Abstract
Breast cancer mortality is usually due to distant recurrence of cancer at an advanced stage of the disease rather than from primary cancer. Therefore, prediction of breast cancer recurrence at the time of diagnosis could lead to advances in personalized treatment of cancer patients in order to prevent risk of recurrence. Two prognostic biomarkers that are currently being used in clinical practice are a 70-gene MammaPrint® signature and a 21-gene Oncotype DX® panel. These assays generate relative risks of recurrence, but they do not provide a ‘yes’ or ‘no’ answer about recurrence in a given patient. These tests include genes that are involved in the cell cycle, invasion, metastasis and angiogenesis related to breast cancer. Emerging evidence suggests that a signature of genes involved in tumor-immune interactions may provide a more accurate prognostic tool. This paper reviews recent advances in the discovery of prognostic biomarkers for breast cancer patients.
Keywords: breast cancer recurrence, cancer testis antigens, HER2, immune function genes, MammaPrint®, myeloid-derived suppressor cells, Oncotype DX®, prognostic biomarkers
Patients with early-stage breast cancer die from tumor relapse rather than from primary cancer. Therefore, prognostic biomarkers and predictors of response to therapy are urgently needed to reduce the incidence of tumor relapse and advanced breast cancer. This review highlights the current developments in the discovery of biomarkers and predictors of response to therapy for breast cancer patients. This will include different subtypes of breast cancer based on the status of hormone receptors, HER2 status, nodal status and status of proliferation and invasion, as well as immune cell infiltrates.
Breast cancer biological subtypes & histological grading can predict risk of tumor recurrence
HER2, estrogen receptor & progesterone receptor
It has been reported that amplification of the HER2 oncogene in 25% of breast cancers is associated with a shorter disease-free and overall survival. Carr et al. conducted retrospective studies on 190 breast cancer patients and showed that HER2 was an independent prognostic biomarker, regardless of tumor grade, estrogen receptor (ER)/progesterone receptor (PR) status and lymph node metastasis [1]. They showed that patients with HER2-positive (HER2+) tumor had a shorter median disease-free interval (22 months) compared with controls (40 months). However, more frequent expression of HER2 in high-grade ductal carcinoma in situ (DCIS) lesions compared with invasive breast cancer raised questions as to the validity of HER2 amplification as an independent prognostic biomarker. Such discordance was addressed by showing that HER2 overexpression in DCIS was associated with an increased risk of rapid progression to invasive disease (2). In fact, invasive foci were detected in HER2+ DCIS at a higher frequency than in HER2-negative (HER2−) DCIS. Such discordance was also addressed by testing a hypothesis that HER2+ DCIS is at a greater risk of progression to HER2− invasive carcinoma under immune pressure. For instance, it was reported that relapse of neu− invasive mammary carcinoma can occur following T-cell-mediated rejection of the neu+ mammary carcinoma (3,4). In addition, presence of the HER2-specific T-cell responses was detected in patients with HER2− breast cancer, and such immune responses were associated with nuclear translocation of IFN-γRα. in the tumor lesions [5]. The authors suggested that presence of HER2-reactive T cells in the absence of the antigen was indicative of HER2+ DCIS in the past and its progression to HER2− breast cancer due to T-cell-mediated HER2 loss. This hypothesis was also supported by the observations showing that HER2-targeted vaccination of DCIS patients resulted in the regression of DCIS lesions as well as HER2 loss following the induction of HER2-specific T-cell responses [6]. Similar observations were made in breast cancer patients whose tumor lost HER2 expression following trastuzumab-based neoadjuvant therapy [7]. However, these reports brought the immune cells into the picture, which further challenged the status of HER2 as an independent prognostic biomarker. In addition, patients with HER2+ but node-negative (node−) breast cancer had a low risk of recurrence at 5-year follow-up whereas those with ER-positive (ER+)/PR-positive breast cancer and HER2 overexpression had a worse disease-free survival [8]. Overall, the prognostic impact of HER2-positivity was lower in node− compared with node-positive (node+) patients. These findings led to the use of multiple tumor biomarkers for breast cancer prognosis, ER/PR status in particular. For instance, patients with triple-negative (ER-negative [ER−]/PR-negative/HER2−) breast cancer are at greater risk of relapse with a one- to three-times greater risk following initial treatment compared with patients who had other subtypes of breast cancer, including patients with HER2+ tumors [9].
Genomic grade index
Histological grading of the tumor is another factor that contributes to predicting risk of breast cancer recurrence. In order to determine whether gene expression profiling can grade tumors more accurately than the conventional histological grade, the genomic grade index (GGI) was developed using microarray analysis [10]. The GGI includes 97 genes that are associated with tumor grade and breast cancer subtypes, and can define patient prognosis and predict chemotherapy sensitivity [11–15]. According to this assay, luminal A and normal-like breast cancer subtypes are categorized as low GGI, whereas HER2, basal-like and luminal B breast cancer subtypes and the subgroup of tumors previously unclassified are categorized as high GGI [16]. In fact, the prognostic value of the genes involved in tumor cell proliferation is better evaluated with GGI than with classic histological grade.
PAM50™
The PAM50 is a quantitative reverse transcriptase-PCR-based assay that measures the expression of 50 genes and five control genes to identify breast cancer subtypes known as luminal A, luminal B, HER2-enriched and basal-like tumors. A comparative analysis of Oncotype DX® and PAM50 showed that high-risk specimens identified by Oncotype DX corresponded to luminal B or basal-like tumors identified by PAM50. In addition, 83% of low-risk specimens were found to be luminal A tumors. In the intermediate-score group, 53 specimens (approximately half) were classified as luminal A, a lower risk of recurrence compared with luminal B, 13 were classified luminal B and another nine fell into two other groups, HER2-enriched and normal-like breast cancers [17]. Although PAM50 gives more prognostic information than clinical factors and immunohistochemistry (IHC), overdependence of PAM50 on proliferation-related genes reduces its accuracy as a standalone prognostic biomarker. Normal tissue or benign tumor tissue sampled concurrently with a malignant tumor is a significant source of bias in the PAM50 genomic predictor [18–21].
IHC4
The IHC4 test provides a prognostic score based on the expression of four IHC markers (ER, PR, HER2 and Ki-67) in tumor biopsy specimens. This assay was validated in ER+ breast cancer patients [22] to identify patients with low risk of relapse who will not benefit from chemotherapy. However, variations in IHC scores reduce the reproducibility of this assay.
A complex of genes expressed by tumor cells & stromal cells can predict breast cancer relapse
An advance in our understanding of the tumor microenvironment led to the development of molecular biomarkers of breast cancer prognosis by focusing on genes that are involved in cell cycle, invasion, metastasis and angiogenesis of breast cancers. This approach stems from the observations showing that intratumor genetic heterogeneity is a key mechanism involved in tumor progression and response to treatment [23]. In addition, clinical studies suggested that a stromal gene expression profile may also predict breast cancer outcome. This knowledge led to the development of two prognostic biomarkers, a 70-gene MammaPrint® signature and a 21-gene Oncotype DX panel, which are currently being used in clinical practice.
70-gene MammaPrint signature
The 70-gene MammaPrint signature (Agendia BV, Amsterdam, The Netherlands) is a fully commercialized microarray-based assay for breast cancer prognosis that requires either fresh-frozen tumor specimens or tissues collected into an RNA preservative solution. This assay has been US FDA-cleared for use in lymph node− and ER+ or ER− breast cancer patients with tumors smaller than 5 cm. It is also being extended to node+ patients [24,25]. In 2007, Agendia received a second clearance from the FDA for a sampling and room temperature shipping procedure using a RNA preserving solution (RNARetain®). The MammaPrint signature is focused on genes associated with cell proliferation, invasion, metastasis, stromal integrity and angiogenesis. The test measures the level of expression of 70 genes in tumor specimens and then uses a specific formula or algorithm to produce a score that determines high risk or low risk of recurrence, without an intermediate-risk group. Each microarray contains three identical sets of the 70 genes to be analyzed. Normalization genes and negative-control genes are also present on each microarray. A low-risk MammaPrint result means a 10% risk of tumor relapse within 10 years without any additional adjuvant treatment. A high-risk MammaPrint result means a 29% chance of tumor relapse within 10 years without any additional adjuvant treatment [26]. Comparative analysis of data obtained from patients with node+ and node− breast cancer showed a similar prognostic value for the MammaPrint test [25].
The MammaPrint assay is largely a prognostic, rather than predictive, assay. However, recent reports suggest a predictive value for the MammaPrint signature [27]. Patients who were identified as high risk by the MammaPrint test and received adjuvant chemotherapy showed an improved relapse-free survival such that only 6% of patients developed tumor relapse within a 5-year follow-up [28]. In fact, adjuvant chemotherapy improved relapse-free survival in 20.7% of the high-risk group. The MINDACT trial is underway to assess the predictive capability of the MammaPrint assay [27]. Node− or node+ patients are eligible for the trial regardless of ER status. Patients who are classified as high risk by using standard clinicopathological factors and MammaPrint receive chemotherapy, while those identified as low risk by both methods receive hormonal therapy as appropriate. Any patients with discordance between standard criteria and the MammaPrint assay will randomly receive either adjuvant chemotherapy or hormonal therapy as clinically appropriate.
The main advantage of MammaPrint is in its ability to identify patients who could be spared unnecessary adjuvant therapy in the low-risk group showing a less-than-10% risk of recurrence within a minimum of 5 years. The 70-gene signature also provides additional information that can help refine the prognostic value of traditional markers. For example, the MammaPrint assay has identified a subgroup of early HER2+ breast cancer with a favorable long-term outcome [29].
21-gene Oncotype DX panel
Oncotype DX is an assay that employs quantitative reverse transcriptase-PCR, using formalin-fixed paraffin-embedded tumor specimens, to determine the expression of a panel of 21 genes, primarily in ER+, lymph node− patients. The Oncotype DX panel includes 16 cancer-related genes, such as groups related to hormone receptors (ER, PR, BCL2 and SCUBE2), proliferation (Ki-67, STK15, SURVIVIN, CYCLIN B1 and MYBL2), invasion (STRMELYSIN3 and CATHEPSIN L2), HER2 (HER2 and GRB7), the macrophage marker CD68, the antiapoptosis gene BAG1 and GSTM1, as well as five reference ‘housekeeping’ genes. A scoring algorithm is used to weigh them relatively with the heaviest weighting for ER- and proliferation-related genes in order to develop a recurrence score. The method applies a Kaplan–Meier estimate to show that the proportion of patients in the low-risk group free of a distant recurrence at 10 years was significantly greater than the proportion of patients identified as high-risk. Multivariate Cox proportional analysis of age, tumor size and recurrence score in relation to the likelihood of distant recurrence were also performed. There are three risk levels based on the recurrence score on a scale of 0–100, which are low risk (<18), intermediate risk (18–30) and high risk (≥31). Oncotype DX was tested prospectively in the NSABP Trial B-14, comprising 2644 patients with ER+, histological node− tumors [30]. Patients were randomized to receive either tamoxifen or placebo. In tamoxifen-treated patients, the 21-gene signature revealed a 5-year recurrence rate of 22.1% for patients with the high-risk score, compared with 2.1% for those with the low-risk score, and 30.5 and 6.8% at 10 years, respectively. Patients with intermediate risk showed a recurrence rate of 14.3% at 10 years.
A retrospective case–control study [31] identified Oncotype DX as a predictive biomarker for hormonal therapy in the NSABP B-14 trial as well as for chemotherapy in the NSABP trial B-20 [32]. The trial showed a large benefit of chemotherapy for patients with a high recurrence score, and minimal benefit for those with a low score. There was no clear-cut benefit for patients with an intermediate recurrence score. The 10-year relapse-free survival was improved from 60 to 88% by adding chemotherapy to hormone therapy in the high-risk group [33]. In fact, adjuvant chemotherapy improved relapse-free survival in 30% of the high-risk group. Further analysis revealed that the 21-gene signature was better than standard clinicopathological variables at predicting recurrence [34].
Oncotype DX is best suited for identifying breast cancers that are less likely to relapse (low-risk group). Despite being prognostic and predictive to chemotherapy, Oncotype DX still returns 40–66% of cases as intermediate risk [35] with no clear data to suggest a benefit of chemotherapy. However, preliminary data suggest that addition of standard clinicopathological variables to the Oncotype DX recurrence score can help reduce the number of cases that fall into the intermediate-risk group.
Both MammaPrint and Oncotype DX panels determine probability of tumor relapse in a given patient. For example, a patient in the high-risk group has approximately a 30% risk of recurrence (29% risk by MammaPrint) or approximately a 70% chance of relapse-free survival, and a patient in the intermediate-risk group has approximately a 14.3% risk of recurrence within 5–10 years. Development of a prognostic biomarker that can identify high-risk and low-risk groups by providing ‘yes’ or ‘no’ answers about tumor relapse would significantly improve management of the disease. Genetic instability and plasticity of tumor cells facilitates constant changes in the tumor cells in response to the host defense system, making it difficult to develop a gold-standard prognostic biomarker that can accurately determine whether a patient would relapse following initial treatment. However, understanding a network of genes that are involved in the tumor–host interactions may dramatically improve our ability to provide ‘yes’ or ‘no’ answers about recurrence.
Recent reports show that manipulation of patient selection resulted in the generation of entirely different signatures that included only 20% of the MammaPrint genes in more than 50% of the patients and appearance of ten new genes that were absent in the MammaPrint gene signature [36]. In addition, 50% of patients who were grouped as intermediate risk by the Oncotype DX were classified as high risk by the 70-gene MammaPrint signature [36]. A comparative analysis of five published breast cancer gene signatures on the same dataset also revealed an outcome prediction agreement of 80% between Oncotype DX and MammaPrint signatures [37].
Stromal gene expression
Both MammaPrint and Oncotype DX panels have been derived from whole tissue consisting of tumor cells and the surrounding stroma. Samples with insufficient tumor cell content are generally excluded from such analyses. This would result in filtered analyses in which key components of the tumor microenvironment, such as tumor-associated stromal cells and infiltrating immune cells, are ignored. Under normal conditions stroma provides a barrier to epithelial cell transformation and growth. During transformation of epithelial cells, stroma undergoes changes that further facilitate cancer progression [38–40]. Tumor stroma is comprised of extracellular matrix and various cell types including fibroblasts, endothelial cells and infiltrating immune cells, which interact with malignant cells via paracrine, physical and hormonal pathways. Early studies showed that the normal mammary microenvironment is capable of converting the malignant cells by triggering differentiation of the cells [41,42]. These data suggest that malignant cells can only thrive in an abnormal microenvironment. To determine the prognostic value of stromal cells, Park and colleagues performed gene expression analysis on stromal cells isolated from breast tissues by laser capture microdissection. Such analysis showed that gene expression signatures derived from whole tumors were different from the signatures derived from the tumor stroma, with the latter showing a strong link to clinical outcome [43]. Tumor stroma from patients with good outcome showed overexpression of immune-related genes such as T-cell and natural killer cell markers with a skewed Th1 profile. By contrast, a diminished expression of the immune-related genes was evident in the tumor stroma from patients with poor outcome. These tumors, instead, showed expression of genes that were involved in hypoxic and angiogenic responses [43]. It was also reported that gene signatures of CD10+ stromal cells are associated with poor prognosis, particularly in HER2+ tumors of patients who were not treated [44]. The signature was also important in differentiating DCIS and associated invasive samples. Although this analysis requires laser capture microdissection of CD10+ cells, which is not a simple procedure for prognosis, it would suggest that tumor stroma should also be targeted in order to improve prognosis in patients with CD10+ tumor stromal signature. More recently, gene expression analyses were performed on stroma-poor fine-needle and stroma-rich core-needle biopsies from breast cancer patients to identify the prognostic value of stroma-associated genes [45]. The study showed that, among highly proliferative cancers (ER− or ER+), a subset of tumors with high expression of a B-cell/plasma cell metagene had a favorable prognosis. This immune function gene signature showed no prognostic value in poorly proliferating cancers. These findings suggest that infiltrating immune cells may have prognostic value in a subset of cancer patients with highly proliferating tumor cells who are at risk of tumor relapse following conventional therapies.
Mammostrat®
The Mammostrat test is a protein-based IHC assay of tumor specimens that employs a set of five biomarkers (SLC7A5, HTF9C, P53, NDRG1 and CEACAM5). These markers are independent of one another and do not directly measure proliferation or ER/PR expression. Therefore, histopathological information about the proliferation and ER/PR status of a tumor can be used for interpretation of Mammostrat results [46]. Increased Mammostrat scores are associated with poor prognosis in ER+ breast cancer patients.
Status of tumor-infiltrating immune cells can predict breast cancer relapse
Breast cancers often express ‘self’ antigens such as HER2 and MUC-1, to which cells of the immune system were tolerized during the thymic selection. However, numerous lines of evidence suggest that cells of the immune system are capable of recognizing and reacting with tumor cells [47]. Breast carcinomas are often infiltrated by inflammatory cells including macrophages and T cells. It was shown that tumor-infiltrating lymphocytes are an invariable feature of breast cancers, such that immune cells were present in all breast cancers examined [48]. In fact, the transition from normal breast to ductal carcinoma is associated with an increased infiltration of mononuclear cells at the parenchyma [49]. Immune-cell infiltrates in the tumor bed or tumor stroma have been studied in the context of protumorigenic inflammation and tumor immunosurveillance. It is now becoming clear that specific infiltrating immune cells have distinct prognostic and predictive value for breast cancer patients. Whereas tumor-infiltrating dendritic cells, M1 macrophages, Th1 cells, CD8+ T cells and natural killer cells are associated with a favorable prognosis, the presence of M2 macrophages, myeloid-derived suppressor cells, Tregs and Th2 cells is associated with poor prognosis in cancer patients. An analysis of three major microarray breast cancer datasets revealed that overexpression of immune response genes in ER− breast cancers was associated with a favorable prognosis [50]. A meta-analysis of 1044 hybridizations showed that tumor infiltration of lymphocytes in ER+ breast cancers was associated with poor prognosis, whereas that in ER− breast cancer was associated with a favorable outcome [51]. Analysis of 1781 primary invasive breast cancers using microarray datasets showed a positive correlation between the expression of T-cell metagene, LCK and a favorable outcome in ER as well as in ER+/HER2+ breast cancers [52]. In a recent study, 1334 breast cancer patients were randomized into training and validation sets in order to determine the prognostic value of CD8+ T-cell infiltrates [53]. The study showed that high total and distant stromal CD8+ T-cell counts were associated with a favorable prognosis.
Emerging evidence suggests that the success of chemotherapy depends on the status of antitumor immune responses. It was recently reported that neoadjuvant chemotherapy altered the immune cell infiltrates by increasing the CD8:CD4 T-cell ratio associated with a favorable outcome [54]. A retrospective study of tumor tissues from 152 breast cancer patients revealed that the CD8+ T-cell infiltrate was an independent predictive factor of response to neoadjuvant chemotherapy [55]. Analysis of a total of 1058 pretherapeutic breast cancer core biopsies from two neoadjuvant studies also showed that the percentage of tumor-associated lymphocytes in breast cancer was an independent predictor of response to neoadjuvant chemotherapy [56].
A signature of genes involved in tumor–immune interactions can predict relapse or no relapse
Given that both tumor cells and infiltrating immune cells have shown prognostic values, understanding a cross-talk between tumor cells and infiltrating immune cells may result in the development of a global prognostic biomarker for breast cancer patients. Currently, there is no prognostic biomarker in clinical practice that can provide ‘yes’ or ‘no’ answers about relapse in a given patient at an early stage of breast cancer, based on clinopathological criteria (including tumor size and invasiveness, and spread to the lymph nodes and distant sites), nor is there any biomarker that can predict who may benefit from active immunotherapy. Several lines of evidence suggest that signatures of the immune response can predict breast cancer outcome. Teschendorff et al. reported that downregulation of a seven-gene signature related to the immune response significantly increased the risk of distant metastasis, independent of lymph node status and lymphocytic infiltration, in ER− breast cancer patients [50]. In addition, microarray data from a number of different carcinomas, including 132 breast cancer patients, showed a prognostic value of immune-related genes, particularly the Th1-associated genes [57]. Rody et al. also reported that T-cell metagenes can predict a favorable prognosis in ER−/HER2+ breast cancer patients [52]. Sabatier et al. used public gene expression and histoclinical data of 2145 early-stage breast cancers and determined that immune function genes – Th1 type in particular – were associated with a good prognosis in basal breast cancers [58]. The immune function signature was different from two other published immune function signatures [50,52], although they still highlight the prognostic value of the immune response genes. The authors have recently identified a network of immune function genes (a five-gene signature) associated with relapse-free survival in the tumor tissue of breast cancer patients, independent of ER and lymph node status, or lymphocytic infiltration [59]. The five-gene prognostic signature was distinct from the 70-gene MammaPrint signature and from the Oncotype DX recurrence score assay panel. Among these genes, IGLL and IGKC had a favorable prognosis. These findings were consistent with previous studies on tumor stroma showing that a B-cell/plasma cell metagene had a favorable prognosis [45]. T-cell-associated genes were also detected in the tumor lesion of patients with relapse-free survival, which is consistent with other reports [50,51,57]. Presence of such immune function genes would suggest the possibility for the expression of highly immunogenic tumor antigens in the tumors of patients who remained relapse free. Among the known tumor antigens, cancer testis antigens (CTAs) hold promise because of their ability to induce strong immune responses. CTAs were first detected in a highly immunogenic cancer, melanoma, where spontaneous regression of cancer was reported. Interestingly, expression of MAGE-4 antigen was reported to be associated with a reduced risk of breast cancer recurrence [60]. The authors recently observed that expression of a panel of CTAs along with a five-gene signature of immune function was restricted to patients who remained relapse-free [PAYNE K, MANJILI MH, UNPUBLISHED DATA]. This observation suggests that patients who are identified as high risk may benefit from an active immunotherapy for the induction of CTA, as well as the triggering of CTA-reactive immune responses.
Conclusion
Characterization of biomarkers for breast cancer prognosis or response to therapy are listed in Table 1. Breast cancer is a multifaceted process and none of the present biomarkers can reliably predict a binary outcome, such as recurrence or no recurrence. Emerging evidence suggests that combined use of the available biomarkers cannot improve the accuracy of prognosis or prediction of response to therapy. This is because each biomarker represents certain sets of genes with specialized function rather than representing a network of genes that are involved in host–tumor interaction. There are also very few common genes between the published gene signatures. For example, SCUBE2, an estrogen-regulated gene, is the only common gene in the 21 genes of the Oncotype DX and the 70 genes of the MammaPrint panel. These findings suggest the need for a more comprehensive and novel biomarker that can improve the accuracy of outcome prediction. Considering the role of the immune response in a favorable prognosis, there is also a need for developing a biomarker that can predict responses to active immunotherapy.
Table 1.
Biomarkers for breast cancer prognosis and prediction of response to therapy.
| Assay | Method | Marker | Prognosis | Predictor | Patient eligibility |
|---|---|---|---|---|---|
| GGI | Microarray | 97 genes | Yes | Yes | Node−/ER+ |
| PAM50™ | qRT-PCR | 55 genes | Yes | Yes | Node−/ER+ |
| IHC4 | IHC | 4 genes | Yes | ND | Node−/ER+ |
| Mammostrat® | IHC | 5 genes | Yes | ND | Node−/ER+ |
| MammaPrint® | Microarray | 70 genes | Yes | Yes | Node±/ER± |
| Oncotype DX® | qRT-PCR | 21 genes | Yes | Yes | Node−/ER+ |
| Immune function genes |
qRT-PCR | 5 immune function genes and CTAs |
In trial | In trial | Node±/ER± |
±: Positive and negative; −: Negative; +: Postitive; CTA: Cancer testis antigen; IHC: Immunohistochemistry; ND: Not determined; qRT: Quantitative reverse transcriptase.
Future perspective
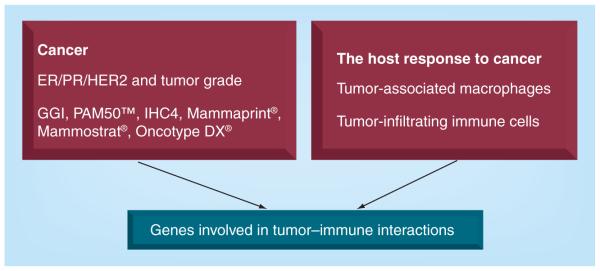
Progress in our understanding of the tumor microenvironment resulted in the development of biomarkers for breast cancer prognosis. Early markers focused on receptors expressed on tumor cells as well as tumor grade. This approach was improved by including an expanded list of genes that were involved in tumor invasion and apoptosis as well as angiogenic factors. A different approach was also developed by focusing on tumor stroma and tumor-infiltrating immune cells. Emerging evidence suggests that identification of genes that are involved in a cross-talk between tumor cells and tumor stroma – particularly host immune responses – may result in the development of a global prognostic biomarker that could also predict responses to therapy (Figure 1).
Figure 1. Development of breast cancer biomarkers.
ER: Estrogen receptor; GGI: Genomic grade index; PR: Progesterone receptor.
Executive summary.
Breast cancer biological subtypes & histological grading can predict risk of tumor recurrence
■ The prognostic impact of HER2 positivity is lower in node-negative compared with node-positive patients. Such limitations led to the use of multiple tumor biomarkers and approaches for breast cancer prognosis, including genomic grade index, PAM50™ and IHC4.
A complex of genes expressed by tumor cells & stromal cells can predict breast cancer relapse
■ The MammaPrint® assay has been US FDA-cleared for use in lymph node-negative and ER-positive or ER-negative breast cancer patients with tumors smaller than 5 cm. It is also being extended to node-positive patients. It is largely a prognostic, rather than predictive, assay. However, recent reports suggest a predictive value for the MammaPrint signature.
■ The main advantage of MammaPrint is in its ability to identify patients who could be spared unnecessary adjuvant therapy in the low-risk group, who show a less than 10% risk of recurrence within a minimum of 5 years. The 70-gene signature also provides additional information that can help refine the prognostic value of traditional markers such as HER2.
■ The Oncotype DX® panel includes 16 cancer-related genes as well as five reference ‘housekeeping’ genes. It serves as a prognostic as well as a predictive biomarker primarily in ER-positive, lymph node-negative patients.
■ Oncotype DX returns 40–66% of cases as intermediate risk with no clear data to suggest a benefit of chemotherapy. However, preliminary data suggest that addition of standard clinicopathological variables to the Oncotype DX recurrence score can help reduce the number of cases that fall into the intermediate-risk group.
■ Gene expression signatures derived from whole tumors are different from the signatures derived from the tumor stroma, with the latter showing a strong link to clinical outcome. Tumor stroma from patients with a good outcome showed overexpression of immune-related genes such as T-cell and natural killer cell markers with a skewed Th1 profile.
The status of tumor-infiltrating immune cells can predict breast cancer relapse
■ Whereas tumor-infiltrating dendritic cells, M1 macrophages, Th1 cells, CD8+ T cells and natural killer cells are associated with a favorable prognosis, presence of M2 macrophages, myeloid-derived suppressor cells, Tregs and Th2 cells is associated with poor prognosis in cancer patients.
■ Emerging evidence suggests that success of chemotherapy depends on the status of the antitumor immune responses.
A signature of genes involved in tumor–immune interactions can predict relapse or no relapse
■ Given that both tumor cells and infiltrating immune cells have shown to have prognostic values, understanding a cross-talk between tumor cells and infiltrating immune cells may result in the development of an improved prognostic biomarker for breast cancer patients.
■ We have recently identified a network of the immune function genes (a five-gene signature) and a panel of cancer testis antigens in the tumor tissue of breast cancer patients who were relapse-free, independent of ER and lymph node status, or lymphocytic infiltration.
Footnotes
Financial & competing interests disclosure
MH Manjili holds a pending patent assigned to Virginia Commonwealth University (VCU) related to [59] entitled “Gene Signatures with Rejection or Recurrence of Cancer” PCT/US2O12/030312. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
■ of interest
■■of considerable interest
- 1.Carr JA, Havstad S, Zarbo RJ, Divine G, Mackowiak P, Velanovich V. The association of HER2/neu amplification with breast cancer recurrence. Arch. Surg. 2000;135(12):1469–1474. doi: 10.1001/archsurg.135.12.1469. [DOI] [PubMed] [Google Scholar]
- 2.Roses RE, Paulson EC, Sharma A, et al. HER2/neu overexpression as a predictor for the transition from in situ to invasive breast cancer. Cancer Epidemiol. Biomarkers Prev. 2009;18(5):1386–1389. doi: 10.1158/1055-9965.EPI-08-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ■. Addressed prognostic significance of HER2 by resolving the discordance between ductal carcinoma in situ and invasive carcinoma in the frequency of HER2 overexpression.
- 3.Kmieciak M, Knutson KL, Dumur CI, Manjili MH. HER2/neu antigen loss and relapse of mammary carcinoma are actively induced by T cell-mediated anti-tumor immune responses. Eur. J. Immunol. 2007;37(3):675–685. doi: 10.1002/eji.200636639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ■. Showed that HER2 is not an independent predictor of recurrence because T-cell responses can induce HER2 loss and result in the relapse of a more aggressive HER2-negative tumor.
- 4.Santisteban M, Reiman JM, Asiedu MK, et al. Immune-induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer Res. 2009;69(7):2887–2895. doi: 10.1158/0008-5472.CAN-08-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kmieciak M, Payne KK, Idowu MO, et al. Tumor escape and progression of HER2/neu negative breast cancer under immune pressure. J. Transl Med. 2011;9:35. doi: 10.1186/1479-5876-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czerniecki BJ, Koski GK, Koldovsky U, et al. Targeting HER2/neu in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion. Cancer Res. 2007;67(4):1842–1852. doi: 10.1158/0008-5472.CAN-06-4038. [DOI] [PubMed] [Google Scholar]
- 7.Mittendorf EA, Wu Y, Scaltriti M, et al. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin. Cancer Res. 2009;15(23):7381–7388. doi: 10.1158/1078-0432.CCR-09-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curigliano G, Viale G, Bagnardi V, et al. Clinical of relevance HER2 overexpression/amplification in patients with small tumor size and node-negative breast cancer. Jsource Clin. Oncol. 2009;27(34)):5693–5699. doi: 10.1200/JCO.2009.22.0962. [DOI] [PubMed] [Google Scholar]
- 9.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin. Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. 15 Pt 1. [DOI] [PubMed] [Google Scholar]
- 10.Sotiriou C, Wirapati P, Loi S, et al. Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J. Natl Cancer Inst. 2006;93:262–272. doi: 10.1093/jnci/djj052. [DOI] [PubMed] [Google Scholar]
- 11.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J. Clin. Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 12.Albain K, Barlow W, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11(1):55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naoi Y, Kishi K, Tanei T, et al. High genomic grade index associated with poor prognosis for lymph node-negative and estrogen receptor-positive breast cancers and with good response to chemotherapy. Cancer. 2011;117(3):472–479. doi: 10.1002/cncr.25626. [DOI] [PubMed] [Google Scholar]
- 14.Sotiriou C, Wirapati P, Loi S, et al. Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J. Natl Cancer Inst. 2006;98(4):262–272. doi: 10.1093/jnci/djj052. [DOI] [PubMed] [Google Scholar]
- 15.Liedtke C, Hatzis C, Symmans WF, et al. Genomic grade index is associated with response to chemotherapy in patients with breast cancer. J. Clin. Oncol. 2009;27(19):3185–3191. doi: 10.1200/JCO.2008.18.5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loi S, Haibe-Kains B, Desmedt C, et al. Definition of clinically distinct molecular subtypes in estrogen receptor-positive breast carcinomas through genomic grade. J. Clin. Oncol. 2007;25:1239–1246. doi: 10.1200/JCO.2006.07.1522. [DOI] [PubMed] [Google Scholar]
- 17.Mallarkey G. Advancing breast cancer therapy by translational research: highlights of the Improving Care and Knowledge through Translational Research (IMPAKT) breast cancer conference. Ther. Adv. Med. Oncol. 2011;3(5):223–227. doi: 10.1177/1758834011413003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elloumi F, Hu Z, Li Y, et al. Systematic bias in genomic classification due to contaminating non-neoplastic tissue in breast tumor samples. BMC Med. Genomics. 2011;4:54. doi: 10.1186/1755-8794-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellis MJ, Suman VJ, Hoog J, et al. Randomized Phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype – ACOSOG Z1031. J. Clin. Oncol. 2011;29(17):2342–2349. doi: 10.1200/JCO.2010.31.6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen TO, Parker JS, Leung S, et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin. Cancer Res. 2010;16(21):5222–5232. doi: 10.1158/1078-0432.CCR-10-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunbier AK, Anderson H, Ghazoui Z, et al. Association between breast cancer subtypes and response to neoadjuvant anastrozole. Steroids. 2011;76(8):736–740. doi: 10.1016/j.steroids.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 22.Cuzick J, Dowsett M, Pineda S, et al. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J. Clin. Oncol. 2011;29(32):4273–4278. doi: 10.1200/JCO.2010.31.2835. [DOI] [PubMed] [Google Scholar]
- 23.Park SY, Gönen M, Kim HJ, Michor F, Polyak K. Cellular and genetic diversity in the progression of in situ human breast carcinomas to an invasive phenotype. J. Clin. Invest. 2010;120(2):636–644. doi: 10.1172/JCI40724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van ’t Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- ■■. Reported on the prognostic value of a 70-gene MammaPrint® signature.
- 25.van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N. Engl. J. Med. 2002;347:1999. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 26.Buyse M, Loi S, van’t Veer L, et al. Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J. Natl Cancer Inst. 2006;98(17):1183–1192. doi: 10.1093/jnci/djj329. [DOI] [PubMed] [Google Scholar]
- 27.Straver ME, Glas AM, Hannemann J, et al. The 70-gene signature as a response predictor for neoadjuvant chemotherapy in breast cancer. Breast Cancer Res. Treat. 2010;119(3):551–558. doi: 10.1007/s10549-009-0333-1. [DOI] [PubMed] [Google Scholar]
- 28.Knauer M, Mook S, Rutgers EJ, et al. The predictive value of the 70-gene signature for adjuvant chemotherapy in early breast cancer. Breast Cancer Res. Treat. 2010;120(3):655–661. doi: 10.1007/s10549-010-0814-2. [DOI] [PubMed] [Google Scholar]
- ■. Shows the predictive value of a 70-gene MammaPrint signature for adjuvant chemotherapy.
- 29.Knauer M, Cardoso F, Wesseling J, et al. Identification of a low-risk subgroup of HER2-positive breast cancer by the 70-gene prognosis signature. Br. J. Cancer. 2010;103(12):1788–1793. doi: 10.1038/sj.bjc.6605916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N. Engl. J. Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 31.Habel LA, Shak S, Jacobs MK, et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res. 2006;8:R25. doi: 10.1186/bcr1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fisher B, Dignam J, Wolmark N, et al. amoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. J. Natl Cancer Inst. 1997;89:1673–1682. doi: 10.1093/jnci/89.22.1673. [DOI] [PubMed] [Google Scholar]
- 33.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J. Clin. Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 34.Bryant J. Oncotype DX correlates more closely with prognosis than adjuvant online. Presented at: 9th International Conference on Primary Therapy of Early Breast Cancer; St Gallen, Switzerland: Jan, 2005. pp. 26–29. [Google Scholar]
- 35.Kelly CM, Krishnamurthy S, Bianchini G, et al. Utility of Oncotype DX risk estimates in clinically intermediate risk hormone receptor-positive, HER2-normal, grade II, lymph node-negative breast cancers. Cancer. 2010;116:5161–5167. doi: 10.1002/cncr.25269. [DOI] [PubMed] [Google Scholar]
- 36.Koscielny S. Critical review of microarray-based prognostic tests and trials in breast cancer. Curr. Opin Obstet. Gynecol. 2008;20:47–50. doi: 10.1097/GCO.0b013e3282f39d9e. [DOI] [PubMed] [Google Scholar]
- 37.Fan C, Oh DS, Wessels L, et al. Concordance among gene-expression-based predictors for breast cancer. N. Engl. J. Med. 2006;355:560–569. doi: 10.1056/NEJMoa052933. [DOI] [PubMed] [Google Scholar]
- 38.Bhowmick NA, Moses HL. Tumor-stroma interactions. Curr. Opin Genet. Dev. 2005;15:97–101. doi: 10.1016/j.gde.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim JB, Stein R, O’Hare MJ. Tumour–stromal interactions in breast cancer: the role of stroma in tumourigenesis. Tumor Biol. 2005;26:173–185. doi: 10.1159/000086950. [DOI] [PubMed] [Google Scholar]
- 40.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu. Rev. Pathol. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 41.DeCosse JJ, Gossens CL, Kuzma JF, Unsworth BR. Breast cancer: induction of differentiation by embryonic tissue. Science. 1973;181:1057–1058. doi: 10.1126/science.181.4104.1057. [DOI] [PubMed] [Google Scholar]
- 42.DeCosse JJ, Gossens C, Kuzma JF, Unsworth BR. Embryonic inductive tissues that cause histologic differentiation of murine mammary carcinoma in vitro. Jsource Natl Cancer Inst. 1975;54:913–922. [PubMed] [Google Scholar]
- 43.Finak G, Bertos N, Pepin F, et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat. Med. 2008;14(5):518–527. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- 44.Desmedt C, Majjaj S, Kheddoumi N, et al. Characterization and clinical evaluation of CD10+ stroma cells in the breast cancer microenvironment. Clin. Cancer Res. 2012;18(4):1004–1014. doi: 10.1158/1078-0432.CCR-11-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bianchini G, Qi Y, Alvarez RH, et al. Molecular anatomy of breast cancer stroma and its prognostic value in estrogen receptor-positive and -negative cancers. J. Clin. Oncol. 2010;28(28):4316–4323. doi: 10.1200/JCO.2009.27.2419. [DOI] [PubMed] [Google Scholar]
- ■. Showed that stromal genes can predict breast cancer recurrence.
- 46.Bartlett JM, Thomas J, Ross DT, et al. Mammostrat as a tool to stratify breast cancer patients at risk of recurrence during endocrine therapy. Breast Cancer Res. 2010;12(4):R47. doi: 10.1186/bcr2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Selleri S, Rumio C, Sabatino M, Marincola FM, Wang E. Tumor microenvironment and the immune response. Surg. Oncol. Clin. N. Am. 2007;16(4):737–753. doi: 10.1016/j.soc.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 48.Georgiannos SN, Renaut A, Goode AW, Sheaff M. The immunophenotype and activation status of the lymphocytic infiltrate in human breast cancers, the role of the major histocompatibility complex in cell-mediated immune mechanisms, and their association with prognostic indicators. Surgery. 2003;134(5):827–834. doi: 10.1016/s0039-6060(03)00292-7. [DOI] [PubMed] [Google Scholar]
- 49.Hussein MR, Hassan HI. Analysis of the mononuclear inflammatory cell infiltrate in the normal breast, benign proliferative breast disease, in situ and infiltrating ductal breast carcinomas: preliminary observations. J. Clin. Pathol. 2006;59(9):972–977. doi: 10.1136/jcp.2005.031252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teschendorff AE, Miremadi A, Pinder SE, Ellis IO, Caldas C. An immune response gene expression module identifies a good prognosis subtype in estrogen receptor negative breast cancer. Genome Biol. 2007;8(8):R157. doi: 10.1186/gb-2007-8-8-r157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calabrò A, Beissbarth T, Kuner R, et al. Effects of infiltrating lymphocytes and estrogen receptor on gene expression and prognosis in breast cancer. Breast Cancer Res. Treat. 2009;116(1):69–77. doi: 10.1007/s10549-008-0105-3. [DOI] [PubMed] [Google Scholar]
- 52.Rody A, Holtrich U, Pusztai L, et al. T-cell metagene predicts a favorable prognosis in estrogen receptor-negative and HER2-positive breast cancers. Breast Cancer Res. 2009;11(2):R15. doi: 10.1186/bcr2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ■. Showed prognostic value of T-cell metagene for breast cancer patients.
- 53.Mahmoud SM, Paish EC, Powe DG, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J. Clin. Oncol. 2011;29(15):1949–1955. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 54.Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. Proc. Natl Acad. Sci. USA. 2012;109(8):2796–2801. doi: 10.1073/pnas.1104303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ladoire S, Mignot G, Dabakuyo S, et al. In situ immune response after neoadjuvant chemotherapy for breast cancer predicts survival. J. Pathol. 2011;224(3):389–400. doi: 10.1002/path.2866. [DOI] [PubMed] [Google Scholar]
- 56.Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J. Clin. Oncol. 2010;28(1):105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 57.Hsu DS, Kim MK, Balakumaran BS, et al. Immune signatures predict prognosis in localized cancer. Cancer Invest. 2010;28(7):765–773. doi: 10.3109/07357900903095755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sabatier R, Finetti P, Cervera N, et al. A gene expression signature identifies two prognostic subgroups of basal breast cancer. Breast Cancer Res. Treat. 2011;126(2):407–420. doi: 10.1007/s10549-010-0897-9. [DOI] [PubMed] [Google Scholar]
- 59.Ascierto ML, Kmieciak M, Idowu MO, et al. A signature of immune function genes associated with recurrence-free survival in breast cancer patients. Breast Cancer Res. Treat. 2012;131(3):871–880. doi: 10.1007/s10549-011-1470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ■■. Shows the prognostic value of immune function genes for breast cancer patients. A five-gene signature of immune function was able to completely segregate high-risk and low-risk patients.
- 60.Bandić D, Juretić A, Šarčević B, et al. Expression and possible prognostic role of MAGE-A4, NY-ESO-1, and HER2 antigens in women with relapsing invasive ductal breast cancer: retrospective immunohistochemical study. Croat. Med. J. 2006;47(1):32–41. [PMC free article] [PubMed] [Google Scholar]