Abstract
Background
Non-typhoidal Salmonella (NTS) is an important public health problem worldwide. Consumption of animal-derived food products and direct and/or indirect contact with animals are the major routes of acquiring infection with NTS. Published information, particularly on the serotype distribution of NTS among human patients with gastroenteritis and associated risk factors, is scarce in Ethiopia. This study investigated the prevalence, risk factors, serotype distribution and antimicrobial susceptibility of Salmonella species among diarrheic out-patients attending health centers in Addis Ababa and patients with various gastrointestinal complaints at Tikur Anbessa Specialized Hospital (TASH).
Methods
Stool samples were cultured for Salmonella species according to the WHO Global Foodborne Infections Network laboratory protocol. Salmonella serotyping was conducted using slide agglutination and microplate agglutination techniques. Antibiotic susceptibility testing was performed using the disk diffusion method according to Clinical and Laboratory Standards Institute guidelines.
Results
A total of 59 (6.2 %) stool samples, out of 957 were culture positive for Salmonella species. Fifty-five (7.2 %) of 765 diarrheic patients from health centers and 4 (2.1 %) of 192 patients from TASH were culture positive for Salmonella species. Multivariable logistic regression analysis after adjusting for all other variables revealed statistically significant association of Salmonella infection with consumption of raw vegetables (OR = 1.91, 95 % CI = 1.29–2.83, χ2 = 4.74, p = 0.025) and symptom of watery diarrhea (OR = 3.3, 95 % CI = 1.23–8.88, χ2 = 10.54, p = 0.005). Eleven serotypes were detected, and the most prominent were S. Typhimurium (37.3 %), S. Virchow (34 %), and S. Kottbus (10.2 %). Other serotypes were S. Miami, S. Kentucky, S. Newport, S. Enteritidis, S. Braenderup, S. Saintpaul, S. Concord and S. V:ROUGH-O. Resistance to three or more antimicrobials was detected in 27 (40.3 %) of the isolates. Resistance to five or more antimicrobials was detected in 17 (25.4 %). Resistance to individual antimicrobials was found at varying proportions: streptomycin (50; 74.6 %), nitrofurantoin (27; 40.3 %), sulfisoxazole (26; 38.8 %), kanamycin (23; 34.3 %), cephalothin (12; 17.9 %), and ampicillin (11; 16.4 %) respectively. Two S. Kentucky, one S. Typhimurium and one S. Concord isolates were multi-drug resistant to more than 10 antimicrobials.
Conclusions
The study demonstrated significant association of Salmonella infection with consumption of raw vegetables. There was no significant association of Salmonella infection with co-occurring parasites. The study also showed the dominance of S. Typhimurium and S. Virchow in primary health care units. Overall, prevalence of MDR was low compared to previous studies. Although their proportion was low, S. Kentucky and S. Concord demonstrated wider spectrum of MDR. Continuous monitoring of circulating serotypes, antimicrobial resistance profile and characterization on molecular resistance determinants is essential for proper treatment of patients and for identifying potential environmental origins of antimicrobial resistance.
Electronic supplementary material
The online version of this article (doi:10.1186/s12879-015-1235-y) contains supplementary material, which is available to authorized users.
Keywords: Non-typhoidal Salmonella, Antimicrobial resistance, Prevalence, Serotype
Background
Non-typhoidal Salmonella (NTS) is an important public health problem worldwide. Globally, NTS is estimated to be responsible for 93.8 million cases of gastroenteritis and 155,000 deaths annually [1]. Unlike typhoid fever, which is mainly limited to developing countries, NTS occurs worldwide. Despite global morbidity, mortality due to NTS infection primarily occurs in the developing world and is related to co-morbidities [2]. Consumption of animal-derived food products and direct and/or indirect contact with animals are the major routes of acquiring infection with NTS [3–6]. NTS can also be transmitted from person to person or from contact with pets such as cats, dogs, rodents, reptiles, or amphibians [3, 4, 7, 8]. Several recent outbreaks have also been associated with consumption of contaminated plant products such as sprouts, tomatoes, fruits, peanuts, and spinach [9–11].
NTS usually causes self-limiting gastroenteritis characterized by diarrhea, abdominal pain and vomiting in people of all ages [12]. However, in children, the elderly and immunocompromised patients, severe invasive disease with complicated extra-intestinal illness, bacteremia and meningitis can be observed [7, 13]. Generally, antibiotic treatment is not necessary for NTS gastroenteritis unless it is invasive salmonellosis or it affects immunocompromised patients [14, 15]. Chemotherapy is believed to prolong the duration of shedding of bacteria and contribute to the development of antimicrobial resistance [16].
The relative importance of major etiologic agents responsible for diarrhea is not well established in Ethiopia. A few studies conducted in different parts of the country have shown that Campylobacter, Salmonella, Shigella and rotavirus are some of the major bacterial and viral pathogens isolated from stool of diarrheic patients, most of them children under 5 years of age [17–20].
Globally, the incidence of Salmonella infection associated with multi-drug resistance (MDR) has increased in the last few decades [21–23]. In Ethiopia, although there are a few studies on prevalence of Salmonella and antimicrobial susceptibility in humans, animals, and food of animal origin, there is no integrated surveillance and monitoring to establish the major serotypes responsible for non-typhoidal salmonellosis in humans and the associated risk factors. Moreover, most of the studies conducted in humans involved pediatric diarrheic patients and the isolates recovered from these patients were not sereotyped [24, 25]. Those that conducted serotyping indicated that Salmonella enterica subspecies enterica serovar Concord (S. Concord) and Salmonella enterica subspecies enterica serovar Typhimurium (S. Typhimurium) were the dominant NTS serotypes isolated from patients with diarrheal illness in Ethiopia [20, 26].
Information on Salmonella serotypes circulating in a given geographic area and their antimicrobial susceptibility is essential for designing appropriate intervention strategy since the outcome of infection varies with serotypes involved and their antimicrobial resistance profile. The objectives of the current study were therefore to investigate the prevalence, serotype distribution and antimicrobial resistance pattern of NTS species among diarrheic patients in 10 primary health centers in Addis Ababa. In addition, patients with gastrointestinal complaints who submitted stool samples to Tikur Anbessa Specialized Hospital (TASH) for bacterial culturing, diagnosis of gastrointestinal parasites and detection of Helicobacter pylori antigen were also included in the study. TASH is national referral hospital giving services mainly to patients from all sub-cities in Addis Ababa and those from outside Addis Ababa. Various patient related variables such as consumption of raw meat, milk and vegetables; patients demographic data; stool consistency; and the occurrence of other pathogens were recorded to analyze for possible association with Salmonella isolation.
Methods
Study design and area
A cross-sectional study was conducted in Addis Ababa, Ethiopia, from May 2013 to January 2014. Addis Ababa is organized under 10 sub-cities and there are 36 government-owned primary health centers in the city. Ten representative government-owned health centers were randomly selected, one from each sub-city. In addition, patients with various gastrointestinal complaints at TASH were also included.
Sample size determination and study subjects
Sample size (n = 576) was calculated based on the previous baseline study in Addis Ababa, with a prevalence of 6.4 % [25] using the formula (Z0/2)/∆)2p(1-p) at 95 % CI, margin of error of 2 %. However, to increase the number of isolates, a total of 765 patients from health centers were included. From each health center, a minimum of 71 and a maximum of 82 diarrheic patients of any age referred for laboratory diagnosis based on stool examination were recruited (n = 765). Diarrhea was defined as the passage of three or more loose or liquid stools per day [27]. In addition, 192 patients who presented with various gastrointestinal complaints (diarrhea, abdominal pain and gastritis) and submitted stool samples to the parasitology and microbiology laboratory at TASH were also included. Only 98 (51 %) of the 192 stool samples from TASH were diarrheic.
Patients’ history, demographic and laboratory data
Information on various potential risk factors was collected including patients’ history such as consumption of raw meat, milk and vegetables during the last 2 weeks by interviewing the patients during sample collection from health centers. All demographic, clinical and laboratory data obtained from study participants at health facilities such as stool consistency and laboratory examination results of stool specimens for other pathogens were recorded. The stool consistency was determined in the health center and hospital laboratories immediately after samples were received according to the Bristol stool consistency scale (type 5, 6 and 7) defined as loose, mucoid and watery, respectively [28]. Analysis for association of Salmonella infection status and various risk factors was conducted only for patients from health centers.
Sample collection, handling and transportation
Stool samples were collected from each study participants in clean screw capped plastic containers and transported to the microbiology laboratory of Aklilu Lemma Institute of Pathobiology (ALIPB) in ice box within 3–4 h of collection for culture and identification of Salmonella species. Sampling was first conducted from the 10 health centers sequentially and finally from TASH.
Microscopic examination of stool specimens
Direct microscopic stool examination was performed for detection of ova and parasites by laboratory technicians in health centers and hospital laboratories where the samples were collected immediately prior to transportation to ALIPB and the laboratory result was recorded.
Culture and identification of Salmonella species
Isolation and identification of Salmonella species was conducted according to World Health Organization (WHO) Global Foodborne Infections Network laboratory protocol [29]. Briefly, 5 g of feces was suspended in 45 ml of buffered peptone water and incubated for 24 h at 37 °C. One hundred μl of this suspension was transferred to 10 ml of Rappaport-Vassiliadis enrichment Broth (RVB), (Oxoid, USA) and incubated for 24 h at 37 °C. One ml of suspension was also transferred to 10 ml of Tetrathionate broth (Oxoid, USA) and incubated for 24 h at 42 °C. The sample from these two broths was streaked on to Xylose Lysine tergitol 4 (XLT-4) selective media and the plates were incubated at 37 °C for 24 h. Presumptive Salmonella colonies were then further investigated biochemically using Triple Sugar Iron (TSI) agar, Urea, Citrate and Lysine Iron Agar (LIA) slants. Those colonies with typical Salmonella biochemical properties were then further confirmed by genus specific PCR [30]. Salmonella recovered from both RVB and TTB of a single patient were first considered as different strains until the isolates were tested for antimicrobial susceptibility. When differences in antimicrobial susceptibility were observed, both isolates were considered as different strains. On the other hand, if the isolates showed similar susceptibility pattern, only one isolate was considered for further analysis.
Salmonella serotyping and phage typing
Salmonella isolates were serotyped and phage-typed at the Public Health Agency of Canada, World Organization for Animal Health (OIÉ) Reference Laboratory for Salmonellosis, Guelph, Ontario, Canada. Briefly, the somatic (O) antigens were determined by slide agglutination tests [31] and flagellar antigens were determined using a microplate agglutination technique [32]. The antigenic formulae of Grimont [33] were used to identify and assign the serotypes of the isolates. Phage typing of S.Typhimurium isolates was performed by the methods developed by Callow [34] and extended by Anderson et al. [35] with reference phages obtained from the Public Health England, Gastrointestinal Bacteria Reference Unit, Colindale, England and the Public Health Agency of Canada, National Laboratory for Enteric Pathogens, Winnipeg, Canada. Salmonella isolates that reacted with the phages but did not conform to any recognized phage type were designated atypical (AT).
Antimicrobial susceptibility testing
Susceptibility of the isolates to a panel of 18 antimicrobials was determined using the Kirby-Bauer disk diffusion method according to the guidelines of the Clinical and Laboratory Standards Institute [36]. The following antimicrobials (Sensi-Discs, Becton, Dickinson and Company, Loveton, USA) and disc potencies (μg) were used: amikacin (30), amoxicillin + clavulanic acid (20/10), ampicillin (10), cefoxitin (30), ceftriaxone (30), cephalothin (30), chloramphenicol (30), ciprofloxacin (5), gentamicin (10), kanamycin (30), nalidixic acid (30), neomycin (30), nitrofurantoin (100), streptomycin (10), sulfisoxazole (1000), sulfamethoxazole + trimethoprim (23.75/1.25), trimethoprim (5) and tetracycline (30). The interpretation of the categories of susceptible, intermediate or resistant was based on the CLSI guidelines [36] and for the purpose of analysis, all readings classified as intermediate were considered as resistant where necessary. Reference strain of Escherichia coli ATCC 25922 was used as a quality control.
Ethical consideration
The study protocol was ethically approved by the Institutional Review Board of College of Health Sciences, Addis Ababa University and National Research Ethics Review Committee (Permit#3-10/474/05 dated 29–03–2015). Individual oral informed consent was obtained from all adult participants and the parents or guardians of all children participated in the study.
Statistical analysis
Data was analyzed using STATA software version 11. Prevalence of Salmonella was calculated as a percentage of Salmonella culture-positive stool samples among the total number of samples examined. Associations of putative risk factors as well as occurrence of protozoan pathogens with Salmonella infection in patients from health centers were assessed using both univariable and multivariable logistic regression, accounting for clustering of patients at the health center level by using cluster-robust standard errors. The variables used in multivariable logistic regression were sex, age, consumption of raw (meat, milk, vegetables), stool consistency, vomiting, and infection status with Entamoeba histolytica and Giardia lamblia. Results were reported statistically significant whenever the p-value was less than 5 %.
Results
Salmonella prevalence and association with patient feeding habits and other factors
All Salmonella isolates recovered from a single patient from both RVB and TTB, though in some cases (11.9 %), differed in antimicrobial resistance pattern; they all belonged to a single serotype. Therefore, only a single serotype per patient was considered for risk factor analysis and serotype distribution analysis, while all isolates (n = 67) were considered for antimicrobial resistance analysis. Seven hundred and sixty-five diarrheic patients from health centers (329 male and 436 female) and 192 patients (101 male and 89 female) from TASH were included in the study. Fifty-five (7.2 %) and 4 (2.1 %) patients from health centers and TASH were culture positive for Salmonella, respectively. The overall prevalence of Salmonella in the current study was 6.2 %. There was no difference in prevalence of Salmonella between male and female patients as well as among different age groups (Tables 1 and 2). Among eating habits of patients, 10.8 % of those consuming raw vegetables were Salmonella culture positive, whereas only 6.1 % of patients with no habit of eating raw vegetables were positive for Salmonella. Multivariable logistic regression analysis after adjusting for all pre-specified variables revealed statistically significant association of Salmonella infection status with consumption of raw vegetables (OR = 1.91, 95 % CI = 1.29–2.83, χ2 = 4.74, p = 0.025) (Table 2).
Table 1.
Frequency of Salmonella infection among diarrheic out-patients attending health centers in Addis Ababa with respect to selected factors
| Factor | Total | No (%) Salmonella positive | P-value |
|---|---|---|---|
| Sex | |||
| Male | 329 | 24(7.3) | 0.92 |
| Female | 436 | 31(7.1) | |
| Consumption of raw meat | |||
| No | 622 | 44(7.1) | 0.80 |
| Yes | 143 | 11(7.7) | |
| Consumption of raw milk | |||
| No | 720 | 51(7.1) | 0.19 |
| Yes | 45 | 4(8.9) | |
| Consumption of raw vegetables | |||
| No | 571 | 34(6.0) | 0.025 |
| Yes | 194 | 21(10.8) | |
| Vomiting | |||
| No | 714 | 51(7.1) | 0.85 |
| Yes | 51 | 4(7.8) | |
| Age | |||
| 0–5 years | 160 | 10(6.3) | |
| 6–18 years | 171 | 12(7.0) | 0.77 |
| 19–45 | 359 | 27(7.5) | |
| >45 | 75 | 6(8) | |
| Stool consistency | |||
| Mucoid | 283 | 10(3.5) | |
| Loose | 117 | 9(7.7) | 0.005 |
| Watery | 365 | 36(9.9) | |
| Entamoeba histolytica infection | |||
| Positive | 145 | 14(9.7) | 0.058 |
| Negative | 620 | 41(6.6) | |
| Giardia lamblia infection | |||
| Positive | 62 | 3(4.8) | 0.46 |
| Negative | 703 | 52(7.4) |
Table 2.
Association of selected risk factors with Salmonella infection in diarrheic out-patients in Addis Ababa health centers
| Variable | Crude odds Ratio and 95 % confidence interval | Adjusted odds Ratio and 95 % confidence interval | ||
|---|---|---|---|---|
| Sex | ||||
| Male | 1.03 | 0.61–1.74 | 1.02 | 0.57–1.85 |
| Female | ||||
| Consumption of raw meat | ||||
| No | 1.1 | 0.58–2.08 | 0.86 | 0.55–1.36 |
| Yes | ||||
| Consumption of raw milk | ||||
| No | 1.28 | 0.53–3.01 | 1.29 | 0.51–3.26 |
| Yes | ||||
| Consumption of raw vegetable | ||||
| No | 1.92 | 1.36–2.70 | 1.91 | 1.29–2.83 |
| Yes | ||||
| Vomiting | ||||
| No | 1.11 | 0.37–3.32 | 0.93 | 0.34–2.52 |
| Yes | ||||
| Age | ||||
| 0–5 years | ||||
| 6–18 years | 1.14 | 0.41–3.13 | 1.03 | 0.38–2.81 |
| 19–45 | 1.22 | 0.53–2.81 | 1.02 | 0.44–2.35 |
| >45 | 1.32 | 0.32–5.57 | 1.27 | 0.33–4.93 |
| Stool consistency | ||||
| Mucoid | ||||
| Loose | 2.28 | 0.90–5.76 | 2.33 | 0.91–5.97 |
| Watery | 2.99 | 1.23–7.27 | 3.05 | 1.22–7.60 |
| Entamoeba histolytica infection | ||||
| Positive | 1.85 | 0.84–4.08 | 1.77 | 0.85–3.67 |
| Negative | ||||
| Giardia lamblia infection | ||||
| Positive | 0.64 | 0.25–1.60 | 0.64 | 0.26–1.56 |
| Negative | ||||
Although there was variability in the detection rate of Salmonella from patients attending different health centers, the difference was not statistically significant (p = 0.29) (Fig. 1). Having watery diarrhea (9.8 %) was significantly associated with Salmonella recovery compared to mucoid (7.7 %) and loose stool (3.5 %) using multivariable logistic regression analysis after adjusting for the other variables (OR = 3.3, 95 % CI = 1.23–8.88, χ2 = 10.54, p = 0.005). Comparison of patients with symptoms of vomiting to those with no vomiting revealed no significant difference in Salmonella culture-positivity (Tables 1 and 2).
Fig. 1.
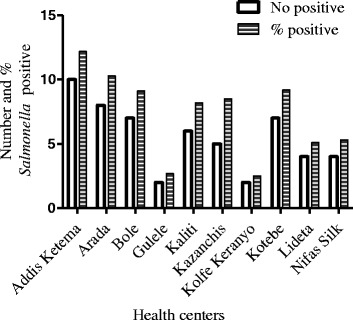
Prevalence of Salmonella among out-patients attending primary health centers in Addis Ababa
Prevalence of ova and parasites and concomitant infection with Salmonella
The most common pathogens detected were Entamoeba histolytica (19 %), Giardia lamblia (8.1 %), egg of Hymenolepis nanna (0.9 %) and egg/larvae of Strongyloides stercoralis (0.7 %) among patients from health centers, whereas only E. histolytica (0.5 %), G. lambia (4.3 %), and egg of Tanea species (1.1 %) were detected in patients from TASH (Table 3). Although Salmonella was commonly detected in patients positive for E. histolytica (9.7 %) compared to negative ones (6.6 %) Salmonella infection was not significantly associated with any of the parasites (Table 2).
Table 3.
Common pathogens detected from stool samples of the study participants
| Health Centers | TASH | |||
|---|---|---|---|---|
| Pathogen infection status | Number examined | Number (%) positive | aNumber examined | Number (%) positive |
| Entamoeba histolytica | 765 | 145(19) | 188 | 1(0.5) |
| Giardia lamblia | 765 | 62(8.1) | 188 | 8(4.3) |
| Hymenolepis nanna | 765 | 7(0.9) | 188 | 0(0) |
| Strongloid worm/eggs | 765 | 5(0.7) | 188 | 0(0) |
| Hook worm | 765 | 3(0.4) | 188 | 0(0) |
| Ascaris egg | 765 | 2(0.3) | 188 | 0(0) |
| Eggs of Tanea | 765 | 0(0) | 188 | 2(1.1) |
aOnly 188 of 192 samples at TASH were examined for ova and parasites
Salmonella Serotype distribution
Overall, 11 different serotypes were recovered, of which the majority were S. Typhimurium (22, 37.3 %) followed by S. Virchow (20, 33.9 %) and S. Kottbus (6, 10.2 %). Other serotypes such as S. Miami (n = 2), S. Kentucky (n = 2), S. Newport (n = 2), S. Enteritidis (n = 1), S. Braenderup (n = 1), S. Saintpaul (n = 1), S. Concord (n = 1), and S. V: ROUGH-O; − :- (n = 1) were also identified (Table 4).
Table 4.
Relative proportion of Salmonella serotypes isolated from diarrheic patients in Addis Ababa and their resistance profile
| Serotype | No. | An | Amp | Amc | C | Cro | Cf | Cip | Fox | Gm | K | Sxt | Tmp | Te | Su | S | Nitro | Na | N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Braenderup | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Concord | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 |
| Enteritidis | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
| Kentucky | 2 | 0 | 2 | 2 | 0 | 0 | 2 | 2 | 1 | 2 | 2 | 0 | 0 | 2 | 2 | 2 | 0 | 2 | 1 |
| Kottbus | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 3 | 0 | 0 | 1 | 4 | 7 | 2 | 1 | 2 |
| Miami | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 3 | 2 | 0 | 0 |
| Newport | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 |
| Sainpaul | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 |
| Typhimurium | 27 | 0 | 6 | 4 | 0 | 1 | 6 | 0 | 0 | 0 | 6 | 1 | 1 | 3 | 13 | 18 | 9 | 1 | 1 |
| V:ROUGH-O;-:- | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Virchow | 21 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 6 | 0 | 0 | 3 | 4 | 14 | 11 | 10 | 3 |
| Total | 67 | 0 | 11 | 8 | 2 | 2 | 12 | 3 | 2 | 5 | 23 | 3 | 3 | 9 | 26 | 50 | 27 | 16 | 9 |
| (%) | 100 | 0 | 16.4 | 11.9 | 3 | 3 | 17.9 | 4.5 | 3 | 7.5 | 34.3 | 4.5 | 4.5 | 13.4 | 38.9 | 74.6 | 40.3 | 23.9 | 13.4 |
An amikacin, Amp ampicillin, Amc amoxicillin and clavulanic acid, Cf cephalothin, C, chloramphenicol, Cro ceftriaxone, Cip ciprofloxacin, Gm gentamicin, K kanamycin, Tmp trimethoprim, Sxt sulfamethoxazole + trimethoprim, Te tetracycline, Su-sulfisoxazole, Nitro nitrofurantoin, Na- nalidixic acid, N neomycin
Antimicrobial Susceptibility
At least one isolate was resistant to all tested antimicrobial agents except amikacin to which all isolates were susceptible. Fifty (74.6 %), 27 (40.3 %), 26 (38.8 %), 23 (34.3 %), 12 (17.9 %), and 11 (16.4 %) of the isolates were resistant to streptomycin, nitrofurantoin, sulfisoxazole, kanamycin, cefalothin, and ampicillin, respectively (Table 4). Nine (13.4 %) of the isolates were pan-susceptible to 18 antimicrobials tested. Six of these isolates were S. Typhimurium, while the remaining 3 were S. Virchow. Resistance to 2 or more antimicrobials was recorded in 64.2 % of the isolates, while resistance to 3 or more antimicrobials was detected in 40.3 % of the total isolates. MDR to 5 or more antimicrobials was detected in 17 (25.4 %) of the isolates. Two strains of S. Kentucky isolated from patients in two different health centers were resistant to 11 antimicrobials. Likewise, one S. Typhimurium phagetype 193 and one S. Concord recovered from two diarrheic children at TASH were resistant to 12 and 13 antimicrobials tested, respectively. In general, 36 different resistance patterns (R-Pattern) were detected among the 67 isolates examined which is an indication of phenotypic diversity of Salmonella strains circulating in the study area. The dominant R-pattern was resistance to a single antimicrobial agent: streptomycin (n = 11) followed by resistance to both streptomycin and nitrofurantoin (6 isolates) (Additional file 1: Table S1).
Association between serotype and antimicrobial resistance
Different serotypes appeared to exhibit differential resistance to some of the antimicrobials tested. For instance, resistance to ampicillin was noted in strains belonging to S. Concord, S. Kentucky, S. Typhimurium, S. V: ROUGH-O;-: and S. Virchow; while S. Kentucky and S. Kottbus were the only serotypes resistant to ciprofloxacin. In other cases, resistance to streptomycin was common among the serotypes (Table 4).
Discussion
In the current study, 7.2 % of patients from the health centers and 2.1 % from TASH were culture positive for Salmonella species. The possible explanation for low prevalence of Salmonella in patients at TASH could be due to health center treated patient referral to this hospital or because not all of the stool samples collected at TASH were from diarrheic patients. The observed heterogeneity of Salmonella infection across health centers goes with the known hygienic levels of residential areas served by these health centers. Addis Ketema and Arada health centers are located at the center of the old Addis Ababa with larger human population and lots of slum areas with poor hygiene compared to Kolfe Keranyo and Gulele health centers, which are located at periphery of the city with relatively better hygienic condition.
The overall prevalence of Salmonella (6.2 %) in the current study is in agreement with previous studies in Addis Ababa (5.3 %) [20] and Jimma hospitals (6.2 %) [37] in pediatric patients. Previous studies in Addis Ababa in adult diarrheic out-patients showed a prevalence of 4.5 % [24] and 6.4 % [25]. Systematic review and meta-analysis of published works in Ethiopia over the period of 38 years has also shown a prevalence of 8.7 % in diarrheic children and 5.7 % in diarrheic adults [38].
Salmonella detection was more common in patients who consume raw vegetables. This suggests that raw vegetables could be one of the major vehicles for Salmonella infection in Addis Ababa. The common vegetables consumed by these patients were lettuce, tomatoes and green peppers. An accumulating body of evidence indicates that vegetables sold in markets could possibly be contaminated with Salmonella species. Indeed, studies conducted in Addis Ababa [39], Jimma (South–west Ethiopia) [40] and Mexico [41] showed that an appreciable percentage of vegetables sold in the respective markets are contaminated with Salmonella species. The use of fecally contaminated water for irrigation and washing of fresh produces can serve as a source of Salmonella contamination for raw vegetables in addition to cross-contamination in the kitchen [42]. More recently, Salmonella has been shown to colonize and be internalized into tomato during pre–and post–harvesting stages [43]. Special attention should, therefore, be paid to reducing the risk of infection of the public with Salmonella from raw vegetables. Appropriate pre–and post–harvest strategies of reducing contamination of vegetables by Salmonella and other enteric pathogens should be implemented.
The dominant pathogens other than Salmonella detected in the current study were E. histolytica followed by G. lamblia. However, no significant association of co-occurrence of parasites with Salmonella infection was detected.
S. Typhimurium followed by S. Virchow and S. Kottubus were the dominant serotypes in the current study unlike the reports by Beyene et al. [20] and Gebre-Yohannes et al. [44] where the dominant serotypes among NTS isolates were S. Concord followed by S. Typhimurium. In the present study, only one S. Concord was isolated from a one year old child at TASH. The difference between the studies could be attributed to differences in patient demography, season and place of collection. Furthermore, differences in dynamics of the sources of infection might have contributed to dominance of different serotypes in the respective studies. A previous study has shown that the epidemiology of NTS is characterized by the temporal dominance of certain successful clones followed by a decline and replacement with another clone [45]. The dominance of S. Typhimurium in the current study is in agreement with studies conducted in other sub-Saharan African countries including Kenya [46] and Congo [47].
Overall, rate of antimicrobial resistance is low in the current study compared to previous investigations. For instance Beyene et al. [20] reported 82.3 % resistance to ampicillin and 78.2 % to ceftriaxone, which is much higher than the current finding of 16.4 % and 3 %, respectively. Only 3 %, 7.5 %, 13.4 %, and 4.5 % of the isolates were resistant to chloramphenicol, gentamicin, tetracycline, and sulfamethoxazole + trimethoprim, respectively in the current study, while 81.4 %, 74.3 %, 39.8 % and 80.5 % of the isolates were resistant to the corresponding antibiotics, respectively, in the previously mentioned study. A separate study reported 59.7 %, 32.3 %, 61.7 %, 29 %, and 51.6 % resistance to ampicillin, cephalothin, chloramphenicol, tetracycline and Sxt, respectively in NTS isolates recovered from hospitalized patients at TASH in Addis Ababa [48]. The reason behind this discrepancy could be differences in serotype composition reported by the studies. As mentioned, the dominant serotype in the study by Beyene et al. [20] was S. Concord. However, only one isolate of this serotype was recovered in the current study. Though S. Concord was resistant to many antimicrobials in both studies, its rarity in the present study might have contributed to the relatively low levels of overall antimicrobial resistance that was observed. Furthermore, the previous studies were conducted in referral hospitals, where patients are admitted after being treated in health centers with various antimicrobials. Thus, patients could have been infected by clonal epidemic MDR strains of S. Concord or other serotypes. Some of the infections in the previous reports also might have been acquired from hospitals by MDR strains. Majority of the isolates in the current study were, however, collected from patients at primary health centers, with minimized prior exposure to antimicrobials. The fact that 2 of the 4 isolates from TSRH in the current study were resistant to 11 and 13 antimicrobials supports this assertion. Similar decrease in prevalence of resistance in NTS in rural district hospital has also been reported from Kenya [49].
Although we have no data on the antimicrobial use for diarrheic patients in the current study, the recent standard treatment guideline prepared by Drug Administration and Control Authority of Ethiopia for severe cases of infectious gastroenteritis including NTS recommends sulfamethoxazole + trimethoprim, ciprofloxacin and chloramphenicol [50]. However, resistance to these antimicrobials was low in Salmonella isolates in the current study probably due to the fact that these isolates were mainly obtained from patients attending primary health centers and were not exposed to prolonged selective pressure of antimicrobials. Drugs like streptomycin, nitrofurantoin, sulfonamides and ampicillin have long been used for management of various infections in the country and high rate of resistance to these drugs might have developed as a consequence of this prolonged use.
Conclusions
There was no significant association of Salmonella infection with co-occurring parasites but there was significant association of Salmonella infection with consumption of raw vegetables. The dominant NTS serotypes at the primary health care units in Addis Ababa were S. Typhimurium, S. Virchow and S. Kottbus with variable antimicrobial resistance phenotypes. Although their proportion was low, S. Kentucky and S. Concord demonstrated extensive MDR. Further characterization on molecular resistance determinants and continuous monitoring of circulating serotypes and antimicrobial resistance profile is recommended. As the extent of MDR appears to be dependent on serotypes involved, it is vital to have at least one national Salmonella reference laboratory to conduct serotyping of Salmonella isolates from all regions of Ethiopia.
Acknowledgments
This study was financially supported by The National Institutes of Health (NIH) Fogarty International Center (grant D43TW008650) to W.A.G./J.S.G., R56AI109002 to J.S.G. and WHO/AGISAR to T.E. We are grateful to Dr Roger P. Johnson and Ann Perets of the Laboratory of Foodborne Zoonoses, Public Health Agency of Canada for serotyping and phage typing of the Salmonella isolates. We are extremely grateful to Dr. Girmay Medhin for the technical contribution to the statistical analysis. We are thankful for cooperation of laboratory technicians at health centers and TASH during sample collection. Technical assistance of Mr. Ephrem Eguale in sample collection was greatly appreciated.
Abbreviations
- ALIPB
Aklilu Lemma Institute of Pathobiology
- MDR
Multi-drug resistance
- NTS
Non-typhoidal Salmonella
- RVB
Rappaport-Vassiliadis Broth
- TASH
Tikur Anbessa Specialized Hospital
- TTB
Tetrathionate broth
- WHO
World Health Organization
Additional file
Resistance pattern of Salmonella serotypes recovered from the study participants. (DOCX 15 kb)
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
TE, WAG, DA, JSG and EE, participated in conception of the study and review of the draft manuscript. TE was involved in sample collection laboratory investigation and preparation of the draft manuscript. HA participated in laboratory work. All authors read and approved the final manuscript.
Contributor Information
Tadesse Eguale, Email: tadesse.eguale@aau.edu.et.
Wondwossen A. Gebreyes, Email: Gebreys.1@osu.edu
Daniel Asrat, Email: asratdan@gmail.com.
Haile Alemayehu, Email: haileale@yahoo.com.
John S. Gunn, Email: John.gunn@Osumc.edu
Ephrem Engidawork, Email: ephrem.engidawork@aau.edu.et.
References
- 1.Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O’Brien SJ, Jones TF, Fazil A, Hoekstra RM, ICoEDBoI Studies The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis. 2010;50(6):882–889. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- 2.Gal-Mor O, Boyle EC, Grassl GA. Same species, different diseases: how and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front Microbiol. 2014;5:391. doi: 10.3389/fmicb.2014.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haeusler GM, Curtis N. Non-typhoidal Salmonella in children: microbiology, epidemiology and treatment. Adv Exp Med Biol. 2013;764:13–26. doi: 10.1007/978-1-4614-4726-9_2. [DOI] [PubMed] [Google Scholar]
- 4.Braden CR. Salmonella enterica serotype Enteritidis and eggs: a national epidemic in the United States. Clin Infect Dis. 2006;43(4):512–517. doi: 10.1086/505973. [DOI] [PubMed] [Google Scholar]
- 5.Fey PD, Safranek TJ, Rupp ME, Dunne EF, Ribot E, Iwen PC, Bradford PA, Angulo FJ, Hinrichs SH. Ceftriaxone-resistant salmonella infection acquired by a child from cattle. N Engl J Med. 2000;342(17):1242–1249. doi: 10.1056/NEJM200004273421703. [DOI] [PubMed] [Google Scholar]
- 6.Jansen A, Frank C, Stark K. Pork and pork products as a source for human salmonellosis in Germany. Berl Munch Tierarztl Wochenschr. 2007;120(7–8):340–346. [PubMed] [Google Scholar]
- 7.Hohmann EL. Nontyphoidal salmonellosis. Clin Infect Dis. 2001;32(2):263–269. doi: 10.1086/318457. [DOI] [PubMed] [Google Scholar]
- 8.Mermin J, Hutwagner L, Vugia D, Shallow S, Daily P, Bender J, Koehler J, Marcus R, Angulo FJ, EIPFW Group Reptiles, amphibians, and human Salmonella infection: a population-based, case–control study. Clin Infect Dis. 2004;38(Suppl 3):S253–261. doi: 10.1086/381594. [DOI] [PubMed] [Google Scholar]
- 9.Bayer C, Bernard H, Prager R, Rabsch W, Hiller P, Malorny B, Pfefferkorn B, Frank C, de Jong A, Friesema I et al.: An outbreak of Salmonella Newport associated with mung bean sprouts in Germany and the Netherlands, October to November 2011. Euro Surveill 2014, 19(1). [DOI] [PubMed]
- 10.Lienemann T, Niskanen T, Guedes S, Siitonen A, Kuusi M, Rimhanen-Finne R. Iceberg lettuce as suggested source of a nationwide outbreak caused by two Salmonella serotypes, Newport and Reading, in Finland in 2008. J Food Prot. 2011;74(6):1035–1040. doi: 10.4315/0362-028X.JFP-10-455. [DOI] [PubMed] [Google Scholar]
- 11.Jackson BR, Griffin PM, Cole D, Walsh KA, Chai SJ. Outbreak-associated Salmonella enterica serotypes and food Commodities, United States, 1998–2008. Emerg Infect Dis. 2013;19(8):1239–1244. doi: 10.3201/eid1908.121511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foley SL, Lynne AM. Food animal-associated Salmonella challenges: pathogenicity and antimicrobial resistance. J Anim Sci. 2008;86(14 Suppl):E173–187. doi: 10.2527/jas.2007-0447. [DOI] [PubMed] [Google Scholar]
- 13.Dutta U, Garg PK, Kumar R, Tandon RK. Typhoid carriers among patients with gallstones are at increased risk for carcinoma of the gallbladder. Am J Gastroenterol. 2000;95(3):784–787. doi: 10.1111/j.1572-0241.2000.01860.x. [DOI] [PubMed] [Google Scholar]
- 14.Van Meervenne E, Botteldoorn N, Lokietek S, Vatlet M, Cupa A, Naranjo M, Dierick K, Bertrand S. Turtle-associated Salmonella septicaemia and meningitis in a 2-month-old baby. J Med Microbiol. 2009;58(Pt 10):1379–1381. doi: 10.1099/jmm.0.012146-0. [DOI] [PubMed] [Google Scholar]
- 15.Collard JM, Place S, Denis O, Rodriguez-Villalobos H, Vrints M, Weill FX, Baucheron S, Cloeckaert A, Struelens M, Bertrand S. Travel-acquired salmonellosis due to Salmonella Kentucky resistant to ciprofloxacin, ceftriaxone and co-trimoxazole and associated with treatment failure. J Antimicrob Chemother. 2007;60(1):190–192. doi: 10.1093/jac/dkm114. [DOI] [PubMed] [Google Scholar]
- 16.Onwuezobe IA, Oshun PO, Odigwe CC. Antimicrobials for treating symptomatic non-typhoidal Salmonella infection. Cochrane Database Syst Rev. 2012;11:CD001167. doi: 10.1002/14651858.CD001167.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lengerh A, Moges F, Unakal C, Anagaw B. Prevalence, associated risk factors and antimicrobial susceptibility pattern of Campylobacter species among under five diarrheic children at Gondar University Hospital, Northwest Ethiopia. BMC Pediatr. 2013;13:82. doi: 10.1186/1471-2431-13-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Debas G, Kibret M, Biadglegne F, Abera B. Prevalence and antimicrobial susceptibility patterns of shigella species at Felege Hiwot Referral Hospital Northwest Ethiopia. Ethiop Med J. 2011;49(3):249–256. [PubMed] [Google Scholar]
- 19.Parashar UD, Burton A, Lanata C, Boschi-Pinto C, Shibuya K, Steele D, Birmingham M, Glass RI. Global mortality associated with rotavirus disease among children in 2004. J Infect Dis. 2009;200(Suppl 1):S9–S15. doi: 10.1086/605025. [DOI] [PubMed] [Google Scholar]
- 20.Beyene G, Nair S, Asrat D, Mengistu Y, Engers H, Wain J. Multidrug resistant Salmonella concord is a major cause of salmonellosis in children in Ethiopia. J Infect Dev Ctries. 2011;5:23–33. doi: 10.3855/jidc.906. [DOI] [PubMed] [Google Scholar]
- 21.van Duijkeren E, Wannet WJ, Houwers DJ, van Pelt W. Antimicrobial susceptibilities of salmonella strains isolated from humans, cattle, pigs, and chickens in the Netherlands from 1984 to 2001. J Clin Microbiol. 2003;41(8):3574–3578. doi: 10.1128/JCM.41.8.3574-3578.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis MA, Hancock DD, Besser TE, Rice DH, Gay JM, Gay C, Gearhart L, DiGiacomo R. Changes in antimicrobial resistance among Salmonella enterica Serovar typhimurium isolates from humans and cattle in the Northwestern United States, 1982–1997. Emerg Infect Dis. 1999;5(6):802–806. doi: 10.3201/eid0506.990610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cailhol J, Lailler R, Bouvet P, La Vieille S, Gauchard F, Sanders P, Brisabois A. Trends in antimicrobial resistance phenotypes in non-typhoid Salmonellae from human and poultry origins in France. Epidemiol Infect. 2006;134(1):171–178. doi: 10.1017/S0950268805004863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashenafi M, Gedebou M. Salmonella and Shigella in adult diarrhoea in Addis Ababa--prevalence and antibiograms. Trans R Soc Trop Med Hyg. 1985;79(5):719–721. doi: 10.1016/0035-9203(85)90201-9. [DOI] [PubMed] [Google Scholar]
- 25.Mache AMY, Cowley C. Salmonella sero groups identified from adult diarrheal out-patients in Addis Ababa, Ethiopia: antibiotic resistance and plasmid profile analysis. East African Med J. 1997;74:183–186. [PubMed] [Google Scholar]
- 26.Gebre-Yohannes A, Mammo K, Wolde H. R-factor-mediated multidrug resistance in Salmonella typhimurium isolates. Ethiop Med J. 1987;25(1):53–54. [PubMed] [Google Scholar]
- 27.WHO: Diarrheal Diseases. In: World Health Organization; Fact sheet No.330; 2013. http://www.who.int/mediacentre/factsheets/fs330/en/. Accessed 10 April 2015.
- 28.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32(9):920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 29.WHO . Isolation of Salmonella spp From Food and Animal Faeces. 5 2010. WHO Global Foodborne Infections Network . Laboratory Protocol. [Google Scholar]
- 30.Cohen ND, Neibergs HL, McGruder ED, Whitford HW, Behle RW, Ray PM, Hargis BM. Genus-specific detection of salmonellae using the polymerase chain reaction (PCR) J Vet Diagn Invest. 1993;5(3):368–371. doi: 10.1177/104063879300500311. [DOI] [PubMed] [Google Scholar]
- 31.Ewing . Serological Identificaiton of Salmonella. 1986. [Google Scholar]
- 32.Shipp CR, Rowe B. A mechanised microtechnique for salmonella serotyping. J Clin Pathol. 1980;33(6):595–597. doi: 10.1136/jcp.33.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grimont P, Weill F-X. Antigenic formulas of Salmonella Serotypes. 9. France: WHO Collaborating Centre for Reference and Research on Salmonella; 2007. [Google Scholar]
- 34.CALLOW BR. A new phage-typing scheme for Salmonella typhi-murium. J Hyg (Lond) 1959;57:346–359. doi: 10.1017/S0022172400020209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson ES, Ward LR, Saxe MJ, de Sa JD. Bacteriophage-typing designations of Salmonella typhimurium. J Hyg (Lond) 1977;78(2):297–300. doi: 10.1017/S0022172400056187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.CLSI: Performance Standards for Antimicrobial Susceptibility Testing: Twenty-third Informational Supplement. Clinical and Laboratory Standards Institute, Wayne, PA; 2013.
- 37.Beyene G, Tasew H. Prevalence of intestinal parasite, Shigella and Salmonella species among diarrheal children in Jimma health center Jimma southwest Ethiopia: a cross sectional study. Ann Clin Microbiol Antimicrob. 2014;13:10. doi: 10.1186/1476-0711-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tadesse G. Prevalence of human Salmonellosis in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. 2014;14:88. doi: 10.1186/1471-2334-14-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guchi B, Ashenafi M. Microbial load, prevalence and antibiograms of Salmonella and Shigella in lettuce and green peppers. Ethiop J Health Sci. 2010;20(1):41–48. doi: 10.4314/ejhs.v20i1.69431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dugassa A, Bacha K, Ketema T. Microbiological Quality and Safety of some selected vegetables sold in Jimma town, SouthWestern Ethiopia. African J Environ Sci Technol. 2014;8(11):633–653. [Google Scholar]
- 41.Quiroz-Santiago C, Rodas-Suárez OR, Carlos RV, Fernández FJ, Quiñones-Ramírez EI, Vázquez-Salinas C. Prevalence of Salmonella in vegetables from Mexico. J Food Prot. 2009;72(6):1279–1282. doi: 10.4315/0362-028x-72.6.1279. [DOI] [PubMed] [Google Scholar]
- 42.Hanning IB, Nutt JD, Ricke SC. Salmonellosis outbreaks in the United States due to fresh produce: sources and potential intervention measures. Foodborne Pathog Dis. 2009;6(6):635–648. doi: 10.1089/fpd.2008.0232. [DOI] [PubMed] [Google Scholar]
- 43.Zheng J, Allard S, Reynolds S, Millner P, Arce G, Blodgett RJ, Brown EW. Colonization and internalization of Salmonella enterica in tomato plants. Appl Environ Microbiol. 2013;79(8):2494–2502. doi: 10.1128/AEM.03704-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gebre-Yohannes A. Salmonella from Ethiopia: prevalent species and their susceptibility to drugs. Ethiop Med J. 1985;23(3):97–102. [PubMed] [Google Scholar]
- 45.Lan R, Reeves PR, Octavia S. Population structure, origins and evolution of major Salmonella enterica clones. Infect Genet Evol. 2009;9(5):996–1005. doi: 10.1016/j.meegid.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 46.Kariuki S, Revathi G, Kariuki N, Kiiru J, Mwituria J, Hart CA. Characterisation of community acquired non-typhoidal Salmonella from bacteraemia and diarrhoeal infections in children admitted to hospital in Nairobi. Kenya BMC Microbiol. 2006;6:101. doi: 10.1186/1471-2180-6-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lunguya O, Lejon V, Phoba MF, Bertrand S, Vanhoof R, Glupczynski Y, Verhaegen J, Muyembe-Tamfum JJ, Jacobs J. Antimicrobial resistance in invasive non-typhoid Salmonella from the Democratic Republic of the Congo: emergence of decreased fluoroquinolone susceptibility and extended-spectrum beta lactamases. PLoS Negl Trop Dis. 2013;7(3):e2103. doi: 10.1371/journal.pntd.0002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolday D. Increase in the incidence of multidrug-resistant salmonellae in Ethiopia. J Antimicrob Chemother. 1998;41(3):421–423. doi: 10.1093/jac/41.3.421. [DOI] [PubMed] [Google Scholar]
- 49.Kariuki S, Revathi G, Kiiru J, Lowe B, Berkley JA, Hart CA. Decreasing prevalence of antimicrobial resistance in non-typhoidal Salmonella isolated from children with bacteraemia in a rural district hospital Kenya. Int J Antimicrob Agents. 2006;28(3):166–171. doi: 10.1016/j.ijantimicag.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 50.DACA . Standard Treatment Guidline for General Hospitals. Addis Ababa: Drug Administration and Control Authority of Ethiopia; 2010. [Google Scholar]


