Abstract
Background
Atrial fibrillation (AF) increases the risk of stroke and death. Data on the predictors for stroke and death in ‘real-world’ AF patients are limited, especially from large prospective Asian cohorts.
Methods
The Fushimi AF Registry is a community-based prospective survey designed to enroll all AF patients who visited the participating medical institutions in Fushimi-ku, Kyoto, Japan. Follow-up data were available for 3,304 patients (median follow-up period 741 days). We explored the predictors for ‘death, stroke, and systemic embolism (SE)’ during follow-up in 1,541 patients not receiving oral anticoagulants (OAC) at baseline.
Results
The mean age was 73.1 ± 12.5 years, and 673 (44%) patients were female. The mean CHADS2 and CHA2DS2-VASc scores were 1.76 and 3.08, respectively. Cumulative events were as follows: stroke/SE in 61 (4%) and death in 230 (15%), respectively. On multivariate analysis, advanced age (hazard ratio (HR): 1.68, 95% confidence interval (CI): 1.24–2.29), underweight (body mass index <18.5 kg/m2) (HR: 1.71, 95% CI: 1.25–2.32), previous stroke/SE/transient ischemic attack (HR: 1.70, 95% CI: 1.25–2.30), heart failure (HR: 1.59, 95% CI: 1.17–2.15), chronic kidney disease (HR: 1.53, 95% CI: 1.16–2.02), and anemia (HR: 2.41, 95% CI: 1.78–3.28) were independent predictors for death/stroke/SE. Cumulative numbers of these 6 risk predictors could stratify the incidence of death/stroke/SE in patients without OAC, as well as those with OAC in our registry.
Conclusions
Advanced age, underweight, previous stroke/SE/transient ischemic attack, heart failure, chronic kidney disease, and anemia were independently associated with the risk of death/stroke/SE in non-anticoagulated Japanese AF patients.
Introduction
Atrial fibrillation (AF) is a common cardiac arrhythmia among the elderly [1], and is a well-established risk factor for stroke/systemic embolism (SE) and death [2, 3]. While many large-scale randomized clinical trials (RCTs) of AF patients were conducted to investigate the efficacy and safety of non-vitamin K antagonist anticoagulants for stroke prevention, they do not necessarily represent patients routinely seen in clinical practice due to trial-specific inclusion/exclusion criteria. Indeed, the original historical RCTs of stroke prevention only randomized <10% of patients screened, and many stroke risk factors were not recorded nor consistently defined [4]. Thus, RCTs should be complemented by ‘real-world’ registries. To date, there have been many reports about risk factors for stroke/SE in patients with AF, but most have been based on the data from RCTs or registries with some inclusion/exclusion criteria, with most large prospective studies being derived from Western countries. Given the global burden of AF, it is important to have ‘real-world’ data on the clinical epidemiology of this common arrhythmia from Asian countries.
Unlike RCTs which are tightly-controlled studies with pre-specified protocols and close follow-up, some deaths could be due to undiagnosed stroke, given the nature of registry studies. Therefore, we defined the primary endpoint as composite of ‘all-cause death, stroke, and SE (death/stroke/SE)’ in the present study. We aimed to test the performance of CHADS2 [5] and CHA2DS2-VASc scores [6], which are well-validated risk stratification schemes for stroke/SE, for the prediction of the composite endpoint, and second, to derive the potential risk factor predictors for the composite endpoint, using a large-scale prospective ‘real-world’ registry of Japanese AF patients.
Methods
The Fushimi AF Registry is a community-based prospective survey of AF patients in Fushimi-ku, Kyoto, Japan [7, 8]. The detailed study design, patient enrollment, participating institution, the definition of the co-morbidities or the measurements, and subjects’ baseline clinical characteristics of the Fushimi AF Registry were previously described (UMIN Clinical Trials Registry: UMIN000005834) [7]. The inclusion criterion for the registry is the documentation of AF on a 12-lead electrocardiogram or Holter monitoring at any time. There are no exclusion criteria for this ‘all-comers’ registry. A total of 79 institutions, all of which are members of Fushimi-Ishikai (Fushimi Medical Association), participated in the registry. The participating institutions comprised 2 cardiovascular centers (National Hospital Organization Kyoto Medical Center and Ijinkai Takeda Hospital), 9 small- and medium-sized hospitals, and 68 primary care clinics. The enrollment of patients was started in March 2011. All of the participating institutions attempted to enroll all consecutive patients with AF under regular outpatient care or hospital admission.
Among the registry participants, we analyzed the patients whose follow-up data were available. We defined the primary endpoint as composite endpoint of ‘death/stroke/SE’ during follow-up period. Stroke was defined as the sudden onset of a focal neurologic deficit in a location consistent with the territory of a major cerebral artery, and it was confirmed by computed tomography or magnetic resonance imaging. SE was defined as an acute vascular occlusion of an extremity or organ.
We defined advanced age as more than or equal to 75 years, underweight as body mass index (BMI) at baseline less than 18.5 kg/m2 according to the World Health Organization criteria [9], and anemia as value of hemoglobin at baseline less than 13 g/dl (male), less than 12 g/dl (female) according to the World Health Organization criteria [10]. Oral anticoagulants (OAC) included warfarin, dabigatran, rivaroxaban, apixaban, and edoxaban.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation (SD), or median and interquartile range. Categorical variables are presented as numbers and percentages. We compared categorical variables using the chi-square test when appropriate; otherwise, we used Fisher’s exact test. We compared continuous variables using Student’s t-test on the basis of the distribution. We stratified the entire cohort by OAC prescription at baseline, and compared the baseline characteristics between patients without OAC and those with it.
To investigate the predictors for death/stroke/SE, we analyzed the patients without OAC at baseline. First, we tested the predictive ability of the CHADS2 and CHA2DS2-VASc scores on the composite endpoint of ‘death/stroke/SE’ in patients without OAC, using a receiver-operator characteristic curve analysis (as a measure of the C-index). Thereafter, we performed univariate and multivariate analysis using Cox proportional hazard models to explore the predictors for composite endpoint of ‘death/stroke/SE’. All clinically relevant potential risk factors for death/stroke/SE were included on multivariate analysis. The patients with at least 1 missing covariate were excluded on multivariate analysis. We also analyzed the patients without OAC throughout follow-up period.
We investigated whether the cumulative number of each risk factors which were significant on multivariate analysis could stratify the incidence of ‘death/stroke/SE’ and ‘stroke/SE’ during the follow-up period. The continuous distribution of cumulative risk factors was then stratified into 5 categories, and the cumulative incidence of death/stroke/SE across these 5 categories were estimated by Kaplan-Meier method, and compared by the log-rank test. We also investigated whether the cumulative number of risk factors could stratify the incidence of events in AF patients taking OAC. Finally, we evaluated the model predictive ability for the cumulative number of risk factors on the composite endpoint of ‘death/stroke/SE’ in patients without OAC and in those with OAC, again using a receiver-operator characteristic curve analysis. We used JMP version 10 (SAS Institute, Cary, NC) to perform all analyses. Two-sided P values less than 0.05 were considered as statistically significant.
Ethics
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki, and was approved by the ethical committees of the National Hospital Organization Kyoto Medical Center and Ijinkai Takeda General Hospital. Since the present research involves an observational study not using human biological specimens, written informed consent was not obtained from each patient for their clinical records to be used in this study, according to the ethical guidelines for epidemiological research issued by the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare, Japan. However, we have published all relevant details regarding this study to be carried out and provide each patient an opportunity to refuse inclusion in this research by posting the details at every participating clinic and at the homepages of our institutions. We also held a public meeting with citizens in Fushimi-ku to demonstrate outlines of the present study. As consent was not obtained, patient records and information was anonymized and de-identified prior to analysis.
Results
A total of 4,115 patients were enrolled by the end of July 2014. Of 3,666 patients who were enrolled one year before (by the end of July 2013), follow-up data (collected every year) were available for 3,304 patients (follow-up rate: 90.1%). Among 3,304 patients, 1,541 patients were not prescribed OAC at baseline, 1,751 patients were prescribed OAC at baseline, and 12 patients’ prescription data were not available. Median follow-up periods were 736 days (interquartile range: 380 days to 1096 days) in patients without OAC, and 748 days (interquartile range: 458 days to 1112 days) in patients with OAC, respectively.
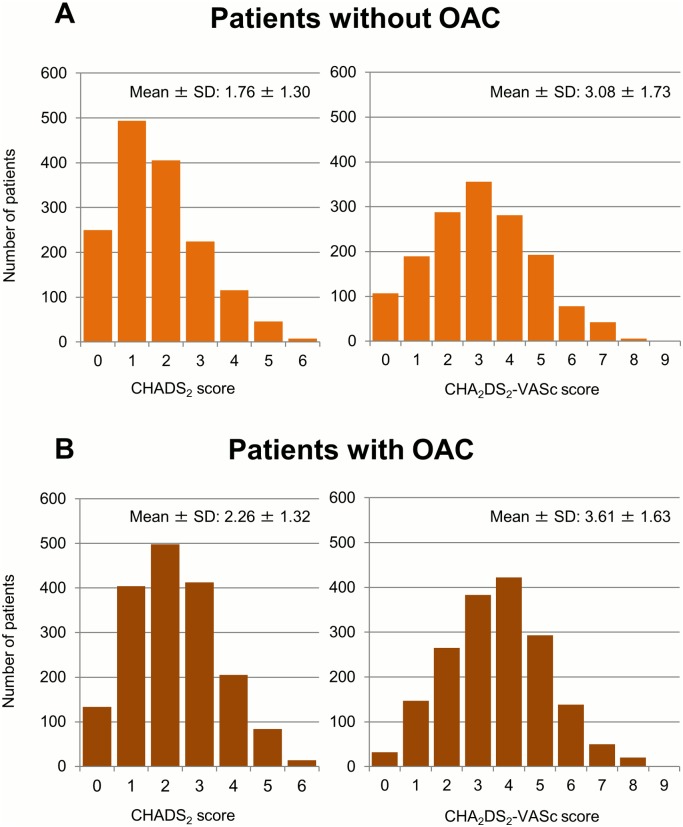
Baseline characteristics, co-morbidities in patients with and without OAC at baseline are shown in Table 1. Patients without OAC were more often female (p<0.01), younger (p = 0.01), and had lower BMI (p<0.01). Previous stroke/SE/transient ischemic attack (TIA), heart failure, hypertension and diabetes mellitus were less common in patients without OAC (all p<0.01), whereas paroxysmal AF and antiplatelet therapy at baseline were more common in patients without OAC (both p<0.01). The mean CHADS2 score and CHA2DS2-VASc score were lower in patients without OAC (both p<0.01) (Fig 1).
Table 1. Comparison of baseline characteristics and co-morbidities between patients with and without OAC at baseline.
| Without OAC | With OAC | p value | |
|---|---|---|---|
| Number | N = 1,541 | N = 1,751 | |
| <Baseline characteristics> | |||
| Female sex | 673 (44%) | 651 (37%) | <0.01 |
| Age (years) | 73.1 ± 12.5 | 74.1 ± 9.2 | 0.01 |
| Advanced age (≥75 years) | 759 (49%) | 929 (53%) | 0.03 |
| Body weight (kg) | 57.9 ± 13.2 | 60.1 ± 13.3 | <0.01 |
| BMI (kg/m2) | 22.7 ± 4.0 | 23.3 ± 4.0 | <0.01 |
| Underweight (BMI <18.5 kg/m2) | 180 (14%) | 138 (9%) | <0.01 |
| Systolic blood pressure (mmHg) | 126.3 ± 19.3 | 123.2 ± 18.6 | <0.01 |
| Diastolic blood pressure (mmHg) | 70.9 ± 13.0 | 70.2 ± 12.7 | 0.10 |
| Heart rate (beats/min) | 78.1 ± 16.0 | 77.4 ± 15.6 | 0.17 |
| Type of atrial fibrillation | |||
| paroxysmal | 963 (63%) | 618 (35%) | |
| Persistent | 111 (7%) | 148 (9%) | <0.01 |
| Permanent | 467 (30%) | 985 (56%) | |
| Antiplatelet therapy at baseline | 535 (35%) | 433 (25%) | <0.01 |
| <Co-morbidities> | |||
| Previous stroke/SE/TIA | 218 (14%) | 466 (27%) | <0.01 |
| Heart failure | 298 (19%) | 580 (33%) | <0.01 |
| Hypertension | 905 (59%) | 1,116 (64%) | <0.01 |
| Diabetes mellitus | 322 (21%) | 442 (25%) | <0.01 |
| Dyslipidemia | 641 (42%) | 785 (45%) | 0.06 |
| Coronary artery disease | 236 (15%) | 251 (14%) | 0.43 |
| Valvular heart disease | 202 (13%) | 389 (22%) | <0.01 |
| Cardiomyopathy | 27 (2%) | 69 (4%) | <0.01 |
| Peripheral artery disease | 58 (4%) | 80 (5%) | 0.25 |
| Chronic kidney disease | 479 (31%) | 661 (38%) | <0.01 |
| Anemia | 552 (39%) | 582 (35%) | 0.07 |
| COPD | 67 (4%) | 95 (5%) | 0.15 |
| History of major bleeding | 44 (3%) | 36 (2%) | 0.14 |
Categorial data are presented as number (%). Continuous data are presented as mean ± standard deviation. OAC: oral anticoagulants, BMI: body mass index, SE: systemic embolism, TIA: transient ischemic attack, COPD: chronic obstructive pulmonary disease.
Fig 1. Distribution of patients for each CHADS2 and CHA2DS2-VASc score.
(A) Patients without oral anticoagulant (OAC). (B) Patients with OAC. SD: standard deviation.
The rates of major clinical events in patients without OAC during follow-up period were as follows: death/stroke/SE in 270 (9.1 per 100 person-years), all-cause death in 230 (7.7 per 100 person-years), non-cardiac death in 205 (6.8 per 100 person-years), and stroke/SE in 61 (2.1 per 100 person-years), respectively (Table 2).
Table 2. Cumulative events and incidence of events during follow-up in patients without OAC at baseline (N = 1,541).
| Cumulative events Number (%) | Incidence of events (/100 person-years) | |
|---|---|---|
| Death/stroke/SE | 270 (18%) | 9.1 |
| All-cause death | 230 (15%) | 7.7 |
| Cardiac death | 25 (2%) | 0.9 |
| Non-cardiac death | 205 (13%) | 6.8 |
| Stroke/SE | 61 (4%) | 2.1 |
| Stroke | 59 (4%) | 2.0 |
| Ischemic stroke | 42 (3%) | 1.4 |
| Hemorrhagic stroke | 17 (1%) | 0.6 |
| SE | 3 (0.2%) | 0.1 |
OAC: oral anticoagulant, SE: systemic embolism.
Predictive abilities of the CHADS2 and CHA2DS2-VASc scores on the composite endpoints of ‘death/stroke/SE’ in patients without OAC were modest, with C-indexes of the CHADS2 and CHA2DS2-VASc scores being 0.67 and 0.67, respectively.
On univariate analysis, following variables were significantly associated with increased risk of death/stroke/SE: advanced age (≥75 years), underweight (BMI <18.5 kg/m2), previous stroke/SE/TIA, heart failure, valvular heart disease, peripheral artery disease, chronic kidney disease (CKD), and anemia. Variables associated with decreased risk of death/stroke/SE were as follows: paroxysmal AF, and dyslipidemia.
Among 1,541 patients without OAC, 233 patients’ BMI data and 111 patients’ laboratory data of hemoglobin were missing. Therefore, we included 1,245 patients without OAC in a multivariate analysis. Advanced age (hazard ratio (HR): 1.68, 95% confidence interval (CI): 1.24–2.29), underweight (HR: 1.71, 95% CI: 1.25–2.32), previous stroke/SE/TIA (HR: 1.70, 95% CI: 1.25–2.30), heart failure (HR: 1.59, 95% CI: 1.17–2.15), CKD (HR: 1.53, 95% CI: 1.16–2.02), and anemia (HR: 2.41, 95% CI: 1.78–3.28) were independent predictors for death/stroke/SE on multivariate analysis (Table 3). We did not investigate the impact of smoking habit or history of malignancy on death/stroke/SE, due to missing data (the number of patients with unknown smoking habit was 507, and we did not collect the status of malignancy).
Table 3. Predictors for the incidence of death/stroke/SE in patients without OAC; Univariate and multivariate analysis.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | p value | HR (95% CI) | p value |
| <Baseline characteristics> | ||||
| Female sex | 1.12 (0.88–1.43) | 0.34 | 0.81 (0.62–1.06) | 0.13 |
| Advanced age (≥75 years) | 2.93 (2.25–3.84) | <0.01 | 1.68 (1.24–2.29) | <0.01 |
| Underweight (BMI <18.5 kg/m2) | 2.76 (2.08–3.64) | <0.01 | 1.71 (1.25–2.32) | <0.01 |
| Paroxysmal atrial fibrillation | 0.59 (0.46–0.75) | <0.01 | 0.84 (0.64–1.11) | 0.22 |
| Antiplatelet therapy at baseline | 1.09 (0.85–1.39) | 0.50 | 0.83 (0.62–1.09) | 0.18 |
| <Co-morbidities> | ||||
| Previous stroke/SE/TIA | 2.41 (1.83–3.14) | <0.01 | 1.70 (1.25–2.30) | <0.01 |
| Heart failure | 3.05 (2.38–3.89) | <0.01 | 1.59 (1.17–2.15) | <0.01 |
| Hypertension | 0.88 (0.70–1.13) | 0.32 | 0.81 (0.62–1.05) | 0.11 |
| Diabetes mellitus | 1.18 (0.89–1.55) | 0.24 | 0.99 (0.72–1.34) | 0.96 |
| Dyslipidemia | 0.69 (0.53–0.88) | <0.01 | 0.90 (0.68–1.19) | 0.48 |
| Coronary artery disease | 1.23 (0.90–1.66) | 0.19 | 0.89 (0.62–1.27) | 0.54 |
| Valvular heart disease | 2.04 (1.51–2.71) | <0.01 | 1.05 (0.75–1.46) | 0.76 |
| Cardiomyopathy | 0.38 (0.06–1.19) | 0.11 | 0.37 (0.06–1.17) | 0.10 |
| Peripheral artery disease | 1.95 (1.17–3.05) | 0.01 | 1.38 (0.80–2.23) | 0.24 |
| Chronic kidney disease | 2.70 (2.13–3.43) | <0.01 | 1.53 (1.16–2.02) | <0.01 |
| Anemia | 3.93 (3.04–5.12) | <0.01 | 2.41 (1.78–3.28) | <0.01 |
| COPD | 1.44 (0.83–2.30) | 0.18 | 1.06 (0.60–1.75) | 0.83 |
| History of major bleeding | 1.62 (0.83–2.82) | 0.15 | 0.84 (0.41–1.52) | 0.58 |
SE: systemic embolism, HR: hazard ratio, CI: confidence interval, BMI: body mass index, TIA: transient ischemic attack, COPD: chronic obstructive pulmonary disease.
In patients without OAC at baseline (n = 1,541), 290 patients (18.8%) started taking OAC during follow-up period. Of patients with OAC at baseline (n = 1,751), 353 patients (20.2%) stopped taking OAC during follow-up. In a sensitivity analysis, we separately analyzed the subgroup of patients who had never taken any OAC throughout the follow-up period (n = 1,251), and our result were consistent, with the following 6 factors (advanced age, underweight, previous stroke/SE/TIA, heart failure, CKD, and anemia) still being independently associated with the incidence of death/stroke/SE on multivariate analysis.
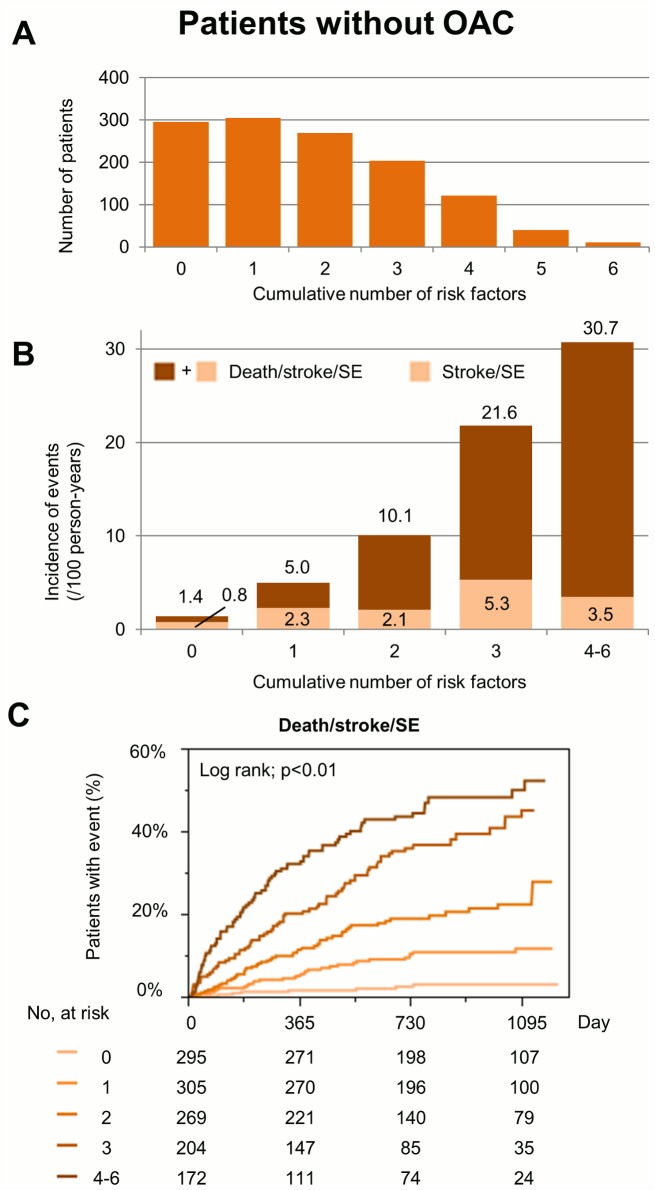
Among the patients without OAC, the numbers of patients and the incidence of death/stroke/SE for each cumulative number of 6 risk factors (advanced age, underweight, previous stroke/SE/TIA, heart failure, CKD, and anemia) are shown in Fig 2A and 2B. Kaplan-Meier curves for the incidence of death/stroke/SE during follow-up between risk categories in patients without OAC are shown in Fig 2C. The cumulative number of these 6 risk factors show a significant gradient for the incidence of these endpoints.
Fig 2.
(A) The number of patients for each cumulative number of risk factors in patients without oral anticoagulant (OAC). (B) Incidence of death/stroke/systemic embolism (SE) during follow-up for each cumulative number of risk factors in patients without OAC. (C) Kaplan-Meier curves for the incidence of events during follow-up in patients without OAC. Risk factors are the following 6 components; advanced age, underweight, previous stroke/SE/TIA, heart failure, CKD, and anemia.
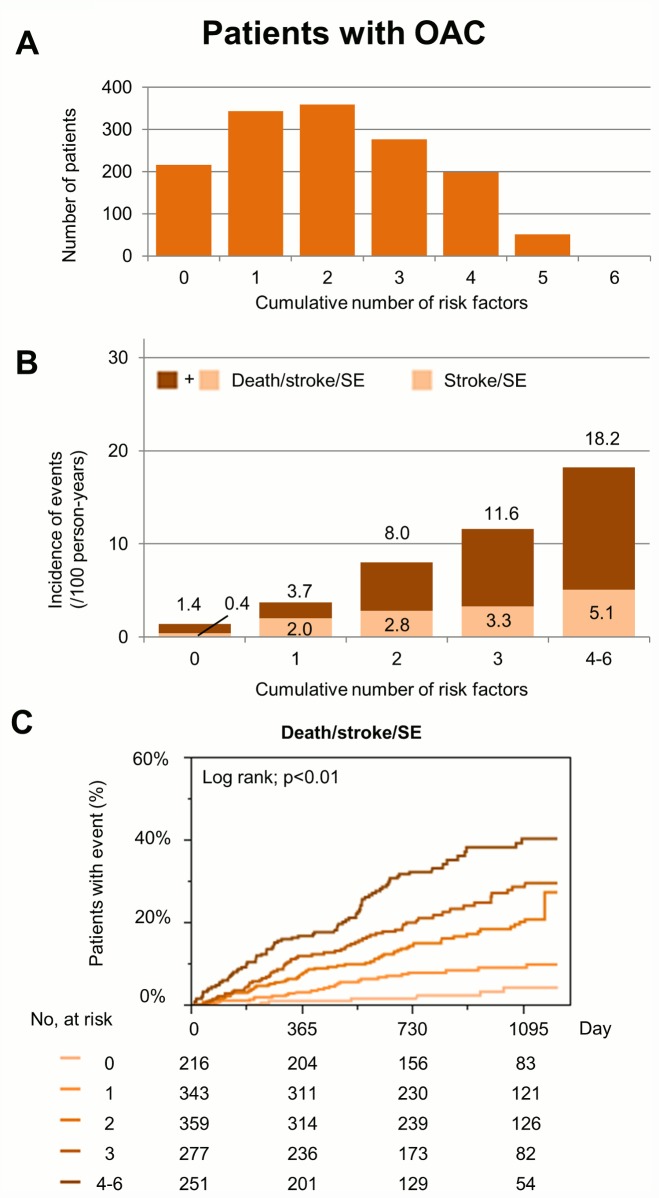
The numbers of patients and incidences of events for each cumulative number of 6 risk factors in patients taking OAC are shown in Fig 3, showing that cumulative number of 6 risk factors could also stratify the incidences of death/stroke/SE and stroke/SE in patients with OAC, as well as those without OAC.
Fig 3.
(A) The number of patients for each cumulative number of risk factors in patients with oral anticoagulant (OAC). (B) Incidence of death/stroke/systemic embolism (SE) during follow-up for each cumulative number of risk factors in patients with OAC. (C) Kaplan-Meier curves for the incidence of events during follow-up in patients with OAC. Risk factors are the following 6 components; advanced age, underweight, previous stroke/SE/TIA, heart failure, CKD, and anemia.
Model predictive ability of the cumulative number of risk factors on the composite endpoint of ‘death/stroke/SE’ was good, with a C-index of 0.76 in patients without OAC, and 0.70 in patients taking OAC, respectively.
Discussion
In this large community-based prospective survey, we have shown that amongst Asian patients with AF, advanced age, underweight, previous stroke/SE/TIA, heart failure, CKD, and anemia were independently associated with the incidence of death/stroke/SE. There was good prediction for endpoint of death/stroke/SE using these 6 variables in patients without OAC, as well as those with OAC. This Japanese study represents one of the largest prospective cohorts of Asian AF patients.
We defined the primary endpoint as composite of death/stroke/SE in the present study. In our cohort of Japanese AF patients, the rate of death was 3 to 4 times higher than the rate of stroke/SE, and the rate of non-cardiac death was higher than previous reports [11, 12]. Some of the ‘non-cardiac deaths’ could be due to undiagnosed stroke, given the nature of registry studies. Also, the RCTs clearly show that anticoagulation (ie. the therapeutic intervention) significantly reduces stroke/systemic embolism (by 64%) and all-cause mortality (by 26%) [13], compared to control/placebo—thus, justifying use of this composite endpoint.
CHADS2/CHA2DS2-VASc scores and death/stroke/SE
In our registry, congestive heart failure, advanced age (≥75 years), and history of stroke/SE/TIA were independent predictors for the incidence of death/stroke/SE. Meanwhile, hypertension, diabetes mellitus, or female sex were not significantly associated with increased risk of death/stroke/SE. They were reported to be risk factors for stroke, but the association has been inconsistent [14, 15]. Well-controlled hypertension and diabetes mellitus may not be a risk factor for adverse events of AF [16]. Moreover, neither hypertension nor diabetes mellitus was associated with the stroke in Chinese AF patients [17], and neither was female sex in Japanese patients [18], suggesting that the risk factors for stroke/SE may possibly differ between Western and Asian countries.
Anemia and death/stroke/SE in AF patients
Anemia is associated with increased mortality and morbidity in various cardiovascular diseases [19–21]. Anemia is also an independent predictor of 1-year all-cause death and re-hospitalization in elderly patients hospitalized with AF [22], and has been associated with major adverse cardiac and cerebrovascular events in patients with AF undergoing percutaneous coronary intervention [23]. A plausible mechanism whereby anemia increases the mortality may be a high risk of coronary ischemic events [19, 20], but cardiac death was relatively small in our registry. Alternatively, the higher rate of all-cause death and cardiovascular events might be related to the higher risk profile in anemic patients and underlying diseases causing anemia. Despite some studies of increased mortality and morbidity in anemic patients with cardiovascular diseases, the results of therapeutic intervention trials targeting anemia have been inconsistent [23]. Although we cannot address whether anemia is a mediator and therefore potentially amenable to therapeutic intervention, or merely a marker of adverse events in AF patients, our data showed that anemia is an independent, and strongest predictor for death/stroke/SE on multivariate analysis.
CKD and death/stroke/SE in AF patients
CKD is considered to be an independent predictor for stroke/SE in AF [24–26], although it does not incrementally add to existing stroke risk scores [27]. CKD is also considered to be an independent predictor for composite endpoints of death/stroke/SE [28, 29]. Multiple possible mechanisms exist for the association between CKD and increased risks of death and cardiovascular diseases; reduced kidney function is associated with increased levels of inflammatory factors, enhanced coagulability, arterial calcification, arterial stiffness, and endothelial dysfunction [30]. Our data show that CKD was independently associated with increased risk of death/stroke/SE in AF patients, consistent with previous studies.
Underweight and death/stroke/SE in AF patients
Being overweight has been associated with a lower risk of all-cause mortality and cardiovascular mortality in AF [31–33], while another study suggested sex-related differences [34]. These studies excluded underweight patients with a BMI <18.5 kg/m2, and the characteristics of underweight patients with AF are unknown. Japanese patients with AF are generally small and lean, and the Fushimi AF Registry contains many frail elderly patients who are underweight. We recently demonstrated that underweight was associated with a higher risk of stroke/SE, despite that the mechanisms remain speculative [35]. Our study showed the association between underweight and death/stroke/SE, but the impact of promoting weight gain in patients with AF is controversial. The recent trial suggests that weight reduction is actually associated with better outcomes in AF [36].
Study limitations
Our study has several limitations. First, our data are based on an observational study and provides only associative evidence, not causative. Second, OAC was prescribed to only 53% of all the participating patients, and 60% of the patients with CHADS2 score ≥2 points in our registry, based on the responsible attending physician. However, OAC is often underused amongst AF patients in daily clinical practice, due to concerns about the bleeding complications [37, 38]. Our registry included many old and frail patients, who were perhaps considered unsuitable for OAC. Third, we did not collect the data of time in therapeutic range in patients with warfarin, and we only stratified entire cohort by OAC prescription at baseline. Indeed, there were some changes in the status of OAC prescription seen during follow-up period. However, our sensitivity analysis still shows that the 6 risk factors remained independently associated with the risk of death/stroke/SE in patients who had never taken any OAC throughout the follow-up period. Whilst exclusion of any OAC use during follow-up may result in conditioning for the future, but despite this limitation, our risk stratification is still predictive of the composite endpoint. Fourth, among 1,541 patients without OAC, 233 patients’ BMI data and 111 patients’ laboratory data of hemoglobin were missing, leading to selection bias. Fifth, the number of patients and incidence of endpoints might be underpowered to detect the impact of some risk factors, such as hypertension and diabetes mellitus. Finally, all enrolled patients were Japanese, thus the body compositions or other factors might differ from other populations. Our risk factors require external validation, with a view to development of risk stratification schemes that can be used in Asian AF patients.
Conclusions
In one of the largest ‘real-world’ non-anticoagulated prospective cohorts of Asian AF patients, we show that advanced age, underweight, previous stroke/SE/TIA, heart failure, CKD, and anemia were independently associated with the incidence of death/stroke/SE. Such risk factors may lead to development of risk stratification schemes for use in Asian AF patients.
Acknowledgments
We sincerely appreciate the help of all the institutions participating in the registry and the clinical research coordinators (Shinagawa T, Mitamura M, Fukahori M, Kimura M, Fukuyama M, Kamata C) for data collection.
Working group members
The following is a list of the institutions participating in the registry.
Chief investigator: Akao M (National Hospital Organization Kyoto Medical Center).
Vice-chief investigator: Chun YH (Ijinkai Takeda General Hospital).
Steering Committee: Esato M (Ijinkai Takeda General Hospital), Abe M (National Hospital Organization Kyoto Medical Center), Tsuji H (Tsuji Clinic), Furuke K (Furuke Clinic).
Statistical Analysis: Wada H (National Hospital Organization Kyoto Medical Center).
Coordinator: Ogawa T (Ogawa Medical Office), Tasato H (Tasato Clinic), Taniguchi Y (Taniguchi Clinic), Nishikawa M (Nishikawa Clinic), Furukawa K (Furukawa Medical Clinic), Kawai C, Hashimoto T, Kanda M (Ijinkai Takeda General Hospital), Tsukahara T, Fukuda S, Nakamura M, Ito T, Hasegawa K (National Hospital Organization Kyoto Medical Center).
Clinical Event Committee: Kawabata Y, Yasuda K, Kameyama K (National Hospital Organization Kyoto Medical Center).
Participating institutions: Department of Cardiology, National Hospital Organization Kyoto Medical Center (Akao M, Abe M, Ogawa H, Masunaga N, Iguchi M, Ishii M, Unoki T, Takabayashi K, Hamatani Y, Yamashita Y, Takagi D, Niki S, An Y, Tezuka Y, Osakada G, Nakashima Y, Kanasaki M, Nakano T, Funatsu J, Nishio M, Takenaka Y); Department of Arrhythmia, Ijinkai Takeda General Hospital (Chun YH, Esato M, Kida Y, Nishina N); Koujinkai Oshima Hospital (Terada K); Divison of Translational Research, National Hospital Organization Kyoto Medical Center (Hasegawa K, Wada H); Kanai Hospital (Nishio M, Kamiya Y, Abe M, Ishii M); Tsuji clinic (Tsuji H); Furukawa Medical Clinic (Furukawa K); Nishikawa Clinic (Nishikawa M); Taniguchi Clinic (Taniguchi Y); Gushiken Clinic (Gushiken T); Fushimi Shimizu Hospital (Hirata Y); Yoda Clinic (Yoda J); Tasato Clinic (Tasato H); Ogawa Medical Office (Ogawa T); Saiwai Hospital (Wakatsuki Y, Yahata M, Higashitani N); Itoh Hemodialysis Clinic (Itoh H); Itoh Clinic (Itoh H, Ohmori Y); Ryokuhoukai Tsuji Clinic (Tsuji K); Kitamura Clinic (Kitamura S); Izumikawa Clinic (Izumikawa F); Hirota Clinic (Hirota N); Kyomachi-Oota Clinic (Oota K); Kouseikai Rehabilitation Clinic (Kou K); Inariyama Hospital (Tanaka T, Iguchi M); Matsushita Clinic (Matsushita N); Kitani Clinic (Kitani K); Kimura Clinic (Kimura F); Hayashi Clinic (Hayashi S); Handa Clinic (Handa S); Soseikai General Hospital (Hasegawa S, Kono T, Otsuka K, Soyama A, Okamoto J, Nakai Y); Asamoto Clinic (Asamoto H); Sugano Clinic (Tanaka H, Murata T); Kyoto Ohashi General Hospital (Kayawake S, Kinoshita Y); Furuke Clinic (Furuke K); Kanehisa Clinic (Asano N); Tahara Clinic (Tahara K); Matsumoto Medical Office (Matsumoto K); Kuroda Clinic (Kuroda O); Ochiai Clinic (Ochiai K, Ochiai J); Fujii Clinic (Fujii M); Kurihara Clinic (Kurihara M); Kuzuyama Clinic (Ito A); Kenkokai Fushimi Clinic (Totsuzaki S); Nakayama Orthopedic Clinic (Nakayama H); Department of Cardiovascular Medicine, Ijinkai Takeda General Hospital (Kawai C, Hashimoto T, Kakio T, Watanabe C, Takeda S, Sasaki Y, Shirasawa K, Beppu K, Inoue T, Shirasaka A, Doi T); Tatsumi Clinic (Ueda T); Oishi Clinic (Oishi M); Koizumi Clinic (Kasahara A); Kishida Clinic (Kishida Su, Kishida Sa); Shibata Clinic (Shibata M); Shimizu Clinic (Shimizu J); Shirasu Clinic (Shirasu M); Fujinokai Clinic (Tateishi S); Tsukuda Clinic (Tsukuda N); Shinseikai Tsuji Clinic (Tsuji K); Nishi Clinic (Nishi T); Nishimura Clinic (Nishimura S); Haba Clinic (Haba T); Higashimae Clinic (Higashimae R); Fujimori Clinic (Fujimori C); Hotta Clinic (Hotta T); Matsui Clinic (Matsui H, Matsui H); Shadan Matsumoto Clinic (Matsumoto H); Maruo Clinic (Maruo N); Misu Clinic (Mikami M); Mekata Clinic (Mekata H); Mori Pediatric Clinic (Mori H); Wakabayashi Clinic (Wakabayashi M); Nakatsugawa Clinic (Sasaki Z); Shiseikai Nishimura Clinic (Nishimura S); Yuge Eye Clinic (Yuge K); Gokita Hospital (Haruta M); Soseikai Clinic (Tsuda E); Toujinkai Hospital (Nishimura M); Kouno Clinic (Kouno T); Matsumura Clinic (Matsumura S); Fujita Clinic (Fujita A); Takayasu Clinic (Takayasu F, Takayasu S), Yano Clinic (Yano Y), Niki Clinic (Niki S), Hasegawa Meiando Clinic (Hasegawa S), Watanabe Medical Clinic (Watanabe T).
Data Availability
All relevant data are within the paper.
Funding Statement
The Fushimi AF Registry is supported by research funding from Boehringer Ingelheim, Bayer Healthcare, Pfizer, Bristol-Myers Squibb, Astellas Pharma, AstraZeneca, Daiichi-Sankyo, Novartis Pharma, MSD, Sanofi-Aventis and Takeda Pharmaceutical. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Inoue H, Fujiki A, Origasa H, Ogawa S, Okumura K, Kubota I, et al. Prevalence of atrial fibrillation in the general population of Japan: an analysis based on periodic health examination. Int J Cardiol. 2009. October 2;137(2):102–7. 10.1016/j.ijcard.2008.06.029 [DOI] [PubMed] [Google Scholar]
- 2. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991. August;22(8):983–8. [DOI] [PubMed] [Google Scholar]
- 3. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998. September 8;98(10):946–52. [DOI] [PubMed] [Google Scholar]
- 4. Lip GY. Stroke and bleeding risk assessment in atrial fibrillation: when, how, and why? Eur Heart J. 2013. April;34(14):1041–9. 10.1093/eurheartj/ehs435 [DOI] [PubMed] [Google Scholar]
- 5. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001. June 13;285(22):2864–70. [DOI] [PubMed] [Google Scholar]
- 6. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010. February;137(2):263–72. 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 7. Akao M, Chun YH, Wada H, Esato M, Hashimoto T, Abe M, et al. Current status of clinical background of patients with atrial fibrillation in a community-based survey: the Fushimi AF Registry. J Cardiol. 2013. April;61(4):260–6. 10.1016/j.jjcc.2012.12.002 [DOI] [PubMed] [Google Scholar]
- 8. Akao M, Chun YH, Esato M, Abe M, Tsuji H, Wada H, et al. Inappropriate Use of Oral Anticoagulants for Patients With Atrial Fibrillation. Circ J. 2014;78(9):2166–2172. [DOI] [PubMed] [Google Scholar]
- 9. Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Arch Intern Med. 1998. September 28;158(17):1855–67. [DOI] [PubMed] [Google Scholar]
- 10. Nutritional anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser. 1968;405:5–37. [PubMed] [Google Scholar]
- 11. Marijon E, Le Heuzey JY, Connolly S, Yang S, Pogue J, Brueckmann M, et al. Causes of death and influencing factors in patients with atrial fibrillation: a competing-risk analysis from the randomized evaluation of long-term anticoagulant therapy study. Circulation. 2013. November 12;128(20):2192–201. 10.1161/CIRCULATIONAHA.112.000491 [DOI] [PubMed] [Google Scholar]
- 12. Lip GY, Laroche C, Ioachim PM, Rasmussen LH, Vitali-Serdoz L, Petrescu L, et al. Prognosis and treatment of atrial fibrillation patients by European cardiologists: one year follow-up of the EURObservational Research Programme-Atrial Fibrillation General Registry Pilot Phase (EORP-AF Pilot registry). Eur Heart J. 2014. December 14;35(47):3365–76. 10.1093/eurheartj/ehu374 [DOI] [PubMed] [Google Scholar]
- 13. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007. June 19;146(12):857–67. [DOI] [PubMed] [Google Scholar]
- 14. Patients with nonvalvular atrial fibrillation at low risk of stroke during treatment with aspirin: Stroke Prevention in Atrial Fibrillation III Study. The SPAF III Writing Committee for the Stroke Prevention in Atrial Fibrillation Investigators. JAMA. 1998. April 22–29;279(16):1273–7. [PubMed] [Google Scholar]
- 15. Petersen P, Kastrup J, Helweg-Larsen S, Boysen G, Godtfredsen J. Risk factors for thromboembolic complications in chronic atrial fibrillation. The Copenhagen AFASAK study. Arch Intern Med. 1990. April;150(4):819–21. [PubMed] [Google Scholar]
- 16. Lip GY, Frison L, Grind M. Effect of hypertension on anticoagulated patients with atrial fibrillation. Eur Heart J. 2007. March;28(6):752–9. [DOI] [PubMed] [Google Scholar]
- 17. Yang YM, Shao XH, Zhu J, Zhang H, Liu Y, Gao X, et al. Risk factors and incidence of stroke and MACE in Chinese atrial fibrillation patients presenting to emergency departments: a national wide database analysis. Int J Cardiol. 2014. May 1;173(2):242–7. 10.1016/j.ijcard.2014.02.040 [DOI] [PubMed] [Google Scholar]
- 18. Inoue H, Atarashi H, Okumura K, Yamashita T, Origasa H, Kumagai N, et al. Impact of gender on the prognosis of patients with nonvalvular atrial fibrillation. Am J Cardiol. 2014. March 15;113(6):957–62. 10.1016/j.amjcard.2013.11.057 [DOI] [PubMed] [Google Scholar]
- 19. Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Borenstein J. Anemia is associated with worse symptoms, greater impairment in functional capacity and a significant increase in mortality in patients with advanced heart failure. J Am Coll Cardiol. 2002. June 5;39(11):1780–6. [DOI] [PubMed] [Google Scholar]
- 20. Muzzarelli S, Pfisterer M. Anemia as independent predictor of major events in elderly patients with chronic angina. Am Heart J. 2006. November;152(5):991–6. [DOI] [PubMed] [Google Scholar]
- 21. Vaglio J, Safley DM, Rahman M, Kosiborod M, Jones P, Thompson R, et al. Relation of anemia at discharge to survival after acute coronary syndromes. Am J Cardiol. 2005. August 15;96(4):496–9. [DOI] [PubMed] [Google Scholar]
- 22. Sharma S, Gage BF, Deych E, Rich MW. Anemia: an independent predictor of death and hospitalizations among elderly patients with atrial fibrillation. Am Heart J. 2009. June;157(6):1057–63. 10.1016/j.ahj.2009.03.009 [DOI] [PubMed] [Google Scholar]
- 23. Puurunen M, Kiviniemi T, Nammas W, Schlitt A, Rubboli A, Nyman K, et al. Impact of anaemia on clinical outcome in patients with atrial fibrillation undergoing percutaneous coronary intervention: insights from the AFCAS registry. BMJ Open. 2014;4(5):e004700 10.1136/bmjopen-2013-004700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zeng WT, Sun XT, Tang K, Mei WY, Liu LJ, Xu Q, et al. Risk of thromboembolic events in atrial fibrillation with chronic kidney disease. Stroke. 2015. January;46(1):157–63. 10.1161/STROKEAHA.114.006881 [DOI] [PubMed] [Google Scholar]
- 25. Piccini JP, Stevens SR, Chang Y, Singer DE, Lokhnygina Y, Go AS, et al. Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R(2)CHADS(2) index in the ROCKET AF (Rivaroxaban Once-daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (AnTicoagulation and Risk factors In Atrial fibrillation) study cohorts. Circulation. 2013. January 15;127(2):224–32. 10.1161/CIRCULATIONAHA.112.107128 [DOI] [PubMed] [Google Scholar]
- 26. Go AS, Fang MC, Udaltsova N, Chang Y, Pomernacki NK, Borowsky L, et al. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation. 2009. March 17;119(10):1363–9. 10.1161/CIRCULATIONAHA.108.816082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Banerjee A, Fauchier L, Vourc'h P, Andres CR, Taillandier S, Halimi JM, et al. Renal impairment and ischemic stroke risk assessment in patients with atrial fibrillation: the Loire Valley Atrial Fibrillation Project. J Am Coll Cardiol. 2013. May 21;61(20):2079–87. 10.1016/j.jacc.2013.02.035 [DOI] [PubMed] [Google Scholar]
- 28. Nakagawa K, Hirai T, Takashima S, Fukuda N, Ohara K, Sasahara E, et al. Chronic kidney disease and CHADS(2) score independently predict cardiovascular events and mortality in patients with nonvalvular atrial fibrillation. Am J Cardiol. 2011. March 15;107(6):912–6. 10.1016/j.amjcard.2010.10.074 [DOI] [PubMed] [Google Scholar]
- 29. Guo Y, Wang H, Zhao X, Zhang Y, Zhang D, Ma J, et al. Relation of renal dysfunction to the increased risk of stroke and death in female patients with atrial fibrillation. Int J Cardiol. 2013. September 30;168(2):1502–8. 10.1016/j.ijcard.2012.12.099 [DOI] [PubMed] [Google Scholar]
- 30. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004. September 23;351(13):1296–305. [DOI] [PubMed] [Google Scholar]
- 31. Badheka AO, Rathod A, Kizilbash MA, Garg N, Mohamad T, Afonso L, et al. Influence of obesity on outcomes in atrial fibrillation: yet another obesity paradox. Am J Med. 2010. July;123(7):646–51. 10.1016/j.amjmed.2009.11.026 [DOI] [PubMed] [Google Scholar]
- 32. Ardestani A, Hoffman HJ, Cooper HA. Obesity and outcomes among patients with established atrial fibrillation. Am J Cardiol. 2010. August 1;106(3):369–73. 10.1016/j.amjcard.2010.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang J, Yang YM, Zhu J, Zhang H, Shao XH, Tian L, et al. Overweight is associated with improved survival and outcomes in patients with atrial fibrillation. Clin Res Cardiol. 2014. July;103(7):533–42. 10.1007/s00392-014-0681-7 [DOI] [PubMed] [Google Scholar]
- 34. Overvad TF, Rasmussen LH, Skjoth F, Overvad K, Lip GY, Larsen TB. Body mass index and adverse events in patients with incident atrial fibrillation. Am J Med. 2013. July;126(7):640.e9–17. [DOI] [PubMed] [Google Scholar]
- 35. Hamatani Y, Ogawa H, Uozumi R, Iguchi M, Yamashita Y, Esato M, et al. Low Body Weight Is Associated With the Incidence of Stroke in Atrial Fibrillation Patients- Insight From the Fushimi AF Registry. Circ J. 2015. April 24;79(5):1009–17. 10.1253/circj.CJ-14-1245 [DOI] [PubMed] [Google Scholar]
- 36. Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX, et al. Long-Term Effect of Goal Directed Weight Management in an Atrial Fibrillation Cohort: A Long-term Follow-Up StudY (LEGACY Study). J Am Coll Cardiol. 2015. March 26;65(20):2159–69. 10.1016/j.jacc.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 37. Kakkar AK, Mueller I, Bassand JP, Fitzmaurice DA, Goldhaber SZ, Goto S, et al. Risk profiles and antithrombotic treatment of patients newly diagnosed with atrial fibrillation at risk of stroke: perspectives from the international, observational, prospective GARFIELD registry. PLoS One. 2013;8(5):e63479 10.1371/journal.pone.0063479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010. July;123(7):638–45 e4. 10.1016/j.amjmed.2009.11.025 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.