Abstract
Identifying the role of GABA neurons in the development of an epileptic state has been particularly difficult in acquired epilepsy, in part because of the multiple changes that occur in such conditions. Although once questioned, there is now considerable evidence for loss of GABA neurons in multiple brain regions in models of acquired epilepsy. This loss can affect several cell types, including both somatostatin- and parvalbumin-expressing interneurons, and the cell type that is most severely affected can vary among brain regions and models. Because of the diversity of GABA neurons in the hippocampus and cerebral cortex, resulting functional deficits are unlikely to be compensated fully by remaining GABA neurons of other subtypes. The fundamental importance of GABA neuron loss in epilepsy is supported by findings in genetic mouse models in which GABA neurons appear to be decreased relatively selectively, and increased seizure susceptibility and spontaneous seizures develop. Alterations in remaining GABA neurons also occur in acquired epilepsy. These include alterations in inputs or receptors that could impair function, as well as morphological reorganization of GABAergic axons and their synaptic connections. Such axonal sprouting could be compensatory if normal circuits are reestablished, but the creation of aberrant circuitry could contribute to an epileptic condition. The functional effects of GABA neuron alterations thus may include not only reductions in GABAergic inhibition but also excessive neuronal synchrony and, potentially, depolarizing GABAergic influences. The combination of GABA neuron loss and alterations in remaining GABA neurons provides likely, though still unproven, substrates for the epileptic state.
Keywords: Inhibition, Plasticity, Seizures, Sprouting, Somatostatin, Parvalbumin
12.1 Introduction
The question of whether GABA neuron loss gives rise to the epileptic state in acquired epilepsy has persisted for many years. Indeed, even the occurrence of GABA neuron loss was questioned at one time. While progress has been made, and a loss of GABA neurons has been convincingly identified in humans with temporal lobe epilepsy (TLE) and in many related animal models, determining the functional consequences of GABA neuron loss in epilepsy remains a major challenge. This review will focus on some of the complexities associated with interneuron loss and their role in epilepsy and suggest that GABA neuron loss could indeed give rise to the epileptic state through both direct and indirect routes.
12.2 Loss of GABA Neurons Is a Consistent Finding in Models of Acquired Epilepsy
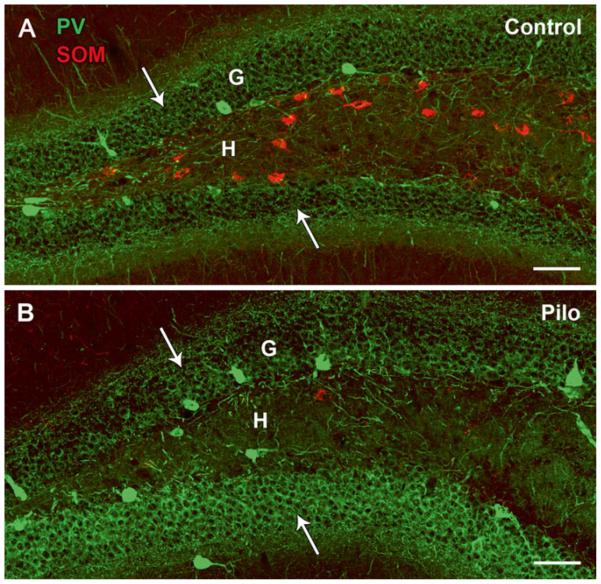
Interneuron loss is one of the most frequently observed alterations in models of TLE, and the consistency of GABA neuron loss provides a solid base for suggesting the potential importance of this alteration in creating an epileptic state. Although loss of GABA neurons has been identified in multiple brain regions, loss of somatostatin (SOM)-expressing GABA neurons in the hilus of the dentate gyrus remains one of the clearest and most consistent findings (Fig. 12.1a, b). Loss of these GABA neurons has been found in virtually all models of acquired epilepsy, including kindling, status epilepticus and traumatic brain injury models [7, 25, 32, 44, 45]. Importantly, loss of SOM/GABA neurons in the dentate hilus is also found in human TLE, as part of the broader loss of neurons in typical hippocampal sclerosis, as well as in pathological conditions with more limited cell loss such as end-folium sclerosis [14, 35, 42, 48, 49].
Fig. 12.1.
Comparisons of somatostatin (SOM)- and parvalbumin (PV)-labeled neurons in the rostral dentate gyrus of control (a) and pilocarpine (Pilo)-treated (b) mice. (a) In the control dentate gyrus, cell bodies of SOM neurons are located primarily within the hilus (H) whereas those of PV neurons are positioned predominantly along the base of the granule cell layer (G). PV-labeled axon terminals are concentrated in perisomatic locations within the granule cell layer (arrows) while SOM terminal fields are located in dendritic regions in the outer molecular layer (not shown). (b) In the pilocarpine-treated mouse at 2 months after status epilepticus, a severe loss of SOM neurons is evident in the hilus whereas many PV neurons and their axon terminals (arrows) in the granule cell layer are preserved. Scale bars, 100 μm
SOM neurons in stratum oriens (s. oriens) of CA1 are also among the vulnerable interneurons in several models of acquired epilepsy [1, 11, 27, 36]. As SOM neurons in both the hilus and s. oriens provide GABAergic innervation of dendrites of granule cells and pyramidal cells, respectively, they are ideally positioned to control excitability of the principal cells directly at the sites of their major excitatory inputs. This pattern of GABA neuron loss has led to the suggestion that interneurons that provide dendritic innervation are more vulnerable to damage in epilepsy than those which provide primarily perisomatic innervation, such as basket cells and axo-axonic cells, many of which express the calcium binding protein parvalbumin (PV). Electrophysiological findings of decreased dendritic inhibition, with preservation of somatic inhibition, in pyramidal cells of CA1 in models of recurrent seizures support this idea [11].
While the distinction between dendritic and perisomatic innervation provides a useful frame-work for considering GABA neuron loss, the differences in vulnerability among the broad types of interneurons are not clear-cut, and additional complexities exist. While PV-expressing neurons are a major source of perisomatic innervation of pyramidal cells in the hippocampus, cholecystokinin (CCK)-expressing interneurons also provide perisomatic innervation of these neurons in CA1 [3, 23]. In a mouse pilocarpine model of recurrent seizures, this CCK innervation appeared to be decreased while the PV innervation was preserved [51]. This could create an imbalance in perisomatic control that could favor synchronizing actions of PV basket cells, with loss of major modulatory inputs from CCK neurons.
Perisomatic innervation includes both basket cells and axo-axonic cells [22], and these cell types could be affected differentially in epilepsy. Decreased innervation of the axon initial segment by PV-expressing axo-axonic neurons has been found in the hippocampus and cerebral cortex in several epilepsy conditions, suggesting that axo-axonic cells could be more vulnerable than basket cells [13, 15, 41].
Thus loss of PV neurons can occur and has been described in the dentate gyrus in several animal models, without distinctions between basket cells and axo-axonic cells [1, 25, 29]. However, when both PV and SOM neurons have been studied in the same animals, the loss of PV-expressing neurons in the dentate gyrus is generally less severe than that of SOM interneurons [7] (Fig. 12.1a, b).
A decrease in numbers of PV-expressing interneurons has now been identified in several other regions of the hippocampal formation where their loss could be particularly important in regulating activity within the broader hippocampal circuit. A significant decrease in PV neurons has been identified in layer II of the entorhinal cortex where loss of these neurons could contribute to increased excitability of the perforant path input to the dentate gyrus [30]. Interestingly, lower densities of PV-containing neurons have also been identified in the subiculum, where their loss could lead to increased excitability of this major output region of the hippocampal formation, a region that is otherwise generally well preserved [2, 16].
Thus decreases in both SOM and PV neurons can occur in epilepsy, and the particular pattern of loss may vary among epilepsy models, species, brain regions and even rostral-caudal levels of the hippocampal formation. The types of GABA neurons that are affected remain important as they will determine the specific functional effects. However, loss of GABA neurons remains a unifying theme.
Despite strong evidence for GABA neuron loss in many brain regions, direct relationships to the epileptic state have been difficult to demonstrate. This could be in part because GABA neuron loss does not occur in isolation in most forms of acquired or lesion-induced epilepsy. In human TLE and related epilepsy models, extensive cell loss can occur in many regions, including CA1 and CA3 as well as the dentate hilus and extra-hippocampal regions. This neuronal loss generally involves both principal cells and interneurons, making it more difficult to link GABA neuron loss directly to development of an epileptic state. However, findings in several genetic mouse models provide support for the importance of GABA neuron loss in the development of epilepsy, and these findings also have relevance for acquired epilepsy.
12.3 Selective Loss of GABA Neurons Can Lead to an Epileptic State in Genetic Models
Some of the strongest evidence for loss of GABA neurons giving rise to the epileptic state has come from genetically-modified mice in which GABA neurons are selectively affected, and increased seizure susceptibility and spontaneous seizures occur. In mice with loss of the Dlx1 gene, a transcription factor that regulates development of GABAergic interneurons originating in the medial ganglionic eminence, there is a time-dependent reduction in the number of interneurons in the cerebral cortex and hippocampus and development of an epilepsy phenotype [10]. SOM and calretinin-expressing neurons were reduced in number whereas PV-expressing neurons appeared to be unaffected. Because the loss of GABA neurons is apparently selective in these mice, the findings provide strong support for loss of GABA neurons giving rise to an epileptic state and also suggest that the loss of GABA neurons does not need to be extensive. In these mice, behavioral seizures were selectively induced by mild stressors by 2 months of age when there was an approximately 22 % reduction in GAD67-labeled neurons in the cerebral cortex and 24 and 29 % reduction in the dentate gyrus and CA1 respectively. Comparable or even greater GABA neuron loss has been observed in the hippocampal formation in models of acquired epilepsy [37, 50].
Similarly, in mice with mutation of the gene encoding urokinase plasminogen activator receptor (uPAR), a key component in hepatocyte growth factor activation and function, interneuron migration is altered, and the mice have a nearly complete loss of PV neurons in the anterior cingulate and parietal cortex [40]. These mice also developed spontaneous myoclonic seizures and increased susceptibility to pharmacologically-induced convulsions. Thus the apparently selective loss of either of two major groups of interneurons supports the importance of GABA neuron loss in the development of epilepsy.
12.4 Remaining GABA Neurons Could Play a Critical Role in Development of Epilepsy
Despite clear evidence for loss of GABA neurons in virtually all models of acquired epilepsy and human TLE, some GABA neurons invariably remain, and alterations in these neurons could contribute to the creation of an epileptic condition. Indeed, it may be difficult to separate the effects of loss of GABA neurons from altered function of remaining neurons as the initial loss of GABA neurons may be a stimulus for the subsequent changes in remaining GABA neurons. Critical changes may include impaired function of remaining GABA neurons and morphological reorganization of remaining interneurons that could lead to altered or aberrant circuitry.
12.4.1 Impaired Function of Remaining Interneurons
Remaining GABA neurons often appear particularly prominent in tissue from animal models of epilepsy, and the preservation of some GABA neurons has suggested that GABA neuron loss may be of limited importance in establishing the epileptic state. However, the function of remaining GABA neurons could be altered, leading to inadequate control of principal cell activity. In the dentate gyrus and hippocampus, the functional state of remaining basket cells has been debated for many years. Specific details of the “dormant basket cell” hypothesis [46, 47] have been questioned, including the role of hilar mossy cell loss in reducing basket cell activity [5, 18]. However, the broad suggestion that basket cells and other GABAergic neurons might be functioning sub-optimally due to decreased or impaired excitatory input remains plausible. Recent studies have identified deficits in basket cell function in the dentate gyrus that could indicate a decrease in excitatory afferent input or reduction of the readily releasable pool of synaptic vesicles, in association with an increased failure rate at basket cell to granule cell synapses [53].
Alterations in the receptors and channels of remaining GABA neurons also could reduce the activity of these neurons. In both the rat and mouse pilocarpine model, expression of the δ subunit of the GABAA receptor is increased in subgroups of GABA neurons in the dentate gyrus [38, 52]. As GABAA receptors expressing the δ subunit are responsible for the majority of tonic inhibition in these neurons [24], an increase in δ subunit expression in interneurons could reduce their excitability and impair inhibitory control of the network [38]. Recent studies have demonstrated that tonic inhibition is indeed enhanced in fast-spiking basket cells of the dentate gyrus at 1 week after pilocarpine-induced status epilepticus [52]. However, additional changes, including decreased KCC2 expression in the basket cells, appeared to compensate partially for the increased tonic inhibition of the basket cells, and dentate excitability was not increased. Nevertheless, simulation studies suggested that the changes in tonic inhibition, in combination with other recognized alterations in dentate gyrus circuitry in epilepsy models, could lead to increased granule cell firing and self-sustained seizure-like activity in a subset of simulated networks [52]. Thus occasional alterations in interneuron activity, when combined with other changes in the network, may be sufficient to overrule compensatory changes and lead to sporadic seizure activity. While regulation of δ subunit-containing GABAA receptors by neurosteroids and other endogenous modulators could play important roles [21], changes in numerous other channels and receptors in remaining GABAergic interneurons could reduce their effectiveness and contribute to the epileptic state.
Functional alterations in interneurons and their relationship to seizure activity are demonstrated convincingly in genetic mouse models in which specific channels have been deleted relatively selectively in interneurons. As a key example, loss of the alpha subunit of the Nav1.1 sodium channel, that is encoded by the SCN1A gene, impairs sodium currents more severely in GABAergic neurons than in pyramidal cells [8, 17, 34]. Such changes limit the ability of the inhibitory interneurons, including PV neurons, to fire action potentials at high frequency, and the animals develop spontaneous generalized seizures.
Similarly, loss of function of the CaV2.1 voltage-gated Ca2+ channel reduces GABA release from cortical PV neurons, and generalized seizures occur in mice with such loss [43]. While decreased expression of this calcium channel was found in both PV and SOM neurons, only the loss in fast spiking, presumably PV, interneurons led to spontaneous seizures. Compensation by N-type Ca2+ channels appeared to maintain function of the SOM interneurons but was insufficient for adequate function of the PV neurons.
Finally, elimination of the voltage-gated potassium channels of the Kv3 subfamily, that are particularly prominent in fast-spiking interneurons in the deep layers of the neocortex, led to an inability of these interneurons to fire at their normal high frequency and an increased susceptibility to seizures [31].
Thus in several genetic models, impairment of fast-spiking PV neurons, particularly a reduction in their ability to fire action potentials at high frequency, can lead to increased seizure susceptibility. Although these functional deficits are induced by specific genetic modifications, similar alterations in remaining GABA neurons may occur in acquired epilepsy, and even small functional impairment in remaining neurons could tip the balance toward seizure activity.
12.4.2 Morphological Reorganization of Remaining Interneurons
Clear demonstrations of loss of GABA neurons in acquired epilepsy have often been obscured by the plasticity of remaining interneurons. Remaining GABA neurons frequently express increased levels of GABA neuron markers, including the mRNA and protein of two isoforms of the GABA synthesizing enzyme, glutamic acid decarboxylase 65 and 67 (GAD65 and GAD67), as well as GABA [9, 19, 20]. Similarly the expression of peptides such as SOM and neuropeptideY (NPY) within specific subclasses of GABA neurons are frequently upregulated [6, 33, 44]. These changes can be substantial and, during the chronic period, labeling of remaining GABA neurons can be quite strong and can suggest that either little loss of GABA neurons has occurred or that axons of remaining GABA neurons have sprouted [12]. It has remained particularly difficult to distinguish morphological growth and reorganization of GABAergic axons from an increase in GABAergic markers within remaining neurons [4, 27].
Additional support for sprouting of existing SOM neurons in the dentate gyrus has been obtained from mice that express enhanced green fluorescent protein (eGFP) in a subgroup of SOM neurons [54]. By studying the labeled interneurons in pilocarpine-treated mice, Buckmaster and colleagues demonstrated that SOM neurons that survive in the ventral (caudal) dentate gyrus can re-innervate the outer half of the dentate gyrus that was partially deafferented by loss of hilar SOM neurons. Such reorganization has generally been presumed to be compensatory as remaining GABA neurons are replacing the innervation of neurons of a similar type and function [26, 54].
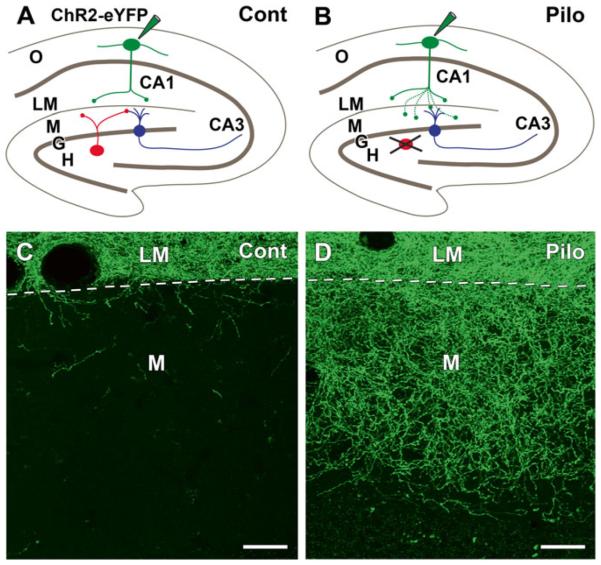
Axonal reorganization of remaining GABA neurons can also create aberrant GABAergic circuitry as has been observed in the rostral dentate gyrus in the pilocarpine mouse model [39]. Apparent reinnervation of the dentate molecular layer was observed during the chronic period, but, in contrast to the previous study, few remaining SOM neurons were found in the rostral hilus. To determine if the innervation could be derived from other sources, SOM neurons in s. oriens of control and pilocarpine-treated SOM-Cre recombinase mice were selectively labeled with a viral vector containing Cre-dependent channel-rhodopsin2 (ChR2) fused to enhanced yellow fluorescent protein (eYFP). In control mice, the axons of many labeled SOM neurons in s. oriens formed a dense plexus of fibers in s. lacunosum-moleculare of CA1 (Fig. 12.2a, c). This plexus was sharply delineated by the hippocampal fissure, and relatively few fibers crossed the fissure to enter the adjacent molecular layer of the dentate gyrus (Fig. 12.2c). In contrast, in pilocarpine-treated mice, an extensive axonal plexus of eYFP-labeled fibers was evident in the outer two-thirds of the dentate gyrus during the chronic period (Fig. 12.2b, d). Thus SOM neurons in s. oriens exhibited an unexpected capacity for morphological growth and reorganization, and created an aberrant circuit between hippocampal interneurons in s. oriens and granule cells of the dentate gyrus. The reorganized axons formed symmetric synaptic contacts with presumptive granule cell dendrites and spines, and optogenetic stimulation demonstrated that activation of the reorganized neurons produced GABAergic inhibition in dentate granule cells [39].
Fig. 12.2.
Axonal reorganization of remaining somatostatin (SOM) neurons in pilocarpine (Pilo)-treated mice at 2 months after status epilepticus, illustrated schematically in (a, b) and in confocal images in (c, d). (a) This schematic illustrates the normal circuitry of SOM neurons in the hilus (red) and s. oriens (green) and the labeling protocol. In a control SOM-Cre mouse, selective labeling of SOM neurons in s. oriens (O) of CA1, by Cre-dependent AAV transfection of ChR2-eYFP, leads to labeling of their axon terminals that are confined to s. lacunosum-moleculare (LM). SOM neurons (red) in the hilus (H) innervate the outer molecular layer (M) of the dentate gyrus where they form synapses with dentate granule cells (G, blue). These hilar SOM neurons are not labeled by the injection in s. oriens. (b) In pilocarpine-treated mice, similar labeling of SOM neurons in s. oriens leads to axonal labeling not only in s. lacunosum-moleculare of CA1 but also in the molecular layer of the dentate gyrus, a region that was previously innervated by vulnerable SOM neurons (red) in the hilus. (c) In a control SOM-Cre mouse, eYFP-labeled axons are concentrated in s. lacunosum-moleculare (LM), and only a limited number of labeled fibers cross the hippocampal fissure (dashed line) to enter the molecular layer (M) of the dentate gyrus. (d) In a similarly transfected pilocarpine-treated mouse, numerous labeled fibers cross the hippocampal fissure and form an extensive plexus in the outer two-thirds of the dentate molecular layer, where they innervate dentate granule cells. Scale bars, 20 μm (Adapted from data in Peng et al. [39])
The in vivo effects of the altered circuit are not known, but it is unlikely to provide normal control of granule cell activity. Because the reorganized fibers originated from GABA neurons in s. oriens of CA1, they would not receive the normal input from dentate granule cells that would be required for efficient feedback inhibition. However, strong activity of CA1 pyramidal cells could potentially activate these SOM neurons and produce inhibitory responses in the granule cells, although through an indirect circuit with presumably altered timing.
These results emphasize that the reemergence of a GABAergic axonal plexus does not necessarily indicate establishment of normal circuitry, and the reorganized circuit could be ineffective in controlling activity of principal cells. Such findings demonstrate yet another way in which GABA neuron loss could lead to altered inhibitory control and thus contribute to an epileptic state.
12.5 Replacement of GABA Neurons Supports Their Functional Importance in Epilepsy
Recent studies of transplantation of GABA neurons in the hippocampal formation of pilocarpine-treated mice support contributions of GABA neuron loss to the epileptic state [28]. After GABA neuron transplantation in the hippocampal formation, the number of spontaneous seizures in these mice was reduced, despite the maintained presence of mossy fiber sprouting in the inner molecular layer and, presumably, loss of mossy cells in the dentate hilus. While these findings are consistent with a loss of GABA neurons leading to the epileptic state, it remains possible that the transplanted GABA neurons could be counteracting other fundamental epilepsy-producing alterations through compensatory increases in inhibition.
12.6 GABA Neuron Loss Has Multiple Effects in Epilepsy
Loss of even a small fraction of GABA neurons can have profound functional effects due to the innervation of numerous principal cells by the expansive axonal plexus of many interneurons. However the effects of an initial loss of GABA neurons could be enhanced further by alterations of remaining GABA neurons. Despite having some basic compensatory effects, the remaining GABA neurons could contribute periodically to the epileptic state through multiple mechanisms. These could include creation of excessive synchronous activity within the network and an inability of aberrant GABAergic circuitry to respond appropriately when increased inhibitory control is required. While still speculative, there is increasing evidence that GABA neuron loss, through both direct and indirect mechanisms, could give rise to the epileptic state.
Acknowledgments
I would like to express my deep gratitude to Phil Schwartzkroin for his generous spirit and support over many years; his insightful and thought-provoking questions that have stimulated and enhanced basic science research in the epilepsy field; and his deep commitment and service to the entire epilepsy community which will continue in many forms.
Other Acknowledgements This work was supported by National Institutes of Health Grant NS075245 and Veterans Affairs Medical Research Funds. I gratefully acknowledge the members of my laboratory, past and present, for their superb work and strong dedication to our studies of GABA neurons and epilepsy.
Abbreviations
- CCK
Cholecystokinin
- eGFP
Enhanced green fluorescent protein
- eYFP
Enhanced yellow fluorescent protein
- GABA
Gamma aminobutyric acid
- GAD
Glutamic acid decarboxylase
- NPY
Neuropeptide Y
- PV
Parvalbumin
- SOM
Somatostatin
- s. oriens
Stratum oriens
- TLE
Temporal lobe epilepsy
- uPAR
Urokinase plasminogen activator receptor
Footnotes
Research Service, Veterans Administration Greater Los Angeles Healthcare System, West Los Angeles, Los Angeles, CA 90073, USA
References
- 1.Andre V, Marescaux C, Nehlig A, Fritschy JM. Alterations of hippocampal GABAergic system contribute to development of spontaneous recurrent seizures in the rat lithium-pilocarpine model of temporal lobe epilepsy. Hippocampus. 2001;11:452–468. doi: 10.1002/hipo.1060. [DOI] [PubMed] [Google Scholar]
- 2.Andrioli A, Alonso-Nanclares L, Arellano JI, DeFelipe J. Quantitative analysis of parvalbumin-immunoreactive cells in the human epileptic hippocampus. Neuroscience. 2007;149:131–143. doi: 10.1016/j.neuroscience.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong C, Soltesz I. Basket cell dichotomy in microcircuit function. J Physiol. 2012;590:683–694. doi: 10.1113/jphysiol.2011.223669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bausch SB. Axonal sprouting of GABAergic interneurons in temporal lobe epilepsy. Epilepsy Behav. 2005;7:390–400. doi: 10.1016/j.yebeh.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 5.Bernard C, Esclapez M, Hirsch JC, Ben-Ari Y. Interneurons are not so dormant in temporal lobe epilepsy: a critical reappraisal of the dormant basket cell hypothesis. Epilepsy Res. 1998;32:93–103. doi: 10.1016/s0920-1211(98)00043-6. [DOI] [PubMed] [Google Scholar]
- 6.Boulland JL, Ferhat L, Tallak Solbu T, Ferrand N, Chaudhry FA, Storm-Mathisen J, Esclapez M. Changes in vesicular transporters for gamma-aminobutyric acid and glutamate reveal vulnerability and reorganization of hippocampal neurons following pilocarpine-induced seizures. J Comp Neurol. 2007;503:466–485. doi: 10.1002/cne.21384. [DOI] [PubMed] [Google Scholar]
- 7.Buckmaster PS, Dudek FE. Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J Comp Neurol. 1997;385:385–404. [PubMed] [Google Scholar]
- 8.Catterall WA, Kalume F, Oakley JC. NaV1.1 channels and epilepsy. J Physiol. 2010;588:1849–1859. doi: 10.1113/jphysiol.2010.187484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavalheiro EA, Fernandes MJ, Turski L, Naffah-Mazzacoratti MG. Spontaneous recurrent seizures in rats: amino acid and monoamine determination in the hippocampus. Epilepsia. 1994;35:1–11. doi: 10.1111/j.1528-1157.1994.tb02905.x. [DOI] [PubMed] [Google Scholar]
- 10.Cobos I, Calcagnotto ME, Vilaythong AJ, Thwin MT, Noebels JL, Baraban SC, Rubenstein JL. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat Neurosci. 2005;8:1059–1068. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- 11.Cossart R, Dinocourt C, Hirsch JC, Merchan-Perez A, DeFelipe J, Ben-Ari Y, Esclapez M, Bernard C. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat Neurosci. 2001;4:52–62. doi: 10.1038/82900. [DOI] [PubMed] [Google Scholar]
- 12.Davenport CJ, Brown WJ, Babb TL. Sprouting of GABAergic and mossy fiber axons in dentate gyrus following intrahippocampal kainate in the rat. Exp Neurol. 1990;109:180–190. doi: 10.1016/0014-4886(90)90072-z. [DOI] [PubMed] [Google Scholar]
- 13.DeFelipe J. Chandelier cells and epilepsy. Brain. 1999;122:1807–1822. doi: 10.1093/brain/122.10.1807. [DOI] [PubMed] [Google Scholar]
- 14.de Lanerolle NC, Kim JH, Robbins RJ, Spencer DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. 1989;495:387–395. doi: 10.1016/0006-8993(89)90234-5. [DOI] [PubMed] [Google Scholar]
- 15.Dinocourt C, Petanjek Z, Freund TF, Ben-Ari Y, Esclapez M. Loss of interneurons innervating pyramidal cell dendrites and axon initial segments in the CA1 region of the hippocampus following pilocarpine-induced seizures. J Comp Neurol. 2003;459:407–425. doi: 10.1002/cne.10622. [DOI] [PubMed] [Google Scholar]
- 16.Drexel M, Preidt AP, Kirchmair E, Sperk G. Parvalbumin interneurons and calretinin fibers arising from the thalamic nucleus reuniens degenerate in the subiculum after kainic acid-induced seizures. Neuroscience. 2011;189:316–329. doi: 10.1016/j.neuroscience.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dutton SB, Makinson CD, Papale LA, Shankar A, Balakrishnan B, Nakazawa K, Escayg A. Preferential inactivation of Scn1a in parvalbumin interneurons increases seizure susceptibility. Neurobiol Dis. 2012;49:211–220. doi: 10.1016/j.nbd.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esclapez M, Hirsch JC, Khazipov R, Ben-Ari Y, Bernard C. Operative GABAergic inhibition in the hippocampal CA1 pyramidal neurons in experimental epilepsy. Proc Natl Acad Sci U S A. 1997;94:12151–12156. doi: 10.1073/pnas.94.22.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esclapez M, Houser CR. Up-regulation of GAD65 and GAD67 in remaining hippocampal GABA neurons in a model of temporal lobe epilepsy. J Comp Neurol. 1999;412:488–505. [PubMed] [Google Scholar]
- 20.Feldblum S, Ackermann RF, Tobin AJ. Long-term increase of glutamate decarboxylase mRNA in a rat model of temporal lobe epilepsy. Neuron. 1990;5:361–371. doi: 10.1016/0896-6273(90)90172-c. [DOI] [PubMed] [Google Scholar]
- 21.Ferando I, Mody I. GABAA receptor modulation by neurosteroids in models of temporal lobe epilepsies. Epilepsia. 2012;53(Suppl 9):89–101. doi: 10.1111/epi.12038. [DOI] [PubMed] [Google Scholar]
- 22.Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 23.Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56:33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABAA receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- 25.Gorter JA, van Vliet EA, Aronica E, Lopes da Silva FH. Progression of spontaneous seizures after status epilepticus is associated with mossy fibre sprouting and extensive bilateral loss of hilar parvalbumin and somatostatin-immunoreactive neurons. Eur J Neurosci. 2001;13:657–669. doi: 10.1046/j.1460-9568.2001.01428.x. [DOI] [PubMed] [Google Scholar]
- 26.Halabisky B, Parada I, Buckmaster PS, Prince DA. Excitatory input onto hilar somatostatin inter-neurons is increased in a chronic model of epilepsy. J Neurophysiol. 2010;104:2214–2223. doi: 10.1152/jn.00147.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houser CR, Esclapez M. Vulnerability and plasticity of the GABA system in the pilocarpine model of spontaneous recurrent seizures. Epilepsy Res. 1996;26:207–218. doi: 10.1016/s0920-1211(96)00054-x. [DOI] [PubMed] [Google Scholar]
- 28.Hunt RF, Girskis KM, Rubenstein JL, Alvarez-Buylla A, Baraban SC. GABA progenitors grafted into the adult epileptic brain control seizures and abnormal behavior. Nat Neurosci. 2013;16:692–697. doi: 10.1038/nn.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi M, Buckmaster PS. Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy. J Neurosci. 2003;23:2440–2452. doi: 10.1523/JNEUROSCI.23-06-02440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar SS, Buckmaster PS. Hyperexcitability, interneurons, and loss of GABAergic synapses in entorhinal cortex in a model of temporal lobe epilepsy. J Neurosci. 2006;26:4613–4623. doi: 10.1523/JNEUROSCI.0064-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lau D, Vega-Saenz de Miera EC, Contreras D, Ozaita A, Harvey M, Chow A, Noebels JL, Paylor R, Morgan JI, Leonard CS, Rudy B. Impaired fast-spiking, suppressed cortical inhibition, and increased susceptibility to seizures in mice lacking Kv3.2 K+ channel proteins. J Neurosci. 2000;20:9071–9085. doi: 10.1523/JNEUROSCI.20-24-09071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowenstein DH, Thomas MJ, Smith DH, McIntosh TK. Selective vulnerability of dentate hilar neurons following traumatic brain injury: a potential mechanistic link between head trauma and disorders of the hippocampus. J Neurosci. 1992;12:4846–4853. doi: 10.1523/JNEUROSCI.12-12-04846.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marksteiner J, Sperk G. Concomitant increase of somatostatin, neuropeptide Y and glutamate decarboxylase in the frontal cortex of rats with decreased seizure threshold. Neuroscience. 1988;26:379–385. doi: 10.1016/0306-4522(88)90155-8. [DOI] [PubMed] [Google Scholar]
- 34.Martin MS, Dutt K, Papale LA, Dube CM, Dutton SB, de Haan G, Shankar A, Tufik S, Meisler MH, Baram TZ, Goldin AL, Escayg A. Altered function of the SCN1A voltage-gated sodium channel leads to gamma-aminobutyric acid-ergic (GABAergic) interneuron abnormalities. J Biol Chem. 2010;285:9823–9834. doi: 10.1074/jbc.M109.078568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mathern GW, Babb TL, Pretorius JK, Leite JP. Reactive synaptogenesis and neuron densities for neuropeptide Y, somatostatin, and glutamate decarboxylase immunoreactivity in the epileptogenic human fascia dentata. J Neurosci. 1995;15:3990–4004. doi: 10.1523/JNEUROSCI.15-05-03990.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morin F, Beaulieu C, Lacaille J–C. Selective loss of GABA neurons in area CA1 of the rat hippocampus after intraventricular kainate. Epilepsy Res. 1998;32:363–369. doi: 10.1016/s0920-1211(98)00033-3. [DOI] [PubMed] [Google Scholar]
- 37.Obenaus A, Esclapez M, Houser CR. Loss of glutamate decarboxylase mRNA-containing neurons in the rat dentate gyrus following pilocarpine-induced seizures. J Neurosci. 1993;13:4470–4485. doi: 10.1523/JNEUROSCI.13-10-04470.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng Z, Huang CS, Stell BM, Mody I, Houser CR. Altered expression of the δ subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J Neurosci. 2004;24:8629–8639. doi: 10.1523/JNEUROSCI.2877-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng Z, Zhang N, Wei W, Huang CS, Cetina Y, Otis TS, Houser CR. A reorganized GABAergic circuit in a model of epilepsy: evidence from optogenetic labeling and stimulation of somatostatin interneurons. J Neurosci. 2013;33:14392–14405. doi: 10.1523/JNEUROSCI.2045-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Powell EM, Campbell DB, Stanwood GD, Davis C, Noebels JL, Levitt P. Genetic disruption of cortical interneuron development causes region- and GABA cell type-specific deficits, epilepsy, and behavioral dysfunction. J Neurosci. 2003;23:622–631. doi: 10.1523/JNEUROSCI.23-02-00622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ribak CE. Axon terminals of GABAergic chandelier cells are lost at epileptic foci. Brain Res. 1985;326:251–260. doi: 10.1016/0006-8993(85)90034-4. [DOI] [PubMed] [Google Scholar]
- 42.Robbins RJ, Brines ML, Kim JH, Adrian T, de Lanerolle NC, Welsh S, Spencer DD. A selective loss of somatostatin in the hippocampus of patients with temporal lobe epilepsy. Ann Neurol. 1991;29:325–332. doi: 10.1002/ana.410290316. [DOI] [PubMed] [Google Scholar]
- 43.Rossignol E, Kruglikov I, van den Maagdenberg AM, Rudy B, Fishell G. Cav2.1. ablation in cortical interneurons selectively impairs fast-spiking basket cells and causes generalized seizures. Ann Neurol. 2013;74:209–222. doi: 10.1002/ana.23913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwarzer C, Williamson JM, Lothman EW, Vezzani A, Sperk G. Somatostatin, neuropeptide Y, neurokinin B and cholecystokinin immunoreactivity in two chronic models of temporal lobe epilepsy. Neuroscience. 1995;69:831–845. doi: 10.1016/0306-4522(95)00268-n. [DOI] [PubMed] [Google Scholar]
- 45.Sloviter RS. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987;235:73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- 46.Sloviter RS. Permanently altered hippocampal structure, excitability, and inhibition after experimental status epilepticus in the rat: the “dormant basket cell” hypothesis and its possible relevance to temporal lobe epilepsy. Hippocampus. 1991;1:41–66. doi: 10.1002/hipo.450010106. [DOI] [PubMed] [Google Scholar]
- 47.Sloviter RS, Zappone CA, Harvey BD, Bumanglag AV, Bender RA, Frotscher M. “Dormant basket cell” hypothesis revisited: relative vulnerabilities of dentate gyrus mossy cells and inhibitory interneurons after hippocampal status epilepticus in the rat. J Comp Neurol. 2003;459:44–76. doi: 10.1002/cne.10630. [DOI] [PubMed] [Google Scholar]
- 48.Sundstrom LE, Brana C, Gatherer M, Mepham J, Rougier A. Somatostatin- and neuropeptide Y-synthesizing neurones in the fascia dentata of humans with temporal lobe epilepsy. Brain. 2001;124:688–697. doi: 10.1093/brain/124.4.688. [DOI] [PubMed] [Google Scholar]
- 49.Swartz BE, Houser CR, Tomiyasu U, Walsh GO, DeSalles A, Rich JR, Delgado-Escueta A. Hippocampal cell loss in posttraumatic human epilepsy. Epilepsia. 2006;47:1373–1382. doi: 10.1111/j.1528-1167.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- 50.Thind KK, Yamawaki R, Phanwar I, Zhang G, Wen X, Buckmaster PS. Initial loss but later excess of GABAergic synapses with dentate granule cells in a rat model of temporal lobe epilepsy. J Comp Neurol. 2010;518:647–667. doi: 10.1002/cne.22235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wyeth MS, Zhang N, Mody I, Houser CR. Selective reduction of cholecystokinin-positive basket cell innervation in a model of temporal lobe epilepsy. J Neurosci. 2010;30:8993–9006. doi: 10.1523/JNEUROSCI.1183-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu J, Proddutur A, Elgammal FS, Ito T, Santhakumar V. Status epilepticus enhances tonic GABA currents and depolarizes GABA reversal potential in dentate fast-spiking basket cells. J Neurophysiol. 2013;109:1746–1763. doi: 10.1152/jn.00891.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang W, Buckmaster PS. Dysfunction of the dentate basket cell circuit in a rat model of temporal lobe epilepsy. J Neurosci. 2009;29:7846–7856. doi: 10.1523/JNEUROSCI.6199-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang W, Yamawaki R, Wen X, Uhl J, Diaz J, Prince DA, Buckmaster PS. Surviving hilar somatostatin interneurons enlarge, sprout axons, and form new synapses with granule cells in a mouse model of temporal lobe epilepsy. J Neurosci. 2009;29:14247–14256. doi: 10.1523/JNEUROSCI.3842-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]