Abstract
Activation of Toll-like receptors has been linked to neuropathic pain and opioid dependence. (+)-Naltrexone acts as a Toll-like receptor 4 (TLR4) antagonist and has been shown to reverse neuropathic pain in rat studies. We designed and synthesized compounds based on (+)-naltrexone and (+)-noroxymorphone and evaluated their TLR4 antagonist activities by their effects on inhibiting lipopolysaccharide (LPS) induced TLR4 downstream nitric oxide (NO) production in microglia BV-2 cells. Alteration of the N-substituent in (+)-noroxymorphone gave us a potent TLR4 antagonist. The most promising analog, (+)-N-phenethylnoroxymorphone ((4S,4aR,7aS,12bR)-4a,9-dihydroxy-3-phenethyl-2,3,4,4a,5,6-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-7(7aH)-one, 1j) showed ~ 75 times better TLR-4 antagonist activity than (+)-naltrexone, and the ratio of its cell viability IC50, a measure of its toxicity, to TLR-4 antagonist activity (140 μM /1.4 μM) was among the best of the new analogs. The 1j was active in vivo; it significantly increased and prolonged morphine analgesia.
Graphical Abstract
INTRODUCTION
Toll-like receptor 4 (TLR4) detects pathogen-associated molecular patterns,1 damage-associated molecular patterns,2, 3 and xenobiotic-associated molecular patterns,4–6 triggering signal transduction cascades culminating in the production of pro-inflammatory mediators.1 These pro-inflammatory factors exaggerate neuronal excitability, thereby contributing to neuropathic pain and drug dependence.7–10 Because of these actions, TLR4 has become an important non-neuronal therapeutic target.3
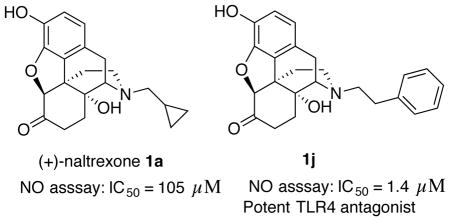
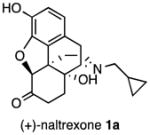
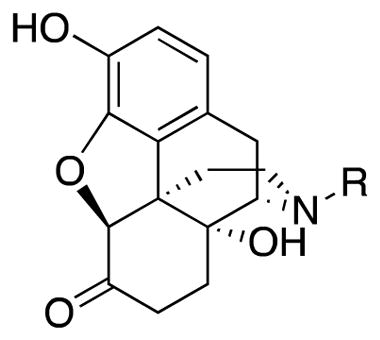
We recently identified naltrexone as a TLR4 antagonist by a variety of biophysical, in vitro, in silico, and in vivo assays.11–13 Naltrexone was found to non-stereoselectively reverse neuropathic pain in a rat model,14 which indicates that this action of naltrexone is independent of opioid receptors. Unlike the pharmacologically active (−)-isomer, (+)-naltrexone15, 16 ((4aR,7aS,12bR)-3-(cyclopropylmethyl)-4a,9-dihydroxy-2,3,4,4a,5,6-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-7(7aH)-one, 1a, Figure 1) does not interact with opioid receptors. Since it cannot act as an opioid antagonist, it does not prevent the beneficial analgesic effect of opioids. Further studies have shown that (+)-naltrexone can reduce opioid- and cocaine-induced conditioned place preference, self-administration, drug-primed reinstatement (relapse), and incubation of craving.4, 17, 18 However, (+)-naltrexone itself does not appear to be the best ligand for treating chronic pain and drug abuse as it has a relatively poor cell viability to TLR4 antagonist ratio (>400/105.5) and is not long-acting in vivo, nor is it particularly potent in its action. Because of this, we have explored the design and synthesis of new ligands based on the (+)-naltrexone molecular template in order to obtain a more promising TLR4 antagonist with an improved cell viability to TLR4 antagonist ratio. In this initial study we evaluated the effect of modification or removal of oxygen atoms in (+)-naltrexone, as well as alteration of the N-substituent. We found that the removal of the furan ring and the ketone from (+)-naltrexone afforded an analog that was ~15 times more potent as a TLR4 antagonist. Most gratifying, the replacement of the N-cyclopropylmethyl substituent in (+)-naltrexone with an N-phenethyl moiety led to analog 1j with ~75 fold better TLR4 antagonist activity than (+)-naltrexone. N-Phenethylnoroxymorphone (4S,4aR,7aS,12bR)-4a,9-dihydroxy-3-phenethyl-2,3,4,4a,5,6- hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-7(7aH)-one (1j), also had relatively low cell cytotoxicity and in vivo efficacy.
Figure 1.
Synthetic targets 2 – 7
RESULTS AND DISCUSSION
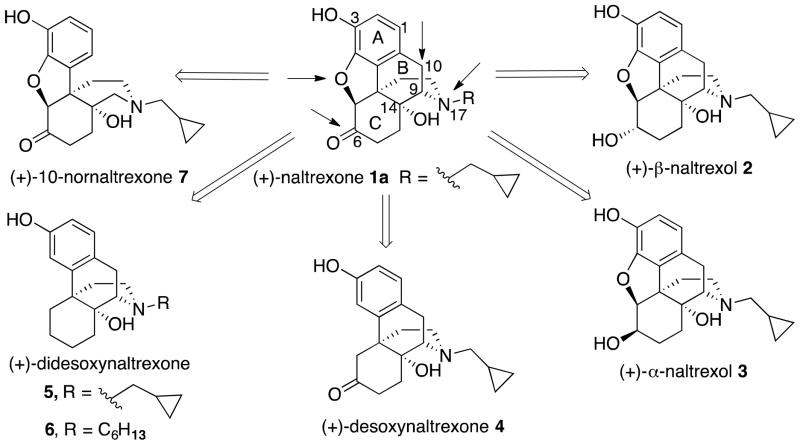
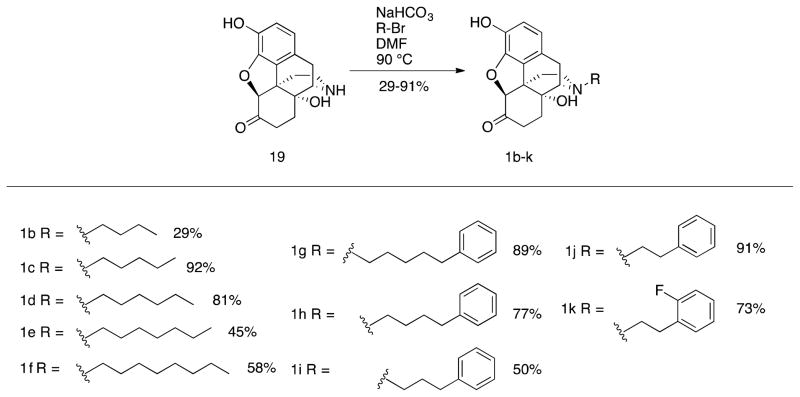
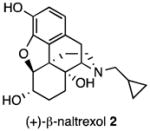
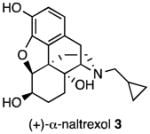
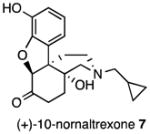
Several logical disconnections and modifications to the structure of (+)-naltrexone 1a (Figure 1) were prepared as structural analogs to establish an initial qualitative SAR. Stereoselective reduction of (+)-naltrexone 1a afforded (+)-β-naltrexol 2 and (+)-α-naltrexol 3. The oxide bridge in (+)-naltrexone 1a was removed to provide (+)-desoxynaltrexone ((4bS,8aR)-11-(cyclopropylmethyl)-3,8a-dihydroxy-8,8a,9,10-tetrahydro-5H-9,4b-(epiminoethano)phenanthren-6(7H)-one, 4), and this structure was further simplified by removing the ketone as well, to afford (+)-didesoxynaltrexone (4bR,8aR)-11-(cyclopropylmethyl)-5,6,7,8,9,10-hexahydro-8aH-9,4b-(epiminoethano)phenanthrene-3,8a-diol, 5). Further, we examined the effect of the N-substituent in (+)-naltrexone by removing the cyclopropylmethyl group and adding various alkyl (e.g., compound 6, Figure 1) and phenylalkyl moieties to the secondary amine. Removal of the C-10 carbon from ring B in (+)-naltrexone, a pentacyclic compound, provided the structural analog (+)-10-nornaltrexone (4aR,7aS,12bR)-3-(cyclopropylmethyl)-4a,9-dihydroxy-2,3,4,4a,5,6-hexahydro-1H-benzofuro[3,2-e]isoquinolin-7(7aH)-one, 7),19 a somewhat less strained tetracyclic compound. The synthesis of 7 was described previously.19
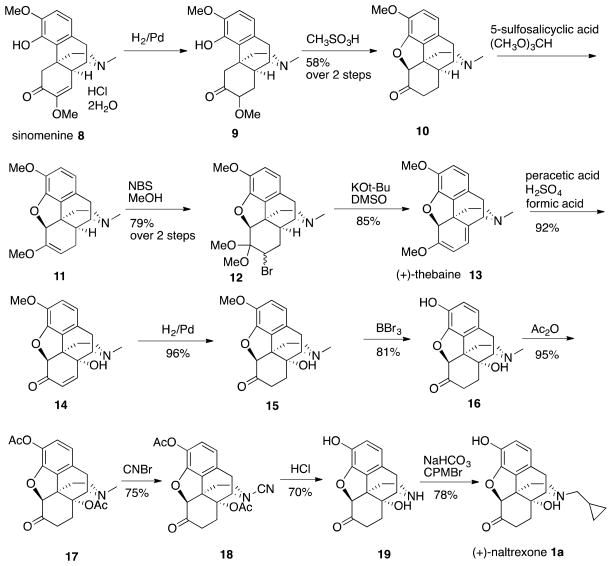
The 12 step synthesis of (+)-naltrexone 1a (Scheme 1) began with hydrogenation using Pd/C to saturate the double-bond in the enol ether present in sinomenine 8, followed by treatment of 9 in a CHCl3 - methanesulfonic acid two-phase system to afford (+)-hydrocodone 10. Formation of the C-6 methyl enol ether in 11 was conducted using the Rapoport et al.20 procedure on our (+)-enantiomer with our critical modification of changing the acid from H2SO4 to 5-sulfosalicylic acid for reasons previously described.15 Bromination of 11 was improved using N-bromosuccinimide (NBS) rather than the reported N-bromoacetamide (NBA) in MeOH20 to give 12. Transformation of bromide 12 into one of our needed intermediates, (+)-noroxymorphone 19, was performed in 7 steps following known procedures,21 with the modification of 3M HCl instead of H2SO4 in the transformation of N-cyano 18 to (+)-noroxymorphone 19. Finally, N-alkylation with cyclopropylmethyl bromide as we have previously described15 afforded our starting material, (+)-naltrexone 1a.
Scheme 1.
Synthesis of (+)-Naltrexone 1a from Commercially Available Sinomenine 8
Stereospecific reduction of (+)-naltrexone to (+)-naltrexols 2 and 3
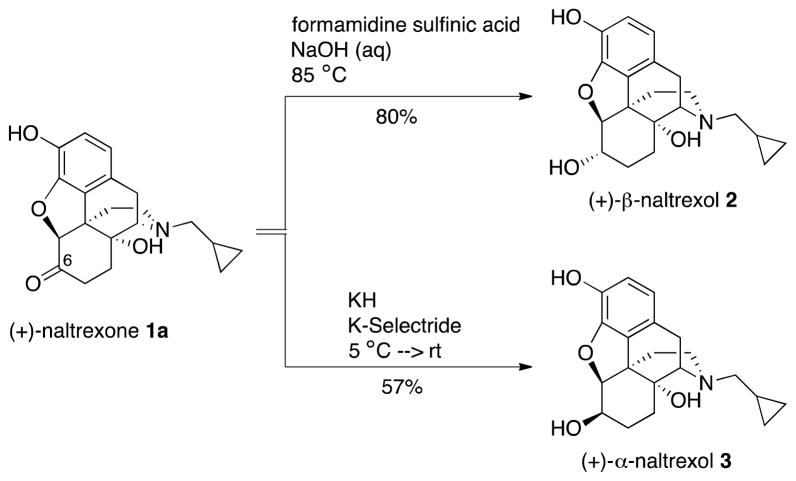
We initially attempted to increase the potency of (+)-naltrexone 1a by determining whether the C-6 keto moiety was necessary for its action; whether a C-6 hydroxyl group might be better. Since it was unpredictable whether that hydroxyl group would be more effective above or below the plane of the ring, we decided to prepare and test both compounds. The stereoselective reduction of (+)-naltrexone 1a to obtain analogs 2 and 3 was performed using procedures that were known to provide the corresponding (−)-isomers.22 The use of formamidine sulfinic acid in aqueous sodium hydroxide on (+)-naltrexone 1a provided (+)-β-naltrexol 2, while reaction with KH and K-Selectride afforded (+)-α-naltrexol 3 (Scheme 2).
Scheme 2.
Stereoselective Reduction of (+)-Naltrexone 1a
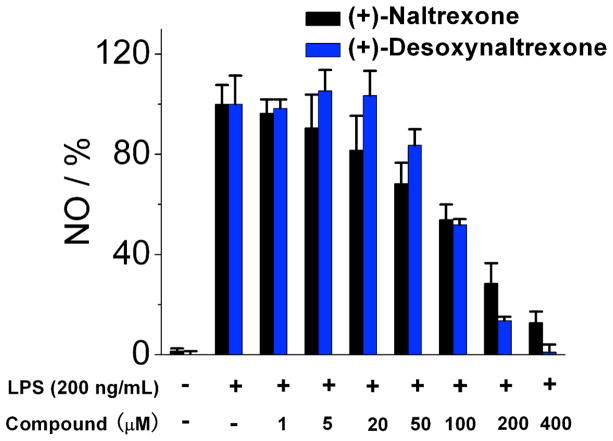
The effect of the (+)-enantiomers 2 and 3 on TLR4 was examined in cell assays. Microglia are the resident cells of the innate immune system in the central nervous system.23 TLR4 is expressed in microglia and its activation is one of the main mechanisms for neuroinflammation.24 We used the microglia BV-2 cell line as the model system of microglia, as they reproduce the primary microglia with high fidelity.25 TLR4 activation induces the production of the downstream inflammatory factor nitric oxide (NO), which is one of the factors contributing to the development of neuropathic pain26 and drug addiction.27, 28 Therefore, NO was used as the readout of lipopolysaccharide (LPS) induced TLR4 activation.13 As shown in Figure 2, (+)-β-naltrexol 2 and (+)-α-naltrexol 3 each showed reduced TLR-4 antagonism in comparison to (+)-naltrexone 1a. (+)-Naltrexone 1a had an IC50 = 105.5 ± 10.1 μM in that assay, whereas (+)-β-naltrexol 2 and (+)-α-naltrexol 3 were less potent with IC50’s = 242.8 ± 21.3 and 143.5 ± 20.8 μM, respectively (Table 1). In contrast, (+)-10-nornaltrexone 719 had an IC50 = 76.2 ± 27.2 μM. Each of these compounds showed low cell cytotoxicity, their IC50 > 400 μM in a cell viability assay. However, the ratio of cytotoxicity to TLR4 antagonism was low (<10) for these analogs, and neither appeared to have great promise.
Figure 2.
(+)-Naltrexone 1a, (+)-β-naltrexol 2 and (+)-α-naltrexol 3 inhibit LPS induced NO over-production in a dose-dependent manner. BV-2 cells were treated with 200 ng/mL LPS and various concentrations of compounds for 24 h. NO in the supernatant was measured by the 2,3-diaminonaphthalene method.6, 29 The NO in the LPS (200 ng/mL) control group was normalized as 100%.
Table 1.
IC50 Values of (+)-Naltrexone 1a, (+)-β-Naltrexol 2, (+)-α-Naltrexol 3, and (+)-10-Nornaltrexone 7
| Compound | IC50 of NO (μM) | IC50 of viability (μM) |
|---|---|---|
|
105.5 ± 10.1 | > 400 |
|
242.8 ± 21.3 | > 400 |
|
143.5 ± 20.8 | > 400 |
|
76.2 ± 27.2 | > 400 |
Removal of Oxygen Functionality
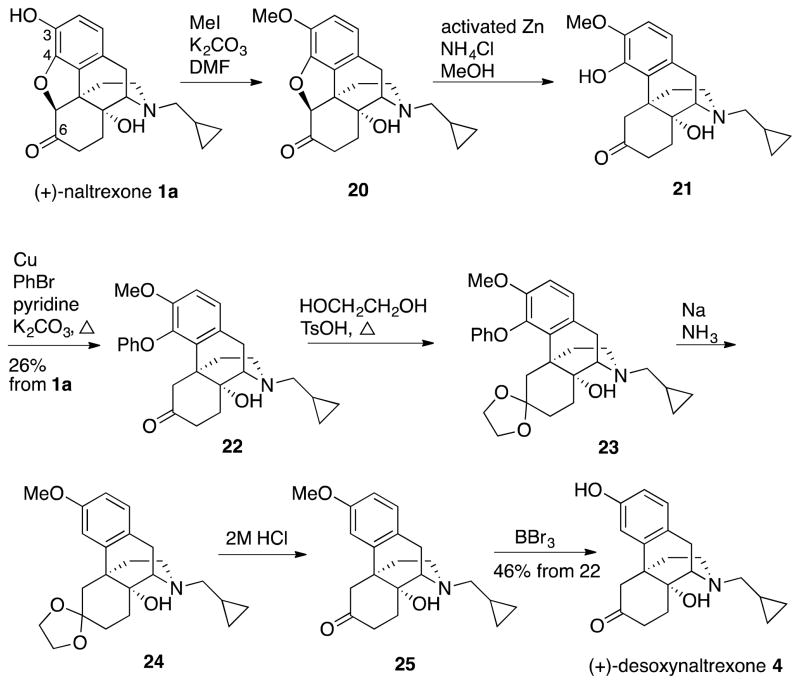
(+)-Desoxynaltrexone 4 (Scheme 3) was prepared using the procedures of Nagase et al.,30, 31 in their synthesis of the (−)-enantiomer of 24 (1a → 24, Scheme 3). This synthesis began with a methylation of the C-3 hydroxy of (+)-naltrexone 1a to arrive at the 3-methoxyether 20, which was subsequently treated with activated zinc and ammonium chloride to open the oxide bridge and afford the C-4 hydroxy compound 21 (Scheme 3). The methoxyphenol 21 was converted to the C-4 phenyl ether 22. Protection of the C-6 ketone in 22 as the cyclic ketal 23 followed by dissolving metal reduction conditions and deprotection of the ketone under acidic conditions provided (+)-O-methyldesoxynaltrexone 25. Finally, O-demethylation of 25 with BBr3 gave (+)-desoxynaltrexone 4.
Scheme 3.
Synthesis of (+)-Desoxynaltrexone 4 from (+)-Naltrexone 1a
As shown in Figure 3, in the NO assay (+)-desoxynaltrexone 4 had an IC50 = 118.1 ± 22.5 μM with an IC50 of cell viability of > 400 μM. (+)-Desoxynaltrexone 4 was a more effective TLR4 antagonist than (+)-naltrexone 1a at concentrations above 100 μM; at lower concentrations, (+)-naltrexone 1a was somewhat more potent. These assays indicated that (+)-desoxynaltrexone 4 would not be a better TLR4 antagonist than (+)-naltrexone 1a.
Figure 3.
(+)-Desoxynaltrexone 4 inhibits LPS induced NO over-production in a dose-dependent manner. (+)-Naltrexone 1a served as a control. BV-2 cells were treated with 200 ng/mL LPS and various concentrations of the test compounds for 24 h. NO in the supernatant was measured by the 2,3-diaminonaphthalene method.6, 29 The NO in the LPS (200 ng/mL) control group was normalized to 100%.
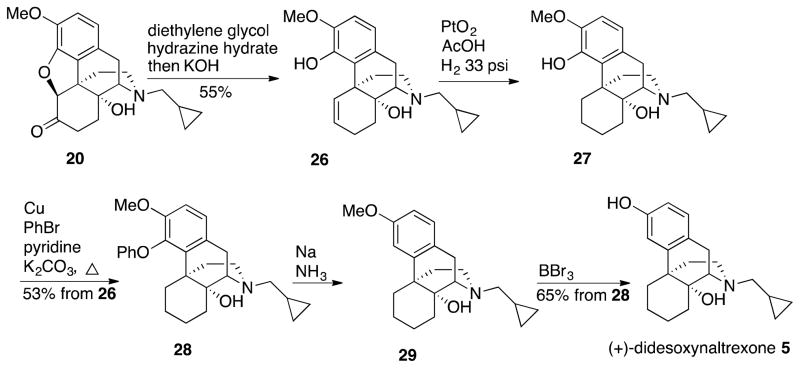
To determine if the C-6 keto moiety in (+)-desoxynaltrexone 4 had an effect on potency, as measured by the NO assay, we prepared (+)-didesoxynaltrexone 5. Synthesis of 5 began with a Wolff-Kishner reduction of the O-methyl ether 20 to give alkenyl phenol 26 as described by Wu et al.,32 which was reduced under H2 atmosphere with PtO2 to afford 27 (Scheme 4). The same synthetic approach utilized in synthesizing 4 (21 → 25, Scheme 3) was then applied to 27 with formation of phenyl ether 28 followed by dissolving metal reduction conditions to provide 29. Finally, an O-demethylation using BBr3 afforded (+)-didesoxynaltrexone 5, the N-cyclopropylmethyl analog of (+)-butorphanol.
Scheme 4.
Synthesis of (+)-Didesoxynaltrexone 5 from (+)-O-Methylnaltrexone 20
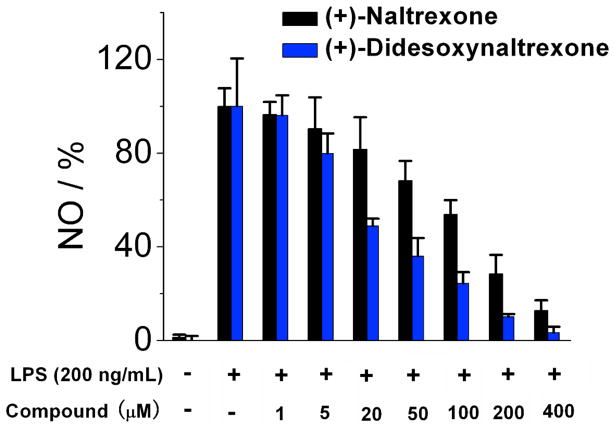
(+)-Didesoxynaltrexone 5 performed better as a TLR-4 antagonist than (+)-naltrexone 1a (Figure 4). The increased antagonism difference became more apparent at the 20 μM testing dose. (+)-Didesoxynaltrexone 5 had an IC50 value in the NO assay of 48.9 ± 6.8 μM with an IC50 of cell viability = 366 ± 78.8 μM. Removal of the C-6 keto moiety from (+)-desoxynaltrexone 4 gave a slightly more potent compound. However, although more potent than both (+)-naltrexone 1a and (+)-desoxynaltrexone 4, the (+)-didesoxynaltrexone 5 was not sufficiently different from either to warrant further work with the compound.
Figure 4.
(+)-Didesoxynaltrexone 5 inhibits LPS induced NO over-production in a dose-dependent manner. (+)-Naltrexone 1a served as the control compound for comparison. BV-2 cells were treated with 200 ng/mL of LPS and various concentrations of test compounds for 24 h. NO in the supernatant was measured by the 2,3-diaminonaphthalene method.6, 29 The NO in the LPS (200 ng/mL) group was normalized as 100%.
Modification of the N-Substituent
The known strong TLR-4 agonist lipopolysaccharide (LPS) contains a lipid A portion which has 6 unsubstituted linear alkanes (11 carbons and greater). The crystal structure of LPS bound to the TLR4/MD2 complex, where MD-2 is the myeloid differentiation factor 2, reported by Park et al.,33 shows 5 of these lipid chains interacting with MD-2 while one of them interacts with TLR4. We thought we would try to mimic the lipid chain structure by introducing a longer or bulkier alkyl chain on the secondary nitrogen in (+)-noroxymorphone 19 (Scheme 1), with the hope that it might have an effect on the potency of these compounds as TLR4 antagonists. To that end, a four-carbon chain was introduced to obtain (+)-N-butylnoroxymorphone 1b, and five to eight-carbon chains were also synthesized (Scheme 5). The compound with the longest chain was (+)-N-octylnoroxymorphone 1f. In addition, to determine the effect of hydrophobicity and bulk on the alkyl chain, (+)-N-(5-phenyl)pentylnoroxymorphone 1g through (+)-N-phenethylnoroxymorphone 1j were prepared. Also, aryl fluoride 1k was synthesized to see if adding an electron-withdrawing substituent on the arene would provide any added benefit.
Scheme 5.
N-Alkyl and N-Arylalkyl Substituents in (+)-Noroxymorphone 1b–1k
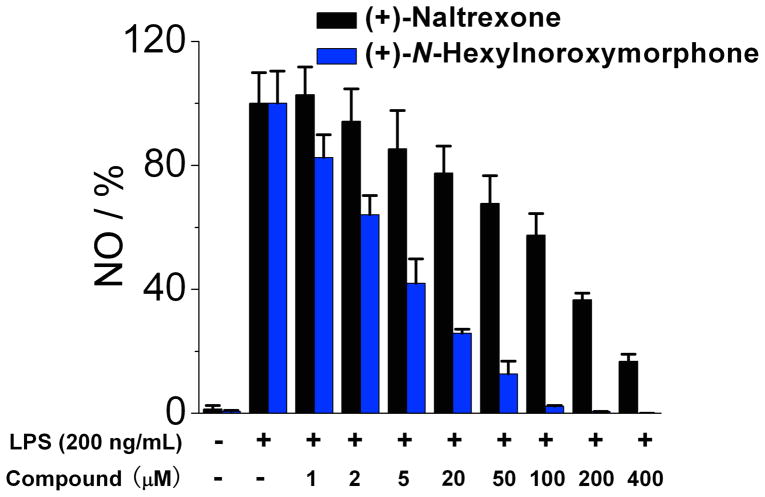
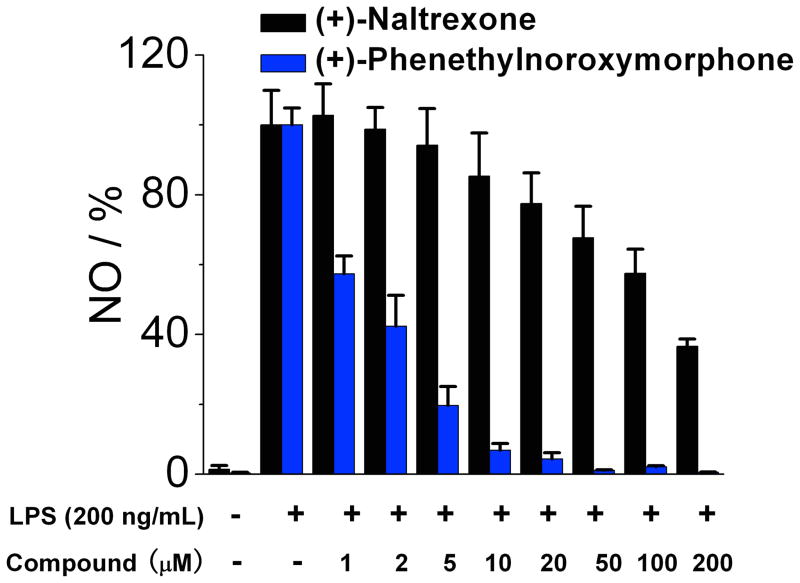
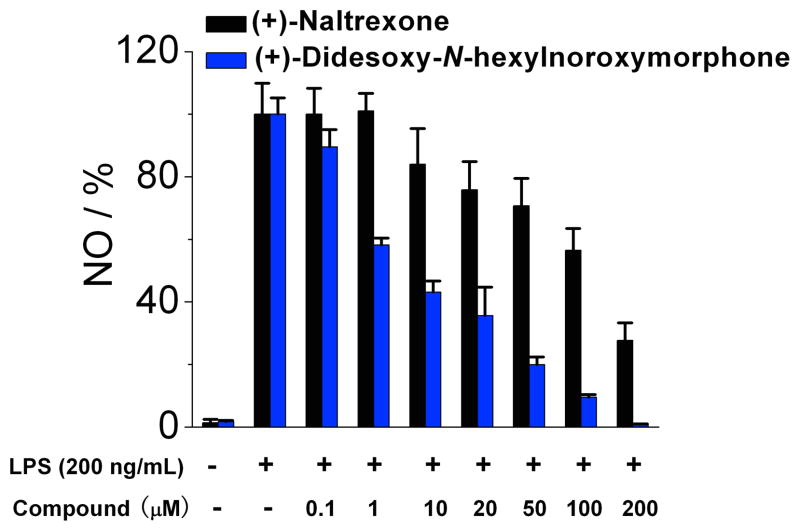
The (+)-N-alkyl- and (+)-N-arylalkyl-noroxymorphone analogs 1b–1k showed increased TLR4 antagonism versus LPS in the BV2 microglial cell assay ranging from 4–88 times greater than (+)-naltrexone 1b (Table 2). A representative example from this group of compounds was that of (+)-N-hexylnoroxymorphone 1d (Figure 5). An increase in the length of the carbon chain from 4 carbons (1b) through 8 carbons (1f) results in an increase in TLR-4 antagonism versus LPS with 1b showing IC50 = 24.8 ± 3.4 μM, and 1f with IC50 = 1.7 ± 0.1 μM (Table 2). This increase in TLR-4 antagonism, however, came at the expense of cell viability where IC50 of viability values > 200 μM and 33.4 ± 0.1 μM for 1b and 1f, respectively. The N-phenylalkyl containing compounds 1g–1k were generally more potent TLR-4 antagonists than the N-alkyl chain compounds 1b–1f (e.g., 1j, Figure 6). The same trend in cell cytotoxicity showing a direct relationship to chain length was observed in this series as well. Although the TLR-4 antagonism remained roughly the same (~ 1.4 μM) for compounds 1g–1j, the ratio of cell toxicity to TLR4 antagonism degenerated as the arylakyl chain length increased. Also, the addition of fluorine to the aromatic ring on the 2-position (1k) resulted in an increase in the cell cytotoxicity by approximately two-fold.
Table 2.
IC50 Values of (+)-N-Alkylnoroxymorphone Analogs 1b–1k.
|
IC50 of NO (μM) | IC50 of viability (μM) |
|---|---|---|
|
|
24.8 ± 3.4 | > 200 |
|
|
7.5 ± 1.7 | > 200 |
|
|
6.7 ± 0.4 | 121.4 ± 7.3 |
|
|
3.0 ± 0.2 | 68.5 ± 2.8 |
|
|
1.7 ± 0.1 | 33.4 ± 1.0 |
|
|
1.7 ± 0.4 | 26.8 ± 0.4 |
|
|
1.2 ± 0.2 | 37.1 ± 1.9 |
|
|
1.4 ± 0.2 | 69.3 ± 3.4 |
|
|
1.4 ± 0.3 | 140.8 ± 5.5 |
|
|
1.8 ± 0.2 | 65.7 ± 3.3 |
Figure 5.
(+)-N-Hexylnoroxymorphone 1d inhibits LPS induced NO over-production in a dose-dependent manner. (+)-Naltrexone 1a served as the control compound for comparison. BV-2 cells were treated with 200 ng/mL LPS and various concentrations of test compounds for 24 h. NO in the supernatant was measured by the 2,3-diaminonaphthalene method.6, 29 The NO in the LPS (200 ng/mL) control group was normalized to 100%.
Figure 6.
(+)-N-Phenethylnoroxymorphone 1j inhibits LPS induced NO over-production in a dose-dependent manner. (+)-Naltrexone 1a served as the control compound for comparison. BV-2 cells were treated with 200 ng/mL LPS and various concentrations of test compounds for 24 h. NO in the supernatant was measured by the 2,3-diaminonaphthalene method.6, 29 The NO in the LPS (200 ng/mL) group was normalized to 100%.
Combination of Oxygen Removal and N-Substitution
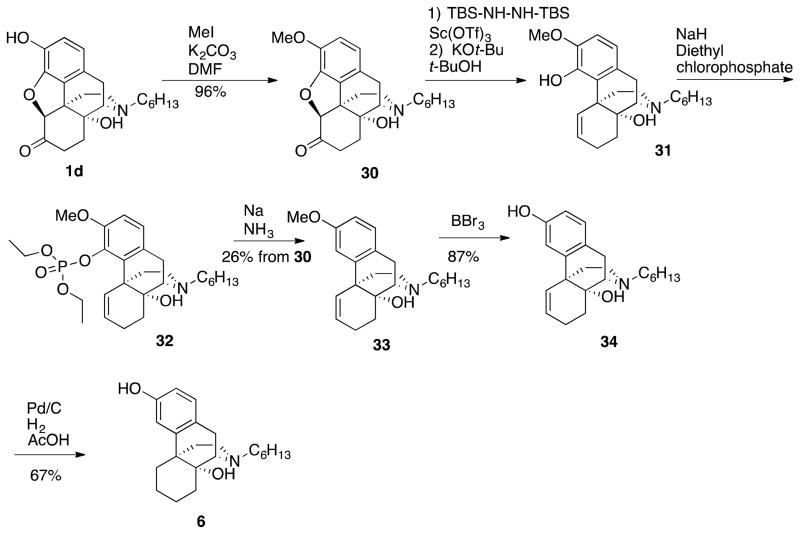
We incorporated in our designed ligand a combination of the benefits of an extended alkyl chain (e.g., as in (+)-N-hexylnoroxymorphone 1d) with the benefits obtained by removal of the oxide bridge and ketone from (+)-naltrexone (that also increased TLR-4 antagonism, as in (+)-didesoxynaltrexone 5), with the hope of obtaining a compound with amplified antagonist potency. Thus, the phenol of (+)-N-hexylnoroxymorphone 1d was methylated (Scheme 6) and the resultant diether 30 was subjected to modified Wolff-Kishner reduction conditions34 to afford monoether 31. Attempts to prepare a phenyl ether from the phenolic hydroxyl in 31 like that of 27 → 28 (Scheme 4) were unsuccessful. Instead, the phosphonate ester 32 (Scheme 6) was synthesized and subsequently treated under dissolving metal reduction conditions to afford the didesoxy compound 33. An O-demethylation of 33 followed by hydrogenation of the alkene with Pd/C provided (+)-didesoxy-N-hexylnoroxymorphone ((4bR,8aR)-11-hexyl-5,6,7,8,9,10-hexahydro-8aH-9,4b-(epiminoethano)phenanthrene-3,8a-diol, 6).
Scheme 6.
Synthesis of (+)-Didesoxy-N-hexylnoroxymorphone 6 from (+)-N-Hexylnoroxymorphone 1d
(+)-Didesoxy-N-hexylnoroxymorphone 6 did have slightly increased TLR-4 antagonism activity (Figure 7) in comparison to both (+)-N-hexylnoroxymorphone 1d and (+)-N-didesoxynaltrexone 5, with an IC50 = 5.2 ± 1.3 μM. However, the increased activity is within the experimental error of the activity of (+)-N-hexylnoroxymorphone 1d itself at 6.7 ± 0.4 μM. In addition, the IC50 of viability assay for (+)-didesoxy-N-hexylnoroxymorphone 6 was found to be 73.4 ± 4.0 μM, while that of (+)-N-hexylnoroxymorphone 1d was 121.4 ± 7.3 μM. We also examined the N-hexyl compound 34 in the BV-2 microglial cell assay, and it was more potent, with an IC50 = 22.5 ± 3.2 μM, and less toxic with an IC50 of viability = 154.7 ± 5.0 μM, although the ratio (~7) was not as good as desired.
Figure 7.
(+)-Didesoxy-N-hexylnoroxymorphone 6 inhibits LPS induced NO over-production in a dose-dependent manner. (+)-Naltrexone 1a served as the control compound for comparison. BV-2 cells were treated with 200 ng/mL LPS and various concentrations of test compounds for 24 h. NO in the supernatant was measured by the 2,3-diaminonaphthalene method.6, 29 The NO in the LPS (200 ng/mL) group was normalized to 100%.
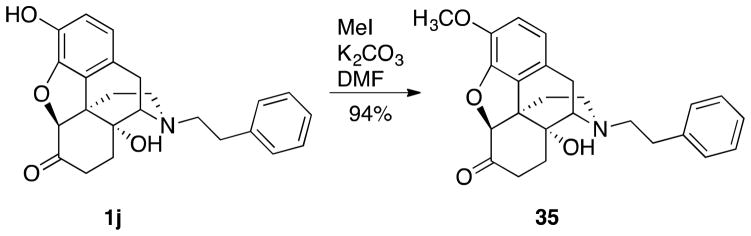
Comparison of Phenol Versus Methyl Ether
All the compounds synthesized and tested up to this point contained a phenol as found in classic agonist and antagonist opiates such as oxymorphone, morphine, and naltrexone. In order to determine if the phenol functionality was necessary for TLR4 activity or whether it had an effect on toxicity, methyl ether 35 ((4aR,7aS,12bR)-4a-hydroxy-9-methoxy-3-phenethyl-2,3,4,4a,5,6-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-7(7aH)-one) was synthesized (Scheme 7) for comparison with the phenol 1j in the BV-2 microglia cell assay. The TLR4 antagonist activity of methyl ether 35 was found to be ~4 fold less (IC50 = 5.7 ± 1.3 μM) than that of phenol 1j (IC50 = 1.4 ± 1.3 μM). In addition, the cell viability assay showed greater cell cytotoxicity for 35 (IC50 of viability = 74.7 ± 3.5 μM) than 1j (IC50 of viability = 140.8 ± 5.5 μM).
Scheme 7.
Synthesis of (+)-N-Phenethylnoroxycodone 35
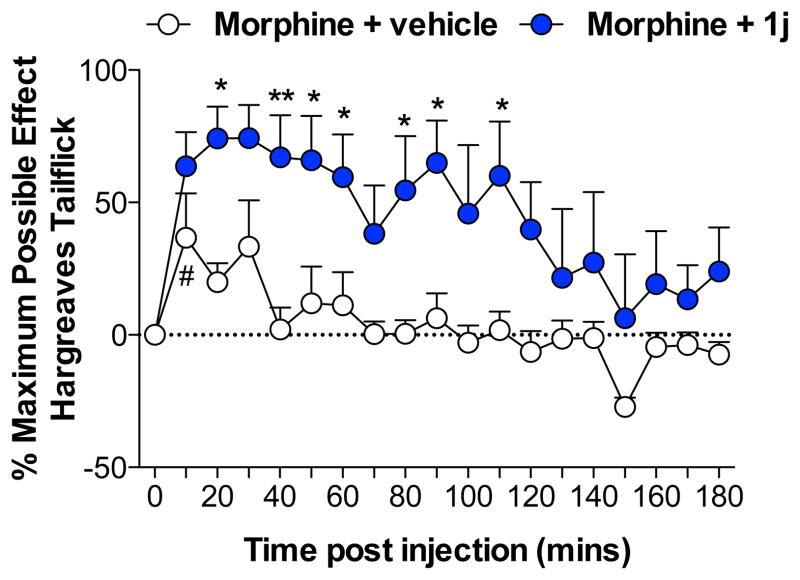
Finally, the in vivo efficacy of (+)-N-phenethylnoroxymorphone (1j) was tested on the Hargreaves thermal hyperalgesia assay.35 Potentiation of morphine analgesia was tested as a clinically relevant outcome, since morphine signaling at TLR4 opposes analgesia in a pro-inflammatory fashion.5, 36 Here, intrathecal morphine (15 μg plus vehicle; open circle) produced significant analgesia to radiant heat, compared to baseline (Fig. 8; P < 0.05). Coadministration of 1j (60 μg, intrathecal; closed circle) significantly increased and prolonged intrathecal morphine analgesia (Figure 8; Drug x Time: F18,180 = 0.83, P = 0.6; Drug: F1,10 = 16.7, P < 0.01; Time: F18,180 = 3.73, P < 0.001).
Figure 8.
Intrathecal coadministration of morphine (15 μg) with 1j (60 μg) produced a significant potentiation of morphine tailflick analgesia. n = 6/group *P < 0.05, **P < 0.01: relative to vehicle; #P < 0.05: relative to baseline.
CONCLUSION
Stereoselective reduction of the ketone of (+)-naltrexone 1a to the (+)-naltrexols 2 and 3 resulted in a slight loss of TLR4 antagonist activity, while removal of the oxide bridge and ketone to give (+)-didesoxynaltrexone 5 afforded an ~15 fold increase in activity relative to (+)-naltrexone 1a. Removal of the bridging benzylic C-10 carbon of (+)-naltrexone resulted in an analog, (+)-10-nornaltrexone 7, with only a slight improvement in TLR4 antagonism. Interestingly, the replacement of the N-cyclopropylmethyl substituent in (+)-naltrexone with a linear alkyl chain (4–8 carbons) resulted in greatly increased activity (~4–62 fold). There was, however, increased cell cytotoxicity, and a poorer toxicity/activity ratio, associated with longer chain length. The N-phenylalkyl analogs were found to be among the most potent TLR-4 antagonists (up to 88 fold compared to (+)-naltrexone). N-Phenethyl through N-phenylpentyl analogs were found to have similar TLR4 antagonism activity (IC50 ≈ 1.4 μM), while the cell cytotoxicity increased with chain length. Finally, it was found that a methyl ether on the aromatic ring, as in 35, decreases TLR4 antagonism and increases toxicity compared with the corresponding phenolic compound. The N-phenethyl-substituted noroxymorphone (1j) was among the most potent compounds found and had the best ratio (~ 100) of TLR4 potent antagonist activity (IC50 = 1.4 ± 0.3 μM), and cell cytotoxicity (IC50 of viability = 140.8 ± 5.5). This analog 1j also showed in vivo efficacy in potentiating morphine analgesia.
EXPERIMENTAL
Cell based characterization
NO assay
NO assays were performed as described previously.6 BV-2 microglia cells were grown in DMEM medium supplemented with 10% FBS. BV-2 cells were detached from the flask by trypsin digestion when ~80% confluence was reached. Cells were seeded at a density of 4×104 cells/well in 96-well plates. After overnight incubation, the medium was aspirated and changed to DMEM (without FBS). LPS (200 ng/mL) and indicated concentration of compounds were added. Following an additional 24 h treatment, 100 μL of media was removed and added to flat black 96-well microfluor plates (Thermo Scientific, MA, USA). Subsequently, 10 μL of 2,3-diaminonaphthalene (0.05 mg/mL in 0.62 M HCl) was added to each well and incubated for 15 min. The reaction was quenched by addition of 5 μL of 3 M NaOH and the plate was read on a Beckman Coulter DTX880 reader (Fullerton, CA, USA) with excitation at 360 nm and emission at 430 nm. Assay of NO in BV-2 microglia cells, rather than assay of HEK293 TLR4 over-expressing cells, was chosen as the HEK293 TLR4 cells can only report activation of NFkappaB signaling pathways so do not appropriately represent the complexity of TLR4 signaling.37, 38
Cell viability assay
Crystal violet staining was used to determine cell viability as described previously.5, 6 After drug treatment, cells were fixed by 3.7% paraformaldehyde (PFA) for 5 min. and then stained by 0.05% crystal violet for 30 min. The plates were subsequently washed 2 times with tap water and dried for 30 min at room temperature. 200 μL of ethanol was added to each well and the plates were shaken for 15 min at room temperature to dissolve the dye. Absorbance at 540 nm was measured with a Beckman Coulter DTX880 reader (Fullerton, CA, USA)
Data analysis
Origin 7.5 (OriginLab Corporation, Northampton, MA, USA) was used for plotting of the data and statistical analysis. Non-linear logistic regression was used to plot and analyze concentration-response curves and to obtain IC50 values. Each experiment was independently repeated at least 3 times with 3–4 biological replicates
In vivo characterization
Animals
Pathogen-free, adult male Sprague Dawley rats were used (300–325 g on arrival; Harlan Labs, Madison, WI), and housed in temperature (23 ± 3 °C) and light (12 h:12 h light:dark cycle; lights on at 0700) controlled rooms with standard rodent chow and water available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Colorado Boulder.
Intrathecal catheter implantation and drug delivery
The method of acute intrathecal drug administration and the construction and implantation of the indwelling intrathecal catheters was based on work described previously.39 In brief, the day prior to experimentation, intrathecal operations were conducted under isoflurane anesthesia by threading sterile polyethylene-10 tubing (PE-10 Intramedic Tubing; Becton Dickinson Primary Care Diagnostics, Sparks, MD, USA) guided by an 18-gauge needle between the L5 and L6 vertebrae. The catheter was inserted such that the proximal catheter tip lay over the lumbosacral enlargement. The needle was removed and the catheter was sutured to the superficial musculature of the lower back. The catheters were pre-loaded with 60 μg of 1j in 1.5% DMSO in 0.9% saline + 15 μg morphine in 0.9% saline or vehicle (1.5% DMSO in 0.9% saline + 15 μg morphine in 0.9% saline) at the distal end in a total volume of 10 μL. The catheters were 90 cm in length, allowing remote drug delivery without touching or otherwise disturbing the rats during the testing. On the day of experimentation, drugs were delivered over 20–30 s.
Hargreaves test for analgesia
Rats received at least three 60 min habituations over successive days to the test environment prior to behavioral testing. Latencies for behavioral response to radiant heat stimuli applied to the tail were assessed using a modified Hargreaves test.35 All testing was conducted blind with respect to group assignment. Briefly, baseline withdrawal values were calculated from an average of three consecutive withdrawal latencies of the tail, measured at 10 min intervals. A short baseline latency (2–3 s) was used to allow quantification of analgesia (lengthening of the latency, relative to baseline, in response to analgesia). A cut-off time of 10 s was imposed to avoid tissue damage. Nociceptive assessments for acute administration experiments were then made at 0 (immediately following remote drug delivery) and every 10 min thereafter for 180 mins.
Statistics and data analysis
The analgesic responses were calculated, as is standard in the pain field, as the % of maximal possible effect (%MPE) using the following equation %MPE = test latency - baseline latency/cutoff – baseline latency x 100. Repeated measures two-way ANOVA with a Holm Sidak posthoc test was used to determine group differences. Repeated measures one-way ANOVA with a Dunnett posthoc test was used to determine changes from baseline. Data are presented as mean ± SEM, and significance was set at P < 0.05.
Chemistry
General Methods
Melting points were determined on a Buchi B-545 instrument and are uncorrected. Proton and carbon nuclear magnetic resonance (1H and 13C NMR) spectra were recorded on a Varian Gemini-400 spectrometer in CDCl3 (unless otherwise noted) with the values given in ppm (TMS as internal standard) and J (Hz) assignments of 1H resonance coupling. Mass spectra (HRMS) were recorded on a VG 7070E spectrometer or a JEOL SX102a mass spectrometer. The optical rotation data were obtained on a PerkinElmer polarimeter model 341. Thin layer chromatography (TLC) analyses were carried out on Analtech silica gel GHLF 0.25 mm plates using various gradients of CHCl3/MeOH containing 1% NH4OH or gradients of EtOAc:n-hexane. Visualization was accomplished under UV light or by staining in an iodine chamber. Flash column chromatography was performed with Fluka silica gel 60 (mesh 220–400). Atlantic Microlabs, Inc., Norcross, GA, or Micro-Analysis, Inc., Wilmington, DE, performed elemental analyses, and the results were within ±0.4% of the theoretical values.
(4S,4aR,7aS,12bR)-3-Butyl-4a,9-dihydroxy-2,3,4,4a,5,6-hexahydro-1H-4,12- methanobenzofuro[3,2-e]isoquinolin-7(7aH)-one (1b)
To a stirred solution of (+)-N-noroxymorphone dihydrate 19 (0.500 g, 1.54 mmol) in DMF (10 mL) in a screw cap vial was added NaHCO3 (0.502 g, 5.98 mmol) and 1-bromobutane (0.365 mL, 3.39 mmol). The vial was capped (the cap was teflon-lined) and heated at 90 °C for 3 h. The reaction mixture was cooled to room temperature, filtered through Celite, and concentrated in vacuo. To the crude residue was added H2O (10 mL), concentrated aq NH4OH (2 mL) and CHCl3 (20 mL). The organics were separated and the aqueous layer was extracted with CHCl3 (20 mL). The combined organics were washed with H2O (5 × 20 mL), dried over Na2SO4, filtered through Celite, and concentrated in vacuo to afford (+)-N-butylnoroxymorphone 1b as a crude white solid. Purification of this solid by SiO2 column chromatography with 10% NH4OH in MeOH/CHCl3 (gradient, 0 → 4%) afforded 1b (0.151 g, 29%) as a white solid. All spectra reported on the free base. [α]20D = + 179.5 (1.1, CHCl3); mp 150–151 °C; IR (thin film) 3184 cm−1; 1H NMR (400 MHz, CDCl3) δ 6.69 (d, J = 8.0 Hz, 1H), 6.54 (d, J = 8.4 Hz, 1H), 6.08 (br s, 2H), 4.73 (s, 1H), 3.08-2.94 (m, 3H), 2.57-2.38 (m, 5H), 2.26 (m, 1H), 2.13 (td, J = 12.0, 3.6 Hz, 1H), 1.86 (m, 1H), 1.61 (td, J = 14.0, 3.2 Hz, 1H), 1.52 (m, 1H), 1.48-1.29 (m, 4H), 0.90 (t, J = 7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 209.8, 143.5, 138.8, 128.9, 123.9, 119.6, 117.9, 90.3, 70.5, 62.6, 53.9, 50.8, 43.4, 36.0, 31.1, 30.5, 29.6, 22.7, 20.3, 13.9; HRMS (TOF MS ES+) calcd for C20H26NO4 (M + H+) 344.1856, found 344.1857. An HCl salt was prepared by dissolving 1b free base in hot i-PrOH (1.0 mL) followed by the addition of concentrated aq HCl (0.10 mL, 3 equiv) and cooling to 5 °C. The crystals were filtered, rinsed with cold i-PrOH, and air-dried to give 1b as its HCl•2H2O salt. Anal. Calcd for C20H30ClNO6 (1b•HCl•2H2O): CHN.
(4S,4aR,7aS,12bR)-4a,9-Dihydroxy-3-pentyl-2,3,4,4a,5,6-hexahydro-1H-4,12- methanobenzofuro[3,2-e]isoquinolin-7(7aH)-one (1c)
The same procedure used for 1b was also used for 1c, with 19 (0.500 g, 1.54 mmol), pentyl bromide (0.421 mL, 3.39 mmol), NaHCO3 (0.502 g, 5.98 mmol), and DMF (10 mL). Purification of the resulting solid by SiO2 column chromatography with 10% NH4OH in MeOH/CHCl3 (gradient, 0 → 4%) afforded N-pentylnoroxymorphone 1c (0.506 g, 92%) as a white solid. All spectra reported on the free base. [α]20 D = + 173.1 (1.2, CHCl3); mp 168–169 °C; IR (thin film) 3181 cm−1; 1H NMR (400 MHz, CDCl3) δ 6.71 (d, J = 8.4 Hz, 1H), 6.58 (d, J = 8.0 Hz, 1H), 4.70 (s, 1H), 3.11-2.88 (m, 3H), 2.59-2.28 (m, 5H), 2.15 (td, J = 12.0, 4.0 Hz, 1H), 1.87 (m, 1H), 1.67-1.45 (m, 3H), 1.37-1.27 (m, 4H), 0.91 (t, J = 7.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 209.7, 143.5, 138.8, 129.0, 124.0, 119.7, 117.8, 90.4, 70.5, 62.7, 54.2, 50.9, 43.4, 36.1, 31.2, 30.5, 29.4, 27.2, 22.7, 22.5, 14.0; HRMS (TOF MS ES+) calcd for C21H28NO4 (M + H+) 358.2013, found 358.2013. An HCl salt was prepared by dissolving 1c free base in hot i-PrOH (3.5 mL) followed by concentrated HCl (0.34 mL, 3 equiv) and cooling to 5 °C. The crystals were filtered, rinsed with cold i-PrOH, and air-dried to give 1c as the HCl salt. Anal. Calcd for C21H28ClNO4 (1c•HCl): CHN.
(4S,4aR,7aS,12bR)-3-Hexyl-4a,9-dihydroxy-2,3,4,4a,5,6-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-7(7aH)-one (1d)
The same procedure used for 1b was also used for 1d, with 19 (3.00 g, 9.25 mmol), 1-bromohexane (2.90 mL, 20.4 mmol), NaHCO3 (3.1 g, 37 mmol), and DMF (60 mL) to afford (+)-N-hexylnoroxymorphone 1d (3.0 g, 87%) as an off-white solid that was used without further purification. All spectra reported on the free base. [α]20 D = + 187.3 (1.1, CHCl3); mp 160–162 °C; IR (thin film) 3190 cm−1; 1H NMR (400 MHz, CDCl3) δ 6.70 (d, J = 8.4 Hz, 1H), 6.55 (d, J = 8.0 Hz, 1H), 6.24 (br s, 1H), 4.75 (s, 1H), 3.09-2.95 (m, 3H), 2.58-2.39 (m, 5H), 2.29 (d, J = 14.8 Hz, 1H), 2.13 (t, J = 11.6, 3.2 Hz, 1H), 1.86 (m, 1H), 1.62 (td, J = 14.0, 3.2 Hz, 1H), 1.53 (m, 1H), 1.48-1.40 (m, 2H), 1.39-1.21 (m, 6H), 0.87 (t, J = 6.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 209.7, 143.5, 138.8, 128.9, 123.9, 119.6, 117.8, 90.3, 70.5, 62.6, 54.2, 50.8, 43.3, 36.0, 31.6, 31.1, 30.5, 27.4, 26.8, 22.7, 22.5, 14.0; HRMS (TOF MS ES+) calcd for C22H30NO4 (M + H+) 372.2169, found 372.2169. An HCl salt was prepared by dissolving 1d free base in hot i-PrOH (21 mL) followed by concentrated HCl (1.9 mL, 3 equiv) and cooling to 5 °C to give 1d as the HCl salt. Anal. Calcd for C22H30ClNO4 (1d•HCl): CHN.
(4S,4aR,7aS,12bR)-3-Heptyl-4a,9-dihydroxy-2,3,4,4a,5,6-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-7(7aH)-one (1e)
The same procedure used for 1b was also used for 1e, with 19 (0.500 g, 1.54 mmol), 1-bromoheptane (0.533 mL, 3.39 mmol), NaHCO3 (0.502 g, 5.98 mmol), and DMF (10 mL). Purification of this solid by SiO2 column chromatography with 10% NH4OH in MeOH/CHCl3 (gradient, 0 → 4%) afforded 1e (0.319 g) that still contained some DMF. To the impure 1e was added i-PrOH (1.9 mL) followed by concentrated aq HCl (0.20 mL). The suspension was cooled to 5 °C, filtered, rinsed with cold i-PrOH (2 × 2 mL), and air-dried to afford pure (+)-N-heptylnoroxymorphone 1e (0.291 g, 45%) as a white HCl•0.3H2O salt. All spectra reported on the free base. [α]20D = + 174.7 (1.2, CHCl3); mp 143–145 °C; IR (thin film) 3170 cm−1; 1H NMR (400 MHz, CDCl3) δ 6.70 (d, J = 8.0 Hz, 1H), 6.54 (d, J = 8.0 Hz, 1H), 6.03 (br s, 2H), 4.73 (s, 1H), 3.08-2.94 (m, 3H), 2.57-2.38 (M, 4H), 2.28 (d, J = 14.4 Hz, 1H), 2.13 (m, 1H), 1.65-1.44 (m, 4H), 1.40-1.20 (m, 6H), 0.86 (t, J = 6.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 209.8, 143.5, 138.8, 128.9, 124.0, 119.7, 117.9, 90.4, 70.5, 62.6, 54.2, 50.9, 43.4, 36.0, 31.7, 31.2, 30.5, 29.1, 27.5, 27.2, 22.7, 22.5, 14.0; HRMS (TOF MS ES+) calcd for C23H32NO4 (M + H+) 386.2326, found 386.2327. Anal. Calcd for C23H32.6ClNO4.3 (1e•HCl•0.3H2O): CHN.
(4S,4aR,7aS,12bR)-4a,9-Dihydroxy-3-octyl-2,3,4,4a,5,6-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-7(7aH)-one (1f)
The same procedure used for 1b was also used for 1f, with 19 (0.500 g, 1.54 mmol), 1-bromooctane (0.589 mL, 3.39 mmol), NaHCO3 (0.502 g, 5.98 mmol), and DMF (10 mL). Purification of this solid by SiO2 column chromatography with 10% NH4OH in MeOH/CHCl3 (gradient, 0 → 4%) afforded 1f (0.414 g) still containing some DMF. To the impure 1f was added i-PrOH (2.5 mL) followed by concentrated aqueous HCl (0.25 mL). The suspension was cooled to 5 °C, filtered, rinsed with cold i-PrOH (2 × 2 mL), and air-dried to afford pure (+)-N-octylnoroxymorphone 1f (0.391 g, 58%) as a white HCl•0.15H2O salt. All spectra reported on the free base. [α]20D = + 156.8 (1.2, CHCl3); mp 139–141 °C; IR (thin film) 3184 cm−1; 1H NMR (400 MHz, CDCl3) δ 6.71 (d, J = 8.4 Hz, 1H), 6.57 (d, J = 8.0 Hz, 1H), 4.71 (s, 1H), 3.10-2.94 (m, 3H), 2.59-2.37 (m, 4H), 2.27 (m, 1H), 2.15 (td, J = 12.0, 3.6 Hz, 1H), 1.87 (m, 1H), 1.66-1.44 (m, 3H), 1.30-1.25 (m, 8H), 0.88 (t, J = 6.6 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 209.8, 143.5, 138.8, 128.9, 123.9, 119.6, 117.9, 90.3, 70.5, 62.6, 54.2, 50.8, 43.3, 36.0, 31.7, 31.2, 30.5, 29.4, 29.1, 27.5, 27.2, 22.7, 22.5, 14.0; HRMS (TOF MS ES+) calcd for C24H34NO4 (M + H+) 400.2482, found 400.2481. Anal. Calcd for C24H34.3ClNO4.15 (1f•HCl•0.15H2O): CHN.
(4S,4aR,7aS,12bR)-4a,9-Dihydroxy-3-(5-phenylpentyl)-2,3,4,4a,5,6-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-7(7aH)-one (1g)
The same procedure used for 1b was also used for 1g, with 19 (0.400 g, 1.24 mmol), 1-bromo-5-phenylpentane (0.504 mL, 2.73 mmol), NaHCO3 (0.402 g, 4.79 mmol), and DMF (8 mL). Purification of this solid by SiO2 column chromatography with 10% NH4OH in MeOH/CHCl3 (gradient, 0 → 4%) afforded (+)-N-phenylpentylnoroxymorphone 1g (0.500 g, 89% corrected for DMF) as an off-white solid. All spectra reported on the free base unless otherwise noted. [α]20 D = + 144.3 (1.2, MeOH, HCl salt); mp of HCl salt dec > 276 °C; IR (thin film) 3286, 1723 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.29-7.25 (m, 2H), 7.19-7.16 (m, 3H), 6.73 (d, J = 8.4 Hz, 1H), 6.57 (d, J = 8.0 Hz, 1H), 6.01 (br s, 2H), 4.70 (s, 1H), 3.09-2.99 (m, 3H), 2.62-2.38 (m, 7H), 2.29 (dt, J = 14.0, 2.8 Hz, 1H), 2.16 (td, 12.0, 3.6 Hz, 1H), 1.88 (m, 1H), 1.67-1.49 (m, 6H), 1.41-1.35 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 210.0, 143.6, 142.5, 139.1, 128.9, 128.4, 128.3, 125.7, 123.8, 119.8, 118.1, 90.3, 70.5, 62.6, 54.2, 50.9, 43.6, 36.6, 36.1, 35.8, 31.3, 30.5, 27.3, 26.9, 22.9; HRMS (TOF MS ES+) calcd for C27H32NO4 (M + H+) 434.23258, found 434.23233. An HCl salt was prepared by dissolving 1g free base in hot i-PrOH (3.5 mL) followed by concentrated aq HCl (0.28 mL, 3 equiv) and cooling to 5 °C. The crystals were filtered, rinsed with cold i-PrOH, and air-dried to give the HCl salt. Anal. Calcd for C27H32ClNO4 (1g•HCl): CHN.
(4S,4aR,7aS,12bR)-4a,9-Dihydroxy-3-(4-phenylbutyl)-2,3,4,4a,5,6-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-7(7aH)-one (1h)
The same procedure used for 1b was also used for 1h, with 19 (0.400 g, 1.24 mmol), 1-bromo-4-phenylbutane (0.479 mL, 2.73 mmol), NaHCO3 (0.402 g, 4.79 mmol), and DMF (8 mL). Purification of this solid by SiO2 column chromatography with 10% NH4OH in MeOH/CHCl3 (gradient, 0 → 4%) afforded (+)-N-phenylbutylnoroxymorphone 1h (0.398 g, 77%) as an off white solid. All spectra reported on the free base. [α]20 D = + 145.8 (1.1, DMSO); mp 223–225 °C; IR (thin film) 3154, 1724 cm−1; 1H NMR (400 MHz, DMSO-D6) δ 9.18 (br s, 1H), 7.27-7.12 (m, 5H), 6.54 (d, J = 8.4 Hz, 1H), 6.48 (d, J = 8.0 Hz, 1H), 4.99 (br s, 1H), 4.72 (s, 1H), 2.97-2.86 (m, 3H), 2.57 (t, J = 7.2 Hz, 2H), 2.48-2.41 (m, 3H), 2.28 (m, 1H), 2.06 (d, J = 14.0 Hz, 1H), 1.93 (m, 1H), 1.73 (m, 1H), 1.61-1.57 (m, 2H), 1.45-1.38 (m, 2H), 1.26 (d, J = 11.2 Hz, 1H); 13C NMR (100 MHz, DMSO-D6) δ 209.1, 143.8, 142.6, 139.8, 129.8, 128.7, 126.1, 123.7, 119.4, 117.6, 89.8, 70.3, 62.4, 53.7, 50.6, 43.6, 36.2, 35.4, 31.5, 30.6, 29.1, 27.0, 22.8; HRMS (TOF MS ES+) calcd for C26H30NO4 (M + H+) 420.21693, found 420.21701. An HCl salt was prepared by dissolving 1h free base in hot EtOH (2.8 mL) with acetic acid (0.80 mL) and concentrated aq HCl (0.23 mL, 3 equiv) and cooling to 5 °C. The crystals were filtered, rinsed with cold i-PrOH, and air-dried to give the HCl•0.5EtOH salt. Anal. Calcd for C27H33ClNO4.5 (1h•HCl•0.5EtOH): CHN.
(4S,4aR,7aS,12bR)-4a,9-Dihydroxy-3-(3-phenylpropyl)-2,3,4,4a,5,6-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-7(7aH)-one (1i)
The same procedure used for 1b was also used for 1i, with 19 (0.400 g, 1.24 mmol), 1-bromo-3-phenylpropane (0.415 mL, 2.73 mmol), NaHCO3 (0.402 g, 4.79 mmol), and DMF (8 mL). Purification of this solid by SiO2 column chromatography with 10% NH4OH in MeOH/CHCl3 (gradient, 0 → 4%) afforded (+)-N-phenylpropylnoroxymorphone 1i (0.430 g, 50% corrected for DMF) as a light orange oil containing ~ 80 mol % DMF. All spectra reported on the free base unless otherwise noted. [α]20D = + 152.5 (1.0, MeOH, HCl•0.25H2O salt); mp of HCl•0.25H2O salt dec > 276 °C; IR (thin film) 3332, 1724 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.28-7.25 (m, 2H), 7.18-7.15 (m, 3H), 6.70 (d, J = 8.4 Hz, 1H), 6.53 (d, J = 8.4 Hz, 1H), 4.65 (s, 1H), 3.06 (m, 3H), 2.65 (t, J = 7.6 Hz, 2H), 2.59-2.46 (m, 4H), 2.38 (t, J = 12.6, 5.0 Hz, 1H), 2.25 (dt, J = 14.4, 3.0 Hz, 1H), 2.13 (td, J = 12.0, 3.6 Hz, 1H), 1.88-1.77 (m, 3H), 1.59 (td, J = 14.0, 3.2 Hz, 1H), 1.51 (dd, J = 12.8, 2.2 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 209.7, 143.6, 141.7, 139.2, 128.9, 128.4, 128.3, 125.9, 123.6, 119.7, 118.1, 90.2, 70.5, 62.8, 53.6, 50.8, 43.5, 36.1, 33.4, 31.2, 30.5, 29.1, 22.9; HRMS (TOF MS ES+) calcd for C25H28NO4 (M + H+) 406.20128, found 406.20123. An HCl salt was prepared by dissolving 1i free base in hot i-PrOH (3.0 mL) followed by concentrated aq HCl (0.26 mL, 3 equiv) and cooling to 5 °C. The crystals were filtered, rinsed with cold i-PrOH, and air-dried to give the HCl•0.25H2O salt. Anal. Calcd for C24H28.5ClNO4.25 (1i•HCl•0.25H2O): CHN.
(4S,4aR,7aS,12bR)-4a,9-Dihydroxy-3-phenethyl-2,3,4,4a,5,6-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-7(7aH)-one (1j)
The same procedure used for 1b was also used for 1j, with 19 (0.500 g, 1.54 mmol), 2-bromoethylbenzene (0.458 mL, 3.39 mmol), NaHCO3 (0.502 g, 5.98 mmol), and DMF (10 mL). Purification of this solid by SiO2 column chromatography with 10% NH4OH in MeOH/CHCl3 (gradient, 0 → 4%) afforded (+)-N-phenethylnoroxymorphone 1j (0.548 g, 91%) as a white solid. All spectra reported on the free base. [α]20D = +139.1 (1.1, CHCl3); mp 80–84 °C; IR (thin film) 3165 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.32 (app t, J = 7.2 Hz, 2H), 7.25-7.19 (m, 3H), 6.71 (d, J = 8.0 Hz, 1H), 6.58 (d, J = 8.0 Hz, 1H), 4.68 (s, 1H), 3.08 (d, J = 18.4 Hz, 1H), 3.03-2.88 (m, 2H), 2.74-2.54 (m, 6H), 2.39-2.17 (m, 3H), 1.83 (m, 1H), 1.63-1.50 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 209.7, 143.5, 139.7, 138.8, 128.9, 128.5, 128.4, 126.2, 123.8, 119.7, 117.9, 90.2, 70.5, 63.5, 55.9, 50.8, 42.9, 36.0, 34.1, 31.1, 30.3, 23.3; HRMS (TOF MS ES+) calcd for C24H26NO4 (M + H+) 392.1856, found 392.1854. An HCl salt was prepared by dissolving 1j free base in hot i-PrOH (3.8 mL) followed by concentrated aq HCl (0.34 mL, 3 equiv) and cooling to 5 °C. The crystals were filtered, rinsed with cold i-PrOH, and air-dried to give the HCl•2H2O salt. Anal. Calcd for C24H30ClNO6 (1j•HCl•2H2O): CHN.
(4S,4aR,7aS,12bR)-3-(2-Fluorophenethyl)-4a,9-dihydroxy-2,3,4,4a,5,6-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-7(7aH)-one (1k)
The same procedure used for 1b was also used for 1k, with 19 (0.500 g, 1.54 mmol), 2-fluorophenethylbromide (0.476 mL, 3.39 mmol), NaHCO3 (0.502 g, 5.98 mmol), and DMF (10 mL). Purification of this solid by SiO2 column chromatography with 10% NH4OH in MeOH/CHCl3 (gradient, 0 → 4%) afforded (+)-N-2-fluorophenethylnoroxymorphone 1k (0.461 g, 73%) as a light yellow solid. All spectra reported on the free base. [α]20D = +132.6 (0.5, CHCl3); mp 70–74 °C; IR (thin film) 3358 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.23-7.18 (m, 2H), 7.10 (d, J = 7.6 Hz, 1H), 7.05 (t, J = 9.2 Hz, 1H), 6.71 (d, J = 8.0 Hz, 1H), 6.58 (d, J = 8.0 Hz, 1H), 3.09-2.57 (m, 9H), 2.40-2.18 (m, 3H), 1.82 (m, 1H), 1.62-1.54 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 209.9, 162.4, 159.9, 143.6, 139.0, 130.83, 130.79, 128.9, 128.1, 128.06, 126.8, 126.6, 124.1, 123.9, 119.8, 118.1, 115.4, 115.2, 90.3, 70.5, 63.9, 54.7, 50.8, 42.9, 36.0, 31.2, 30.4, 27.7, 23.5; HRMS (TOF MS ES+) calcd for C24H25NO4F (M + H+) 410.1762, found 410.1762. An HCl salt was prepared by dissolving 1k free base in hot i-PrOH (3.2 mL) followed by concentrated aq HCl (0.27 mL, 3 equiv) and cooling to 5 °C. The crystals were filtered, rinsed with cold i-PrOH, and air-dried to give the HCl•0.5H2O salt. Anal. Calcd for C24H26ClFNO4.5 (1k•HCl•0.5H2O): CHN.
(4S,4aR,7S,7aS,12bR)-3-(Cyclopropylmethyl)-2,3,4,4a,5,6,7,7a-octahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinoline-4a,7,9-triol (2)
The procedures that de Costa et al. used on the enantiomer were followed.22 To a stirring solution of (+)-naltrexone 1a free base (1.00 g, 2.93 mmol) in 0.533 M NaOH (20 mL) was added a solution of formamidine sulfinic acid (1.01 g, 11.7 mmol) in 0.533 M NaOH (20 mL) dropwise over 15 min. The reaction mixture was warmed to 85 °C and maintained at that temperature for 1 h and 15 min. The reaction mixture was cooled to ~ 5 °C and a saturated NH4Cl aqueous solution (40 mL) followed by CHCl3 (40 mL) were added. The aqueous fraction was extracted with CHCl3 (3 × 100 mL), washed with H2O (100 mL), filtered through Celite, and the combined organics were concentrated in vacuo. The crude residue was dried under high vacuum at 100 °C for 14 h to give crude 2 (0.805 g, 80%). All spectral data matched those reported by de Costa et al. on the enantiomer.22 [α]20 D = +196.1 (1.0, CHCl3); mp 185–186 °C. To the crude free base 2 (0.805 g) was added EtOAc (8 mL) and (1R,3S)-(+)-camphoric acid (0.469 g, 2.34 mmol). The mixture was heated to solution, and cooled to room temperature causing salt formation. The salt crystals were filtered and rinsed with EtOAc (8 mL) to afford 2 (0.902 g) as the (1R, 3S)-(+)-camphoric acid • H2O. Anal. Calcd for (C30H43NO9) (2•camphoric acid•H2O): CHN.
(4S,4aR,7R,7aS,12bR)-3-(Cyclopropylmethyl)-2,3,4,4a,5,6,7,7a-octahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinoline-4a,7,9-triol (3)
The procedures were followed that de Costa et al. used on the enantiomer.22 To a stirred solution of (+)-naltrexone 1a free base (1.00 g, 2.93 mmol) in THF (30 mL) was added 35% KH in mineral oil (0.675 mL, 5.86 mmol). The reaction mixture was cooled to 5 °C and K-Selectride (4.40 mL, 4.40 mmol, 1.0M in THF) was added dropwise over 20 min. After stirring at 5 °C for 20 min, the reaction mixture was warmed to room temperature. After stirring at room temperature for 4 h, H2O (5 mL) was added and the reaction mixture was concentrated in vacuo to remove organics. Additional H2O (10 mL) was added followed by 3M HCl to arrive at a pH of ~ 3. The aqueous mixture was washed with Et2O (3 × 20 mL), basified with concentrated aqueous NH4OH to a pH of ~ 9.5, and extracted with CHCl3 (3 × 30 mL). The combined organics were filtered through Celite and concentrated in vacuo to afford a crude solid (0.90 g). This crude solid was recrystallized from EtOAc (6 mL) to give 3 (0.570 g, 57%) as a white solid. All spectral data matched those reported by de Costa et al. on the enantiomer.22 [α]20D = +202.1 (0.7, MeOH); mp 210–211 °C.
To 3 (0.478 g) was added EtOAc (5 mL) and (1R,3S)-(+)-camphoric acid (0.279 g, 1.39 mmol). The mixture was heated to solution and subsequently cooled to room temperature, causing salt formation. The salt crystals were filtered and rinsed with EtOAc (5 mL) to afford 3 (0.254 g) as the (1R, 3S)-(+)-camphoric acid • 0.7H2O. Anal. Calcd for C30H42.4NO8.7 (3•camphoric acid•0.7H2O): CHN
(4bS,8aR,9S)-11-(Cyclopropylmethyl)-3,8a-dihydroxy-8,8a,9,10-tetrahydro-5H-9,4b-(epiminoethano)phenanthren-6(7H)-one (4)
The procedure of Nagase et al.30 on the (−)-isomer was followed for the synthesis of 20 from 1a. To a stirred solution of (+)-naltrexone 1a free base (2.20 g, 6.45 mmol) was added DMF (60 mL), K2CO3 (1.84 g, 13.3 mmol), and MeI (0.484 mL, 7.76 mmol). After stirring at room temperature for 18 h, the reaction mixture was filtered through Celite and concentrated in vacuo. To the crude mixture was added H2O (20 mL) and Et2O (20 mL). The aq layer was extracted with Et2O (3 × 20 mL) and the combined organics were washed with H2O (5 × 20 mL), filtered through Celite, and concentrated in vacuo to afford 20 (1.98 g) as a white solid which was used without further purification.
The sequence from 20 → 24 followed the procedures of Nagase et al.31 To a solution of crude 20 (1.98, 5.57 mmol) in MeOH (50 mL) was added NH4Cl (2.98 g, 55.7 mmol). The reaction mixture was heated to reflux and activated Zn (2.55 g, 39.0 mmol) was added in 3 portions spaced 1 h apart. After refluxing for a total of 3 h, the reaction mixture was cooled to room temperature and filtered through Celite, rinsing with MeOH (50 mL). The filtrate was concentrated in vacuo and 3M NaOH (25 mL) was added followed by 10% MeOH in CHCl3 (50 mL). The aqueous layer was extracted with 10% MeOH in CHCl3 (2 × 50 mL), and the combined organics were dried over Na2SO4, filtered and concentrated to afford 21 as a white solid which was used without further purification.
To a solution of the crude 21 in pyridine (20 mL) was added concentrated NH4OH (2.30 g, 16.7 mmol), Cu powder (0.372 g, 5.85 mmol), and bromobenzene (2.93 mL, 27.9 mmol). The reaction mixture was heated to reflux. After refluxing for 14 h, the reaction mixture was cooled to room temperature, filtered through Celite and concentrated in vacuo to give crude 22 as an oil. This crude oil was purified by SiO2 column chromatography with 10% NH4OH in MeOH/CHCl3 (gradient, 0 → 8%) to afford 22 (0.740 g, 26% from 1a) as a white solid. Spectral data matched those reported by Nagase et al.,31 on the enantiomer.
To a stirred solution of 22 (0.491 g, 1.13 mmol) in toluene (11 mL) was added ethylene glycol (0.630 mL, 11.3 mmol) and p-TsOH•H2O (0.344 g, 1.81 mmol). The reaction mixture outfitted with a Dean-Stark apparatus was brought to reflux. After refluxing for 4 h, the reaction mixture was cooled to room temperature and aqueous saturated NaHCO3 (15 mL) followed by K2CO3 were added to bring the mixture to pH ~ 9.5. The aqueous layer was extracted with CHCl3 (3 × 15 mL), filtered through Celite, and concentrated in vacuo to afford crude 23 (0.512 g) as a white solid, which was used without further purification.
To a stirred solution of crude 23 (0.512 g) in NH3 (liquid) (6 mL) and THF (6 mL) at −78 °C, was added Na (0.062 g, 2.7 mmol) to give a blue solution. The reaction mixture was maintained at −78 °C and additional Na pieces were added to maintain the blue color over the course of 3 h. After 3 h at −78 °C, EtOH (2 mL) was added and the reaction mixture was warmed to room temperature. Brine (20 mL) and toluene (20 mL) were added to the reaction mixture. The aqueous layer was extracted with toluene (3 × 20 mL) and the combined organics were dried over Na2SO4, filtered through Celite and concentrated in vacuo to afford crude 24 (0.318 g), which was used without further purification.
To crude 24 (0.318 g) was added 2M HCl (8 mL) and the reaction mixture was heated to reflux. After refluxing for 5 h, the reaction mixture was cooled to room temperature and concentrated aqueous NH4OH (3 mL) and H2O (10 mL) were added. The aqueous mixture was extracted with CHCl3 (3 × 25 mL) and the combined organics were dried over Na2SO4, filtered through Celite and concentrated in vacuo to afford crude 25 (0.270 g) which was used without further purification.
To a stirred solution of crude 25 (0.270 g) in CH2Cl2 (8 mL) at 0 °C was bubbled HCl gas until pH ~ 3. A solution of BBr3 (0.374 mL, 3.95 mmol) in CH2Cl2 (2 mL) was added dropwise. The reaction mixture was warmed to room temperature. After stirring at room temperature for 1.5 h, the reaction mixture was cooled to 0 °C and H2O (10 mL) was added dropwise. The reaction mixture was warmed to room temperature. After stirring 30 min at room temperature, the CH2Cl2 was distilled off and the remaining aqueous solution was refluxed for an additional 30 min. The reaction mixture was then cooled to 0 °C and concentrated aq NH4OH was added to a pH ~ 9.5. The aqueous mixture was extracted with 10% EtOH in CHCl3 (5 × 20 mL) and the combined organics were filtered through Celite and concentrated in vacuo to afford 4 as a crude solid. This crude solid was purified by SiO2 column chromatography with 10% NH4OH in MeOH/CHCl3 (gradient, 0 → 5%) to afford 4 (0.171 g, 46% from 22) as a white solid. All spectra reported on the free base. [α]20 D = +162.1 (1.1, CHCl3); IR (thin film) 3180 cm−1; 1H NMR (400 MHz, CDCl3) δ 6.89 (d, J = 8.4 Hz, 1H), 6.86 (s, 1H), 6.63 (d, J = 8.0 Hz, 1H), 6.23 (br s, 2H), 3.13 (d, J = 6.0 Hz, 1H), 3.06 (d, J = 5.6 Hz, 1H), 3.02 (d, J = 10.0 Hz, 1H), 2.83-2.69 (m, 3H), 2.58 (m, 1H), 2.38 (d, J = 6.4 Hz, 2H), 2.17-2.07 (m, 3H), 1.91-1.77 (m, 2H), 1.16 (m, 1H), 0.84 (m, 1H), 0.50 (d, J = 7.6 Hz, 2H), 0.11 (d, J = 4.0 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 212.1, 155.0, 140.1, 128.5, 126.1, 114.1, 112.4, 69.0, 59.6, 59.1, 46.3, 45.4, 43.2, 37.4, 36.7, 31.6, 24.3, 9.3, 3.8, 3.7; HRMS (TOF MS ES+) calcd for C20H26NO3 (M + H+) 328.1907, found 328.1909.
To 4 (0.171 g) was added Et2O (2 mL) and MeOH (15 drops). Anhydrous HCl gas was bubbled through the solution causing salt formation. The salt crystals were filtered, rinsed with Et2O (2 mL), and air dried to afford 4 (0.090 g) as the HCl • 0.5H2O salt mp 194-200 °C. Anal. Calcd for (C20H27ClNO3.5): CHN.
(4bR,8aR,9S)-11-(Cyclopropylmethyl)-6,7,8,8a,9,10-hexahydro-5H-9,4b-(epiminoethano)phenanthrene-3,8a-diol (5)
To a stirred solution of 28 (0.522 g, 1.24 mmol) in NH3 (liquid) (6 mL) and THF (6 mL) at −78 °C was added freshly cut Na pieces (0.086 g, 3.72 mmol) during which time a blue solution color persisted. Additional small pieces of Na were added to maintain the blue color of the solution over the course of 4 h while maintaining the temperature at −78 °C. After 4 h, EtOH (2 mL) was added and the reaction mixture was allowed to warm to room temperature. To the reaction mixture was added saturated NaCl (10 mL) and the aq layer was extracted with CHCl3 (3 × 15 mL). The combined organic fractions were dried over Na2SO4, filtered through Celite, and concentrated in vacuo to afford 29 (0.323 g) as a crude white solid that was used without further purification.
To the crude 29 (0.323 g) was added CH2Cl2 (10 mL), and anhydrous HCl was bubbled through the solution for 5 min. The reaction mixture was cooled to 0 °C and a solution of BBr3 (0.51 mL, 5.4 mmol) in CH2Cl2 (5 mL) was added dropwise. After addition, the reaction mixture was warmed to room temperature, stirred for 1.5 h and cooled to 0 °C. To the mixture was added H2O (10 mL), the CH2Cl2 was distilled off, and the reaction mixture was refluxed for 30 min. The solution was cooled to 0 °C, concentrated NH4OH (1 mL) was added and the aqueous layer was extracted with CHCl3/EtOH (3 × 10 mL, 5:1). The combined organics were dried over Na2SO4, filtered through Celite, and concentrated in vacuo to afford 5 as a crude white solid. Purification of this crude solid by SiO2 column chromatography with 10% NH4OH in MeOH/CHCl3 (gradient, 0 → 8%) afforded 5 (0.254 g, 65% from 28) as a white solid. [α]20D = +98.0 (1.0, CHCl3); mp 80–86 °C; IR (thin film) 3275 cm−1; 1H NMR (400 MHz, CD3OD) δ 6.93 (d, J = 7.2 Hz, 1H), 6.73 (s, 1H), 6.60 (d, J = 6.8 Hz, 1H), 4.94 (s, 2H), 3.04 (d, J = 18.4 Hz, 1H), 2.91 (s, 1H), 2.58 (d, J = 8.8 Hz, 1H), 2.52 (m, 1H), 2.49-2.32 (m, 2H), 2.15-1.76 (m, 5H), 1.50-1.37 (m, 5H), 1.03 (d, J = 12.0 Hz, 1H), 0.87 (m, 1H), 0.53 (d, J = 6.8 Hz, 2H), 0.15 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 157.8, 144.1, 130.2, 129.0, 115.2, 113.5, 72.2, 62.9, 61.1, 46.6, 43.4, 39.0, 33.5, 32.1, 26.3, 23.6, 23.5, 11.2, 5.3, 5.1; HRMS (TOF MS ES+) calcd for C20H28NO2 (M + H+) 314.2115, found 314.2114.
Anhydrous HCl gas was bubbled through a solution of 5 (0.254 g) in a mixture of Et2O (2 mL) and MeOH (0.2 mL). The HCl salt of 5 precipitated almost immediately. It was filtered and rinsed with Et2O (3 mL) to give 5 as an HCl•0.5 MeOH salt, mp (dec) >270 °C. Anal. Calcd for C20.5H30ClNO2.5 (5•HCl•0.5 MeOH): CHN.
(4bR,8aR,9S)-11-Hexyl-6,7,8,8a,9,10-hexahydro-5H-9,4b-(epiminoethano)phenanthrene-3,8a-diol (6)
To a solution of 34 (0.31 g, 0.90 mmol) in EtOH (8 mL) and AcOH (1 mL) was added 5% Pd/C Escat 103 (0.030 g). The reaction mixture was subjected to an atmosphere of H2 (33 psi) for 14 h on a Parr hydrogenation apparatus. After 14 h, H2O (10 mL) and concentrated aq NH4OH (2 mL) were added, followed by CHCl3 (15 mL). The aqueous layer was extracted with CHCl3 (3 × 15 mL), and the combined organics were dried over Na2SO4, filtered through Celite, and concentrated in vacuo to afford 6 as a crude yellow oil. Purification of this oil by SiO2 column chromatography with 10% NH4OH in MeOH/CHCl3 (gradient, 0 → 3%) afforded 6 (0.206 g, 67%) as a light yellow foam. All spectra reported on the free base. [α]20D = +67.7 (1.0, CHCl3); mp 108–110 °C; IR (thin film) 3289 cm−1; 1H NMR (400 MHz, CDCl3) δ 6.93 (d, J = 8.0 Hz, 1H), 6.77 (s, 1H), 6.64 (d, J = 7.6 Hz, 1H), 5.49 (br s, 2H), 3.02 (d, J = 17.6 Hz, 1H), 2.77-2.68 (m, 2H), 2.43-2.40 (m, 3H), 2.10-1.75 (m, 5H), 1.51-1.20 (m, 12H), 1.02 (d, J = 12.8 Hz, 1H), 0.86 (t, J = 6.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 154.7, 142.5, 128.3, 127.7, 113.2, 112.0, 70.3, 61.4, 54.3, 44.3, 41.5, 37.0, 31.7, 31.5, 30.2, 27.6, 27.0, 24.8, 22.6, 21.7, 21.6, 14.0; HRMS (TOF MS ES+) calcd for C22H34NO2 (M + H+) 344.2584, found 344.2584. An HCl salt was prepared by dissolving 6 free base in hot i-PrOH (1.4 mL) followed by concentrated aq HCl (0.15 mL, 3 equiv) and cooling to 5 °C. The resultant salt was filtered, rinsed with cold i-PrOH, and dried in a vacuum oven at room temperature. Anal. Calcd for C22H34ClNO2 (6•HCl): CHN.
(4bR,8aR)-11-(Cyclopropylmethyl)-3-methoxy-4-phenoxy-6,7,8,8a,9,10-hexahydro-5H-9,4b-(epiminoethano)phenanthren-8a-ol (28)
To a stirred solution of 20 (1.63 g, 4.59 mmol) in diethylene glycol (16 mL) was added hydrazine hydrate (1.10 mL, 22.7 mmol). The reaction mixture was heated to 70 °C and maintained at that temperature for 1 h. After 1 h, a solution of KOH (1.80 g, 32.1 mmol) in H2O (0.9 mL) was added and the solution was heated to 130 °C. After heating at 130 °C for 2 h, the reaction mixture was cooled to room temperature and H2O (15 mL) was added. The mixture was extracted with Et2O (3 × 15 mL), washed with saturated NaCl (30 mL), dried over Na2SO4, filtered, and concentrated in vacuo to afford 26 as a crude oil. Purification of this oil by SiO2 column chromatography with 10% NH4OH in MeOH/CHCl3 (gradient, 0 → 3.5%) afforded 26 (0.859 g, 55%) as a colorless oil. Spectra matched that reported by Wu et al.40
To a solution of 26 (0.808 g, 2.37 mmol) in EtOH (20 mL) and AcOH (2 mL) was added PtO2 (150 mg, 0.661 mmol). The reaction mixture was subjected to an atmosphere of H2 (33 psi) while shaking for 3 h in a Parr hydrogenator. After 3h, H2O (20 mL) was added followed by concentrated aqueous NH4OH to obtain a pH of ~ 9.5. The reaction mixture was concentrated in vacuo to remove the organics and the aqueous layer was extracted with CHCl3/EtOH (3 × 20 mL, 10:1), dried over Na2SO4, filtered, and concentrated in vacuo to afford crude 27 (0.675 g), which was used without further purification.
To the stirred solution of crude 27 (0.675 g) in pyridine (20 mL) was added K2CO3 (0.814 g, 5.88 mmol), Cu powder (0.132 g, 2.06 mmol), and bromobenzene (1.03 mL, 9.80 mmol). The reaction mixture was refluxed for 36 h, cooled to room temperature, filtered through Celite rinsing with CHCl3, and concentrated in vacuo to give a crude tacky orange solid. Purification of the crude solid by SiO2 column chromatography with 10% NH4OH in MeOH/CHCl3 (gradient, 0 → 5%) afforded 28 (0.522 g, 53% from 26) as a tacky orange solid. [α]20D = +56.6 (1.6, CHCl3); IR (thin film) 3426 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.21 (app t, J = 7.8 Hz, 2H), 6.97 (d, J = 8.4 Hz, 1H), 6.93 (t, J = 8.0 Hz, 1H), 6.83 (d, J = 8.4 Hz, 1H), 6.70 (d, J = 8.0 Hz, 1H), 4.47 (br s, 1H), 3.65 (s, 3H), 3.01 (m, 1H), 2.93-2.88 (m, 2H), 2.67 (d, J = 13.6 Hz, 1H), 2.51 (dd, J = 11.6, 4.0 Hz, 1H), 2.34 (m, 1H), 2.33 (t, J = 6.4 Hz, 1H), 2.09 (td, J = 11.8, 2.4 Hz, 1H), 1.87-1.73 (m, 3H), 1.33 (m, 1H), 1.15 (d, J = 13.2 Hz, 1H), 0.82 (m, 1H), 0.49 (d, J = 8.0 Hz, 2H), 0.10 (d, J = 4.4 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 157.8, 150.8, 141.3, 135.4, 131.0, 129.0, 124.5, 120.9, 114.5, 110.5, 69.5, 60.3, 59.2, 55.8, 44.7, 42.6, 33.8, 32.0, 29.9, 25.4, 22.9, 21.9, 9.4, 3.8, 3.7; HRMS (TOF MS ES+) calcd for C27H34NO3 (M + H+) 420.2533, found 420.2533.
(4S,4aR,7aS,12bR)-3-Hexyl-4a-hydroxy-9-methoxy-2,3,4,4a,5,6-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-7(7aH)-one (30)
To a stirred solution of (+)-N-hexylnoroxymorphone 1d (3.0 g, 8.08 mmol) in DMF (50 mL) was added K2CO3 (2.50 g, 18.1 mmol) and iodomethane (0.659 mL, 10.6 mmol). The reaction mixture was stirred at room temperature for 48 h, filtered through Celite, and concentrated in vacuo. To the crude residue was added H2O (50 mL), concentrated aq NH4OH (5 mL) and CHCl3 (50 mL). The organics were separated and the aqueous layer extracted with CHCl3 (50 mL). The combined organics were washed with H2O (5 × 40 mL), dried over Na2SO4, filtered through Celite, and concentrated in vacuo to afford 30 (2.98 g, 96%) as a colorless oil that was used without further purification. An analytical sample was prepared by purification with SiO2 column chromatography with 10% NH4OH in MeOH/CHCl3 (gradient, 0 → 4%). [α]20D = + 161.9 (2.0, CHCl3); IR (thin film) 3377 cm−1; 1H NMR (400 MHz, CDCl3) δ 6.66 (d, J = 8.4 Hz, 1H), 6.58 (d, J = 8.0 Hz, 1H), 5.10 (br s, 1H), 4.62 (s, 1H), 3.85 (s, 3H), 3.06 (d, J = 18.4 Hz, 1H), 2.97 (td, J = 14.4, 4.8 Hz, 1H), 2.91 (m, 1H), 2.58-2.31 (m, 1H), 2.25 (dt, J = 14.4, 3.0 Hz, 1H), 2.10 (td, J = 12.4, 3.6 Hz, 1H), 1.83 (m, 1H), 1.62-1.42 (m, 4H), 1.34-1.23 (m, 6H), 0.86 (t, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 208.4, 144.8, 142.8, 129.4, 124.9, 119.2, 114.8, 90.2, 70.1, 62.7, 56.7, 54.2, 50.7, 43.3, 36.0, 31.6, 31.3, 30.6, 27.4, 26.8, 22.7, 22.5, 13.9; HRMS (TOF MS ES+) calcd for C23H32NO4 (M + H+) 386.2326, found 386.2326.
(4bS,8aR,9S)-11-Hexyl-3-methoxy-8,8a,9,10-tetrahydro-7H-9,4b-(epiminoethano)phenanthren-8a-ol (33)
To a stirred solution of 30 (2.783 g, 7.22 mmol) in CHCl3 (60 mL) in a screw-cap vial was added 1,2-bis(tert-butyldimethylsilyl)hydrazine34 (4.87 g, 18.8 mmol) and the vial was capped (teflon-lined). After stirring at room temperature for 18 h, hexanes (120 mL) were added and the resultant suspension was filtered through Celite rinsing with hexanes (2 × 60 mL). The filtrate was concentrated in vacuo and hexanes (120 mL) were added to the residue (part 1). This was added to a DMSO/tBuOH/KOtBu mixture that had been prepared in a separate flask (DMSO (60 mL) and t-butanol (1.4 mL) were added to potassium tert-butoxide (16.2 g, 144 mmol) and stirred for 30 min), and the mixture was stirred for 30 min. The reaction mixture was then added to a chilled saturated NaHCO3/H2O (1:1, 500 mL) solution. The aqueous mixture was extracted with 1:1 EtOAc/hexanes (3 × 500 mL). The organics were combined and washed with H2O (5 × 250 mL), dried over Na2SO4, filtered through Celite, and concentrated in vacuo to give crude 31 (1.31 g) that was used without further purification.
To a stirred suspension of 60% NaH in mineral oil (0.282 g, 7.05 mmol) in THF (30 mL) at 5 °C was added dropwise a solution of crude 31 (1.31 g) in THF (5 mL). The reaction mixture was stirred at 5 °C and diethylchlorophosphate (0.918 mL, 6.35 mmol) was added dropwise. After stirring at 5 °C for 1 h, saturated NH4Cl (35 mL) and CHCl3 (60 mL) were added. The aqueous layer was extracted with CHCl3 (3 × 60 mL) and the combined organic solution was dried over Na2SO4, filtered through Celite, and concentrated in vacuo to afford crude 32 (2.35 g), which was used without further purification.
To a stirred solution of crude 32 (2.35 g) in NH3 (liquid) and THF (15 mL) at −78 °C was added freshly cut Na (0.90 g, 3.9 mmol). After stirring 30 min at −78 °C, an additional 0.90 g of Na was added and the reaction continued to stir for another 15 min, at which time the starting material was consumed as indicated by TLC. MeOH (1 mL) was added to the reaction mixture, the solution was warmed to room temperature, and H2O (20 mL) and concentrated aq NH4OH (1 mL) were added. The aqueous layer was extracted with CHCl3 (3 × 20 mL). The organic solution was dried over Na2SO4, filtered through Celite, and concentrated in vacuo to afford 33 (1.36 g) as a yellow oil. Purification of this oil by SiO2 column chromatography with 10% NH4OH in MeOH/CHCl3 (gradient, 0 → 4%) afforded 33 (0.675 g, 26% from 30) as a light yellow oil. All spectra reported on the free base. [α]20 D = +25.7 (1.1, CHCl3); IR (thin film) 3418 cm−1; 1H NMR (400 MHz, CDCl3) δ 6.96 (d, J = 8.4 Hz, 1H), 6.71 (d, J = 2.4 Hz, 1H), 6.66 (dd, J = 8.4, 2.4 Hz, 1H), 5.95 (d, J = 10.0 Hz, 1H), 5.84 (dt, J = 7.2, 3.2 Hz, 1H), 3.76 (s, 3H), 3.07 (d, J = 18.0 Hz, 1H), 2.82 (d, J = 5.6 Hz, 1H), 2.76 (dd, J = 18.0, 6.0 Hz, 1H), 2.51-2.38 (m, 4H), 2.23-1.98 (m, 3H), 1.69 (m, 1H), 1.54-1.43 (m, 3H), 1.38, 1.23 (m, 7H), 0.88 (t, J = 6.6 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 157.8, 145.8, 131.5, 127.6, 127.0, 126.0, 112.4, 111.3, 68.7, 61.5, 55.0, 54.0, 44.1, 41.5, 35.0, 31.6, 27.8, 27.5, 26.9, 24.6, 22.8, 22.5, 14.0; HRMS (TOF MS ES+) calcd for C23H34NO2 (M + H+) 356.2584, found 356.2584.
(4bS,8aR,9S)-11-Hexyl-8,8a,9,10-tetrahydro-7H-9,4b-(epiminoethano)phenanthrene-3,8a-diol (34)
To a solution of 33 (0.575 g, 1.62 mmol) in CHCl3 (14 mL) at −78 °C was added a solution of BBr3 (0.77 mL, 8.1 mmol) in CHCl3 (2 mL) dropwise. The reaction mixture was warmed to room temperature over the course of 1h and then H2O (15 mL) was added dropwise. The CHCl3 layer was distilled off and the resulting aqueous solution was refluxed for 30 min. The reaction mixture was cooled to ~ 5 °C, and concentrated aqueous NH4OH (1mL) and CHCl3 (20 mL) were added. The aqueous layer was extracted with CHCl3 (3 × 20 mL) and the combined organics were dried over Na2SO4, filtered through Celite, and concentrated in vacuo to afford 34 (0.479 g, 87%) as an off-white solid. All spectra reported on the free base. [α]20D = +35.6 (1.0, CHCl3); mp 137-139 °C; IR (thin film) 3293 cm−1; 1H NMR (400 MHz, CDCl3) δ 6.88 (d, J = 8.4 Hz, 1H), 6.67 (d, J = 2.0 Hz, 1H), 6.60 (dd, J = 8.2, 2.0 Hz, 1H), 5.90 (d, J = 10.0 Hz, 1H), 5.80 (d, J = 9.6 Hz, 1H), 5.43 (br s, 2H), 3.04 (d, J = 18.4 Hz, 1H), 2.84 (d, J = 5.6 Hz, 1H), 2.75 (dd, J = 18.0, 5.8 Hz, 1H), 2.50-2.37 (m, 4H), 2.21-2.09 (m, 2H), 1.99 (m, 1H), 1.70 (m, 1H), 1.53 (dd, J = 12.8, 6.4 Hz, 1H), 1.37-1.19 (m, 7H), 0.87 (t, J = 6.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 154.3, 145.7, 131.5, 127.7, 126.9, 125.4, 113.8, 113.3, 69.3, 61.5, 54.1, 44.1, 41.4, 35.0, 31.6, 27.7, 27.6, 26.9, 24.6, 22.7, 22.6, 14.0; HRMS (TOF MS ES+) calcd for C22H32NO2 (M + H+) 342.2428, found 342.2428. An HCl salt was prepared by dissolving 34 free base in hot i-PrOH (3.4 mL) followed by concentrated aq HCl (0.34 mL, 3 equiv) and cooling to 5 °C. The resultant salt was filtered, rinsed with cold i-PrOH, and dried in a vacuum oven at room temperature. Anal. Calcd for C22H31ClNO2 (34•HCl): CHN.
(4aR,7aS,12bR)-4a-Hydroxy-9-methoxy-3-phenethyl-2,3,4,4a,5,6-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-7(7aH)-one (35)
The same procedure as 30 with 1j HCl•2H2O (0.200 g, 0.431 mmol), K2CO3 (0.298 g, 2.16 mmol), iodomethane (0.040 mL, 0.65 mmol), and DMF (4 mL) afforded 35 (0.164 g, 94%) as a white solid. All spectra reported on the free base. [α]20D = + 164.4 (1.3, CHCl3); mp 146–148 °C; IR (thin film) 3404 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.35-7.32 (m, 2H), 7.25-7.20 (m, 3H), 6.70 (d, J = 8.4 Hz, 1H), 6.62 (d, J = 8.0 Hz, 1H), 4.78 (br s, 1H), 4.64 (s, 1H), 3.90 (s, 3H), 3.10 (d, J = 18.4 Hz, 1H), 2.98 (td, J = 14.0, 5.0 Hz, 1H), 2.91 (d, J = 6.0 Hz, 1H), 2.84-2.58 (m, 5H), 2.38-2.17 (m, 3H), 1.82 (m, 1H), 1.63-1.51 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 208.3, 144.8, 142.7, 139.6, 129.3, 128.4, 128.3, 126.1, 124.7, 119.2, 114.7, 90.1, 70.1, 63.5, 56.6, 55.8, 50.5, 42.7, 35.9, 34.0, 31.2, 30.3, 23.2; HRMS (TOF MS ES+) calcd for C25H28NO4 (M + H+) 406.20128, found 406.20109. An HCl salt was prepared by dissolving 35 free base in THF (1.1 mL) followed by concentrated aq HCl (0.10 mL, 3 equiv) and cooling to 5 °C, i-PrOH (1.1 mL) was added and gave crystals that were filtered, rinsed with cold i-PrOH, and air-dried to give the HCl•1.5H2O. Anal. Calcd for C25H31ClNO5.5 (35•HCl•1.5H2O): CHN.
Acknowledgments
The work of the Drug Design and Synthesis Section was supported by the NIH Intramural Research Programs of the National Institute on Drug Abuse (NIDA) and the National Institute of Alcohol Abuse and Alcoholism. The work on in vitro characterization was supported by a grant from the Bioscience Discovery Evaluation Program of the state of Colorado and grants NIH GM103843 and GM101279, and a NHMRC CJ Martin Fellowship [IDD: 1054091] to P.M.G. The authors express their gratitude to Noel Whittaker, Mass Spectrometry Facility NIDDK, for mass spectral data.
Abbreviations
- TLR4
Toll-like receptor 4
- NO
nitric oxide
- LPS
lipopolysaccharides
- DMEM
Dulbecco’s Modified Eagle Medium
- FBS
fetal bovine serum
Footnotes
The authors declare no competing financial interests.
Supporting Information
1H and 13C NMRs of novel compounds can be found in the supporting information along with the 1H NMRs of known compounds. The microanalytical data are presented in tabular form. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, Lu B, Chavan S, Rosas-Ballina M, Al-Abed Y, Akira S, Bierhaus A, Erlandsson-Harris H, Andersson U, Tracey KJ. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Nat Acad Sci. 2010;107:11942–11947. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X, Smith C, Yin H. Targeting Toll-like receptors with small molecule agents. Chem Soc Rev. 2013;42:4859–4866. doi: 10.1039/c3cs60039d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hutchinson MR, Northcutt AL, Hiranita T, Wang X, Lewis SS, Thomas J, van Steeg K, Kopajtic TA, Loram LC, Sfregola C, Galer E, Miles NE, Bland ST, Amat J, Rozeske RR, Maslanik T, Chapman TR, Strand KA, Fleshner M, Bachtell RK, Somogyi AA, Yin H, Katz JL, Rice KC, Maier SF, Watkins LR. Opioid activation of Toll-like receptor 4 contributes to drug reinforcement. J Neurosci. 2012;32:11187–11200. doi: 10.1523/JNEUROSCI.0684-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Loram LC, Ramos K, de Jesus AJ, Thomas J, Cheng K, Reddy A, Somogyi AA, Hutchinson MR, Watkins LR, Yin H. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Natl Acad Sci US A. 2012;109:6325–6330. doi: 10.1073/pnas.1200130109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Grace PM, Pham MN, Cheng K, Strand KA, Smith C, Li J, Watkins LR, Yin H. Rifampin inhibits Toll-like receptor 4 signaling by targeting myeloid differentiation protein 2 and attenuates neuropathic pain. FASEB J. 2013;27:2713–2722. doi: 10.1096/fj.12-222992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discovery. 2014;13:533–548. doi: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014;14:217–231. doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coller JK, Hutchinson MR. Implications of central immune signaling caused by drugs of abuse: Mechanisms, mediators and new therapeutic approaches for prediction and treatment of drug dependence. Pharmacol Ther. 2012;134:219–245. doi: 10.1016/j.pharmthera.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Hutchinson MR, Shavit Y, Grace PM, Rice KC, Maier SF, Watkins LR. Exploring the neuroimmunopharmacology of opioids: An integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev. 2011;63:772–810. doi: 10.1124/pr.110.004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR. Opioid-induced glial activation: Mechanisms of activation and implications for opioid analgesia, dependence, and reward. Sci World J. 2007;7:98–111. doi: 10.1100/tsw.2007.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bachtell RK, Hutchinson MR, Wang X, Rice K, Maier SF, Watkins LR. Targeting the toll of drug abuse: The translational potential of toll-like receptor 4. CNS Neurol Disord-DR. 2014 doi: 10.2174/1871527314666150529132503. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Zhang Y, Hutchinson MR, Rice KC, Yin H, Watkins LR. Pharmacological characterization of the opioid inactive isomers (+)-naltrexone and (+)-naloxone as Toll-like receptor 4 antagonists. Br J Pharmacol. 2014 doi: 10.1111/bph.13394. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier SF, Rice KC, Watkins LR. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of Toll-like receptor 4 (TLR4) Eur J Neurosci. 2008;28:20–29. doi: 10.1111/j.1460-9568.2008.06321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rice KC, Newman AH. US 5,668,285. Total synthesis of northebaine, normorphine, noroxymorphone enantiomers and derivatives via N-Nor intermediates. 1997
- 16.Iijima I, Minamikawa J, Rice KC, Jacobson AE. Synthesis and antinociceptive activity of 7-methoxycodeine. J Med Chem. 1978;21:1320–1322. doi: 10.1021/jm00210a031. [DOI] [PubMed] [Google Scholar]
- 17.Theberge FR, Li X, Kambhampati S, Pickens CL, St Laurent R, Bossert JM, Baumann MH, Hutchinson MR, Rice KC, Watkins LR, Shaham Y. Effect of chronic delivery of the Toll-like receptor 4 antagonist (+)-naltrexone on incubation of heroin craving. Biol Psychiatry. 2013;73:729–737. doi: 10.1016/j.biopsych.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Northcutt A, Hutchinson M, Wang X, Baratta MV, Hiranita T, Cochran TA, Pomerenze MB, Galer E, Kopajtic TA, Li CM, Amat J, Larsen G, Cooper DC, Huang Y, O’Neill CE, Yin H, Zahniser NR, Katz JL, Rice KC, Maier SF, Bachtell RK, Watkins LR. DAT isn’t all that: cocaine reward and reinforcement require Toll-like receptor 4 signaling. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selfridge B, Deschamps J, Jacobson A, Rice K. Synthesis of enantiopure 10-nornaltrexones in the search for Toll-like receptor 4 antagonists and opioid ligands. J Org Chem. 2014;79:5007–18. doi: 10.1021/jo500568s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rapoport H, Lovell CH, Reist HR, Warren ME. The synthesis of thebaine and northebaine from codeinone dimethyl ketal. J Am Chem Soc. 1967;89:1942–1947. doi: 10.1021/ja00984a032. [DOI] [PubMed] [Google Scholar]
- 21.Iijima I, Minamikawa J, Jacobson AE, Brossi A, Rice KC. Studies in the (+)- morphinan series. 5. Synthesis and biological properties of (+)-naloxone. J Med Chem. 1978;21:398–400. doi: 10.1021/jm00202a018. [DOI] [PubMed] [Google Scholar]
- 22.de Costa BR, Iadarola MJ, Rothman RB, Berman KF, George C, Newman AH, Mahboubi A, Jacobson AE, Rice KC. Probes for narcotic receptor mediated phenomena .18. Epimeric 6α-iodo-3,14-dihydroxy-17-(cyclopropylmethyl)-4,5α-epoxymorphinans and 6β-iodo-3,14-dihydroxy-17-(cyclopropylmethyl)-4,5α-epoxymorphinans as potential ligands for opioid receptor single photon emission computed tomography - synthesis, evaluation, and radiochemistry of 125I-6β-iodo-3,14-dihydroxy-17-(cyclopropylmethyl)-4,5-epoxymorphinan. J Med Chem. 1992;35:2826–2835. doi: 10.1021/jm00093a016. [DOI] [PubMed] [Google Scholar]
- 23.Neumann H, Wekerle H. Brain microglia: Watchdogs with pedigree. Nat Neurosci. 2013;16:253–255. doi: 10.1038/nn.3338. [DOI] [PubMed] [Google Scholar]
- 24.Hutchinson MR, Watkins LR. Why is neuroimmunopharmacology crucial for the future of addiction research? Neuropharmacology. 2014;76(Part B):218–227. doi: 10.1016/j.neuropharm.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henn A, Lund S, Hedtjarn M, Schrattenholz A, Porzgen P, Leist M. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. ALTEX. 2009;26:83–94. doi: 10.14573/altex.2009.2.83. [DOI] [PubMed] [Google Scholar]
- 26.Schmidtko A, Tegeder I, Geisslinger G. No NO, no pain? The role of nitric oxide and cGMP in spinal pain processing. Trends Neurosci. 2009;32:339–346. doi: 10.1016/j.tins.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Tayfun Uzbay I, Oglesby MW. Nitric oxide and substance dependence. Neurosci Biobehav Rev. 2001;25:43–52. doi: 10.1016/s0149-7634(00)00049-x. [DOI] [PubMed] [Google Scholar]
- 28.Toda N, Kishioka S, Hatano Y, Toda H. Modulation of opioid actions by nitric oxide signaling. Anesthesiology. 2009;110:166–181. doi: 10.1097/ALN.0b013e31819146a9. [DOI] [PubMed] [Google Scholar]
- 29.Misko TP, Schilling RJ, Salvemini D, Moore WM, Currie MG. A fluorometric assay for the measurement of nitrite in biological samples. Anal Biochem. 1993;214:11–16. doi: 10.1006/abio.1993.1449. [DOI] [PubMed] [Google Scholar]
- 30.Nagase H, Watanabe A, Harada M, Nakajima M, Hasebe K, Mochizuki H, Yoza K, Fujii H. Novel synthesis of a 1,3,5-trioxazatriquinane skeleton using a nitrogen clamp. Org Lett. 2008;11:539–542. doi: 10.1021/ol8024988. [DOI] [PubMed] [Google Scholar]
- 31.Nagase H, Yamamoto N, Nemoto T, Yoza K, Kamiya K, Hirono S, Momen S, Izumimoto N, Hasebe K, Mochizuki H, Fujii H. Synthesis of a stable iminium salt and propellane derivatives. J Org Chem. 2008;73:8093–8096. doi: 10.1021/jo801276z. [DOI] [PubMed] [Google Scholar]
- 32.Wu H, Thatcher LN, Bernard D, Parrish DA, Deschamps JR, Rice KC, MacKerell AD, Coop A. Position of coordination of the lithium ion determines the regioselectivity of demethylations of 3,4-dimethoxymorphinans with L-selectride. Org Lett. 2005;7:2531–2534. doi: 10.1021/ol050433c. [DOI] [PubMed] [Google Scholar]
- 33.Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 34.Furrow ME, Myers AG. Practical procedures for the preparation of N-tertbutyldimethylsilylhydrazones and their use in modified Wolff—Kishner reductions and in the synthesis of vinyl halides and gem-dihalides. J Am Chem Soc. 2004;126:5436–5445. doi: 10.1021/ja049694s. [DOI] [PubMed] [Google Scholar]
- 35.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 36.Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler J, Hughes TS, Landgraf KE, Chan S, Fong S, Phipps S, Falke JJ, Leinwand LA, Maier SF, Yin H, Rice KC, Watkins LR. Evidence that opioids may have Toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010;24:83–95. doi: 10.1016/j.bbi.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watkins LR, Wang X, Mustafa S, Hutchinson MR. In vivo veritas: (+)- Naltrexone’s actions define translational importance: A letter in response to Skolnick et al. ‘Translational potential of naloxone and naltrexone as TLR4 antagonists’. Trends Pharmacol Sci. 2014;35:432–433. doi: 10.1016/j.tips.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Skolnick P, Davis H, Arnelle D, Deaver D. Translational potential of naloxone and naltrexone as TLR4 antagonists. Trends Pharmacol Sci. 2014;35:431–432. doi: 10.1016/j.tips.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 39.Milligan ED, Hinde JL, Mehmert KK, Maier SF, Watkins LR. A method for increasing the viability of the external portion of lumbar catheters placed in the spinal subarachnoid space of rats. J Neurosci Methods. 1999;90:81–86. doi: 10.1016/s0165-0270(99)00075-8. [DOI] [PubMed] [Google Scholar]
- 40.Wu HF, Bernard D, Chen WB, Strahan GD, Deschamps JR, Parrish DA, Lewis JW, MacKerell AD, Coop A. Functionalization of the 6,14-bridge of the orvinols. 2. Preparation of 18- and 19-hydroxyl-substituted thevinols and their treatment with benzyl bromide. J Org Chem. 2005;70:1907–1910. doi: 10.1021/jo048388u. [DOI] [PubMed] [Google Scholar]