Abstract
Mesenchymal stromal cells (MSCs) support the growth and differentiation of normal hematopoietic stem cells (HSCs). Here we studied the ability of MSCs to support the growth and survival of leukemic stem cells (LSCs) in vitro. Primary leukemic blasts isolated from the peripheral blood of 8 patients with acute myeloid leukemia (AML) were co-cultured with equal numbers of irradiated MSCs derived from unrelated donor bone marrow, with or without cytokines for up to 6 weeks. Four samples showed CD34+CD38− predominance, and four were predominantly CD34+CD38+. CD34+ CD38− predominant leukemia cells maintained the CD34+ CD38− phenotype and were viable for 6 weeks when co-cultured with MSCs compared to co-cultures with cytokines or medium only, which showed rapid differentiation and loss of the LSC phenotype. In contrast, CD34+ CD38+ predominant leukemic cells maintained the CD34+CD38+ phenotype when co-cultured with MSCs alone, but no culture conditions supported survival beyond 4 weeks. Cell cycle analysis showed that MSCs maintained a higher proportion of CD34+ blasts in G0 than leukemic cells cultured with cytokines. AML blasts maintained in culture with MSCs for up to 6 weeks engrafted NSG mice with the same efficiency as their non-cultured counterparts, and the original karyotype persisted after co-culture. Chemosensitivity and transwell assays suggest MSCs provide pro-survival benefits to leukemic blasts through cell-cell contact. We conclude that MSCs support long-term maintenance of LSCs in vitro. This simple and inexpensive approach will facilitate basic investigation of LSCs and enable screening of novel therapeutic agents targeting LSCs.
INTRODUCTION
Acute myeloid leukemia (AML) is the best studied human tumor for cancer stem cell research and has contributed to our understanding of the cancer stem cell model [1-3]. Since leukemic stem cells (LSCs) are considered to be associated with relapse, chemotherapy resistance and overall poor prognosis [4, 5], targeting LSCs has been proposed as a promising strategy for cure of AML [6-8]. Immuno-deficient mouse xenotransplantation models are the gold standard assay for LSCs, and SCID leukemia-initiating cells (SL-ICs) are thought to possess the functional and molecular characteristics of LSCs [2, 3]. However SL-ICs in primary human AML are rare and heterogeneous [4, 5, 9], therefore patient-derived human primary AML samples are not always sufficient to allow hematopoietic reconstitution in xenotransplantation model. Development of an in vitro LSC culture system would facilitate further advances in cancer stem cell research and assist in the development of novel therapeutic targets of LSCs[10]. Mesenchymal stromal cells (MSCs) are known to support the growth and differentiation of normal hematopoietic stem cells (HSCs) [11-13] through various molecular signals such as CXCL12-mediated CXCR4 signaling and Wnt-induced β-catenin signaling. Murine stromal cell lines and growth factors have been used to expand primary human AML blasts in vitro, however the leukemic blasts eventually lost their original CD34+CD38− phenotype in long-term in vitro culture systems[14, 15]. We hypothesized that human MSCs may also provide a survival benefit for LSCs since they share similar molecular signatures with normal HSCs [4, 5], and MSC co-culture systems can be utilized for long-term maintenance of LSCs in vitro even without growth factors. Furthermore, understanding relationships between AML and its stromal niche is of importance for defining mechanisms of leukemic persistence and preventing leukemic relapse. Here we co-cultured human primary leukemic blasts with unrelated bone marrow (BM) derived human MSCs and characterized the phenotype and function of leukemic blasts and their ability to engraft in a xenotransplantation mouse model.
MATERIALS AND METHODS
Primary Leukemic Samples
Peripheral blood samples were collected from eight patients with AML (mean age 53, range 23-74: Table 1). Written informed consent was obtained from the patients and healthy volunteers in accordance with the Declaration of Helsinki for the use of samples for research according to the requirements of the Institutional Review Board of the National Heart, Lung, and Blood Institute and MD Anderson Cancer Center. Cells were thawed in human cell culture medium [RPMI 1640 (Life Technologies, Carlsbad, CA) supplemented with 10% human AB serum (Gemini Bio-Products, West Sacrament, CA), 2mM L-glutamine, 100U/mL penicillin and 100 microgram/mL streptomycin (Life Technologies, Carlsbad, CA)].
Table 1.
Characteristics of AML patients
| UPN | Age | Sex | AML Subtype | Karyotype | Status at sample collection | Leukemia Phenotype |
|---|---|---|---|---|---|---|
| UPN1 | 71 | F | AML/MDS | 5q−, 7q−, 8+ | Relapse | CD34+CD38− predominant |
| UPN2 | 74 | M | AML/MDS | Normal | Refractory to chemotherapy | CD34+CD38− predominant |
| UPN3 | 43 | F | AML/MDS | 7q− | Evolved from MDS | CD34+CD38− predominant |
| UPN4 | 77 | F | AML/MPD-PV | t(2;6),del(3), −4 | De novo | CD34+CD38− predominant |
| UPN5 | 42 | M | AML-FLT3ITD | Normal | Relapse | CD34+CD38+ predominant |
| UPN6 | 23 | M | AML/MDS | Complex | Relapse | CD34+CD38+ predominant |
| UPN7 | 39 | M | M5 | 7q− | Refractory to chemotherapy | CD34+CD38+ predominant |
| UPN8 | 57 | M | AML/MDS | 7q− | De novo | CD34+CD38+ predominant |
Abbreviations: AML, acute myeloid leukemia; LSC, leukemic stem cell; MDS, myelodysplastic syndrome; MPD, myeloproliferative disease; PV, polycythemia vera
MSC isolation, culture and expansion
After obtaining informed consents, BM aspirates were collected from healthy volunteers in the Department of Transfusion Medicine, National Institutes of Health. The BM aspirates were plated in 75cm2 flask in MSC medium consisting of MEMα (Life Technologies, Carlsbad, CA) supplemented with 20% fetal bovine serum (Sigma-Aldrich, St. Louis, MO), and 1% L-glutamine (Life Technologies, Carlsbad, CA). Non-adherent cells were removed after 24 hours, and the adherent cells were cultured for approximately 14 days with twice weekly MSC medium changes. The cells were harvested using 0.05% trypsin-EDTA (Life Technologies, Carlsbad, CA) when 70% confluence was achieved and used for further expansion. The cells were plated at a density of 4 ×103/cm2 in four-layer cell factory flasks (Thermo Scientific Nunc™ Cell Factory™ Systems, Waltham, MA) in MSC medium. Serial passages were obtained once the cells reached 70% confluence and subsequently expanded MSCs were harvested and cryopreserved in liquid nitrogen. Passage 4 MSCs were thawed in human cell culture medium and were irradiated with 50Gy. The cells were then plated at selected density in flat bottom plates one day before co-culture experiments to allow reticular network formation.
Isolation of primary leukemic cells and co-culture with MSCs
Cells from primary leukemic samples were stained with antibodies to CD34-APC (clone 581, BD Biosciences, San Jose, CA), and lineage antibodies, including CD2 (clone TS1/8, Biolegend, San Diego, CA), CD3 (clone S4.1PB), CD14 (clone clone TüK4), and CD19 (clone SJ25-C1)-Pacific Blue (Invitrogen, Carlsbad, CA), as well as Propidium Iodide (PI: Molecular Probes, Eugene, OR). Lineage negative (Lin-) CD34+ cells were sorted on FACSAria II cell sorter (BD, Franklin Lakes, NJ) and 2.5 ×105 cells were co-cultured with an equal number of irradiated MSCs in 24-well flat bottom plates with or without cytokines (150 ng/ml FLT3-ligand, 150 ng/ml Stem cell factor (SCF), 50ng/ml Interleukin-3 (IL-3)). In control wells, Lin-CD34+ cells were cultured without MSC support in the presence or absence of the same cytokines. In all wells, culture media were replaced twice weekly. In transwell assays, sorted Lin-CD34+ cells were placed in the transwell insert (Costar Transwell® Permable Supports: 0.4μm pore size) with or without MSCs plated in the lower compartment.
Leukemic phenotype and cell cycle analysis
The phenotype of cultured cells was analyzed weekly using fluorescently-conjugated monoclonal antibodies against CD38-FITC (clone IM0775U), CD34-PECy7 (clone 8G12), CD11b-APCCy7 (clone ICRF44), CD123-PECy5 (clone 9F5), CD45-V500 (clone HI30), in addition to the lineage panel (CD2, CD3, CD14, CD19-Pacific Blue). Cells were also stained with Annexin V-APC (BD Biosciences, San Jose, CA) and PI and the proportion of viable, non-apoptotic cells was evaluated in Annexin V negative and PI negative populations. Admixed CD45 negative MSCs were easily distinguished from the CD45 positive leukemic cells. Stained cells were acquired on a FACS Canto II (BD Biosciences). For cell cycle analysis, at least 1 million viable cells were first stained with CD34-PECy7, CD45-APC (BD Biosciences) for 15 minutes at room temperature. After washing, the cells were incubated at 37°C, 5% CO2 for 45 minutes in 2uM Hoechst 33342 solution (Molecular Probes, Eugene, OR). Pyronin Y 1μg/ml (Polysciences, Inc. Warrington, PA) was added and incubation continued for another 45 minutes at 37°C, 5% CO2. After washing, PI was added to the cells before acquiring on an LSRII (BD, Franklin Lakes, NJ). All flow cytometry data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR). The fraction of cells in the G0, G1, S-G2-M phases of the cell cycle was determined by gating on cell populations defined by their DNA and RNA contents.
Transplantation of leukemic cells into immune deficient mice
Non-obese diabetic/severe combined immunodeficiency IL-2Rγnull (NSG) mice were purchased from Jackson Laboratories (Bar Harbor, ME) and maintained under specific pathogen-free conditions. 6-8 week old mice (2-5 mice/group) were sublethally irradiated (300cGy) 18-24 hours before transplantation. For limiting dilution analysis, specific CD34+ cell doses of primary leukemic samples ranging from 5×103 to 5×105 were injected into at least 5 NSG mice per dose. To evaluate the engraftment capacity of MSC co-cultured cells, CD45+ leukemic cells were sorted 4-6 weeks after co-culture, and injected into irradiated mice through tail-vein injection. Equivalent numbers of CD34+ leukemic cells freshly sorted from primary leukemic samples were used as controls. At week 8 after transplantation, mice were sacrificed and BM cells were harvested from both femurs and tibias. Cells were stained with human-CD45-PE, CD34-PECy7, CD38-FITC, CD123-PECy5, CD90-APC, CD45RA- and human leukemia engraftment was defined as a threshold of 0.1% human CD45+CD34+ cells in total viable BM cells. The animal experiments were approved by the Animal Care and Use Committee of the National Institutes of Health.
Cytogenetic analysis and Spectral Karyotyping (SKY)
Samples from the original uncultured Lin−CD34+ sorted leukemic blasts, as well as from Lin−CD45+ leukemic blasts sorted 4-6 weeks after MSC co-culture or 2 months after NSG transplantation were used for metaphase preparations. After mitotic arrest with colcemid (0.015 lg/mL, 2–4 hours) (GIBCO, Gaithersburg, MD) and hypotonic treatment (0.075mol/L KCl, 20 min, 37°C), the cells were fixed with methanol–acetic acid (3:1).[16] Spectral Karyotyping (Applied Spectral Imaging INC, Carlsbad, CA) and DAPI banding techniques were performed to identify structural and numerical chromosome aberrations and clonal evolution during culture. Clones were defined and karyotypes were designated according to the ISCN (2009).[17]
Chemosensitivity assay to cytarabine
Lin−CD34+ leukemic blasts were first labeled with CFSE (Vybrant® CFDA SE Cell Tracer Kit, Life Technologies, NY) according to the manufacturer's instructions. CFSE-labeled CD34+ blasts (2 ×104 cells) were co-cultured with or without an equal number of irradiated MSCs (2 ×104 cells) in 96 well flat bottom plates. Serial dilutions of cytarabine (Bedford Laboratories, OH) from 10μM to 0.5μM were added to each well, and the cells were incubated at 37°C, 5% CO2 for 48 hours. Leukemic blasts were then harvested from each well and the percentage of Annexin V- and PI-negative viable cells was determined in CFSE-labeled cells using FACS Fortessa. Relative viability was calculated using the formula: % relative viability = 100-(a/b ×100) where a is the percentage of viable cells with or without MSCs in different doses of cytarabine and b is the percentage viable cells co-culture with MSCs in the absence of cytarabine.
Statistical analysis
Data were analyzed with Prism Version 5.04 (GraphPad Software, Inc. La Jolla, CA). Results were considered statistically significant if a p value was found to be less than 0.05 based on one-way ANOVA analysis and paired t-test. The frequency of LSCs was calculated using L-Calc software (StemSoft Software Inc., Vancouver, CA).
RESULTS
Primary leukemic cell surface phenotypes are heterogeneous
We characterized surface markers of eight AML blasts on the basis of CD34 and CD38 expression within the lineage (CD2/CD3/CD14/CD19) negative population. Primary leukemic samples were phenotypically heterogeneous as previously reported [4, 9]. Based on the proportions of cells co-staining for CD34 and CD38, we classified leukemic samples as CD34+ CD38− predominant leukemias (n=4) and CD34+CD38+ predominant leukemias (n=4)(Supplementary Figure 1). The latter group included both CMP- and GMP-like leukemias (Supplementary Figure 2).
Co-culture with MSCs maintains viable leukemic blasts in vitro
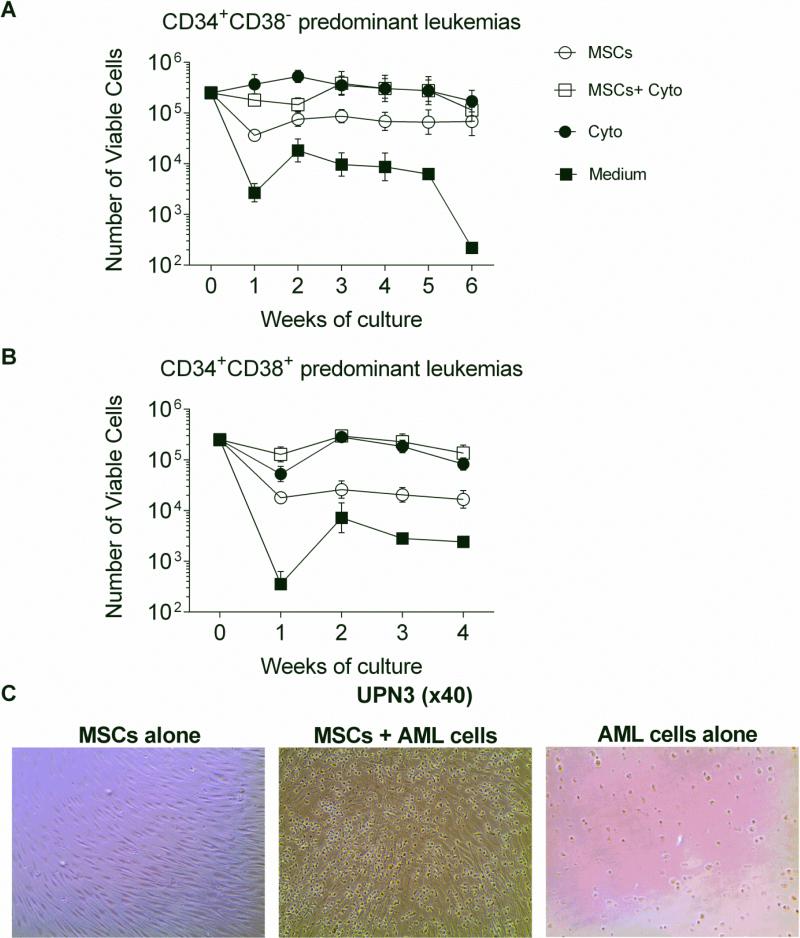
Sorted CD34+ blasts from primary leukemias were cultured in different conditions as follows: 1) co-culture with MSCs; 2) co-culture with MSCs in a cytokine cocktail consisting of Flt3L, SCF, and IL-3; 3) culture in a cytokine cocktail alone; or 4) culture in human cell culture medium only (medium alone) . All leukemic blasts retained significantly higher viable absolute counts after at least 4 weeks when co-cultured with MSCs (38±17× 103 cells: p=0.01), MSCs+cytokines (193±108× 103 cells: p=0.04), or cytokines (163±83× 103 cells: p=0.01) compared to medium alone (5±3× 103 cells) except one outlier sample (UPN1) which maintained viable cells even in medium alone (Supplementary Figure3). MSCs maintained all CD34+ CD38− predominant leukemic cells viable for up to 6 weeks (Figure 1A and Figure 1C). In contrast, CD34+ CD38+ predominant leukemic samples remained viable for only 4 weeks when co-cultured with cytokines with or without MSCs (Figure 1B).
Figure 1. Numbers of viable AML blasts generated during culture.
(A) Trend of absolute numbers of viable LSC-like leukemic blasts excluding UPN1 (separately shown in Supplementary Figure 2). Better viability of leukemic blasts was observed when co-cultured with MSCs, MSCs supplemented with cytokines (MSCs + Cyto) (IL-3, Flt3-ligand, and SCF), cytokines alone (Cyto) in contrast to medium only (Medium). (B) Trend of absolute numbers of viable non-LSC-like leukemic blasts. (C) Microscopic appearance of leukemic blasts co-cultured with MSCs. Representative images for UPN3 (CD34+ CD38− predominant leukemia) indicating preserved morphology of leukemic blasts cultured for 2 weeks in the presence (middle panel) or absence (right panel) of MSCs.
Co-culture with MSCs maintains the surface phenotype of the primary leukemias in vitro
We evaluated the cell surface phenotype of cultured leukemic cells weekly in each culture condition. CD34+ CD38− predominant leukemic samples (n=4) maintained the original CD34+CD38− phenotype when co-cultured with MSCs at 6 weeks, in contrast to cultures with MSCs+cytokines (p=0.03), cytokines (p=0.03), or medium alone (p=0.03) which showed rapid decline of the CD34+ CD38− phenotype within two weeks (Figure 2A). CD34+ CD38+ predominant leukemias (n=4) also maintained their CD34+CD38+ phenotype when co-cultured with MSCs at 2 weeks compared with MSCs+cytokines (p=0.05), cytokines (p=0.03), or medium alone (p=0.03) (Figure 2B). Absolute numbers of CD34+CD38− cells were highest in MSCs or MSCs+cytokines conditions in all CD34+ CD38− predominant leukemias (Figure 2C). Similarly, absolute numbers of CD34+CD38+ cells were highest in MSCs or MSCs+cytokines conditions in all CD34+ CD38+ predominant leukemias (Figure 2D). Cells cultured in a cytokine cocktail lost CD34 expression rapidly, suggesting differentiation of the leukemic blasts, while cells co-cultured with MSCs remained undifferentiated as shown in Figure 2E. The total number of viable leukemic cells and the persistence of the original leukemic phenotype were significantly diminished when leukemic cells were separated from MSCs in a transwell culture system as compared to co-culture conditions allowing direct cell-to-cell contact between MSCs and leukemic blasts (Supplementary Figure 4).
Figure 2. Phenotype of AML blasts during culture.
(A) Percentage of CD34+CD38− cells in CD34+ CD38− predominant leukemic samples during culture with MSCs, MSCs with cytokines (MSCs + Cyto), cytokines alone (Cyto), or medium alone (Medium). CD34+ CD38− predominant leukemias (average %CD34+CD38− 72.5%; range 55.6-90.0% at day 0) maintained a higher proportion of CD34+CD38− cells when co-cultured with MSCs compared to other culture conditions. (B) Percentage of CD34+CD38+ cells in CD34+ CD38+ predominant (average %CD34+CD38+ 55.6%; range 30.3- 89.9% at day 0) leukemic samples during culture. CD34+ CD38+ predominant leukemias maintained a higher proportion of CD34+CD38+ cells when co-cultured with MSCs. (C) Trend of absolute numbers of viable CD34+CD38− cells in CD34+ CD38− predominant leukemic blasts excluding UPN1 (separately shown in Supplementary Figure 2). (D) Trend of absolute numbers of viable CD34+CD38+ cells in CD34+ CD38+ predominant leukemic blasts. (E) Representative immunophenotype data of CD45+ leukemic blasts from patient UPN4 (CD34+ CD38− predominant leukemia) and UPN6 (CD34+ CD38+ predominant leukemia) during culture. Co-culture with MSCs favored persistence of the original phenotype compared to other culture conditions.
Leukemic blasts remained quiescent when co-cultured with MSCs
Cell cycle analysis was performed 1 and 2 weeks after initiation of in vitro culture. A higher proportion of CD45+CD34+ leukemic blasts remained in G0 when co-cultured with MSCs (average 51.1±5.1% for all 8 leukemias) compared to cultures in either MSCs+cytokines (34.8±5.1%; p=0.01) or cytokines (34.2±4.3%; p=0.05, Figures 3A and 3B) with. The G0/G1 ratio was also higher when leukemic cells were co-cultured with MSCs (2.0±0.36) compared to cultures in either MSCs+cytokines (0.99±0.25; p=0.01) or cytokines (0.99±0.28; p=0.05). Absolute numbers of G0 cells were not significantly different with or without MSCs (Figure 3C and D). These findings indicate that co-culture with MSCs favored quiescence in leukemic blasts and minimized cell cycling compared to culture conditions utilizing cytokines.
Figure 3. Cell cycle analysis of AML blasts during culture.
(A) Representative cell cycle analysis of UPN2 (CD34+ CD38− predominant leukemia) 1 week after culture with MSCs, MSCs with cytokines (MSCs + Cyto), or cytokines alone (Cyto). (B) Summary of cell cycle analysis before co-culture and 1 week after co-culture (n=8). A higher proportion of cells remained in G0 when co-cultured with MSCs compared to other culture conditions using cytokines. (C) Trend of absolute numbers of CD34+ CD38− predominant leukemic cells in the G0 phase of the cell cycle. (D) Trend of absolute numbers of CD34+ CD38+ predominant leukemic cells in the G0 phase of the cell cycle.
CD34+ CD38− predominant leukemic blasts contain higher frequencies of leukemia initiating cells engrafting in NSG mice
The frequency of leukemia initiating cells in the primary leukemias was evaluated by limiting dilution analysis using two representative samples, UPN1 (CD34+ CD38− predominant leukemia) and UPN6 (CD34+ CD38+ predominant leukemia). Eight weeks after injecting a specific dose of sorted leukemic blasts (5×103 to 5×105), both leukemias successfully engrafted as defined by more than 0.1% CD45+ CD34+ cells in the bone marrow. The calculated frequency of leukemia initiating cells (LIC) was superior in the CD34+ CD38− predominant leukemic sample tested (1 LIC per 2.7× 103, 95% CI: 1.6-4.6 ×103) compared to the CD34+ CD38+ predominant leukemic sample studied (1 LIC per 3.8× 105, 95% CI: 2.3-6.4 ×105) (p=0.0003).
Leukemic blasts in long-term MSC co-culture systems successfully engraft in NSG mice
We repeated xenotransplantation experiments to confirm the persistence of leukemia initiating cells after MSC co-culture. We cultured UPN1 and UPN6 on MSCs for 6 weeks and 4 weeks respectively, and then injected equivalent numbers of sorted CD45+ leukemic blasts from MSC co-cultures and non-cultured CD34+ leukemic blasts. Both leukemias successfully engrafted in NSG mice in 8 weeks achieving equivalent proportions of CD45+ cell engraftment compared to freshly sorted leukemic cells (UPN1: p=0.54 and UPN6: p=0.99 respectively: Figure 4A). Engrafted cells retained the original phenotypes in both CD34+ CD38− predominant and CD34+ CD38+ predominant samples (Figure 4B). These results confirmed the maintenance of leukemia initiating cells after long-term in vitro co-culture with MSCs.
Figure 4. Transplantation of AML blasts in NSG mice.
(A) Summary (post MSCs, n=5 mice; uncultured, n=2 mice) and (B) representative experiment of human cell engraftment in the bone marrow of NSG mice 8 weeks after transplantation. Both leukemic blasts harvested 4 and 6 weeks after MSC co-culture, respectively, maintained their ability to engraft compared to uncultured blasts derived from the original leukemic samples. The engrafted cells retained the original phenotypes regarding to CD34 and CD38 expression.
Maintenance of the leukemic clones after co-culture with MSCs
The original chromosomal aberrations found in UPN1 and UPN6 were monitored at different time points: 1) in fresh CD34+ sorted blasts prior to MSC co-culture; 2) in CD45+ sorted blasts 6 weeks after MSC co-culture; 3) in CD45+ blasts sorted from the bone marrow of NSG mice 8 weeks after transplantation. The chromosomal abnormalities in the original samples persisted after MSC co-culture and engraftment of MSC co-cultured cells in NSG mice; however other chromosomal aberrations were found (Figures 5A and 5B).
Figure 5. Cytogenetic analysis of leukemic blasts.
(A) Representative images of spectral karyotype analysis (SKY) and (B) cytogenetic abnormalities from clones found in leukemic blasts. For both UPN1 blasts (CD34+ CD38− predominant leukemia) and UPN6 blasts (CD34+ CD38+ predominant leukemia), the original chromosomal abnormalities (upper panels) persisted in CD45+ blasts sorted 6 weeks after MSC co-culture (middle panels) as well as in CD45+ blasts sorted from the bone marrow of NSG mice 8 weeks after transplantation (lower panels). Additional chromosomal aberrations were also detected.
MSCs promote chemotherapy resistance in leukemic blasts
To evaluate the impact of MSCs on the function of leukemic blasts, we performed a chemosensitivity assay to cytarabine. Six primary leukemic blast samples (Lin−CD34+ cells from CD34+ CD38− predominant (n=3) and CD34+ CD38+ predominant leukemias (n=3)) were incubated for 48 hrs with serial dilutions of cytarabine with or without MSCs. The viability of leukemic blasts was significantly higher with less apoptotic cells when co-cultured with MSCs (p=0.0009: Figure 6). This finding indicates that MSCs provide pro-survival benefit, protecting primary leukemic cells from chemotherapy.
Figure 6. Chemosensitivity of leukemic blasts to cytarabine.
Cells from six primary leukemic samples were cultured for 48 hours with or without MSCs in the presence of different doses of cytarabine. Data are represented as a relative percentage of viability compared MSC co-cultured blasts. The viability of leukemic blasts was significantly higher when co-cultured with MSCs.
DISCUSSION
BM-derived MSCs represent part of the multicellular stromal support that creates a milieu to maintain HSC function and allow growth and differentiation of hematopoietic progenitors. The ease with which MSCs can be expanded in vitro facilitates the study of the interaction of MSCs with hematopoietic progenitors. MSCs have been successfully employed to increase the hematopoietic stem and progenitor cell numbers in cord blood for transplantation [11]. A current interest is whether the marrow milieu is also supportive to AML stem cells and represents a sanctuary for persistence of LSCs after remission. Understanding the nature of the stroma-LSC interactions would inform more effective therapy to eliminate residual leukemia persisting after induction chemotherapy. Furthermore an in vitro stromal/LSC co-culture system that replicates favorable conditions for LSC persistence would serve as a more relevant model for testing agents active against LSCs.
In this study, we demonstrated that unrelated allogeneic human MSCs supported a LSC phenotype in a largely quiescent state in a long-term vitro culture system. We then confirmed the persistence of LIC after 6 weeks co-culture with MSCs using the NSG mouse xenotransplant model. Our results also revealed that MSCs, in contrast to cocktails of stem cell growth factors or medium alone, support survival of quiescent LSCs with minimal outgrowth of more differentiated leukemic populations. Of interest, for one of 8 leukemic samples studied, LSCs were maintained in culture for up to 6 weeks even without MSC support. Variability between leukemic samples has been described before [14]. While the mechanisms underlying the atypical in vitro growth pattern of leukemic blasts in this patient remain unclear, we have observed increased telomere length in this sample compared to leukemic cells derived from age-matched patients (unpublished data). The maintenance of viable leukemic blasts in a stromal co-culture system is consistent with the previous findings observed by van Gosliga et al[15] using murine MS5 stromal cell lines and by Klco et al[14] using human HS27 stromal cell lines to expand primary AML blasts. However, both groups combined MSCs with growth factors (SCF, IL-3, Flt-3 ligand +/− TPO), and one study demonstrated loss of the CD34+ cell population after long-term co-culture [15]. While the distinct stromal cells utilized in each study may account for the differences observed, our data suggest that addition of growth factors during co-culture results in depletion of LSCs by promoting their differentiation in vitro.
We also demonstrated that the protective effect on LSCs requires direct cell-to-cell contact since a transwell culture system failed to replicate the findings in vitro. The nature of the interactions between MSCs and leukemic blasts that favor survival, quiescence and maintenance of the original phenotype remains to be elucidated. Despite the prolonged culture (and in keeping with the preservation of quiescence) we found that the clonality of the original leukemic population was largely preserved by MSCs. This ability of MSCs to preserve the original leukemic phenotype was observed more significantly in CD34+ CD38− predominant leukemias than CD34+ CD38+ predominant leukemias. The non-LSC-like leukemias had two logs fewer leukemia initiating cells than the LSC samples, thus the preferential pro-survival effects of MSCs in vitro may be largely restricted to preserving leukemic stem cells. Further studies to identify the mechanisms of LSC support by MSCs should shed light on the role of MSCs in the marrow milieu.
While remission induction has continued to improve in patients with AML, disease relapse remains a major limitation for cure. We showed that leukemic blasts maintained on MSCs were more resistant to cytotoxicity from cytarabine, a widely used anthracycline to induce remission in AML. Currently allogeneic stem cell transplantation represents one of the most reliable strategies for eliminating residual disease through a graft-versus-leukemia (GVL) effect. Leukemic cells capable of initiating leukemia in a xenograft model are currently the best descriptor of the cell type responsible for leukemic relapse after chemotherapy. Our in vitro model could be used to study interactions of alloreactive lymphocytes with GVL-like effects on LSCs as well as to explore new agents with specificity for quiescent LSCs. While we show differences in the supportive capacity of MSCs for CD34+ CD38− predominant and CD34+ CD38+ predominant leukemias, additional studies will be needed to clarify the nature of MSC interactions with specific AML subtypes, and to correlate LSC maintenance in vitro with relapse probability in vivo.
CONCLUSION
Co-culture of leukemic blasts with MSCs represents a simple approach to maintain leukemia initiating cells in vitro. This culture system will permit exploration of the protective effects of the marrow microenvironment on leukemic stem cells and serve as an improved method to explore therapeutic approaches targeting the leukemic stem cell.
Supplementary Material
HIGHLIGHTS.
- Unrelated human MSCs support the maintenance of LSCs in a quiescent state in vitro.
- LSCs co-cultured with MSCs engrafted NSG mice without losing the efficiency.
- MSCs provided chemo-protection to primary LSCs.
- Such in vitro culture systems would provide a simple and inexpensive approach to investigate LSCs.
ACKNOWLEDGEMENTS
We thank M Franco Colon, and F Chinian (Hematology Branch, NHLBI, NIH, USA) for their technical support. We also thank all patients who donated samples for this study.
FUNDIND
This research was supported by the Intramural Research Programs of the National Heart, Lung, and Blood Institute and the National Human Genome Research Institute, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS:
Study concept and design (S.I, A.J.B., A.L., J.J.M.): In vitro experiment and data collection (S.I., A.L., J.J.M., S.M., P.M., A.D., E.P., K.K., N.H.): Analysis and interpretation of data (S.I., A.J.B., P.L., J.J.M., A.L.): Drafting of the manuscript (S.I, A.L., K.R., P.M., P.L., J.J.M., A.J.B.) Clinical sample and clinical data collection (S.I., K.R., A.J.B.): Obtained funding and study supervision (A.J.B., A.L.).
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
All authors declare no conflict of interest.
REFERENCES
- 1.Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112:4793–4807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- 2.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia M, Bonnet D, Murdoch B, Gan OI, Dick JE. A newly discovered class of human hematopoietic cells with SCID-repopulating activity. Nat Med. 1998;4:1038–1045. doi: 10.1038/2023. [DOI] [PubMed] [Google Scholar]
- 4.Eppert K, Takenaka K, Lechman ER, Waldron L, Nilsson B, van Galen P, Metzeler KH, Poeppl A, Ling V, Beyene J, Canty AJ, Danska JS, Bohlander SK, Buske C, Minden MD, Golub TR, Jurisica I, Ebert BL, Dick JE. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat Med. 2011;17:1086–1093. doi: 10.1038/nm.2415. [DOI] [PubMed] [Google Scholar]
- 5.Gentles AJ, Plevritis SK, Majeti R, Alizadeh AA. Association of a leukemic stem cell gene expression signature with clinical outcomes in acute myeloid leukemia. JAMA. 2010;304:2706–2715. doi: 10.1001/jama.2010.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saito Y, Yuki H, Kuratani M, Hashizume Y, Takagi S, Honma T, Tanaka A, Shirouzu M, Mikuni J, Handa N, Ogahara I, Sone A, Najima Y, Tomabechi Y, Wakiyama M, Uchida N, Tomizawa-Murasawa M, Kaneko A, Tanaka S, Suzuki N, Kajita H, Aoki Y, Ohara O, Shultz LD, Fukami T, Goto T, Taniguchi S, Yokoyama S, Ishikawa F. A Pyrrolo-Pyrimidine Derivative Targets Human Primary AML Stem Cells in Vivo. Sci Transl Med. 2013;5:181ra152. doi: 10.1126/scitranslmed.3004387. [DOI] [PubMed] [Google Scholar]
- 7.Saito Y, Kitamura H, Hijikata A, Tomizawa-Murasawa M, Tanaka S, Takagi S, Uchida N, Suzuki N, Sone A, Najima Y, Ozawa H, Wake A, Taniguchi S, Shultz LD, Ohara O, Ishikawa F. Identification of therapeutic targets for quiescent, chemotherapy-resistant human leukemia stem cells. Sci Transl Med. 2010;2:17ra19. doi: 10.1126/scitranslmed.3000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Misaghian N, Ligresti G, Steelman LS, Bertrand FE, Basecke J, Libra M, Nicoletti F, Stivala F, Milella M, Tafuri A, Cervello M, Martelli AM, McCubrey JA. Targeting the leukemic stem cell: the Holy Grail of leukemia therapy. Leukemia. 2009;23:25–42. doi: 10.1038/leu.2008.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarry JE, Murphy K, Perry R, Sanchez PV, Secreto A, Keefer C, Swider CR, Strzelecki AC, Cavelier C, Recher C, Mansat-De Mas V, Delabesse E, Danet-Desnoyers G, Carroll M. Human acute myelogenous leukemia stem cells are rare and heterogeneous when assayed in NOD/SCID/IL2Rgammac-deficient mice. J Clin Invest. 2011;121:384–395. doi: 10.1172/JCI41495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennedy JA, Barabe F. Investigating human leukemogenesis: from cell lines to in vivo models of human leukemia. Leukemia. 2008;22:2029–2040. doi: 10.1038/leu.2008.206. [DOI] [PubMed] [Google Scholar]
- 11.de Lima M, McNiece I, Robinson SN, Munsell M, Eapen M, Horowitz M, Alousi A, Saliba R, McMannis JD, Kaur I, Kebriaei P, Parmar S, Popat U, Hosing C, Champlin R, Bollard C, Molldrem JJ, Jones RB, Nieto Y, Andersson BS, Shah N, Oran B, Cooper LJ, Worth L, Qazilbash MH, Korbling M, Rondon G, Ciurea S, Bosque D, Maewal I, Simmons PJ, Shpall EJ. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med. 2012;367:2305–2315. doi: 10.1056/NEJMoa1207285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10:709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Robinson SN, Ng J, Niu T, Yang H, McMannis JD, Karandish S, Kaur I, Fu P, Del Angel M, Messinger R, Flagge F, de Lima M, Decker W, Xing D, Champlin R, Shpall EJ. Superior ex vivo cord blood expansion following co-culture with bone marrow-derived mesenchymal stem cells. Bone Marrow Transplant. 2006;37:359–366. doi: 10.1038/sj.bmt.1705258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klco JM, Spencer DH, Lamprecht TL, Sarkaria SM, Wylie T, Magrini V, Hundal J, Walker J, Varghese N, Erdmann-Gilmore P, Lichti CF, Meyer MR, Townsend RR, Wilson RK, Mardis ER, Ley TJ. Genomic impact of transient low-dose decitabine treatment on primary AML cells. Blood. 2013;121:1633–1643. doi: 10.1182/blood-2012-09-459313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Gosliga D, Schepers H, Rizo A, van der Kolk D, Vellenga E, Schuringa JJ. Establishing long-term cultures with self-renewing acute myeloid leukemia stem/progenitor cells. Exp Hematol. 2007;35:1538–1549. doi: 10.1016/j.exphem.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Schrock E, du Manoir S, Veldman T, Schoell B, Wienberg J, Ferguson-Smith MA, Ning Y, Ledbetter DH, Bar-Am I, Soenksen D, Garini Y, Ried T. Multicolor spectral karyotyping of human chromosomes. Science. 1996;273:494–497. doi: 10.1126/science.273.5274.494. [DOI] [PubMed] [Google Scholar]
- 17.International Standing Committee on Human Cytogenetic Nomenclature. Shaffer LG, Slovak ML, Campbell LJ. ISCN 2009 : an international system for human cytogenetic nomenclature (2009) Karger; Basel: 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.