Abstract
Background
Recent studies suggest atrial fibrillation (AF) is an independent risk factor for coronary heart disease (CHD).
Aims
To determine if alterations in hemostasis or inflammation explain the association between AF and CHD.
Methods
C-reactive protein (CRP), D-dimer, factor VIII, and fibrinogen were measured in incident CHD cases (n=647) and a stratified cohort random sample (CRS, n=1,104) between 2003 and 2007 from the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Using a case-cohort approach, Cox models examined whether inflammation or hemostasis biomarkers explained the association between AF and CHD.
Results
In participants free of CHD at baseline, 12.2% of CHD cases and 7.1% of the CRS had AF. Over a median follow-up of 4.4 years, all biomarkers were associated with an increased risk of CHD in those with and those without AF after adjusting for CHD risk factors. The association of D-dimer with CHD was greater in those with AF (HR 2.52, 95% CI=1.49, 4.26) than those without AF (HR 1.34, 95% CI=1.12, 1.61) (p-interaction=0.02). Similar interactions were not observed for the other biomarkers.
Conclusions
Our results suggest that alterations in D-dimer, a marker of hemostasis, explain the association between AF and CHD. Potentially, D-dimer is a useful biomarker to assess CHD risk in persons with AF.
Keywords: atrial fibrillation, coronary disease, biological markers
INTRODUCTION
Atrial fibrillation (AF) affects approximately 3 million Americans and the prevalence is projected to increase to ~5.6 million over the next 30 years.1,2 For unknown reasons, AF recently has been implicated as an independent risk factor for cardiovascular mortality and fatal and non-fatal coronary heart disease (CHD).3–7
Increased levels of inflammation and hemostasis biomarkers are associated with AF and CHD.8–12 Persons with AF who have increased fibrin-fragment D-dimer (D-dimer) levels have a higher risk of stroke and CHD events.8–10 Similarly, increased levels of circulating C-reactive protein (CRP) are associated with an increased risk of stroke, vascular events, and mortality in persons with AF.11,12 Potentially, alterations in inflammation or hemostasis explain the increased CHD risk in those with AF.
Using data from the REasons for Geographic And Racial Differences in Stroke (REGARDS) study, we examined whether CRP, D-dimer, factor VIII, or fibrinogen were associated with CHD in individuals with AF and whether these biomarkers modified the association between AF and CHD. We hypothesized that the CHD risk in persons with AF would either be modified or dependent on elevated biomarkers of inflammation or hemostasis. For this analysis, we assumed that D-dimer and factor VIII were representative biomarkers of hemostasis, and that CRP and fibrinogen were markers of inflammation.13 By understanding what drives the increased CHD risk in AF, future investigators will be able to develop strategies to assess CHD risk and reduce the burden of CHD in persons with AF.
METHODS
Study Population
REGARDS is a prospective cohort study designed to identify causes of regional and racial disparities in stroke mortality in the contiguous United States. Between January 2003 and October 2007, a total of 30,239 participants were recruited by over-sampling blacks and residents of the southeastern stroke belt region (North Carolina, South Carolina, Georgia, Alabama, Mississippi, Tennessee, Arkansas, and Louisiana). Demographic information, medical history, and oral informed consent were obtained using a computer-assisted telephone interview followed by an in-home visit for written informed consent, physical examination, and fasting phlebotomy 3 to 4 weeks after the telephone interview. The study received institutional review board approval from all participating institutions. Details of the study design have been published previously.14
We examined whether inflammatory or hemostasis biomarkers were associated with CHD in individuals with AF and whether these biomarkers modified the association between AF and CHD. Participants with AF were identified at baseline and followed for subsequent CHD events. CRP, D-dimer, factor VIII, and fibrinogen were measured using a stratified case-cohort design.15 This analysis included all CHD cases (n=647) occurring between 2003 and 2009 (December 31, 2009). These participants were followed at 6 month intervals by telephone for the detection of incident CHD events, including possible cases. In addition, a cohort random sample (CRS; n=1,104) was selected and stratified to ensure representation across age, race, and sex.16 Of these, 224 individuals with prevalent CHD at baseline were excluded and 883 participants remained in the CRS.
Coronary Heart Disease Events
CHD was defined as the composite of definite and probable myocardial infarction (MI) and definite and probable acute cardiac death. Events were adjudicated by an expert panel and details of the CHD adjudication process in REGARDS have been described.17 Cases were assigned to 2 independent adjudicators and disagreements were adjudicated by committee review. The test for agreement between adjudicators yielded a κ level >0.80 for the presence of definite or probable MI and definite or probable acute cardiac death.
Guided by current expert opinion, medical records for suspected cases of MI were obtained and reviewed for the presence of the following: presence of signs or symptoms suggestive of ischemia; a rising and/or falling pattern in cardiac enzyme levels (troponin or CK-MB) over 6 or more hours with a peak level ≥2 times the upper limit of normal; and electrocardiogram changes consistent with ischemia or MI, guided by the Minnesota code and classified as evolving diagnostic, positive, nonspecific, or not consistent with ischemia.18,19 Definite cases of MI included those with diagnostic enzymes or electrocardiogram. Probable MI included cases with elevated but not diagnostic enzymes with a positive but non-diagnostic electrocardiogram, or a positive electrocardiogram in the presence of ischemic signs or symptoms if cardiac enzyme data were missing.
Acute cardiac death was ascertained using the following sources of data: interviews with proxies or next of kin about circumstances immediately prior to death, autopsy reports, hospital records, death certificates, or National Death Index data. For hospitalized events, the underlying cause was definite or probable if the death occurred within 28 days of hospital admission in definite or probable MI cases; postmortem findings were consistent with MI within 28 days; or the death occurred within 6 hours of hospital admission with symptoms and/or signs consistent with cardiac etiology and other confirmatory data (e.g., cardiac enzymes or electrocardiographic data). For non-hospitalized events, the cause of death was definite or probable acute CHD if the death included one of the following: sudden cardiac death; documented definite or probable MI within 28 days of death and no evidence of a non-coronary etiology; autopsy evidence of recent coronary occlusion or MI within 28 days of death; history of CHD and/or documented cardiac pain within 72 hours preceding death and no evidence of a non-coronary etiology; or autopsy evidence of chronic CHD (e.g., coronary atherosclerosis and myocardial scarring).
Laboratory
Blood was collected during the in-home examination after an 8- to 10-hour fast and sample processing and validation of the laboratory data have been reported.20 Fasting morning blood samples were obtained and after centrifugation, serum, plasma, and the cell layer were shipped overnight with 2 frozen gel ice packs to the University of Vermont Laboratory for Clinical Biochemistry Research. Upon receipt, samples were catalogued and re-centrifuged at 4 °C for 30,000 g-minutes, then stored at -80 °C. The following analytes were used in this analysis: total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, fasting glucose, CRP, D-dimer, factor VIII, fibrinogen, serum creatinine, and urinary albumin and creatinine from a spot urine specimen.20,21
Covariates
Age, sex, race (black or white), smoking status, and region of residence were self-reported. Smoking was defined as current, never, or former. Diabetes was classified as present if the fasting glucose level was ≥126 mg/dL (or non-fasting glucose ≥200 mg/dL in those failing to fast) or if participants reported the use of insulin or oral hypoglycemic medications. The current use of aspirin, statins, warfarin, and antihypertensive and lipid-lowering medications was self-reported. Body mass index was computed as the weight in kilograms divided by the square of the height in meters. After each participant rested for 5 minutes in a seated position, blood pressure was measured using a sphygmomanometer. Two values were obtained following a standardized protocol and averaged. Baseline renal disease was assessed by serum creatinine measurements and estimated glomerular filtration rate (eGFR) was estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. eGFR was dichotomized at 60 mL/min per 1.73 m2. Additionally, urine albumin-to-creatinine ratio (ACR) was calculated. Baseline cardiovascular disease was confirmed by self-reported history of stroke or peripheral arterial disease. AF was identified at baseline by the study-scheduled electrocardiogram recorded during the in-home visit and also from participant self-reported history during the computer-assisted telephone survey.22
Statistics
Biomarkers with a skewed distribution by visual inspection (CRP and D-dimer) were log-transformed for all analyses. Categorical variables were reported as frequency and percentage while continuous variables were recorded as mean ± standard deviation (SD), accounting for sample weighting. Statistical significance for categorical variables was tested using the chi-square procedure and the student’s t-test for continuous variables. Weighted linear regression was used to determine if CRP, D-dimer, factor VIII, or fibrinogen were associated with baseline AF. Cox regression was used to compute hazard ratios (HR) with robust sandwich estimators to compute the 95% confidence intervals (CI) for the association between AF and CHD. Model 1 included basic demographics (age, sex, race, region of residence, education, and income). Model 2 included Model 1 covariates plus smoking, total cholesterol, HDL cholesterol, systolic blood pressure, diabetes, cardiovascular disease, aspirin, statins, warfarin, and antihypertensive medications. A sensitivity analysis was performed with markers of renal dysfunction (eGFR <60 mL/min per 1.73 m2 and log ACR) and inflammation (CRP) to ensure these factors did not alter the relationship between AF and CHD. We then individually added each hemostasis or inflammation biomarker to the model and calculated the percent change in the HR for CHD. If the percent change was ≥10% in the main analysis, a formal mediation analysis was performed. To further explore CHD risk in those with AF, interactions between AF and each biomarker were tested using interaction terms. Subgroup analyses stratified by race and sex also were performed. Statistical significance was defined as p <0.05. SAS Version 9.3 (Cary, NC) was used for all analyses. In this analysis, participants were excluded if they had baseline CHD, missing AF data, or missing biomarker data. Participants lost to follow-up were censored at last contact.
RESULTS
After excluding 10 participants with missing baseline data regarding AF and CHD, 12.2% (79/647) of CHD cases and 7.2% (weighted, 63/883) of the CRS had AF. Baseline characteristics of the CHD group and the CRS are shown in Table 1.
Table 1.
Baseline Characteristics
| Characteristic | CHD Cases (n=647)
|
P-value* | Cohort Random Sample (n=883)
|
P-value* | ||
|---|---|---|---|---|---|---|
| AF (n=79) | No AF (n=568) | AF (n= 63 | 1741) | No AF (n= 820 | 22,419) | |||
| Age, mean (SD), years | 71 (10) | 68 (9) | 0.002 | 65 (9) | 64 (9) | 0.56 |
| Male (%) | 35 (44) | 365 (64) | <0.001 | 19 | 384 (22) | 392 | 966 (43) | 0.02 |
| Black (%) | 36 (46) | 247 (43) | 0.73 | 32 | 764 (44) | 414 | 9497 (39) | 0.04 |
| Region | ||||||
| Stroke belt (%) | 30 (38) | 230 (40) | 32 | 977 (56) | 282 | 7673 (34) | ||
| Stroke buckle (%) | 11 (14) | 97 (17) | 6 | 199 (11) | 144 | 3983 (18) | ||
| Rest of nation (%) | 38 (48) | 241 (42) | 0.59 | 25 | 6565 (32) | 394 |10,762 (48) | 0.02 |
| Body mass index, mean (SD), kg/m2 | 30.7 (7) | 29.1 (6) | 0.07 | 29.0 (6) | 29.3 (6) | 0.74 |
| Smoking | ||||||
| Current (%) | 6 (8) | 126 (22) | 10 | 260 (15) | 116 | 2967 (13) | ||
| Never (%) | 37 (47) | 210 (37) | 26 | 687 (39) | 417 | 11,430 (51) | ||
| Former (%) | 36 (46) | 232 (41) | 0.10 | 27 | 794 (46) | 286 | 7961 (36) | 0.30 |
| Diabetes (%) | 24 (32) | 175 (33) | 0.89 | 18 | 672 (39) | 155 | 4214 (20) | 0.003 |
| Systolic blood pressure, mean (SD), mm Hg | 134 (19) | 135 (20) | 0.81 | 129 (15) | 127(16) | 0.38 |
| Total cholesterol, mean (SD), mg/dL | 188 (40) | 194 (42) | 0.27 | 188 (39) | 193 (38) | 0.33 |
| HDL cholesterol, mean (SD), mg/dL | 49 (13) | 48 (15) | 0.32 | 51 (19) | 53 (16) | 0.52 |
| LDL cholesterol, mean (SD), mg/dL | 110 (33) | 118 (38) | 0.07 | 111 (35) | 114 (32) | 0.48 |
| Hypertension (%) | 64 (83) | 312 (57) | <0.001 | 35 | 977 (59) | 398 | 10,775 (50) | 0.21 |
| Statin use (%) | 25 (32) | 154 (27) | 0.40 | 18 | 567 (33) | 203 | 6032 (27) | 0.42 |
| Aspirin use (%) | 37 (47) | 239 (42) | 0.42 | 32 | 1017 (58) | 288 | 8479 (38) | 0.010 |
| Warfarin use (%) | 15 (19) | 15 (3) | <0.001 | 16 | 298 (17) | 9 | 261 (1) | <0.001 |
| Log[CRP], mean (SD), mg/L | 1.18 (1.12) | 1.04 (1.19) | 0.36 | 0.97 (1.01) | 0.73 (1.13) | 0.09 |
| Log[D-dimer], mean (SD), mg/L | −0.49 (0.83) | −0.58 (0.84) | 0.41 | −0.93 (0.77) | −0.89 (0.83) | 0.72 |
| Factor VIII, mean (SD), mg/L | 141 (52) | 135 (58) | 0.42 | 126 (35) | 117 (40) | 0.04 |
| Fibrinogen, mean (SD), mg/L | 442 (109) | 417 (118) | 0.09 | 412 (74) | 386 (101) | 0.01 |
| Serum creatinine, mean (SD), mg/dL | 1.00 (0.36) | 1.14 (1.11) | 0.03 | 0.79 (0.19) | 0.88 (0.31) | <0.001 |
| Albumin-to-creatinine ratio, mean (SD), mg/g | 94 (337) | 129 (484) | 0.45 | 168 (620) | 26 (101) | 0.13 |
Statistical significance for continuous variables was tested using the student’s t-test procedure and for categorical variables the chi-square method was used.
AF=atrial fibrillation; CHD=coronary heart disease; CRP=C-reactive protein; HDL=high-density lipoprotein; LDL=low-density lipoprotein; SD=standard deviation.
Table 2 presents the association of inflammation and hemostasis biomarkers with AF. Accounting for age, sex, race, region of residence, body mass index, aspirin use, statin use, and warfarin use, mean levels of factor VIII and fibrinogen were 10.2 mg/L (95% CI=1.1, 19.3) and 27.4 mg/L (95% CI=9.7, 45.0) higher in participants with baseline AF than those without baseline AF, respectively. No baseline association was observed between CRP or D-dimer and AF.
Table 2.
Baseline Association of Biomarkers with AF*
| Biomarker | β (95%CI) | P-value |
|---|---|---|
| Log[CRP] | 0.09 (−0.15, 0.33) | 0.45 |
| Log[D-dimer] | 0.08 (−0.09, 0.25) | 0.34 |
| Factor VIII | 10.2 (1.1, 19.3) | 0.03 |
| Fibrinogen | 27.4 (9.7, 45.0) | 0.002 |
Adjusted for age, sex, race, region, body mass index, aspirin use, statin use, and warfarin use.
AF=atrial fibrillation; CI=confidence interval; CRP=C-reactive protein
The association of AF with CHD is shown in Table 3. Over a median follow-up of 4.4 years, AF was associated with an increased risk of CHD in all models. In the basic model adjusted for age, sex, race, region of residence, and education, AF was associated with an increased risk of CHD (HR=2.18, 95%CI=1.36, 3.48). A similar result was obtained with further adjustment for covariates included in Model 2 (HR=2.08, 95%CI=1.15, 3.74). The inclusion of markers of renal dysfunction and CRP did not alter the association of AF with CHD (Model 3: HR=2.08, 95%CI=1.10, 3.93). Differences were not observed, when the analysis was stratified by race and sex (Table 3).
Table 3.
Risk of CHD with AF
| All | Race | Sex | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| White | Black | P-interaction | Male | Female | P-interaction | ||
| Model 1* | 2.18 (1.36, 3.48) | 2.08 (1.09, 3.98) | 2.30 (1.20, 4.40) | 0.83 | 2.49 (1.18, 5.26) | 1.94 (1.08, 3.49) | 0.61 |
| Model 2† | 2.08 (1.15, 3.74) | 1.87 (0.82, 4.25) | 2.29 (1.09, 4.79) | 0.70 | 1.78 (0.71, 4.49) | 2.32 (1.18, 4.58) | 0.63 |
| + log[CRP] HR difference (95% CI)‡ | 1.98 (1.08, 3.63) | 1.82 (0.77, 4.28) | 2.14 (0.98, 4.66) | 0.77 | 1.82 (0.70, 4.70) | 2.10 (1.03, 4.31) | 0.80 |
| + log[D-dimer] HR difference (95% CI)‡ | 2.29 (1.28, 4.13) | 2.25 (0.99, 5.14) | 2.33 (1.10, 4.93) | 0.95 | 2.11 (0.85, 5.27) | 2.43 (1.22, 4.84) | 0.80 |
| + factor VIII HR difference (95% CI)‡ | 1.86 (1.00, 3.49) | 1.98 (0.87, 4.52) | 1.75 (0.74, 4.15) | 0.83 | 1.40 (0.50, 3.89) | 2.27 (1.12, 4.60) | 0.41 |
| + fibrinogen HR difference (95% CI)‡ | 1.96 (1.08, 3.56) | 1.67 (0.72, 3.89) | 2.30 (1.10, 4.80) | 0.55 | 1.74 (0.68, 4.45) | 2.15 (1.08, 4.30) | 0.70 |
| + log[CRP], log[D-dimer], factor VIII, fibrinogen | 2.00 (1.08, 3.77) | 2.29 (0.98, 5.32) | 1.79 (0.77, 4.20) | 0.68 | 1.57 (0.57, 4.36) | 2.38 (1.16, 4.88) | 0.49 |
Adjusted for age, sex, race, region, education, income.
Adjusted for Model 1 covariates plus smoking, total cholesterol, HDL cholesterol, systolic blood pressure, diabetes, cardiovascular disease, aspirin, statins, warfarin, and antihypertensive medications.
HR difference presented as % change in HR for CHD as follows: ((HRAF – HRAF with biomarker)/(HRafib – 1) * 100%).
AF=atrial fibrillation; CHD=coronary heart disease; CI=confidence interval; HDL=high-density lipoprotein; HR=hazard ratio; CRP=C-reactive protein.
The addition of factor VIII and fibrinogen individually attenuated the HR in Model 2 by more than 10% and a formal mediation analysis was performed (Table 3). However, the median percent changes in the HRs for factor VIII (19%, 95%CI=−35%, 60%) and fibrinogen (9.9%, 95%CI=−19%, 40%) were not significant.
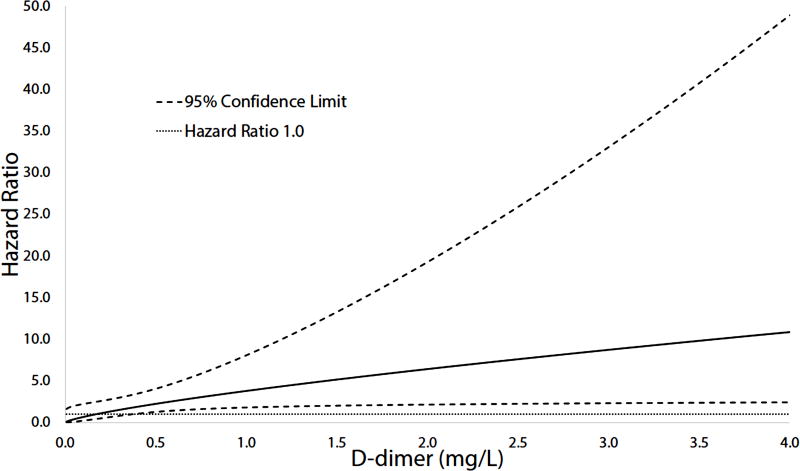
A significant interaction between D-dimer and AF was observed with the association of D-dimer with CHD being stronger in those with AF (adjusted HR=2.52, 95%CI=1.49, 4.26) than those without AF (adjusted HR=1.34, 95%CI=1.12, 1.61) (p-interaction=0.02) (Table 4). The risk of CHD at various D-dimer values for those with AF is shown in Figure 1. Furthermore, the association of AF with CHD was dependent on high D-dimer values. The CHD risk for those with AF compared with participants without AF among those with D-dimer levels above the median value (0.18 mg/L) was nearly 3-fold (HR=2.83, 95%CI=1.59, 5.04) greater than those below the median value (HR=1.27, 95%CI=0.06, 25.45). Similar interactions were not observed for CRP, factor VIII, and fibrinogen.
Table 4.
Risk of CHD with Biomarkers by AF
| Biomarker* | AF | No AF | P-Interaction |
|---|---|---|---|
| Log[CRP] (per SD unit = 1.13) | 1.13 (0.68, 1.90) | 1.37 (1.16, 1.62) | 0.49 |
| Log [D-dimer] (per SD unit = 0.83) | 2.52 (1.49, 4.26) | 1.34 (1.12, 1.61) | 0.02 |
| Factor VIII (per SD unit = 40) | 1.16 (0.85, 1.59) | 1.49 (1.27, 1.75) | 0.15 |
| Fibrinogen (per SD unit = 101) | 2.02 (1.01, 4.05) | 1.26 (1.06, 1.51) | 0.18 |
Adjusted for age, sex, race, region, education, income, smoking, total cholesterol, HDL cholesterol, systolic blood pressure, diabetes, cardiovascular disease, aspirin, statins, warfarin, and antihypertensive medications.
AF=atrial fibrillation; CHD=coronary heart disease; CRP=C-reactive protein; HDL=high-density lipoprotein; SD=standard deviation.
Figure 1.
Association of AF with CHD across D-dimer levels.
AF=atrial fibrillation; CHD=coronary heart disease.
DISCUSSION
In this analysis from REGARDS, the association of AF with CHD was dependent on D-dimer levels, a biomarker of hemostasis. Our results suggest that derangements in hemostasis are a key marker for CHD risk in those with AF and potentially D-dimer is able to assess CHD risk in this population.
The increased risk of CHD events in persons with AF was first explored in the REduction of Atherothrombosis for Continued Health (REACH) Registry in which those with AF were shown to have higher rates of fatal and non-fatal cardiovascular outcomes.3 Recent data also have shown that AF is strongly associated with future CHD events, independent of traditional CHD risk factors.4–7 Extending previous research, our data suggest that alterations in hemostasis, as measured through D-dimer, play a key role in the increased CHD risk among persons with AF. Similar interactions were not observed for CRP, factor VIII, and fibrinogen. This likely is due to D-dimer being a more sensitive marker of hemostasis activation in AF that is relevant to the pathophysiology of acute CHD events.
The association between hypercoagulability and AF has been previously described.23 The most frequent clinical manifestation of this association is atrial thrombogenesis and further embolization which often leads to stroke or other thromboembolic complications.24 Data from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial and other studies have shown that elevated D-dimer are observed in those with AF and in AF patients who are high risk for stroke.25–27 Our findings show that the association between AF and CHD potentially is driven by higher D-dimer levels, suggesting that alterations in hemostasis explain the increased CHD risk observed in AF. We are not aware of other research showing that the increased CHD risk observed among those with AF likely is attributed to alterations in hemostasis. The fact that D-dimer levels are reduced among AF patients who receive warfarin therapy and the risk of CHD is reduced in AF patients who use vitamin K antagonists further supports this observation.5,28
Dabigatran, a novel oral anticoagulant approved to prevent stroke and systemic embolization in AF patients, has been linked to an increased risk of CHD compared with warfarin in the RE-LY trial.29,30 In contrast, agents that inhibit factor Xa (e.g., rivaroxaban and apixaban) are not associated with an increased risk of MI compared with warfarin.31,32 The association of direct thrombin inhibitors with CHD compared with warfarin was not seen in trials of dabigatran to treat venous thrombosis, a population of presumably low CHD risk.33,34 In aggregate, these data suggest that medications which affect hemostasis impact CHD risk and our results confirm these findings in persons with AF. Due to the increased CHD risk in AF, studies are needed to determine the most efficacious anticoagulation treatment to reduce the likelihood of further CHD events in this population. Additionally, the role of D-dimer in AF management and risk prediction of adverse cardiovascular events, such as CHD, merits further exploration.
Inflammation, coagulation, and fibrinolysis are implicated in the pathophysiology of CHD.35–38 These processes are related to the acute plaque rupture and subsequent thrombosis that is observed during acute MI and CHD death. Additionally, AF is associated with increased levels of inflammation, implicating this process as a confounding factor.39,40 However, our data suggest that the association of AF with CHD is dependent on abnormalities in hemostasis and coagulation instead of inflammation. This is supported by recent data showing that the association between AF and MI is limited to NSTEMI.7 This would suggest that that alterations in hemostasis and thrombotic risk in AF lead to partial coronary occlusion that results in myocardial injury with increased oxygen demand (e.g., demand ischemia) rather than acute atherosclerotic plaque rupture.
This study should be interpreted in the context of several limitations. Cases of AF were ascertained by self-reported history and baseline electrocardiogram data. Self-reported AF cases subjected our analysis to recall bias. However, we have shown that self-reported AF in our cohort serves as a reliable and strong predictor of stroke events that can be used interchangeably or in combination with electrocardiogram-detected AF as a predictor of stroke.22 Potentially, non-permanent cases of AF were missed as we relied on single electrocardiogram measurements. Due to the small number of CHD events, we were unable to stratify our analysis by MI type (e.g., STEMI vs. NSTEMI). Despite the number of CHD cases, AF is a not a common risk factor and we were unable to detect significant mediation for the biomarkers examined. A larger study would be needed to address this question. Overall, these limitations would bias our results towards the null rather than inflating the association. Furthermore, although we adjusted for potential confounders, we acknowledge that residual confounding remains a possibility.
In conclusion, we have demonstrated that that the association of AF with CHD is dependent on D-dimer levels, a marker of hemostasis. Potentially, anticoagulation strategies in AF have implications beyond stroke prevention to decrease CHD risk. Future studies are needed to clarify the role of D-dimer in CHD prediction among those with AF and to determine which anticoagulation therapies are able to reduce the CHD burden in this population.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
FUNDING
This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service, K08HL096841 and R01HL080477 from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Additional funding was provided by an investigator-initiated grant-in-aid from Amgen Corporation for measurement of urinary biomarkers within the cohort. Amgen played no role in the study design, collection, analysis and interpretation of data for this manuscript.
Footnotes
DISCLOSURES
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 3.Goto S, Bhatt DL, Rother J, et al. Prevalence, clinical profile, and cardiovascular outcomes of atrial fibrillation patients with atherothrombosis. Am Heart J. 2008;156(5):855–863. 863 e852. doi: 10.1016/j.ahj.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 4.Conen D, Chae CU, Glynn RJ, et al. Risk of death and cardiovascular events in initially healthy women with new-onset atrial fibrillation. JAMA. 2011;305(20):2080–2087. doi: 10.1001/jama.2011.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soliman EZ, Safford MM, Muntner P, et al. Atrial Fibrillation and the Risk of Myocardial Infarction. JAMA Intern Med. 2014;174(1):107–114. doi: 10.1001/jamainternmed.2013.11912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Neal WT, Sangal K, Zhang ZM, Soliman EZ. Atrial fibrillation and incident myocardial infarction in the elderly. Clin Cardiol. 2014;37(12):750–755. doi: 10.1002/clc.22339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soliman EZ, Lopez F, O'Neal WT, et al. Atrial Fibrillation and Risk of ST-Segment Elevation versus Non-ST Segment Elevation Myocardial Infarction: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2015 doi: 10.1161/CIRCULATIONAHA.114.014145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahe I, Bergmann JF, Chassany O, et al. A multicentric prospective study in usual care: D-dimer and cardiovascular events in patients with atrial fibrillation. Thromb Res. 2012;129(6):693–699. doi: 10.1016/j.thromres.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Sadanaga T, Sadanaga M, Ogawa S. Evidence that D-dimer levels predict subsequent thromboembolic and cardiovascular events in patients with atrial fibrillation during oral anticoagulant therapy. J Am Coll Cardiol. 2010;55(20):2225–2231. doi: 10.1016/j.jacc.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 10.Vene N, Mavri A, Kosmelj K, Stegnar M. High D-dimer levels predict cardiovascular events in patients with chronic atrial fibrillation during oral anticoagulant therapy. Thromb Haemost. 2003;90(6):1163–1172. doi: 10.1160/TH03-06-0363. [DOI] [PubMed] [Google Scholar]
- 11.Lip GY, Patel JV, Hughes E, Hart RG. High-sensitivity C-reactive protein and soluble CD40 ligand as indices of inflammation and platelet activation in 880 patients with nonvalvular atrial fibrillation: relationship to stroke risk factors, stroke risk stratification schema, and prognosis. Stroke. 2007;38(4):1229–1237. doi: 10.1161/01.STR.0000260090.90508.3e. [DOI] [PubMed] [Google Scholar]
- 12.Hermida J, Lopez FL, Montes R, Matsushita K, Astor BC, Alonso A. Usefulness of high-sensitivity C-reactive protein to predict mortality in patients with atrial fibrillation (from the Atherosclerosis Risk In Communities [ARIC] Study) Am J Cardiol. 2012;109(1):95–99. doi: 10.1016/j.amjcard.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kritchevsky SB, Cesari M, Pahor M. Inflammatory markers and cardiovascular health in older adults. Cardiovasc Res. 2005;66(2):265–275. doi: 10.1016/j.cardiores.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 14.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 15.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol. 1999;52(12):1165–1172. doi: 10.1016/s0895-4356(99)00102-x. [DOI] [PubMed] [Google Scholar]
- 16.Zakai NA, Judd SE, Alexander K, et al. ABO blood type and stroke risk: the REasons for Geographic And Racial Differences in Stroke Study. J Thromb Haemost. 2014;12(4):564–570. doi: 10.1111/jth.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Safford MM, Brown TM, Muntner PM, et al. Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308(17):1768–1774. doi: 10.1001/jama.2012.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation. 2007;116(22):2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 19.Prineas RJ, Crow RS, Blackburn HW. The Minnesota code manual of electrocardiographic findings : standards and procedures for measurement and classification. 2. London: Springer; 2010. [Google Scholar]
- 20.Gillett SR, Boyle RH, Zakai NA, McClure LA, Jenny NS, Cushman M. Validating laboratory results in a national observational cohort study without field centers: the Reasons for Geographic and Racial Differences in Stroke cohort. Clin Biochem. 2014;47(16–17):243–246. doi: 10.1016/j.clinbiochem.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peralta CA, Shlipak MG, Judd S, et al. Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA. 2011;305(15):1545–1552. doi: 10.1001/jama.2011.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soliman EZ, Howard G, Meschia JF, et al. Self-reported atrial fibrillation and risk of stroke in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke. 2011;42(10):2950–2953. doi: 10.1161/STROKEAHA.111.621367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choudhury A, Lip GY. Atrial fibrillation and the hypercoagulable state: from basic science to clinical practice. Pathophysiol Haemost Thromb. 2003;33(5–6):282–289. doi: 10.1159/000083815. [DOI] [PubMed] [Google Scholar]
- 24.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 25.Lip GY, Lowe GD, Rumley A, Dunn FG. Fibrinogen and fibrin D-dimer levels in paroxysmal atrial fibrillation: evidence for intermediate elevated levels of intravascular thrombogenesis. Am Heart J. 1996;131(4):724–730. doi: 10.1016/s0002-8703(96)90278-1. [DOI] [PubMed] [Google Scholar]
- 26.Sadanaga T, Kohsaka S, Ogawa S. D-dimer levels in combination with clinical risk factors can effectively predict subsequent thromboembolic events in patients with atrial fibrillation during oral anticoagulant therapy. Cardiology. 2010;117(1):31–36. doi: 10.1159/000319626. [DOI] [PubMed] [Google Scholar]
- 27.Hijazi Z, Oldgren J, Siegbahn A, Granger CB, Wallentin L. Biomarkers in atrial fibrillation: a clinical review. Eur Heart J. 2013;34(20):1475–1480. doi: 10.1093/eurheartj/eht024. [DOI] [PubMed] [Google Scholar]
- 28.Nakatani Y, Mizumaki K, Nishida K, et al. Anticoagulation control quality affects the D-dimer levels of atrial fibrillation patients. Circ J. 2012;76(2):317–321. doi: 10.1253/circj.cj-11-0885. [DOI] [PubMed] [Google Scholar]
- 29.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 30.Hohnloser SH, Oldgren J, Yang S, et al. Myocardial ischemic events in patients with atrial fibrillation treated with dabigatran or warfarin in the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) trial. Circulation. 2012;125(5):669–676. doi: 10.1161/CIRCULATIONAHA.111.055970. [DOI] [PubMed] [Google Scholar]
- 31.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 32.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 33.Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342–2352. doi: 10.1056/NEJMoa0906598. [DOI] [PubMed] [Google Scholar]
- 34.Schulman S, Kakkar AK, Goldhaber SZ, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129(7):764–772. doi: 10.1161/CIRCULATIONAHA.113.004450. [DOI] [PubMed] [Google Scholar]
- 35.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350(14):1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 36.Danesh J, Whincup P, Walker M, et al. Fibrin D-dimer and coronary heart disease: prospective study and meta-analysis. Circulation. 2001;103(19):2323–2327. doi: 10.1161/01.cir.103.19.2323. [DOI] [PubMed] [Google Scholar]
- 37.Cushman M, Lemaitre RN, Kuller LH, et al. Fibrinolytic activation markers predict myocardial infarction in the elderly. The Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1999;19(3):493–498. doi: 10.1161/01.atv.19.3.493. [DOI] [PubMed] [Google Scholar]
- 38.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101(15):1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 39.Aviles RJ, Martin DO, Apperson-Hansen C, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108(24):3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 40.Hatzinikolaou-Kotsakou E, Tziakas D, Hotidis A, et al. Relation of C-reactive protein to the first onset and the recurrence rate in lone atrial fibrillation. Am J Cardiol. 2006;97(5):659–661. doi: 10.1016/j.amjcard.2005.09.104. [DOI] [PubMed] [Google Scholar]