Introduction
Emergence of pathogenic viruses is of great concern, although the underlying mechanisms for emergence remain often poorly understood. RNA viruses are frequently implicated and recent examples include viruses within Orthomyxoviridae, Flaviviridae, or Coronaviridae [1–3]. Within Caliciviridae, the Lagovirus genus is particularly intriguing because it has generated viruses of exceptional pathogenicity on several occasions within the past 40 years. The genus Lagovirus encompasses two pathogenic viruses, Rabbit hemorrhagic disease virus (RHDV) affecting European rabbit (Oryctolagus cuniculus), and European brown hare syndrome virus (EBHSV) affecting Brown, Mountain, and Italian hare (Lepus europaeus, L. timidus, and L. corsicanus) [4]. These two viruses show a similar structure and ~70% homology [5–15]. They cause two distinct diseases, RHD (rabbit hemorrhagic disease) and EBHS (European brown hare syndrome), that emerged in the late 1970s to early 1980s [16–17]. Both cause high mortalities and impose a heavy economic burden on the rabbit industry and game animal management. They have also contributed to declines of wild leporid populations throughout Europe, resulting in major ecological impact in natural ecosystems where leporids are key species [18–25].
RHD was first detected in China in 1984, apparently in rabbits imported from Germany [16], suggesting that RHDV originated in Europe. Rabbit lagoviruses consist of pathogenic viruses (RHDV) and related, but genetically divergent, nonpathogenic viruses [26–34]. Phylogenetic analyses of pathogenic RHDV strains show three distinct groups: the classic RHDV with the genogroups G1–G5 [27,35–55], the antigenic variant RHDVa/G6 [35,56–61], and RHDV2/RHDVb [62–77]. The RHDV and RHDVa are phylogenetically related and differ from RHDV2 by more than 15% in nucleotide diversity. The RHDV strains have emerged at different times: RHDV was first isolated in 1984 [16] and RHDV2 in 2010 [62]. RHDV2 was identified in France and has since spread throughout other Western European countries, replacing the circulating strains in France and the Iberian Peninsula [64,67,71,76], while in Italy, it currently cocirculates with the “original” strains [64].
EBHS was first reported in Sweden in 1980 [17] and later found in other European countries [11,78–85]. It may have emerged earlier, as suggested by descriptions of hares with lesions consistent with the disease in 1976 in England [86]. Otherwise, antibodies against the virus have also been found in archived sera [87], and the virus was detected by RT-PCR in samples collected in Sweden before 1980 [88].
Two competing hypotheses can be put forward to explain RHDV and EBHSV origin and the emergence of RHDV2: 1) the evolution from pre-existing nonpathogenic viruses circulating in European leporids; 2) a species jump. The first hypothesis is shared by several authors and originates from the detection of anti-RHDV antibodies in rabbit blood samples collected before the first documented outbreak in Europe and Australia [89–92] and later in the characterization of different nonpathogenic viruses in European rabbits [26–34]. However, this hypothesis has not been confirmed and fails to explain the abrupt emergence of high pathogenicity on several occasions in a short period of time. Notably, the pathogenic and nonpathogenic viruses are phylogenetically separated and display ~20% of nucleotide divergence in the capsid gene [26–34], suggesting that the pathogenic forms did not directly originate from the nonpathogenic ones. Nevertheless, nonpathogenic strains have not been exhaustively characterized in European leporids, which may explain why the ancestors of pathogenic strains have not yet been found. The second hypothesis involves a species jump from species sympatric with European leporids, either native or previously introduced. Among these species, Eastern cottontail (Sylvilagus floridanus), a leporid native to North America, would constitute a worthwhile species. Although no data is available on the presence of original lagoviruses, they likely would have caused asymptomatic infection in its natural host, with a course of infections similar to what occurs with myxoma virus, benign in Sylvilagus species, but lethal in the European rabbit following a species jump [93]. Indeed, it is intriguing that both RHDV and EBHSV emerged at around the same time, coinciding with the introduction of the Eastern cottontail in Europe. Large numbers of introductions of Eastern cottontails by hunters occurred in Europe from the 1960s, but because they were illegal, these introductions are poorly documented. The first known introduction attempt from the United States was in 1966 in Italy. This was followed by massive introductions involving thousands of animals, especially in Italy and France. It is highly likely that localized introductions still occur, as suggested by the existence of cottontail breeders in France.
In the Po valley in Italy, where Eastern cottontails are invasive and widespread, a serological study showed that 18% and 33% of them carry antibodies detected by both anti-EBHSV and anti-RHDV serological tests, proving the susceptibility of the species to lagoviruses [94]. Moreover, recent documentation of RHDV strains in Iberian hares (L. granatensis) with lesions compatible with RHD [95], demonstration of the capacity of RHDV2 to infect Sardinian Cape and Italian hares (L. capensis mediterraneus and L. corsicanus, respectively) causing RHDV-like disease [65,70], and the experimental infection of cottontails by EBHSV [94], show the feasibility of species jumps of lagoviruses between leporid species.
We therefore suggest that European leporids carry lagoviruses of two distinct origins (Fig 1): nonpathogenic strains that have evolved with these species for a long time and a second group including pathogenic strains that possibly emerged following species jumps from S. floridanus and that have then evolved in European leporids. Pathogenic strains may be pure cottontail viruses or recombinants of cottontail viruses and nonpathogenic viruses of European leporid species. Indeed, recombination in RHDV is reported [96,97], and the recent documentation of recombination events between genome regions encoding the capsid and VP10 structural proteins of RHDV2 and the nonstructural proteins from nonpathogenic or pathogenic G1 lagoviruses suggests that recombination could have had an important role in the lagovirus evolution [74].
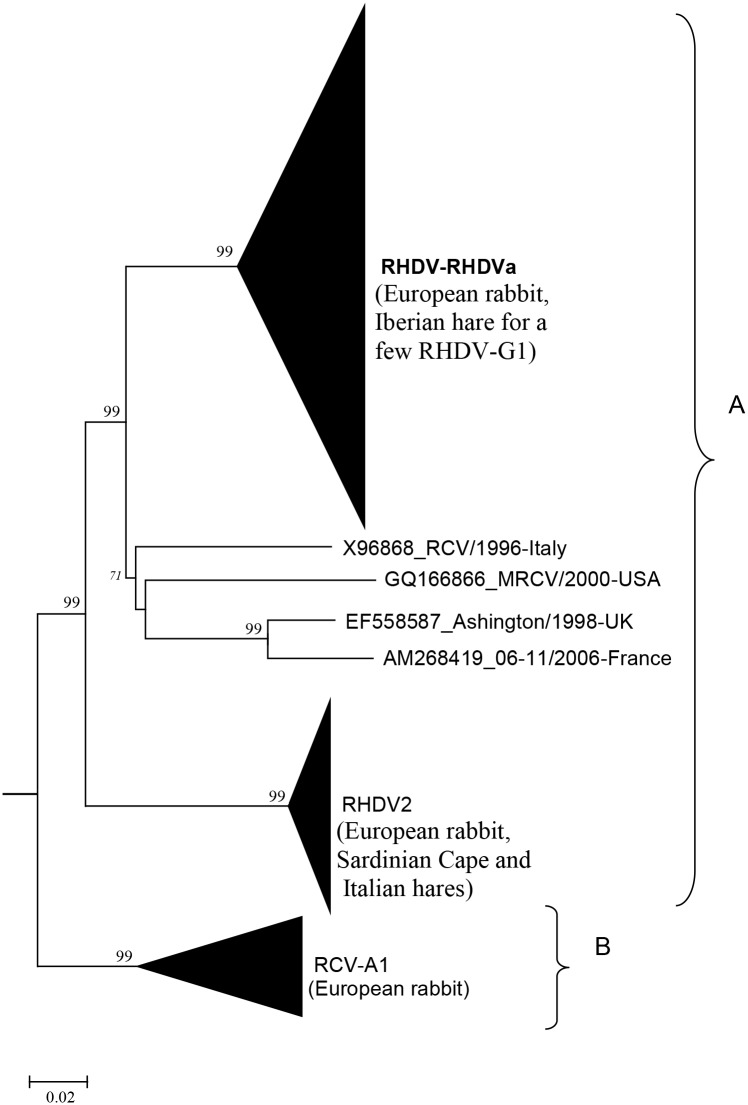
Fig 1. Possible origin of European rabbit (O. cuniculus) lagoviruses according to the hypothesis of a species jump.
A) Lagoviruses that may share common ancestors following several species jump(s), B) Nonpathogenic viruses that have evolved in European rabbit for a long time. Phylogenetic tree (Neighbor-joining method) derived from 303 rabbit lagovirus sequences of the VP60 gene available on public databases (May 2015). The pathogenic RHDV, RHDVa, RHDV2, and the nonpathogenic RCV-A1 branches are collapsed; the name of the leporid species where these strains were isolated is given in brackets. X96868_RCV/1996-Italy, GQ166866_MRCV/2000-USA, EF558587_Ashington/1998-UK, and AM268419_06-11/2006-France are nonpathogenic strains isolated in the European rabbit. Percentage greater than 70% of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) are given at major branch nodes. The EBHSV strain GD (Z69620) was used as an outgroup to root the tree. Similar clustering was observed in several recent works [63,64,66,70,74].
Evaluation of potential emergence of new pathogenic lagoviruses from the nonpathogenic strains circulating among native European leporid species and Sylvilagus species, as well as characterization of the genetic determinisms of pathogenicity, are key to identify the mechanisms of disease emergence and may help to evaluate the possibility of emergence of other highly pathogenic lagoviruses.
Funding Statement
The authors are grateful to the ERA-Net anihwa (Animal Health and Welfare), a Coordination Action funded under the European Commission’s ERA-Net scheme within the Seventh Framework Programme (Contract No. 291815), for having retained the project ECALEP to come as part of the 2nd join call involving 15 European countries for the next three years. The ECALEP project is funded by the ANR (France, contracts ANR-14-ANWA-0004-01, ANR-14-ANWA-0004-02, ANR-14-ANWA-0004-03, ANR-14-ANWA-0004-04), the Ministry of Health, Dep. for Veterinary Public Health, Nutrition & Food Safety (Italy) and the Research council FORMAS (Sweden, contract FORMAS 221-2014-1841). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bengis RG, Leighton FA, Fischer JR, Artois M, Mörner T, Tate CM. The roleof wildlife in emerging and re-emerging zoonoses. Rev Sci Tech. 2004;23: 497–511. [PubMed] [Google Scholar]
- 2. Gautret P, Gray GC, Charrel RN, Odezulu NG, Al-Tawfiq JA, Zumla A, Memish ZA. Emerging viral respiratory tract infections—environmental risk factors and transmission. Lancet Infect Dis. 2014;14: 1113–1122. 10.1016/S1473-3099(14)70831-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mandl JN, Ahmed R, Barreiro LB, Daszak P, Epstein JH, Virgin HW, Feinberg MB. Reservoir host immune responses to emerging zoonotic viruses. Cell. 2015;160: 20–35. 10.1016/j.cell.2014.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abrantes J, van der Loo W, Le Pendu J, Esteves PJ. Rabbit haemorrhagic disease (RHD) and rabbit haemorrhagic disease virus (RHDV): a review. Veterinary Research. 2011;43: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Capucci L, Scicluna MT, Lavazza A. Diagnosis of viral haemorrhagic disease of rabbits and the European brown hare syndrome. Rev Sci Tech. 1991;10: 347–370. [DOI] [PubMed] [Google Scholar]
- 6. Marcato PS, Benazzi C, Vecchi G, Galeotti M, Della Salda L, Sarli G, Lucidi P. Clinical and pathological features of viral haemorrhagic disease of rabbits and the European brown hare syndrome. Rev Sci Tech. 1991;10: 371–392. [DOI] [PubMed] [Google Scholar]
- 7. Chasey D, Lucas M, Westcott D, Williams M. European brown hare syndrome in the U.K.; a calicivirus related to but distinct from that of viral haemorrhagic disease in rabbits. Arch Virol. 1992;124: 363–370. [DOI] [PubMed] [Google Scholar]
- 8. Fuchs A, Weissenbock H. Comparative histopathological study of rabbit haemorrhagic disease (RHD) and European brown hare syndrome (EBHS). J Comp Pathol 1992;107: 103–113. [DOI] [PubMed] [Google Scholar]
- 9. Wirblich C, Meyers G, Ohlinger VF, Capucci L, Eskens U, Haas B, Thiel HJ. European brown hare syndrome virus: relationship to rabbit hemorrhagic disease virus and other caliciviruses. J Virol. 1994;68: 5164–5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lavazza A, Scicluna MT, Capucci L. Susceptibility of hares and rabbits to the European brown hare syndrome virus (EBHSV) and rabbit haemorrhagic disease virus (RHDV) under experimental conditions. Zentralbl Veterinarmed B. 1996;43: 401–410. [DOI] [PubMed] [Google Scholar]
- 11. Le Gall G, Huguet S, Vende P, Vautherot J-F, Rasschaert D. European brown hare syndrome virus: molecular cloning and sequencing of the genome. J Gen Virol. 1996;77: 1693–1697. [DOI] [PubMed] [Google Scholar]
- 12. Nowotny N, Bascunana CR, Ballagi-Pordany A, Gavier-Widen D, Uhlen M, Belak S. Phylogenetic analysis of rabbit haemorrhagic disease and European brown hare syndrome viruses by comparison of sequences from the capsid protein gene. Arch. Virol. 1997;142: 657–673. [DOI] [PubMed] [Google Scholar]
- 13. Le Gall-Reculé G, Zwingelstein F, Laurent S, Portejoie Y, Rasschaert D. Molecular epidemiology of European brown hare syndrome virus in France between 1989 and 2003. Arch. Virol. 2006;151: 1713–1721. [DOI] [PubMed] [Google Scholar]
- 14. Lopes AM, Gavier-Widén D, Le Gall-Reculé G, Esteves PJ, Abrantes J. Complete coding sequences of European brown hare syndrome virus (EBHSV) strains isolated in 1982 in Sweden. Archives of Virology 2013;158: 2193–2196. 10.1007/s00705-013-1714-7 [DOI] [PubMed] [Google Scholar]
- 15. Lopes AM, Capucci L, Gavier-Widén D, Le Gall-Reculé G, Brocchi E, Barbieri I, Quéméner A, Le Pendu J, Geoghegan JL, Holmes EC, Esteves PJ, Abrantes J. Molecular evolution and antigenic variation of European brown hare syndrome virus (EBHSV). Virology 2014;468–470:104–12. 10.1016/j.virol.2014.08.002 [DOI] [PubMed] [Google Scholar]
- 16. Liu SJ, Xue HP, Pu BQ, Qian NH. A new viral disease in rabbit. Anim Husb Vet Med. 1984;16: 253–255. [Google Scholar]
- 17. Gavier-Widén D, Mörner T. Epidemiology and diagnosis of the European brown hare syndrome in Scandinavian countries: a review. Rev Sci Tech. 1991;10: 453–458. [DOI] [PubMed] [Google Scholar]
- 18. Xu WY. Viral haemorrhagic disease of rabbits in the People’s Republic ofChina: epidemiology and virus characterisation. Rev Sci Tech 1991;10: 393–408. [PubMed] [Google Scholar]
- 19. Gregg DA, House C, Meyer R, Berninger M. Viral haemorrhagic disease of rabbits in Mexico: epidemiology and viral characterization. Rev Sci Tech 1991;10: 435–451. [DOI] [PubMed] [Google Scholar]
- 20. Mitro S, Krauss H. Rabbit hemorrhagic disease: a review with special reference to its epizootiology. Eur J Epidemiol. 1993;9: 70–78. [DOI] [PubMed] [Google Scholar]
- 21. Villafuerte R, Calvete C, Blanco JC, Lucientes J. Incidence of viral hemorrhagic disease in wild rabbit populations in Spain. Mammalia. 1995;59: 651–660. [Google Scholar]
- 22. Marchandeau S, Chantal J, Portejoie Y, Barraud S, Chaval Y. Impact of viral haemorrhagic disease on a wild population of European rabbits in France. Journal of Wildlife Diseases. 1998;34: 429–435. [DOI] [PubMed] [Google Scholar]
- 23. Marchandeau S, Chaval Y, Le Goff E. Prolonged decline in the abundance of wild European rabbits Oryctolagus cuniculus and high immunity level over three years following the arrival of rabbit haemorrhagic disease. Wildlife Biology. 2000;6: 141–147. [Google Scholar]
- 24. Delibes-Mateos M, Delibes M, Ferreras P, Villafuerte R. Key role of European rabbits in the conservation of the Western Mediterranean basin hotspot. Conserv Biol. 2008;22: 1106–17. 10.1111/j.1523-1739.2008.00993.x [DOI] [PubMed] [Google Scholar]
- 25. Delibes-Mateos M, Ferreira C, Carro F, Escudero MA, Gortázar C. Ecosystem effects of variant rabbit hemorrhagic disease virus, Iberian Peninsula. Emerg Infect Dis. 2014;20: 2166–2168. 10.3201/eid2012.140517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Capucci L, Fusi P, Lavazza A, Pacciarini ML, Rossi C. Detection and preliminary characterization of a new rabbit calicivirus related to rabbit hemorrhagic disease virus but nonpathogenic. J Virol. 1996;70: 8614–8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moss SR, Turner SL, Trout RC, White PJ, Hudson PJ, Desai A, Armesto M, Forrester NL, Gould EA. Molecular epidemiology of Rabbit haemorrhagic disease virus. J Gen Virol. 2002; 83: 2461–2467. [DOI] [PubMed] [Google Scholar]
- 28. Forrester NL, Trout RC, Gould EA. Benign circulation of rabbit haemorrhagic disease virus on Lambay Island, Eire. Virology. 2007;358: 18–22. [DOI] [PubMed] [Google Scholar]
- 29. Forrester NL, Boag B, Buckley A, Moureau G, Gould EA. Co-circulation of widely disparate strains of Rabbit haemorrhagic disease virus could explain localized epidemicity in the United Kingdom. Virology. 2009;393: 42–48. 10.1016/j.virol.2009.07.008 [DOI] [PubMed] [Google Scholar]
- 30. Strive T, Wright JD, Robinson AJ. Identification and partial characterization of a new Lagovirus in Australian wild rabbits. Virology. 2009;384: 97–105. 10.1016/j.virol.2008.11.004 [DOI] [PubMed] [Google Scholar]
- 31. Bergin IL, Wise AG, Bolin SR, Mullaney TP, Kiupel M, Maes RK. Novel calicivirus identified in rabbits, Michigan, USA. Emerg. Infect. Dis. 2009;15: 1955–1962. 10.3201/eid1512.090839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Abrantes J, Esteves PJ. Not-so-novel Michigan rabbit calicivirus. Emerg Infect Dis. 2010;16:1331–1332. 10.3201/eid1608.091803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jahnke M, Holmes EC, Kerr PJ, Wright JD, Strive T. Evolution and phylogeography of the nonpathogenic calicivirus RCV-A1 in wild rabbits in Australia. 2010;84: 12397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Le Gall-Recule G, Zwingelstein F, Fages MP, Bertagnoli S, Gelfi J, Aubineau J, Roobrouck A, Botti G, Lavazza A, Marchandeau S. Characterisation of a non-pathogenic and non-protective infectious rabbit lagovirus related toRHDV. Virology. 2011;410: 395–402. 10.1016/j.virol.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 35. Le Gall-Recule G, Zwingelstein F, Laurent S, de Boisseson C, Portejoie Y, Rasschaert D. Phylogenetic analysis of rabbit haemorrhagic disease virus in France between 1993 and 2000, and the characterisation of RHDV antigenic variants. Arch Virol; 2003;148: 65–81. [DOI] [PubMed] [Google Scholar]
- 36. Gould AR, Kattenbelt JA, Lenghaus C, Morrissy C, Chamberlain T, Collins BJ, Westbury HA. The complete nucleotide sequence of rabbit haemorrhagic disease virus (Czech strain V351): use of the polymerase chain reaction to detect replication in Australian vertebrates and analysis of viral population sequence variation. Virus Res. 1997;47: 7–17. [DOI] [PubMed] [Google Scholar]
- 37. Muller A, Freitas J, Silva E, Le Gall-Recule G, Zwingelstein F, Abrantes J, Esteves PJ, Alves PC, van der Loo W, Kolodziejek J, Nowotny N, Thompson G. Evolution of rabbit haemorrhagic disease virus (RHDV) in the European rabbit (Oryctolagus cuniculus) from the Iberian Peninsula. Vet Microbiol. 2009;135: 368–373. 10.1016/j.vetmic.2008.09.057 [DOI] [PubMed] [Google Scholar]
- 38. Milton ID, Vlasak R, Nowotny N, Rodak L, Carter MJ. Genomic 3’ terminalsequence comparison of three isolates of rabbit haemorrhagic disease virus. FEMS Microbiol Lett. 1992;72: 37–42. [DOI] [PubMed] [Google Scholar]
- 39. Boga JA, Casais R, Marin MS, Martin-Alonso JM, Carmenes RS, Prieto M, Parra F. Molecular cloning, sequencing and expression in Escherichia coliof the capsid protein gene from rabbit haemorrhagic disease virus (Spanish isolate AST/89). J Gen Virol. 1994;75: 2409–2413. [DOI] [PubMed] [Google Scholar]
- 40. Parra F, Boga JA, Marin MS, Casais R. The amino terminal sequence of VP60 from rabbit hemorrhagic disease virus supports its putative subgenomic origin. Virus Res. 1993, 27: 219–228. [DOI] [PubMed] [Google Scholar]
- 41. Le Gall G, Arnauld C, Boilletot E, Morisse JP, Rasschaert D. Molecular epidemiology of rabbit haemorrhagic disease virus outbreaks in France during 1988 to 1995. J Gen Virol. 1998;79: 11–16. [DOI] [PubMed] [Google Scholar]
- 42. Matiz K, Ursu K, Kecskemeti S, Bajmocy E, Kiss I. Phylogenetic analysis of rabbit haemorrhagic disease virus (RHDV) strains isolated between 1988 and 2003 in eastern Hungary. Arch. Virol. 2006;151: 1659–1666. [DOI] [PubMed] [Google Scholar]
- 43. Tian L, Liao J, Li JW, Zhou WR, Zhang XL, Wang HN. Isolation and identification of a non-haemagglutinating strain of rabbit hemorrhagic disease virus from China and sequence analysis for the VP60 Gene. Virus Genes. 2007;35: 745–752. [DOI] [PubMed] [Google Scholar]
- 44. Guittre C, Baginski I, Le Gall G, Prave M, Trepo C, Cova L. Detection of rabbit haemorrhagic disease virus isolates and sequence comparison of the N-terminus of the capsid protein gene by the polymerase chain reaction. Res Vet Sci. 1995;58: 128–132. [DOI] [PubMed] [Google Scholar]
- 45. Rasschaert D, Huguet S, Madelaine MF, Vautherot JF. Sequence and genomic organization of a rabbit hemorrhagic disease virus isolated from a wild rabbit. Virus Genes. 1995;9: 121–132. [DOI] [PubMed] [Google Scholar]
- 46. Viaplana E, Villaverde A. Microheterogeneity of p60 capsid protein andthe encoding gene among contemporary isolates of rabbit hemorrhagic disease virus. Virus Genes. 1996;12: 189–192. [DOI] [PubMed] [Google Scholar]
- 47. Asgari S, Hardy JR, Cooke BD. Sequence analysis of rabbit haemorrhagic disease virus (RHDV) in Australia: alterations after its release. Arch. Virol. 1999;144: 135–145. [DOI] [PubMed] [Google Scholar]
- 48. van de Bildt MW, van Bolhuis GH, van Zijderveld F, van Riel D, Drees JM, Osterhaus AD, Kuiken T. Confirmation and phylogenetic analysis of rabbit hemorrhagic disease virus in free-living rabbits from the Netherlands. J Wildl Dis. 2006;42: 808–812. [DOI] [PubMed] [Google Scholar]
- 49. Yang L, Wang F, Hu B, Xue J, Hu Y, Zhou B, Wang D, Xu W. Development of an RT-PCR for rabbit hemorrhagic disease virus (RHDV) and the epidemiology of RHDV in three eastern provinces of China. J. Virol. Methods 2008;151: 24–29. 10.1016/j.jviromet.2008.04.003 [DOI] [PubMed] [Google Scholar]
- 50. Hukowska-Szematowicz B, Pawlikowska M, Deptula W. Genetic variability of Czech and German RHD virus strains. Pol. J. Microbiol. 2009;58: 237–245. [PubMed] [Google Scholar]
- 51. Kerr PJ, Kitchen A, Holmes EC. Origin and phylodynamics of rabbit hemorrhagic disease virus. J Virol. 2009;83: 12129–12138. 10.1128/JVI.01523-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Alda F, Gaitero T, Suárez M, Merchán T, Rocha G, Doadrio I. Evolutionary history and molecular epidemiology of rabbit haemorrhagic disease virus in the Iberian Peninsula and Western Europe. BMC Evol Biol. 2010;10: 347 10.1186/1471-2148-10-347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Abrantes J, Lopes AM, Esteves PJ. Complete genomic sequences of rabbit hemorrhagic disease virus G1 strains isolated in the European rabbit original range. J Virol. 2012;86: 13886 10.1128/JVI.02683-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Esteves PJ, Lopes AM, Magalhães MJ, Pinheiro A, Gonçalves D, Abrantes J. Rabbit hemorrhagic disease virus detected in Pico, Azores, Portugal, revealed a unique endemic strain with more than 17 years of independent evolution. Viruses. 2014; 6: 2698–2707. 10.3390/v6072698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kovaliski J, Sinclair R, Mutze G, Peacock D, Strive T, Abrantes J, Esteves PJ, Holmes EC. Molecular epidemiology of Rabbit Haemorrhagic Disease Virus in Australia: when one became many. Mol Ecol. 2014;23: 408–20 10.1111/mec.12596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Capucci L, Fallacara F, Grazioli S, Lavazza A, Pacciarini ML, Brocchi E. A further step in the evolution of rabbit hemorrhagic disease virus: the appearance of the first consistent antigenic variant. Virus Res. 1998; 58: 115–126. [DOI] [PubMed] [Google Scholar]
- 57. Farnos O, Rodriguez D, Valdes O, Chiong M, Parra F, Toledo JR, Fernandez E,Lleonart R, Suarez M. Molecular and antigenic characterization of rabbit hemorrhagic disease virus isolated in Cuba indicates a distinct antigenic subtype. Arch Virol. 2007;152: 1215–1221. [DOI] [PubMed] [Google Scholar]
- 58. Schirrmeier H, Reimann I, Kollner B, Granzow H. Pathogenic, antigenic and molecular properties of rabbit haemorrhagic disease virus (RHDV) isolated from vaccinated rabbits: detection and characterization of antigenic variants. Arch Virol. 1999;144: 719–735. [DOI] [PubMed] [Google Scholar]
- 59. McIntosh MT, Behan SC, Mohamed FM, Lu Z, Moran KE, Burrage TG, Neilan JG, Ward GB, Botti G, Capucci L, Metwally SA. A pandemic strain of calicivirus threatens rabbit industries in the Americas. Virol J. 2007;4: 96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Oem JK, Lee KN, Roh IS, Lee KK, Kim SH, Kim HR, Park CK, Joo YS. Identification and characterization of rabbit hemorrhagic disease virus genetic variants isolated in Korea. J Vet Med Sci 2009;71: 1519–1523. [DOI] [PubMed] [Google Scholar]
- 61. Abrantes J, Lopes AM, Dalton KP, Parra F, Esteves PJ. Detection of RHDVa on the Iberian Peninsula: isolation of an RHDVa strain from a Spanish rabbitry. Arch Virol. 2014;159: 321–326. 10.1007/s00705-013-1808-2 [DOI] [PubMed] [Google Scholar]
- 62. Le Gall-Reculé G, Zwingelstein F, Boucher S, Le Normand B, Plassiart G, Portejoie Y, Decors A, Bertagnoli S, Guérin JL, Marchandeau S. Detection of a new variant of rabbit haemorrhagic disease virus in France. Vet Rec. 2011;168: 137–138. [DOI] [PubMed] [Google Scholar]
- 63. Dalton KP, Nicieza I, Balseiro A, Muguerza MA, Rosell JM, Casais R, Álvarez ÁL, Parra F. Variant rabbit hemorrhagic disease virus in young rabbits, Spain. Emerg Infect Dis. 2012;18: 2009–2012. 10.3201/eid1812.120341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Le Gall-Reculé G, Lavazza A, Marchandeau S, Bertagnoli S, Zwingelstein F, Cavadini P, Martinelli N, Lombardi G, Guérin JL, Lemaitre E, Decors A, Boucher S, Le Normand B, Capucci L. Emergence of a new lagovirus related to Rabbit Haemorrhagic Disease Virus. Vet Res. 2013;44: 81 10.1186/1297-9716-44-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Puggioni G, Cavadini P, Maestrale C, Scivoli R, Botti G, Ligios C, Le Gall-Reculé G, Lavazza A, Capucci L. The new French 2010 Rabbit Hemorrhagic Disease Virus causes an RHD-like disease in the Sardinian Cape hare (Lepus capensis mediterraneus).Vet Res. 2013;44: 96 10.1186/1297-9716-44-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Abrantes J, Lopes AM, Dalton KP, Melo P, Correia JJ, Ramada M, Alves PC, Parra F, Esteves PJ. New variant of rabbit hemorrhagic disease virus, Portugal, 2012–2013. Emerg Infect Dis. 2013;19: 1900–1902. 10.3201/eid1911.130908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dalton KP, Nicieza I, Abrantes J, Esteves PJ, Parra F. Spread of new variant RHDV in domestic rabbits on the Iberian Peninsula. Vet Microbiol. 2014;169: 67–73. 10.1016/j.vetmic.2013.12.015 [DOI] [PubMed] [Google Scholar]
- 68. Baily JL, Dagleish MP, Graham M, Maley M, Rocchi MS. RHDV variant 2 presence detected in Scotland. Vet Rec. 2014;174: 411. [DOI] [PubMed] [Google Scholar]
- 69. Simpson V, Everest D, Westcott D. RHDV variant 2 and Capillaria hepatica infection in rabbits. Vet Rec. 2014;174: 486. [DOI] [PubMed] [Google Scholar]
- 70. Camarda A, Pugliese N, Cavadini P, Circella E, Capucci L, Caroli A, Legretto M, Mallia E, Lavazza A. Detection of the new emerging rabbit haemorrhagic disease type 2 virus (RHDV2) in Sicily from rabbit (Oryctolagus cuniculus) and Italian hare (Lepus corsicanus). Res Vet Sci. 2014;97: 642–645. 10.1016/j.rvsc.2014.10.008 [DOI] [PubMed] [Google Scholar]
- 71. Lopes AM, Correia J, Abrantes J, Melo P, Ramada M, Magalhães MJ, Alves PC, Esteves PJ. Is the new variant RHDV replacing genogroup 1 in Portuguese wild rabbit populations? Viruses. 2014:7: 27–36. 10.3390/v7010027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dalton KP, Abrantes J, Lopes AM, Nicieza I, Álvarez ÁL, Esteves PJ, Parra F Complete genome sequence of two rabbit hemorrhagic disease virus variant b isolates detected on the Iberian Peninsula. Arch. Virol. 2015; 160:877–881. 10.1007/s00705-014-2329-3 [DOI] [PubMed] [Google Scholar]
- 73. Westcott DG, Choudhury B. Rabbit haemorrhagic disease virus 2-like variant in Great Britain. Vet Rec. 2015;176: 74 10.1136/vr.102830 [DOI] [PubMed] [Google Scholar]
- 74. Lopes AM, Dalton KP, Magalhães MJ, Parra F, Esteves PJ, Holmes EC, Abrantes J. Full genomic analysis of new variant Rabbit Hemorrhagic Disease Virus (RHDVb) revealed multiple recombination events. J Gen Virol. 2015;96: 1309–1319. 10.1099/vir.0.000070 [DOI] [PubMed] [Google Scholar]
- 75. Duarte M, Henriques M, Barros SC, Fagulha T, Ramos F, Luís T, Fevereiro M, Benevides S, Flor L, Barros SV, Bernardo S. Detection of RHDV variant 2 in the Azores. Vet Rec. 2015;176: 130 10.1136/vr.h497 [DOI] [PubMed] [Google Scholar]
- 76. Calvete C, Sarto P, Calvo AJ, Monroy F, Calvo JH. Could the new Rabbit haemorrhagic disease virus variant (RHDVb) be fully replacing classical RHD strains in the Iberian Peninsula? World Rabbit Sci. 2014;22: 91. [Google Scholar]
- 77. Almeida T, Lopes AM, Magalhães MJ, Neves F, Pinheiro A, Gonçalves D, Leitão M, Esteves PJ, Abrantes J. Tracking the evolution of the G1/RHDVb recombinant strains introduced from the Iberian Peninsula to the Azores islands, Portugal. Infection, Genetics and Evolution 2015;34: 307–313. 10.1016/j.meegid.2015.07.010 [DOI] [PubMed] [Google Scholar]
- 78. Poli A, Nigro M, Gallazzi D, Siroli G, Lavazza A, Gelmetti D. Acute hepatosis in the European Brown Hare (Lepus europaeus) in Italy. J Wild Dis. 1991;27: 621–629. [DOI] [PubMed] [Google Scholar]
- 79. Zanni ML, Benassi ML, Scicluna MT, Lavazza A, Capucci L. Clinical evolution and diagnosis of an episode of European Brown Hare Syndrome (EBHS) in hares reared in captivity. Rev. sci. tech. Off. int. Epiz. 1993;12: 931–940. [DOI] [PubMed] [Google Scholar]
- 80. Scicluna MT, Capucci L, Lavazza A. European brown hare syndrome in northern Italy: results of a virological and serological survey. Rev. sci. tech. Off. int. Epiz. 1994;13: 893–904. [DOI] [PubMed] [Google Scholar]
- 81. Billinis C, Psychas V, Tontis DK, Spyrou V, Birtsas PK, Sofia M, Likotrafitis F, Maslarinou OM, Kanteres D. European brown hare syndrome in wild European brown hares from Greece. J. Wildl. Dis. 2005;41: 783–786. [DOI] [PubMed] [Google Scholar]
- 82. Chasey D, Duff P. European brown hare syndrome and associated virus particles in the UK. Vet. Rec. 1990;126: 623–624. [PubMed] [Google Scholar]
- 83. Frölich K, Fickel J, Ludwig A, Lieckfeldt D, Streich WJ, Jurčík R, Slamečka J, Wibbelt G. New variants of European brown hare syndrome virus strains in free ranging European brown hares (Lepus europaeus) from Slovakia. J. Wildl. Dis. 2007;43: 89–96. [DOI] [PubMed] [Google Scholar]
- 84. Frölich K, Meyer HHD, Pielowski Z, Ronsholt L, Seck-Lanzendorf Sv, Stolte M. European brown hare syndrome in free-ranging hares in Poland. J. Wildl. Dis. 1996;32: 280–285. [DOI] [PubMed] [Google Scholar]
- 85. Lavazza A, Vecchi G. Osservazioni su alcuni episodi di mortalità nelle lepri. Evidenziazione al microscopio elettronico di una particella virale. Nota preliminare. Selezione Veterinaria. 1989;30: 461–467. [Google Scholar]
- 86. Duff JP, Chasey D, Munro R, Wooldridge M. European brown hare syndrome in England. Vet. Rec. 1994; 134: 669–673. [DOI] [PubMed] [Google Scholar]
- 87.Duff JP, Whitwell K, Chasey D. The emergence and epidemiology of European brown hare syndrome in the UK, Proceedings of the First International Symposium on Caliciviruses, United Kingdom, 1997;pp. 176–181.
- 88. Ros Bascuñana C, Nowotny N, Belák S. Detection and differentiation of rabbit hemorrhagic disease and European brown hare syndrome viruses by amplification of VP60 genomic sequences from fresh and fixed tissue specimens. J. Clin. Microbiol. 1997;35: 2492–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rodak L, Smid B, Valicek L, Vesely T, Stepanek J, Hampl J, Jurak E. Enzyme-linked immunosorbent assay of antibodies to rabbit haemorrhagic disease virus and determination of its major structural proteins. J. Gen. Virol. 1990;71: 1075–1080. [DOI] [PubMed] [Google Scholar]
- 90. O'Keefe JS, Tempero JE, Motha MXJ, Hansen MF, Atkinsona PH. Serology of rabbit haemorrhagic disease virus in wild rabbits before and after relase of the virus in New Zealand. Vet Microbiol. 1999;66: 29–40. [DOI] [PubMed] [Google Scholar]
- 91. Cooke BD, Robinson AJ, Merchant JC, Nardin A, Capucci L. Use of ELISAs in field studies of rabbit haemorrhagic disease (RHD) in Australia. Epidemiol Infect. 2000;124: 563–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Robinson AJ, Kirkland PD, Forrester RI, Capucci L, Cooke BD, Philbey AW. Serological evidence for the presence of a calicivirus in Australian wild rabbits, Oryctolagus cuniculus, before the introduction of rabbit haemorrhagic disease virus (RHDV): its potential influence on thespecificity of a competitive ELISA for RHDV. Wildl Res. 2002;29: 655–662. [Google Scholar]
- 93. Fenner F, Ratcliffe FN. Myxomatosis; Cambridge University Press; 1965; Cambridge, UK. [Google Scholar]
- 94. Lavazza A, Cavadini P, Barbieri I, Tizzani P, Pinheiro A, Abrantes J, Esteves PJ, Grilli G, Gioia E, Zanoni M, Meneguz P, Guitton JS, Marchandeau S, Chiari M, Capucci L. Field and experimental data indicate that the eastern cottontail (Sylvilagus floridanus) is susceptible to infection with European brown hare syndrome (EBHS) virus and not with rabbit haemorrhagic disease (RHD) virus. Vet Res. 2015; 46: 13 10.1186/s13567-015-0149-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lopes AM, Marques S, Silva E, Magalhaes MJ, Pinheiro A, Alves PC, Le Pendu J, Esteves PJ, Thompson G, Abrantes J. Detection of RHDV strains in the Iberian hare (Lepus granatensis): earliest evidence of rabbit lagovirus cross-species infection. Vet Res. 2014;45: 94 10.1186/s13567-014-0094-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Abrantes J, Esteves PJ, van der Loo W. Evidence for recombination in the major capsid gene VP60 of the rabbit haemorrhagic disease virus (RHDV). Archives of Virology 2008;153: 329–335. 10.1007/s00705-007-1084-0 [DOI] [PubMed] [Google Scholar]
- 97. Forrester NL, Moss SR, Turner SL, Schirrmeier H, Gould EA. Recombination in Rabbit haemorrhagic disease virus: possible impact on evolution and epidemiology. Virology 2008;376: 390–396. 10.1016/j.virol.2008.03.023 [DOI] [PubMed] [Google Scholar]