the China Scholarship Council (201408310049).
3. Results
3.1. Significantly Increased FFA Levels Were Observed in Overweight/Obese Subjects With T2D
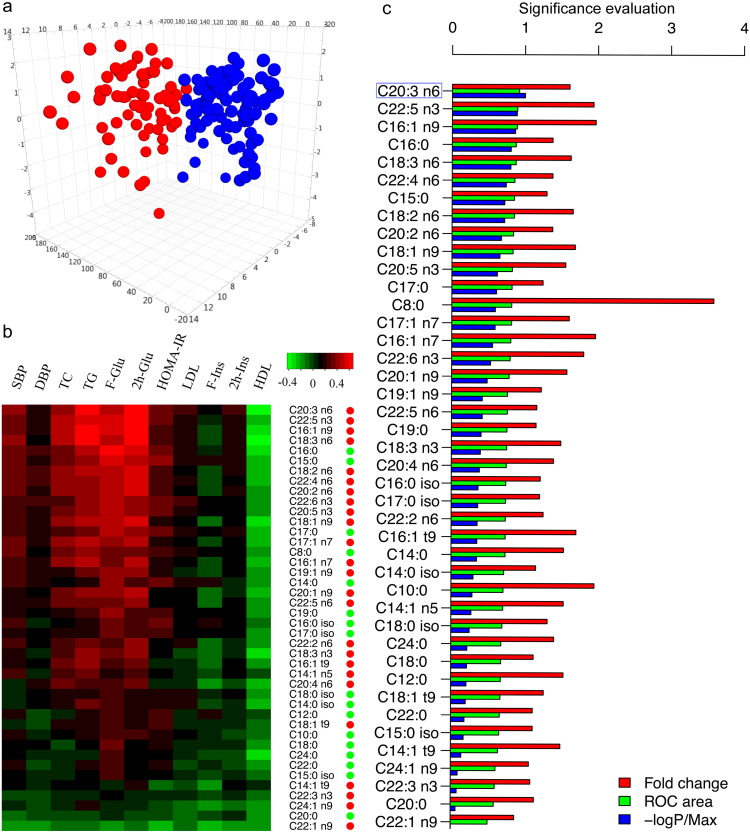
In the cross-sectional study, comprising 132 normal-weight (NW), 107 healthy overweight/obese subjects (HO), and 73 overweight/obese subjects diagnosed with T2D (here referred to as unhealthy obese subjects, UO), we observed that there were significant differences among the three groups according to BMI and key metabolic markers (Table 1). From our metabolic analysis, NW and HO groups shared similar FFA profiles due to their healthy metabolic status; however, the FFA profiles were significantly elevated in the UO group compared to the HO or NW group (Supplementary Table S2). Using a multivariate analysis model (i.e., OPLS-DA), we also noticed that individuals in the UO group were well separated from those in the HO group based on their FFA levels (Fig. 2a). The Spearman correlation analysis indicated their close correlations between FFAs and metabolic markers, and most of UFAs showed strong and positive correlations with them, e.g., glucose and HOMA-IR levels (Fig. 2b). The power of individual FFA in discriminating unhealthy subjects from their healthy counterparts was further compared according to their p-values from Mann–Whitney test, fold change ratios and calculated ROC areas (Fig. 2c). Among them, DGLA (C20:3 n6) stood out due to the highest ROC value (0.92, 95% CI: 0.88–0.96) and the smallest p value 1.09 × 10− 21.
Table 1.
The clinical characteristics and metabolic markers of NW, HO and UO subjects in the cross-sectional study.
| Name | NW | HO | UO | P1 | P2 | P3 |
|---|---|---|---|---|---|---|
| Male (female) | 47(85) | 39(68) | 37(36) | |||
| 2h-glucose (mmol/L) | 5.55 ± 0.09 | 5.74 ± 0.1 | 14.88 ± 0.5 | 1.52E-01 | 3.76E-32 | 8.69E-30 |
| Fasting glucose (mmol/L) | 5 ± 0.04 | 5.14 ± 0.03 | 8.04 ± 0.3 | 1.55E-03 | 1.25E-28 | 2.56E-25 |
| 0.5h-glucose (mmol/L) | 8.18 ± 0.14 | 8.55 ± 0.14 | 13.34 ± 0.41 | 3.42E-02 | 1.49E-24 | 5.71E-22 |
| TG (mmol/L) | 0.82 ± 0.02 | 0.99 ± 0.03 | 2.38 ± 0.17 | 4.99E-05 | 9.26E-26 | 1.31E-18 |
| TC (mmol/L) | 4.28 ± 0.05 | 4.26 ± 0.05 | 5.44 ± 0.11 | 6.82E-01 | 1.64E-16 | 1.93E-16 |
| Age (years) | 45.721 ± 0.852 | 46.269 ± 0.788 | 57.111 ± 0.846 | 7.72E-01 | 1.42E-14 | 9.01E-15 |
| SBP (mmHg) | 114.61 ± 0.95 | 116.01 ± 1.06 | 137.54 ± 2.57 | 3.48E-01 | 1.16E-16 | 4.48E-14 |
| HOMA-IR | 1.3 ± 0.05 | 2.09 ± 0.1 | 3.89 ± 0.24 | 5.32E-11 | 2.32E-25 | 6.30E-12 |
| HOMA-β | 80.4 ± 3.16 | 115.8 ± 6.48 | 61.64 ± 4.21 | 5.96E-07 | 2.60E-04 | 8.91E-12 |
| 0.5h-insulin (μU/mL) | 50.17 ± 2.14 | 67.75 ± 3.51 | 38.76 ± 3.58 | 1.80E-05 | 3.62E-05 | 7.89E-11 |
| 2h-insulin (μU/mL) | 27.83 ± 1.47 | 36.82 ± 2.32 | 69.67 ± 5.9 | 1.27E-03 | 1.34E-14 | 1.63E-08 |
| LDL (mmol/L) | 2.47 ± 0.04 | 2.69 ± 0.04 | 3.29 ± 0.1 | 5.51E-04 | 3.15E-12 | 1.26E-07 |
| DBP (mmHg) | 72.21 ± 0.67 | 74.6 ± 0.82 | 81.91 ± 1.63 | 5.97E-03 | 9.29E-09 | 3.74E-05 |
| Waist (cm) | 72.72 ± 0.4 | 87.9 ± 0.75 | 92.39 ± 0.82 | 2.86E-35 | 7.56E-32 | 1.01E-04 |
| Uric acid (μmol/mL) | 274.47 ± 5.17 | 297.16 ± 7.29 | 328.95 ± 7.78 | 3.03E-02 | 1.13E-08 | 1.05E-03 |
| HDL (mmol/L) | 1.63 ± 0.03 | 1.4 ± 0.03 | 1.27 ± 0.03 | 3.27E-08 | 4.53E-15 | 1.34E-03 |
| Fasting insulin (μU/mL) | 5.78 ± 0.23 | 9.07 ± 0.41 | 11.03 ± 0.6 | 5.70E-11 | 6.25E-16 | 8.67E-03 |
| ALT (U/L) | 15.21 ± 0.6 | 19.13 ± 0.81 | 22.92 ± 1.19 | 7.59E-05 | 3.62E-09 | 1.44E-02 |
| BMI (kg/m2) | 20.51 ± 0.06 | 27.11 ± 0.18 | 27.68 ± 0.3 | 2.78E-40 | 2.28E-32 | 1.37E-01 |
| Creatinine | 65.45 ± 1.2 | 65.21 ± 1.37 | 64.05 ± 1.72 | 7.60E-01 | 4.35E-01 | 6.12E-01 |
| AST (U/L) | 19.7 ± 0.4 | 20.27 ± 0.5 | 20.7 ± 0.66 | 3.49E-01 | 2.02E-01 | 7.02E-01 |
| Urea | 4.87 ± 0.1 | 4.85 ± 0.11 | 4.81 ± 0.1 | 7.58E-01 | 7.77E-01 | 9.97E-01 |
Note: values represent means ± SEM. P1, P2, P3 values are calculated using Mann–Whitney U test to compare the FFA differences between NW and HO, NW and UO, HO and UO, respectively. The variables are ordered by P3 values.
Fig. 2.
FFA analysis in the cross-sectional study. (a) The 3D OPLS-DA scores plot showing the groupings of HO (blue), and UO (red) individuals based on their FFA profiles. (b) Heat map of correlation coefficients between FFAs and metabolic markers. FFAs are ordered by their average correlations with metabolic markers. FFAs belonging to UFA or SFA group are indicated by red or green dots. (c) Bar plots of the fold changes, ROC areas, and normalized p values (− log p/maximum) calculated between HO and UO group.
3.2. A Panel of FFAs Were Predictive of the Risk of Developing Future MS in Overweight/Obese Subjects
The longitudinal study consisted of 62 healthy obese subjects selected from the SHDS study. Its purpose was to further examine the performance of these differential FFAs found in the cross-sectional study in predicting the risk of future MS. The selected 62 overweight subjects had normal metabolic markers at baseline and 50 of them developed MS (UO) while 12 remained healthy (HO) according to their re-evaluation after ten years. There were no differences between these two groups at baseline according to their metabolic markers. However, the baseline serum levels of six FFAs (i.e., five UFAs and one SFA) were significantly increased or decreased in the UO group (Table 2). Logistic regression models adjusting for age, sex, BMI, HOMA-IR and fasting glucose were fitted. This result further confirmed that the baseline concentrations of these FFAs are predictive of the development of future MS in these subjects, including the most significant UFA found in the cross-sectional study, DGLA.
Table 2.
The baseline clinical characteristics, metabolic markers, and FFA levels of participants in the longitudinal study.
| Name | HO | UO | FC | P1 | OR (95% CI) | P2 |
|---|---|---|---|---|---|---|
| Male (female) | 1(11) | 15(35) | ||||
| Age | 39.92 ± 3.66 | 43.94 ± 1.83 | 1.02 | 5.80E-01 | ||
| BMI | 26.88 ± 0.47 | 26.89 ± 0.37 | 0.99 | 4.87E-01 | ||
| SBP | 109.22 ± 3.78 | 115.57 ± 1.7 | 1.05 | 3.74E-01 | ||
| DBP | 72.31 ± 2.55 | 74.33 ± 0.77 | 1 | 7.87E-01 | ||
| Fasting glucose | 4.87 ± 0.12 | 4.7 ± 0.06 | 0.96 | 2.30E-01 | ||
| 2h-glucose | 5.1 ± 0.19 | 5.26 ± 0.16 | 0.99 | 7.01E-01 | ||
| Fasting insulin | 7.4 ± 0.85 | 7.1 ± 0.48 | 0.85 | 4.65E-01 | ||
| 2h-insulin | 43.6 ± 11.43 | 39.42 ± 3.51 | 0.91 | 9.50E-01 | ||
| HOMA-IR | 1.61 ± 0.19 | 1.48 ± 0.1 | 0.83 | 3.83E-01 | ||
| TG | 0.94 ± 0.09 | 1.14 ± 0.04 | 1.28 | 4.99E-02 | ||
| TC | 4.17 ± 0.13 | 4.08 ± 0.07 | 0.93 | 4.98E-01 | ||
| HDL | 1.31 ± 0.06 | 1.34 ± 0.03 | 1.01 | 5.09E-01 | ||
| LDL | 2.74 ± 0.13 | 2.67 ± 0.06 | 0.92 | 5.04E-01 | ||
| SFA | 164.16 ± 20.52 | 166.65 ± 7.08 | 1.21 | 5.04E-01 | ||
| C8:0 | 0.14 ± 0.02 | 0.13 ± 0.02 | 0.68 | 2.28E-01 | ||
| C10:0 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.91 | 5.39E-01 | ||
| C12:0 | 0.23 ± 0.04 | 0.24 ± 0.02 | 0.99 | 9.93E-01 | ||
| C14:0 | 10.87 ± 0.71 | 9.99 ± 0.22 | 0.89 | 1.73E-01 | ||
| C15:0 | 1.47 ± 0.13 | 1.41 ± 0.05 | 1.07 | 9.79E-01 | ||
| C16:0 | 74.74 ± 11.05 | 82.59 ± 4.69 | 1.27 | 2.81E-01 | ||
| C17:0 | 3.31 ± 0.25 | 3.28 ± 0.09 | 1.1 | 4.93E-01 | ||
| C18:0 | 69.35 ± 8.64 | 65.12 ± 2.28 | 1.15 | 7.15E-01 | ||
| C19:0 | 0.39 ± 0.03 | 0.38 ± 0.01 | 1.05 | 9.22E-01 | ||
| C20:0 | 0.91 ± 0.06 | 0.86 ± 0.02 | 1 | 4.28E-01 | ||
| C22:0 | 0.07 ± 0.01 | 0.07 ± 0 | 0.9 | 5.51E-01 | ||
| C24:0 | 0.01 ± 0 | 0.01 ± 0 | 0.52 | 1.58E-02 | 0.19 (0.06–0.66) | 8.19E-03 |
| C14:0 iso | 0.15 ± 0.03 | 0.16 ± 0.01 | 0.98 | 9.64E-01 | ||
| C15:0 iso | 0.94 ± 0.07 | 0.89 ± 0.03 | 1.02 | 5.51E-01 | ||
| C16:0 iso | 0.67 ± 0.06 | 0.64 ± 0.02 | 1.04 | 9.50E-01 | ||
| C17:0 iso | 0.51 ± 0.03 | 0.51 ± 0.02 | 1.02 | 9.79E-01 | ||
| C18:0 iso | 0.33 ± 0.03 | 0.3 ± 0.01 | 1.04 | 6.75E-01 | ||
| MUFA | 26.06 ± 4.23 | 33.98 ± 2.01 | 1.57 | 2.78E-02 | ||
| C14:1 n5 | 0.4 ± 0.09 | 0.45 ± 0.04 | 1.02 | 3.14E-01 | ||
| C16:1 n7 | 1.97 ± 0.28 | 2.63 ± 0.14 | 1.46 | 3.04E-02 | 4.32 (1.39–13.43) | 1.16E-02 |
| C16:1 t9 | 0.1 ± 0.01 | 0.11 ± 0.01 | 0.81 | 7.02E-01 | ||
| C17:1 n7 | 0.25 ± 0.04 | 0.32 ± 0.02 | 1.43 | 7.33E-02 | ||
| C18:1 n9 | 21.82 ± 3.73 | 28.9 ± 1.78 | 1.61 | 2.10E-02 | 1.10 (1.01–1.20) | 2.85E-02 |
| C18:1 t9 | 0.3 ± 0.03 | 0.26 ± 0.01 | 0.82 | 1.62E-01 | ||
| C19:1 n9 | 0.06 ± 0.01 | 0.07 ± 0 | 1.33 | 1.15E-01 | ||
| C20:1 n9 | 0.65 ± 0.11 | 0.74 ± 0.05 | 1.16 | 1.62E-01 | ||
| C22:1 n9 | 0.5 ± 0.11 | 0.49 ± 0.06 | 0.83 | 8.52E-01 | ||
| C24:1 n9 | 0.02 ± 0.01 | 0.03 ± 0 | 0.93 | 7.02E-01 | ||
| n − 3 PUFA | 26.68 ± 3.37 | 32.92 ± 1.72 | 1.18 | 1.42E-01 | ||
| C18:3 n3 | 0.65 ± 0.12 | 0.7 ± 0.04 | 1.29 | 2.97E-01 | ||
| C20:5 n3 | 2.23 ± 0.43 | 2.81 ± 0.28 | 1.33 | 4.28E-01 | ||
| C22:3 n3 | 0.01 ± 0 | 0.02 ± 0 | 1.44 | 3.40E-01 | ||
| C22:5 n3 | 0.17 ± 0.02 | 0.26 ± 0.03 | 1.45 | 2.78E-02 | 3.98 (1.03–15.32) | 4.49E-02 |
| C22:6 n3 | 23.62 ± 2.87 | 29.13 ± 1.42 | 1.16 | 1.46E-01 | ||
| n − 6 PUFA | 67.75 ± 11.7 | 93.57 ± 8.04 | 1.54 | 4.90E-02 | ||
| C18:2 n6 | 46.93 ± 8.92 | 62.72 ± 5.14 | 1.43 | 6.78E-02 | ||
| C18:3 n6 | 2.85 ± 0.39 | 3.48 ± 0.15 | 1.26 | 1.11E-01 | ||
| C20:2 n6 | 0.73 ± 0.09 | 0.96 ± 0.05 | 1.51 | 2.10E-02 | 1.34 (1.02–1.77) | 3.61E-02 |
| C20:3 n6 | 1.72 ± 0.33 | 2.80 ± 0.27 | 1.81 | 1.03E-02 | 2.21 (1.02–4.82) | 4.52E-02 |
| C20:4 n6 | 6.85 ± 0.91 | 9.71 ± 1.62 | 1.29 | 6.26E-02 | ||
| C22:2 n6 | 0.03 ± 0 | 0.03 ± 0 | 1.12 | 3.14E-01 | ||
| C22:4 n6 | 0.19 ± 0.03 | 0.27 ± 0.02 | 1.39 | 2.31E-02 | ||
| C22:5 n6 | 1.11 ± 0.14 | 1.53 ± 0.11 | 1.27 | 5.54E-02 |
Note: values represent means ± SEM. The concentration unit of FFAs is μg/mL. FC values are fold changes ratios of medians in UO over HO group. P1 values are calculated using Mann Whitney-U test, and highlighted in bold if p < 0.05. OR (95% CI) are odd ratios (95% confidence intervals) for metabolic syndrome from logistic regression models. These models are adjusted for age, sex, BMI, HOMA-IR, and fasting glucose. P2 values are calculated from logistic regression models.
3.3. Metabolic Surgery Produced Significant Changes of UFA Profiles That Also Predicted T2D Remission in Obese Subjects
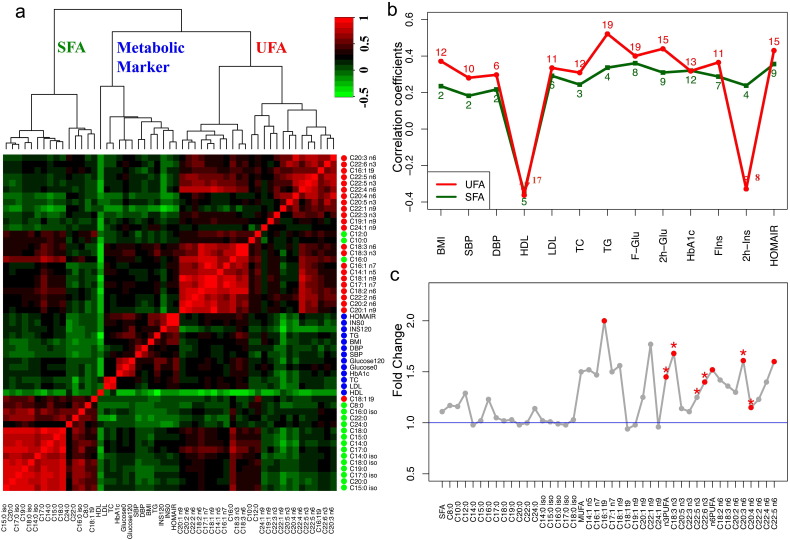
Among 40 subjects, 95% of them (n = 38) showed diabetes remission as defined by a normal glycated hemoglobin concentration (HbA1c < 6.5%) in the absence of medication within the first year. At the 2nd year follow-up examination, of the 38 subjects that achieved T2D remission, 28 subjects continued in remission while the remaining 10 patients suffered recurrence of T2D. First, we studied the 38 individuals who received diabetes remission within one year in order to correlate their FFA levels with improved metabolic markers. The key metabolic markers of these subjects returned to normal levels at 3 months after surgery and maintained the normal levels at 6 and 12 months (Table 3). While mild changes in SFA levels were observed in the participants, UFAs were progressively and significantly decreased during the 12 months after surgery. Particularly, MUFAs and n − 6 PUFAs decreased concurrently with key metabolic markers, e.g., TC, TG, HbA1c, glucose, insulin and HOMA-IR (Supplementary Table S3 and Figure S1A–E). Then we calculated the pairwise correlation coefficients between circulating FFAs and metabolic markers. Based on the hierarchical clustering of their correlations, SFAs, UFAs and metabolic markers formed three major clusters (Fig. 3a) while UFAs had closer correlations with metabolic markers. To compare the correlations of SFAs and UFAs with each metabolic marker, we selected their largest coefficient with statistical significance to represent their strongest relationship with each metabolic marker. The result showed that UFAs had stronger correlations with metabolic markers compared to SFAs (Fig. 3b). Also, the number of individual UFAs that had statistically significant correlations with each metabolic marker is more than that of SFAs, as indicated in Fig. 3b.
Table 3.
The clinical characteristics and metabolic markers of obese subjects before and after metabolic surgery.
| Baseline | Month 1 | Month 3 | Month 6 | Month 12 | p Value | |
|---|---|---|---|---|---|---|
| Male (female) | 18 (20) | 18 (20) | 18 (20) | 18 (20) | 18 (20) | / |
| Age (years) | 44.40 ± 1.96 | 44.40 ± 1.96 | 44.40 ± 1.96 | 44.40 ± 1.96 | 44.40 ± 1.96 | / |
| BMI (kg/m2) | 32.25 ± 0.67 | 28.04 ± 0.58** | 25.84 ± 0.55** | 24.59 ± 0.48** | 24.37 ± 0.43** | 1.93E-15 |
| HOMA-IR | 8.55 ± 1.23 | 4.14 ± 0.73** | 2.04 ± 0.26** | 1.73 ± 0.19** | 1.77 ± 0.19** | 2.30E-15 |
| Total FFA (μmol/L) | 509.92(27.59) | 861 (41.17)** | 534.59(42.11) | 377.84 (20.50)** | 428.21 (29.56)* | 5.59E-15 |
| Waist (cm) | 107.33 ± 2.18 | 95.97 ± 1.65** | 89.83 ± 1.7** | 86.24 ± 1.54** | 85.63 ± 1.37** | 1.26E-13 |
| Fasting insulin (μU/mL) | 25.05 ± 3.69 | 12.92 ± 1.72** | 7.94 ± 0.86** | 7.02 ± 0.7** | 6.99 ± 0.68** | 1.31E-12 |
| 2h-insulin (μU/mL) | 108.26 ± 13.7 | 20.94 ± 2.9** | 24.02 ± 3.66** | 31.21 ± 5.37** | 23.09 ± 3.14** | 5.68E-12 |
| TG (mmol/L) | 2.15 ± 0.2 | 1.5 ± 0.11* | 1.22 ± 0.09** | 1.09 ± 0.06** | 0.97 ± 0.06** | 6.81E-11 |
| 2h-glucose (mmol/L) | 12.03 ± 0.64 | 7.41 ± 0.41** | 7.12 ± 0.48** | 6.73 ± 0.47** | 6.7 ± 0.44** | 2.26E-09 |
| HDL (mmol/L) | 1.03 ± 0.03 | 0.97 ± 0.03* | 1.03 ± 0.03 | 1.16 ± 0.03* | 1.26 ± 0.04** | 4.20E-08 |
| Fasting glucose (mmol/L) | 7.84 ± 0.4 | 6.77 ± 0.3* | 5.7 ± 0.23** | 5.45 ± 0.17** | 5.69 ± 0.18** | 5.31E-08 |
| HbA1c (%) | 7.53 ± 0.28 | 6.68 ± 0.19* | 5.96 ± 0.16** | 5.81 ± 0.12** | 5.89 ± 0.16** | 6.50E-08 |
| TC (mmol/L) | 5.18 ± 0.17 | 4.91 ± 0.17 | 4.32 ± 0.13** | 4.24 ± 0.12** | 4.28 ± 0.13** | 1.36E-05 |
| SBP (mmHg) | 137.34 ± 2.93 | 129.17 ± 2.72 | 124.45 ± 2.33* | 121.11 ± 2.28** | 119.97 ± 1.99** | 2.40E-05 |
| DBP (mmHg) | 86.94 ± 1.99) | 81.58 ± 1.56* | 80.52 ± 1.81* | 76.24 ± 1.62** | 77.82 ± 1.39** | 1.34E-04 |
| LDL (mmol/L) | 3.18 ± 0.15) | 3.14 ± 0.15 | 2.62 ± 0.11* | 2.53 ± 0.12* | 2.48 ± 0.1** | 1.34E-04 |
| HOMA-β | 158.55 ± 27.6 | 93.22 ± 10.27 | 95.89 ± 10.77 | 91.81 ± 11.12 | 77.52 ± 7.91* | 3.26E-01 |
Note: values represent means ± SEM. Symbols * (p < 0.05) or ** (p < 0.001) indicate that the significant difference between a follow-up time and baseline using Mann–Whitney U test. p Values are calculated to test the differences among five time points using Kruskal–Wallis test, and highlighted in bold if p < 0.05. The variables are ordered by p values. HOMA-IR is calculated by the formula: [fasting insulin (μU/mL) × fasting glucose (mmol/L)] / 22.5. HOMA-β is calculated by the formula: [20 × fasting insulin (μU/mL)]% / [fasting glucose (mmol/L) − 3.5].
Fig. 3.
FFA analysis in the metabolic surgery study. (a) Heat map of correlation coefficients between circulating FFAs, as well as between FFAs and metabolic markers. The color of dots represents different classes of variables: UFA (red), metabolic markers (blue) and SFA (green). (b) Line plot of the largest correlation coefficient between representatives SFA/UFA with each metabolic marker. The single number highlighted in red or green indicates the number of UFAs or SFAs that had statistically significant correlations with metabolic markers (p < 0.05). (c) Fold change plot of baseline FFAs (REG/HG). FFAs labeled with red dots were significantly different between two groups (p < 0.05). Symbol * indicates statistical significance from logistical regression analysis (p < 0.05).
Two years after surgery, a total of 10 individuals had diabetes recurrence (recurrence group, REG), 2 had T2D initially without remission (also in the REG) and 28 subjects received complete diabetes remission (healthy group, HG). The HG group had lower HbA1c level at baseline and experienced more weight loss during the two years, compared to the REG group (Supplementary Table S4). In addition, a group of UFAs were significantly elevated in the REG group while SFA levels were comparable between two groups (Fig. 3c). The logistic regression models were fitted to assess the associations between baseline FFA levels and future recurrence/remission of diabetes after surgery, adjusting for age, sex, BMI, HbA1c, weight loss and HOMA-IR. Five UFAs were determined to be potential predictive markers for a therapeutic response to metabolic surgery (Supplementary Table S5).
3.4. Dietary Intervention Resulted in Significant Improvements in Clinical Markers and 9 UFAs
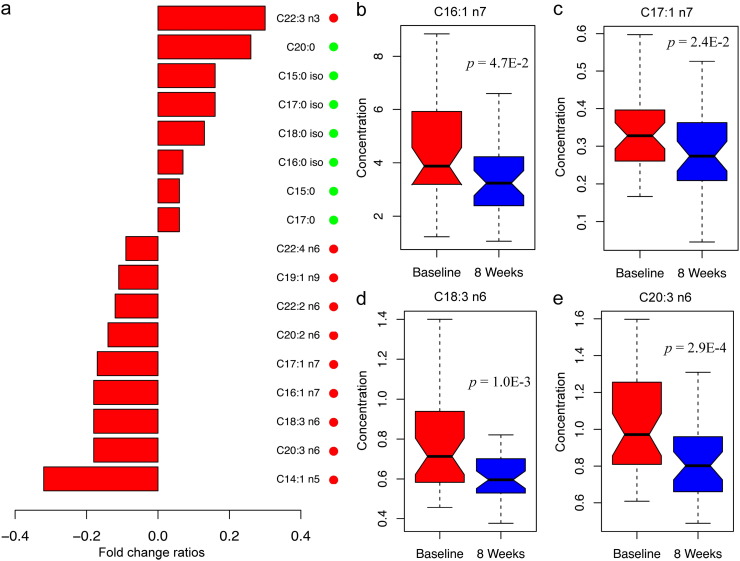
In the dietary intervention study, 38 obese subjects participated in an eight-week, very low carbohydrate diet (VLCD) intervention and showed beneficial effects, such as significant improvement of clinical characteristics and metabolic markers, including BMI, waist circumference, blood pressure, TG, and HOMA-IR (Supplementary Table S6). Similar to the results from the metabolic surgery study, the overall UFA levels, MUFA and n − 6 PUFAs in particular, were decreased after intervention. In contrast, seven SFAs and an n − 3 PUFA were increased significantly (Fig. 4a). Among nine UFAs of statistical significance, we noticed that four of them were also associated with improved metabolic markers in the metabolic surgery study, including PA (C16:1 n7), heptadecenoic acid (C17:1 n7, HA), γ-linolenic acid (C18:3 n6, GLA), and DGLA (Fig. 4b–e).
Fig. 4.
FFA analysis of obese subjects in the dietary intervention study. (a) Bar plot of fold change ratios of significant FFAs (p < 0.05), which were calculated by dividing the median level of each FFA at the 8th week over the baseline level − 1. Positive and negative values represent elevation and depletion of FFA concentrations after intervention. FFAs belonging to UFA or SFA group are indicated by red or green dots. (b–e) Box plots of four UFAs at baseline and 8 weeks. The unit of FFA concentrations is μg/mL.
4. Discussion
Using a targeted mass spectrometry metabolomics approach, we analyzed serum samples of a total of 452 subjects from four independent studies and identified a panel of UFAs whose fasting serum concentrations at routine examination delineates the metabolic status of obese individuals. In the cross-sectional study, FFA levels were found significantly elevated in overweight/obese subjects with T2D compared to their healthy counterparts. A panel of UFAs, DGLA in particular, was closely associated with metabolic markers, and was a significant marker to distinguish metabolically healthy and unhealthy individuals. We also analyzed the baseline FFAs of subjects from a longitudinal study, and found that fasting concentrations of a similar panel of UFAs were elevated up to 10 years before the onset of MS. Finally, in the two weight loss intervention studies, obese participants experienced significant beneficial effects including weight loss and improved metabolic characteristics. A panel of UFAs decreased significantly in response to the surgical or dietary interventions.
UFAs, as compared to SFAs, were decreased more significantly after weight loss interventions, and increased more significantly in obese subjects with MS. Additionally, UFA signatures were more closely associated with metabolic markers. To date, SFAs have been generally thought to have detrimental effects on health. However, conflicting evidence was reported regarding the effects of high SFA intake on the risk of diabetes (Micha and Mozaffarian, 2010). A large cohort study recently suggested that different individual plasma phospholipid SFAs were not homogeneous and associated with incident T2D in opposite ways (Forouhi et al., 2014). They reported that even-chain SFAs (those containing an even number of carbon atoms) including palmitic acid (C16:0) were positively associated and odd-chain (those containing an odd number of carbon atoms) and longer-chain SFAs (those with 13 to 21 carbon atoms) were inversely associated with incident diabetes. In our study, most SFAs did not show strong correlations with metabolic markers, except C16:0 that was decreased significantly in obese individuals after metabolic surgery, and increased significantly in the UO group of the cross-sectional study. Palmitic acid has long been thought to be the major culprit of insulin resistance (Reynoso et al., 2003, Mordier and Iynedjian, 2007). Palmitate in plasma phospholipid and erythrocyte membranes phospholipid was prospectively associated with higher diabetes risk in several longitudinal studies (Ma et al., 2015, Wang et al., 2003, Patel et al., 2010). Increased amounts of FFAs, especially palmitate from either dietary intake or adipose tissue lipolysis, are delivered into the liver cells via specific fatty acid transport proteins (FATPs) in their cell membranes (Supplementary Figure S2A). If palmitate is not oxidized and used for energy then it is used as a substrate for de novo lipogenesis (DNL), a metabolic pathway that is normally inhibited and usually contributes only 5% of the stored triglycerides in the liver and which increases to 30% in MS. In obese individuals, FATPs are upregulated in the liver and diminished in the adipose tissue (Gruben et al., 2014, Perry et al., 2014).
Compared to SFAs, convincing evidence observed in our study was that UFAs are more closely associated with metabolic status in obese individuals. The fluctuations of circulating FFAs in obese phenotypes may be due to the different FA mobilization mechanisms from adipose tissue to blood, where SFAs were found much less mobilized than PUFAs (Connor et al., 1996). Our findings particularly highlight a panel of UFAs that was consistently associated with metabolic status in obese individuals across four independent studies. Two UFAs, PA and DGLA may be potential inflammation markers in predicting the risk of developing MS and monitoring the metabolic status among overweight/obese individuals. PA has been proposed as a lipid-controlling hormone (lipokine) used by adipose tissue to communicate with distant organs and regulate systemic metabolic homeostasis (Cao et al., 2008). Increased levels of plasma PA indicate an increase in stearoyl-CoA desaturase (SCD1) activity (increased DNL) in the liver as it is virtually absent in the diet and thus can be used as a marker for upregulation of this usually inhibited hepatic lipid metabolic pathway (Supplementary Figure S2B) (Gong et al., 2011). Increased DNL means increased formation of diacylglycerol (DAG), which, in turn, contributes to inflammation via release of arachidonic acid (AA) from the plasma membrane. DGLA is a key player in the synthetic pathway for pro-inflammatory series 2 prostaglandins and leukotrienes and elevated levels of this PUFA may contribute to the inflammatory phenotype in obesity/MS (Supplementary Figure S2C–D). Recently, it has been proposed that the distinction between HO and UO groups is related to the degree of chronic inflammation present (Perreault et al., 2014, Steffen et al., 2012a). This has led to several studies comparing inflammation markers such as levels of TNF-α, adiponectin, leptin, resistin, C-reactive protein, plasminogen activator inhibitor-1 and complement component c3, between HO and UO populations (Steffen et al., 2012a, Phillips and Perry, 2013). The major conclusion derived from these studies was that no significant differences were seen for inflammation markers between HO and NW subjects but inflammation markers were found to be elevated in the UO groups. A previous study on 2848 adults found that obese individuals had significantly higher levels of n − 6 PUFAs (e.g. DGLA, GLA, and AA) compared to normal and overweight subjects, and DGLA showed strong associations with inflammatory and endothelial activation markers in obesity, e.g. IL-6 and sICAM-1 (Steffen et al., 2012). It was also reported that a high proportion of DGLA in serum cholesterol ester was associated with high concentrations of C-reactive protein, which is a sensitive marker of low-grade inflammation and associated with insulin resistance and T2D (Kurotani et al., 2012).
To examine the effects of gender, age and BMI factors on FFA metabolism, we performed subgroup analysis on all of the participants in the cross-sectional study. First, we found three FFAs, i.e., C12:0, C18:1 t9 and C22:6 n3, were significantly increased in females (n = 85) compared to males (n = 47) in the NW group (Supplementary Figure S3A–C). Second, ten SFAs were consistently increased with age in females only (Supplementary Figure S4). Third, in terms of the BMI factor, we analyzed male subjects only who were able to keep relatively stable FFA levels over time, and found that three FFAs, i.e., C18:0, C14:1 n5 and C22:6 n3 were significantly different between normal weight (n = 47) and overweight/obesity (n = 39) (Supplementary Figure S3D–F). Thus, we further confirmed that these confounding factors do not affect our findings of UFAs in evaluating and predicting the metabolic status in obesity.
Key strengths of the present study are its comprehensive design to study the associations of circulating FFA levels with metabolic phenotypes among several groups of obese participants. In addition, with a complete panel of key metabolic markers measured for all the participants, we were able to compare the FFA profiles with the metabolic status of the study participants. The limitation of the present study is the medium-sized sample sets in the longitudinal study, due to the strict inclusion criteria that all the participants should have complete clinical records and BMI ≥ 25 at baseline when they were healthy and at a 10-year follow-up time. Future studies examining inflammatory marker association with the FFA profiles discussed in this manuscript are being pursued.
Author contributions
WJ and WPJ were principal investigators of this study. WJ and WPJ designed the research. YN performed the statistical analysis and drafted the manuscript. XM, YB, CW, CH and HY were responsible for sample collection and explanation. WJ, CR, LL, YS, and HY revised the manuscript. TC and YZ did the initial data processing and quality control assessment. MS prepared documents for sample preparation and instrument analysis. MS and GX provided valuable suggestions in preparing the manuscript. LZ and AZ were responsible for the measurement of free fatty acids.
Conflict of interest
The authors report no potential conflicts of interest relevant to this article.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2015.09.004.
Contributor Information
Wei Jia, Email: wjia@cc.hawaii.edu.
Weiping Jia, Email: wpjia@sjtu.edu.cn.
Appendix A. Supplementary data
Supplementary figures and tables.
References
- Bao Y.Q., Ma X.J., Yang R. Inverse relationship between serum osteocalcin levels and visceral fat area in Chinese men. J. Clin. Endocrinol. Metab. 2013;98(1):345–351. doi: 10.1210/jc.2012-2906. [DOI] [PubMed] [Google Scholar]
- Bei-Fan Z., Cooperative Meta-Analysis Group of Working Group on Obesity in C Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults: study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Asia Pac. J. Clin. Nutr. 2002;8(11 Suppl.):S685–S693. [PubMed] [Google Scholar]
- Boden G. Effects of free fatty acids (FFA) on glucose metabolism: significance for insulin resistance and type 2 diabetes. Exp. Clin. Endocrinol. Diabetes. 2003;111(3) doi: 10.1055/s-2003-39781. (121–124) [DOI] [PubMed] [Google Scholar]
- Boden G. Obesity, insulin resistance and free fatty acids. Curr. Opin. Endocrinol. Diabetes Obes. 2011;18(2):139–143. doi: 10.1097/MED.0b013e3283444b09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G., Shulman G.I. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur. J. Clin. Investig. 2002;32:14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- Cao H., Gerhold K., Mayers J.R., Wiest M.M., Watkins S.M., Hotamisligil G.S. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134(6):933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles M.A., Eschwege E., Thibult N. The role of non-esterified fatty acids in the deterioration of glucose tolerance in Caucasian subjects: results of the Paris Prospective Study. Diabetologia. 1997;40(9):1101–1106. doi: 10.1007/s001250050793. [DOI] [PubMed] [Google Scholar]
- Connor W.E., Lin D.S., Colvis C. Differential mobilization of fatty acids from adipose tissue. J. Lipid Res. 1996;37(2):290–298. [PubMed] [Google Scholar]
- Fabbrini E., Sullivan S., Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51(2):679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forouhi N.G., Koulman A., Sharp S.J. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol. 2014;2(10):810–818. doi: 10.1016/S2213-8587(14)70146-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J., Campos H., McGarvey S., Wu Z., Goldberg R., Baylin A. Adipose tissue palmitoleic acid and obesity in humans: does it behave as a lipokine? Am. J. Clin. Nutr. 2011;93(1):186–191. doi: 10.3945/ajcn.110.006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruben N., Shiri-Sverdlov R., Koonen D.P., Hofker M.H. Nonalcoholic fatty liver disease: a main driver of insulin resistance or a dangerous liaison? Biochim. Biophys. Acta. 2014;1842(11):2329–2343. doi: 10.1016/j.bbadis.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Gu Y.J., Zhao A.H., Huang F.J. Very Low carbohydrate diet significantly alters the serum metabolic profiles in obese subjects. J. Proteome Res. 2013;12(12):5801–5811. doi: 10.1021/pr4008199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y., Yu H., Li Y. Beneficial effects of an 8-week, very low carbohydrate diet intervention on obese subjects. Evid. Based Complement. Alternat. Med. 2013;2013:760804. doi: 10.1155/2013/760804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer M., Stamatakis E. Metabolically healthy obesity and risk of all-cause and cardiovascular disease mortality. J. Clin. Endocrinol. Metab. 2012;97(7):2482–2488. doi: 10.1210/jc.2011-3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia W.P., Pang C., Chen L. Epidemiological characteristics of diabetes mellitus and impaired glucose regulation in a Chinese adult population: the Shanghai Diabetes Studies, a cross-sectional 3-year follow-up study in Shanghai urban communities. Diabetologia. 2007;50(2):286–292. doi: 10.1007/s00125-006-0503-1. [DOI] [PubMed] [Google Scholar]
- Kahn S.E., Hull R.L., Utzschneider K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- Karelis A.D., St-Pierre D.H., Conus F., Rabasa-Lhoret R., Poehlman E.T. Metabolic and body composition factors in subgroups of Obesity: what do we know? J. Clin. Endocrinol. Metab. 2004;89(6):2569–2575. doi: 10.1210/jc.2004-0165. [DOI] [PubMed] [Google Scholar]
- Kramer C.K., Zinman B., Retnakaran R. Are metabolically healthy overweight and obesity benign conditions?: a systematic review and meta-analysis. Ann. Intern. Med. 2013;159(11):758–769. doi: 10.7326/0003-4819-159-11-201312030-00008. [DOI] [PubMed] [Google Scholar]
- Kurotani K., Sato M., Ejima Y. High levels of stearic acid, palmitoleic acid, and dihomo-gamma-linolenic acid and low levels of linoleic acid in serum cholesterol ester are associated with high insulin resistance. Nutr. Res. 2012;32(9):669–675. doi: 10.1016/j.nutres.2012.07.004. e3. [DOI] [PubMed] [Google Scholar]
- Kusunoki J., Kanatani A., Moller D.E. Modulation of fatty acid metabolism as a potential approach to the treatment of obesity and the metabolic syndrome. Endocrine. 2006;29(1):91–100. doi: 10.1385/ENDO:29:1:91. [DOI] [PubMed] [Google Scholar]
- Ma W., Wu J.H., Wang Q. Prospective association of fatty acids in the de novo lipogenesis pathway with risk of type 2 diabetes: the Cardiovascular Health Study. Am. J. Clin. Nutr. 2015;101(1):153–163. doi: 10.3945/ajcn.114.092601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micha R., Mozaffarian D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: a fresh look at the evidence. Lipids. 2010;45(10):893–905. doi: 10.1007/s11745-010-3393-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordier S., Iynedjian P.B. Activation of mammalian target of rapamycin complex 1 and insulin resistance induced by palmitate in hepatocytes. Biochem. Biophys. Res. Commun. 2007;362(1):206–211. doi: 10.1016/j.bbrc.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Pankow J.S., Duncan B.B., Schmidt M.I. Fasting plasma free fatty acids and risk of type 2 diabetes — the atherosclerosis risk in communities study. Diabetes Care. 2004;27(1):77–82. doi: 10.2337/diacare.27.1.77. [DOI] [PubMed] [Google Scholar]
- Pankow J.S., Duncan B.B., Schmidt M.I. Fasting plasma free fatty acids and risk of type 2 diabetes: the ARIC Study. Circulation. 2004;109(7) [Google Scholar]
- Paolisso G., Tataranni P.A., Foley J.E., Bogardus C., Howard B.V., Ravussin E. A high concentration of fasting plasma non-esterified fatty acids is a risk factor for the development of NIDDM. Diabetologia. 1995;38(10):1213–1217. doi: 10.1007/BF00422371. [DOI] [PubMed] [Google Scholar]
- Patel P.S., Sharp S.J., Jansen E. Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes: a pilot study in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. Am. J. Clin. Nutr. 2010;92(5):1214–1222. doi: 10.3945/ajcn.2010.29182. [DOI] [PubMed] [Google Scholar]
- Perreault M., Zulyniak M.A., Badoud F. A distinct fatty acid profile underlies the reduced inflammatory state of metabolically healthy obese individuals. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0088539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R.J., Samuel V.T., Petersen K.F., Shulman G.I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510(7503):84–91. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips C.M., Perry I.J. Does inflammation determine metabolic health status in obese and nonobese adults? J. Clin. Endocrinol. Metab. 2013;98(10):E1610–E1619. doi: 10.1210/jc.2013-2038. [DOI] [PubMed] [Google Scholar]
- Puttmann M., Krug H., Vonochsenstein E., Kattermann R. Fast Hplc determination of serum-free fatty-acids in the picomole range. Clin. Chem. 1993;39(5):825–832. [PubMed] [Google Scholar]
- Reynoso R., Salgado L.M., Calderon V. High levels of palmitic acid lead to insulin resistance due to changes in the level of phosphorylation of the insulin receptor and insulin receptor substrate-1. Mol. Cell. Biochem. 2003;246(1–2):155–162. [PubMed] [Google Scholar]
- Stefan N., Haring H.U., Hu F.B., Schulze M.B. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013;1(2):152–162. doi: 10.1016/S2213-8587(13)70062-7. [DOI] [PubMed] [Google Scholar]
- Steffen B.T., Steffen L.M., Tracy R. Obesity modifies the association between plasma phospholipid polyunsaturated fatty acids and markers of inflammation: the Multi-Ethnic Study of Atherosclerosis. Int. J. Obes. 2012;36(6):797–804. doi: 10.1038/ijo.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Onge M.P., Janssen I., Heymsfield S.B. Metabolic syndrome in normal-weight Americans: new definition of the metabolically obese, normal-weight individual. Diabetes Care. 2004;27(9):2222–2228. doi: 10.2337/diacare.27.9.2222. [DOI] [PubMed] [Google Scholar]
- Trufelli H., Famiglini G., Termopoli V., Cappiello A. Profiling of non-esterified fatty acids in human plasma using liquid chromatography-electron ionization mass spectrometry. Anal. Bioanal. Chem. 2011;400(9):2933–2941. doi: 10.1007/s00216-011-4955-x. [DOI] [PubMed] [Google Scholar]
- Wang L., Folsom A.R., Zheng Z.J., Pankow J.S., Eckfeldt J.H., Investigators A.S. Plasma fatty acid composition and incidence of diabetes in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Clin. Nutr. 2003;78(1):91–98. doi: 10.1093/ajcn/78.1.91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures and tables.