Abstract
In the past, partially HLA-mismatched related donor, or HLA-haploidentical, blood or marrow transplantation (haploBMT), for hematologic malignancies has been complicated by unacceptably high incidences of graft rejection or GvHD resulting from intense bi-directional alloreactivity. Administration of high doses of cyclophosphamide early after haploBMT selectively kills proliferating, alloreactive T cells while sparing non-alloreactive T cells responsible for immune reconstitution and resistance to infection. In the clinic, haploBMT with high-dose, post-transplantation cyclophosphamide is associated with acceptably low incidences of fatal graft rejection, GvHD and non-relapse mortality, and provides an acceptable treatment option for hematologic malignancies patients lacking suitably HLA-matched donors. HaploBMT with PTCy is now being investigated as a treatment of hemoglobinopathy and as a method for inducing tolerance to solid organs transplanted from the same donor. Ongoing and future clinical trials will establish the hierarchy of donor preference for hematologic malignancy patients lacking an HLA-matched sibling.
INTRODUCTION
Allogeneic blood or marrow transplantation, or alloBMT, is an accepted and potentially curative therapy for patients with hematologic malignancies that cannot be cured by standard chemotherapy alone. An important therapeutic component of alloBMT is the ‘graft-versus-tumor’ effect mediated by donor T cells reactive to host histocompatibility Ags. Historically, the best results of alloBMT have been obtained when the donor is an HLA-matched sibling or an unrelated donor who is matched to the recipient at each of eight high-expression HLA molecules: both alleles at each of HLA-A, -B, -C and -DRB1. Unfortunately, as many as half of patients in need of hematopoietic stem cell transplantation (HSCT) do not have an HLA-matched sibling or unrelated donor. Alternative stem cell sources such as umbilical cord blood or partially HLA-mismatched, or HLA-haploidentical, related donors have recently emerged to fill the void for patients lacking HLA-matched donors. Potential HLA-haploidentical donors include a patient’s biological parents or children, and each sibling or half-sibling of a patient has a 50% chance of being HLA-haploidentical. Thus, it is highly likely that any patient has an HLA-haploidentical donor within the immediate family. Advantages of the HLA-haploidentical option include several potential donors per patient, rapid graft acquisition time and availabililty of the donor to donate lymphocytes in the case of post-transplantation relapse. The major disadvantage of the HLA-haploidentical option is intense bi-directional alloreactivity that occurs as a result of HLA mismatch, resulting in historically high incidences of graft rejection1 and severe GvHD2 T-cell depletion of an HLA-haploidentical graft reduces the risk of GvHD but at the expense of a higher incidence of graft rejection3 and susceptibility to opportunistic infection.4 As leaving all T cells in the HLA-haploidentical graft resulted in excessive rates of severe GVHD, and removing all T cells from the graft resulted in unacceptable rates of graft failure or opportunistic infection, there has been a pressing need to develop methods to selectively remove alloreactive T cells from both the donor and recipient while sparing the donor lymphocytes that are responsible for providing immune reconstitution and resistance to infection. High-dose, post-transplantation cyclophosphamide (PTCy) was developed as a method of selective in vivo allodepletion5 for HLA-haploidentical stem cell transplantation and is the subject of this review.
Immunobiology of the allogeneic response
A cardinal feature of the immune response is the high frequency of T cells reactive to allogeneic MHC molecules. Whereas the frequency of T cells that react to a specific foreign peptide or a non-MHC, or minor, histocompatibility antigen is on the order of 10−4 to 10−5, ~1–10% of all T cells in an immunocompetent animal will react to an allogeneic MHC molecule, and the vast majority of T cells will react to at least one of a panel of several allogeneic stimulators. The explanation for the high frequency of T cells reactive to allogeneic MHC molecules is still debated, but a plausible hypothesis is that an MHC molecule can bind to any of several distinct peptides, leading to many unique shapes that can be recognized by many different T cells.6 In addition to the degeneracy of peptide binding by MHC molecules, the phenomenon of immunologic cross-reactivity also contributes to the intensity of the response to allogeneic MHC.7 Experiments have shown that virus infection leads to the proliferation and clonal expansion of T cells that also proliferate or lyse allogeneic, uninfected stimulator cells.8 Thus, T cells can differentiate to memory cells as a result of infection, but such memory cells can also respond to MHC-disparate stimulator cells. This phenomenon creates a challenge in clinical transplantation because memory T cells are much more resistant to tolerance induction than are their naive counterparts.9 The clinical manifestations of the high frequency of HLA-alloreactive T cells and the significant proportion of memory T cells are a higher incidence of graft rejection and severe acute GVHD after partially HLA-mismatched as opposed to HLA-matched alloBMT.
PTCy: preclinical data
Administration of immunosuppressive drugs is required after T cell replete alloBMT, whether HLA-matched or not, because without these drugs the risks of graft failure, severe acute GvHD and nonrelapse mortality are prohibitive. The gold standard of immunosuppression in HLA-matched BMT has been a calcineurin inhibitor, such as cyclosporine or tacrolimus, plus methotrexate, mycophenolate mofetil or sirolimus. The combination of cyclosporine plus methotrexate has not been adequate for HLA-haploidentical BMT, as the incidences of severe GvHD, graft failure and non-relaspe mortality were considered unacceptable.2 In contrast, PTCy has been associated with acceptably low incidences of these complications, as detailed below.
High-dose PTCy is essentially an example of the general strategy of drug-induced immunological tolerance as first described by Schwartz and Dameshek in 1959.10 Their experiments employed 6-mercaptopurine as the tolerance-inducing drug, but Berenbaum tested a variety of agents for immunologic tolerance induction and found cyclophosphamide to be well-tolerated and highly effective.11 The pioneering experiments of Schwartz and Dameshek had used abrogation of Ab production as the read-out of tolerance, but Berenbaum examined the effect of cyclophosphamide on the survival of skin allografts in rats. Contrary to expectations, the drug was more effective at prolonging MHC-disparate skin graft survival when given after as opposed to before transplantation. Median survival of skin grafts was ~ 12 days in untreated rats versus 16–19 days in rats treated with Cyclophosphamide on day 3 after transplantation. However, grafts were ultimately rejected in all animals, indicating that PTCy alone is inadequate to induce tolerance across an MHC barrier.
A series of elegant experiments by Mayumi et al.12 established that it is possible to induce tolerance to minor histocompatibility Ags (miHAs) in mice by a simple strategy comprising the i.v. infusion of a high dose of MHC-matched, miHA-mismatched spleen cells followed by the i.p. injection of cyclophosphamide ≥150 mg/kg from 48 to 72 h after spleen cell infusion.12 Tolerance to miHAs was associated with a low level of donor hematopoietic chimerism, the absence of recipient cytotoxicity against donor cells and indefinite survival of donor strain but not third party strain skin grafts. Thus, tolerance was exquisitely specific to the Ags present on the donor cells. The authors proceeded to define the optimal protocol for tolerance induction, including a requirement for ≥50 million donor spleen cells to be given intravenously, and administration of a ≥150 mg/kg cyclophosphamide given in the precise time window of 48–72 h after the i.v. injection of the donor spleen cells.13 Administration of Cyclophosphamide either 24h or 72 h after i.v. infusion of miHA-incompatible cells failed to result in low-level chimerism or tolerance induction. Interestingly, if the spleen cell donors were primed against host miHAs by an i.p. injection of host spleen cells 1 week before i.v. donor cell administration and PTCy, full donor hematopoietic chimerism was achieved but the recipient animals died of GvHD.14 This result suggests that miHA-primed, effector T cells are resistant to PTCy and can cause lethal GvHD following adoptive transfer.
The same group also performed extensive mechanistic studies of Cyclophosphamide-induced tolerance to miHAs, concluding that tolerance is induced via the peripheral destruction of alloreactive (host-versus-graft as well as graft-versus-host-reactive) T cells and by the generation of intrathymic mixed chimerism resulting in clonal deletion of host-versus-graft reactive T cells, but at later stages the thymic chimerism is lost and tolerance is maintained by a peripheral suppressive mechanism.15–21
As mentioned above, the simple protocol of high-dose spleen cell infusion followed in 48–72 h by high-dose cyclophosphamide administration failed to induce tolerance to MHC-incompatible cells. However, Mayumi and Good22 were able to achieve tolerance across an MHC barrier in mice by adding pretransplant administration of anti-lymphocyte serum to allogeneic spleen cell infusion followed by PTCy. In this model system, all three components of the therapy were required for tolerance induction, and a high number of donor hematopoietic cells were required for tolerance. Colson and Ildstad23 likewise achieved tolerance across the MHC barrier by treating the transplant recipient with ≥500 cGy TBI before bone marrow infusion and PTCy,23 and the dose of TBI required for tolerance could be lowered to 300 cGy if the recipient was also treated with antilymphocyte serum before transplantation.24 Luznik et al.5 achieved mixed hematopoietic chimerism and tolerance across MHC barriers by conditioning mice with fludarabine and TBI followed by BMT and administration of PTCy. Not only did PTCy prevent graft rejection in this model, it also mitigated GvHD in mice given lethal conditioning and transfused with MHC-mismatched bone marrow and spleen cells. These results demonstrate that PTCy inhibits alloreactivity in both the host-versus-graft (rejection) and graft-versus-host directions. Another important feature of this regimen is that it is truly nonmyeloablative, as reconstitution of autologous hematopoiesis occurred in all animals that received conditioning and ‘posttransplantation’ cyclophosphamide but did not receive an allograft.
Post-transplantation cyclophosphamide in the clinic
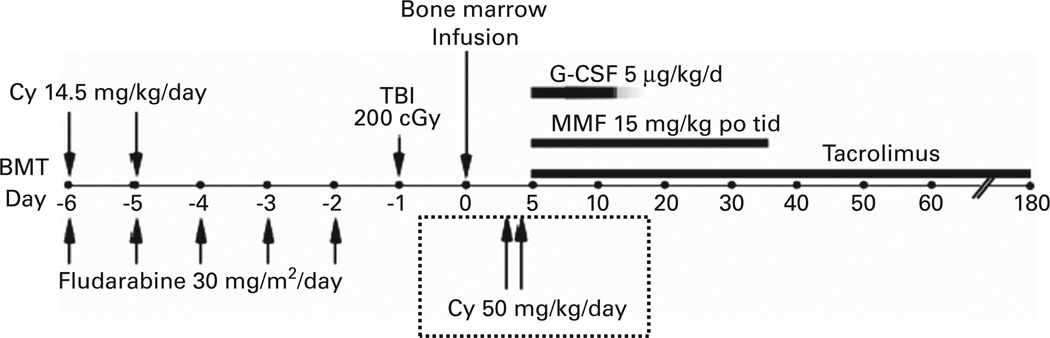
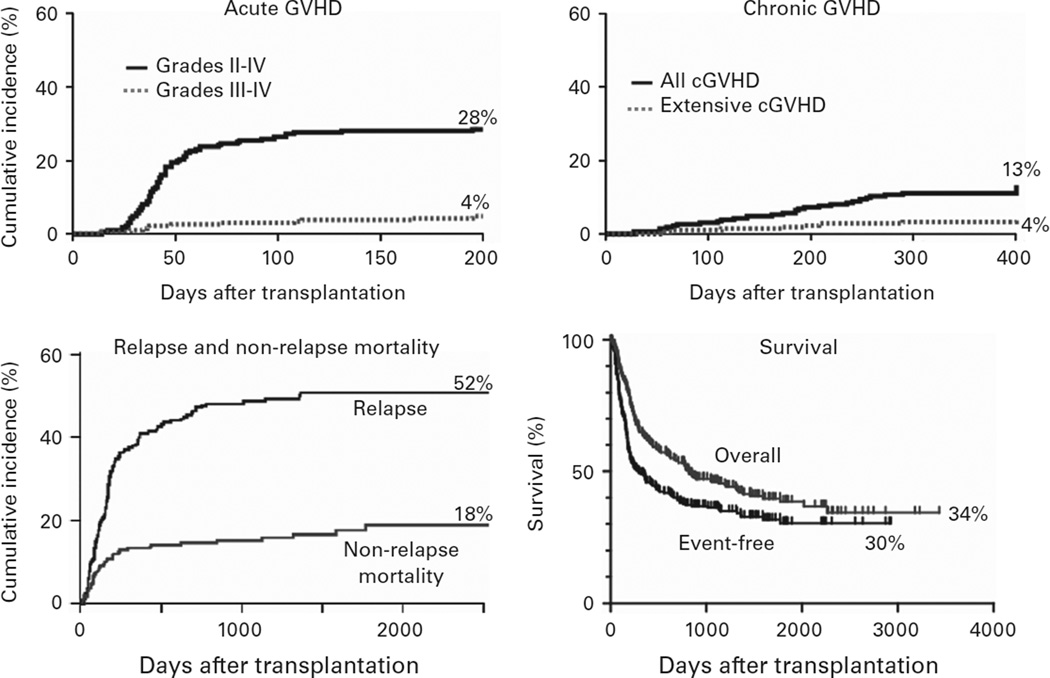
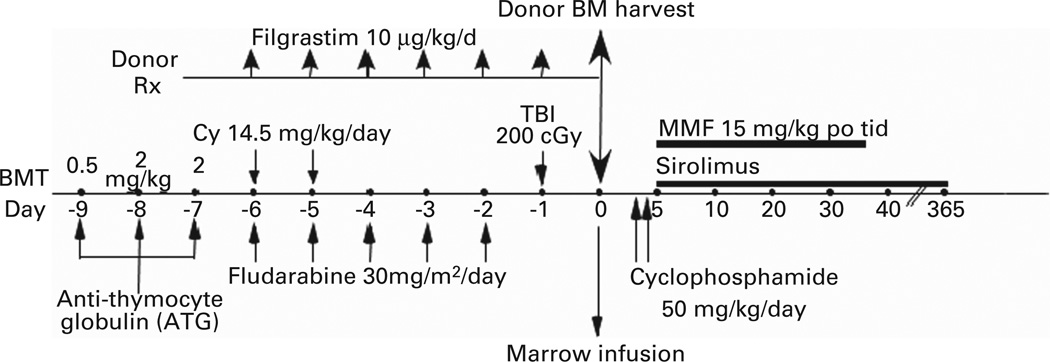
The regimen developed by Luznik et al.25 was adapted to the clinic for the treatment of patients with hematologic malignancies (Figure 1).25 Three hundred and seventy-five patients at the Sidney Kimmel Comprehensive Cancer Center (SKCCC) at Johns Hopkins have been treated with this regimen between 2004 and 2012. Patients were eligible for treatment if they were referred for allogeneic stem cell transplantation and lacked an HLA-matched sibling but had a partially HLA-mismatched first degree relative, including parents, children, siblings or half siblings. HLA typing was performed at high resolution at HLA-A, -B, -C, -DRB1 and -DQB1. To be considered as HLA-haploidentical, a donor had to be matched at high resolution for at least one allele of each of these loci. Among 236 patients transplanted between 2004 and 2009, the median patient age was 52 and the median donor age was 42. Forty-eight percent of donors were siblings or half-siblings, 35% were children and 17% were parents of the patient. Graft failure in the absence of relapsed disease occurred in 19 out of 228 evaluable patients (8.3%), but nearly all patients experiencing graft failure had recovery of autologous hematopoiesis, confirming that the regimen is non-ablative. Major outcomes of transplantation for these 236 patients are shown in Figure 2. The safety of the procedure is quite remarkable. The cumulative incidences of grades II-IV and III-IV acute GVHD by day 200 after transplantation were 28 and 4%, respectively, similar to the incidences of acute GVHD after reduced intensity conditioning and HLA-matched BMT.26 The incidences of chronic GVHD and non-relapse mortality were also acceptably low. Relapse exceeded 50%, but it cannot be determined whether this apparently high incidence of relapse is due to suppression of a graft-versus-tumor effect by PTCy or due to the generally poor risk profile of patients undergoing this procedure at our institution.
Figure 1.
Treatment schema for nonmyeloablative, HLA-haploidentical bone marrow transplantation (haploBMT) with high-dose, posttransplantation cyclophosphamide (PTCy). Cy = cyclophosphamide; MMF = mycophenolate mofetil.
Figure 2.
Major outcomes of non-myeloablative, haploBMT with PTCy. OS = overall survival.
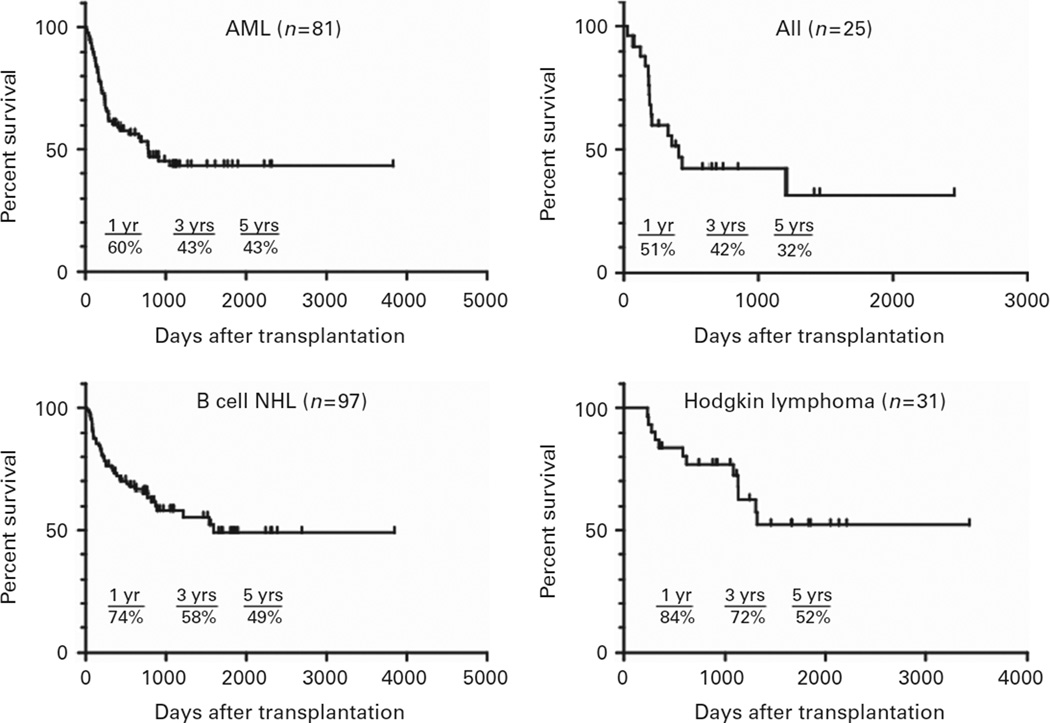
Figure 3 shows overall survival for patients with acute leukemia or lymphoma who received reduced intensity, HLA-haploidentical BMT as recently as 2012. Each curve appears to achieve a plateau, with 5-year survivals of 32% for patients with ALL, 43% for patients with AML, 49% for patients with B-cell non-Hodgkin lymphoma and 52% for patients with Hodgkin lymphoma. Twenty-two of the 31 patients with Hodgkin lymphoma had experienced disease relapse after prior autologous stem cell transplantation, demonstrating that haploidentical BMT is an effective salvage therapy for patients with chemotherapy-resistant disease.
Figure 3.
Survival after nonmyeloablative, haploBMT with PTCy, by tumor histology. NHL = non-Hodgkin lymphoma.
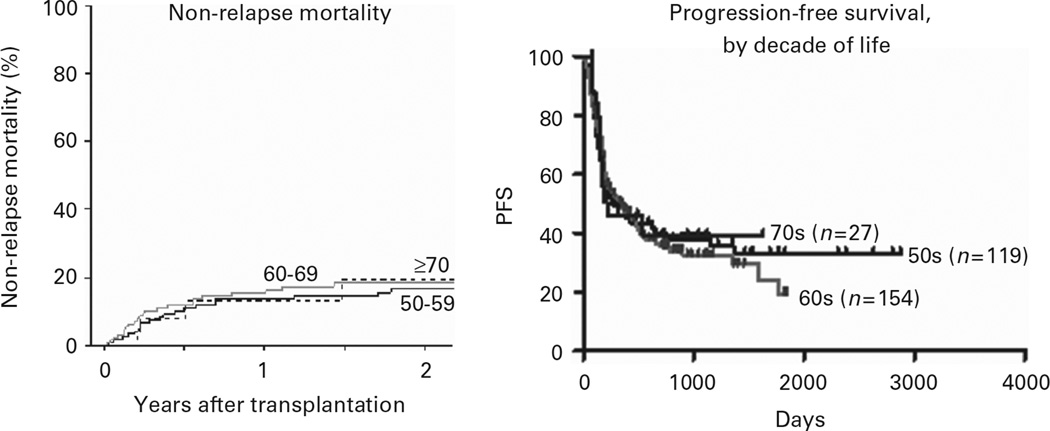
HLA-haploidentical BMT was originally developed as a therapeutic alternative for hematologic malignancy patients who were referred for transplantation but who lacked an HLA-matched sibling or suitably matched unrelated donor. With the favorable toxicity profile described above, we have extended this option to older adults with hematologic malignancies. Patients up to the age of 75 have been eligible if they lack HLA-matched sibling donor and meet eligibility criteria for organ function, including a left ventricular ejection fraction ≥35%, forced expiratory volume in 1 s and forced vital capacity >40% of predicted, bilirubin ≤3.0 mg/dL (except for patients with Gilbert’s disease), and alanine and aspartate transaminases less than five times the upper limit of normal. We have recently analyzed the outcomes of reduced intensity, haploidentical BMT with PTCy according to patient age by decade for patients 50 and above (Figure 4).27 The left panel shows that non-relapse mortality was no higher among patients 70 years of age and above compared with patients in their 50 s or 60 s. PFS also did not differ significantly between those over 70 versus younger patients (right panel). As a significant fraction of hematologic malignancy patients are above the age of 70, the tolerability of haploBMT with PTCy should broaden the application of alloBMT.
Figure 4.
Non-relapse mortality (left) and PFS (right) after nonmyeloablative haploBMT with PTCy, by patient age (decades).
HaploBMT with PTCy for sickle cell disease
The improved safety of alloBMT in general and haploBMT in particular has facilitated the application of the procedure to treat patients with non-malignant disorders. Sickle cell disease (SCD) is a severely disabling and life shortening hemoglobinopathy resulting from a mutation in the gene encoding the hemoglobin beta chain. The disease is readily curable by myeloablative conditioning and transplantation of stem cells from an unaffected, HLA-matched sibling.28 Unfortunately, an HLA-matched sibling is available for only 18% of SCD patients, and many patients decline myeloablative BMT because of the substantial risks of toxicity, especially chronic GvHD.29 The reduced intensity, HLA-haploidentical BMT platform offers three significant advantages for the treatment of SCD: (1) near universal availability of donors, since biologic parents of the patient are guaranteed to be HLA-haploidentical and are also heterozygous for the sickle cell mutation; (2) ability to reconstitute autologous hematopoiesis in the event of graft failure; and (3) a low incidence of chronic GVHD, which patients perceive as the most unacceptable complication of alloBMT.29 The treatment schema shown in Figure 1 was applied to SCD patients receiving HLA-matched sibling or HLA-haploidentical bone marrow and PTCy for GVHD prophylaxis. Three changes have been made as the treatment protocol has evolved: (1) sirolimus was substituted for tacrolimus because of the occurrence of posterior reversible encephalopathy syndrome, a potential complication of calcineurin inhibitors such as tacrolimus or cyclosporine; (2) anti-thymocyte globulin has been added to the conditioning of the patient to reduce the risk of graft failure; and (3) donors are treated with filgrastim before bone marrow harvest to increase the marrow nucleated cell dose, again to overcome graft rejection.22,30,31 Our current conditioning regimen is shown in Figure 5. We have reported the outcomes of RIC alloBMT in 17 patients with SCD, 14 of whom received bone marrow from HLA-haploidentical first degree relatives and 3 of whom received bone marrow from an HLA-matched sibling.32 All three recipients of HLA-matched sibling bone marrow have experienced engraftment and are stable mixed hematopoietic chimeras on continuing single agent immunosuppression. In contrast, graft failure has occurred in six of fourteen recipients of HLA-haploidentical bone marrow. Of the eight patients with stable donor chimerism, seven evolved to full donor hematopoietic chimerism and one patient has very low levels of donor chimerism (<10% donor) with ongoing sickle hematopoiesis. At the time of reporting, six of the seven patients with full donor hematopoietic chimerism had successfully discontinued immunosuppressive medications with no evidence of GVHD, while the seventh patient was still on single agent sirolimus <1 year after transplantation. Importantly, among these 17 patients there was only one case of grade I acute GVHD, which resolved without treatment, no mortality and no serious long-term morbidities attributable to transplantation. Reduced intensity, HLA-haploidentical BMT with PTCy is therefore a valid therapeutic option for patients with SCD who lack an HLA-matched sibling or unrelated donor. Graft failure remains a significant risk. More research is required to develop methods of overcoming graft rejection, especially for patients with SCD who have been heavily sensitized to HLA alloantigens via repeated blood transfusions.
Figure 5.
Treatment schema for nonmyeloablative, haploBMT with PTCy for patients with sickle cell disease. BMT = bone marrow transplantation; BM = bone marrow; Cy = cyclophosphamide; MMF = mycophenolate mofetil.
HLA-haploidentical BMT plus PTCy for induction of tolerance to solid organ allografts
The pioneering studies by Owen,33 and Medawar and his colleagues34,35 established that hematopoietic chimerism, the stable presence of blood cells from a genetically distinct donor, was associated with tolerance to grafts of solid organs from the same donor. Indeed, case reports have shown that patients who develop kidney or liver failure after undergoing allogeneic stem cell transplantation for a hematologic disorder can be successfully transplanted from the same donor and weaned off immunosuppression without experiencing graft rejection.36 Kidney transplantation is an established and effective treatment for patients with end-stage renal failure. Such patients must take immunosuppressive drugs for the remainder of their lives to prevent acute graft rejection. Despite significant advances in pharmacologic immunosuppression, there is still a 4% per year rate of graft loss from chronic rejection, and these drugs are associated with significant long-term complications such as infection, cancer and heart disease. There is significant interest in leveraging hematopoietic chimerism to induce tolerance to solid organ allografts, thereby permitting discontinuation of immunosuppressive medications.
Leventhal et al.37 have utilized high-dose PTCy (50 mg/kg i.v. on day 3) to facilitate induction of tolerance in combined kidney/hematopoietic stem cell transplantation from fully or partially HLA-mismatched unrelated donors. Our group is adapting the regimen shown in Figure 5, with the only modifications being that the kidney is transplanted along with the bone marrow on day 0, tacrolimus, MMF and steroids are substituted for sirolimus and MMF, and tapering of immunosuppression is commenced at 6 months after combined transplantation with the goal of complete discontinuation by 1 year after transplantation. This trial of BMT and high-dose PTCy for chimerism induction and renal allograft tolerance (ACCEPTOR; clinicaltrials.gov #NCT02029638) is sponsored by the US National Institutes of Health via the Immune Tolerance Network and accrued its first patient in February, 2014. The primary objective of this trial is to assess the ability of BMT and high-dose PTCy to induce renal allograft tolerance and thus enable discontinuation of immunosuppressive therapy in haploidentical living related donor renal transplant recipients.
HLA-haploidentical stem cell transplantation: where do we go from here?
The haploidentical option for hematopoietic stem cell (HSC) transplantation has always been attractive from the standpoint of donor availability, donor motivation, potential for combined donation of HSCs and a solid organ, and availability of the original donor to donate lymphocytes in the event of post-transplantation relapse of the underlying malignancy. The only drawback to haploidentical BMT has been intense bi-directional alloreactivity, which made this therapeutic option unacceptable in the past.2,3,38 We believe that PTCy reduces alloreactivity to the point that HLA-haploidentical BMT can now be performed with safety and efficacy equivalent, at least, to the alternative graft sources-unrelated donor umbilical cord blood, and mismatched adult unrelated donors. It is even possible that haploBMT with PTCy is as safe and effective as 8/8 matched unrelated donor or even HLA-matched sibling BMT. The US Blood and Marrow Transplant Clinical Trials Network (BMT CTN) is conducting a randomized, phase III trial of reduced intensity conditioning and transplantation of either double unrelated donor umbilical cord blood versus HLA-haploidentical bone marrow for patients with hematologic malignancies (clinicaltrials.gov identifier NCT01597778). Patients on the haploidentical BMT arm receive PTCy. Ancillary studies are designed to compare the cost-effectiveness of each approach and to characterize post-transplant immune reconstitution. Registry studies are also underway through the Center for International Blood and Marrow Transplant Research to compare the outcomes of haploBMT with PTCy to 8/8 matched unrelated donor transplantation for patients with acute myeloid leukemia. These studies will be important to determine the relative safety and efficacy of different graft sources for hematopoietic stem cell transplantation.
As the safety of haploidentical SCT improves, new possibilities emerge. For example, cadaveric liver transplantation is an accepted treatment of hepatocellular carcinoma, but its application is limited severely by the dearth of livers from cadaveric donors. Living related donor partial liver transplantation can be performed, but pharmacologic immunosuppression appears to increase the risk of cancer relapse within the transplanted liver. Combined HLA-haploidentical partial liver/bone marrow transplantation offers an intriguing possibility for increasing the supply of transplantable liver tissue for treatment of hepatocellular carcinoma, achieving solid organ allograft tolerance, and maintaining immune surveillance against relapse by discontinuing immunosuppressive medications.
Another potential area of application of haploidentical BMT is in the treatment of chronic viral infections or cancers, such as human papillomavirus (HPV)-associated cancers, that result from such infections. For example, HPV-specific T cells can be expanded from a healthy first-degree relative, whether HLA-matched or -haploidentical, and infused after alloBMT from the same donor for a patient with advanced, HPV-associated cervical or oropharyngeal cancer. Although such an approach is not unique to HLA-haploidentical donors and recipients, the haploidentical option nearly guarantees that such a treatment would be possible, especially in the child to parent direction.
In summary, HLA-haploidentical BMT with high-dose, PTCy is becoming a safe and effective treatment for patients with hematologic malignancies or hemoglobinopathies and for the induction of tolerance to transplants of solid organs from the same donor. In the immediate future, research should focus on defining the optimal graft source for a patient in need of allogeneic stem cell transplantation. At the same time, there is the potential to expand the application of HLA-haploidentical stem cell transplantation for the treatment of solid tumor malignancies or for the eradication of chronic viral infections or their complications.
ACKNOWLEDGEMENTS
This work was supported by P01 CA15396 from the National Cancer Institute and by Grant #117279 from the Immune Tolerance Network/Benaroya Research Institute.
This article was published as part of a supplement, supported by WIS-CSP Foundation, in collaboration with Gilead, Milteny Biotec, Gamida cell, Adienne Pharma and Biotech, Medac hematology, Kiadis Pharma, Almog Diagnostic.
Footnotes
CONFLICT OF INTEREST
The author declares no conflict of interest.
REFERENCES
- 1.Anasetti C, Amos D, Beatty PG, Appelbaum FR, Bensinger W, Buckner CD, et al. Effect of HLA compatibility on engraftment of bone marrow transplants in patients with leukemia or lymphoma. N Engl J Med. 1989;320:197–204. doi: 10.1056/NEJM198901263200401. [DOI] [PubMed] [Google Scholar]
- 2.Anasetti C, Beatty PG, Storb R, Martin PJ, Mori M, Sanders JE, et al. Effect of HLA incompatibility on graft-versus-host disease, relapse, and survival after marrow transplantation for patients with leukemia or lymphoma. Hum Immunol. 1990;29:79–91. doi: 10.1016/0198-8859(90)90071-v. [DOI] [PubMed] [Google Scholar]
- 3.Ash RC, Horowitz MM, Gale RP, van Bekkum DW, Casper JT, Gordon-Smith EC, et al. Bone marrow transplantation from related donors other than HLA- identical siblings: effect of T cell depletion. Bone Marrow Transplant. 1991;7:443–452. [PubMed] [Google Scholar]
- 4.Aversa F, Tabilio A, Velardi A, Terenzi A, Falzetti F, Ruggeri L, et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype [see comments] N Engl J Med. 1998;339:1186–1193. doi: 10.1056/NEJM199810223391702. [DOI] [PubMed] [Google Scholar]
- 5.Luznik L, Jalla S, Engstrom LW, Iannone R, Fuchs EJ. Durable engraftment of major histocompatibility complex-incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and post-transplantation cyclophosphamide. Blood. 2001;98:3456–3464. doi: 10.1182/blood.v98.12.3456. [DOI] [PubMed] [Google Scholar]
- 6.Matzinger P, Bevan MJ. Hypothesis: why do so many lymphocytes respond to major histocompatibility antigens? Cell Immunol. 1977;29:1–5. doi: 10.1016/0008-8749(77)90269-6. [DOI] [PubMed] [Google Scholar]
- 7.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111:1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finberg R, Burakoff SJ, Cantor H, Benacerraf B. Biological significance of alloreactivity: T cells stimulated by Sendai virus-coated syngeneic cells specifically lyse allogeneic target cells. Proc Natl Acad Sci USA. 1978;75:5145–5149. doi: 10.1073/pnas.75.10.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nadazdin O, Boskovic S, Murakami T, Tocco G, Smith RN, Colvin RB, et al. Host alloreactive memory T cells influence tolerance to kidney allografts in nonhuman primates. Sci Transl Med. 2011;3:86ra51. doi: 10.1126/scitranslmed.3002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz R, Dameshek W. Drug-induced immunological tolerance. Nature. 1959;183:1682–1683. doi: 10.1038/1831682a0. [DOI] [PubMed] [Google Scholar]
- 11.Berenbaum MC, Brown IN. Dose-response relationships for agents inhibiting the immune response. Immunology. 1964;7:65–71. [PMC free article] [PubMed] [Google Scholar]
- 12.Mayumi H, Himeno K, Shin T, Nomoto K. Drug-induced tolerance to allografts in mice. VI. Tolerance induction in H-2-haplotype-identical strain combinations in mice. Transplantation. 1985;40:188–194. doi: 10.1097/00007890-198508000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Mayumi H, Himeno K, Tokuda N, Nomoto K. Drug-induced tolerance to allografts in mice. VII. Optimal protocol and mechanism of cyclophosphamide-induced tolerance in an H-2 haplotype-identical strain combination. Transplant Proc. 1986;18:363–369. [PubMed] [Google Scholar]
- 14.Mayumi H, Himeno K, Tanaka K, Tokuda N, Fan JL, Nomoto K. Drug-induced tolerance to allografts in mice. XII. The relationships between tolerance, chimerism, and graft-versus-host disease. Transplantation. 1987;44:286–290. doi: 10.1097/00007890-198708000-00021. [DOI] [PubMed] [Google Scholar]
- 15.Eto M, Mayumi H, Tomita Y, Yoshikai Y, Nishimura Y, Maeda T, et al. Specific destruction of host-reactive mature T cells of donor origin prevents graft-versus-host disease in cyclophosphamide- induced tolerant mice. J Immunol. 1991;146:1402–1409. [PubMed] [Google Scholar]
- 16.Eto M, Mayumi H, Tomita Y, Yoshikai Y, Nomoto K. Intrathymic clonal deletion of V beta 6+ T cells in cyclophosphamide-induced tolerance to H-2-compatible, Mls-disparate antigens. J Exp Med. 1990;171:97–113. doi: 10.1084/jem.171.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eto M, Mayumi H, Tomita Y, Yoshikai Y, Nishimura Y, Nomoto K, et al. Sequential mechanisms of cyclophosphamide-induced skin allograft tolerance including the intrathymic clonal deletion followed by late breakdown of the clonal deletion. J Immunol. 1990;145:1303–1310. [PubMed] [Google Scholar]
- 18.Eto M, Mayumi H, Tomita Y, Yoshikai Y, Nishimura Y, Nomoto K, et al. The requirement of intrathymic mixed chimerism and clonal deletion for a long-lasting skin allograft tolerance in cyclophosphamide-induced tolerance. Eur J Immunol. 1990;20:2005–2013. doi: 10.1002/eji.1830200919. [DOI] [PubMed] [Google Scholar]
- 19.Eto M, Mayumi H, Tomita Y, Yoshikai Y, Nishimura Y, Nomoto K. Sequential mechanisms of cyclophosphamide-induced skin allograft tolerance including the intrathymic clonal deletion followed by late breakdown of the clonal deletion. J Immunol. 1990;145:1303–1310. [PubMed] [Google Scholar]
- 20.Maeda T, Eto M, Nishimura Y, Nomoto K, Kong YY. Direct evidence for clonal destruction of allo-reactive T cells in the mice treated with cyclophosphamide after allo-priming. Immunology. 1993;78:113–121. [PMC free article] [PubMed] [Google Scholar]
- 21.Maeda T, Eto M, Nishimura Y, Nomoto K, Kong YY. Role of peripheral hemopoietic chimerism in achieving donor- specific tolerance in adult mice. J Immunol. 1993;150:753–762. [PubMed] [Google Scholar]
- 22.Mayumi H, Good RA. Long-lasting skin allograft tolerance in adult mice induced across fully allogeneic (multimajor H-2 plus multiminor histocompatibility) antigen barriers by a tolerance-inducing method using cyclophosphamide. J Exp Med. 1989;169:213–238. doi: 10.1084/jem.169.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colson YL, Wren SM, Schuchert MJ, Patrene KD, Johnson PC, Boggs SS, et al. A nonlethal conditioning approach to achieve durable multilineage mixed chimerism and tolerance across major, minor, and hematopoietic histocompatibility barriers. J Immunol. 1995;155:4179–4188. [PubMed] [Google Scholar]
- 24.Colson YL, Li H, Boggs SS, Patrene KD, Johnson PC, Ildstad ST, et al. Durable mixed allogeneic chimerism and tolerance by a nonlethal radiation-based cytoreductive approach. J Immunol. 1996;157:2820–2829. [PubMed] [Google Scholar]
- 25.Luznik L, O'Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Storb R, Gyurkocza B, Storer BE, Sorror ML, Blume K, Niederwieser D, et al. Graft-versus-host disease and graft-versus-tumor effects after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2013;31:1530–1538. doi: 10.1200/JCO.2012.45.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasamon YL, Prince G, Bolaños-Meade J, Tsai H-L, Luznik L, Ambinder RF, et al. Encouraging outcomes in older patients (pts) following nonmyeloablative (NMA) haploidentical blood or marrow transplantation (haploBMT) with high-dose posttransplantation cyclophosphamide (PT/Cy) Blood. 2013;122:158. [Google Scholar]
- 28.King AS. Evidence-based focused review of the status of hematopoietic stem cell transplantation as treatment of sickle cell disease and thalassemia. Blood. 2014;123:3089–3094. doi: 10.1182/blood-2013-01-435776. [DOI] [PubMed] [Google Scholar]
- 29.Chakrabarti S, Bareford D. A survey on patient perception of reduced-intensity transplantation in adults with sickle cell disease. Bone Marrow Transplant. 2007;39:447–451. doi: 10.1038/sj.bmt.1705622. [DOI] [PubMed] [Google Scholar]
- 30.Bachar-Lustig E, Rachamim N, Li HW, Lan F, Reisner Y. Megadose of T cell-depleted bone marrow overcomes MHC barriers in sublethally irradiated mice. Nat Med. 1995;1:1268–1273. doi: 10.1038/nm1295-1268. [DOI] [PubMed] [Google Scholar]
- 31.Wekerle T, Kurtz J, Ito H, Ronquillo JV, Dong V, Zhao G, et al. Allogeneic bone marrow transplantation with co-stimulatory blockade induces macro-chimerism and tolerance without cytoreductive host treatment. Nat Med. 2000;6:464–469. doi: 10.1038/74731. [DOI] [PubMed] [Google Scholar]
- 32.Bolaños-Meade J, Fuchs EJ, Luznik L, Lanzkron SM, Gamper CJ, Jones RJ, et al. HLA-haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood. 2012;120:4285–4291. doi: 10.1182/blood-2012-07-438408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Owen RD. Immunogenetic consequences of vascular anastomoses between bovine twins. Science. 1945;102:400–401. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- 34.Anderson D, Billingham RE, Lampkin GH, Medawar PB. The use of skin grafting to distinguish between monozygotic and dizygotic twins in cattle. Heredity. 1951;5:379–397. [Google Scholar]
- 35.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 36.Koenecke C, Hertenstein B, Schetelig J, van Biezen A, Dammann E, Gratwohl A, et al. Solid organ transplantation after allogeneic hematopoietic stem cell transplantation: a retrospective, multicenter study of the EBMT. Am J Transplant. 2010;10:1897–1906. doi: 10.1111/j.1600-6143.2010.03187.x. [DOI] [PubMed] [Google Scholar]
- 37.Leventhal J, Abecassis M, Miller J, Gallon L, Ravindra K, Tollerud DJ, et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med. 2012;4:124ra28. doi: 10.1126/scitranslmed.3003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szydlo R, Goldman JM, Klein JP, Gale RP, Ash RC, Bach FH, et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol. 1997;15:1767–1777. doi: 10.1200/JCO.1997.15.5.1767. [DOI] [PubMed] [Google Scholar]