Abstract
We characterized 12 clinical isolates of Klebsiella oxytoca with the extended-spectrum β-lactamase (ESBL) phenotype (high minimum inhibitory concentration [MIC] values of ceftriaxone) recovered over 9 months at a university hospital in Japan. To determine the clonality of the isolates, we used pulsed-field gel electrophoresis (PFGE), multi-locus sequence typing (MLST), and PCR analyses to detect bla RBI, which encodes the β-lactamase RbiA, OXY-2-4 with overproduce-type promoter. Moreover, we performed the isoelectric focusing (IEF) of β-lactamases, and the determination of the MICs of β-lactams including piperacillin/tazobactam for 12 clinical isolates and E. coli HB101 with pKOB23, which contains bla RBI, by the agar dilution method. Finally, we performed the initial screening and phenotypic confirmatory tests for ESBLs. Each of the 12 clinical isolates had an identical PFGE pulsotype and MLST sequence type (ST9). All 12 clinical isolates harbored identical bla RBI. The IEF revealed that the clinical isolate produced only one β-lactamase. E. coli HB101 (pKOB23) and all 12 isolates demonstrated equally resistance to piperacillin/tazobactam (MICs, >128 μg/ml). The phenotypic confirmatory test after the initial screening test for ESBLs can discriminate β-lactamase RbiA-producing K. oxytoca from β-lactamase CTX-M-producing K. oxytoca. Twelve clinical isolates of K. oxytoca, which were recovered from an outbreak at one university hospital, had identical genotypes and produced β-lactamase RbiA that conferred resistance to piperacillin/tazobactam. In order to detect K. oxytoca isolates that produce RbiA to promote research concerning β-lactamase RbiA-producing K. oxytoca, the phenotypic confirmatory test after the initial screening test for ESBLs would be useful.
Introduction
Klebsiella oxytoca, a member of the Enterobacteriaceae, is a Gram-negative opportunistic pathogen that causes pneumonia, bacteraemia, urinary tract infections, and enterocolitis [1, 2]. The chromosome of K. oxytoca typically encodes a class A β-lactamase designated OXY (previously called K1 or KOXY) [3]. K. oxytoca strains, which overproduce OXY due to a point mutation in the promoter region that confers resistance to broad-spectrum β-lactams, aztreonam (ATM) as well as to β-lactamase inhibitors, were reported approximately 24 years ago [4–10]. There are recent reports of K. oxytoca isolates that produce plasmid-encoded β-lactamases, including extended-spectrum β-lactamases (ESBLs) and carbapenemases [11–14]. A recent nosocomial outbreak caused by K. pneumoniae carbapenemase (KPC)-producing K. oxytoca isolates was reported as well [15, 16].
Although research has focused on carbapenemase-producing K. oxytoca isolates, K. oxytoca strains that produce ESBLs or overproduce OXY must not be overlooked. The β-lactamase OXY group comprises the OXY-1, OXY-2, OXY-3, OXY-4, OXY-5 and OXY-6 subgroups [17–19]. Strains that overproduce the chromosomally encoded β-lactamase OXY are resistant to all β-lactamase inhibitors [9, 20, 21]. For example, we earlier reported that, in Japan, a variant of OXY with an overproduce-type promoter that drives the expression of the β-lactamase, RbiA (accession number D84548, OXY-2-4), shows resistance to β-lactamase inhibitors [20]. The combination of piperacillin, a penicillin antibiotic, and tazobactam, a β-lactamase inhibitor (TZP), is now widely used in Japan, because most Klebsiella species are susceptible to TZP [22].
We experienced an outbreak caused by K. oxytoca with the ESBL phenotype (high minimum inhibitory concentration [MIC] value of ceftriaxone [CRO]) at a university hospital in Japan. Here, we report the characterization of clinical isolates of K. oxytoca derived from this outbreak over a period of 9 months.
Materials and Methods
Ethics statement
We used clinical information concerning clinical isolates analyzed in this study. All the clinical information was approved by the ethical committee of the Aichi Medical University Graduate School of Medicine.
Clinical information
This outbreak was declared in June 2009 and containment of the outbreak was declared in December 2010. The outbreak has been ended by enforcing strict hand hygiene, strict contact precaution and promotion of antimicrobial stewardship. K. oxytoca clinical isolates NUBL-1520, 1521, 1522, 1523, 1524, 1525, 1526, 1527, 1528, 1529, 1530, and 1531 were recovered from 8 different patients at one university hospital in Aichi, Japan from 2009 June to 2010 February (Table 1). All the patients were inpatients, admitted at the identical ward of neurosurgery, for various operations. The outcomes of all the patients were survival or change of hospital.
Table 1. Clinical information concerning Klebsiella oxytoca NUBL1520-1531.
| Clinical isolates | Patient | Age (yr.) | Sex | Isolation date (mo./day/yr.) | Specimen | Underlying disease | Judgment of infection | Treatment for infection |
|---|---|---|---|---|---|---|---|---|
| NUBL1520 | Patient A | 72 | F | 6/5/2009 | Sputum | Hypertension, diabetes | Colonization | N.A. |
| NUBL1521 | Patient A | 6/9/2009 | IHC | Colonization | N.A. | |||
| NUBL1522 | Patient B | 56 | M | 7/4/2009 | Urine | Hypertension | Colonization | N.A. |
| NUBL1523 | Patient C | 75 | F | 7/22/2009 | Urine | Diabetes | Colonization | N.A. |
| NUBL1524 | Patient C | 7/22/2009 | Sputum | Colonization | N.A. | |||
| NUBL1525 | Patient B | 8/17/2009 | Sputum | Colonization | N.A. | |||
| NUBL1526 | Patient D | 46 | M | 9/25/2009 | Pus | N.A. | PSSTI | MEPM |
| NUBL1527 | Patient E | 56 | M | 9/24/2009 | Sputum | N.A. | Colonization | N.A. |
| NUBL1528 | Patient F | 37 | M | 9/28/2009 | Sputum | N.A. | Pneumonia | DRPM |
| NUBL1529 | Patient G | 17 | F | 9/28/2009 | Sputum | N.A. | Pneumonia | DRPM |
| NUBL1530 | Patient H | 73 | F | 2/15/2010 | Urine | Hypertension, diabetes | Colonization | N.A. |
| NUBL1531 | Patient H | 2/15/2010 | Sputum | Pneumonia | MEPM |
Abbreviations: F, female; M, male; IHC, intravenous hyperalimentation catheter; N.A., not applicable; PSSTI, postoperative skin and soft-tissue infection; MEPM, meropenem; DRPM, doripenem.
Clinical isolates
NUBL-1521 was isolated from an intravenous hyperalimentation catheter, NUBL-1522, 1523 and 1530 were isolated from urine samples and NUBL-1526 was isolated from pus. All other isolates were recovered from sputum (Table 1).
Plasmid vectors
The plasmid pKOB23 [20] harbors the bla RBI gene of K. oxytoca SB23, which is carried by the pMK16 cloning vector.
Reagents
Ampicillin (AMP) and cefotaxime (CTX) were purchased from Wako Pure Chemical Industries, LTD. Piperacillin (PIP) and tazobactam were purchased from LKT Laboratories, Inc. Imipenem (IPM) was purchased from Ark Pharm. The disks used for Screening and Confirmatory Tests for ESBLs contained the antibiotics as follows: cefpodoxime (CPD), ATM, CRO, ceftazidime (CAZ), and CTX disks were purchased from Becton, Dickinson and Company. Clavulanic acid (CLA) was purchased from Wako Pure Chemical Industries, LTD.
Pulsed-field gel electrophoresis (PFGE)
Plugs were prepared using suspensions of clinical isolates with an optical density of 0.8; these plugs had treated with 2 mg/ml of lysozyme solution at 37°C for 6 h and 1 mg/ml of proteinase K solution at 55°C for 8 h. The digested plugs were incubated with XbaI (Takara). We performed PFGE for 24 h using a CHEF-DR III System (BioRad). Gels were stained with 0.5 μg/ml of ethidium bromide for 1 h.
Multi-locus sequence typing (MLST)
We performed MLST analysis of the K. oxytoca isolates as described previously [23]. We isolated chromosomal DNA using a Wizard Genomic DNA Purification Kit (Promega). The seven housekeeping genes were amplified using PCR with the high-fidelity PrimeSTAR HS DNA polymerase (Takara). Nucleotide sequences were determined using an Applied Biosystems 3130xl Genetic Analyzer or an Applied Biosystems 3730xl DNA Analyzer and BigDye Terminator V3.1. We determined the sequence type (ST) using the K. oxytoca MLST website (http://pubmlst.org/koxytoca/).
PCR detection of β-lactamase RbiA gene
We performed the chromosomal DNA isolation from K. oxytoca NUBL-1520, 1521, 1522, 1523, 1524, 1525, 1526, 1527, 1528, 1529, 1530 and 1531, using Wizard Genomic DNA Purification Kit (Promega). We performed PCR reaction using the purified chromosomal DNA as templates, high fidelity DNA polymerase, PrimeSTAR HS DNA polymerase (Takara), and previously described primers, OXY-383 and OXY-S [7]. The nucleotide sequences of the amplicons were determined as described above.
Isoelectric focusing (IEF) of β-lactamases
To extract β-lactamases from the clinical isolate NUBL-1520, we performed a freeze-thaw procedure [24] and subjected the resulting supernatant to IEF using an Invitrogen system. IEF was conducted for 1 h at 100 V, 2 h at 200 V and 30 min at 500 V. The β-lactamase in the gel was detected using 0.05% nitrocefin solution [25].
Determination of MICs
The MICs of AMP, PIP, TZP, CTX, and IPM were determined according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) using the agar dilution method [26]. E. coli ATCC 25922 and E. coli ATCC 35218 strains served as controls.
Screening and Confirmatory Tests for ESBLs
We performed the disk diffusion method recommended by CLSI called the Screening and Confirmatory Tests for ESBLs [26]. In the Initial Screen Test, we used CPD, and CRO, and ATM disks. For K. oxytoca, the breakpoints of the CPD zone, CRO zone, and ATM zone are ≤17 mm, ≤25 mm, and ≤27 mm, respectively. According to the CLSI, “Zones above may indicate ESBL production.” In Phenotypic Confirmatory Test, we used CAZ, CAZ-CLA, CTX, and CTX-CLA disks. Confirmatory testing requires the use of both CAZ and CTX, alone and in combination with CLA. According to the CLSI, “a ≥5 mm increase in the zone diameter for either antimicrobial agent tested in combination with CLA vs. its zone when tested alone = ESBL.” We used NUBL-793 and 810, which have been already confirmed as β-lactamase CTX-M-producing K. oxytoca.
Results
MICs at a clinical setting
The MICs for the 12 clinical isolates of K. oxytoca determined at a microbiological laboratory of a university hospital are shown in Table 2. All isolates were resistant to cefazolin, cefotiam, and CRO. At first, the laboratory technicians missed the high MIC values of sultamicillin and cefoperazone/sulbactam. Therefore, they suspected these clinical isolates as ESBL-producing K. oxytoca, because of their high MIC values of CRO.
Table 2. MIC values of K. oxytoca NUBL1520-1531 determined at a microbiological laboratory of a university hospital.
| Clinical isolates | MIC [μg/ml] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SBTPC | CFZ | CTM | CFP/SUL | CAZ | CRO | CZOP | CFPN | IPM | LVX | FOF | SXT | |
| NUBL1520 | >32 | >16 | 16 | >16/16 | ≤0.5 | 32 | 32 | ≤1 | ≤0.5 | 4 | 128 | ≤0.25/4.75 |
| NUBL1521 | >32 | >16 | >32 | >16/16 | 2 | >32 | 32 | 2 | ≤0.5 | >4 | >128 | ≤0.25/4.75 |
| NUBL1522 | >32 | >16 | 32 | >16/16 | 2 | 32 | 8 | ≤1 | ≤0.5 | >4 | 128 | ≤0.25/4.75 |
| NUBL1523 | >32 | >16 | 16 | >16/16 | 1 | >32 | >32 | ≤1 | ≤0.5 | >4 | >128 | ≤0.25/4.75 |
| NUBL1524 | >32 | >16 | 16 | >16/16 | 1 | >32 | >32 | ≤1 | ≤0.5 | >4 | >128 | ≤0.25/4.75 |
| NUBL1525 | >32 | >16 | 16 | >16/16 | 1 | 32 | >32 | ≤1 | ≤0.5 | >4 | >128 | ≤0.25/4.75 |
| NUBL1526 | >32 | >16 | 32 | >16/16 | 1 | 16 | >32 | ≤1 | ≤0.5 | >4 | >128 | ≤0.25/4.75 |
| NUBL1527 | >32 | >16 | >32 | >16/16 | 1 | >32 | >32 | ≤1 | ≤0.5 | >4 | 128 | ≤0.25/4.75 |
| NUBL1528 | >32 | >16 | >32 | >16/16 | 4 | >32 | 32 | >8 | ≤0.5 | 4 | >128 | ≤0.25/4.75 |
| NUBL1529 | >32 | >16 | >32 | >16/16 | 1 | >32 | >32 | ≤1 | ≤0.5 | >4 | 128 | ≤0.25/4.75 |
| NUBL1530 | >32 | >16 | 32 | >16/16 | 1 | 16 | ≤1 | ≤1 | ≤0.5 | >4 | >128 | ≤0.25/4.75 |
| NUBL1531 | >32 | >16 | 32 | >16/16 | 1 | 16 | ≤1 | ≤1 | ≤0.5 | >4 | >128 | ≤0.25/4.75 |
Abbreviations: MIC, minimum inhibitory concentration; SBTPC, sultamicillin; CFZ, cefazolin; CTM, cefotiam; CFP, cefoperazone; SUL, sulbactam; CAZ, ceftazidime; CRO, ceftriaxone; CZOP, cefozopran; CFPN, cefcapene; IPM, imipenem; LVX, levofloxacin; FOF, fosfomycin; SXT, trimethoprim-sulfamethoxazole.
PFGE analysis
All clinical isolates exhibited the identical pulsotype (Fig 1), suggesting that they possessed identical genotypes, which indicates that the outbreak was caused by the same clinical isolate.
Fig 1. Pulsed-field gel electrophoresis (PFGE) analysis of 12 clinical isolates of K. oxytoca.
M, size markers.
MLST
All 12 clinical isolates were ST9, indicating that they possessed the identical genotype.
PCR detection of the β-lactamase RbiA gene
Because of the high MIC values of sultamicillin and cefoperazone/sulbactam and our previous findings that the β-lactamase RbiA confers resistance to β-lactamase inhibitors upon K. oxytoca [20], we performed PCR and nucleotide sequence analyses to detect bla RBI and found the bla RBI sequences of all isolates were identical (Accession Number D84548), including the –35 and –10 regions, the Shine-Dalgarno sequence, and the coding region. This supports the clonal origin of the 12 clinical isolates.
IEF analysis of β-lactamases
To determine the number of β-lactamases produced by the clinical isolates, we performed IEF (Fig 2). A single band was detected at pH 5.6, suggesting that NUBL1520 produces ‘only one’ β-lactamase and supporting that only one β-lactamase produced by NUBL1520 is β-lactamase RbiA [20, 27].
Fig 2. Isoelectric focusing (IEF) of the β-lactamase of clinical isolate NUBL1520.
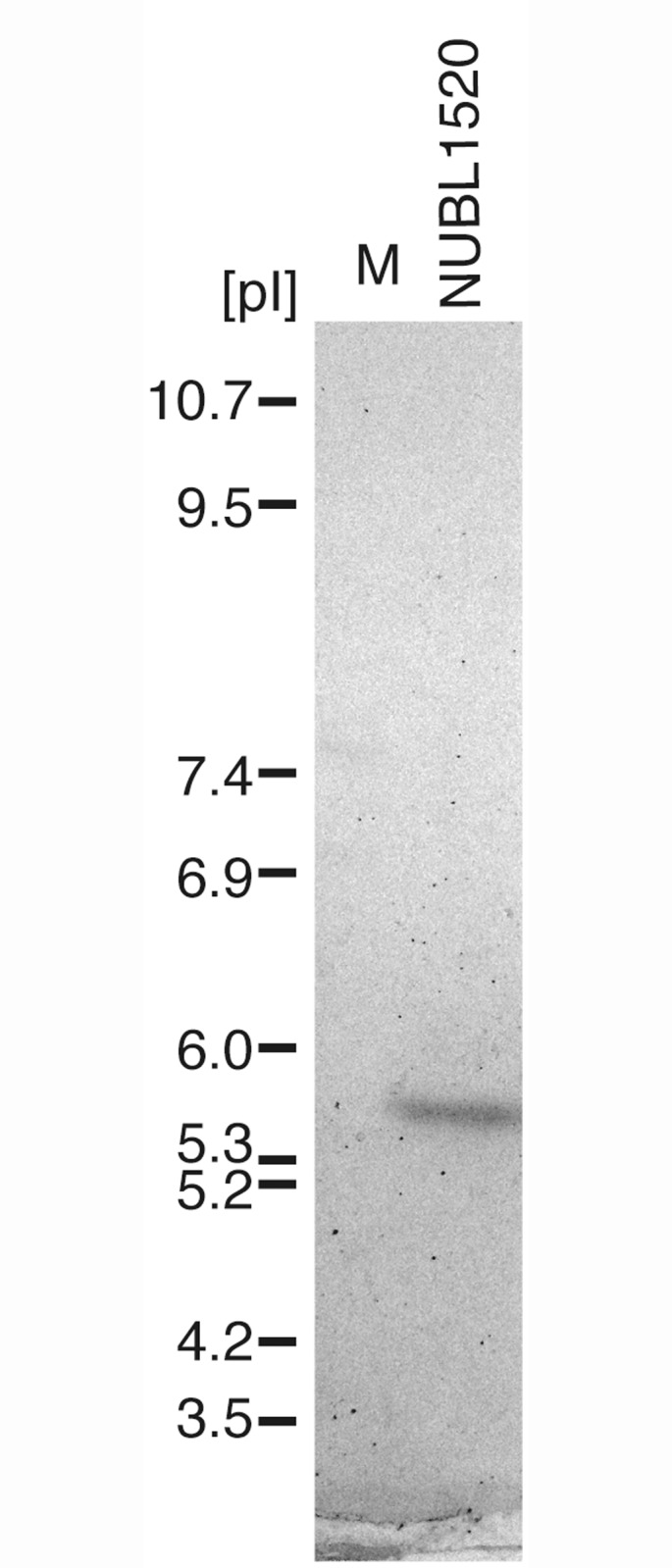
M, pI markers.
Determination of MICs
Although the wide use of TZP started recently in Japan and there are a few reports concerning TZP resistant K. oxytoca that produce OXY-2 type β-lactamase [12, 28], it remained to be determined whether K. oxytoca strains that produce RbiA are resistant to TZP. Therefore, we determined the MICs of five β-lactams, including TZP, for the 12 clinical isolates and E. coli HB101 (pKOB23) that harbors bla RBI. All isolates were resistant to AMP, PIP and TZP, exhibited intermediate or resistance to CTX and were susceptible to IPM (Table 3). E. coli HB101 (pKOB23) and all 12 isolates demonstrated equally resistance to TZP (MICs, >128 μg/ml), and E. coli HB101 (pMK16) showed susceptibility to TZP (MIC, 1 μg/ml), suggesting that β-lactamase RbiA confers to resistance to TZP in 12 clinical isolates of K. oxytoca.
Table 3. MIC values of β-lactams for K. oxytoca NUBL1520-1531, E. coli HB101 (pKOB23), and E. coli HB101 (pMK16) determined using the agar dilution method.
| Clinical isolates, Strains | MICs [μg/ml] | ||||
|---|---|---|---|---|---|
| AMP | PIP | TZP | CTX | IPM | |
| NUBL 1520 | >128 | >128 | >128 | 8 | 0.5 |
| NUBL 1521 | >128 | >128 | >128 | 16 | 1 |
| NUBL 1522 | >128 | >128 | >128 | 4 | 0.25 |
| NUBL 1523 | >128 | >128 | >128 | 4 | 0.12 |
| NUBL 1524 | >128 | >128 | >128 | 4 | 0.12 |
| NUBL 1525 | >128 | >128 | >128 | 2 | 0.12 |
| NUBL 1526 | >128 | >128 | >128 | 2 | 0.12 |
| NUBL 1527 | >128 | >128 | >128 | 4 | 0.5 |
| NUBL 1528 | >128 | >128 | >128 | 4 | 0.25 |
| NUBL 1529 | >128 | >128 | >128 | 4 | 0.25 |
| NUBL 1530 | >128 | >128 | >128 | 2 | 0.12 |
| NUBL 1531 | >128 | >128 | >128 | 4 | 0.25 |
| E. coli HB101 (pKOB23) | >128 | >128 | >128 | 8 | 0.25 |
| E. coli HB101 (pMK16) | 8 | 4 | 1 | 0.06 | 0.25 |
Abbreviations: MIC, minimum inhibitory concentration; AMP, ampicillin; PIP, piperacillin; TZP, piperacillin-tazobactam; CTX, cefotaxime; IPM, imipenem.
Initial Screening and Confirmatory Tests for ESBLs
Although it was previously reported that many β-lactamase K1-overproducing K. oxytoca strains show false-positive in ESBL tests [29], no data were available indicating whether the initial screening and confirmatory tests for ESBLs recommended by CLSI detect K. oxytoca clinical isolates that produce RbiA. Therefore, we tested the clinical isolates along with the control strains K. oxytoca NUBL793 and NUBL810 that produce CTX-M. In the initial screening test, the diameters of inhibition surrounding the CPD, ATM, and CRO disks in plates containing NUBL793, NUBL810 as well as those of all clinical isolates were less than the cut-off values recommended by CLSI, suggesting that all clinical isolates may produce ESBLs (Table 4). In the phenotypic confirmatory test, NUBL793 and NUBL810 showed an obvious increase (≥5 mm) of the diameters of the CTX-CLA and CTX disks; however, none of the 12 clinical isolates showed the necessary increase of the diameters between CTX-CLA disk and CTX alone disk, and CAZ-CLA disk and CAZ alone disk (Table 5), suggesting that the phenotypic confirmatory test discriminates K. oxytoca strains that produce RbiA from those that produce CTX-M.
Table 4. Initial Screening Tests for ESBLs.
| Strains and clinical isolates | CPD 10 μg | ATM 30 μg | CRO 30 μg |
|---|---|---|---|
| NUBL793 (K. oxytoca CTX-M1) | 8 | 12 | 10 |
| NUBL810 (K. oxytoca CTX-M2) | 6 | 20 | 12 |
| NUBL1520 | 16 | 13 | 15 |
| NUBL1521 | 8 | 6 | 9 |
| NUBL1522 | 12 | 7 | 14 |
| NUBL1523 | 13 | 8 | 15 |
| NUBL1524 | 13 | 8 | 15 |
| NUBL1525 | 13 | 8 | 14 |
| NUBL1526 | 13 | 9 | 15 |
| NUBL1527 | 14 | 8 | 15 |
| NUBL1528 | 17 | 17 | 19 |
| NUBL1529 | 15 | 9 | 16 |
| NUBL1530 | 13 | 9 | 15 |
| NUBL1531 | 14 | 10 | 18 |
For K. oxytoca, the breakpoints of the CPD, CRO, and ATM zones are ≤17 mm, ≤25 mm, and ≤27 mm, respectively. According to the CLSI, “Zones above may indicate ESBL production.”
Abbreviations: ESBL, extended-spectrum β-lactamase; CPD, cefpodoxime; ATM, aztreonam; CRO, ceftriaxone.
Table 5. Phenotypic Confirmatory Tests for ESBLs.
| Clinical isolates, Strains | CAZ 30 μg | CAZ-CLA 30/10 μg | CTX 30 μg | CTX-CLA 30/10 μg |
|---|---|---|---|---|
| NUBL793 (K.oxytoca CTX-M1) | 26 | 27 | 18 | 23 |
| NUBL810 (K. oxytoca CTX-M2) | 24 | 30 | 15 | 28 |
| NUBL1520 | 27 | 27 | 22 | 24 |
| NUBL1521 | 20 | 21 | 13 | 12 |
| NUBL1522 | 21 | 20 | 21 | 21 |
| NUBL1523 | 21 | 21 | 23 | 23 |
| NUBL1524 | 21 | 21 | 21 | 22 |
| NUBL1525 | 21 | 21 | 23 | 23 |
| NUBL1526 | 23 | 23 | 23 | 22 |
| NUBL1527 | 23 | 24 | 24 | 25 |
| NUBL1528 | 28 | 30 | 26 | 27 |
| NUBL1529 | 26 | 24 | 24 | 25 |
| NUBL1530 | 22 | 22 | 23 | 23 |
| NUBL1531 | 25 | 26 | 25 | 27 |
Confirmatory testing requires the use of both CAZ and CTX, alone and in combination with CLA. According to the CLSI, “a ≥5 mm increase in the zone diameter for either antimicrobial agent tested in combination with CLA vs. its zone when tested alone = ESBL.”
Abbreviations: ESBL, extended-spectrum β-lactamase; CAZ, ceftazidime; CLA, clavulanic acid; CTX, cefotaxime.
Discussion
We show here that 12 clinical isolates of K. oxytoca, which we recovered from an outbreak at one university hospital, had identical genotypes and produced β-lactamase RbiA that conferred resistance to TZP. Moreover, we demonstrated that the phenotypic confirmatory test after the initial screening test for ESBLs recommended by CLSI is useful for discriminating K. oxytoca clinical isolates that produce RbiA from those that produce CTX-M. It has been reported previously that some ESBL-producing clinical isolates of Klebsiella spp. are resistant to TZP [30, 31], and that only 12 of 25 Enterobacteriaceae strains producing ESBLs are susceptible to TZP [32]. However, although several studies have examined ESBL-producing clinical isolates [33], the number of reports concerning K. oxytoca clinical isolates producing β-lactamase RbiA is limited. Therefore, in order to promote research on K. oxytoca clinical isolates producing β-lactamase RbiA, it is important to discriminate K. oxytoca clinical isolates that produce RbiA from ESBL-producing K. oxytoca clinical isolates and to characterize K. oxytoca clinical isolates producing β-lactamase RbiA.
TZP is often prescribed for patients treated in the hospital studied here (data not shown). It is possible that the outbreak described here was caused by selection by TZP of K. oxytoca strains that produce RbiA. Moreover, the amount of TZP prescribed in Japan may be increasing in concert with the increase in ESBL-producing Enterobacteriaceae. Therefore, it is reasonable to assume that outbreaks similar to that described here will occur again.
It is difficult to readily discriminate between ESBL-producing K. oxytoca strains and those that produce RbiA because of the ESBL-phenotype (high MIC values of CRO et al.) of the latter. However, we show here that K. oxytoca clinical isolates that produce RbiA are resistant to TZP. Moreover, in our hands, the confirmatory test after the initial screening test for ESBLs recommended by CLSI were useful for discriminating between the two K. oxytoca phenotypes. In order to detect K. oxytoca isolates that produce RbiA to promote research concerning β-lactamase RbiA-producing K. oxytoca, the phenotypic confirmatory test after the initial screening test for ESBLs would be useful.
Acknowledgments
We thank all the members of Prof. Arakawa’s Laboratory for technical advice and discussions.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by grants H21-Shinkou-Ippan-008 and H24-Shinkou-Ippan-010 from the Ministry of Health, Labour and Welfare of Japan and, in part, by a Grant-in-Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- 1. Högenauer C, Langner C, Beubler E, Lippe IT, Schicho R, Gorkiewicz G, et al. Klebsiella oxytoca as a causative organism of antibiotic-associated haemorrhagic colitis. N Engl J Med. 2006; 355: 2418–2426. [DOI] [PubMed] [Google Scholar]
- 2. Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998; 11: 589–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arakawa Y, Ohta M, Kido N, Mori M, Ito H, Komatsu T, et al. Chromosomal β-lactamase of Klebsiella oxytoca, a new class A enzyme that hydrolyzes broad-spectrum β-lactam antibiotics. Antimicrob Agents Chemother. 1989; 33: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fournier B, Arlet G, Lagrange PH, Philippon A. Klebsiella oxytoca: resistance to aztreonam by overproduction of the chromosomally encoded β-lactamase. FEMS Microbiol Lett. 1994; 116: 31–36. [DOI] [PubMed] [Google Scholar]
- 5. Fournier B, Lu CY, Lagrange PH, Krishnamoorthy R, Philippon A. Point mutation in the Pribnow box, the molecular basis of β-lactamase overproduction in Klebsiella oxytoca . Antimicrob Agents Chemother. 1995; 39: 1365–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fournier B, Lagrange PH, Philippon A. β-lactamase gene promoters of 71 clinical strains of Klebsiella oxytoca . Antimicrob Agents Chemother. 1996; 40: 460–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fournier B, Roy PH. Variability of chromosomally encoded β-lactamases from Klebsiella oxytoca . Antimicrob Agents Chemother. 1996; 41: 1641–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fournier B, Gravel A, Hooper DC, Roy PH. Strength and regulation of the different promoters for chromosomal β-lactamases of Klebsiella oxytoca . Antimicrob Agents Chemother. 1999; 43: 850–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sirot D, Labia R, Pouedras P, Chanal-Claris C, Cerceau C, Sirot J. Inhibitor-resistant OXY-2-derived β-lactamase produced by Klebsiella oxytoca . Antimicrob Agents Chemother. 1998; 42: 2184–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu SW, Dornbusch K, Kronvall G. Genetic characterization of resistance to extended-spectrum β-lactams in Klebsiella oxytoca isolates recovered from patients with septicaemia at hospitals in the Stockholm area. Antimicrob Agents Chemother. 1999; 43: 1294–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marchese A, Arlet G, Schito GC, Lagrange PH, Philippon A. Characterization of FOX-3, an AmpC-type plasmid-mediated β-lactamase from an Italian isolate of Klebsiella oxytoca . Antimicrob Agents Chemother. 1998; 42: 464–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Decré D, Burghoffer B, Gautier V, Petit JC, Arlet G. Outbreak of multi-resistant Klebsiella oxytoca involving strains with extended-spectrum β-lactamases and strains with extended-spectrum activity of the chromosomal β-lactamase. J Antimicrob. Chemother. 2004; 54: 881–888. [DOI] [PubMed] [Google Scholar]
- 13. Livermore DM, Yuan M. Antibiotic resistance and production of extended-spectrum β-lactamases amongst Klebsiella spp. from intensive care units in Europe. J Antimicrob Chemother. 1996; 38: 409–424. [DOI] [PubMed] [Google Scholar]
- 14. Yigit H, Queenan AM, Rasheed JK, Biddle JW, Domenech-Sanchez A, Alberti S, et al. Carbapenem-resistant strain of Klebsiella oxytoca harboring carbapenem-hydrolysing β-lactamase KPC-2. Antimicrob Agents Chemother. 2003; 47: 3881–3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoenigl M, Valentin T, Zarfel G, Wuerstl B, Leitner E, Salzer HJ, et al. Nosocomial outbreak of Klebsiella pneumoniae carbapenemase-producing Klebsiella oxytoca in Austria. Antimicrob Agents Chemother. 2012; 56: 2158–2161. 10.1128/AAC.05440-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leitner E, Zarfel G, Luxner J, Herzog K, Pekard-Amenitsch S, Hoenigl M, et al. Contaminated handwashing sinks as the source of a clonal outbreak of KPC-2-producing Klebsiella oxytoca on a hematology ward. Antimicrob Agents Chemother. 2015; 59: 714–716. 10.1128/AAC.04306-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fournier B, Roy PH, Lagrange PH, Philippon A. Chromosomal β-lactamase genes of Klebsiella oxytoca are divided into two main groups, bla OXY-1 and bla OXY-2 . Antimicrob Agents Chemother. 1996; 40: 454–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Granier SA, Leflon-Guibout V, Goldstein FW, Nicolas-Chanoine MH. New Klebsiella oxytoca β-lactamase genes bla OXY-3 and bla OXY-4 and a third genetic group of K. oxytoca based on bla OXY-3 . Antimicrob Agents and Chemother. 2003; 47: 2922–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fevre C, Jbel M, Passet V, Weill FX, Grimont PA, Brisse S. Six groups of the OXY β-lactamase evolved over millions of years in Klebsiella oxytoca . Antimicrob Agents Chemother. 2005; 49: 3453–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kimura K, Arakawa Y, Ohsuka S, Ito H, Suzuki K, Kurokawa H, et al. Molecular aspects of high-level resistance to sulbactam-cefoperazone in Klebsiella oxytoca clinical isolates. Antimicrob Agents Chemother. 1996; 40:1988–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Livermore DM. β-Lactamases in laboratory and clinical resistance. Clin Microbiol Rev. 1995; 8: 557–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen HY, Bonfiglio G, Allen M, Piper D, Edwardson T, McVey D, et al. Multicentre survey of the in-vitro activity of piperacillin/tazobactam against bacteria from hospitalized patients in the British Isles. J Antimicrob Chemother. 1993; 32: 247–266. [DOI] [PubMed] [Google Scholar]
- 23. Herzog KA, Schneditz G, Leitner E, Feieri G, Hoffmann KM, Zollner-Schwetz I, et al. Genotypes of Klebsiella oxytoca isolates from patients with nosocomial pneumonia are distinct from those of isolates from patients with antibiotic-associated haemorrhagic colitis. J Clin Microbiol. 2014; 52:1607–1616. 10.1128/JCM.03373-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sykes RB, Bonner DP, Bush K, Georgopapadakou NH. Azthreonam (SQ 26,776), a synthetic monobactam specifically active against aerobic gram-negative bacteria. Antimicrob Agents Chemother. 1982; 21: 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu SW, Dornbusch K, Kronvall G, Norgren M. Characterization and nucleotide sequence of a Klebsiella oxytoca cryptic plasmid encoding a CMY-type β-lactamase: confirmation that the plasmid-mediated cephamycinase originated from the Citrobacter freundii AmpC β-lactamase. Antimicrob Agents Chemother. 1999; 43: 1350–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twentieth Informational Supplement M100-S20. Wayne, PA: Clinical and Laboratory Standards Institute; 2010. [Google Scholar]
- 27. Matthew M, Harris AM. Identification of β-lactamases by analytical isoelectric focusing: correlation with bacterial taxonomy. J Gen Microbiol. 1976; 94: 55–67. [DOI] [PubMed] [Google Scholar]
- 28. Zárate MS, Gales AC, Picão RC, Pujol GS, Lanza A, Smayevsky J. Outbreak of OXY-2-producing Klebsiella oxytoca in a renal transplant unit. J Clin Microbiol. 2008; 46: 2099–2101. 10.1128/JCM.00194-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Potz NAC, Colman M, Warner M, Reynolds R, Livermore DM. False-positive extended-spectrum β-lactamase test for Klebsiella oxytoca strains hyperproducing K1 β-lactamase. J Antimicrob Chemother. 2004; 53: 545–547. [DOI] [PubMed] [Google Scholar]
- 30. Edelstein M, Pimkin M, Palagin I, Edelstein I, Stratchounski L. Prevalence and molecular epidemiology of CTX-M extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in Russian hospital. Antimicrob Agents Chemother. 2003; 47: 3724–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thomson KS, Moland ES. Cefepime, piperacillin-tazobactam, and the inoculum effect in tests with extended-spectrum β-lactamase-producing Enterobacteriaceae . Antimicrob Agents Chemother. 2001; 45: 3548–3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li H, Estabrook M, Jacoby GA, Nichols WW, Testa RT, Bush K. In vitro susceptibility of characterized β-lactamase-producing strains tested with avibactam combinations. Antimicrob Agents Chemother. 2015; 59: 1789–1793. 10.1128/AAC.04191-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pitout JD, Laupland KB. Extended-spectrum β-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008; 8: 159–166. 10.1016/S1473-3099(08)70041-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.



