Abstract
As more evidence points to a clear role for astrocytes in synaptic processing, synaptogenesis and cognition, continuing research on astrocytic function could lead to strategies for neurodegenerative disease prevention. Reactive astrogliosis results in astrocyte proliferation early in injury and disease states and is considered neuroprotective, indicating a role for astrocytes in disease etiology. This review describes the different types of human cortical astrocytes and the current evidence regarding adult cortical astrogenesis in injury and degenerative disease. A role for disrupted astrogenesis as a cause of cortical degeneration, with a focus on the tauopathies and synucleinopathies, will also be considered.
Keywords: astrocyte, gliogenesis, neurogenesis, gliosis, Alzheimer’s disease, dementia with Lewy bodies
Introduction
Mature astrocytes in the cerebral cortex are capable of local proliferation under certain conditions, with indications that their progeny may resume normal physiological function over time.1,2 It is known that injury and degeneration can cause reactive astrogliosis and subsequent astrocyte proliferation, although the exact molecular stimulus of proliferation is currently unknown.3 The process whereby reactive astrocytes proliferate was originally considered detrimental, as after injury or stroke, glial scar formation inhibited axonal regrowth in the area.4 However, reactive astrogliosis and astrocyte proliferation are now understood to be neuroprotective, provide factors to promote cell survival, and in severe lesions in injury and degeneration seal off an area of robust necrosis.1,5,6
One of the hallmarks of neurodegenerative disease is synapse loss,7 which occurs early in disease and has long been associated with cognitive decline in cortical dementias, such as Alzheimer’s disease (AD), Parkinson’s disease dementia, and dementia with Lewy bodies (DLBs).7–9 Protoplasmic astrocytes in the cortical gray matter are closely associated with synapses and synapse monitoring.10–12 Recent evidence points to astrocytes in the cortex as contributors to synaptogenesis in response to neuronal communication13,14 and responsible for general control of synapse number.15
Astrocytes are responsible for a variety of homeostatic functions that can lead to neurodegeneration if unchecked. Astrocytes supply neurons with glutathione precursors and protect neurons from cell death as a result of reactive oxygen species production.16 Clearance of toxins from the parenchyma occurs through astrocytes via the glymphatic system.17–19 Astrocytes also remove glutamate through glutamate transporters to avoid excitotoxicity and neuronal cell death.20 It is no longer disputed that astrocytes express the same transmitters and receptors as neurons, are active contributors in central nervous system communication,21 and maintain ionic and osmotic homeostasis in the brain.22
Astrocytes monitor and regulate cerebral blood flow in the cortex.23,24 This is particularly significant as local astrogenesis occurs in the cortex perivascularly,2 and vascular defects are commonly associated with cortical dementia.25 Astrocytes also remove and clear amyloid-β from the extracellular space before the accumulation of the protein into amyloid plaques, the pathological hallmark of AD.17
Indeed, because of the ubiquitous number of functions involved, there is increasing evidence that many neurodegenerative diseases are astrocytic in nature,26–29 and it is becoming clear that astrocytes are an important avenue for the treatment and prevention of neurodegenerative disease.30,31 Because of the ability for proliferation, and if cortical dementias have an astrocytic cause, astrogenesis could lead to an understanding of prevention through regeneration, or a disruption of astrogenesis could be involved in disease etiology. Here we review human cortical astrocytes and conditions that lead to cortical astrogenesis, with a special focus on neurodegenerative disease.
Types of Human Cortical Astrocytes
Protoplasmic astrocytes
Human cortical astrocytes are pleomorphic, and the morphology and density of astrocytes differ between and within brain regions.32,33 Human protoplasmic astrocytes are the most abundant astrocyte in the human cortex and reside in layers II–VI.34 Astrocytes in the human cortex increase their cell body size and projections in response to perturbations in the extracellular and neuronal environment, and the result is the upregulation of intermediate filament glial fibrillary acidic protein (GFAP), which is a distinguishing factor of astrocyte classification.35 The hypertrophic morphology is termed reactive astrogliosis and results in local proliferation of a subset of protoplasmic astrocytes.36
Contrary to the appearance associated with a GFAP-immunostained astrocyte, or those drawn based on classic silver stain, dye injection studies reveal their shape and processes to have a “bushy” appearance.30 These bushy-like endfeet and extensive projections allow protoplasmic astrocytes to make connections with 270,000–2 million synapses in the human brain compared to 20,000–120,000 synapses in the rodent models.37 Human cortical protoplasmic astrocytes form independent signaling domains with nonoverlapping territories, are the most complex compared to other primates and rodents, have 2.55 times larger diameter than the rodent, equating to 16.5 times great volume, and signal via calcium wave over 5 times faster.37 Protoplasmic astrocytes also make contact via their end feet with perivascular processes.38,39
Human cortical protoplasmic astrocytes communicate via calcium signaling through end feet gap junctions to other astrocytes adjacent to their domains.37 The communication results in the release of transmitter to the extracellular space and can happen in response to extracellular transmitter communication from neurons and other astrocytes.40,41 It has been demonstrated that the transmitters ATP and glutamate stimulate calcium signaling in human cortical astrocytes.37
Interlaminar astrocytes
In primates, a distinct astrocyte cell type with a stellate morphology has been described in layer I—the molecular layer of the cortex—that does not appear in other mammals.42–45 In the prosimian Microcebus murinus, its length is shorter and it is found in fewer numbers, suggesting the increasing importance of the interlaminar astrocyte in primate species with a more recent common ancestor with humans.46 However, in other new- and old-world monkeys, including humans, their numbers are similar. Interlaminar astrocytes send a process that extends into and ends in layers III and IV from the molecular layer, making contact with other cells and blood vessels, which does not respect specific domains as is seen with protoplasmic astrocytes.37,46 Calcium wave signaling was shown to transmit down this projecting process for long distance communication.37
Varicose projection astrocytes
The least studied and most recently discovered type of astrocyte in the human cortex resides in layers V–VI and extends long processes up to layers III, IV, and V, making numerous cellular contacts and blood vessel projections.37 Varicose projection astrocytes are completely unique to the hominid and have not been seen in other primates or in rodents.32,37 The function of these cells, much similar to that of interlaminar astrocytes, is uncertain at the moment. However, when human glial progenitor cells were grafted into the forebrains of mice, they differentiated into interlaminar and varicose projection astrocytes and had increased learning and memory capabilities compared to murine astrocytes. This was evidenced behaviorally, as well as on the cellular level, through neuronal long-term potentiation studies.47 Although the tauopathies, synucleinopathies, and other cortical dementias appear to be more prevalent in humans than other mammals,48 interlaminar and varicose projection astrocytes have not been researched in degenerative disease and are interesting avenues for future studies. Because of the unique primate nature of interlaminar and varicose projection astrocytes, experimental studies in rodent models described here specifically consider cortical protoplasmic astrocytes, which are the only subtype of astrocyte currently described in the rodent cortical gray matter. In human and primate experiments, protoplasmic astrocytes are also the predominant cell discussed, unless otherwise indicated.
Cortical Reactive Astrogliosis and Astrogenesis
Protoplasmic astrocytes
Reactive astrogliosis is typically defined as the change in morphology of astrocytes and subsequent upregulation of proteins involved in neuroprotection near injury, ischemia, or neurodegeneration.49 However, due to the nature of the cortex, astrocytes are consistently responding to complex changes and subjected to stimuli that can produce astrogliosis, so even subtle perturbations in the nervous system are likely to produce a local reaction.50 This can happen along a graded continuum of a series of responses, where it is unknown what series of signals produce local astrogenesis.51 Reactive astrogliosis has most been studied after injury and lesion, and during these insults, astrocyte proliferation is observed, a process traditionally termed astrocytosis.2
Reactive astrogliosis in injury and disease states was originally considered detrimental because in severe cases a GFAP+ glial scar forms and sends signals that inhibit neurite outgrowth;52 however, it is now established to play a neuroprotective role.6 Proliferating astrocytes appear to be crucial to the recovery after injury, as transgenic experiments that eliminated astrocyte proliferation demonstrated a larger lesion-reduced scar formation and persistent blood-brain barrier dysfunction.53 In vitro, reactive astrocytes formed neurospheres and have shown stem cell potential.1,54 In vivo, the cortex appears to foster a gliogenic environment, as ectopic grafting of cells with immature neuronal precursor markers into the cortex reverted back to oligodendrocyte or astrocyte phenotypes.55
An increased expression of GFAP is typically used as a marker of cortical reactive astrogliosis, but this is not indicative of proliferation, but rather an upregulation of the protein in response to insult.49 In milder insults and distal to the injury site, astrocytes can exhibit reactivity, which has been termed “isomorphic,” and are predominantly devoted to promoting neurite growth, synaptogenesis, and neuronal modeling.49 “Anisomorphic” astrocyte morphology with overlapping domains and proliferation appears to occur in more severe injury,49 although proliferation occurs along a continuum, with graded increases from mild to severe, and the exact molecular stimulus is currently unknown.50
Genetic lineage tracing demonstrated that reactive protoplasmic astrocytes in the cortex began to proliferate within 3–5 days of injury, with half of them reentering the cell cycle up to a week after injury. Known progenitor cell markers, such as nestin, DSD1 proteoglycan, and CD15, are upregulated, and protoplasmic astrocytes isolated from injured tissue also produce neurospheres in vitro.1,56,57 After controlled cortical impact, it was shown that astrocytes proliferate at all three stages after injury, with 70% of proliferating cells staining for GFAP.58 In hypoxic-ischemic stroke model of the cortex, GFAP colocalized with bromodeoxyuridine (BrdU), demonstrating astrocyte proliferation in this experimental model as well.59 Astrocytes have been shown to increase Notch-1 expression to induce proliferation, which reduced proliferation when blocked.60 GFAP-CreER-Notch-1-cKO mice also exhibited a defect in astrocyte proliferation in adults after ischemic injury.61
Proliferation vs. astrogenesis
Although certain cortical reactive astrocytes can proliferate, it remains to be seen whether the progeny can differentiate into a nonreactive physiological state. Recently, it was shown through genetic fate-mapping studies and live imaging that 45% of proliferating protoplasmic astrocytes after stab wound in the mouse cortex had soma in direct contact with blood vessels. With unique juxtaposition on blood vessels, the cells proliferate and maintain the bushy morphology of functional astrocytes in response to injury.2 The cells divided only once, creating two daughter cells after injury, and can function for many weeks after division, indicating astrogenesis after proliferation, even while retaining the bushy reactive morphology. Interestingly, these cells were typically further from the injury site. Cells adjacent to the stab lesion did not appear to proliferate. Instead, they became reactive and exhibited a polar morphology, by extending long processes to the injury site.2 Proliferating astrocytes also did not seem to contribute to the glial scar, indicating in this study that GFAP+ cells in the scar must be derived from another source (Fig. 1).2
Figure 1.
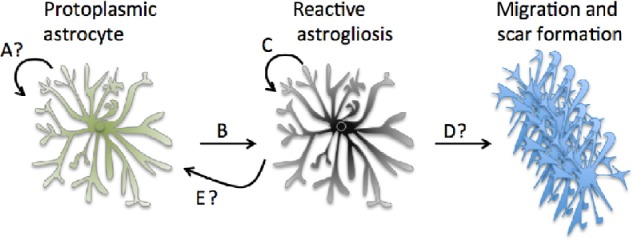
Local astrogenesis from protoplasmic astrocytes. (A) It is unclear to what extent local cortical astrogenesis occurs in the healthy brain. (B) Reactive astrogliosis occurs as an astrocytic response to changes in the extracellular environment—in some cases this can lead to proliferation in C. (D) It is generally believed that protoplasmic astrocytes contribute to the glial scar when reactive in injury, although recent evidence indicates that the GFAP+ cells in the scar may derive from a different source.2 (E) Recent evidence indicates that reactive astrocytes can retain their physiological function after proliferation, although the molecular process and time course are still unclear.2
NG2 cells (synantocytes)
In vitro studies demonstrate that two other cortical cell types, namely neural/glial antigen 2 (NG2) proteoglycan-expressing cells and blood vessel lining pericytes, can differentiate into astrocytes.62,63 In vivo studies on the ability for pericytes to differentiate into astrocytes, however, have not been studied in the cortex. Additionally, NG2 cells in the cortex were long considered to be a subset of astrocytes because of their morphology and functionality.64 However, it is now known they are not a subset of astrocytes, as the mature NG2 cell is functionally different and does not express many typical astrocyte markers.65 NG2 cells are also the main oligodendrocyte precursor in the cortex, which is distinct from mature cortical astrocyte stem cell function in vivo.65 They have a long cell cycle of one month in the adult human cortex and consistently regenerate to contribute to the oligodendroglial pool.66,67
NG2 cells also proliferate after injury and have been shown in severe cases to differentiate into astrocytes in the cortex and contribute to the glial scar and neuroprotection.68–71 NG2 cells are the second type of cell to proliferate after injury to the brain,66 after microglia, which are recruited to the injury site by astrocytes.72 After recent studies on astrocyte proliferation demonstrated they did not incorporate into the scar in stab wound studies, it is possible NG2 progenitors differentiate into scar-forming GFAP+ cells and contribute more than thought to the scar. Early genetic fate-mapping studies also confirmed that NG2 cells differentiate into astrocytes in the cortex after injury.73 Immunohistochemical studies showed that 5–8 days after injury, 20% of GFAP+ cells colocalize with NG2, indicating that NG2 progenitors differentiate into reactive astrocytes under certain conditions.69 Another study showed that in the cortex, within a week after injury, a small population of NG2 cells will express vimentin and nestin, two immature astrocyte markers.71
However, there is some conflicting evidence for their astrocyte lineage, as other studies demonstrated that NG2 cells do not appear to be proliferating into astrocytes.74 In the spinal cord of amyotrophic lateral sclerosis (ALS) mice, it was determined through fate-mapping studies that NG2+ cells were committed to an oligodendrocyte fate postnatally.75 However, another study showed that genetic fate-mapping studies indicated that NG2 cells could become astrocytes in postnatal development.76 Further studies demonstrated that a subset of NG2 cells can indeed differentiate into protoplasmic astrocytes postnatally; however, by postnatal day 60, no new astrocytes were born from NG2 cells.77 Although most evidence points to astrocyte and NG2 cell proliferation in injury conditions,78 there is currently no indication that NG2 cells can become mature functional protoplasmic astrocytes (Fig. 2).
Figure 2.
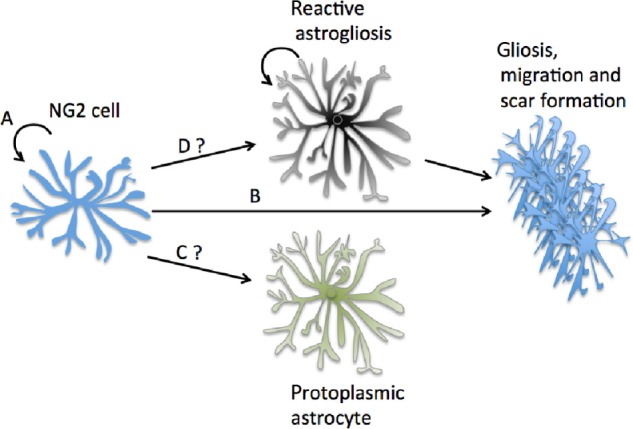
NG2 cells and cortical astrogenesis. NG2 cells divide monthly as indicated in A and are known to become reactive and contribute to the glial scar in B. (C) Many early lines of evidence on NG2 cells pointed to them as an astrocyte precursor cell in the adult cortex. Recent fate-mapping studies have shown that may not be the case.74 (D) It is also unsure whether they can become reactive astrocytes under severe conditions as was previously believed and then proliferated.74
Adult stem cells in germinal layers
While experimental evidence indicates that mature cortical protoplasmic astrocytes can proliferate in certain conditions, one question is whether it is possible that cells can migrate from germinal niches, such as the ventricular–subventricular zone (V-SVZ)79,80 or the subgranular zone (SGZ) in the hippocampus to contribute to the cortical astrocyte population. GFAP+ adult neural stem cells with astrocyte properties in the subventricular zone of mammals have been shown to differentiate into neuronal and astrocytic precursors and migrate to other areas of the brain, most notably the olfactory bulb.81–85 However, focal ischemia of the striatum adjacent to the V-SVZ produced mainly glial lineages, with 60% astrocytes from neural stem cells.86 Similarly, in rodents, GFAP+ astrocytes in the SGZ of the hippocampus can give rise to new functioning neurons and mature astrocytes.87
After birth, in the human brain prior to 18 months, proliferating cells in the V-SVZ migrate to the prefrontal cortex with an astrocytic fate instead of a neuronal one, before subsiding, with astrocytes proliferating in the cortex locally afterward.88 In piglets by postnatal day 7, it was seen that few, if any, proliferating V-SVZ cells colocalized with immature neurons.89
After cortical injury, nestin+ cells that did not express GFAP were shown to migrate and become new ipsilateral astrocytes to the injury site.90 Also, in normal conditions, V-SVZ fate-mapped nestin+ cells become astrocytes in the corpus callosum but did not appear to contribute to astrogenesis in the cortex.91
Interestingly, it was recently shown with tamoxifen-induced nestin-Cretm 4 lineage tracing that the majority of cells that contribute to a cortical injury site are produced through astrogenesis, with cells deriving from a V-SVZ lineage.92 Some cells from the V-SVZ contributed to the glial scar and were also high thrombospondin-producing cells, a protein released by astrocytes known to induce synaptogenesis (Fig. 3).14 KO mice for thrombospondin 4 caused alterations in the glial scar and increased microvascular hemorrhage.92
Figure 3.
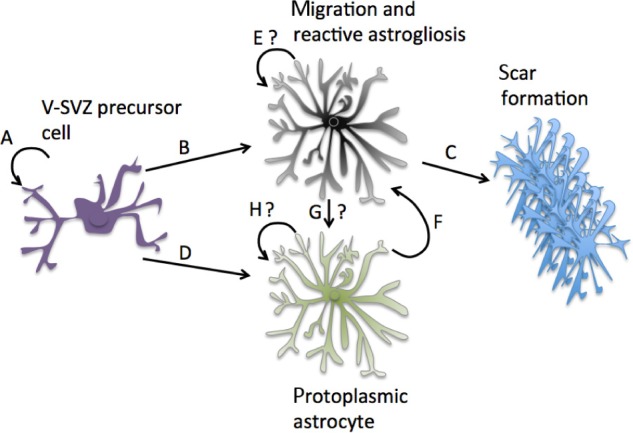
V-SVZ adult stem cells and cortical astrogenesis. V-SVZ adult stem cells are regenerating cells (A) that have recently shown to proliferate into protective reactive astrocytes after injury (B) and contribute to the glial scar in the cortex (C).92 (D) It is also known that they can contribute to adult astrogenesis. However, it is unclear whether the reactive astrocytes they produce can proliferate locally (E), although this proliferation likely occurs if derived locally from a mature astrocyte (F). It is also uncertain whether reactive astrocytes produced by the V-SVZ can become mature protoplasmic astrocytes (G), which may also be able to proliferate (H).
In the hippocampus, it is known that neural precursor cells in the SGZ contribute to the mature astrocyte population in CA1 under normal physiological conditions and that this process is disrupted in injury and disease.93 However, although of interest because of known hippocampal degeneration in AD, the evidence is scant for astrocyte proliferation from the SGZ to the cortex.94 In aging mice, division happened less frequently, and GFAP-expressing cells began to exhibit characteristics of reactive astrogliosis.95 Immediately after injury to the cortex, astrocyte proliferation occurred locally in the hippocampus without migration to the cortex.96
Cortical Astrogenesis in Other Conditions
Aged and normal healthy cortex
It appears that the astrocyte capability of reactivity and proliferation is inherent to astrocytic cell function. During development, astrocytes derive from radial glial cells, but postnatally, in the mouse cortex, they proliferate locally and incorporate into functional units with defined astrocyte regions.97 Because of the astrocyte ability to respond to even slight perturbations in the parenchyma,50 studies on cortical astrogenesis in “normal” healthy adult brains or aged cortex might reveal the mechanisms of astrocytic proliferation.
Early studies of aged human brains are somewhat conflicting because gray matter human astrocytes were designated as “fibrous” astrocytes in many cases. Fibrous astrocytes currently refer to astrocytes residing in white matter tracts, but before this designation, protoplasmic or interlaminar astrocytes were determined to be “fibrous” in some studies if they were labeled immunohistochemically with GFAP.98 It is believed that astrocytes undergo a change in morphology in aging, and originally, it was thought that astrocyte reactivity was increased in aging, as well as consequential astrogenesis, whereby an increase was seen on an average of >20% of astrocytes in the cortex of aged brains.99,100 Furthermore, in the aged rat cortex, a 20%–22% increase in astrocytes and pericytes was shown.101
However, it must also be remembered that an increase in GFAP expression of cortical protoplasmic astrocytes is not indicative of proliferation102 but traditionally a marker of reactive astrogliosis.6 One study analyzed the brains of several aged controls with no neurodegenerative disease diagnosis and noticed an increase in the GFAP expression of the cortex, in both the molecular layer and cellular layer.103 As the interlaminar astrocyte is unique to primates in the molecular layer, it is likely that interlaminar astrocytes undergo reactive astrogliosis in response to aging. Additionally, in cellular layers II–VI, increased GFAP expression was associated with perivascular location and typically, in duplicate, indicating possible proliferation in aging.103 In rats, increased GFAP expression was also noticed in the aging cortex.104
In the entorhinal cortex of aged mice, an area that degenerates early in AD a decrease in GFAP expression and astrocyte atrophy was observed.105 Also, in another human study of female brains, aged 65–75, 76–85, and 94–105 years of age, it was observed that there was no change in neuron or astrocyte numbers.106 Additionally, other sources indicate no increase in the amount of astrocytes in the cortex.107 In rats, an electron microscopic study concluded that there was an increase in the number of astrocytes in aging animals compared to controls.108 Nonneuronal cells stained with cresyl violet were also increased in the parietal cortex of aged rats compared to controls.109
Studies of nonnervous system origin cancer patients injected with BrdU demonstrated that new cells formed in the cortex were nonneuronal with a small subset colocalizing with GFAP <0.5 cell/mm3, indicating the prevalence of astrogenesis in the noninjured nondegenerating adult cortex.110 Neurons, however, did not colocalize with BrdU, indicating that new neurons were not formed in the cortex in the lifespan of the organism and that proliferation was strictly glial.110
In rhesus monkeys, neuronal cell loss was not observed in the cortex of aged monkeys, and astrocytes had exhibited an increase in cellular inclusions. The older monkeys had significant memory impairment compared to the younger monkeys.111 However, in subsequent studies, they noticed that only microglia increased in numbers with aging.112 Although many studies have indicated that increased GFAP staining correlates with age, indicating an increase in astrocyte reactivity,113 studies on astrogenesis in healthy human cortex are few.
Learning, exercise, and environmental enrichment
Environmental enrichment has been known to increase cell division in the adult brain because a study by Altman and Das in the 1960s demonstrated a significant increase in gliogenesis in the brains of rats.114 The cell type was not determined, and they noticed increased cell division in all the areas of the white matter of the coronal radiations. Cell division occurred in the gray matter as well, but this was not statistically studied.114
The hippocampus is currently the region of the brain with the most evidence for increased astrogenesis in environmental enrichment conditions, where astrocytes from the SGZ proliferated into mature astrocytes in the CA1 region.93 Cells were shown to be mature and distinct from the GFAP+ progenitor cells where they arose in the SGZ.115 Cells in the SGZ that are GFAP+ can also differentiate into neurons,116 and this is increased in environmental enrichment and learning conditions.117 GFAP expression and increased size and complexity of astrocytes were seen in the dentate gyrus after physical activity and environmental enrichment.118
There is also evidence for cortical astrogenesis in environmental enrichment, as in the motor cortex of mice, a noticeable increase in astrogenesis was observed, without an increase in oligodendrocytes and with no new neurons formed.119 Additionally, operant conditioning tasks showed that astrogenesis occurred in the prefrontal cortex and that learning maintained cell survival, whereas if learning did not occur, new cells were not maintained.120 Voluntary exercise also resulted in a 3× increase in astrogenesis compared to normal controls in the medial prefrontal cortex of mouse brains.121 Although the lineage of the proliferating astrocytes in the pre-frontal cortex of the mouse brain has not yet been studied, they appear to be from local progenitors or originating from cells in the V-SVZ.
Cortical spreading depression
Studies have shown that cortical spreading depression (CSD) can cause the proliferation of astrocytes in cortical regions. This is preceded by cortical spreading depolarization, which is associated with migraine, stroke, and epilepsy and results in the excitation spread of coordinated neuronal firing.122 CSD results in an increase in a number of dividing cells that coexpress GFAP.123–126 Cortical brain slice preparation demonstrated the origin of the cells were NG2 cells differentiating into astrocytes.127 In the entorhinal cortex, a robust increase in cell proliferation to CSD remained astrogenic, and no subsequent cortical neurogenesis was observed.128 CSD shifted the relative frequencies of glial cells from NG2 cells to astrocytes and microglia.128 Nestin+ astrocytes were also increased after CSD in the cortex.97
Cortical Astrocytes and Neurodegeneration
Tauopathies and amyloid-β
Reactive astrogliosis as described by hypertrophy and subsequent proliferation is found in chronic neurodegenerative lesions.6,49,50 In human, similar to what was studied in aging brains, where an increase in GFAP+ expression of protoplasmic astrocytes was noticed in cortical layers II–VI after age 70, a much larger increase in GFAP+ cells was seen, which was greater than four times in the cellular layer of patients diagnosed with AD compared to age-matched controls.98
Amyloid precursor protein (APP) when cleaved by beta-secretase 1 (BACE-1) and gamma secretase produces amyloid-β1–40 and amyloid-β1–42, peptides that accumulate in amyloid plaques in neurodegenerative disease.129 Additionally, amyloid-β1–42 is closely associated with disease130 and has been shown to preferentially stimulate astrogenesis from human embryonic neural stem cells in vitro,131 while BACE-1 null mice show diminished astrogenesis in the hippocampus.132 In vitro, the amyloid-β1–42 peptide treatment of postnatal primary mouse astrocytes increased proliferation133 and was shown to disrupt calcium signaling between astrocytes, which is also diminished in disease progression.134,135 APP has been shown to stimulate astrogenesis in development as well.136
Amyloid-β1–40 and amyloid-β1–42 are cleared from the extracellular space by astrocytes through the glymphatic system.17 It was observed that astrocytes surrounding plaques increase the expression of GFAP and vimentin; however, in GFAP and vimentin KO mice, the plaque load was not diminished, but lysosomal and inflammation genes increased expression.137
Importantly, researchers observed that proliferative circumferential reactive astrogliosis around amyloid-β plaques correlated with cognitive scores in disease.138 Synapse loss in the cortex also occurs early on in disease and correlates with cognitive decline,7–9 and astrocytes contribute to the regulation of synaptogenesis.15 This lack of circumferential astrogliosis also correlated with apolipoprotein E ε4 genotype, a known genetic precursor for late-onset AD,138 which is a risk factor after early life incidence of head injury, another known stimulator of astrogenesis.139 Astrocytes are the predominant apolipoprotein E-producing cell in the cortex,140 and the protein appears to be involved in cholesterol transport via lipid rafts, a contributor to synaptogenesis.141
In the TgCRND8 mouse AD model, it was observed that GFAP+ cells colabeled with BrdU in aged mice, indicating a proliferative response.142 However, when proliferating cells in another mouse model of AD, the APPswe/PS1dE9 (APPPS1) transgenic mice were studied; microglia were the main proliferating cell type. GFAP+ reactive astrocytes were not proliferative around the plaque, compared to those in injury, where reactive astrocytes are prevalent and begin to proliferate, as the severity of the injury increases.143
In vitro, neurospheres indicative of stem cell properties have been produced from astrocytes after injury in APPPS1 mice. Also, it has been shown that 2.7% of cortical proliferating cells were astrocytes, which account for only about 1.1% of the astrocytes in the cortex.144 Many of the cells were microglia and NG2 cells. Many astrocytes not proliferating also produce immature cell markers, such as nestin, DSD1, and tenascin-C, which are upregulated in reactive astrocytes.144 However, it appears that sonic hedgehog signaling is responsible for astrocyte proliferation from reactive astrocytes.144
Glial atrophy has been shown in the cortex of the APPPS1 AD mouse.145 In AD transgenic mice, the hippocampus exhibited extensive reactive astrogliosis, but not the entorhinal cortex where astrocyte atrophy was observed, which is one of the areas of the brain to exhibit selective early vulnerability in AD.146 Additionally, astrocyte atrophy was observed in medial prefrontal cortex in AD transgenics.147
Additionally, neurofibrillary tangles formed as a result of hyperphosphorylated tau protein aggregation are observed in AD neurodegeneration and can occur in cortical tauopathies independent of plaque formation.148 In other tauopathies, such as frontotemporal dementia, astrocyte apoptosis preceded neuronal apoptosis in the disease progression.149 In a transgenic model of tauopathy, there was an age-related increase in tau accumulation in astrocytes, which is similar to what is seen in neurons in disease.150 A reduction of astrocyte glutamate transporter 1 was observed in corticobasal degeneration, and a tau mouse model from the GFAP promoter demonstrated similar vascular defects and neurofibrillary tangle formation in disease states.151 The hyperphosphorylation of tau, and accumulation within astrocyte end feet processes was also observed to contribute to vascular defects in corticobasal degeneration and progressive supranuclear palsy.152 In many cases, AD and other tauopathies can be thought of as a cerebrovascular disease and have been considered as such, where it has been estimated that as many as 84% of cases show both morphologies.153
Finally, early on in AD, it is noticed that many genes and proteins involved in cell cycle stimulation are upregulated.154 This has been considered from a neuronal perspective, with a hypothesis that cell cycle reentry and dysfunction in neurons lead to degeneration.155,156 However, because of the known proliferative nature of astrocytes, cell cycle biomarkers in astrocytes in disease states provide a future avenue for study.
Synucleinopathies
Synucleinopathies are characterized by the accumulation of protein α-synuclein (α-syn) in Lewy Bodies inclusions.157–159 α-Syn is abundantly expressed at neuronal synapses160–162 and can be released extracellularly as a possible signaling protein as evidenced by its binding to postsynaptic protein and ability to be transferred to neighboring neurons to form Lewy body inclusions.163–165 Extracellular α-syn has also been shown to assimilate in human cortical astrocytes in vivo and in vitro.166–168 Common synucleinopathies affecting the cortex are multiple system atrophy (MSA), Parkinson’s disease dementia, and DLBs.169
Genes involved in familial Parkinson’s disease, such as Pink1, Parkin, DJ-1, and LRRK2, are specifically expressed by astrocytes and shown to produce proteins associated with lipid rafts.170,171 Pink1, Parkin, DJ-1, and LRRK2 were also shown to be involved in cell cycle regulation.172 Additionally, human cortical astrocytes in culture treated with α-syn revealed apolipoprotein Eredistribution to the cytoplasm and an increase in GFAP+ astrocytes.167 α-Syn signaling to astrocytes at the synapse was also shown to be increased in the songbirds developing song control system and demonstrates a possible involvement in neuroplasticity.160
In midbrain regions affected early in Parkinson’s disease, reactive astrogliosis in the substantia nigra was similar to normal control tissue, whereas in another synucleinopathy, multiple system atrophy, reactive astrogliosis was increased in the substantia nigra.173 However, in the frontal cortex in both Parkinson’s disease and multiple system atrophy, an increase in reactive astrogliosis as marked by GFAP, vimentin, and heat shock protein-27 immunoreactivity was observed.173 Additionally, astrocyte and microglia marker YKL-40 were significantly reduced in the cerebral spinal fluid of patients with synucleinopathies (PD, MSA, and DLB) compared to tauopathies, where the reduction was significant compared to controls but higher than the synucleinopathies.174
A model of MSA demonstrated that α-syn can induce reactive astrogliosis in the frontal and visual cortex of human brain via astrocyte proximity to accumulated α-syn inclusions.175 In other GFAP and vimentin expression studies, it was observed that, unlike that in AD, cortical reactive astrogliosis does not correlate with cognitive decline in Parkinson’s disease dementia compared to normal controls.176,177 In vivo, it appears that there is early dysfunction in astrocytes in disease progression, as there is an indication that cortical protoplasmic astrocytes become nonreactive and susceptible to α-syn accumulation while recruiting microglia to attack the affected neurons.178 Selective expression of A53T mutant α-syn in astrocytes also resulted in aggressive disease progression in mice.179
Recently, in human neuropathological studies γ-synuclein (γ-syn), another member of the synuclein family, was shown to be expressed in cellular inclusions along with α-syn.180 γ-Syn is upregulated in glioblastomas181 and is known to be involved in cell cycle regulation.182 An increase in the expression of γ-syn was also seen along with α-syn in the cerebral spinal fluid of patients diagnosed with synucleinopathy and vascular disease,183 and a mouse model overexpressing γ-syn demonstrated widespread neuropathy.184
Conclusion
Although the general notion is that astrocytes achieve a quiescent mature cell fate in adulthood, the physiology of astrocytes in the brain during neuronal communication and neuronal dysfunction allows for them to be dynamic in their response, whereby they undergo reactive astrogliosis and proliferation to protect the neuronal environment. The exact type of perturbations on the molecular level that stimulate proliferation is currently unknown. It is also unknown to what extent over time reactive astrocytes can then resume normal function after proliferation.
Additionally, fate-mapping studies have provided clearer observations on the lineage of cells proliferating in the cortex in disease and injury states, but the evidence is still murky. The contribution and function of cells arising from local proliferation is yet to be determined. Also, the function in early disease states of reactive astrocytes, in addition to biomarkers for the manipulation of astrocytic mechanisms in disease, will be useful. In particular, due to upregulated cell cycle markers and for cell replacement, neurogenesis has been studied in injury and disease cause and prevention; however, because of the inherent proliferative abilities of adult astrocytes compared to neurons, as well as neuroprotective functions, studies on astrogenesis could provide insights into disease onset.
Because much of the early research in neurodegenerative disease has focused on the response of astrocytes to neuronal degeneration, there are many avenues to consider for the study of astrocyte involvement in the cause of degenerative disease. For instance, it is now becoming clear that astrocyte dysfunction can lead to synaptic loss, neurodegeneration, and protein accumulation in the form of Lewy bodies, neurofibrillary tangles, and amyloid plaques. Therefore, an exploration of astrogenesis, in the normal aging brain and neurodegenerative disease, could provide fruitful studies on the cause and prevention of degenerative diseases of the brain.
Acknowledgments
The authors would like to specially thank Irene Luccia Pearl and Xavier Vagus for their support and help with manuscript preparation.
Footnotes
ACADEMIC EDITOR: Lora Talley Watts, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 984 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Wrote the first draft of the manuscript: TCM. Contributed to the writing of the manuscript: TCM and AOK. Agree with manuscript results and conclusions: TCM and AOK. Jointly developed the structure and arguments for the paper: TCM and AOK. Made critical revisions and approved final version: AOK. All authors reviewed and approved the final manuscript.
REFERENCES
- 1.Buffo A, Rite I, Tripathi P, et al. Origin and progeny of reactive gliosis: a source of multipotent cells in the injured brain. Proc Natl Acad Sci U S A. 2008;105(9):3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bardehle S, Krüger M, Buggenthin F, et al. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat Neurosci. 2013;16(5):580–586. doi: 10.1038/nn.3371. [DOI] [PubMed] [Google Scholar]
- 3.Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;6(11):1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- 4.Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49(6):377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- 5.Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81(2):229–248. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terry RD, Masliah E, Salmon DP, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30(4):572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 8.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298(5594):789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 9.Jellinger KA. Morphological substrates of dementia in parkinsonism—a critical update. J Neural Transm Suppl. 1997;51:57–82. doi: 10.1007/978-3-7091-6846-2_6. [DOI] [PubMed] [Google Scholar]
- 10.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22(5):208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 11.Haydon PG. Glia: listening and talking to the synapse. Nat Rev Neurosci. 2001;2(3):185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- 12.Verkhratsky A, Nedergaard M. Astroglial cradle in the life of the synapse. Philos Trans R Soc Lond B Biol Sci. 2014;369(1654):20130595. doi: 10.1098/rstb.2013.0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mauch DH, Nägler K, Schumacher S, et al. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294(5545):1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- 14.Christopherson KS, Ullian EM, Stokes CC, et al. Thrombospondins are astrocytesecreted proteins that promote CNS synaptogenesis. Cell. 2005;120(3):421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 15.Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291(5504):657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- 16.Dringen R, Gutterer JM, Hirrlinger J. Glutathione metabolism in brain— metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem. 2000;267(16):4912–4916. doi: 10.1046/j.1432-1327.2000.01597.x. [DOI] [PubMed] [Google Scholar]
- 17.Iliff JJ, Lee H, Yu M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123(3):1299–1309. doi: 10.1172/JCI67677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.lliff JJ, Nedergaard M. Is there a cerebral lymphatic system? Stroke. 2013;44(6):S93–S95. doi: 10.1161/STROKEAHA.112.678698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang L, Kress BT, Weber HJ, et al. Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer. J Transl Med. 2013;1:11. doi: 10.1186/1479-5876-11-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothstein JD, Dykes-Hoberg M, Pardo CA, et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16(3):675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 21.Kimelberg HK. Receptors on astrocytes—what possible functions? Neurochem Int. 1995;26(1):27–40. doi: 10.1016/0197-0186(94)00118-e. [DOI] [PubMed] [Google Scholar]
- 22.Kimelberg HK. Water homeostasis in the brain: basic concepts. Neuroscience. 2004;129(4):851–860. doi: 10.1016/j.neuroscience.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 23.Takano T, Tian GF, Peng W, et al. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9(2):260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 24.Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86(3):1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- 25.Mirra SS, Heyman A, McKeel D, et al. The consortium to establish a registry for Alzheimer’s-disease (CERAD). 2. Standardization of the neuropathologic assessment of Alzheimer’s-disease. Neurology. 1991;41(4):479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 26.Verkhratsky A, Parpura V, Pekna M, Pekny M, Sofroniew M. Glia in the pathogenesis of neurodegenerative diseases. Biochem Soc Trans. 2014;42(5):1291–1301. doi: 10.1042/BST20140107. [DOI] [PubMed] [Google Scholar]
- 27.Verkhratsky A, Sofroniew MV, Messing A, et al. Neurological diseases as primary gliopathies: a reassessment of neurocentrism. ASN Neuro. 2012;4(3):e00082. doi: 10.1042/AN20120010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez-Arellano JJ, Parpura V, Zorec R, Verkhratsky A. Astrocytes in physiological aging and Alzheimer’s disease. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 29.De Keyser J, Mostert JP, Koch MW. Dysfunctional astrocytes as key players in the pathogenesis of central nervous system disorders. J Neurol Sci. 2008;267(1–2):3–16. doi: 10.1016/j.jns.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 30.Kimelberg HK, Nedergaard M. Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics. 2010;7(4):338–353. doi: 10.1016/j.nurt.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng L, Parpura V, Verkhratsky A. EDITORIAL neuroglia as a central element of neurological diseases: an underappreciated target for therapeutic intervention. Curr Neuropharmacol. 2014;12(4):303–307. doi: 10.2174/1570159X12999140829152550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oberheim NA, Goldman SA, Nedergaard M. Heterogeneity of astrocytic form and function. Methods Mol Biol. 2012;814:23–45. doi: 10.1007/978-1-61779-452-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Marques J, Lopez-Mascaraque L. Clonal identity determines astrocyte cortical heterogeneity. Cereb Cortex. 2013;23(6):1463–1472. doi: 10.1093/cercor/bhs134. [DOI] [PubMed] [Google Scholar]
- 34.Luse SA. Electron microscopic observations of the central nervous system. J Biophys Biochem Cytol. 1956;2(5):531–542. doi: 10.1083/jcb.2.5.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bignami A, Eng LF, Dahl D, Uyeda CT. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972;43(2):429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- 36.Robel S, Berninger B, Gotz M. The stem cell potential of glia: lessons from reactive gliosis. Nat Rev Neurosci. 2011;12(2):88–104. doi: 10.1038/nrn2978. [DOI] [PubMed] [Google Scholar]
- 37.Oberheim NA, Takano T, Han X, et al. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29(10):3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26(10):523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Anderson CM, Nedergaard M. Astrocyte-mediated control of cerebral microcirculation. Trends Neurosci. 2003;26(7):340–344. doi: 10.1016/S0166-2236(03)00141-3. author reply 344–34. [DOI] [PubMed] [Google Scholar]
- 40.Pasti L, Volterra A, Pozzan T, Carmignoto G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci. 1997;17(20):7817–7830. doi: 10.1523/JNEUROSCI.17-20-07817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamatemediated astrocyte-neuron signalling. Nature. 1994;369(6483):744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 42.Colombo JA, Yanez A, Puissant V, Lipina S. Long, interlaminar astroglial cell processes in the cortex of adult monkeys. J Neurosci Res. 1995;40(4):551–556. doi: 10.1002/jnr.490400414. [DOI] [PubMed] [Google Scholar]
- 43.Colombo JA, Gayol S, Yanez A, Marco P. Immunocytochemical and electron microscope observations on astroglial interlaminar processes in the primate neocortex. J Neurosci Res. 1997;48(4):352–357. [PubMed] [Google Scholar]
- 44.Colombo JA, Lipina S, Yanez A, Puissant V. Postnatal development of interlaminar astroglial processes in the cerebral cortex of primates. Int J Dev Neurosci. 1997;15(7):823–833. doi: 10.1016/s0736-5748(97)00043-9. [DOI] [PubMed] [Google Scholar]
- 45.Colombo JA, Reisin HD. Interlaminar astroglia of the cerebral cortex: a marker of the primate brain. Brain Res. 2004;1006(1):126–131. doi: 10.1016/j.brainres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Colombo JA, Hartig W, Lipina S, Bons N. Astroglial interlaminar processes in the cerebral cortex of prosimians and old world monkeys. Anat Embryol. 1998;197(5):369–376. doi: 10.1007/s004290050147. [DOI] [PubMed] [Google Scholar]
- 47.Han X, Chen M, Wang F, et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell. 2013;12(3):342–353. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toledano A, Alvarez MI. Lesions and dysfunctions of the nucleus basalis as Alzheimer’s disease models: general and critical overview and analysis of the long-term changes in several excitotoxic models. Curr Alzheimer Res. 2004;1(3):189–214. doi: 10.2174/1567205043332117. [DOI] [PubMed] [Google Scholar]
- 49.Verkhratsky A, Butt A. Glial Physiology and Pathophysiology. Wiley-Blackwell; Hoboken, New Jersey: 2013. [Google Scholar]
- 50.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32(12):638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sofroniew MV. Astrogliosis. In: Barres BA, Freeman MR, Stevens B, editors. Glia. 1st ed. New York, NY: Cold Spring Harbor Laboratory Press; 2015. pp. 107–122. [Google Scholar]
- 52.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5(2):146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 53.Bush TG, Puvanachandra N, Horner CH, et al. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23(2):297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- 54.Sirko S, Neitz A, Mittmann T, et al. Focal laser-lesions activate an endogenous population of neural stem/progenitor cells in the adult visual cortex. Brain. 2009;132(pt 8):2252–2264. doi: 10.1093/brain/awp043. [DOI] [PubMed] [Google Scholar]
- 55.Seidenfaden R, Desoeuvre A, Bosio A, Virard I, Cremer H. Glial conversion of SVZ-derived committed neuronal precursors after ectopic grafting into the adult brain. Mol Cell Neurosci. 2006;32(1–2):187–198. doi: 10.1016/j.mcn.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 56.Robel S, Mori T, Zoubaa S, et al. Conditional deletion of beta1-integrin in astroglia causes partial reactive gliosis. Glia. 2009;57(15):1630–1647. doi: 10.1002/glia.20876. [DOI] [PubMed] [Google Scholar]
- 57.Gotz M, Sirko S, Beckers J, Irmler M. Reactive astrocytes as neural stem or progenitor cells: In vivo lineage, In vitro potential, and genome-wide expression analysis. Glia. 2015;63(8):1452–1468. doi: 10.1002/glia.22850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Susarla BT, Villapol S, Yi JH, Geller HM, Symes AJ. Temporal patterns of cortical proliferation of glial cell populations after traumatic brain injury in mice. ASN Neuro. 2014;6(3):159–170. doi: 10.1042/AN20130034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sizonenko SV, Camm EJ, Dayer A, Kiss JZ. Glial responses to neonatal hypoxicischemic injury in the rat cerebral cortex. Int J Dev Neurosci. 2008;26(1):37–45. doi: 10.1016/j.ijdevneu.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Y, He K, Wang F, Li X, Liu D. Notch-1 signaling regulates astrocytic proliferation and activation after hypoxia exposure. Neurosci Lett. 2015;603:12–18. doi: 10.1016/j.neulet.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 61.LeComte MD, Shimada IS, Sherwin C, Spees JL. Notch1-STAT3-ETBR signaling axis controls reactive astrocyte proliferation after brain injury. Proc Natl Acad Sci U S A. 2015;112(28):8726–8731. doi: 10.1073/pnas.1501029112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Magnus T, Coksaygan T, Korn T, et al. Evidence that nucleocytoplasmic Olig2 translocation mediates brain-injury-induced differentiation of glial precursors to astrocytes. J Neurosci Res. 2007;85(10):2126–2137. doi: 10.1002/jnr.21368. [DOI] [PubMed] [Google Scholar]
- 63.Dore-Duffy P, Cleary K. Morphology and properties of pericytes. Methods Mol Biol. 2011;686:49–68. doi: 10.1007/978-1-60761-938-3_2. [DOI] [PubMed] [Google Scholar]
- 64.Nishiyama A, Yang Z, Butt A. Astrocytes and NG2-glia: what’s in a name? J Anat. 2005;207(6):687–693. doi: 10.1111/j.1469-7580.2005.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Butt AM, Hamilton N, Hubbard P, Pugh M, Ibrahim M. Synantocytes: the fifth element. J Anat. 2005;207(6):695–706. doi: 10.1111/j.1469-7580.2005.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simon C, Gotz M, Dimou L. Progenitors in the adult cerebral cortex: cell cycle properties and regulation by physiological stimuli and injury. Glia. 2011;59(6):869–881. doi: 10.1002/glia.21156. [DOI] [PubMed] [Google Scholar]
- 67.Psachoulia K, Jamen F, Young KM, Richardson WD. Cell cycle dynamics of NG2 cells in the postnatal and ageing brain. Neuron Glia Biol. 2009;5(3–4):57–67. doi: 10.1017/S1740925X09990354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rhodes KE, Raivich G, Fawcett JW. The injury response of oligodendrocyte precursor cells is induced by platelets, macrophages and inflammation-associated cytokines. Neuroscience. 2006;140(1):87–100. doi: 10.1016/j.neuroscience.2006.01.055. [DOI] [PubMed] [Google Scholar]
- 69.Zhao JW, Raha-Chowdhury R, Fawcett JW, Watts C. Astrocytes and oligodendrocytes can be generated from NG2+ progenitors after acute brain injury: intracellular localization of oligodendrocyte transcription factor 2 is associated with their fate choice. Eur J Neurosci. 2009;29(9):1853–1869. doi: 10.1111/j.1460-9568.2009.06736.x. [DOI] [PubMed] [Google Scholar]
- 70.Rhodes KE, Moon LD, Fawcett JW. Inhibiting cell proliferation during formation of the glial scar: effects on axon regeneration in the CNS. Neuroscience. 2003;120(1):41–56. doi: 10.1016/s0306-4522(03)00285-9. [DOI] [PubMed] [Google Scholar]
- 71.Alonso G. NG2 proteoglycan-expressing cells of the adult rat brain: possible involvement in the formation of glial scar astrocytes following stab wound. Glia. 2005;49(3):318–338. doi: 10.1002/glia.20121. [DOI] [PubMed] [Google Scholar]
- 72.Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8(6):752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 73.Tatsumi K, Takebayashi H, Manabe T, et al. Genetic fate mapping of Olig2 progenitors in the injured adult cerebral cortex reveals preferential differentiation into astrocytes. J Neurosci Res. 2008;86(16):3494–3502. doi: 10.1002/jnr.21862. [DOI] [PubMed] [Google Scholar]
- 74.Richardson WD, Young KM, Tripathi RB, McKenzie I. NG2-glia as multipotent neural stem cells: fact or fantasy? Neuron. 2011;70(4):661–673. doi: 10.1016/j.neuron.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68(4):668–681. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135(1):145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- 77.Zhu X, Hill RA, Dietrich D, Komitova M, Suzuki R, Nishiyama A. Age-dependent fate and lineage restriction of single NG2 cells. Development. 2011;138(4):745–753. doi: 10.1242/dev.047951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burns KA, Murphy B, Danzer SC, Kuan CY. Developmental and post-injury cortical gliogenesis: a genetic fate-mapping study with Nestin-CreER mice. Glia. 2009;57(10):1115–1129. doi: 10.1002/glia.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Crouch EE, Liu C, Silva-Vargas V, Doetsch F. Regional and stage-specific effects of prospectively purified vascular cells on the adult V-SVZ neural stem cell lineage. J Neurosci. 2015;35(11):4528–4539. doi: 10.1523/JNEUROSCI.1188-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bayraktar OA, Fuentealba LC, Alvarez-Buylla A, Rowitch DH. Astrocyte development and heterogeneity. In: Barres BA, Freeman MR, Stevens B, editors. Glia. New York, NY: Cold Spring Harbor Laboratory Press; 2015. pp. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci U S A. 1999;96(20):11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6):703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 83.Lim DA, Alvarez-Buylla A. Adult neural stem cells stake their ground. Trends Neurosci. 2014;37(10):563–571. doi: 10.1016/j.tins.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124(3):319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 85.Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137(4):433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- 86.Li L, Harms KM, Ventura PB, Lagace DC, Eisch AJ, Cunningham LA. Focal cerebral ischemia induces a multilineage cytogenic response from adult subventricular zone that is predominantly gliogenic. Glia. 2010;58(13):1610–1619. doi: 10.1002/glia.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21(18):7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sanai N, Nguyen T, Ihrie RA, et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478(7369):382–386. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koob AO, Harris BT, Duhaime AC. Cellular genesis in the postnatal piglet. Int J Dev Neurosci. 2008;26(6):641–646. doi: 10.1016/j.ijdevneu.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 90.Holmin S, Almqvist P, Lendahl U, Mathiesen T. Adult nestin-expressing sub-ependymal cells differentiate to astrocytes in response to brain injury. Eur J Neurosci. 1997;9(1):65–75. doi: 10.1111/j.1460-9568.1997.tb01354.x. [DOI] [PubMed] [Google Scholar]
- 91.Sohn J, Orosco L, Guo F, et al. The subventricular zone continues to generate corpus callosum and rostral migratory stream astroglia in normal adult mice. J Neurosci. 2015;35(9):3756–3763. doi: 10.1523/JNEUROSCI.3454-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Benner EJ, Luciano D, Jo R, et al. Protective astrogenesis from the SVZ niche after injury is controlled by Notch modulator Thbs4. Nature. 2013;497(7449):369–373. doi: 10.1038/nature12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kronenberg G, Wang LP, Geraerts M, et al. Local origin and activity-dependent generation of nestin-expressing protoplasmic astrocytes in CA1. Brain Struct Funct. 2007;212(1):19–35. doi: 10.1007/s00429-007-0141-5. [DOI] [PubMed] [Google Scholar]
- 94.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Capilla-Gonzalez V, Cebrian-Silla A, Guerrero-Cazares H, Garcia-Verdugo JM, Quinones-Hinojosa A. Age-related changes in astrocytic and ependymal cells of the subventricular zone. Glia. 2014;62(5):790–803. doi: 10.1002/glia.22642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chirumamilla S, Sun D, Bullock MR, Colello RJ. Traumatic brain injury induced cell proliferation in the adult mammalian central nervous system. J Neurotrauma. 2002;19(6):693–703. doi: 10.1089/08977150260139084. [DOI] [PubMed] [Google Scholar]
- 97.Ge WP, Miyawaki A, Gage FH, Jan YN, Jan LY. Local generation of glia is a major astrocyte source in postnatal cortex. Nature. 2012;484(7394):376–380. doi: 10.1038/nature10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schechter R, Yen SH, Terry RD. Fibrous Astrocytes in senile dementia of the Alzheimer type. J Neuropathol Exp Neurol. 1981;40(2):95–101. doi: 10.1097/00005072-198103000-00002. [DOI] [PubMed] [Google Scholar]
- 99.Salminen A, Ojala J, Kaarniranta K, Haapasalo A, Hiltunen M, Soininen H. Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. Eur J Neurosci. 2011;34(1):3–11. doi: 10.1111/j.1460-9568.2011.07738.x. [DOI] [PubMed] [Google Scholar]
- 100.Wang SM, Lee YC, Ko CY, et al. Increase of zinc finger protein 179 in response to CCAAT/enhancer binding protein delta conferring an antiapoptotic effect in astrocytes of Alzheimer’s disease. Mol Neurobiol. 2015;51(1):370–382. doi: 10.1007/s12035-014-8714-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Peinado MA, Quesada A, Pedrosa JA, et al. Quantitative and ultrastructural changes in glia and pericytes in the parietal cortex of the aging rat. Microsc Res Tech. 1998;43(1):34–42. doi: 10.1002/(SICI)1097-0029(19981001)43:1<34::AID-JEMT6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 102.Boda E, Buffo A. Glial cells in non-germinal territories: insights into their stem/progenitor properties in the intact and injured nervous tissue. Arch Ital Biol. 2010;148(2):119–136. [PubMed] [Google Scholar]
- 103.Hansen LA, Armstrong DM, Terry RD. An immunohistochemical quantification of fibrous astrocytes in the aging human cerebral cortex. Neurobiol Aging. 1987;8(1):1–6. doi: 10.1016/0197-4580(87)90051-0. [DOI] [PubMed] [Google Scholar]
- 104.Rozovsky I, Finch CE, Morgan TE. Age-related activation of microglia and astrocytes: in vitro studies show persistent phenotypes of aging, increased proliferation, and resistance to down-regulation. Neurobiol Aging. 1998;19(1):97–103. doi: 10.1016/s0197-4580(97)00169-3. [DOI] [PubMed] [Google Scholar]
- 105.Rodriguez JJ, Yeh CY, Terzieva S, Olabarria M, Kulijewicz-Nawrot M, Verkhratsky A. Complex and region-specific changes in astroglial markers in the aging brain. Neurobiol Aging. 2014;35(1):15–23. doi: 10.1016/j.neurobiolaging.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 106.Fabricius K, Jacobsen JS, Pakkenberg B. Effect of age on neocortical brain cells in 90+ year old human females—a cell counting study. Neurobiol Aging. 2013;34(1):91–99. doi: 10.1016/j.neurobiolaging.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 107.Pelvig DP, Pakkenberg H, Stark AK, Pakkenberg B. Neocortical glial cell numbers in human brains. Neurobiol Aging. 2008;29(11):1754–1762. doi: 10.1016/j.neurobiolaging.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 108.Vaughan DW, Peters A. Neuroglial cells in the cerebral cortex of rats from young adulthood to old age: an electron microscope study. J Neurocytol. 1974;3(4):405–429. doi: 10.1007/BF01098730. [DOI] [PubMed] [Google Scholar]
- 109.Peinado MA, Quesada A, Pedrosa JA, et al. Light microscopic quantification of morphological changes during aging in neurons and glia of the rat parietal cortex. Anat Rec. 1997;247(3):420–425. doi: 10.1002/(SICI)1097-0185(199703)247:3<420::AID-AR14>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 110.Bhardwaj RD, Curtis MA, Spalding KL, et al. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci U S A. 2006;103(33):12564–12568. doi: 10.1073/pnas.0605177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Peters A, Leahu D, Moss MB, McNally KJ. The effects of aging on area 46 of the frontal cortex of the rhesus monkey. Cereb Cortex. 1994;4(6):621–635. doi: 10.1093/cercor/4.6.621. [DOI] [PubMed] [Google Scholar]
- 112.Peters A, Josephson K, Vincent SL. Effects of aging on the neuroglial cells and pericytes within area 17 of the rhesus monkey cerebral cortex. Anat Rec. 1991;229(3):384–398. doi: 10.1002/ar.1092290311. [DOI] [PubMed] [Google Scholar]
- 113.Cotrina ML, Nedergaard M. Astrocytes in the aging brain. J Neurosci Res. 2002;67(1):1–10. doi: 10.1002/jnr.10121. [DOI] [PubMed] [Google Scholar]
- 114.Altman J, Das GD. Autoradiographic examination of the effects of enriched environment on the rate of glial multiplication in the adult rat brain. Nature. 1964;204:1161–1163. doi: 10.1038/2041161a0. [DOI] [PubMed] [Google Scholar]
- 115.Steiner B, Kronenberg G, Jessberger S, Brandt MD, Reuter K, Kempermann G. Differential regulation of gliogenesis in the context of adult hippocampal neurogenesis in mice. Glia. 2004;46(1):41–52. doi: 10.1002/glia.10337. [DOI] [PubMed] [Google Scholar]
- 116.Ihrie RA, Alvarez-Buylla A. Cells in the astroglial lineage are neural stem cells. Cell Tissue Res. 2008;331(1):179–191. doi: 10.1007/s00441-007-0461-z. [DOI] [PubMed] [Google Scholar]
- 117.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 118.Sampedro-Piquero P, De Bartolo P, Petrosini L, Zancada-Menendez C, Arias JL, Begega A. Astrocytic plasticity as a possible mediator of the cognitive improvements after environmental enrichment in aged rats. Neurobiol Learn Mem. 2014;114:16–25. doi: 10.1016/j.nlm.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 119.Ehninger D, Kempermann G. Regional effects of wheel running and environmental enrichment on cell genesis and microglia proliferation in the adult murine neocortex. Cereb Cortex. 2003;13(8):845–851. doi: 10.1093/cercor/13.8.845. [DOI] [PubMed] [Google Scholar]
- 120.Rapanelli M, Frick LR, Zanutto BS. Learning an operant conditioning task differentially induces gliogenesis in the medial prefrontal cortex and neurogenesis in the hippocampus. PLoS One. 2011;6(2):e14713. doi: 10.1371/journal.pone.0014713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mandyam CD, Wee S, Eisch AJ, Richardson HN, Koob GF. Methamphetamine self-administration and voluntary exercise have opposing effects on medial pre-frontal cortex gliogenesis. J Neurosci. 2007;27(42):11442–11450. doi: 10.1523/JNEUROSCI.2505-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brennan KC, Beltran-Parrazal L, Lopez-Valdes HE, Theriot J, Toga AW, Charles AC. Distinct vascular conduction with cortical spreading depression. J Neurophysiol. 2007;97(6):4143–4151. doi: 10.1152/jn.00028.2007. [DOI] [PubMed] [Google Scholar]
- 123.Urbach A, Bruehl C, Witte OW. Microarray-based long-term detection of genes differentially expressed after cortical spreading depression. Eur J Neurosci. 2006;24(3):841–856. doi: 10.1111/j.1460-9568.2006.04862.x. [DOI] [PubMed] [Google Scholar]
- 124.Holmin S, von Gertten C, Sandberg-Nordqvist AC, Lendahl U, Mathiesen T. Induction of astrocytic nestin expression by depolarization in rats. Neurosci Lett. 2001;314(3):151–155. doi: 10.1016/s0304-3940(01)02292-3. [DOI] [PubMed] [Google Scholar]
- 125.Kraig RP, Dong LM, Thisted R, Jaeger CB. Spreading depression increases immunohistochemical staining of glial fibrillary acidic protein. J Neurosci. 1991;11(7):2187–2198. doi: 10.1523/JNEUROSCI.11-07-02187.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xue JH, Yanamoto H, Nakajo Y, et al. Induced spreading depression evokes cell division of astrocytes in the subpial zone, generating neural precursor-like cells and new immature neurons in the adult cerebral cortex. Stroke. 2009;40(11):e606–e613. doi: 10.1161/STROKEAHA.109.560334. [DOI] [PubMed] [Google Scholar]
- 127.Tamura Y, Eguchi A, Jin G, Sami MM, Kataoka Y. Cortical spreading depression shifts cell fate determination of progenitor cells in the adult cortex. J Cereb Blood Flow Metab. 2012;32(10):1879–1887. doi: 10.1038/jcbfm.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Urbach A, Brueckner J, Witte OW. Cortical spreading depolarization stimulates gliogenesis in the rat entorhinal cortex. J Cereb Blood Flow Metab. 2015;35(4):576–582. doi: 10.1038/jcbfm.2014.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vassar R, Bennett BD, Babu-Khan S, et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286(5440):735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 130.Näslund J, Haroutunian V, Mohs R, et al. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA. 2000;283(12):1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- 131.Lee IS, Jung K, Kim IS, Park KI. Amyloid-beta oligomers regulate the properties of human neural stem cells through GSK-3beta signaling. Exp Mol Med. 2013;45:e60. doi: 10.1038/emm.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hu X, He W, Luo X, Tsubota KE, Yan R. BACE1 regulates hippocampal astrogenesis via the Jagged1-Notch pathway. Cell Rep. 2013;4(1):40–49. doi: 10.1016/j.celrep.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Casal C, Serratosa J, Tusell JM. Effects of beta-AP peptides on activation of the transcription factor NF-kappaB and in cell proliferation in glial cell cultures. Neurosci Res. 2004;48(3):315–323. doi: 10.1016/j.neures.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 134.Lim D, Ronco V, Grolla AA, Verkhratsky A, Genazzani AA. Glial calcium signalling in Alzheimer’s disease. Rev Physiol Biochem Pharmacol. 2014;167:45–65. doi: 10.1007/112_2014_19. [DOI] [PubMed] [Google Scholar]
- 135.Chow SK, Yu D, Macdonald CL, Buibas M, Silva GA. Amyloid beta-peptide directly induces spontaneous calcium transients, delayed intercellular calcium waves and gliosis in rat cortical astrocytes. ASN Neuro. 2010;2(1):e00026. doi: 10.1042/AN20090035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Trazzi S, Fuchs C, Valli E, Perini G, Bartesaghi R, Ciani E. The amyloid precursor protein (APP) triplicated gene impairs neuronal precursor differentiation and neurite development through two different domains in the Ts65Dn mouse model for Down syndrome. J Biol Chem. 2013;288(29):20817–20829. doi: 10.1074/jbc.M113.451088. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 137.Kamphuis W, Kooijman L, Orre M, Stassen O, Pekny M, Hol EM. GFAP and vimentin deficiency alters gene expression in astrocytes and microglia in wild-type mice and changes the transcriptional response of reactive glia in mouse model for Alzheimer’s disease. Glia. 2015;63(6):1036–1056. doi: 10.1002/glia.22800. [DOI] [PubMed] [Google Scholar]
- 138.Mathur R, Ince PG, Minett T, MRC Cognitive Function and Ageing Neuropathology Study Group et al. A reduced astrocyte response to beta-amyloid plaques in the ageing brain associates with cognitive impairment. PLoS One. 2015;10(2):e0118463. doi: 10.1371/journal.pone.0118463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mahley RW, Weisgraber KH, Huang YD. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology including Alzheimer’s disease. Proc Natl Acad Sci U S A. 2006;103(15):5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pitas RE, Boyles JK, Lee SH, Foss D, Mahley RW. Astrocytes synthesize apolipoprotein-E and metabolize apolipoprotein E-containing lipoproteins. Biochim Biophys Acta. 1987;917(1):148–161. doi: 10.1016/0005-2760(87)90295-5. [DOI] [PubMed] [Google Scholar]
- 141.Pfrieger FW. Role of cholesterol in synapse formation and function. Biochim Biophys Acta. 2003;1610(2):271–280. doi: 10.1016/s0005-2736(03)00024-5. [DOI] [PubMed] [Google Scholar]
- 142.Luccarini I, Grossi C, Traini C, Fiorentini A, Dami T, Casamenti F, editors. Abeta plaque-associated glial reaction as a determinant of apoptotic neuronal death and cortical gliogenesis: a study in APP mutant mice. Neurosci Lett. 2012;506(1):94–99. doi: 10.1016/j.neulet.2011.10.056. [DOI] [PubMed] [Google Scholar]
- 143.Kamphuis W, Orre M, Kooijman L, Dahmen M, Hol EM. Differential cell proliferation in the cortex of the APPswePS1dE9 Alzheimer’s disease mouse model. Glia. 2012;60(4):615–629. doi: 10.1002/glia.22295. [DOI] [PubMed] [Google Scholar]
- 144.Sirko S, Behrendt G, Johansson PA, et al. Reactive glia in the injured brain acquire stem cell properties in response to sonic hedgehog. [Corrected] Cell Stem Cell. 2013;12(4):426–439. doi: 10.1016/j.stem.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 145.Verkhratsky A, Marutle A, Rodriguez-Arellano JJ, Nordberg A. Glial asthenia and functional paralysis: a new perspective on neurodegeneration and Alzheimer’s Disease. Neuroscientist. 2014;21(5):562–568. doi: 10.1177/1073858414547132. [DOI] [PubMed] [Google Scholar]
- 146.Yeh CY, Vadhwana B, Verkhratsky A, Rodriguez JJ. Early astrocytic atrophy in the entorhinal cortex of a triple transgenic animal model of Alzheimer’s disease. ASN Neuro. 2011;3(5):271–279. doi: 10.1042/AN20110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kulijewicz-Nawrot M, Verkhratsky A, Chvatal A, Sykova E, Rodriguez JJ. Astrocytic cytoskeletal atrophy in the medial prefrontal cortex of a triple transgenic mouse model of Alzheimer’s disease. J Anat. 2012;221(3):252–262. doi: 10.1111/j.1469-7580.2012.01536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee VMY, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 149.Broe M, Kril J, Halliday GM. Astrocytic degeneration relates to the severity of disease in frontotemporal dementia. Brain. 2004;127(pt 10):2214–2220. doi: 10.1093/brain/awh250. [DOI] [PubMed] [Google Scholar]
- 150.Forman MS, Lal D, Zhang B, et al. Transgenic mouse model of tau pathology in astrocytes leading to nervous system degeneration. J Neurosci. 2005;25(14):3539–3550. doi: 10.1523/JNEUROSCI.0081-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dabir DV, Robinson MB, Swanson E, et al. Impaired glutamate transport in a mouse model of tau pathology in astrocytes. J Neurosci. 2006;26(2):644–654. doi: 10.1523/JNEUROSCI.3861-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shibuya K, Yagishita S, Nakamura A, Uchihara T. Perivascular orientation of astrocytic plaques and tuft-shaped astrocytes. Brain Res. 2011;1404:50–54. doi: 10.1016/j.brainres.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 153.Attems J, Jellinger KA. The overlap between vascular disease and Alzheimer’s disease—lessons from pathology. BMC Med. 2014;12:206. doi: 10.1186/s12916-014-0206-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Raina AK, Zhu X, Smith MA. Alzheimer’s disease and the cell cycle. Acta Neurobiol Exp. 2004;64(1):107–112. doi: 10.55782/ane-2004-1496. [DOI] [PubMed] [Google Scholar]
- 155.Yurov YB, Vorsanova SG, Iourov IY. The DNA replication stress hypothesis of Alzheimer’s disease. Scientific World Journal. 2011;11:2602–2612. doi: 10.1100/2011/625690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Moh C, Kubiak JZ, Bajic VP, Zhu X, Smith MA, Lee HG. Cell cycle deregulation in the neurons of Alzheimer’s disease. Results Probl Cell Differ. 2011;53:565–576. doi: 10.1007/978-3-642-19065-0_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 158.Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci Lett. 1998;251(3):205–208. doi: 10.1016/s0304-3940(98)00504-7. [DOI] [PubMed] [Google Scholar]
- 159.Wakabayashi K, Matsumoto K, Takayama K, Yoshimoto M, Takahashi H. NACP, a presynaptic protein, immunoreactivity in Lewy bodies in Parkinson’s disease. Neurosci Lett. 1997;239(1):45–48. doi: 10.1016/s0304-3940(97)00891-4. [DOI] [PubMed] [Google Scholar]
- 160.Clayton DF, George JM. The synucleins: a family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci. 1998;21(6):249–254. doi: 10.1016/s0166-2236(97)01213-7. [DOI] [PubMed] [Google Scholar]
- 161.Iwai A, Masliah E, Yoshimoto M, et al. The precursor protein of non-A-beta component of Alzheimer’s-disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14(2):467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- 162.Jakes R, Spillantini MG, Goedert M. Identification of two distinct synucleins from human brain. FEBS Lett. 1994;345(1):27–32. doi: 10.1016/0014-5793(94)00395-5. [DOI] [PubMed] [Google Scholar]
- 163.Lee HJ, Bae EJ, Lee SJ. Extracellular alpha—synuclein-a novel and crucial factor in Lewy body diseases. Nat Rev Neurol. 2014;10(2):92–98. doi: 10.1038/nrneurol.2013.275. [DOI] [PubMed] [Google Scholar]
- 164.Koob AO, Shaked GM, Bender A, Bisquertt A, Rockenstein E, Masliah E. Neurogranin binds alpha-synuclein in the human superior temporal cortex and interaction is decreased in Parkinson’s disease. Brain Res. 2014;1591:102–110. doi: 10.1016/j.brainres.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Desplats P, Lee HJ, Bae EJ, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106(31):13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lee HJ, Suk JE, Patrick C, et al. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010;285(12):9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Koob AO, Paulino AD, Masliah E. GFAP reactivity, apolipoprotein E redistribution and cholesterol reduction in human astrocytes treated with alpha-synuclein. Neurosci Lett. 2010;469(1):11–14. doi: 10.1016/j.neulet.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Braak H, Sastre M, Del Tredici K. Development of alpha-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta Neuropathol. 2007;114(3):231–241. doi: 10.1007/s00401-007-0244-3. [DOI] [PubMed] [Google Scholar]
- 169.Fujiwara H, Hasegawa M, Dohmae N, et al. Alpha-synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4(2):160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 170.Kim KS, Kim JS, Park JY, et al. DJ-1 associates with lipid rafts by palmitoylation and regulates lipid rafts-dependent endocytosis in astrocytes. Hum Mol Genet. 2013;22(23):4805–4817. doi: 10.1093/hmg/ddt332. [DOI] [PubMed] [Google Scholar]
- 171.Bandopadhyay R, Kingsbury AE, Cookson MR, et al. The expression of DJ-1 (PARK7) in normal human CNS and idiopathic Parkinson’s disease. Brain. 2004;127:420–430. doi: 10.1093/brain/awh054. [DOI] [PubMed] [Google Scholar]
- 172.O’Flanagan CH, O’Neill C. PINK1 signalling in cancer biology. Biochim Biophys Acta. 2014;1846(2):590–598. doi: 10.1016/j.bbcan.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 173.Tong J, Ang LC, Williams B, et al. Low levels of astroglial markers in Parkinson’s disease: relationship to alpha-synuclein accumulation. Neurobiol Dis. 2015;82:243–253. doi: 10.1016/j.nbd.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Olsson B, Constantinescu R, Holmberg B, Andreasen N, Blennow K, Zetterberg H. The glial marker YKL-40 is decreased in synucleinopathies. Mov Disord. 2013;28(13):1882–1885. doi: 10.1002/mds.25589. [DOI] [PubMed] [Google Scholar]
- 175.Radford R, Rcom-H’cheo-Gauthier A, Wong MB, et al. The degree of astrocyte activation in multiple system atrophy is inversely proportional to the distance to alpha-synuclein inclusions. Mol Cell Neurosci. 2015;65:68–81. doi: 10.1016/j.mcn.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 176.van den Berge SA, Kevenaar JT, Sluijs JA, Hol EM. Dementia in Parkinson’s disease correlates with alpha-Synuclein pathology but not with cortical astrogliosis. Parkinsons Dis. 2012;2012:420957. doi: 10.1155/2012/420957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.George JM, Jin H, Woods WS, Clayton DF. Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron. 1995;15(2):361–372. doi: 10.1016/0896-6273(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 178.Halliday GM, Stevens CH. Glia: initiators and progressors of pathology in Parkinson’s disease. Mov Disord. 2011;26(1):6–17. doi: 10.1002/mds.23455. [DOI] [PubMed] [Google Scholar]
- 179.Gu XL, Long CX, Sun L, Xie C, Lin X, Cai H. Astrocytic expression of Parkinson’s disease-related A53T alpha-synuclein causes neurodegeneration in mice. Mol Brain. 2010;3:12. doi: 10.1186/1756-6606-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Surgucheva I, Newell KL, Burns J, Surguchov A. New alpha- and gamma-synuclein immunopathological lesions in human brain. Acta Neuropathol Commun. 2014;2:132. doi: 10.1186/s40478-014-0132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fung KM, Rorke LB, Giasson B, Lee VM, Trojanowski JQ. Expression of alpha-, beta-, and gamma-synuclein in glial tumors and medulloblastomas. Acta Neuropathol. 2003;106(2):167–175. doi: 10.1007/s00401-003-0718-x. [DOI] [PubMed] [Google Scholar]
- 182.Surguchov A, Palazzo RE, Surgucheva I. Gamma synuclein: subcellular localization in neuronal and non-neuronal cells and effect on signal transduction. Cell Motil Cytoskeleton. 2001;49(4):218–228. doi: 10.1002/cm.1035. [DOI] [PubMed] [Google Scholar]
- 183.Mukaetova-Ladinska EB, Milne J, Andras A, et al. Alpha- and gamma-synuclein proteins are present in cerebrospinal fluid and are increased in aged subjects with neurodegenerative and vascular changes. Dement Geriatr Cogn Disord. 2008;26(1):32–42. doi: 10.1159/000141039. [DOI] [PubMed] [Google Scholar]
- 184.Ninkina N, Peters O, Millership S, Salem H, van der Putten H, Buchman VL. Gamma-synucleinopathy: neurodegeneration associated with overexpression of the mouse protein. Hum Mol Genet. 2009;18(10):1779–1794. doi: 10.1093/hmg/ddp090. [DOI] [PMC free article] [PubMed] [Google Scholar]


