Abstract
Many microbial pathogens subvert proteoglycans for their adhesion to host tissues, invasion of host cells, infection of neighbouring cells, dissemination into the systemic circulation, and evasion of host defence mechanisms. Where studied, specific virulence factors mediate these proteoglycan–pathogen interactions, which are thus thought to affect the onset, progression and outcome of infection. Proteoglycans are composites of glycosaminoglycan (GAG) chains attached covalently to specific core proteins. Proteoglycans are expressed ubiquitously on the cell surface, in intracellular compartments, and in the extracellular matrix. GAGs mediate the majority of ligand-binding activities of proteoglycans, and many microbial pathogens elaborate cell-surface and secreted factors that interact with GAGs. Some pathogens also modulate the expression and function of proteoglycans through known virulence factors. Several GAG-binding pathogens can no longer attach to and invade host cells whose GAG expression has been reduced by mutagenesis or enzymatic treatment. Furthermore, GAG antagonists have been shown to inhibit microbial attachment and host cell entry in vitro and reduce virulence in vivo. Together, these observations underscore the biological significance of proteoglycan–pathogen interactions in infectious diseases.
Proteoglycans are expressed on the cell surface, in intracellular compartments, and in the extracellular matrix (ECM) in mammals. The chemical nature of the glycosaminoglycan (GAG) chains attached to core proteins defines proteoglycans as heparan sulfate proteoglycans (HSPGs), chondroitin sulfate proteoglycans (CSPGs), dermatan sulfate proteoglycans (DSPGs), or keratan sulfate proteoglycans (KSPGs). Some are hybrid proteoglycans, carrying both heparan sulfate (HS) and chondroitin sulfate (CS) chains. Studies on GAGs and proteoglycans date back to 1916 when a medical student trying to isolate a procoagulant molecule from liver extracts unexpectedly isolated a potent anticoagulant. This anticoagulant is now known as heparin, a highly sulfated version of HS. HS was first recognised as a mere contaminant in the heparin preparation, but was later distinguished from heparin by the difference in the extent of sulfation and greater structural variability.
For a long time, biological functions of GAGs and proteoglycans were largely speculative. However, studies during the past several decades have revealed critical biological functions of GAGs and proteoglycans in modulating molecular and cellular interactions pertinent to development and disease, including infection. Accumulating evidence indicates that many viral, bacterial and parasitic pathogens subvert proteoglycans at various stages during the course of infection. This review provides an overview of the major mechanisms whereby pathogens exploit proteoglycans to promote infection, using prototypical examples of each. The review also evaluates the potential implications of proteoglycan-based therapies as novel approaches to prevent, attenuate, halt or reverse the course of infectious diseases.
Primer on proteoglycan biology
Structure
A proteoglycan consists of a core protein and one or several covalently attached GAG chains, which are unbranched polysaccharides composed of repeating disaccharide units. In most proteoglycans, GAGs make up more than 50% of the total molecular mass and mediate the biological functions. GAG biosynthesis is initiated with the formation of a covalent bond between the reducing end of a xylosyl (Xyl) residue and the hydroxyl moiety of certain serine residues within a Ser-Gly dipeptide sequence often repeated two or more times in the core protein. This is followed by formation of the -GlcA-Gal-Gal-Xyl tetrasaccharide linkage domain (where GlcA is glucuronic acid, and Gal is galactose), polymerisation of a characteristic disaccharide unit, and modification of the newly synthesised polysaccharide chain, with each step catalysed by specific enzymes. GAGs are defined by the nature (composition and chemical linkage) of the repeating disaccharide unit, which comprises a hexosamine [e.g. N-acetylglucosamine (GlcNAc), N-acetylgalactosamine (GalNac)] and uronic acid [e.g. GlcA, iduronic acid (IdoA)]. The signature disaccharide unit of HS/heparin is GlcAβ1→ 4GlcNAcα1→4, CS is GlcAβ1→3GalNAcβ1→4, dermatan sulfate (DS) is IdoAβ1→3GalNAcβ1→4, keratan sulfate (KS) is Galβ1→4GalNAcβ1→3, and hyaluronan (HA) is GlcAβ1→3GlcNAcβ1→4. The chemical structure of HS is shown in Figure 1. Except for HA, GAGs are modified in the Golgi complex by several sulfation and epimerisation reactions. Because the polymerisation and modification reactions do not go to completion, the biosynthetic process generates an exceptionally diverse array of GAG structures.
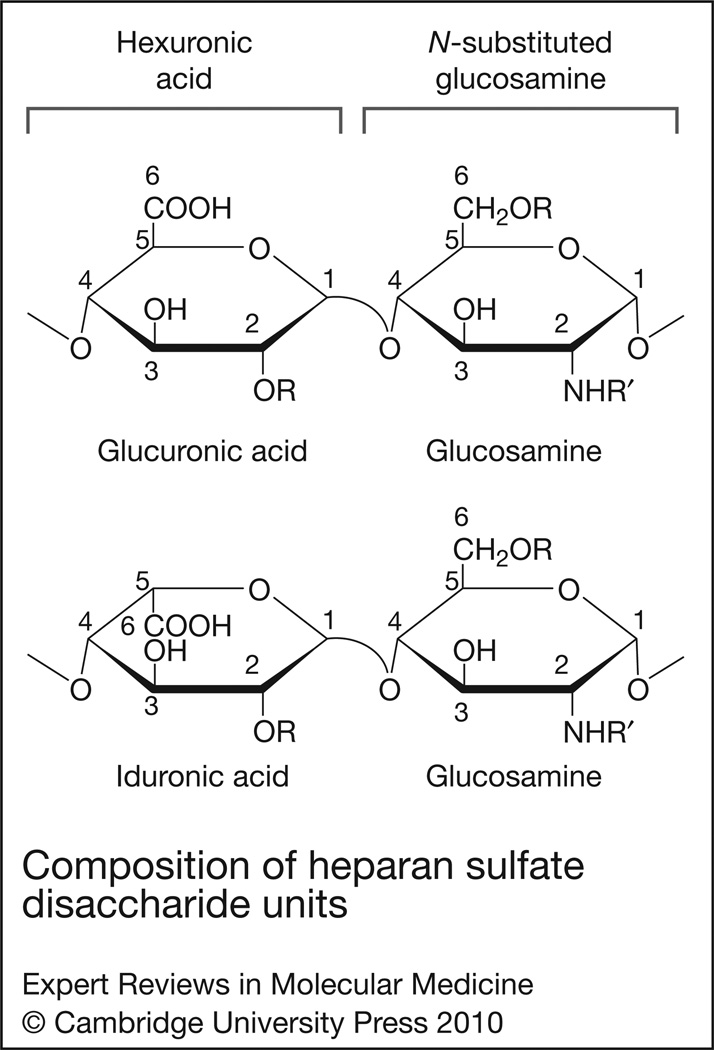
Figure 1. Composition of heparan sulfate disaccharide units.
Heparan sulfate is composed of repeating disaccharide units of hexuronic acid (either glucuronic or iduronic acid) alternating with an N-substituted (R′ = acetate or sulfate) or unsubstituted (R′ = H) glucosamine. Both N-acetylated and N-sulfated glucosamine are commonly found in mature HS chains, whereas N-unsubstituted glucosamine is not. Glucosamine can also be sulfated at the C-6 and C-3 position, although 3-O-sulfated glucosamine is rare in HS. The hexuronic acids can be sulfated at the C-2 position, and this is a common modification in iduronic acid (2-O-sulfated iduronic acid). In general, the unique sulfation pattern dictates the ligand-binding specificity of HS.
All GAGs, except for HA, are found covalently linked to specific core proteins in vivo as proteoglycans. The spatial and temporal expression patterns of proteoglycans are primarily dictated by the core proteins. Cell-surface proteoglycans include the type I transmembrane syndecans (SDC1–4), NG2 (CSPG4), CD44, betaglycan (TGFBR3) and thrombomodulin (THBD), and glycosylphosphatidylinositol (GPI)-anchored glypicans (GPC1–6) (Refs 1, 2, 3, 4, 5). ECM proteoglycans include: perlecan (HSPG2), agrin (AGRN), bamacan (SMC3) and type XVIII collagen in basement membranes; small leucine-rich proteoglycans, such as decorin (DCN), biglycan (BGN), fibromodulin (FMOD), lumican (LUM) and keratocan (KERA); and HA-binding versican (VCAN), aggrecan (ACAN) and brevican (BCAN), among others (Refs 6, 7). Serglycin (SRGN), decorated with highly sulfated heparin and CS chains, is expressed in intracellular vesicles of mast cells (Ref. 8). As shown in Figure 2, proteoglycans have distinct structural designs and GAG attachment sites. For example, syndecan core proteins contain an extracellular domain extended in conformation due to a high proline content, whereas glypican extracellular domains are globular due to several intramolecular disulfide bonds formed by the highly conserved cysteine residues. Perlecan structure resembles pearls on a string by rotary shadowing electron microsopy. HS chains are attached distal to the plasma membrane on all syndecans and CS chains are also attached proximal to the cell surface on some syndecans (e.g. syndecan-1). GAG attachment sites in NG2 (CS) and CD44 (HS/CS) are located in the middle portion of the core protein, and those of glypicans (HS), thrombomodulin (CS) and betaglycan (HS/CS) are proximal to the cell surface. HS chains in perlecan are attached at the N-terminus. The cytoplasmic domain of the transmembrane proteoglycans also mediates several important biological functions. The highly conserved, short cytoplasmic domain of syndecans contains several signalling and scaffolding motifs, such as three highly conserved tyrosines and one highly conserved serine, and a C-terminal PDZ-binding domain. The CD44 cytoplasmic domain contains an ezrin–radixin–moesin (ERM) motif that might link surface CD44 to the actin cytoskeleton.
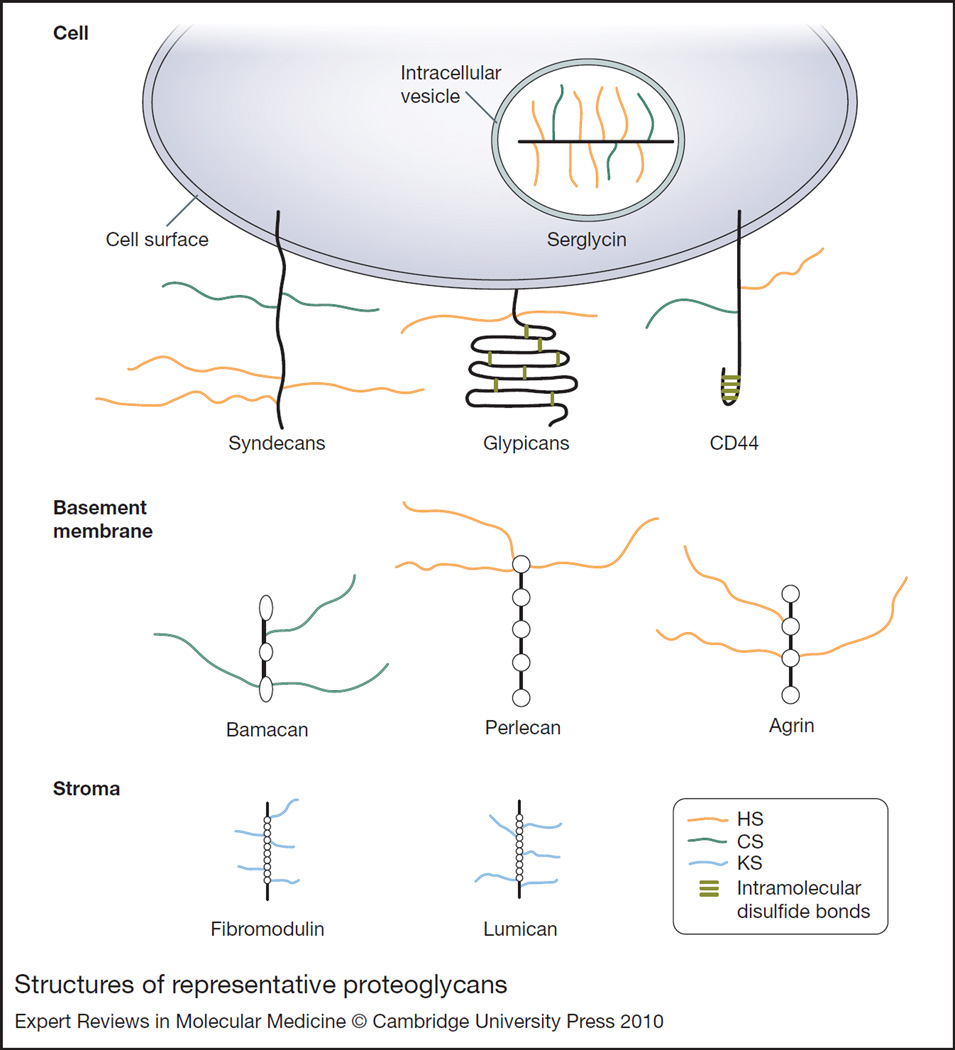
Figure 2. Structures of representative proteoglycans.
The structures of several cell-surface (syndecans, glypicans, CD44), intracellular (serglycin), and matrix (bamacan, perlecan, agrin, fibromodulin, lumican) proteoglycans are shown. Serglycin is decorated with highly sulfated HS (i.e. heparin) and CS chains, whereas syndecans and CD44 harbour HS and CS chains. Glypicans, perlecan and agrin contain HS chains, and bamacan contains CS chains. The small leucine-rich proteoglycans fibromodulin and lumican are decorated with KS chains. These proteoglycans interact with various ligands through their glycosaminoglycan chains. Abbreviations: CD, cluster of differentiation; CS, chondroitin sulfate; HS, heparan sulfate; KS, keratan sulfate.
Biological functions and implications for microbial pathogenesis
Proteoglycans, especially those harbouring the structurally diverse HS chains, bind to and regulate a multitude of biological molecules through their GAG chains. The list includes growth factors, cytokines, chemokines, proteinases, antimicrobial factors, ECM components, and many more (Refs 1, 4, 9, 10). Although cell-surface proteoglycans can serve as primary receptors for some ligands, in most cases cell-surface proteoglycans function as coreceptors that capture ligands and facilitate the encounter between ligands and their respective signalling receptors. This also holds for proteoglycan interaction with microbial pathogens, with the majority of HS-binding microbial pathogens using cell-surface HSPGs as coreceptors to facilitate their interaction with secondary internalisation receptors. Proteoglycans can also regulate protein–protein interactions by affecting the stability, conformation and oligomerisation state of either ligand or receptor, and some microbial adhesins and secreted virulence factors subvert these mechanisms to enhance pathogenic activities. Furthermore, proteoglycans can function as soluble molecules because they can be released from the cell surface or ECM by proteolytic cleavage. Once solubilised, proteoglycans show functions similar to or distinct from their immobilised counterparts, and certain bacterial pathogens are known to exploit soluble proteoglycans to inhibit host defence mechanisms. Since GAG fine structures dictate the ligand-binding activities of proteoglycans, regulation of GAG biosynthetic enzymes is also thought to modulate proteoglycan functions; however, it is not known if microbial pathogens possess the means to modulate the expression or function of these host enzymes.
Proteoglycans in microbial pathogenesis and host defence
Proteoglycans in viral attachment and internalisation
Herpes simplex virus
With respect to microbial pathogenesis, proteoglycans were first identified as coreceptors that primarily concentrate pathogens on host cell surfaces, increasing the pathogen’s ability to bind to specific secondary receptors (Fig. 3a). Most viruses bind to the HS moiety of cell-surface HSPGs to enhance viral attachment and subsequent internalisation (Refs 11, 12). The best-studied example is provided by the alphaherpesviruses herpes simplex virus (HSV) serotypes 1 and 2. HSV glycoproteins gB and gC bind to cell-surface HSPGs and mediate the initial attachment to target host cells (Refs 13, 14, 15), which are primarily epithelial cells of the skin, cornea, and urogenital system. Although specific HSPGs that mediate HSV attachment have not been identified, the observed target-cell specificity suggests that syndecan-1, the predominant HSPG of epithelial cells, is a likely candidate. Binding to cell-surface HSPGs concentrates HSV virions at the surface and facilitates the interaction of HSV gD with secondary entry receptors, such as herpes virus entry mediator (HVEM/TNFRSF14) and nectin-1 and −2 (PVRL1 and PVRL2) (Refs 13, 14, 15). Engagement of entry receptors by gD induces fusion of the viral envelope with the host plasma membrane, leading to internalisation of HSV virions. Interestingly, gD can also use a rare 3-O-sulfated form of HS as an entry receptor (Refs 16, 17). These findings suggest that cell-surface HSPGs may serve as direct internalisation receptors for HSV in some tissues rich in 3-O-sulfated HS.
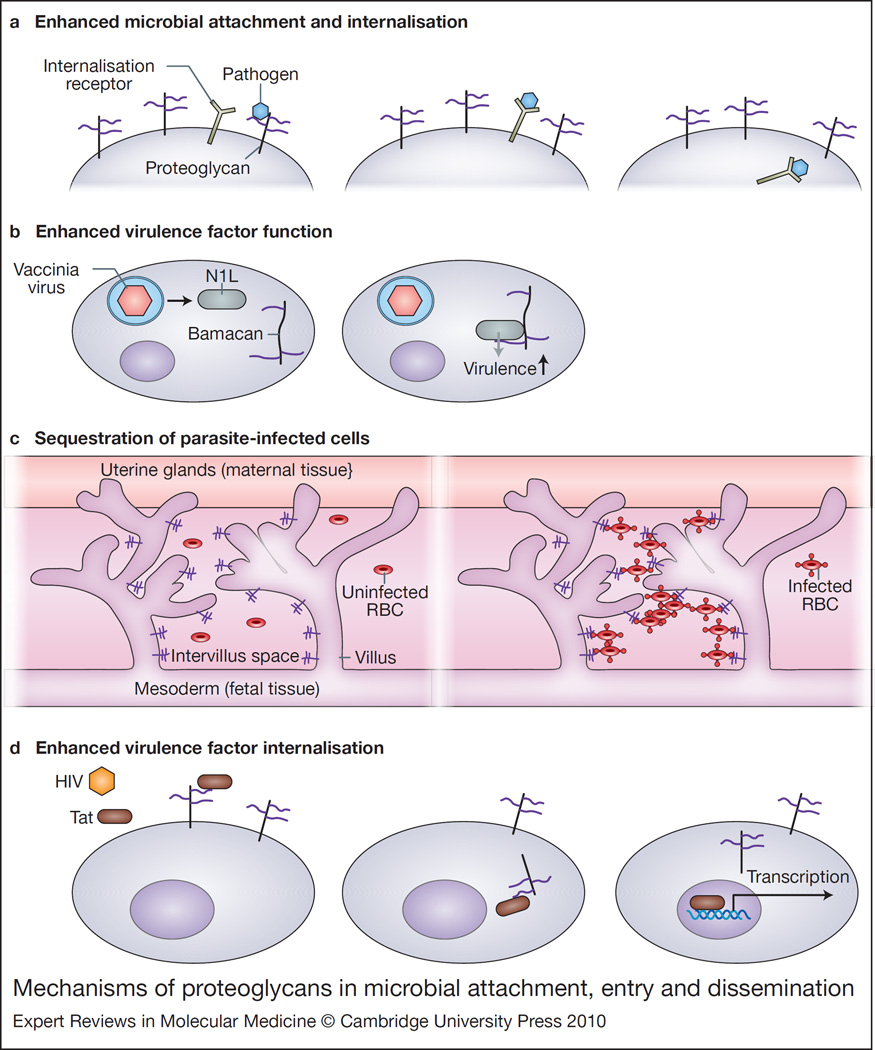
Figure 3. Mechanisms of proteoglycans in microbial attachment, entry and dissemination.
(a) Enhanced microbial attachment and internalisation. Pathogens use proteoglycans as coreceptors to increase pathogen concentration on the cell surface, facilitating binding to specific secondary receptors. This binding often results in internalisation of the pathogen. (b) Enhanced virulence factor function. Vaccinia virus produces the virulence factor N1L after internalisation. N1L binds to the CSPG bamacan, resulting in improved viral growth in vitro and neurovirulence in vivo. (c) Sequestration of parasite-infected cells. Placental tissue expresses a CS-A (purple), which binds Plasmodium falciparum-infected red blood cells, leading to their sequestration and clinical manifestations including anaemia. (d) Enhanced virulence factor internalisation. HIV Tat binds to cell-surface HSPGs and is then internalised where it can activate transcription. Abbreviations: CS-A, chondroitin sulfate A; CSPG, chondroitin sulfate proteoglycan; HIV, human immunodeficiency virus; HSPG, heparan sulfate proteoglycan; RBC, red blood cell; Tat, transactivator of transcription.
Human immunodeficiency virus
Human immunodeficiency virus (HIV), the aetiologic agent of acquired immune deficiency syndrome (AIDS), binds to cell-surface HSPGs of macrophages, dendritic cells, endothelial cells and epithelial cells through gp120 (Refs 18, 19, 20, 21). Syndecans are apparently the major cell-surface HSPG receptors because HIV does not attach to K562 cells deficient in syndecans, but does attach when K562 cells are transfected with syndecan-1 (Ref. 21). HIV also shows increased binding to Burkitt lymphoma-derived Namalwa B cells transfected with syndecan-1, −2, −3 or −4 (Ref. 19). Furthermore, syndecan-3 has been shown to be the major HIV-1 attachment receptor on dendritic cells (Ref. 20). These data suggest that several syndecans expressed in a cell-specific manner may promote HIV attachment. In addition, because target cells of HIV also express other HSPGs, these observations suggest that syndecans harbour HS structures essential for gp120 binding or syndecans are expressed in a cellular niche where gp120 preferentially binds to them. HIV entry receptors include CD4, DC-SIGN (CD209) and mannose receptors, and syndecan-3 has been shown to stabilise the captured virus and enhance viral interaction with DC-SIGN (Ref. 20). Whether syndecans increase the interaction of HIV with other entry receptors remains to be examined. HIV-1 can also bind to the basement membrane HSPG agrin through the envelope glycoprotein gp41 (Ref. 22). Here, gp41 binds specifically to agrin tethered to the apical epithelial cell surface, reinforcing the interaction of gp41 with its epithelial receptor galactosyl ceramide. Together, these data suggest that HIV possesses multiple mechanisms to bind to HSPGs, and the HSPG interaction is critical for HIV pathogenesis.
Human papillomavirus
Human papillomavirus (HPV) types 11 (Ref. 23), 16, 33 and 39 (Ref. 24) use cell-surface HSPGs as low-affinity attachment receptors to facilitate their interaction with high-affinity internalisation receptors. HPVs infect skin and mucosal epithelial cells and manipulate the host cell cycle to cause both benign and malignant epithelial tumours. Several studies suggest a mechanism where binding of the major capsid protein L1 to cell-surface HSPGs leads to a conformational change in the minor capsid protein L2, exposing residues in L2 that bind to a secondary internalisation receptor. Consistent with this mechanism, HPV pseudovirions with L1 and L2 have increased infectivity over those with only L1 (Refs 25, 26, 27). In addition, neither anti-L2 neutralising antibody (Refs 28, 29, 30) nor deletion of either the N- or C-terminus of L2 can block initial virion attachment to the host cell surface (Ref. 31). Studies using HPV16 demonstrated that the L2 residues responsible for facilitating infection (amino acid residues 13–31) are displayed on the capsid surface only when L1 binds to HSPGs (Refs 32, 33). How this is accomplished is not precisely understood, but a recent study showed that cyclophilin B present on the cell surface in association with HSPGs facilitates conformational changes of L2 (Ref. 34). Whether cell-surface cyclophilin B serves as an internalisation receptor for HPV remains to be determined.
Vaccinia virus
Vaccinia virus, an enveloped, double-stranded DNA virus in the poxvirus family, is able to infect many different cell types. Initial work demonstrated that the vaccinia protein A27L binds to cell-surface HSPGs, and soluble heparin inhibits viral attachment to host cells (Ref. 35). Other poxviruses, including cowpox virus, rabbitpox virus, Shope fibroma virus and myxoma virus, also bind to HSPGs, suggesting that HS binding may be a general mechanism of attachment for this group of viruses (Ref. 35). Further studies showed that a series of 12 positively charged amino acids in the N-terminal domain of A27L mediate binding to HSPGs, and this interaction allows for subsequent A27L-mediated cell fusion (Ref. 36). Interestingly, vaccinia proteins A34 and B5 have also been shown to mediate virus binding to GAGs on the host cell surface, and these interactions induce nonfusogenic dissolution of the outer viral membrane (Ref. 37). This is an important mechanism that allows fusion of the inner viral membrane with the plasma membrane and penetration of the virus core into the cytoplasm. Other studies have identified another GAG-binding vaccinia protein, DL8, which binds to CS and has a role in virus entry (Ref. 38). Deletion of DL8 significantly diminished infectivity of virions, confirming the importance of DL8–CS interaction in vaccinia infection (Ref. 38).
In addition, a recent study suggested that binding of vaccinia to the core protein portion of the basement-membrane-associated CS proteoglycan bamacan might be important for its neurovirulence (Ref. 39) (Fig. 3b). Vaccinia N1L protein binds to the C-terminal 227 amino acids of bamacan (Ref. 39), whose expression is increased in brain tissues (Ref. 40). Increased expression of bamacan in vitro results in improved viral growth (Ref. 39). Furthermore, N1L, which is produced only after viral infection of a host cell, is responsible for neurovirulence, possibly including neurological complications after vaccination with vaccinia. Thus, N1L binding to bamacan may lead to downstream effects of this virulence factor, such as influencing cytokine (Ref. 41) and ATP levels (Ref. 42) and inhibiting Toll-like receptor mediated NF-κB signalling (Ref. 43).
Proteoglycans in bacterial and parasitic attachment and internalisation
Listeria monocytogenes
Several bacterial pathogens similarly bind to the GAG moiety of proteoglycans to promote their attachment and internalisation. Listeria monocytogenes is a Gram-positive, intracellular food-borne pathogen that crosses the intestinal mucosa and enters the systemic circulation where it can induce sepsis and meningitis in immunocompromised hosts. The L. monocytogenes internalin protein A (InlA) binds to E-cadherin (Ref. 44) and the complex is internalised through caveolin or clathrin-coated pits (Ref. 45). In contrast to the monospecific InlA–E-cadherin interaction, internalin B (InlB) is known to bind to three receptors: the receptor for complement factor C1q, the hepatocyte growth factor receptor MET, and cell-surface HSPGs. C1q receptor was identified as an InlB receptor by affinity chromatography (Ref. 46). Although the mechanism is not fully understood, C1q receptor mediates the uptake of L. monocytogenes in professional phagocytes. In contrast, the InlB–MET interaction mediates bacterial internalisation in hepatocytes by inducing the mono-ubiquitination of MET and subsequent clathrin-dependent endocytosis (Ref. 47). Binding of InlB to cell-surface HSPGs is thought to facilitate internalisation through MET by clustering both InlB and MET at the cell surface (Ref. 48) or by stabilising the InlB–MET complex during bacterial internalisation (Ref. 49). Specific HSPGs that mediate these processes have not been identified.
Neisseria gonorrhoeae
In the case of the Gram-negative bacterium Neisseria gonorrhoeae, cell-surface HSPGs appear to serve as both coreceptors and direct internalisation receptors. Along with chlamydial infection, gonorrhoeae is one of the two most common bacterial sexually transmitted diseases in the USA. Available data suggest that binding of N. gonorrhoeae Opa protein to syndecan-1 and −4 is important for pathogenesis as overexpression of syndecan-1 or −4 in HeLa cells increases N. gonorrhoeae infection of these cells (Ref. 50). However, N. gonorrhoeae attaches to but does not invade HeLa cells expressing syndecan-1 or −4 constructs lacking the cytoplasmic domain. Furthermore, a syndecan-4 mutant lacking the dimerisation motif in the cytoplasmic domain that binds to protein kinase C (PKC) and phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2), and a syndecan-4 mutant lacking the invariant C-terminal Glu-Phe-Tyr-Ala PDZ-binding domain does not support gonococcal invasion (Ref. 50). These findings suggest that both syndecan-1 and −4 can serve as direct internalisation receptors for N. gonorrhoeae and that intracellular signalling mediated by the cytoplasmic domain of syndecans is essential. Consistent with this mechanism, N. gonorrhoeae binding to cell-surface HSPGs induces the generation of phosphatidylcholine-specific phospholipase C, diacylglycerol, acidic sphingomyelinase, and ceramide (Ref. 51). However, how engagement of the HS moiety of syndecans by N. gonorrhoeae leads to core-protein-mediated signalling is not known.
N. gonorrhoeae can also utilise cell-surface HSPGs as coreceptors to facilitate fibronectin-mediated internalisation in HEp-2 human laryngeal carcinoma cells (Ref. 52). Here, gonococci use HSPGs to bind to fibronectin, and use fibronectin as a molecular bridge to bind to β1 integrins, which then mediate N. gonorrhoeae internalisation. Consistent with this mechanism, RGD-containing peptides, fibronectin fragments, and blocking antibodies against α5β1 integrin inhibit fibronectin-mediated uptake of MS11-OpaA, and fibronectin is unable to enhance gonococcal entry into cells that have been treated with heparinase III (Ref. 52).
Borrelia burgdorferi
The spirochaete that causes Lyme disease, Borrelia burgdorferi, expresses two adhesins that bind the DSPG decorin (Ref. 53). The physiological significance of this interaction is underscored by the observation that mice deficient in decorin resist Borrelia-induced arthritis, but are as susceptible as wild-type mice to Borrelia infection of other sites including heart and bladder (Ref. 54). Longer-term studies suggest that in decorin-rich tissues, such as joint and skin, Borrelia decorin-binding protein A (DbpA) expression and decorin binding may prevent immune-mediated clearance of spirochaetes (Ref. 55). The same protection from immune clearance was not seen in tissues with low levels of decorin, such as heart and bladder (Ref. 55).
Parasites
Several parasites also bind to proteoglycans to promote their initial attachment and entry. Examples include Plasmodium spp. (Ref. 56), Leishmania spp. (Ref. 57), and Trypanosoma cruzi (Ref. 58). Plasmodium falciparum, the most virulent parasite that causes malaria, is thought to cause end-organ damage by sequestration of infected red blood cells (IRBCs) in the microvasculature (Ref. 59). P. falciparum binds to multiple adhesion molecules on the host cell surface, and this ability allows the pathogen to switch its mode of attachment as the host develops neutralising antibodies to specific adhesins with repeated infection (Ref. 60). A unique case arises in pregnant women, in that the placenta expresses a new receptor for IRBCs: chondroitin sulfate A (CS-A) (Ref. 61). Studies have shown that IRBCs bind to CS-A in fetal placental tissues (Refs 60, 62). In the absence of a pre-existing host immune response that can block IRBC binding to the CS-A receptor in the placenta, parasites can multiply at will, leading to IRBC sequestration in the placenta and influx of inflammatory cells (Fig. 3c).
Proteoglycans in virulence factor internalisation
Proteoglycans can also serve as receptors for certain secreted virulence factors. Examples include HIV transactivator of transcription (Tat) and dengue virus nonstructural protein-1 (NS1). Tat is a small cationic polypeptide that is released from HIV-infected cells (Ref. 63). Tat has important roles in the biological effects of HIV on cells lacking CD4. Tat is thought to be a neurotoxin involved in the pathogenesis of AIDS dementia (Ref. 64) and involved in tumourigenesis by inducing the replication of Kaposi-sarcoma-associated herpes virus (Ref. 65). Four classes of receptors are known to interact with Tat: αvβ3 integrin (Ref. 66); FLT1 and FLK1/KDR (vascular endothelial growth factor receptors) (Ref. 67); chemokine receptors CCR2, CCR3 and CXCR4 (Refs 68, 69); and HSPGs (Ref. 70). Tat binding to HSPGs is necessary for Tat internalisation (Ref. 71) and subsequent activation of transcription (Fig. 3d). Overproduction of CS cannot compensate for a lack of HS, indicating that Tat specifically binds to HSPGs (Ref. 71). The interaction between Tat and HSPGs depends on the size, and degree and type of sulfation, of HS (Refs 72, 73). As has been described for interactions of HSPG with growth factors, HSPG binding leads to Tat oligomerisation, which facilitates tyrosine kinase receptor dimerisation and signalling (Ref. 73). HS/heparin also protects Tat from proteolytic degradation (Ref. 74). The cell line WiDr, which lacks all HSPGs except perlecan, is permissive for Tat internalisation (Ref. 75), suggesting that perlecan is one of the HSPG receptors.
Dengue virus NS1 is a secreted glycoprotein that accumulates on the plasma membrane of target host cells. Dengue virus is a mosquitoborne RNA virus of the Flaviviridae family. Immune recognition of NS1 on endothelial cells has been proposed as a mechanism for vascular injury and leakage during severe dengue virus infection. NS1 secreted from infected cells has been shown to bind to cell-surface HS and CS-E of uninfected cells (Ref. 76). Consistent with these data, studies with CHO cells established that specific HS and CS structures modified by 2-O- and 3-O-sulfotransferases promote NS1 binding to cell surfaces (Ref. 76). Interestingly, NS1 binds preferentially to cultured human microvascular endothelial cells over aortic or umbilical cord vein endothelial cells (Ref. 76). Whether this is due to specific HS or CS structures awaits precise comparison of GAG structures on endothelial cells from different tissues.
Proteoglycans in microbial dissemination
The final step of infection where proteoglycans are known to play a role is dissemination. A key role for HSPGs in HIV-1 dissemination has been identified. Dendritic cells are the first cells to encounter HIV-1 at the mucosal surface during sexual transmission (Ref. 77). Dendritic cells bind HIV (Ref. 78) and transfer it to T cells (Ref. 79), where rapid viral expansion occurs. Syndecan-3 has been identified as a dendritic-cell-specific HSPG receptor for HIV-1 (Ref. 20). Syndecan-3 and DC-SIGN together prolong the infectivity of HIV-1, increase infectivity of dendritic cells in cis, and promote transmission to T cells. Thus, syndecan-3 at mucosal surfaces is important for pathogen transmission to T cells in which the virus can explosively replicate and disseminate. Interestingly, syndecan-2 may also function in the transmission of HIV to T cells (Ref. 19), suggesting that syndecans possess the HS structure required for this activity. Furthermore, cell-surface HSPGs might influence HIV dissemination by facilitating the interaction of infected lymphoid cells with the endothelium through Tat. Here, HSPGs on lymphoid and endothelial cells simultaneously bind to Tat homodimers, leading to the formation of HSPG–Tat–Tat–HSPG quaternary complexes that physically link lymphoid cells to the endothelium, promoting their extravasation (Ref. 80).
Mycobacterium tuberculosis, the causative agent of tuberculosis, continues to be a major global pathogen infecting an estimated third of the world’s population. In pulmonary tuberculosis, the primary target host cell in early infection is the alveolar macrophage. However, the role of alveolar epithelial cells in disseminated tuberculosis is becoming more evident. The heparin-binding haemagglutinin adhesin (HBHA) has been identified as an epithelial cell adhesin for M. tuberculosis and other pathogenic Mycobacteria (Ref. 81). Deletion of HBHA decreases the ability of M. tuberculosis to attach to type II alveolar epithelial cells in vitro (Ref. 82). Moreover, studies in a mouse model of intranasal tuberculosis demonstrated that HBHA deletion has no effect on the initial pulmonary colonisation, but HBHA deletion significantly decreases the bacteria’s ability to disseminate (Ref. 82). This difference was not seen when wild-type and HBHA-deficient M. tuberculosis strains were injected intravenously into mice, suggesting that HBHA does not affect the ability to colonise or replicate in distant organs. Thus, these findings suggest that HBHA is specifically needed for dissemination of primary pulmonary tuberculosis (Ref. 82). How HBHA mediates dissemination is incompletely understood, but HBHA binding to epithelial HSPGs has been shown to lead to reorganisation of the actin cytoskeleton, leaving the tight junctions between cells intact, and trigger endocytosis (Ref. 83). The resulting vesicles containing M. tuberculosis are transported across the cytoplasm of the epithelial cell to the basal surface, facilitating dissemination (Ref. 83).
In addition to InlB, the surface protein ActA of L. monocytogenes binds to cell-surface HSPGs (Ref. 84). ActA is best known for its capacity to manipulate the actin cytoskeleton to allow bacterial migration within and between host cells (Refs 85, 86, 87). ActA binding to HSPGs is thought to affect the invasion of epithelial cells, possibly through microvilli (Ref. 88). In support of this, coinstillation of heparin during oral inoculation of mice with L. monocytogenes did not alter the bacterial load in the caecum, but did decrease extraintestinal dissemination (Ref. 89). Although the mechanism is incompletely understood, the ability of ActA to interact with HSPGs may have important ramifications regarding the tissue specificity and dissemination of listerial infection.
The tissue-specific expression and modification of HS may also have important implications in the tissue tropism and dissemination of P. falciparum. Infected mosquitoes inject P. falciparum sporozoites into the skin of a mammalian host, and the sporozoites travel through the bloodstream to the liver where they invade hepatocytes and are transformed into extraerythrocytic forms. Each extraerythrocytic form releases thousands of merozoites, which enter the blood stream and infect erythrocytes, causing the symptoms of malarial disease, such as anaemia, fever, arthralgia and, in severe cases, coma and death. To disseminate from the skin to the liver, P. falciparum apparently detects the different degree of HS sulfation in tissues that it encounters through circumsporozoite protein (CSP), the major surface protein of circumsporozoites. CSP binds to low-sulfated HSPGs on dermal and endothelial cells, but only invades cells with highly sulfated HSPGs, such as those of hepatocytes, allowing P. falciparum sporozoites to migrate through tissues and specifically infect the liver (Ref. 90).
Mechanisms of proteoglycans in pathogen evasion of host defence
Cationic antimicrobial peptides, including defensins and cathelicidins, are important components of the innate immune response to many pathogens (Ref. 91). These peptides act by disrupting the lipid membranes of various classes of pathogens including Gram-positive and Gram-negative bacteria, and parasites and fungi (Ref. 91). Given the highly positive charge of these peptides, it has been hypothesised that pathogens might exploit negatively charged GAGs to neutralise the effect of cationic antimicrobial peptides. Indeed, DS and HS, but not CS, were able to bind to α-defensin and inhibit α-defensin-mediated killing of Pseudomonas aeruginosa, Enterococcus faecalis and Streptococcus pyogenes (Ref. 92). Furthermore, P. aeruginosa, E. faecalis and S. pyogenes were found to secrete proteinases that degrade the DSPG decorin and release soluble DS fragments (Ref. 92). Together, these data suggest that several Gram-positive and Gram-negative bacteria exploit decorin to promote their survival by secreting proteinases that degrade decorin, releasing soluble DS fragments that inhibit cationic antimicrobial peptides (Fig. 4a).
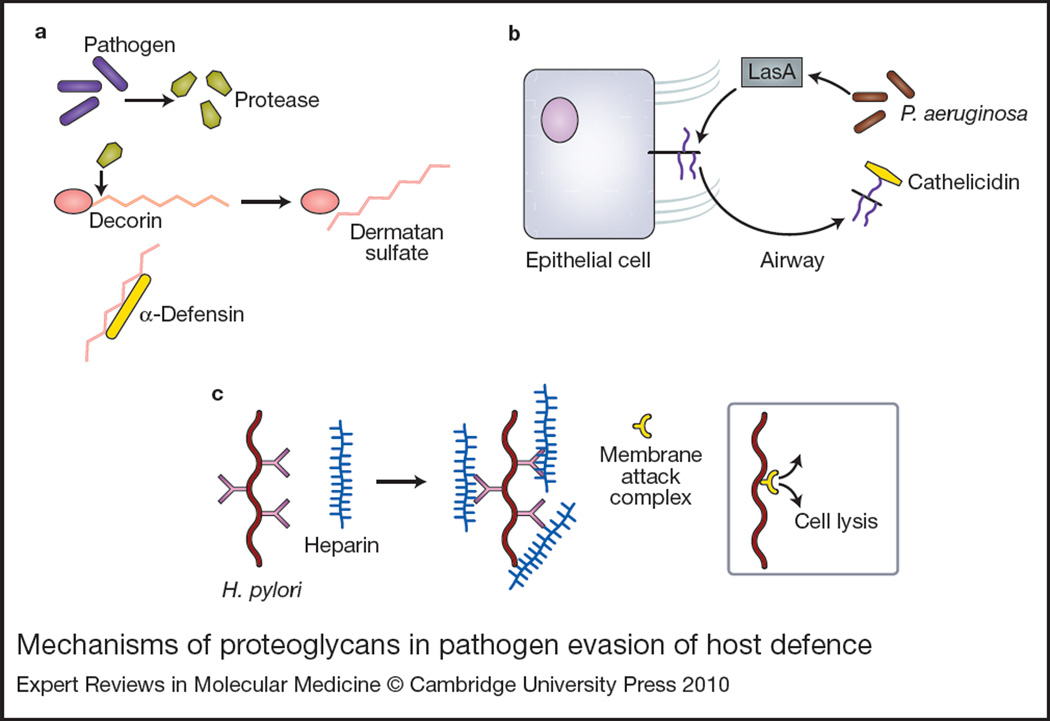
Figure 4. Mechanisms of proteoglycans in pathogen evasion of host defence.
(a) Pathogens including Pseudomonas aeruginosa secrete proteases that cleave decorin, releasing dermatan sulfate. Dermatan sulfate binds to and neutralises cationic antimicrobial peptides such as α-defensin. (b) P. aeruginosa virulence factor LasA usurps the host cell machinery to enhance syndecan-1 shedding into the airspace. Shed syndecan-1 can bind and neutralise antimicrobial peptides such as cathelicidins. (c) Helicobacter pylori binds heparin, enhancing its ability to resist complement-mediated killing by preventing assembly of the membrane attack complex.
Similarly, P. aeruginosa, Staphylococcus aureus and Streptococcus pneumoniae are able to induce the release of syndecan-1 ectodomains from the cell surface, which can inhibit innate immune mechanisms (Refs 93, 94, 95, 96). Here, bacterial pathogens secrete virulence factors that stimulate the host cell’s shedding mechanism where endogenous metalloproteinases cleave the core protein to release the ectodomain of syndecan-1 as an intact HSPG from the cell surface. Shed syndecan-1 binds to and inhibits antimicrobial peptides, such as cathelicidins and potentially other host defence factors (e.g. collectins), in an HS-dependent manner (Fig. 4b). Consistent with this mechanism, the virulence of P. aeruginosa is significantly reduced in syndecan-1-null mice compared with wild-type mice in models of intranasal lung infection (Ref. 95) and burn injury infection (Ref. 97). Furthermore, administration of HS or purified shed syndecan-1 increases bacterial virulence in the resistant syndecan-1-null mice, whereas metalloproteinase inhibitors reduce virulence in the susceptible wild-type mice. Together, these findings suggest that subversion of syndecan-1 shedding to inhibit antimicrobial peptides is an important pathogenic mechanism utilised by distinct bacterial pathogens. Interestingly, bacterial pathogens appear to specifically target shed syndecan-1 to promote their pathogenesis. How this is accomplished and whether shed forms of other HSPGs are exploited remain to be determined.
Helicobacter pylori is a spiral-shaped Gram-negative pathogen that colonises the gastric mucosa of approximately half of the human population. H. pylori infection has been associated with diseases including peptic ulcers and some gastric cancers (Ref. 98). H. pylori binds specifically to heparin and HS even in the presence of pepsin or under low pH conditions (Ref. 99). The H. pylori–HSPG interaction has been shown to promote bacterial attachment (Ref. 100), but other roles for HSPGs have emerged. Heparin/HS enhances the ability of H. pylori to resist phagocytosis in the presence of untreated and heat-treated serum (Ref. 101), suggesting that HS interferes with the phagocytosis of H. pylori mediated by the classical and alternative complement pathways (Fig. 4c). This mechanism may partly explain how H. pylori establishes chronic infection because one explanation for the persistence of H. pylori in patients is its ability to resist phagocytosis (Ref. 102). It remains to be determined whether other bacterial pathogens utilise this HS-dependent mechanism to evade serum-mediated killing mechanisms and cause chronic infections.
Implications of proteoglycan-based antimicrobial therapy
As discussed above, proteoglycans are exploited in a diverse array of pathogenic mechanisms. Moreover, genetically distinct viral, bacterial and parasitic pathogens utilise similar mechanistic approaches to subvert proteoglycans to promote their infection, indicating that subversion of proteoglycans is a broadly used pathogenic strategy. These features suggest that the benefits of developing proteoglycan-based antimicrobial therapies may be great. Several classes of natural and synthetic compounds that interfere with proteoglycan functions have been identified and they include: (1) GAG mimetics that compete with endogenous GAGs; (2) cationic compounds that bind to and inhibit GAGs; (3) inhibitors of GAG biosynthesis; and (4) enzymes that digest the polysaccharide backbone of GAGs or remove sulfate residues of GAGs. Some of these have been tested against proteoglycan– pathogen interactions and have been shown to inhibit pathogenesis both in vitro and in vivo (Table 1).
Table 1.
Proteoglycan-based antagonists of microbial pathogenesis
| Antagonist | Pathogen interaction | Refs |
|---|---|---|
| Engineered GAGs | ||
| Engineered E.coli K5 polysaccharide | Inhibits infection and dissemination of HIV, HSV-1, HSV-2 and HPV | 114 |
| Chemoenzymatically modified HS oligosaccharides | Inhibits HSV-1 entry | 112 |
| Periodate-depolymerised heparin | Disrupts P. falciparum-induced rosette formation; reverses sequestration | 176 |
| Polysulfated compounds | ||
| Carrageenans | Blocks dengue virus attachment and entry; inhibits HSV replication | 122, 177 |
| PI-88 (phosphomanno pentaose sulfate) | Blocks HSV-1 cell–cell spread; attenuates dengue and flaviviral encephalitis in mice | 131, 132 |
| Rhamnan | Inhibits HSV-1, HCMV and HIV-1 replication | 178 |
| Curdlan sulfate | Blocks P. falciparum rosette formation; blocks P. falciparum growth in vitro | 179, 180 |
| Polysulfonated compounds | ||
| Suramin (polysulfonate pharmaceutical) | Blocks HCV; therapeutic drug for human African trypanosomiasis | 132, 181 |
| PSS [poly(sodium-4-styrene sulfonate)] | Inhibits attachment of C. trachomatis, N. gonorrhoeae, HSV-1 and HSV-2 | 138 |
| PRO 2000 (naphthalene sulfonate polymer) | Inhibits HSV binding, entry, and cell–cell spread | 136, 182 |
| Small anionic tetrapyrroles (sulfonated porphyrin) | Blocks HIV cell fusion, gp120–CD4 binding, and transmission of cell-associated HIV | 183, 184, 185, 186 |
| Cationic compounds | ||
| Lactoferrin (iron-binding glycoprotein) | Blocks HSV-1 and HSV-2 attachment; decreases HCV load in chronic hepatitis C patients | 187 |
| Lactoferricin (lactoferrin derivative) | Blocks attachment of HSV-1 and HSV-2 | 144 |
| Surfen (bis-2-methyl-4-amino- quinolyl-6-carbamide) | Blocks HSV-1 infection in vitro | 149 |
| DSTP 27 (dispirotripiperazine derivative) | Prevents binding of HS-binding pathogens to HSPG receptors | 150 |
Abbreviations: E. coli, Escherichia coli; C. trachomatis, Chlamydia trachomatis; GAG, glycosaminoglycan; HCMV, human cytomegalovirus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HPV, human papillomavirus; HS, heparan sulfate; HSPG, heparan sulfate proteoglycan; HSV, herpes simplex virus; P. falciparum, Plasmodium falciparum.
GAG mimetics
Soluble HS and heparin have been shown to inhibit infection of host cells by HS-binding pathogens, such as HSV (Refs 13, 14, 15), hepatitis B virus (Refs 103, 104), HPV (Ref. 24), L. monocytogenes (Ref. 89), Staphylococcus spp. (Ref. 105), Streptococcus spp. (Ref. 105), and Chlamydia spp. (Refs 106, 107, 108), among many others. These findings suggest that along with pharmaceutical heparin, HS and its derivatives are potential candidates for therapy against a wide range of HS-binding pathogens. The major drawbacks of using heparin is that it is a potent anticoagulant that can cause excessive bleeding when misused, and it also has the potential to cause immunopathological manifestations, such as heparin-induced thrombocytopaenia. Relative to heparin, HS is an inefficient anticoagulant and, as a ubiquitous molecule, it is unlikely to be immunogenic. However, because macromolecular HS can bind to and regulate many biological molecules, usage of intact HS as a therapeutic agent could also cause unforeseen adverse effects. These features of HS/heparin have served as a basis for defining the minimum active structure for a given biological function, and this effort has been successful in the development of several HS mimetics specific for a particular function. For instance, based on the finding that induced expression of 3-O-sulfotransferases can convert resistant CHO-K1 cells to susceptibility to HSV-1 entry (Refs 16, 109, 110, 111), a heparin octasaccharide modified with 3-O-sulfotransferases that inhibits HSV-1 entry was successfully synthesised (Ref. 112). These data suggest that GAG mimetics with selective antimicrobial activities can be synthesised in vitro with a chemoenzymatic approach.
In addition to synthesising defined HS mimetics from HS oligosaccharides and polysaccharides, attempts have been made to synthesise structurally defined K5, a nonsulfated capsular polysaccharide of Escherichia coli with the same structure as that of the HS/heparin precursor N-acetyl heparosan (Ref. 113). K5 synthesis can be controlled and modified to enhance binding specificity to a particular ligand. A variety of K5-derived compounds have been studied with regards to their ability to inhibit cellular infection by viruses including HIV, HSV-1 and −2, and HPV (Ref. 114). Highly sulfated K5 derivatives, with a pI higher than that of heparin, inhibited HIV infection and replication in host cells in culture (Refs 115, 116). Three K5 derivatives have also been shown to inhibit HIV Tat binding to cell-surface HSPGs and the subsequent internalisation and transcription activation (Ref. 117). Further, N- and O-sulfated K5 and O-sulfated and epimerised K5 inhibited infection of Vero cells by HSV-1 and −2 by impairing the attachment and entry into cells and by reducing cell–cell spread of virus particles (Ref. 118). In addition, N- and O-sulfated K5 and highly O-sulfated K5 inhibited infection of host cells by HPV-6, −16 and −18 (Ref. 119). Importantly, all of these K5 derivatives inhibited cellular infection at concentrations that were not cytototoxic to host cells. These data suggest that specifically engineered K5 derivatives are promising therapeutic agents against a broad range of diseases caused by HS-binding pathogens.
Other polysulfated and polysulfonated GAG mimetics also bind to GAG-binding sites in proteins and can function as antagonists of proteoglycan–pathogen interactions (Table 1). The polysulfated compounds with antimicrobial activity include carrageenans (Refs 120, 121, 122, 123, 124), fucoidan (Refs 120, 125, 126, 127), dextran sulfate (Refs 120, 126, 128, 129, 130), phosphomanno pentaose sulfate (PI-88) (Refs 130, 131, 132), and pentosan polysulfate (PPS) (Refs 120, 126, 130). The polysulfonated compounds with antimicrobial activity include suramin (Refs 103, 133, 134, 135) and poly(sodium 4-styrene sulfonate) (PSS) (Refs 129, 136, 137, 138). In fact, suramin is the first-line therapy for human African trypanosomiasis, a potentially fatal disease caused by the parasite Trypanosoma brucei. However, the efficacy and toxicity in vivo are not known for most of these compounds. The polysulfated and polysulfonated compounds are thought to interfere with GAG interactions that are required for attachment to host cells and/or entry. Interestingly, some of these compounds may possess additional antimicrobial activities. PI-88 has different effects on infection in vitro than larger compounds such as unfractionated heparin. Heparin-like compounds are known to inhibit HSV-1 and −2 infection by blocking attachment to host cells, but have limited activity against cell–cell spread of infection (Ref. 139); in contrast, PI-88 has weak activity against preventing HSV-1 or −2 attachment, but can significantly inhibit viral spread from cell to cell (Ref. 131). Furthermore, in mouse models of dengue virus and flaviviral encephalitis, PI-88, but not suramin or PPS, significantly improved disease outcome (Ref. 132), suggesting that in vitro activity does not always predict in vivo therapeutic success. PI-88 is currently under clinical trials in cancer therapy, and results from Phase I studies identified thrombocytopaenia and pulmonary embolism as dose-limiting side effects (Ref. 140).
Cationic compounds
Another approach in inhibiting proteoglycan– pathogen interactions is usage of cationic peptides and proteins that bind to the highly negative GAG chains. Examples include lactoferrin, protamine, and Arg- and Lys-rich peptides such as synthetic polylysine and natural cationic antimicrobial peptides. Lactoferrin, an iron-binding glycoprotein of the transferrin family (Ref. 141), has multiple cationic domains in its N-terminus, through which it can bind to GAGs (Ref. 142). Trypsin digestion of lactoferrin yields a cleavage product consisting of the cationic N-terminus of lactoferrin called lactoferricin (Ref. 143). Lactoferricin shares many of the same antiviral properties of lactoferrin. Both were examined for their ability to inhibit HSV-1 and −2 infection and shown to prevent the initial attachment in an HSPG-dependent manner (Ref. 144). In addition, lactoferricin had an inhibitory effect on later stages of infection by a mechanism that is currently unknown (Ref. 144). Initial human studies using lactoferrin in the treatment of hepatitis C infection were promising, with one study showing that high-dose bovine lactoferrin (3.6 g/day) decreased hepatitis C virus RNA load in the blood of patients with chronic hepatitis C, although no improvement in transaminases was seen (Ref. 145). However, in clinical trials to evaluate the efficacy of lactoferrin in combination therapy with interferon a (Ref. 146) or with interferon a and ribavirin (Ref. 147) in patients with chronic hepatitis C infection, no added benefit was seen with lactoferrin.
Protamine is a cationic peptide that binds to and neutralises GAGs, and is used as a neutralising agent of pharmaceutical heparin (Ref. 148). Protamine has been shown to inhibit the attachment of HS-binding viruses to cell-surface HSPGs (Ref. 103). Furthermore, protamine can inhibit pathogenesis by inhibiting the anti-host-defence activity of solubilised HSPGs. As described above, certain bacterial pathogens induce the shedding of syndecan-1 and utilise the anti-host-defence activity of shed syndecan-1 to enhance their virulence. Thus, in a mouse model of intranasal P. aeruginosa infection, coinoculation of protamine significantly reduced the bacterial burden in the lung and spleen (Ref. 95). Although protamine has antibacterial activities at high concentrations, growth and viability of P. aeruginosa were not affected by the low-dose protamine tested in this study, suggesting that protamine specifically inhibits P. aeruginosa infection by interfering with the anti-host-defence activities of shed syndecan-1.
The aminoquinuride surfen (bis-2-methyl-4-amino-quinolyl-6-carbamide) is another small-compound antagonist of GAGs. Surfen binds to various GAGs in vitro with a preference for binding heparin then DS then HS then CS, an order indicating its preference for highly negatively charged GAGs (Ref. 149). Although the mechanism is incompletely understood, it is thought that positively charged aminoquinoline moieties of surfen interact with the negatively charged sulfate and carboxyl groups of GAGs (Ref. 149). Surfen has been shown to inhibit cell attachment and infection of CHO cells by HSV-1 in vitro, suggesting that it inhibits the HSV–HSPG interaction (Ref. 149).
Derivatives of dispirotripiperazine (DSTP), such as the N,N’-bisheteryl derivative DSTP 27, are another family of small-compound inhibitors of GAG-binding pathogens. DSTP 27 prevents host-cell attachment of a variety of HS-binding pathogens, including pseudorabiesvirus, HSV-1 and −2, HIV and respiratory syncytial virus (RSV) (Ref. 150). The inability of DSTP 27 to inhibit infection with varicella–zoster virus or Epstein–Barr virus – herpes viruses that do not use HSPGs as initial attachment receptors – suggests that DSTP 27 specifically interferes with HSPG interactions (Ref. 150). The ability of DSTP 27 to prevent attachment persists even after its removal, suggesting a very high affinity of DSTP 27 for HSPGs. Interestingly, DSTP 27 has been shown to have antiviral activities against HPV when applied several hours before or after infection of cells (Ref. 151). Pretreatment of cells with DSTP 27 prevents infection through binding to cell-surface HSPGs and blocking the initial attachment of HPV. However, when administered postinfection, DSTP 27 appears to inhibit infection by blocking the transfer of HPV to a non-HSPG receptor, which induces virion internalisation through a pathway that does not favour infection (Ref. 151).
Inhibitors of GAG biosynthesis
Several chemical inhibitors of GAG synthesis have been tested for their inhibitory effects on infection of host cells in culture. Xylosides containing various hydrophobic aglycone moieties resemble xylose attached to a proteoglycan core protein. Thus, xylosides, such as β-d-xyloside, can function as false acceptors for the formation of the linkage region and subsequent elongation of GAG chains on proteoglycans, and serve as chemical inhibitors of GAG biosynthesis. Xylosides have been used to study mechanisms of GAG synthesis in vitro (Refs 152, 153) and in vivo (Refs 154, 155, 156), but not in the context of infection in vivo. Because xylosides serve as effective primers for CS and DS, and to a lesser extent for HS, toxicity and lack of specificity are a major hindrance for usage in vivo. However, efforts are being made to synthesise selective xyloside inhibitors of GAG synthesis (Ref. 157), which may be tested in in vivo models of infection.
By contrast, chlorate inhibits the extent of GAG sulfation by blocking the formation of the universal sulfate donor PAPS (3′-phosphoadenosine-5′-phosphosulfate). Treatment of host cells with sodium chlorate has been shown to inhibit infection by several viruses (Refs 24, 103, 131, 158), bacteria (Ref. 159) and parasites (Ref. 160), confirming the importance of GAG sulfation in facilitating microbial pathogenesis. However, because of its strong oxidative properties and inhibitory effects on sulfation of not only GAGs but also of other glycans and tyrosines, sodium chlorate is likely toxic in vivo. In fact, one study found that oral administration of sodium chlorate causes nephrotoxicity through formation of methaemoglobins in rabbits (Ref. 161).
Several studies have indicated that proteoglycan–pathogen interactions are mediated by specific GAG modifications. For example, both dengue virus NS1 (Ref. 76) and HSV gD (Refs 16, 17) bind to 3-O-sulfated HS, which leads to internalisation of ligand. Thus, inhibition of specific GAG modifications may reduce the toxicity associated with therapeutic approaches targeting GAG biosynthesis. For instance, gene knockdown techniques (e.g. RNA interference) against specific GAG biosynthetic enzymes administered in vivo should, in theory, selectively reduce the content of a particular modification and inhibit key steps of pathogenesis. Several function-perturbing antibodies that bind to specific domains in GAGs have also been generated (Refs 162, 163). However, these selective approaches to inhibit GAG biosynthesis have not yet been tested in vivo in the context of infectious diseases.
Enzymes that digest the polysaccharide backbone or remove sulfate residues of GAGs
Enzymes such as bacterial heparinases and mammalian heparanases that digest the polysaccharide backbone of HS, and endosulfatases that remove sulfate residues from GAGs, are potential candidates for proteoglycan-based antimicrobial therapeutics. Flavobacterium heparinases are HS lyases that selectively digest sulfated HS domains (heparinase I), low sulfated domains (heparinase III), or both domains (heparinase II) (Ref. 164). In vitro studies showed that treatment of host cells with heparinases inhibits attachment or entry by several HS-binding viral (Refs 20, 24, 104, 111), bacterial (Refs 84, 165, 166) and parasitic (Ref. 167) pathogens. In vivo studies of heparinases in infectious diseases are limited, but topical administration of heparinase III prior to genital HPV-16, −31 or −5 pseudovirus infection in mice has been shown to inhibit infection by at least 89% (Ref. 168), suggesting the in vivo feasibility of heparinase therapy.
Mammalian heparanase is an endoglycosidase that degrades HS. Heparanases have been implicated in a variety of pathologies, such as cancer and inflammatory diseases (Refs 169, 170, 171). Endosulfatases edit the sulfated HS structures by removing a subset of 6-O-sulfate groups within the highly sulfated HS domains (Refs 172, 173). By doing so, they regulate the capacity of HSPGs to bind to HS-binding molecules, such as fibroblast growth factor 2 (Ref. 174) and hepatocyte growth factor (Ref. 175). In light of the inhibitory effects of bacterial heparinases and the requirement of particular sulfate modifications in microbial attachment and entry, it is plausible that heparanases and endosulfatases can also inhibit infection caused by HS-binding pathogens. However, the feasibility of these enzymes as antimicrobial agents has yet to be studied both in vitro and in vivo.
Summary
Proteoglycan–pathogen interactions illustrate the multiple roads taken by pathogens to establish a successful infection. Proteoglycans influence key processes at several steps of pathogenesis, such as adhesion to and invasion of host cells, dissemination into the systemic circulation, and evasion of host defence mechanisms. Given these central roles, it is not surprising that proteoglycans are subverted by a wide variety of viral, bacterial and parasitic pathogens. Studies during the past several decades have revealed that pathogens primarily exploit endogenous functions of proteoglycans, such as serving as a cell-surface coreceptor, enhancing receptor–ligand interactions by inducing a conformational change of ligands or receptors, and mediating the internalisation of ligands. However, proteoglycan roles in transmitting ligands to neighbouring cells (e.g. HIV), mediating dissemination and invasion of distant tissues (e.g. P. falciparum), and inhibiting host defence mechanisms as soluble proteoglycans (e.g. shed syndecan-1) were unexpected. It will be interesting to examine if these previously unknown functions of proteoglycans are important in endogenous processes, such as tumour cell metastasis and protection from inflammatory tissue damage. The subversion of endogenous proteoglycan functions, along with the fact that essential mammalian genes do not evolve as rapidly as those of microbes, suggest that pathogens will not rapidly develop resistance to therapeutic agents that target proteoglycan functions. Thus, in theory, the opportunities to develop antimicrobial agents that are directed against critical propathogenic functions of proteoglycans are great. However, care must be taken in this approach because interfering with a physiological molecule and its mechanisms can potentially have dire consequences. Additional studies directed at defining the key features of proteoglycan–pathogen interactions and evaluating the in vivo efficacy of proteoglycan antagonists should provide a foundation for the development of novel proteoglycan-based antimicrobial therapies.
Acknowledgments
Acknowledgements and funding
We thank the peer reviewers for their helpful comments. We apologise to many authors for not citing work that may have been relevant, because of space limitations. Our research described in this review was supported by NIH grants HL81474 and HL94613 (P.W.P.), and RR17665 (A.H.B.).
References
- 1.Bernfield M, et al. Functions of cell surface heparan sulfate proteoglycans. Annual Review of Biochemistry. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 2.Filmus J, Selleck SB. Glypicans: proteoglycans with a surprise. Journal of Clinical Investigation. 2001;108:497–501. doi: 10.1172/JCI13712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartmann U, Maurer P. Proteoglycans in the nervous system–the quest for functional roles in vivo. Matrix Biology. 2001;20:23–35. doi: 10.1016/s0945-053x(00)00137-2. [DOI] [PubMed] [Google Scholar]
- 4.Park PW, Reizes O, Bernfield M. Cell surface heparan sulfate proteoglycans: selective regulators of ligand-receptor encounters. Journal of Biological Chemistry. 2000;275:29923–29926. doi: 10.1074/jbc.R000008200. [DOI] [PubMed] [Google Scholar]
- 5.Taylor KR, Gallo RL. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. FASEB Journal. 2006;20:9–22. doi: 10.1096/fj.05-4682rev. [DOI] [PubMed] [Google Scholar]
- 6.Schaefer L, Iozzo RV. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. Journal of Biological Chemistry. 2008;283:21305–21309. doi: 10.1074/jbc.R800020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Current Opinion in Cell Biology. 2002;14:617–623. doi: 10.1016/s0955-0674(02)00375-7. [DOI] [PubMed] [Google Scholar]
- 8.Kolset SO, Tveit H. Serglycin- structure and biology. Cellular and Molecular Life Sciences. 2008;65:1073–1085. doi: 10.1007/s00018-007-7455-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kreuger J, et al. Interactions between heparan sulfate and proteins: the concept of specificity. Journal of Cell Biology. 2006;174:323–327. doi: 10.1083/jcb.200604035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rostand KS, Esko JD. Microbial adherence to and invasion through proteoglycans. Infection and Immunity. 1997;65:1–8. doi: 10.1128/iai.65.1.1-8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shukla D, Spear PG. Herpesviruses and heparan sulfate:an intimate relationship in aid of viral entry. Journal of Clinical Investigation. 2001;108:503–510. doi: 10.1172/JCI13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spillmann D. Heparan sulfate: anchor for viral intruders? Biochimie. 2001;83:811–817. doi: 10.1016/s0300-9084(01)01290-1. [DOI] [PubMed] [Google Scholar]
- 13.Feyzi E, et al. Structural requirement of heparan sulfate for interaction with herpes simplex virus type I virions and isolated glycoprotein C. Journal of Biological Chemistry. 1997;272:24850–24857. doi: 10.1074/jbc.272.40.24850. [DOI] [PubMed] [Google Scholar]
- 14.Laquerre S, et al. Heparan sulfate proteoglycan binding by herpes simplex virus type 1 glycoproteins B and C, which differ in their contributions to virus attachment, penetration, and cell-to-cell spread. Journal of Virology. 1998;72:6119–6130. doi: 10.1128/jvi.72.7.6119-6130.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spear PG, et al. Heparan sulfate glycosaminoglycans as primary cell surface receptors for herpes simplex virus. Advances in Experimental Medicine and Biology. 1992;313:341–353. doi: 10.1007/978-1-4899-2444-5_33. [DOI] [PubMed] [Google Scholar]
- 16.O’Donnell CD, et al. A role for heparan sulfate 3-O-sulfotransferase isoform 2 in herpes simplex virus type 1 entry and spread. Virology. 2006;346:452–459. doi: 10.1016/j.virol.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Shukla D, et al. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell. 1999;99:13–22. doi: 10.1016/s0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]
- 18.Argyris EG, et al. Human immunodeficiency virus type 1 enters primary human brain microvascular endothelial cells by a mechanism involving cell surface proteoglycans independent of lipid rafts. Journal of Virology. 2003;77:12140–12151. doi: 10.1128/JVI.77.22.12140-12151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bobardt MD, et al. Syndecan captures, protects, and transmits HIV to T lymphocytes. Immunity. 2003;18:27–39. doi: 10.1016/s1074-7613(02)00504-6. [DOI] [PubMed] [Google Scholar]
- 20.de Witte L, et al. Syndecan-3 is a dendritic cell-specific attachment receptor for HIV-1. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19464–19469. doi: 10.1073/pnas.0703747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saphire AC, et al. Syndecans serve as attachment receptors for human immunodeficiency virus type 1 on macrophages. Journal of Virology. 2001;75:9187–9200. doi: 10.1128/JVI.75.19.9187-9200.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alfsen A, et al. HIV-1-infected blood mononuclear cells form an integrin- and agrin-dependent viral synapse to induce efficient HIV-1 transcytosis across epithelial cell monolayer. Molecular Biology of the Cell. 2005;16:4267–4279. doi: 10.1091/mbc.E05-03-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joyce JG, et al. The L1 major capsid protein of human papillomavirus type 11 recombinant virus-like particles interacts with heparin and cell-surface glycosaminoglycans on human keratinocytes. Journal of Biological Chemistry. 1999;274:5810–5822. doi: 10.1074/jbc.274.9.5810. [DOI] [PubMed] [Google Scholar]
- 24.Giroglou T, et al. Human papillomavirus infection requires cell surface heparan sulfate. Journal of Virology. 2001;75:1565–1570. doi: 10.1128/JVI.75.3.1565-1570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roden RB, et al. Positively charged termini of the L2 minor capsid protein are necessary for papillomavirus infection. Journal of Virology. 2001;75:10493–10497. doi: 10.1128/JVI.75.21.10493-10497.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Unckell F, Streeck RE, Sapp M. Generation and neutralization of pseudovirions of human papillomavirus type 33. Journal of Virology. 1997;71:2934–2939. doi: 10.1128/jvi.71.4.2934-2939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeager MD, et al. Neutralization of human papillomavirus (HPV) pseudovirions: a novel and efficient approach to detect and characterize HPV neutralizing antibodies. Virology. 2000;278:570–577. doi: 10.1006/viro.2000.0674. [DOI] [PubMed] [Google Scholar]
- 28.Roden RB, et al. Papillomavirus L1 capsids agglutinate mouse erythrocytes through a proteinaceous receptor. Journal of Virology. 1995;69:5147–5151. doi: 10.1128/jvi.69.8.5147-5151.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roden RB, et al. Interaction of papillomaviruses with the cell surface. Journal of Virology. 1994;68:7260–7266. doi: 10.1128/jvi.68.11.7260-7266.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roden RB, et al. Neutralization of bovine papillomavirus by antibodies to L1 and L2 capsid proteins. Journal of Virology. 1994;68:7570–7574. doi: 10.1128/jvi.68.11.7570-7574.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang R, et al. Cell surface-binding motifs of L2 that facilitate papillomavirus infection. Journal of Virology. 2003;77:3531–3541. doi: 10.1128/JVI.77.6.3531-3541.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawana K, et al. A surface immuno determinant of human papillomavirus type 16 minor capsid protein L2. Virology. 1998;245:353–359. doi: 10.1006/viro.1998.9168. [DOI] [PubMed] [Google Scholar]
- 33.Kawana K, et al. Common neutralization epitope in minor capsid protein L2 of human papillomavirus types 16 and 6. Journal of Virology. 1999;73:6188–6190. doi: 10.1128/jvi.73.7.6188-6190.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bienkowska-Haba M, Patel HD, Sapp M. Target cell cyclophilins facilitate human papillomavirus type 16 infection. PLoS Pathogens. 2009;5:e1000524. doi: 10.1371/journal.ppat.1000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung CS, et al. A27L protein mediates vaccinia virus interaction with cell surface heparan sulfate. Journal of Virology. 1998;72:1577–1585. doi: 10.1128/jvi.72.2.1577-1585.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsiao JC, Chung CS, Chang W. Cell surface proteoglycans are necessary for A27L protein-mediated cell fusion: identification of the N-terminal region of A27L protein as the glycosaminoglycan-binding domain. Journal of Virology. 1998;72:8374–8379. doi: 10.1128/jvi.72.10.8374-8379.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Law M, et al. Ligand-induced and nonfusogenic dissolution of a viral membrane. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5989–5994. doi: 10.1073/pnas.0601025103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsiao JC, Chung CS, Chang W. Vaccinia virus envelope D8L protein binds to cell surface chondroitin sulfate and mediates the adsorption of intracellular mature virions to cells. Journal of Virology. 1999;73:8750–8761. doi: 10.1128/jvi.73.10.8750-8761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohan KV, Zhang CX, Atreya CD. The proteoglycan bamacan is a host cellular ligand of vaccinia virus neurovirulence factor N1L. Journal of Neurovirology. 2009;15:229–237. doi: 10.1080/13550280902913636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghiselli G, Siracusa LD, Iozzo RV. Complete cDNA cloning, genomic organization, chromosomal assignment, functional characterization of the promoter, and expression of the murine Bamacan gene. Journal of Biological Chemistry. 1999;274:17384–17393. doi: 10.1074/jbc.274.24.17384. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Z, et al. The vaccinia virus N1L protein influences cytokine secretion in vitro after infection. Annals of the New York Academy of Sciences. 2005;1056:69–86. doi: 10.1196/annals.1352.005. [DOI] [PubMed] [Google Scholar]
- 42.Abrahams MR, et al. The vaccinia virus N1L ORF may encode a multifunctional protein possibly targeting different kinases, one of which influences ATP levels in vivo. Annals of the New York Academy of Sciences. 2005;1056:87–99. doi: 10.1196/annals.1352.006. [DOI] [PubMed] [Google Scholar]
- 43.DiPerna G, et al. Poxvirus protein N1L targets the I-kappaB kinase complex, inhibits signaling to NF-kappaB by the tumor necrosis factor superfamily of receptors, and inhibits NF-kappaB and IRF3 signaling by toll-like receptors. Journal of Biological Chemistry. 2004;279:36570–36578. doi: 10.1074/jbc.M400567200. [DOI] [PubMed] [Google Scholar]
- 44.Mengaud J, et al. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 45.Bonazzi M, et al. Successive post-translational modifications of E-cadherin are required for InlA-mediated internalization of Listeria monocytogenes. Cellular Microbiology. 2008;10:2208–2222. doi: 10.1111/j.1462-5822.2008.01200.x. [DOI] [PubMed] [Google Scholar]
- 46.Braun L, Ghebrehiwet B, Cossart P. gC1q–R/p32, a C1q–binding protein, is a receptor for the InlB invasion protein of Listeria monocytogenes. EMBO Journal. 2000;19:1458–1466. doi: 10.1093/emboj/19.7.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veiga E, Cossart P. Listeria hijacks the clathrin-dependent endocytic machinery to invade mammalian cells. Nature Cell Biology. 2005;7:894–900. doi: 10.1038/ncb1292. [DOI] [PubMed] [Google Scholar]
- 48.Jonquieres R, Pizarro-Cerda J, Cossart P. Synergy between the N- and C-terminal domains of InlB for efficient invasion of non-phagocytic cells by Listeria monocytogenes. Molecular Microbiology. 2001;42:955–965. doi: 10.1046/j.1365-2958.2001.02704.x. [DOI] [PubMed] [Google Scholar]
- 49.Banerjee M, et al. GW domains of the Listeria monocytogenes invasion protein InlB are required for potentiation of Met activation. Molecular Microbiology. 2004;52:257–271. doi: 10.1111/j.1365-2958.2003.03968.x. [DOI] [PubMed] [Google Scholar]
- 50.Freissler E, et al. Syndecan-1 and syndecan-4 can mediate the invasion of OpaHSPG-expressing Neisseria gonorrhoeae into epithelial cells. Cellular Microbiology. 2000;2:69–82. doi: 10.1046/j.1462-5822.2000.00036.x. [DOI] [PubMed] [Google Scholar]
- 51.Grassmé H, et al. Acidic sphingomyelinase mediates entry of N. gonorrhoeae into nonphagocytic cells. Cell. 1997;91:605–615. doi: 10.1016/s0092-8674(00)80448-1. [DOI] [PubMed] [Google Scholar]
- 52.van Putten JP, Duensing TD, Cole RL. Entry of OpaA+ gonococci into HEp-2 cells requires concerted action of glycosaminoglycans, fibronectin and integrin receptors. Molecular Microbiology. 1998;29:369–379. doi: 10.1046/j.1365-2958.1998.00951.x. [DOI] [PubMed] [Google Scholar]
- 53.Guo BP, et al. Decorin-binding adhesins from Borrelia burgdorferi. Molecular Microbiology. 1998;30:711–723. doi: 10.1046/j.1365-2958.1998.01103.x. [DOI] [PubMed] [Google Scholar]
- 54.Brown EL, et al. Resistance to Lyme disease in decorin-deficient mice. Journal of Clinical Investigation. 2001;107:845–852. doi: 10.1172/JCI11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liang FT, et al. Protective niche for Borrelia burgdorferi to evade humoral immunity. American Journal of Pathology. 2004;165:977–985. doi: 10.1016/S0002-9440(10)63359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pancake SJ, et al. Malaria sporozoites and circumsporozoite proteins bind specifically to sulfated glycoconjugates. Journal of Cell Biology. 1992;117:1351–1357. doi: 10.1083/jcb.117.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Love DC, Esko JD, Mosser DM. A heparin-binding activity on Leishmania amastigotes which mediates adhesion to cellular proteoglycans. Journal of Cell Biology. 1993;123:759–766. doi: 10.1083/jcb.123.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ortega-Barria E, Pereira ME. A novel T. cruzi heparin-binding protein promotes fibroblast adhesion and penetration of engineered bacteria and trypanosomes into mammalian cells. Cell. 1991;67:411–421. doi: 10.1016/0092-8674(91)90192-2. [DOI] [PubMed] [Google Scholar]
- 59.Sherman IW, Eda S, Winograd E. Cytoadherence and sequestration in Plasmodium falciparum: defining the ties that bind. Microbes and Infection. 2003;5:897–909. doi: 10.1016/s1286-4579(03)00162-x. [DOI] [PubMed] [Google Scholar]
- 60.Muthusamy A, et al. Chondroitin sulfate proteoglycan but not hyaluronic acid is the receptor for the adherence of Plasmodium falciparum-infected erythrocytes in human placenta, and infected red blood cell adherence upregulates the receptor expression. American Journal of Pathology. 2007;170:1989–2000. doi: 10.2353/ajpath.2007.061238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 62.Muthusamy A, et al. Plasmodium falciparum-infected erythrocytes adhere both in the intervillous space and on the villous surface of human placenta by binding to the low-sulfated chondroitin sulfate proteoglycan receptor. American Journal of Pathology. 2004;164:2013–2025. doi: 10.1016/S0002-9440(10)63761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Noonan D, Albini A. From the outside in: extracellular activities of HIV Tat. Advances in Pharmacology. 2000;48:229–250. doi: 10.1016/s1054-3589(00)48008-7. [DOI] [PubMed] [Google Scholar]
- 64.Dewhurst S, Gelbard HA, Fine SM. Neuropathogenesis of AIDS. Molecular Medicine Today. 1996;2:16–23. doi: 10.1016/1357-4310(96)88754-5. [DOI] [PubMed] [Google Scholar]
- 65.Zeng Y, et al. Intracellular Tat of human immunodeficiency virus type 1 activates lytic cycle replication of Kaposi’s sarcoma-associated herpesvirus: role of JAK/STAT signaling. Journal of Virology. 2007;81:2401–2417. doi: 10.1128/JVI.02024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Urbinati C, et al. alpha(v)beta3-integrin-dependent activation of focal adhesion kinase mediates NF-kappaB activation and motogenic activity by HIV-1 Tat in endothelial cells. Journal of Cell Science. 2005;118:3949–3958. doi: 10.1242/jcs.02518. [DOI] [PubMed] [Google Scholar]
- 67.Albini A, et al. The angiogenesis induced by HIV-1 tat protein is mediated by the Flk-1/KDR receptor on vascular endothelial cells. Nature Medicine. 1996;2:1371–1375. doi: 10.1038/nm1296-1371. [DOI] [PubMed] [Google Scholar]
- 68.Albini A, et al. HIV-1 Tat protein mimicry of chemokines. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:13153–13158. doi: 10.1073/pnas.95.22.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xiao H, et al. Selective CXCR4 antagonism by Tat: implications for in vivo expansion of coreceptor use by HIV-1. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11466–11471. doi: 10.1073/pnas.97.21.11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rusnati M, Presta M. HIV-1 Tat protein: a target for the development of anti-AIDS therapies. Drugs of the Future. 2002;27:481–493. [Google Scholar]
- 71.Tyagi M, et al. Internalization of HIV-1 tat requires cell surface heparan sulfate proteoglycans. Journal of Biological Chemistry. 2001;276:3254–3261. doi: 10.1074/jbc.M006701200. [DOI] [PubMed] [Google Scholar]
- 72.Rusnati M, et al. Interaction of HIV-1 Tat protein with heparin. Role of the backbone structure, sulfation, and size. Journal of Biological Chemistry. 1997;272:11313–11320. doi: 10.1074/jbc.272.17.11313. [DOI] [PubMed] [Google Scholar]
- 73.Rusnati M, et al. Multiple interactions of HIV-1 Tat protein with size-defined heparin oligosaccharides. Journal of Biological Chemistry. 1999;274:28198–28205. doi: 10.1074/jbc.274.40.28198. [DOI] [PubMed] [Google Scholar]
- 74.Chang HC, et al. HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS. 1997;11:1421–1431. doi: 10.1097/00002030-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 75.Argyris EG, et al. The perlecan heparan sulfate proteoglycan mediates cellular uptake of HIV-1 Tat through a pathway responsible for biological activity. Virology. 2004;330:481–486. doi: 10.1016/j.virol.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 76.Avirutnan P, et al. Secreted NS1 of dengue virus attaches to the surface of cells via interactions with heparan sulfate and chondroitin sulfate E. PLoS Pathogens. 2007;3:1798–1812. doi: 10.1371/journal.ppat.0030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Patterson BK, et al. Susceptibility to human immunodeficiency virus-1 infection of human foreskin and cervical tissue grown in explant culture. American Journal of Pathology. 2002;161:867–873. doi: 10.1016/S0002-9440(10)64247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trumpfheller C, et al. Cell type-dependent retention and transmission of HIV-1 by DC-SIGN. International Immunology. 2003;15:289–298. doi: 10.1093/intimm/dxg030. [DOI] [PubMed] [Google Scholar]
- 79.Cameron PU, et al. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science. 1992;257:383–387. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]
- 80.Urbinati C, et al. HIV-1 Tat and heparan sulfate proteoglycan interaction: a novel mechanism of lymphocyte adhesion and migration across the endothelium. Blood. 2009;114:3335–3342. doi: 10.1182/blood-2009-01-198945. [DOI] [PubMed] [Google Scholar]
- 81.Menozzi FD, et al. Molecular characterization of the mycobacterial heparin-binding hemagglutinin, a mycobacterial adhesin. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:12625–12630. doi: 10.1073/pnas.95.21.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pethe K, et al. The heparin-binding haemagglutinin of M. tuberculosis is required for extrapulmonary dissemination. Nature. 2001;412:190–194. doi: 10.1038/35084083. [DOI] [PubMed] [Google Scholar]
- 83.Menozzi FD, et al. Mycobacterium tuberculosis heparin-binding haemagglutinin adhesin (HBHA) triggers receptor-mediated transcytosis without altering the integrity of tight junctions. Microbes and Infection. 2006;8:1–9. doi: 10.1016/j.micinf.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 84.Alvarez-Dominguez C, et al. Host cell heparan sulfate proteoglycans mediate attachment and entry of Listeria monocytogenes, and the listerial surface protein ActA is involved in heparan sulfate receptor recognition. Infection and Immunity. 1997;65:78–88. doi: 10.1128/iai.65.1.78-88.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lasa I, Cossart P. Actin-based bacterial motility: towards a definition of the minimal requirements. Trends in Cell Biology. 1996;6:109–114. doi: 10.1016/0962-8924(96)81001-4. [DOI] [PubMed] [Google Scholar]
- 86.Portnoy DA, et al. Molecular determinants of Listeria monocytogenes pathogenesis. Infection and Immunity. 1992;60:1263–1267. doi: 10.1128/iai.60.4.1263-1267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sheehan B, et al. Molecular and genetic determinants of the Listeria monocytogenes infectious process. Current Topics in Microbiology and Immunology. 1994;192:187–216. doi: 10.1007/978-3-642-78624-2_9. [DOI] [PubMed] [Google Scholar]
- 88.Suarez M, et al. A role for ActA in epithelial cell invasion by Listeria monocytogenes. Cellular Microbiology. 2001;3:853–864. doi: 10.1046/j.1462-5822.2001.00160.x. [DOI] [PubMed] [Google Scholar]
- 89.Henry-Stanley MJ, et al. Role of heparan sulfate in interactions of Listeria monocytogenes with enterocytes. Medical Microbiology and Immunology. 2003;192:107–115. doi: 10.1007/s00430-002-0165-7. [DOI] [PubMed] [Google Scholar]
- 90.Coppi A, et al. Heparan sulfate proteoglycans provide a signal to Plasmodium sporozoites to stop migrating and productively invade host cells. Cell Host and Microbe. 2007;2:316–327. doi: 10.1016/j.chom.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends in Immunology. 2009;30:131–141. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schmidtchen A, Frick I, Björck L. Dermatan sulfate is released by proteinases of common pathogenic bacteria and inactivates antibacterial alpha-defensin. Molecular Microbiology. 2001;39:708–713. doi: 10.1046/j.1365-2958.2001.02251.x. [DOI] [PubMed] [Google Scholar]
- 93.Chen Y, et al. Streptococcus pneumoniae sheds syndecan-1 ectodomains through ZmpC, a metalloproteinase virulence factor. Journal of Biological Chemistry. 2007;282:159–167. doi: 10.1074/jbc.M608542200. [DOI] [PubMed] [Google Scholar]
- 94.Park PW, et al. Activation of syndecan-1 ectodomain shedding by Staphylococcus aureus alpha-toxin and beta-toxin. Journal of Biological Chemistry. 2004;279:251–258. doi: 10.1074/jbc.M308537200. [DOI] [PubMed] [Google Scholar]
- 95.Park PW, et al. Exploitation of syndecan-1 shedding by Pseudomonas aeruginosa enhances virulence. Nature. 2001;411:98–102. doi: 10.1038/35075100. [DOI] [PubMed] [Google Scholar]
- 96.Park PW, et al. Syndecan-1 shedding is enhanced by LasA, a secreted virulence factor of Pseudomonas aeruginosa . Journal of Biological Chemistry. 2000;275:3057–3064. doi: 10.1074/jbc.275.5.3057. [DOI] [PubMed] [Google Scholar]
- 97.Haynes A, 3rd, et al. Syndecan-1 shedding contributes to Pseudomonas aeruginosa sepsis. Infection and Immunity. 2005;73:7914–7921. doi: 10.1128/IAI.73.12.7914-7921.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cover TL, Blaser MJ. Helicobacter pylori in health and disease. Gastroenterology. 2009;136:1863–1873. doi: 10.1053/j.gastro.2009.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ascencio F, Fransson L, Wadström T. Affinity of the gastric pathogen Helicobacter pylori for the N-sulphated glycosaminoglycan heparan sulfate. Journal of Medical Microbiology. 1993;38:240–244. doi: 10.1099/00222615-38-4-240. [DOI] [PubMed] [Google Scholar]
- 100.Chmiela M, et al. The role of heparan sulphate-binding activity of Helicobacter pylori bacteria in their adhesion to murine macrophages. APMIS: Acta Pathologica Microbiologica et Immunologica Scandinavica. 1995;103:469–474. doi: 10.1111/j.1699-0463.1995.tb01133.x. [DOI] [PubMed] [Google Scholar]
- 101.Dubreuil JD, et al. Effect of heparin binding on Helicobacter pylori resistance to serum. Journal of Medical Microbiology. 2004;53:9–12. doi: 10.1099/jmm.0.05389-0. [DOI] [PubMed] [Google Scholar]
- 102.Chmiela M, Lelwala-Guruge J, Wadstrom T. Interaction of cells of Helicobacter pylori with human polymorphonuclear leucocytes: possible role of haemagglutinins. FEMS Immunology and Medical Microbiology. 1994;9:41–48. doi: 10.1111/j.1574-695X.1994.tb00472.x. [DOI] [PubMed] [Google Scholar]
- 103.Schulze A, Gripon P, Urban S. Hepatitis B virus infection initiates with a large surface protein-dependent binding to heparan sulfate proteoglycans. Hepatology. 2007;46:1759–1768. doi: 10.1002/hep.21896. [DOI] [PubMed] [Google Scholar]
- 104.Leistner CM, Gruen-Bernhard S, Glebe D. Role of glycosaminoglycans for binding and infection of hepatitis B virus. Cellular Microbiology. 2008;10:122–133. doi: 10.1111/j.1462-5822.2007.01023.x. [DOI] [PubMed] [Google Scholar]
- 105.Henry-Stanley MJ, et al. Ability of the heparan sulfate proteoglycan syndecan-1 to participate in bacterial translocation across the intestinal epithelial barrier. Shock. 2005;24:571–576. doi: 10.1097/01.shk.0000184286.95493.78. [DOI] [PubMed] [Google Scholar]
- 106.Davis CH, Wyrick PB. Differences in the association of Chlamydia trachomatis serovar E and serovar L2 with epithelial cells in vitro may reflect biological differences in vivo. Infection and Immunity. 1997;65:2914–2924. doi: 10.1128/iai.65.7.2914-2924.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Taraktchoglou M, et al. Infectivity of Chlamydia trachomatis serovar LGV but not E is dependent on host cell heparan sulfate. Infection and Immunity. 2001;69:968–976. doi: 10.1128/IAI.69.2.968-976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yan Y, et al. Inhibitory effect of heparan sulfate-like glycosaminoglycans on the infectivity of Chlamydia pneumoniae in HL cells varies between strains. Microbes and Infection. 2006;8:866–872. doi: 10.1016/j.micinf.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 109.Xia G, et al. Heparan sulfate 3-O-sulfotransferase isoform 5 generates both an antithrombin-binding site and an entry receptor for herpes simplex virus, type 1. Journal of Biological Chemistry. 2002;277:37912–37919. doi: 10.1074/jbc.M204209200. [DOI] [PubMed] [Google Scholar]
- 110.Xu D, et al. Characterization of heparan sulphate 3-O-sulphotransferase isoform 6 and its role in assisting the entry of herpes simplex virus type 1. Biochemical Journal. 2005;385:451–459. doi: 10.1042/BJ20040908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tiwari V, et al. Role for 3-O-sulfated heparan sulfate as the receptor for herpes simplex virus type 1 entry into primary human corneal fibroblasts. Journal of Virology. 2006;80:8970–8980. doi: 10.1128/JVI.00296-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Copeland R, et al. Using a 3-O-sulfated heparin octasaccharide to inhibit the entry of herpes simplex virus type 1. Biochemistry. 2008;47:5774–5783. doi: 10.1021/bi800205t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vann WF, et al. The structure of the capsular polysaccharide (K5 antigen) of urinary-tract-infective Escherichia coli 010:K5:H4. A polymer similar to desulfo-heparin. European Journal of Biochemistry. 1981;116:359–364. doi: 10.1111/j.1432-1033.1981.tb05343.x. [DOI] [PubMed] [Google Scholar]
- 114.Rusnati M, et al. Sulfated K5 Escherichiacoli polysaccharide derivatives: A novel class of candidate antiviral microbicides. Pharmacology and Therapeutics. 2009;123:310–322. doi: 10.1016/j.pharmthera.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 115.Cicala C, et al. R5 and X4 HIV envelopes induce distinct gene expression profiles in primary peripheral blood mononuclear cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3746–3751. doi: 10.1073/pnas.0511237103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pacciarini F, et al. Sulfated K5 Escherichia coli polysaccharide derivatives inhibit human immunodeficiency type-1 (HIV-1) infection: candidate microbicides to prevent sexual HIV transmission. New Microbiologica. 2004;27:5–9. [PubMed] [Google Scholar]
- 117.Urbinati C, et al. Chemically sulfated Escherichia coli K5 polysaccharide derivatives as extracellular HIV-1 Tat protein antagonists. FEBS Letters. 2004;568:171–177. doi: 10.1016/j.febslet.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 118.Pinna D, et al. Inhibition of herpes simplex virus types 1 and 2 in vitro infection by sulfated derivatives of Escherichia coli K5 polysaccharide. Antimicrobial Agents and Chemotherapy. 2008;52:3078–3084. doi: 10.1128/AAC.00359-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lembo D, et al. Sulfated K5 Escherichia coli polysaccharide derivatives as wide-range inhibitors of genital types of human papillomavirus. Antimicrobial Agents and Chemotherapy. 2008;52:1374–1381. doi: 10.1128/AAC.01467-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Baba M, et al. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrobial Agents and Chemotherapy. 1988;32:1742–1745. doi: 10.1128/aac.32.11.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Buck CB, et al. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathogens. 2006;2:e69. doi: 10.1371/journal.ppat.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Talarico LB, Damonte EB. Interference in dengue virus adsorption and uncoating by carrageenans. Virology. 2007;363:473–485. doi: 10.1016/j.virol.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 123.Utt M, Wadstrom T. Identification of heparan sulphate binding surface proteins of Helicobacter pylori: inhibition of heparan sulphate binding with sulphated carbohydrate polymers. Journal of Medical Microbiology. 1997;46:541–546. doi: 10.1099/00222615-46-7-541. [DOI] [PubMed] [Google Scholar]
- 124.Adams Y, et al. Carrageenans inhibit the in vitro growth of Plasmodium falciparum and cytoadhesion to CD36. Parasitology Research. 2005;97:290–294. doi: 10.1007/s00436-005-1426-3. [DOI] [PubMed] [Google Scholar]
- 125.Romanos MT, et al. A sulphated fucan from the Laminaria abyssalis inhibits the human T cell lymphotropic virus type 1-induced syncytium formation in HeLa cells. Antiviral Chemistry and Chemotherapy. 2002;13:219–221. doi: 10.1177/095632020201300402. [DOI] [PubMed] [Google Scholar]
- 126.Fallgren C, Andersson A, Ljungh A. The role of glycosaminoglycan binding of staphylococci in attachment to eukaryotic host cells. Current Microbiology. 2001;43:57–63. doi: 10.1007/s002840010260. [DOI] [PubMed] [Google Scholar]
- 127.Ortega-Barria E, Boothroyd JC. A Toxoplasma lectin-like activity specific for sulfated polysaccharides is involved in host cell infection. Journal of Biological Chemistry. 1999;274:1267–1276. doi: 10.1074/jbc.274.3.1267. [DOI] [PubMed] [Google Scholar]
- 128.Ying C, et al. Sulphated and sulphonated polymers inhibit the initial interaction of hepatitis B virus with hepatocytes. Antiviral Chemistry and Chemotherapy. 2002;13:157–164. doi: 10.1177/095632020201300302. [DOI] [PubMed] [Google Scholar]
- 129.Christensen ND, et al. Papillomavirus microbicidal activities of high-molecular-weight cellulose sulfate, dextran sulfate, and polystyrene sulfonate. Antimicrobial Agents and Chemotherapy. 2001;45:3427–3432. doi: 10.1128/AAC.45.12.3427-3432.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Adams Y, et al. Inhibition of Plasmodium falciparum growth in vitro and adhesion to chondroitin-4-sulfate by the heparan sulfate mimetic PI-88 and other sulfated oligosaccharides. Antimicrobial Agents and Chemotherapy. 2006;50:2850–2852. doi: 10.1128/AAC.00313-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nyberg K, et al. The low molecular weight heparan sulfate-mimetic, PI-88, inhibits cell-to-cell spread of herpes simplex virus. Antiviral Research. 2004;63:15–24. doi: 10.1016/j.antiviral.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 132.Lee E, et al. Antiviral effect of the heparan sulfate mimetic, PI-88, against dengue and encephalitic flaviviruses. Antiviral Research. 2006;69:31–38. doi: 10.1016/j.antiviral.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 133.Aguilar JS, Rice M, Wagner EK. The polysulfonated compound suramin blocks adsorption and lateral difusion of herpes simplex virus type-1 in vero cells. Virology. 1999;258:141–151. doi: 10.1006/viro.1999.9723. [DOI] [PubMed] [Google Scholar]
- 134.Clanton DJ, et al. Novel sulfonated and phosphonated analogs of distamycin which inhibit the replication of HIV. Antiviral Research. 1995;27:335–354. doi: 10.1016/0166-3542(95)00017-g. [DOI] [PubMed] [Google Scholar]
- 135.Fleck SL, et al. Suramin and suramin analogues inhibit merozoite surface protein-1 secondary processing and erythrocyte invasion by the malaria parasite Plasmodium falciparum. Journal of Biological Chemistry. 2003;278:47670–47677. doi: 10.1074/jbc.M306603200. [DOI] [PubMed] [Google Scholar]
- 136.Cheshenko N, et al. Candidate topical microbicides bind herpes simplex virus glycoprotein B and prevent viral entry and cell-to-cell spread. Antimicrobial Agents and Chemotherapy. 2004;48:2025–2036. doi: 10.1128/AAC.48.6.2025-2036.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Taylor DL, et al. Potent inhibition of human immunodeficiency virus by MDL 101028, a novel sulphonic acid polymer. Antiviral Research. 1995;28:159–173. doi: 10.1016/0166-3542(95)00046-o. [DOI] [PubMed] [Google Scholar]
- 138.Herold BC, et al. Poly(sodium 4-styrene sulfonate): an effective candidate topical antimicrobial for the prevention of sexually transmitted diseases. Journal of Infectious Diseases. 2000;181:770–773. doi: 10.1086/315228. [DOI] [PubMed] [Google Scholar]
- 139.Vaheri A. Heparin and related polyionic substances as virus inhibitors. 1964. APMIS: Acta Pathologica Microbiologica et Immunologica Scandinavica. 2007;115:565–570. doi: 10.1111/j.1600-0463.2007.apm_686a.x. discussion 571-562. [DOI] [PubMed] [Google Scholar]
- 140.Basche M, et al. A phase I biological and pharmacologic study of the heparanase inhibitor PI-88 in patients with advanced solid tumors. Clinical Cancer Research. 2006;12:5471–5480. doi: 10.1158/1078-0432.CCR-05-2423. [DOI] [PubMed] [Google Scholar]
- 141.Metz-Boutigue MH, et al. Human lactotransferrin: amino acid sequence and structural comparisons with other transferrins. European Journal of Biochemistry. 1984;145:659–676. doi: 10.1111/j.1432-1033.1984.tb08607.x. [DOI] [PubMed] [Google Scholar]
- 142.Wu H, Monroe DM, Church FC. Characterization of the glycosaminoglycan-binding region of lactoferrin. Archives of Biochemistry and Biophysics. 1995;317:85–92. doi: 10.1006/abbi.1995.1139. [DOI] [PubMed] [Google Scholar]
- 143.Tomita M, et al. Potent antibacterial peptides generated by pepsin digestion of bovine lactoferrin. Journal of Dairy Science. 1991;74:4137–4142. doi: 10.3168/jds.S0022-0302(91)78608-6. [DOI] [PubMed] [Google Scholar]
- 144.Andersen JH, et al. Anti-HSV activity of lactoferrin and lactoferricin is dependent on the presence of heparan sulphate at the cell surface. Journal of Medical Virology. 2004;74:262–271. doi: 10.1002/jmv.20171. [DOI] [PubMed] [Google Scholar]
- 145.Iwasa M, et al. Lactoferrin inhibits hepatitis C virus viremia in chronic hepatitis C patients with high viral loads and HCV genotype 1b. American Journal of Gastroenterology. 2002;97:766–767. doi: 10.1111/j.1572-0241.2002.05573.x. [DOI] [PubMed] [Google Scholar]
- 146.Hirashima N, et al. A randomized controlled trial of consensus interferon with or without lactoferrin for chronic hepatitis C patients with genotype 1b and high viral load. Hepatology Research. 2004;29:9–12. doi: 10.1016/j.hepres.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 147.Ishibashi Y, et al. Randomized placebo-controlled trial of interferon alpha-2b plus ribavirin with and without lactoferrin for chronic hepatitis C. Hepatology Research. 2005;32:218–223. doi: 10.1016/j.hepres.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 148.Guidry JR, Raschke RA, Morkunas AR. Toxic effects of drugs used in the ICU. Anticoagulants and thrombolytics. Risks and benefits. Critical Care Clinics. 1991;7:533–554. [PubMed] [Google Scholar]
- 149.Schuksz M, et al. Surfen, a small molecule antagonist of heparan sulfate. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:13075–13080. doi: 10.1073/pnas.0805862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schmidtke M, et al. Binding of a N,N’-bisheteryl derivative of dispirotripiperazine to heparan sulfate residues on the cell surface specifically prevents infection of viruses from different families. Virology. 2003;311:134–143. doi: 10.1016/s0042-6822(03)00166-1. [DOI] [PubMed] [Google Scholar]
- 151.Selinka HC, et al. Inhibition of transfer to secondary receptors by heparan sulfate-binding drug or antibody induces noninfectious uptake of human papillomavirus. Journal of Virology. 2007;81:10970–10980. doi: 10.1128/JVI.00998-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kanwar YS, et al. Effect of beta-D-xyloside on the glomerular proteoglycans. I. Biochemical studies. Journal of Cell Biology. 1984;99:715–722. doi: 10.1083/jcb.99.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Galligani L, et al. Stimulation of synthesis of free chondroitin sulfate chains by beta-D-xylosides in cultured cells. Journal of Biological Chemistry. 1975;250:5400–5406. [PubMed] [Google Scholar]
- 154.Sobue M, et al. beta-D-xylosides and their analogues as artificial initiators of glycosaminoglycan chain synthesis. Aglycone-related variation in their effectiveness in vitro and in ovo. Biochemical Journal. 1987;241:591–601. doi: 10.1042/bj2410591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gibson KD, Segen BJ, Audhya TK. The effect of beta-D-xylosides on chondroitin sulphate biosynthesis in embryonic chicken cartilage in the absence of protein synthesis inhibitors. Biochemical Journal. 1977;162:217–233. doi: 10.1042/bj1620217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rada JA, et al. Inhibition of scleral proteoglycan synthesis blocks deprivation-induced axial elongation in chicks. Experimental Eye Research. 2002;74:205–215. doi: 10.1006/exer.2001.1113. [DOI] [PubMed] [Google Scholar]
- 157.Victor XV, et al. Investigating the elusive mechanism of glycosaminoglycan biosynthesis. Journal of Biological Chemistry. 2009;284:25842–25853. doi: 10.1074/jbc.M109.043208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Barth H, et al. Cellular binding of hepatitis C virus envelope glycoprotein E2 requires cell surface heparan sulfate. Journal of Biological Chemistry. 2003;278:41003–41012. doi: 10.1074/jbc.M302267200. [DOI] [PubMed] [Google Scholar]
- 159.Baron MJ, et al. Alpha C protein of group B Streptococcus binds host cell surface glycosaminoglycan and enters cells by an actin-dependent mechanism. Journal of Biological Chemistry. 2004;279:24714–24723. doi: 10.1074/jbc.M402164200. [DOI] [PubMed] [Google Scholar]
- 160.Oliveira FO, Jr, et al. Trypanosoma cruzi heparin-binding proteins and the nature of the host cell heparan sulfate-binding domain. Microbial Pathogenesis. 2008;44:329–338. doi: 10.1016/j.micpath.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 161.Steffen C, Wetzel E. Chlorate poisoning: mechanism of toxicity. Toxicology. 1993;84:217–231. doi: 10.1016/0300-483x(93)90118-c. [DOI] [PubMed] [Google Scholar]
- 162.Ten Dam GB, et al. 3-O-sulfated oligosaccharide structures are recognized by anti-heparan sulfate antibody HS4C3. Journal of Biological Chemistry. 2006;281:4654–4662. doi: 10.1074/jbc.M506357200. [DOI] [PubMed] [Google Scholar]
- 163.van Kuppevelt TH, et al. Generation and application of type-specific anti-heparan sulfate antibodies using phage display technology. Further evidence for heparan sulfate heterogeneity in the kidney. Journal of Biological Chemistry. 1998;273:12960–12966. doi: 10.1074/jbc.273.21.12960. [DOI] [PubMed] [Google Scholar]
- 164.Liu D, et al. Tumor cell surface heparan sulfate as cryptic promoters or inhibitors of tumor growth and metastasis. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:568–573. doi: 10.1073/pnas.012578299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.de Vries FP, et al. Neisseria meningitidis producing the Opc adhesin binds epithelial cell proteoglycan receptors. Molecular Microbiology. 1998;27:1203–1212. doi: 10.1046/j.1365-2958.1998.00763.x. [DOI] [PubMed] [Google Scholar]
- 166.Moelleken K, Hegemann JH. The Chlamydia outer membrane protein OmcB is required for adhesion and exhibits biovar-specific differences in glycosaminoglycan binding. Molecular Microbiology. 2008;67:403–419. doi: 10.1111/j.1365-2958.2007.06050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rathore D, et al. Molecular mechanism of host specificity in Plasmodium falciparum infection: role of circumsporozoite protein. Journal of Biological Chemistry. 2003;278:40905–40910. doi: 10.1074/jbc.M306250200. [DOI] [PubMed] [Google Scholar]
- 168.Johnson KM, et al. Role of heparan sulfate in attachment to and infection of the murine female genital tract by human papillomavirus. Journal of Virology. 2009;83:2067–2074. doi: 10.1128/JVI.02190-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Reiland J, et al. Heparanase degrades syndecan-1 and perlecan heparan sulfate: functional implications for tumor cell invasion. Journal of Biological Chemistry. 2004;279:8047–8055. doi: 10.1074/jbc.M304872200. [DOI] [PubMed] [Google Scholar]
- 170.van den Hoven MJ, et al. Heparanase in glomerular diseases. Kidney International. 2007;72:543–548. doi: 10.1038/sj.ki.5002337. [DOI] [PubMed] [Google Scholar]
- 171.Yang Y, et al. Heparanase enhances syndecan-1 shedding: a novel mechanism for stimulation of tumor growth and metastasis. Journal of Biological Chemistry. 2007;282:13326–13333. doi: 10.1074/jbc.M611259200. [DOI] [PubMed] [Google Scholar]
- 172.Ai X, et al. Substrate specificity and domain functions of extracellular heparan sulfate 6-O-endosulfatases, QSulf1 and QSulf2. Journal of Biological Chemistry. 2006;281:4969–4976. doi: 10.1074/jbc.M511902200. [DOI] [PubMed] [Google Scholar]
- 173.Morimoto-Tomita M, et al. Cloning and characterization of two extracellular heparin-degrading endosulfatases in mice and humans. Journal of Biological Chemistry. 2002;277:49175–49185. doi: 10.1074/jbc.M205131200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang S, et al. QSulf1, a heparan sulfate 6-O-endosulfatase, inhibits fibroblast growth factor signaling in mesoderm induction and angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4833–4838. doi: 10.1073/pnas.0401028101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lai J, et al. Loss of HSulf-1 up-regulates heparin-binding growth factor signaling in cancer. Journal of Biological Chemistry. 2003;278:23107–23117. doi: 10.1074/jbc.M302203200. [DOI] [PubMed] [Google Scholar]
- 176.Vogt AM, et al. Release of sequestered malaria parasites upon injection of a glycosaminoglycan. PLoS Pathogens. 2006;2:e100. doi: 10.1371/journal.ppat.0020100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Carlucci MJ, Scolaro LA, Damonte EB. Inhibitory action of natural carrageenans on Herpes simplex virus infection of mouse astrocytes. Chemotherapy. 1999;45:429–436. doi: 10.1159/000007236. [DOI] [PubMed] [Google Scholar]
- 178.Lee JB, et al. Antiviral activities against HSV-1, HCMV, and HIV-1 of rhamnan sulfate from Monostroma latissimum. Planta Medica. 1999;65:439–441. doi: 10.1055/s-2006-960804. [DOI] [PubMed] [Google Scholar]
- 179.Kyriacou HM, et al. In vitro inhibition of Plasmodium falciparum rosette formation by Curdlan sulfate. Antimicrobial Agents and Chemotherapy. 2007;51:1321–1326. doi: 10.1128/AAC.01216-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Havlik I, Rovelli S, Kaneko Y. The effect of curdlan sulphate on in vitro growth of Plasmodium falciparum. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1994;88:686–687. doi: 10.1016/0035-9203(94)90230-5. [DOI] [PubMed] [Google Scholar]
- 181.Garson JA, et al. Suramin blocks hepatitis C binding to human hepatoma cells in vitro. Journal of Medical Virology. 1999;57:238–242. [PubMed] [Google Scholar]
- 182.Keller MJ, et al. PRO 2000 gel inhibits HIV and herpes simplex virus infection following vaginal application: a double-blind placebo-controlled trial. Journal of Infectious Diseases. 2006;193:27–35. doi: 10.1086/498533. [DOI] [PubMed] [Google Scholar]
- 183.Vzorov AN, et al. Inactivation of human immunodeficiency virus type 1 by porphyrins. Antimicrobial Agents and Chemotherapy. 2002;46:3917–3925. doi: 10.1128/AAC.46.12.3917-3925.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Vzorov AN, et al. Parameters of inhibition of HIV-1 infection by small anionic microbicides. Antiviral Research. 2007;73:60–68. doi: 10.1016/j.antiviral.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 185.Vzorov AN, et al. Prevention of HIV-1 infection by phthalocyanines. Antiviral Research. 2003;59:99–109. doi: 10.1016/s0166-3542(03)00035-4. [DOI] [PubMed] [Google Scholar]
- 186.Dixon DW, et al. Sulfonated naphthyl porphyrins as agents against HIV-1. Journal of Inorganic Biochemistry. 2005;99:813–821. doi: 10.1016/j.jinorgbio.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 187.Marchetti M, et al. Inhibition of herpes simplex virus infection by lactoferrin is dependent on interference with the virus binding to glycosaminoglycans. Virology. 2004;318:405–413. doi: 10.1016/j.virol.2003.09.029. [DOI] [PubMed] [Google Scholar]
Further reading, resources and contacts
- Shukla D, et al. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell. 1999;99:13–22. doi: 10.1016/s0092-8674(00)80058-6. This paper elegantly showed that heparan sulfate (HS) modified by a subset of 3-O-sulfotransferase isoforms binds to HSV-1 gD and mediates viral entry. These data suggest that certain modifications might enable heparan sulfate proteoglycans (HSPGs) to function as both attachment and internalisation receptors for pathogens.
- Reeves EP, et al. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–297. doi: 10.1038/416291a. This paper described a novel function of intracellular proteoglycans in modulating bacterial killing mechanisms of neutrophils. The study showed that upon neutrophil activation, a surge of K+ ions enter granules to compensate for the accumulation of anionic charge provided by the influx of reactive oxygen species. The rise in ionic strength activates cationic granule proteins, such as elastase and cathepsin G, by releasing them from the anionic proteoglycan matrix, enabling them to kill phagocytosed bacteria. These data show that proteoglycans can also modulate intracellular host defence mechanisms against pathogens.
- Hayashida A, et al. Staphylococcus aureus beta-toxin induces acute lung injury through syndecan-1. American Journal of Pathology. 2009;174:509–518. doi: 10.2353/ajpath.2009.080394. This paper showed for the first time in vivo that a bacterial virulence factor causes inflammatory tissue damage by dysregulating the capacity of syndecan-1 to moderate neutrophil infiltration.
- Baleux F, et al. A synthetic CD4-heparan sulfate glycoconjugate inhibits CCR5 and CXCR4 HIV-1 attachment and entry. Nature Chemical Biology. 2009;5:743–748. doi: 10.1038/nchembio.207. This paper described a chemical approach to synthesise a CD 4-mimetic peptide linked to an HS dodecasaccharide. The study showed that the linkage between the CD4 mimetic and HS derivative provides strong cooperative effects, resulting in low-nanomolar antiviral activity against both CCR5- and CXCR4-tropic HIV-1 strains. The study demonstrates the feasibility of and efficacy of a new type of inhibitor, which has the unique ability to target simultaneously two critical and highly conserved regions of gp120.
- Vivès RR, Lortat-Jacob H, Fender P. Heparan sulphate proteoglycans and viral vectors: ally or foe? Current Gene Therapy. 2006;6:35–44. doi: 10.2174/156652306775515565. This review provides an overview on the multiple roles of HSPGs during viral infection, with a special focus on viruses used as gene delivery vectors. It evaluates various strategies that have been developed to potentiate the advantages or to overcome the drawbacks resulting from viral vector interaction with HS.
- Pyong Woo Park’s. URL: http://www.childrenshospital.org/cfapps/research/data_admin/Site2568/mainpageS2568P0.html.