Abstract
Successful adoptive T cell therapy (ACT) requires the ability to activate tumor-specific T cells with the ability to traffic to the tumor site and effectively kill their target as well as persist over time. We hypothesized that ACT using marrow-infiltrating lymphocytes (MILs) in multiple myeloma (MM) could impart greater antitumor immunity in that they were obtained from the tumor microenvironment. We describe the results from the first clinical trial using MILs in MM. Twenty-five patients with either newly diagnosed or relapsed disease had their MILs harvested, activated and expanded, and subsequently infused on the third day after myeloablative therapy. Cells were obtained and adequately expanded in all patients with anti-CD3/CD28 beads plus interleukin-2, and a median of 9.5 × 108 MILs were infused. Factors indicative of response to MIL ACT included (i) the presence of measurable myeloma-specific activity of the ex vivo expanded product, (ii) low endogenous bone marrow T cell interferon-γ production at baseline, (iii) a CD8+ central memory phenotype at baseline, and (iv) the generation and persistence of myeloma-specific immunity in the bone marrow at 1 year after ACT. Achieving at least a 90% reduction in disease burden significantly increased the progression-free survival (25.1 months versus 11.8 months; P = 0.01). This study demonstrates the feasibility and efficacy of MILs as a form of ACT with applicability across many hematologic malignancies and possibly solid tumors infiltrating the bone marrow.
INTRODUCTION
Myeloablative chemotherapy is an accepted therapy for many hematologic malignancies including multiple myeloma (MM), albeit with minimal evidence of long-term cures (1). However, we and others have previously shown that the myeloablative therapy also provides an ideal platform for the superimposition of immune-based therapies (2–4). Specifically, the lymphopenia resulting from high-dose chemotherapy facilitates homeostatic lymphocytic proliferation, eliminates tolerogenic antigen-presenting cells (APCs), and induces cytokine release that generates a more favorable environment for adoptive T cell therapy (ACT). Indirect evidence that the immune system can contribute to the clinical benefits of high-dose chemotherapy was shown with early lymphoid recovery, resulting in improved clinical outcomes in patients with myeloma, lymphoma, and acute myeloid leukemia undergoing an autologous stem cell transplant (SCT) (5–7). Furthermore, these improved outcomes in myeloma were associated directly with the dose of autologous lymphocytes infused from the apheresis product (8). Together, these data support the hypothesis that antitumor immunity can have clinically measurable benefits and advance the question of how to harness such immunity to augment the efficacy of currently available therapies.
The ability to eradicate measurable disease with ACT requires T cells to be appropriately activated and present in sufficient numbers, have appreciable antitumor activity, home to the tumor site, effectively kill the tumor upon encounter, and persist over time. Stimulation with paramagnetic beads to which anti-CD3 and anti-CD28 are bound can effectively reverse an anergic (tolerant) state, generate activated T cells, and expand their numbers (9). Although bead-bound anti-CD3 and anti-CD28 provide a straightforward and robust T cell amplification in vitro, a major limitation of this approach is the nonspecific stimulation of the entire T cell repertoire without enrichment of tumor-specific T cells. One strategy to augment the tumor specificity of ACT is to use a T cell population with greater endogenous tumor specificity. Such an enrichment accounts for the considerable antitumor activity of ACT using tumor-infiltrating lymphocytes (TILs) from metastatic melanoma (10). However, TILs are present only in a subset of patients with metastatic melanoma, and of those, successful TIL preparations can be achieved in only 60 to 70% of patients with harvestable tumor, which limits the general applicability of such an approach (11). We hypothesized that because the bone marrow (BM) is the tumor microenvironment for many hematologic malignancies such as MM, marrow-infiltrating lymphocytes (MILs) could be harnessed to generate tumor-specific T cell therapy for these specific cancers. In contrast to TILs, MILs are present in all patients, can be obtained with a simple bedside procedure, and can be rapidly expanded in all patients.
In hematologic malignancies, the BM represents not only the site of disease but also a distinctive immunologic microenvironment. Even in solid tumors, evidence exists that BM-derived T cells can be enriched in memory or effector memory T cells. The immune component within the BM is a reservoir of antigen-experienced T cells for both tumor-specific T cells in host with early-stage breast cancer as well as vaccine-primed T cells (12). In the BM, memory CD4 cells are in close contact with interleukin-7 (IL-7)–producing stromal cells but not necessarily maintained by either IL-7 or IL-15 (13), and memory CD8 cells are recruited and maintained through the persistence of antigen expression and effective antigen presentation, as well as in response to proliferative signals from IL-7 and IL-15 (14, 15). Hence, the heightened tumor specificity of MILs in this setting is likely due to the presence of tumor as a source of antigen, whereas their persistence is maintained through the immune interactions with stromal elements, cytokines, and APCs capable of effective antigen presentation in this environment.
We have previously shown that anti-CD3/anti-CD28 ex vivo activated MILs have several essential properties for ACT. Upon activation, they demonstrate significant tumor specificity compared to their peripheral blood lymphocyte counterparts, target a broad range of antigens present on both the mature MM plasma cells and their clonogenic precursors, and effectively kill MM plasma cells (16). Similar to TILs, MILs have a greater endogenous polyclonal antigenic specificity than peripheral lymphocytes. In contrast to TILs, MILs are present in all patients and are obtained from a more immune-responsive microenvironment (17). Hence, MILs represent a promising tumor-specific approach to ACT for hematologic malignancies with BM involvement.
Here, we describe the first clinical study using adoptive therapy with MILs. In addition to determining the feasibility and toxicity of administering ex vivo activated MILs in myeloma patients receiving myeloablative chemotherapy, we demonstrate a direct correlation between the tumor specificity of both the ex vivo expanded/activated MILs and the BM T cells obtained at various time points after transplant with clinical outcomes. We also attempt to identify criteria indicative of clinical responses and disease progression.
RESULTS
Patient characteristics
A total of 25 patients were enrolled. All but three patients received MILs: one patient relapsed between the time of consent signing and collection of the MILs, one patient relapsed between the MIL collection and SCT, and one patient withdrew consent. No enrolled patient was excluded from MIL therapy because of failure to expand the MIL product or to meet release criteria. Table 1 shows the main characteristics of the patients. The median age of the patients was 56 years (range, 30 to 71 years); 64% were male and 82% were white. Patients who had achieved a complete remission (CR) to their last treatment were excluded from the study. For all patients, the median number of previous therapies was 2.13. Forty-five percent of patients were heavily pretreated and had previously relapsed, with a median number of previous therapies of 3.2 (range, 2 to 6). Patients received MILs when they obtained the maximal response to the last therapy. Previous treatment regimens contained thalidomide, lenalidomide, bortezomib, and dexamethasone but were not specified in the protocol.
Table 1.
Patient characteristics.
| Value | Percent | |
|---|---|---|
| Age (years) | 56 (30–71) | |
| Race | ||
| White | 18 | 81 |
| African American | 3 | 14 |
| Hispanic | 1 | 5 |
| Sex | ||
| Male | 14 | 64 |
| Female | 8 | 36 |
| Myeloma subtype | ||
| IgG | 13 | 60 |
| IgA | 9 | 40 |
| Number of previous therapies | ||
| 1 | 12 | 55 |
| 2 | 4 | 18 |
| 3 | 3 | 14 |
| >4 | 3 | 14 |
| Response before BMT | ||
| CR | 0 | 0 |
| VGPR | 3 | 14 |
| PR | 14 | 64 |
| MR | 2 | 8 |
| SD | 3 | 14 |
| Response to BMT | ||
| CR | 7 | 32 |
| PR | 6 | 27 |
| SD | 6 | 27 |
| PD | 3 | 14 |
BMT, bone marrow transplantation; IgA, immunoglobulin A; IgG, immunoglobulin G; MR, minor response; VGPR, very good partial response.
Study design and clinical outcomes
This was a phase 1 study designed to examine the overall feasibility, safety, and efficacy of MILs when administered to patients with MM undergoing an autologous peripheral SCT. The sample size was determined on the basis of the ability to establish the toxicity and response rates. Stopping rules were only considered in the event of significant toxicity as defined in the protocol. All patients who received MILs were considered in the analysis of the data.
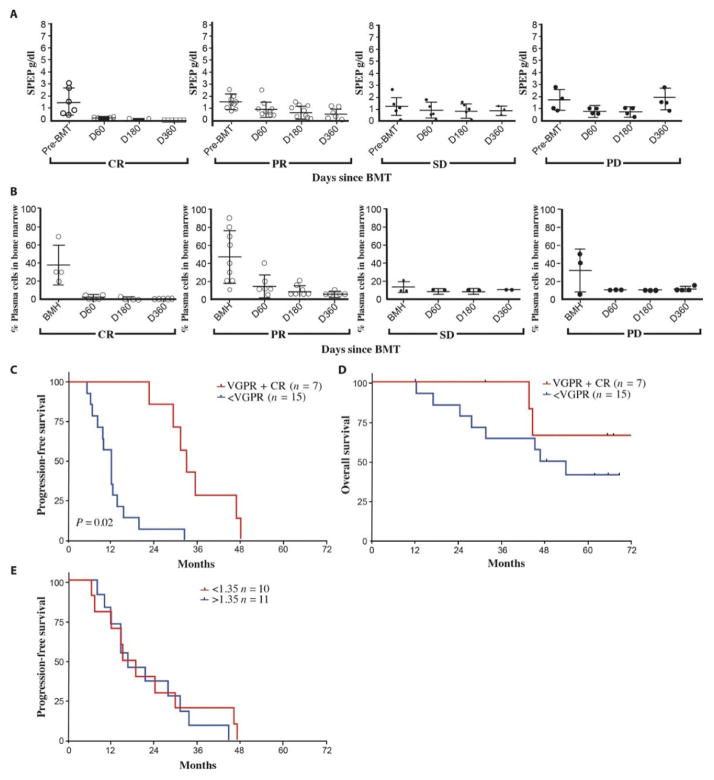
Patients received a CD34-selected stem cell product after high-dose melphalan, with a median number of infused CD34+ cells of 7.39 × 106/kg (03.3 × 106 to 24.2 × 106/kg) (Fig. 1). The CD34+ purity was 99.0% in the CD34-selected products, and the median number of T cells accompanying the graft was 6.65 × 106 total cells or 0.042% of the total infused product. This was done in an effort to measure the direct therapeutic effect of the ex vivo expanded MILs and to minimize the contribution of the T cells in the CD34-mobilized stem product. Other than this modification, patients received a standard melphalan-200 transplant. MILs were infused on day +3 in all patients with an average total T cell dose of per ideal body weight of 2.84 × 107/kg (1.29 × 107 to 6.80 × 107/kg). The patients entering into the trial all had measurable disease with a median serum M-spike of 1.44 (0.07 to 3.2) and a BM plasmacytosis of 32.3% (0 to 90%) at the time of MIL harvest. The percentage of plasma cells in the BM at harvest was 37.5% for patients who ultimately achieved a CR, 46.6% for partial response (PR), 13.3% for stable disease (SD), and 31.2% for patients who developed progressive disease (PD). There was no direct correlation between disease burden at the time of MIL harvest and clinical outcomes as measured by either monoclonal protein (Fig. 2A) or percent tumor in the BM (Fig. 2B) on day 360 after transplant. However, the overall response to treatment before transplant did correlate with outcome. Patients who achieved at least a >90% reduction in disease burden had a longer progression-free survival (PFS) and overall survival (OS) compared to those who did not (17.2 months versus 9.7 months; P = 0.02; PFS) (not reached versus 31.5 months; P = 0.02; OS).
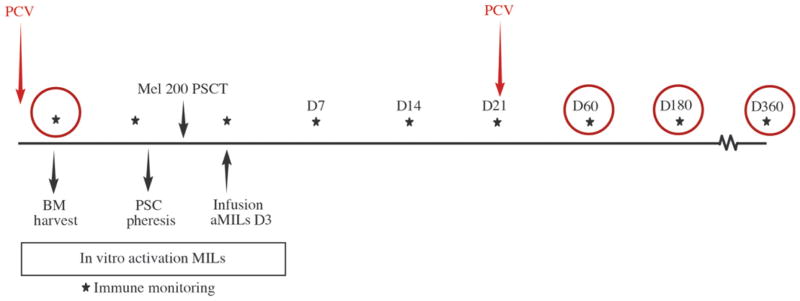
Fig. 1. Study schema.
A PCV was given 2 weeks before BM was obtained for MIL expansion. Patients then underwent a standard stem cell mobilization with cyclophosphamide and G-CSF. A melphalan-200 preparative regimen (Mel 200) was given in preparation for the SCT. The activated MILs (aMILs) were infused on day (D) 3 after transplant. A second PCV was given on day +21. Immune monitoring of the BM occurred with the BM harvest and on days 60, 180, and 360 as indicated by the circles. PSCT, peripheral SCT.
Fig. 2. Clinical data.
(A) Serum protein electrophoresis (SPEP) analysis. Disease burden was measured by SPEP (g/dl) at bone marrow harvest (BMH) and on days 60, 180, and 360. CR, n = 6; PR, n = 7; SD, n = 5; PD, n = 4. (B) Percentage plasma cells in BM. The percent of plasma cells in BM biopsies was determined at the indicated time points. (C) PFS. PFS was analyzed comparing patients who achieved a VGPR or better versus those who achieved less than a VGPR (P = 0.02, Student’s t test). (D) OS. OS was analyzed comparing patients who achieved a VGPR or better versus those who achieved less than a VGPR. (E) PFS and MIL dosage. PFS was analyzed comparing patients who received an MIL dose of 1.35 × 108 cells to those who received less than 1.35 × 108 cells.
One distinguishing feature of MILs compared to peripheral blood lymphocytes (PBLs) was the increased expression of CXCR4 (16). Expression of this chemokine receptor could be responsible for the SDF-1–CXCR4 interaction between the BM stroma and T cells that ultimately facilitates trafficking of MILs to the BM and increases the anti-tumor benefit of the ACT. Exogenous granulocyte colony-stimulating factor (G-CSF) reduces SDF-1 expression in the BM, which could reduce the tropism of MILs to the marrow (18). The concern for the potential negative impact of G-CSF on MIL trafficking provided the rationale for not treating with G-CSF after transplant. The median time to achieving an absolute neutrophil count >500 cells/μl was 17.9 days (range, 12 to 32 days). Several studies using activated peripheral lymphocytes have reported a profound lymphocytosis of >3000 lymphocytes/μl within the first 21 days after transplant (19–21). However, despite the observations that early lymphoid reconstitution in standard autologous transplants correlated with better disease-related outcomes (5), no such relationship has been reported in the setting of ACT. No noteworthy lymphocytosis was observed with MILs in this study. On day 14, the median lymphocyte count was 886 cells/μl (range, 138 to 2397 cells/μl) and peaked at 1925 cells/μl (range, 349 to 3500 cells/μl) by day 28. A lymphocyte count >500/μl or even >1000/μl on day 14 did not correlate with PFS or OS in this study.
The overall clinical response for patients achieving a PR or better in this study was 54% with CR 27%, PR 27%, SD 23%, and PD 14%. PFS was associated with the depth of response that was achieved. Patients who achieved at least a 90% reduction in disease burden had a significantly longer PFS of 25.1 months versus 11.8 months (P = 0.01), but showed no difference in OS (Fig. 2, C and D). Furthermore, there was no difference in PFS based on the total dose of MILs given to the patients (Fig. 2E).
MIL product
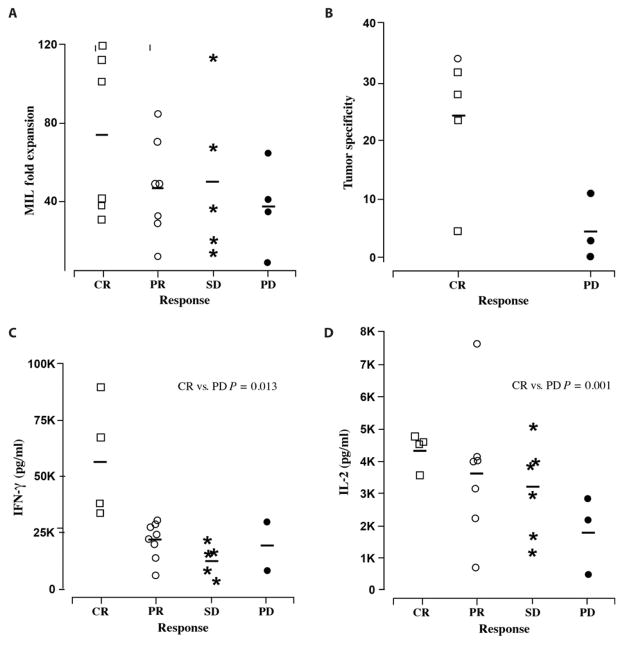
An advantage of MILs is the generation of polyclonal, endogenous, tumor specificity that is not achievable with the ex vivo expansion of PBLs, which lack intrinsic tumor specificity. In light of this intrinsic property, one major goal of this study was to determine whether any properties of the expanded product were associated with clinical outcomes. MILs were expanded in static culture conditions for 7 days. This time frame was previously chosen to maximize the number of tumor-specific T cells and not necessarily the total T cell dose considering the maximal tumor specificity between 7 and 9 days of expansion. The average fold expansion was 48.5 (6.9 to 112.3). Fold expansion (Fig. 3A) and tumor specificity (Fig. 3B) trends were greater in patients with better clinical outcomes, although they were not statistically significant with the limited number of patients evaluated. These data would suggest that the in vitro tumor specificity assay of the expanded MIL product could serve as a potential biomarker of clinical efficacy. With the Wilcoxon signed rank test, higher interferon-γ (IFN-γ) levels from the activated MIL product supernatant were associated with an increased likelihood of achieving a CR (P = 0.013) (Fig. 3C) as did IL-2 levels (P = 0.001) (Fig. 3D). Of the other cytokines examined, tumor necrosis factor–α (TNF-α) showed a trend toward greater secretion in patients achieving a CR versus PD. No statistically significant differences were observed with IL-1β, IL-8, IL-10, IL-13, IL-17, or GM-CSF (granulocyte-macrophage CSF) (Table 2). The biologic relevance of IFN-γ and TNF-α production by ex vivo activated T cells was recently confirmed with IL-21–treated cells (22).
Fig. 3. MILs clinical product.
(A) MILs fold expansion. Fold expansion of MIL products was analyzed according to disease response (CR, n = 6; PR, n = 7; SD, n = 5; PD, n = 4). (B) Tumor specificity of MIL product. The tumor specificity of the MIL products was measured by CD3+/CFSElow/IFN-γ–producing cells comparing patients who achieved a CR (n = 5) versus those who had PD (n = 3). (C) IFN-γ–activated MIL clinical product supernatant. IFN-γ (pg/ml) was measured in the activated MIL clinical product supernatant. Data were analyzed according to disease response. With a Kruskal-Wallis test and Wilcoxon sum rank, the patients who achieved a CR (n = 4) had significantly more IFN-γ in the product supernatant than those with PD (n = 3) (P = 0.013). (D) IL-2 MIL product supernatant. IL-2 was measured in the MIL product supernatant. Data were analyzed according to disease response. With a Kruskal-Wallis test and Wilcoxon sum rank, patients who achieved a CR (n = 4) had significantly more IL-2 than those with PD (n = 3) (P = 0.001).
Table 2. Cytokine production in the supernatant of the MIL post-expansion product (mean ± SE).
MIP-1β, macrophage inflammatory protein–1β.
| Cytokine (pg/ml) | CR (n = 4) | PR (n = 8) | SD (n = 5) | PD (n = 2) |
|---|---|---|---|---|
| IL-1β | 18.9 (17.3) | 16.3 (8.4) | 16.8 (12.2) | 28.8 (27.0) |
| IL-2* | 4,396.8 (571.4) | 3,412.2 (2076.0) | 3,392.7 (1463.7) | 1,684.7 (1597.8) |
| IL-4 | 7.7 (1.4) | 8.3 (1.3) | 9.2 (2.4) | 14.0 (9.3) |
| IL-5 | 125.0 (103.7) | 201.7 (216.3) | 518.2 (601.3) | 359.4 (415.3) |
| IL-6 | 447.3 (296.2) | 424.5 (168.4) | 346.2 (292.8) | 663.8 (541.3) |
| IL-8 | 9,044.8 (6025.1) | 9,521.7 (4230.9) | 8,460.9 (6888.1) | 10,815.6 (1755.2) |
| IL-10 | 20.5 (5.0) | 17.5 (8.6) | 23.8 (15.9) | 28.6 (30.1) |
| IL-12 | 23.3 (3.6) | 20.8 (8.6) | 20.8 (10.0) | 34.0 (28.1) |
| IL-13 | 679.6 (514.3) | 420.8 (271.8) | 1,254.3 (1650.3) | 1,551.3 (2039.5) |
| IL-17 | 64.0 (12.9) | 65.6 (45.4) | 56.1 (25.0) | 118.2 (97.5) |
| GM-CSF | 5,275.0 (1510.1) | 5,172.7 (362.5) | 5,320.1 (710.9) | 8,663.5 (5902.9) |
| IFN-γ† | 57,195.0 (26626.3) | 21,846.8 (8657.8) | 12,911.4 (7073.2) | 18,878.1 (15091.2) |
| MIP-1β | 2,316.0 (1325.8) | 1,536.7 (1156.5) | 1,951.5 (2055.7) | 552.6 (n = 1) |
| TNF-α | 59.5 (42.2) | 36.3 (32.2) | 41.4 (39.4) | 13.2 (8.5) |
P = 0.001.
P = 0.013.
Correlation of anti-myeloma response with functional immunity
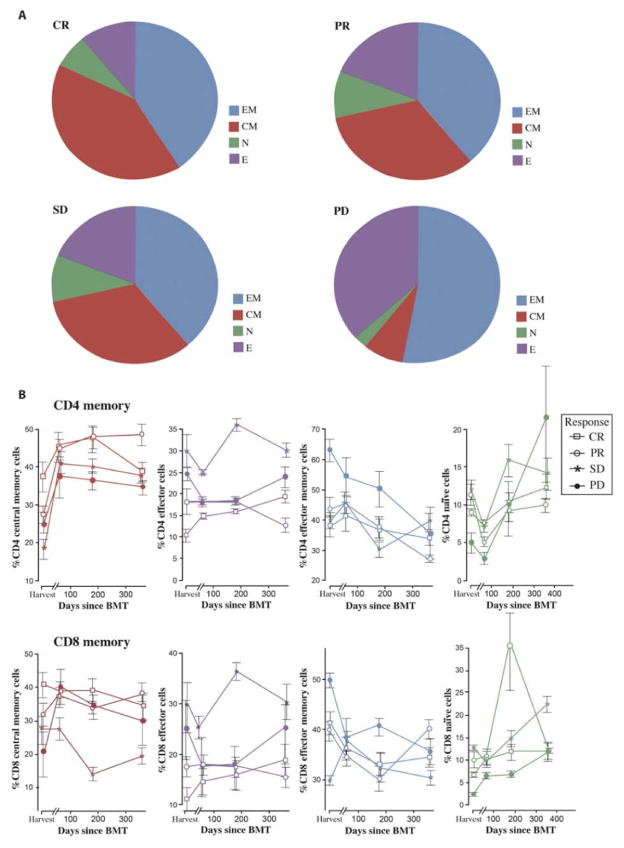
The major focus of this study was not only to use T cells obtained from the BM as a source of tumor-specific effector cells but also to assess the immune responses within the tumor microenvironment at various time points after ACT and to correlate these with clinical response. One goal was to determine whether the baseline immune status of the host could potentially predict response to treatment. Baseline in this study is defined as the time of the harvest. A central memory (TCM) CD8+ phenotype of MILs at baseline was associated with achieving a CR after transplant (Fig. 4A), and this persisted over time (Fig. 4C). In contrast, patients with PD showed more effector T cells (TE) at baseline, which was associated with the higher amounts of endogenous IFN-γ present in the BM plasma at harvest (Table 3). After treatment, the BM plasma cytokines showed increases in IFN-γ and decreases in IL-6 by day 360 for patients in a CR. There were no appreciable changes in cytokine patterns for IL-8, GM-CSF, or IL-17.
Fig. 4. Memory subsets.
(A) T cell memory subsets at baseline. CD8 memory subsets of MILs were measured by staining CD8/CD45RO/CD62L. Subsets are as follows: naïve cells (N) are CD45RO−/CD62L−; effector cells (E) are CD45RO−/CD62L+; effector memory cells (EM) are CD45RO+/CD62L−; and central memory cells (CM) are CD45RO+/CD62L+. Data were analyzed according to disease response. CR, n = 6; PR, n = 7; SD, n = 5; PD, n = 4. (B) T cell memory subsets after MIL infusion. CD4 (upper row) and CD8 (lower row) memory cell subsets in the BM were plotted for each patient at harvest and on days 60, 180, and 360 according to disease response (CR, n = 6; PR, n = 7; SD, n = 5; PD, n = 4).
Table 3. In vivo cytokine production (mean ± SE).
MCP-1, monocyte chemoattractant protein–1.
| CR (n= 3)
|
PD (n= 3)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| BMH | D60 | D180 | D360 | BMH | D60 | D180 | D360 | |
| IL-6* | 88.7 (66.3) | 27.9 (15.6) | 7.0 (4.0) | 2.2* (0.6) | 87.1 (32.3) | 13.7 (5.6) | 38.3 (25.3) | 122.8* (6.5) |
| IL-8 | 53.1 (26.9) | 66.2 (47.9) | 8.6 (0.9) | 6.5 (2.3) | 33.6 (15.2) | 15.8 (7.6) | 10.9 (2.7) | 8.1 (5.3) |
| IL-17 | 18.9 (3.7) | 12.5 (3.3) | 5.9 (3.0) | 4.7 (2.0) | 29.3 (15.4) | 10.5 (2.0) | 12.6 (3.5) | 5.3 (1.9) |
| GM-CSF | 62.7 (26.4) | 106.2 (18.5) | 61.8 (21.1) | 59.1 (11.9) | 93.4 (10.1) | 81.1 (13) | 94.3 (9.8) | 67.0 (24.6) |
| IFN-γ† | 21.1† (5.6) | 84.9 (38.3) | 9.4 (8.4) | 119.3 (80.9) | 107.3† (30.0) | 20.9 (0.9) | 15.3 (2.9) | 30.5 (24.7) |
| MCP-1 | 94.6 (10.7) | 451 (312.9) | 88.9 (36.3) | 109 (23.8) | 193.3 (17.0) | 380 (150.2) | 302.0 (53.9) | 119.9 (46.5) |
| MIP-1β | 215.4 (107.8) | 86.3 (20.4) | 59.2 (24.3) | 66.4 (17) | 219.4 (110.8) | 64.4 (10.6) | 76.2 (21.8) | 42.9 (5.5) |
P = 0.0001.
P = 0.022.
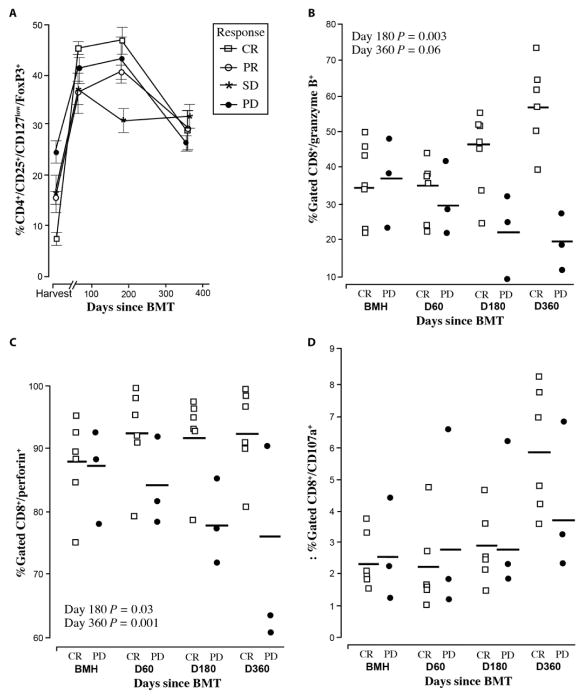
T cell subsets were also examined. Controversy still exists regarding the role of regulatory T cells (Tregs) in myeloma. For our analysis, Tregs were defined as CD4+/CD25+/CD127low/FoxP3+ and were analyzed within the BM compartment. As shown in Fig. 5A, the baseline percent-age of Tregs was lower (6.8% versus 24.6%) in patients who achieved a CR compared to those with PD. However, the final percentage on day 360 was surprisingly similar in all groups, with the CR patients showing the greatest increase from 6.8 to 28.5% as compared to a change from 24.6 to 26.5% for patients with PD. Nonetheless, we hypothesize that these results suggest that Treg depletion of MILs might enhance efficacy or even the ex vivo expansion of MILs considering the direct relationship between the higher Treg percentage and lower expansion of the MIL products in the patients who demonstrated PD (Fig. 3A).
Fig. 5. Peritransplant immune responses.
(A) Tregs. Tregs as measured by CD4+/CD25+/FoxP3+ were measured at harvest and on days 60, 180, and 360. Data were analyzed according to disease response. CR, n = 6; PR, n = 7; SD, n = 5; PD, n = 4. (B) CD8+ granzyme B+ expression on marrow-derived T cells was analyzed in patients achieving a CR or PD by flow cytometry examining CD8 and granzyme B production. Student’s t test showed that statistical significance was reached at days 180 and 360 (P = 0.003 and 0.006, respectively). CR, n = 6; PD, n = 3. (C) CD8+ perforin+ marrow-derived T cells were analyzed by flow cytometry in patients who either achieved a CR or had PD. CD8 perforin production with Student’s t test statistical significance P = 0.03 on day 180 and P = 0.01 on day 360. CR, n = 6; PD, n = 3. (D) CD8+CD107a+ marrow-derived T cells were analyzed by flow cytometry in patients who either achieved a CR or had PD. With a Student’s t test, these data were not statistically significant.
CD8 cells were stained for perforin, granzyme B, and CD107a as a marker of cytotoxic specific degranulation (23). At baseline, there were no statistically significant (Student’s t test) differences between patients who achieved a CR compared to those with PD (Fig. 5B). By day 360, differences became apparent. In CR patients, CD8 cytotoxicity was globally increased. Specifically, granzyme B expression increased by 35.1% every 6 months, perforin expression remained unchanged, and CD107a increased by 31.8% the first 6 months and 101% by 1 year. In contrast, PD patients showed a decrease in cytolytic activity. Granzyme B decreased by 33.1%, perforin decreased by 12.7%, but an increase, albeit small, in CD107a expression was seen at 12 months.
Immune responses to pneumococcal conjugate vaccine
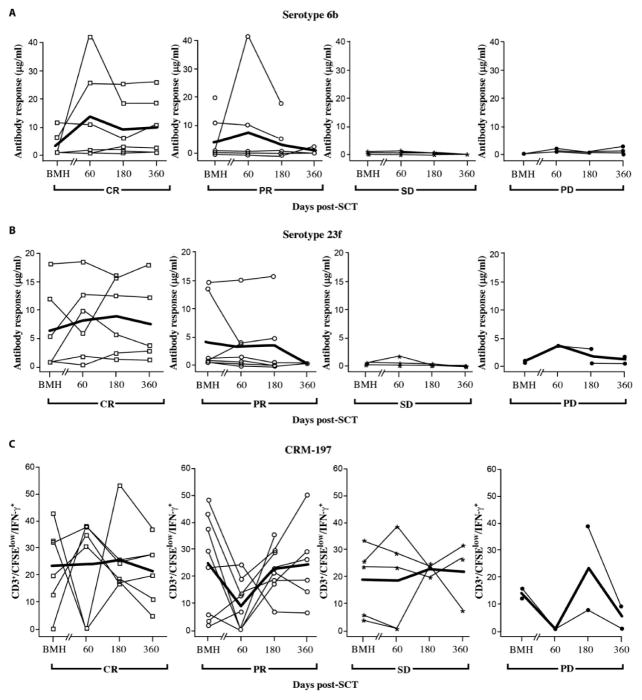
A major objective of this first study using MILs for ACT was to determine the ability of these cells to survive and impart measurable antigen-specific immunity upon transfer. In light of the fact that these ex vivo activated MILs were not gene-modified and thus could not be distinguished from the endogenous T cell repertoire in the host after myeloablation, the patients were given two pneumococcal conjugate vaccine (PCV) vaccinations: the first was administered 2 weeks before the collection of MILs, and the second on day +21 after treatment. The rationale was first to determine whether antigen-specific immunity could be generated and adoptively transferred by ex vivo activated MILs in MM patients and second to assess the persistence of this immunity. This vaccine was chosen because of its ability to prime both humoral responses to several pneumococcal specific antigens and a cellular response to the carrier molecule CRM-197, a modified diphtheria toxin. The pneumococcal specific humoral responses show a trend toward better responses in the CR patients and worst responses in the PD patients. However, only the antibody responses to 23F achieved measurable titers that achieved statistical significance from baseline to day 360 when comparing the CR group to the PD group (P = 0.027) (Fig. 6, A and B). Elevated antibody titers at harvest can be explained by both the presence of preexisting immunity in the patients and the fact that these antibody responses are being measured in the BM where the first time point was actually 2 weeks after the pretransplant PCV vaccination and not before vaccination.
Fig. 6. PCV serologic and T cell responses.
Plasma from the BM at the indicated time points before and after MIL infusion were used to quantify humoral responses. (A and B) 6B (A) and 23F (B) antibody titers after PCV vaccination. Data were graphed per patient and analyzed according to disease response. CR, n = 6; PR, n = 7; SD, n = 5; PD, n = 4. (C) CRM-197–specific T cell responses. T cell responses to CRM-197 were measured in the BM by quantifying CD3+/CFSElow/IFN-γ+ T cells. The positive responses at BMH were a result of the administration of PCV 2 weeks before the harvest. Data were analyzed according to disease response groups.
T cell responses to the PCV carrier molecule CRM-197 were also examined. In light of the fact that the patients transplanted in this study received a CD34-enriched stem cell product (and thus T cell–reduced), the demonstration of CRM-197 T cell immunity would most likely reflect vaccine priming and persistence of MILs after adoptive transfer. Although statistically significant differences were not appreciated between the patients in CR versus PD, the persistence of these responses suggested that substantial fraction of the infused MILs persisted up to 1 year (Fig. 6C).
Tumor-specific immunity in the BM
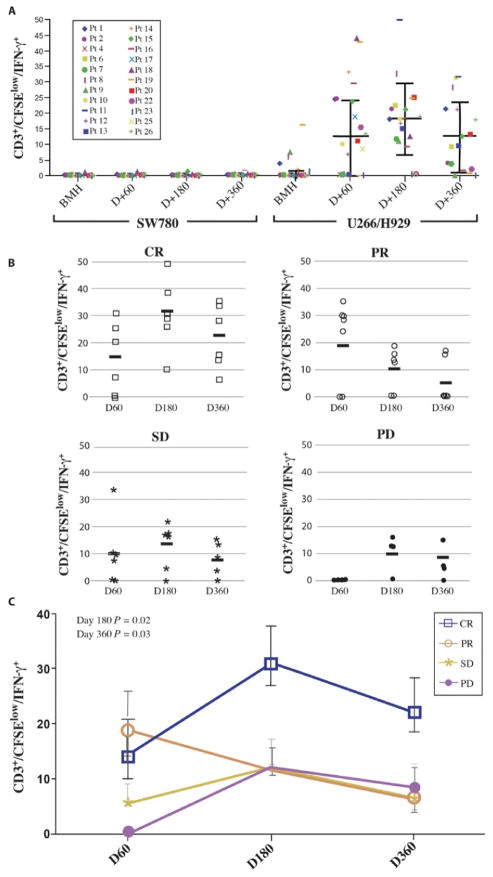
A major limitation of many immune-based studies lies in the lack of correlation between immune responses and clinical outcomes. To address this concern, we examined the immune response in the tumor microenvironment by using T cells from the BM obtained at the indicated time points. We have previously shown that the antigen-specific T cell responses are greater when analyzed in the BM as compared to the blood (24). Myeloma-specific immunity was minimal at baseline as expected, peaked at day 180, but was surprisingly still present and only modestly reduced at 1 year after transplant (Fig. 7A). Differences in antitumor immunity were also evident between patients. One group of patients failed to ever demonstrate detectable myeloma-specific immunity, whereas in another cohort, greater than 30% of their CD3+/CFSElow cells produced IFN-γ in response to myeloma lysate suggestive of a potent anti-myeloma response. These differences in myeloma-specific immunity were associated with clinical outcomes. The greatest myeloma-specific immunity was observed in those who achieved a CR (Fig. 7B). Patients who achieved a PR, SD, or PD to therapy all had similar amounts of tumor specificity. These data demonstrate several important properties that MILs have in the setting of ACT. Specifically, they can prime and adoptively transfer both vaccine-specific and polyclonal, anti-myeloma immunity, effectively traffic to the BM, and persist over time.
Fig. 7. Tumor-specific responses.
(A) Tumor-specific responses over time. Myeloma-specific immunity was measured by pulsing BM that was CFSE-labeled with tumor lysate and analyzing CD3+/CFSElow/IFN-γ+ cells as the antigen-specific cells. CR, n = 6; PR, n = 7; SD, n = 5; PD, n = 4. (B) Tumor-specific fold change over time by disease response. The fold change of CD3+/CFSElow/IFN-γ+ cells was measured from baseline over time and analyzed. (C) Tumor-specific fold change over time by disease response summary. The fold change of CD3+/CFSElow/IFN-γ+ cells was measured from baseline over time and analyzed according to disease response. With the Wilcoxon sum rank at days 180 and 360, the patients achieving a CR had statistically significantly greater fold increase in tumor-specific T cells than those in all other response groups (P = 0.02 and 0.03, respectively).
DISCUSSION
This study is the first demonstration of the feasibility and efficacy of ACT using MILs. We also demonstrate a strong correlation between clinical outcome and tumor-specific activity, as well as persistence of tumor-specific T cells in the BM after ACT. The results presented here underpin a randomized multicenter clinical trial currently under way to assess efficacy of this ACT approach relative to autologous transplant alone.
The correlation between the tumor specificity upon ex vivo expansion and clinical outcomes can potentially result in a biomarker in patient selection, which could predict clinical efficacy in those patients who go on to receive this product. It also suggests that this could be a straightforward readout for future strategies to optimize ex vivo MIL expansion. Indeed, we have already introduced various modifications in the MIL processing based on expansion of tumor-specific T cells ex vivo. The rationale behind the development of MILs lies in several of their features. The broad antigenic specificity of these activated cells, the ability to traffic to the BM upon infusion, and their persistence over time underscore their distinct properties. While initially envisioning the use of MILs merely as a source of tumor-specific T cells, we must also consider the immunologic properties of the BM immune environment. It is rich in APCs that likely play an important role in enriching for antigen-experienced T cells and maintaining endogenous T cell proliferation (17, 25), as well as enrichment of central memory T cells (12).
The presence of TCM CD8+ phenotype was associated with the highest likelihood of achieving a CR. This supports studies by others whereby central memory T cells impart better and more durable immunity, as well as have greater persistence in vivo, better antigen recall responses, and improved trafficking to secondary lymphoid organs and, in our case, also to the BM compared to effector memory T cells (26, 27). We failed to observe any correlation between naïve CD8+ T cells and response and did not examine the presence of the stem cell memory (TSCM) population, both of which have been associated with long-term T cell persistence. However, in light of the data showing minimal numbers of TSCM in the BM (28), we would not expect these cells to correlate with clinical outcomes in our MIL patients. Other features of CD8+ cells correlating with CR included increases in granzyme B, CD107a, and persistent perforin expression.
A major difference and potential benefit of MILs compared to PBLs is the greater tumor specificity of MILs that is observed after anti-CD3/CD28 ex vivo activation. This was measurable in all patients enrolled on this study but has never been observed with activated PBLs in our preclinical studies (16). Significant variability in the tumor specificity was observed with ex vivo, activated MILs. This tumor specificity was associated with clinical outcomes (Fig. 7), suggesting that MILs are capable of both trafficking to the BM and exerting a cytotoxic effect upon their encounter with tumor—both critical features for successful ACT.
MILs differ from TILs in that they come from very different compartments and in feasibility of standardized product production for ACT. In contrast to TILs, MILs could be harvested in all patients, and an average of 3.3 × 109 cells were obtained with a 200-ml bedside BM collection. The manufacturing of the expanded product was done with a 7-day process, and T cells were expanded in all patients. The easy access to the tumor site, the absence of the need for surgical removal of the tissue containing the T cells, the numbers of cells obtained with the harvest, and the ability to expand products in all patients with a relatively short process at a reasonable cost contrast sharply with several limitations of TIL ACT.
The marked recent successes in ACT have been observed with chimeric antigen receptor (CAR)–modified T cells. This approach has generated enthusiasm largely based on the rather impressive results observed with the CD19 CAR T cells treating both adult chronic lymphocytic leukemia and pediatric acute lymphoblastic leukemia patients with relapsed/refractory disease (29–31). These results have clearly pointed to the ability to achieve clinically meaningful remissions targeting a single surface antigen. In myeloma, several surface antigens are currently being explored including CS-1 (32) and B cell maturation antigen (BCMA) (33). Targeting of a single antigen runs the risk of ultimately developing antigen escape variants at relapse. One distinctive feature of MILs compared to PBLs, which are currently used in most ACT studies, is the broader endogenous antigenic repertoire. Also, on the basis of their distinct functional capacity compared with PBL, MILs could potentially be harnessed as a better source of T cells for CAR-based therapies.
Patients in a CR at the time of their MIL harvest were excluded from the study to ensure the presence of antigen during the activation and expansion process. The underlying rationale for this was that the presence of tumor during T cell expansion maintains a level of antigenic specificity that generates a more tumor-specific product. We have, in fact, previously demonstrated that the greatest tumor specificity of the MIL product was obtained when these T cells were grown in the presence of tumor (16). Removing MILs from the BM microenvironment before T cell activation significantly reduced the tumor specificity. However, factors mediating this enhanced tumor specificity extended beyond the mere presence of tumor because activation of PBLs in the presence of tumor was incapable of achieving the level of tumor specificity that was observed with activated MILs—a factor that further underscores substantial differences between PBLs and MILs. Our manufacturing approach was developed to maximize the endogenous properties of the BM and generate the greatest possible tumor specificity per T cell.
A major limitation of many immunotherapeutic studies has been the lack of correlation between clinical outcomes and immune responsiveness. One factor contributing to this discrepancy may be related to performing the immune studies in an irrelevant tissue compartment. To address this issue, we performed all the immune analysis using only BM obtained at various time points throughout the study. Furthermore, the assays were performed ex vivo to best assess the true immune status. One major concern in this first study using MILs was the persistence of the cells upon infusion. The absence of any specific gene marker limited our ability to directly track the fact of adoptively transferred MILs. However, the patients were vaccinated with PCV 2 weeks before the MIL harvest and again on day 21 after transplant. Hence, it is reasonable to assume that the vast majority of the CRM-197+ T cells were MILs that were primed with the preharvest vaccine, adoptively transferred, and further augmented with the early post-transplant vaccine—a time in which T cell reconstitution from either the host or the stem cell graft is negligible. The presence of CRM-197+ T cells with the MIL harvest and the relatively unvaried persistence of those T cells out to 1 year are suggestive of the persistence of MILs (Fig. 6E). In light of these findings, the tumor specificity data are most likely a reflection of the function of the infused activated MILs. The surprising findings were both the apparent correlation between high levels of tumor-specific immunity and CR as well as the persistence of that response (Fig. 7B). We observed a 25% incidence of grade 1 to 2 autologous skin graft-versus-host disease (GVHD), which is slightly higher than the 16% reported with activated peripheral lymphocytes (20). As with other reported studies, autologous GVHD did not appear to correlate with an improved antitumor effect (34).
Clinically, this protocol enrolled a rather high-risk group of patients defined by the fact that none were in CR, 45% had previously relapsed disease, and the median number of previous therapies was 2.13. One of the best predictors to achieving a CR after transplant is being in a CR at the time of transplant (35, 36), and that specific group was intentionally excluded from the study. Furthermore, the tumor concentration at BM harvest (average of 33% plasma cells) was substantially greater than would be expected in a patient population entering transplant.
In conclusion, this study shows that MILs can be effectively used as a source of tumor-specific T cells for adoptive cell therapy especially in hematologic malignancies. Furthermore, it highlights several baseline parameters in the MIL product and in the host that could potentially predict clinical outcomes, which, if confirmed in larger studies, could serve as potential biomarkers to determine patient eligibility and possibly evaluate the quality of the product. However, durability of the clinical response was less than one would ultimately wish to achieve. This may be due to having failed to achieve the threshold dose of T cell necessary to impart a persistent response or the loss of tumor specificity over time, or a function of the high-risk population enrolled. Hence, approaches to address these issues include modification of our manufacturing processes from static to dynamic culture systems to obtain greater numbers and potentially also adding a tumor vaccine in the peritransplant setting to both increase the precursor frequency of tumor-specific MILs and maintain tumor specificity after transplant.
METHODS
Study design
This was a phase 1 study designed to examine the overall feasibility, safety, and efficacy of MILs when administered to patients with MM undergoing an autologous peripheral SCT. The sample size was determined on the basis of the ability to establish the toxicity and response rates. Stopping rules were only considered in the event of significant toxicity as defined in the protocol. All patients who received MILs were considered in the analysis of the data. Immune monitoring time points are shown in Fig. 1, and the experiments were performed in triplicate.
Patients
Study participants were at least 18 years old with a diagnosis of active, symptomatic myeloma requiring treatment (Durie-Salmon II or III). Patients were eligible for the study if they had never previously received an autologous SCT and had measurable disease at the time of the MIL harvest as determined by the presence of a monoclonal spike in their serum and/or urine coupled with a detectable presence of clonotypic plasma cells in the BM. Patients with amyloidosis or plasma cell leukemia were excluded, as were patients with active autoimmune disease requiring systemic treatment. Patients who completed their first line of chemotherapy as well as patients with relapsed disease receiving subsequent lines of therapy were eligible for the study as long as they had never previously undergone an autologous SCT. MILs could be harvested either before treatment for newly diagnosed patients or before the SCT. Eligibility criteria included the following: a total neutrophil count >1000/mm3, platelets >75,000/mm3, Eastern Cooperative Oncology Group (ECOG) performance status >2, left ventricular ejection fraction >45%, adequate pulmonary function with an FEV1 (forced expiratory volume in 1 s) and FVC (forced vital capacity) >70, bilirubin <2.0, transaminases <2 times the upper limit of normal, and serum creatinine <2.0. Patients were required to be HIV1- and HIV2-negative as well as human T cell leukemia virus (HTLV) 1– and HTLV2-negative. All patients gave written informed consent in accordance with the Declaration of Helsinki. The study was approved by the Institutional Review Board of Johns Hopkins University and registered with ClinicalTrials.gov NCT00566098.
Clinical trial
The schema for the clinical trial is shown in Fig. 1. Patients who met the initial eligibility criteria received a pretransplant PCV (Prevnar) 2 weeks before undergoing a BM collection of 200 ml for MIL expansion. Peripheral stem cells were mobilized and collected with cyclophosphamide (3000 mg/m2, intravenously) followed by G-CSF (10 μg/kg, subcutaneously) starting on the day after the cyclophosphamide through the day of stem cell collection. High flow pheresis was done to obtain a minimum of 5 × 106 CD34+ cells/kg. CD34+ stem cells were then isolated using a Miltenyi CliniMACS. The preparative regimen consisted of melphalan (200 mg/m2) given over 2 days followed by the stem cell infusion on day 0. The cryopreserved activated MIL product was administered on day +3 after transplant as a single infusion. No G-CSF was permitted after transplant. Patient received PCV again on day +21 after transplantation. BM biopsies were performed on days +60, +180, and +360 as per standard institutional practices.
MIL collection
Patients had their MILs collected either at diagnosis, before treatment (n = 4), or before transplant (n = 18). To minimize any effects of chemotherapy on the ability to expand the MILs, the chemotherapy was discontinued 3 weeks before the MIL collection. The collection of MILs was an outpatient, bedside procedure in which the patients were given conscious sedation consisting of intravenous fentanyl and midazolam as per standard practice and titrated to patient comfort. The posterior iliac crests were sterilely prepped and draped, and the collection was performed by two operators drawing 20 ml of BM into syringes containing 2 ml of heparin (1:1000) for a total of 200 ml.
MIL expansion
The collected BM was kept at room temperature overnight and subsequently depleted of red cells using the COBE Spectra and then frozen. At the time of cell expansion, the MILs were thawed and stimulated with ClinExVivo CD3/CD28 obtained from Life Technologies at a 3:1 bead–to–T cell ratio. The expansions were done in static cultures with American Fluoroseal Corporation bags for 7 days with the addition of IL-2 (200 IU/ml). The MILs were subsequently harvested and subjected to magnetic bead depletion using the CliniMACS bead removal system. The expanded MIL product was frozen and subsequently thawed at the bedside at the time of infusion. The product was administered, after premedication with acetaminophen and diphenhydramine, through standard blood tubing without an additional filter at a rate of 10 ml/min. The average dose of MILs infused was 9.5 × 108 cells (range, 4.1 × 108 to 2.2 × 109).
Luminex cytokine analysis
Luminex analysis was performed on the cell culture medium obtained on the final day of the MIL expansion, as well as from the plasma obtained from the BM aspirates at the indicated time points before and after transplant. For analysis of the MIL expansion product, the medium was removed from the culture and centrifuged. For the BM plasma, the BM obtained at the time of harvest was centrifuged at 1500 rpm within 2 hours of collection and the plasma was collected. The Luminex 17-plex protocol was followed per the manufacturer’s recommendations. The cytokines analyzed included IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-13, IL-17, G-CSF, GM-CSF, IFN-γ, MCP-1, MIP-1β, and TNF-α. The results were plotted against clinical outcomes.
Immune monitoring analysis
Tumor specificity assay
BM-derived T cells obtained at the indicated time points before and after transplant were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen), incubated for 10 min at 37°C, and washed per the manufacturer’s recommendations. Autologous BM cells were pulsed either in AIM-V medium alone or with SW780 (bladder carcinoma cell line) lysate or H929 + U266 (myeloma cell line) lysates, respectively. After 5 days, tumor specificity was determined by staining the cells with anti-CD3 (BD Biosciences) and IFN-γ (eBioscience) and by analyzing them with flow cytometry. Data were collected on the Gallios flow cytometer (Beckman Coulter) and analyzed with Kaluza software (Beckman Coulter). Tumor-specific cells were scored as CD3+/CFSElow/IFN-γ+.
CRM-197 T cell responses
BM cells were thawed in AIM-V medium (Invitrogen), labeled with CFSE (Invitrogen), incubated for 10 min at 37°C, and then washed according to the manufacturers’ recommendations. CRM-197 responses were determined by adding the diphtheria toxin, CRM-197 (Sigma) (10 μg/ml), or medium alone for 5 days at 37°C. After culture, cells were stained extracellularly with anti-CD3 (BD Biosciences) and intracellularly with anti–IFN-γ(eBioscience) before analysis by flow cytometry. Data were acquired on a Gallios flow cytometer and analyzed using Kaluza software. Antigen-specific T cells were identified as CD3+/CFSElow/IFN-γ+ T cells.
Immune reconstitution phenotype
BM and/or peripheral blood cells were stained extracellularly with CD3, CD4, CD8, CD25, CD45RO, CD62L, CD69, PD1, PDL1, CD14, CD15, HLA-DR, CD124, CD19, CD56, CD38, and CD138 and intracellularly for FoxP3 and IFN-γ(all antibodies were obtained from either BD Biosciences or Beckman Coulter). Cells were run on the Gallios flow cytometer (Beckman Coulter), and data were analyzed using Kaluza software (Beckman Coulter).
Statistics
The Kruskal-Wallis test, followed by the Wilcoxon rank sum, was used to determine statistical significance from baseline (BMH) to each successive time points (days 60, 180, and 360) in the four clinical outcome groups (CR, PR, SD, and PD). Results are reported as means ± SEM. Two-tailed Student’s t test was performed to compare the mean of two groups. Kaplan-Meir analysis was performed followed by log-rank test for statistical significance. Significance was assumed at P < 0.05. All analyses were performed using functions and methods implemented in the R statistical environment.
Acknowledgments
We would like to thank the patients and their families for their willingness to participate in this study, K. Sheldon for the data management in the study, E. Griffin for his processing and storage of the blood and BM samples used in the study, A. Casildo for her technical assistance with the assays, and K. Maly for her assistance in the preparation of the manuscript. We also thank the clinical staff of the BMT program at the Johns Hopkins Sidney Kimmel Cancer Center for the outstanding clinical care provided to the patients.
Funding: This study was supported by the Commonwealth Fund, 5P01 CA015396, and P30 CA006973, as well as the Baca and Morisi Funds.
Footnotes
Author contributions: K.A.N. and I.B.: MIL concept and design, MIL clinical optimization, immunoassay design, assay performance, assay analysis, and manuscript preparation; J.D., M.V.L., S.F., and J.B.: MIL clinical expansion; A.F.: study research nurse; A.E.: BM harvests for MIL collection; C.A.H., L.L., W.M., J.P., E.F., R.F.A., and R.J.J.: patient care; C.E. and G.L.R.: statistical analysis; L.R.: immunoassay performance and assay analysis; D.P.: immunoassay design, assay analysis, and manuscript preparation.
Competing interests: The authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, Casassus P, Maisonneuve H, Facon T, Ifrah N, Payen C, Bataille R. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 2.Borrello I, Sotomayor EM, Rattis FM, Cooke SK, Gu L, Levitsky HI. Sustaining the graft-versus-tumor effect through posttransplant immunization with granulocyte-macrophage colony-stimulating factor (GM-CSF)–producing tumor vaccines. Blood. 2000;95:3011–3019. [PubMed] [Google Scholar]
- 3.Rapoport AP, Stadtmauer EA, Aqui N, Badros A, Cotte J, Chrisley L, Veloso E, Zheng Z, Westphal S, Mair R, Chi N, Ratterree B, Pochran MF, Natt S, Hinkle J, Sickles C, Sohal A, Ruehle K, Lynch C, Zhang L, Porter DL, Luger S, Guo C, Fang HB, Blackwelder W, Hankey K, Mann D, Edelman R, Frasch C, Levine BL, Cross A, June CH. Restoration of immunity in lymphopenic individuals with cancer by vaccination and adoptive T-cell transfer. Nat Med. 2005;11:1230–1237. doi: 10.1038/nm1310. [DOI] [PubMed] [Google Scholar]
- 4.Dummer W, Niethammer AG, Baccala R, Lawson BR, Wagner N, Reisfeld RA, Theofilopoulos AN. T cell homeostatic proliferation elicits effective antitumor auto-immunity. J Clin Invest. 2002;110:185–192. doi: 10.1172/JCI15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porrata LF, Gertz MA, Inwards DJ, Litzow MR, Lacy MQ, Tefferi A, Gastineau DA, Dispenzieri A, Ansell SM, Micallef IN, Geyer SM, Markovic SN. Early lymphocyte recovery predicts superior survival after autologous hematopoietic stem cell transplantation in multiple myeloma or non-Hodgkin lymphoma. Blood. 2001;98:579–585. doi: 10.1182/blood.v98.3.579. [DOI] [PubMed] [Google Scholar]
- 6.Joao C, Porrata LF, Inwards DJ, Ansell SM, Micallef IN, Johnston PB, Gastineau DA, Markovic SN. Early lymphocyte recovery after autologous stem cell transplantation predicts superior survival in mantle-cell lymphoma. Bone Marrow Transplant. 2006;37:865–871. doi: 10.1038/sj.bmt.1705342. [DOI] [PubMed] [Google Scholar]
- 7.Porrata LF, Litzow MR, Tefferi A, Letendre L, Kumar S, Geyer SM, Markovic SN. Early lymphocyte recovery is a predictive factor for prolonged survival after autologous hematopoietic stem cell transplantation for acute myelogenous leukemia. Leukemia. 2002;16:1311–1318. doi: 10.1038/sj.leu.2402503. [DOI] [PubMed] [Google Scholar]
- 8.Porrata LF, Inwards DJ, Ansell SM, Micallef IN, Johnston PB, Gastineau DA, Litzow MR, Winters JL, Markovic SN. Early lymphocyte recovery predicts superior survival after autologous stem cell transplantation in non-Hodgkin lymphoma: A prospective study. Biol Blood Marrow Transplant. 2008;14:807–816. doi: 10.1016/j.bbmt.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine BL, Bernstein WB, Connors M, Craighead N, Lindsten T, Thompson CB, June CH. Effects of CD28 costimulation on long-term proliferation of CD4+ T cells in the absence of exogenous feeder cells. J Immunol. 1997;159:5921–5930. [PubMed] [Google Scholar]
- 10.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: A clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen LT, Yen PH, Nie J, Liadis N, Ghazarian D, Al-Habeeb A, Easson A, Leong W, Lipa J, McCready D, Reedijk M, Hogg D, Joshua AM, Quirt I, Messner H, Shaw P, Crump M, Sharon E, Ohashi PS. Expansion and characterization of human melanoma tumor-infiltrating lymphocytes (TILs) PLOS One. 2010;5:e13940. doi: 10.1371/journal.pone.0013940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feuerer M, Beckhove P, Bai L, Solomayer EF, Bastert G, Diel IJ, Pedain C, Oberniedermayr M, Schirrmacher V, Umansky V. Therapy of human tumors in NOD/SCID mice with patient-derived reactivated memory T cells from bone marrow. Nat Med. 2001;7:452–458. doi: 10.1038/86523. [DOI] [PubMed] [Google Scholar]
- 13.Lantz O, Grandjean I, Matzinger P, Di Santo JP. γ chain required for naïve CD4+ T cell survival but not for antigen proliferation. Nat Immunol. 2000;1:54–58. doi: 10.1038/76917. [DOI] [PubMed] [Google Scholar]
- 14.Mazo IB, Honczarenko M, Leung H, Cavanagh LL, Bonasio R, Weninger W, Engelke K, Xia L, McEver RP, Koni PA, Silberstein LE, von Andrian UH. Bone marrow is a major reservoir and site of recruitment for central memory CD8+ T cells. Immunity. 2005;22:259–270. doi: 10.1016/j.immuni.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Becker TC, Coley SM, Wherry EJ, Ahmed R. Bone marrow is a preferred site for homeostatic proliferation of memory CD8 T cells. J Immunol. 2005;174:1269–1273. doi: 10.4049/jimmunol.174.3.1269. [DOI] [PubMed] [Google Scholar]
- 16.Noonan K, Matsui W, Serafini P, Carbley R, Tan G, Khalili J, Bonyhadi M, Levitsky H, Whartenby K, Borrello I. Activated marrow-infiltrating lymphocytes effectively target plasma cells and their clonogenic precursors. Cancer Res. 2005;65:2026–2034. doi: 10.1158/0008-5472.CAN-04-3337. [DOI] [PubMed] [Google Scholar]
- 17.Feuerer M, Beckhove P, Garbi N, Mahnke Y, Limmer A, Hommel M, Hammerling GJ, Kyewski B, Hamann A, Umansky V, Schirrmacher V. Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nat Med. 2003;9:1151–1157. doi: 10.1038/nm914. [DOI] [PubMed] [Google Scholar]
- 18.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fujii N, Sandbank J, Zipori D, Lapidot T. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 19.Laport GG, Levine BL, Stadtmauer EA, Schuster SJ, Luger SM, Grupp S, Bunin N, Strobl FJ, Cotte J, Zheng Z, Gregson B, Rivers P, Vonderheide RH, Liebowitz DN, Porter DL, June CH. Adoptive transfer of costimulated T cells induces lymphocytosis in patients with relapsed/refractory non-Hodgkin lymphoma following CD34+-selected hematopoietic cell transplantation. Blood. 2003;102:2004–2013. doi: 10.1182/blood-2003-01-0095. [DOI] [PubMed] [Google Scholar]
- 20.Rapoport AP, Stadtmauer EA, Aqui N, Vogl D, Chew A, Fang HB, Janofsky S, Yager K, Veloso E, Zheng Z, Milliron T, Westphal S, Cotte J, Huynh H, Cannon A, Yanovich S, Akpek G, Tan M, Virts K, Ruehle K, Harris C, Philip S, Vonderheide RH, Levine BL, June CH. Rapid immune recovery and graft-versus-host disease–like engraftment syndrome following adoptive transfer of costimulated autologous T cells. Clin Cancer Res. 2009;15:4499–4507. doi: 10.1158/1078-0432.CCR-09-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rapoport AP, Aqui NA, Stadtmauer EA, Vogl DT, Fang HB, Cai L, Janofsky S, Chew A, Storek J, Akpek G, Badros A, Yanovich S, Tan MT, Veloso E, Pasetti MF, Cross A, Philip S, Murphy H, Bhagat R, Zheng Z, Milliron T, Cotte J, Cannon A, Levine BL, Vonderheide RH, June CH. Combination immunotherapy using adoptive T-cell transfer and tumor antigen vaccination on the basis of hTERT and survivin after ASCT for myeloma. Blood. 2011;117:788–797. doi: 10.1182/blood-2010-08-299396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chapuis AG, Ragnarsson GB, Nguyen HN, Chaney CN, Pufnock JS, Schmitt TM, Duerkopp N, Roberts IM, Pogosov GL, Ho WY, Ochsenreither S, Wolfl M, Bar M, Radich JP, Yee C, Greenberg PD. Transferred WT1-reactive CD8+ T cells can mediate antileukemic activity and persist in post-transplant patients. Sci Transl Med. 2013;5:174ra127. doi: 10.1126/scitranslmed.3004916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 24.Noonan K, Rudraraju L, Ferguson A, Emerling A, Pasetti MF, Huff CA, Borrello I. Lenalidomide-induced immunomodulation in multiple myeloma: Impact on vaccines and antitumor responses. Clin Cancer Res. 2012;18:1426–1434. doi: 10.1158/1078-0432.CCR-11-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Rosa F, Pabst R. The bone marrow: A nest for migratory memory T cells. Trends Immunol. 2005;26:360–366. doi: 10.1016/j.it.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, Waldmann TA, Restifo NP. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lugli E, Dominguez MH, Gattinoni L, Chattopadhyay PK, Bolton DL, Song K, Klatt NR, Brenchley JM, Vaccari M, Gostick E, Price DA, Waldmann TA, Restifo NP, Franchini G, Roederer M. Superior T memory stem cell persistence supports long-lived T cell memory. J Clin Invest. 2013;123:594–599. doi: 10.1172/JCI66327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, Milone MC, Levine BL, June CH. Chimeric antigen receptor–modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maus MV, Grupp SA, Porter DL, June CH. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood. 2014;123:2625–2635. doi: 10.1182/blood-2013-11-492231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska M, Qu J, Wasielewska T, He Q, Fink M, Shinglot H, Youssif M, Satter M, Wang Y, Hosey J, Quintanilla H, Halton E, Bernal Y, Bouhassira DC, Arcila ME, Gonen M, Roboz GJ, Maslak P, Douer D, Frattini MG, Giralt S, Sadelain M, Brentjens R. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra225. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chu J, Deng Y, Benson DM, He S, Hughes T, Zhang J, Peng Y, Mao H, Yi L, Ghoshal K, He X, Devine SM, Zhang X, Caligiuri MA, Hofmeister CC, Yu J. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia. 2014;28:917–927. doi: 10.1038/leu.2013.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carpenter RO, Evbuomwan MO, Pittaluga S, Rose JJ, Raffeld M, Yang S, Gress RE, Hakim FT, Kochenderfer JN. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res. 2013;19:2048–2060. doi: 10.1158/1078-0432.CCR-12-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holmberg L, Kikuchi K, Gooley TA, Adams KM, Hockenbery DM, Flowers ME, Schoch HG, Bensinger W, McDonald GB. Gastrointestinal graft-versus-host disease in recipients of autologous hematopoietic stem cells: Incidence, risk factors, and outcome. Biol Blood Marrow Transplant. 2006;12:226–234. doi: 10.1016/j.bbmt.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 35.Lahuerta JJ, Mateos MV, Martinez-Lopez J, Rosinol L, Sureda A, de la Rubia J, Garcia-Larana J, Martinez-Martinez R, Hernandez-Garcia MT, Carrera D, Besalduch J, de Arriba F, Ribera JM, Escoda L, Hernandez-Ruiz B, Garcia-Frade J, Rivas-Gonzalez C, Alegre A, Blade J, San Miguel JF. Influence of pre- and post-transplantation responses on outcome of patients with multiple myeloma: Sequential improvement of response and achievement of complete response are associated with longer survival. J Clin Oncol. 2008;26:5775–5782. doi: 10.1200/JCO.2008.17.9721. [DOI] [PubMed] [Google Scholar]
- 36.Sonneveld P, Goldschmidt H, Rosinol L, Blade J, Lahuerta JJ, Cavo M, Tacchetti P, Zamagni E, Attal M, Lokhorst HM, Desai A, Cakana A, Liu K, van de Velde H, Esseltine DL, Moreau P. Bortezomib-based versus nonbortezomib-based induction treatment before autologous stem-cell transplantation in patients with previously untreated multiple myeloma: A meta-analysis of phase III randomized, controlled trials. J Clin Oncol. 2013;31:3279–3287. doi: 10.1200/JCO.2012.48.4626. [DOI] [PubMed] [Google Scholar]