Abstract
Primary embryonic hippocampal neurons can develop morphologically and functionally in culture but do not survive more than a few weeks. It has been reported that basic fibroblast growth factor (bFGF) promotes the survival of and neurite elongation from fetal hippocampal neurons. We report that bFGF, in a dose-dependent manner, can induce the survival (50 pg to 1 ng/ml) and proliferation (10-20 ng/ml) of embryonic hippocampal progenitor neurons in vitro. In serum-free medium containing high concentrations of bFGF, neurons not only proliferated (4-day doubling time) and differentiated morphologically but also could be passaged and grown as continuous cell lines. The neuronal nature of the proliferating cells was positively established by immunostaining with several different neuron-specific markers and by detailed ultrastructural analyses. The proliferative effect of bFGF was used to generate nearly pure neuronal cell cultures that can be passaged, frozen, thawed, and cultured again. Neurons have been maintained > 5 months in culture. The ability to establish long-term primary neuronal cultures offers the possibility that clonal lines of distinct neuronal cell types may be isolated from specific areas of the central nervous system. Such long-term neuronal cultures should prove valuable in studying neurons at the individual cell level and also in exploring interactions between neurons in vitro. The observed dose dependence raises the possibility that cell survival and proliferation in vivo may be influenced by different levels of bFGF.
Full text
PDF
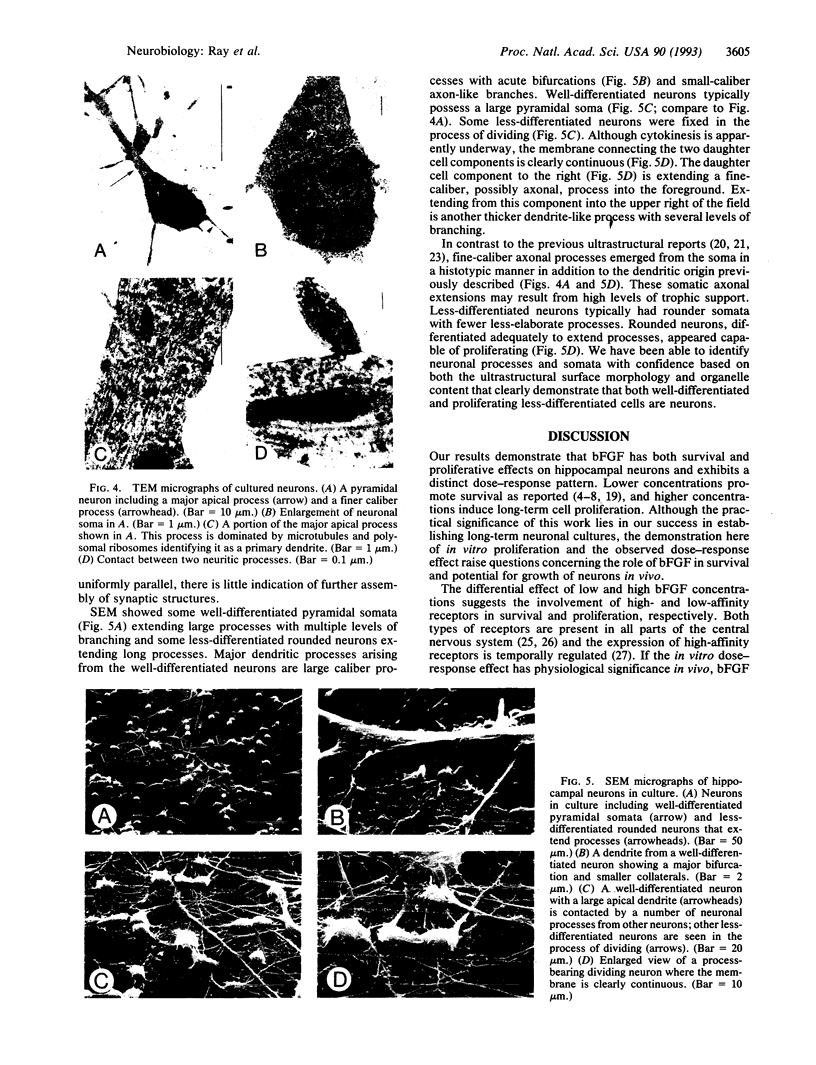

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banker G. A., Cowan W. M. Further observations on hippocampal neurons in dispersed cell culture. J Comp Neurol. 1979 Oct 1;187(3):469–493. doi: 10.1002/cne.901870302. [DOI] [PubMed] [Google Scholar]
- Banker G. A., Cowan W. M. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977 May 13;126(3):397–342. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
- Banker G. A. Trophic interactions between astroglial cells and hippocampal neurons in culture. Science. 1980 Aug 15;209(4458):809–810. doi: 10.1126/science.7403847. [DOI] [PubMed] [Google Scholar]
- Barnes D., Sato G. Methods for growth of cultured cells in serum-free medium. Anal Biochem. 1980 Mar 1;102(2):255–270. doi: 10.1016/0003-2697(80)90151-7. [DOI] [PubMed] [Google Scholar]
- Besnard F., Perraud F., Sensenbrenner M., Labourdette G. Effects of acidic and basic fibroblast growth factors on proliferation and maturation of cultured rat oligodendrocytes. Int J Dev Neurosci. 1989;7(4):401–409. doi: 10.1016/0736-5748(89)90061-0. [DOI] [PubMed] [Google Scholar]
- Bottenstein J. E., Sato G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979 Jan;76(1):514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottenstein J. E., Skaper S. D., Varon S. S., Sato G. H. Selective survival of neurons from chick embryo sensory ganglionic dissociates utilizing serum-free supplemented medium. Exp Cell Res. 1980 Jan;125(1):183–190. doi: 10.1016/0014-4827(80)90202-5. [DOI] [PubMed] [Google Scholar]
- Bulloch K., Stallcup W. B., Cohn M. A new method for the establishment of neuronal cell lines from the mouse brain. Life Sci. 1978 Feb;22(6):495–504. doi: 10.1016/0024-3205(78)90430-7. [DOI] [PubMed] [Google Scholar]
- Bulloch K., Stallcup W. B., Cohn M. The derivation and characterization of neuronal cell lines from rat and mouse brain. Brain Res. 1977 Oct 21;135(1):25–36. doi: 10.1016/0006-8993(77)91049-6. [DOI] [PubMed] [Google Scholar]
- Cattaneo E., McKay R. Proliferation and differentiation of neuronal stem cells regulated by nerve growth factor. Nature. 1990 Oct 25;347(6295):762–765. doi: 10.1038/347762a0. [DOI] [PubMed] [Google Scholar]
- Deloulme J. C., Baudier J., Sensenbrenner M. Establishment of pure neuronal cultures from fetal rat spinal cord and proliferation of the neuronal precursor cells in the presence of fibroblast growth factor. J Neurosci Res. 1991 Aug;29(4):499–509. doi: 10.1002/jnr.490290410. [DOI] [PubMed] [Google Scholar]
- Ferrari G., Minozzi M. C., Toffano G., Leon A., Skaper S. D. Basic fibroblast growth factor promotes the survival and development of mesencephalic neurons in culture. Dev Biol. 1989 May;133(1):140–147. doi: 10.1016/0012-1606(89)90305-9. [DOI] [PubMed] [Google Scholar]
- Gensburger C., Labourdette G., Sensenbrenner M. Brain basic fibroblast growth factor stimulates the proliferation of rat neuronal precursor cells in vitro. FEBS Lett. 1987 Jun 8;217(1):1–5. doi: 10.1016/0014-5793(87)81230-9. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Neufeld G., Schweigerer L. Fibroblast growth factor. Mol Cell Endocrinol. 1986 Aug;46(3):187–204. doi: 10.1016/0303-7207(86)90001-8. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Shooter E. M. The nerve growth factor: biochemistry, synthesis, and mechanism of action. Annu Rev Neurosci. 1980;3:353–402. doi: 10.1146/annurev.ne.03.030180.002033. [DOI] [PubMed] [Google Scholar]
- Grothe C., Otto D., Unsicker K. Basic fibroblast growth factor promotes in vitro survival and cholinergic development of rat septal neurons: comparison with the effects of nerve growth factor. Neuroscience. 1989;31(3):649–661. doi: 10.1016/0306-4522(89)90430-2. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. Developmental neurobiology and the natural history of nerve growth factor. Annu Rev Neurosci. 1982;5:341–362. doi: 10.1146/annurev.ne.05.030182.002013. [DOI] [PubMed] [Google Scholar]
- Morrison R. S., Sharma A., de Vellis J., Bradshaw R. A. Basic fibroblast growth factor supports the survival of cerebral cortical neurons in primary culture. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7537–7541. doi: 10.1073/pnas.83.19.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M., Drago J., Bartlett P. F. Fibroblast growth factor stimulates the proliferation and differentiation of neural precursor cells in vitro. J Neurosci Res. 1990 Apr;25(4):463–475. doi: 10.1002/jnr.490250404. [DOI] [PubMed] [Google Scholar]
- Peacock J. H., Rush D. F., Mathers L. H. Morphology of dissociated hippocampal cultures from fetal mice. Brain Res. 1979 Jun 22;169(2):231–246. doi: 10.1016/0006-8993(79)91027-8. [DOI] [PubMed] [Google Scholar]
- Pennypacker K., Fischer I., Levitt P. Early in vitro genesis and differentiation of axons and dendrites by hippocampal neurons analyzed quantitatively with neurofilament-H and microtubule-associated protein 2 antibodies. Exp Neurol. 1991 Jan;111(1):25–35. doi: 10.1016/0014-4886(91)90047-g. [DOI] [PubMed] [Google Scholar]
- Perraud F., Besnard F., Pettmann B., Sensenbrenner M., Labourdette G. Effects of acidic and basic fibroblast growth factors (aFGF and bFGF) on the proliferation and the glutamine synthetase expression of rat astroblasts in culture. Glia. 1988;1(2):124–131. doi: 10.1002/glia.440010204. [DOI] [PubMed] [Google Scholar]
- Powell P. P., Finklestein S. P., Dionne C. A., Jaye M., Klagsbrun M. Temporal, differential and regional expression of mRNA for basic fibroblast growth factor in the developing and adult rat brain. Brain Res Mol Brain Res. 1991 Aug;11(1):71–77. doi: 10.1016/0169-328x(91)90023-q. [DOI] [PubMed] [Google Scholar]
- Reynolds B. A., Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992 Mar 27;255(5052):1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Richards L. J., Kilpatrick T. J., Bartlett P. F. De novo generation of neuronal cells from the adult mouse brain. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8591–8595. doi: 10.1073/pnas.89.18.8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
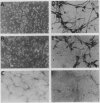
- Rothman S., Cowan W. M. A scanning electron microscope study of the in vitro development of dissociated hippocampal cells. J Comp Neurol. 1981 Jan 1;195(1):141–155. doi: 10.1002/cne.901950108. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Gray A., Berman C., Dull T. J. Human beta-nerve growth factor gene sequence highly homologous to that of mouse. Nature. 1983 Jun 30;303(5920):821–825. doi: 10.1038/303821a0. [DOI] [PubMed] [Google Scholar]
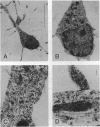
- Walicke P. A., Baird A. Internalization and processing of basic fibroblast growth factor by neurons and astrocytes. J Neurosci. 1991 Jul;11(7):2249–2258. doi: 10.1523/JNEUROSCI.11-07-02249.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walicke P. A., Baird A. Trophic effects of fibroblast growth factor on neural tissue. Prog Brain Res. 1988;78:333–338. doi: 10.1016/s0079-6123(08)60301-5. [DOI] [PubMed] [Google Scholar]
- Walicke P. A. Basic and acidic fibroblast growth factors have trophic effects on neurons from multiple CNS regions. J Neurosci. 1988 Jul;8(7):2618–2627. doi: 10.1523/JNEUROSCI.08-07-02618.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walicke P. A., Feige J. J., Baird A. Characterization of the neuronal receptor for basic fibroblast growth factor and comparison to receptors on mesenchymal cells. J Biol Chem. 1989 Mar 5;264(7):4120–4126. [PubMed] [Google Scholar]
- Walicke P., Cowan W. M., Ueno N., Baird A., Guillemin R. Fibroblast growth factor promotes survival of dissociated hippocampal neurons and enhances neurite extension. Proc Natl Acad Sci U S A. 1986 May;83(9):3012–3016. doi: 10.1073/pnas.83.9.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanaka A., Johnson E. M., Jr, Milbrandt J. Localization of FGF receptor mRNA in the adult rat central nervous system by in situ hybridization. Neuron. 1990 Sep;5(3):267–281. doi: 10.1016/0896-6273(90)90164-b. [DOI] [PubMed] [Google Scholar]
- di Porzio U., Daguet M. C., Glowinski J., Prochiantz A. Effect of striatal cells on in vitro maturation of mesencephalic dopaminergic neurones grown in serum-free conditions. Nature. 1980 Nov 27;288(5789):370–373. doi: 10.1038/288370a0. [DOI] [PubMed] [Google Scholar]