Abstract
Objective
In vivo Corneal Confocal Microscopy (IVCCM) is a validated, non-invasive test for diabetic sensorimotor polyneuropathy (DSP) detection, but its utility is limited by the image analysis time and expertise required. We aimed to determine the inter- and intra-observer reproducibility of a novel automated analysis program compared to manual analysis.
Methods
In a cross-sectional diagnostic study, 20 non-diabetes controls (mean age 41.4±17.3y, HbA1c 5.5±0.4%) and 26 participants with type 1 diabetes (42.8±16.9y, 8.0±1.9%) underwent two separate IVCCM examinations by one observer and a third by an independent observer. Along with nerve density and branch density, corneal nerve fibre length (CNFL) was obtained by manual analysis (CNFLMANUAL), a protocol in which images were manually selected for automated analysis (CNFLSEMI-AUTOMATED), and one in which selection and analysis were performed electronically (CNFLFULLY-AUTOMATED). Reproducibility of each protocol was determined using intraclass correlation coefficients (ICC) and, as a secondary objective, the method of Bland and Altman was used to explore agreement between protocols.
Results
Mean CNFLManual was 16.7±4.0, 13.9±4.2 mm/mm2 for non-diabetes controls and diabetes participants, while CNFLSemi-Automated was 10.2±3.3, 8.6±3.0 mm/mm2 and CNFLFully-Automated was 12.5±2.8, 10.9 ± 2.9 mm/mm2. Inter-observer ICC and 95% confidence intervals (95%CI) were 0.73(0.56, 0.84), 0.75(0.59, 0.85), and 0.78(0.63, 0.87), respectively (p = NS for all comparisons). Intra-observer ICC and 95%CI were 0.72(0.55, 0.83), 0.74(0.57, 0.85), and 0.84(0.73, 0.91), respectively (p<0.05 for CNFLFully-Automated compared to others). The other IVCCM parameters had substantially lower ICC compared to those for CNFL. CNFLSemi-Automated and CNFLFully-Automated underestimated CNFLManual by mean and 95%CI of 35.1(-4.5, 67.5)% and 21.0(-21.6, 46.1)%, respectively.
Conclusions
Despite an apparent measurement (underestimation) bias in comparison to the manual strategy of image analysis, fully-automated analysis preserves CNFL reproducibility. Future work must determine the diagnostic thresholds specific to the fully-automated measure of CNFL.
Introduction
Diabetic sensory polyneuropathy (DSP) is characterized by progressive, symmetrical and length-dependent loss of function to peripheral nerves resulting at least partially from chronic hyperglycemia. [1] DSP is estimated to be present in 50% of patients with type 1 diabetes (T1DM), and the presence of DSP exaggerates the risk of ulceration, infection and amputation. [2] Currently there is no effective disease-modifying treatment for DSP and intervention is aimed primarily at controlling the intensity and duration of hyperglycemic exposure. However, the selection of patients at the highest clinical need for intensified glycemic intervention could be informed by objective evidence for the presence of early neuropathy. [2]
Accurate diagnosis of DSP requires identifying abnormal electrodiagnostic data in combination with neuropathic signs and symptoms. [3, 4] This definition correlates well with large-fibre dysfunction and is thus effective at detecting the later stages of neuropathy. Identification of the earliest process of peripheral nerve injury requires the existence of a reliable and valid test for detecting damage to small, thinly- and un-myelinated nerve fibres as damage to these fibres precedes large fibre impairment. [5–7] The gold-standard for measuring small fibre neuropathy is the morphological assessment of intra-epidermal nerve fibres through skin biopsies, but its invasive nature makes it less practical for routine screening purposes. [8] To fill this gap, in vivo corneal confocal microscopy (IVCCM) has emerged as a tool for detecting early morphological alterations in the small nerve fibres, sampled non-invasively by imaging the nerve fibre plexus contained in the transparent cornea of the eye. These fibres arise from the ophthalmic division of the trigeminal nerve. Their morphological features correlate well with those in the intra-epithelial layer of skin, and cross-sectional and longitudinal studies of their diagnostic performance for DSP have for the most part been validated. [9–20] However, utility is substantially limited by the time and expertise required for image analysis.
To address this need, investigators from the University of Manchester have developed a tool capable of automated analysis of individual images, which holds the promise of eliminating the need for trained image analyst personnel and reducing the time that it takes to analyse a single image from 10–20 minutes to several seconds. [21] Ultimately, knowledge translation of IVCCM into clinical practice requires assurance of its reliability in the context of the complex methodologically-sound studies designed to appropriately evaluate reproducibility.
By way of an existing image repository from a previous detailed reproducibility study, in which each participant was repeatedly examined three times in a single day, we aimed to systematically determine the inter- and intra-observer reproducibility of a semi- and fully-automated image analysis protocol compared to that of the existing manual analysis reference standard. As a secondary objective, we aimed to explore correlation and agreement between protocols. [22]
Methods
Study Participants
Twenty-six type 1 diabetes participants and twenty non-diabetes controls, previously examined in a study of reproducibility between 2008 and 2010, were randomly selected from The Toronto Neuropathy Cohort study. Details of the nested study and the cohort study have previously been described. [22] In brief, participants with type 1 diabetes and non-diabetes controls were recruited from Endocrinology and Neurology clinics at the University Health Network, Toronto, Ontario, Canada. Controls were ascertained from spouses, family and friends of these participants. Inclusion required being 18 years of age or greater, and the absence of non-diabetes neuropathy. The study protocol was approved by the University Health Network research ethics board (approval number 08-0717-A), all participants provided written informed consent and the study was conducted in accordance with the Declaration of Helsinki.
Clinical and Electrophysiological Variables
All participants underwent clinical evaluation, electrophysiological testing, and laboratory testing on the same day. As per research guidelines, DSP case definition required presence of at least one electrophysiological parameter abnormality in each of the peroneal and sural nerves, and the presence of one neuropathic sign or symptom. [3, 22–24] Electrophysiological parameters included amplitude potential and conduction velocity of the peroneal and sural nerve as well as the f-wave latency for the peroneal nerve. Age- and height-adjusted criteria for sural and peroneal parameters were applied and each parameter was scored as normal or abnormal based on laboratory reference values. [25] Research staff conducting the electrophysiological and clinical exams were blinded to the participants’ IVCCM results. All testing procedures were done in accordance with the standards of the American Association for Neuromuscular and Electrodiagnostic Medicine as well as the Canadian Society of Clinical Neurophysiology as previously described for this cohort. [26]
In Vivo Corneal Confocal Microscopy Procedure
We performed bilateral examinations of the nerve plexus adjacent to the corneal Bowman’s layer using the Rostock cornea module of the Heidelberg Tomograph III using the 300μm field of view lens (Heidelberg Engineering, Smithfield, RI, USA) according to previously-published methods. [22, 23, 27] In brief, the technician administered a topical anaesthetic to the participant’s eye to temporarily suppress the corneal blink reflex. Then, a gel was used to form a bridge between the cornea and the sterile cap of the microscope lens. A 670 nm red wavelength diode laser illuminated the ocular structures and aided the technician in targeting the cornea’s apex. The volume scan mode was used to automatically capture 40 0.3 by 0.3 mm images that spanned a total depth of 50 μm at 1.3 μm increments. This was done twice per eye and then repeated on the contralateral eye. One examination yielded 80 images per eye (160 images in total), and each participant underwent three examinations, two by a single examiner and a third by an independent examiner. All examinations were done within a three hour period of each other. This allowed for systematic evaluation of both inter- and intra-observer reproducibility. Three distinct protocols for quantifying IVCCM parameters were then applied: manual image analysis, semi-automated image analysis and fully-automated image analysis.
Manual Image Analysis
A research technician visually examined the 80 images obtained per eye and selected the image which had the highest density of nerve fibres and was of high technical quality with respect to focus and contrast. This was repeated for the participant’s other eye such that a total of two images were selected per participant. The selected images were then analysed using CCMetrics, developed by the University of Manchester group. This involved manually tracing and placing cursor marks over the fibres and branches with a graphic pen tablet which allowed for quantification of corneal nerve fibre length (CNFL, measured in units of mm/mm2), corneal nerve fibre density (CNFD, measured in units of fibres/mm2) and corneal nerve branch density (CNBD, measured in units of branches/mm2). [28] Nerve branches included all branches from main nerve fibres, and any subsequent branches attached to those. The CNFL, CNFD and CNBD obtained from both eyes were averaged to determine the participant’s overall corneal nerve parameters. We note that the results of a reproducibility analysis using this manual protocol have been previously published, and we present them as a means of comparison with the new methods described below.
Semi-Automated Image Analysis
In the semi-automated analysis protocol the two images per participant were selected in the same way as they were selected in the manual image analysis protocol. Parameter quantification was accomplished using ACCMetrics, an automated software for IVCCM image analysis also developed the University of Manchester group. [21, 29] ACCMetrics detects low contrast nerve fibres amongst image noise. The software took approximately 15 seconds to analyse one image and unlike CCMetrics, required no preliminary, manual image tracing. ACCMetrics was used to analyse the two images chosen per participant, and the overall CNFL, CNFD and CNBD were obtained by averaging the results of these two images.
Fully-Automated Image Analysis
A research technician visually examined the two sets of 40 images obtained per eye, selecting one set for analysis. Within the selected set, the images (between 5 and 15) that included the nerve plexus layer were selected by the technician and all were analysed by ACCMetrics. The image with the highest CNFL value was chosen per eye and the results were averaged to obtain the participant’s overall CNFL, CNFD and CNBD.
Statistical Methods
Statistical analysis was performed using SAS 9.2 (SAS Institute, Cary, NC). Baseline characteristics of the non-diabetes controls, participants with type 1 diabetes without DSP, and participants with type 1 diabetes with DSP were compared using the χ2-test or Fisher's exact test (for smoking prevalence), and one way ANOVA (for continuous variables). Inter- and intra-observer reproducibility of each IVCCM parameter was assessed using the intraclass correlation coefficients ICC(2,1), as per the notation Shrout and Fleiss. [30] As described in our previous work, [22] the ICC(2,1) was used since it produces less biased and more conservative estimates of reproducibility and reliability compared to the other classes described by Shrout and Fleiss. This method also has high generalizability as it assumes that all participants are rated by the same raters from a random subset of the population of all possible raters. [22] ICC >0.80 were considered very good, 0.61–0.80 good, 0.41–0.60 moderate, 0.21–0.40 fair, and ICC <0.21 poor. [31] ICC were compared using their 95% confidence intervals (CI). [32] We performed two stratified analyses to explore the reproducibility within subgroups of the cohort: The first was an evaluation of ICC within the subgroup with DSP, and the second was an evaluation of the subgroup with CNFL values below 12.3mm/mm2, a threshold that represents the 2.5th percentile of the distribution observed in a previous study of non-diabetes controls using the manual analysis protocol. [33] This threshold approximates the lower extreme of the normal CNFL distribution values reported in a second, independent publication. [34] As a secondary objective, correlation and agreement between the protocols were assessed using Pearson correlation coefficients and the method of Bland & Altman, respectively. [35] Both absolute and percentage differences were used in the calculation of these metrics, and the 95%CI of the differences were reported. An alpha level of 0.05 was used for all comparisons.
Results
Clinical characteristics of the 46 study participants categorized as non-diabetes controls (n = 20), diabetes without DSP (n = 13), and diabetes with DSP (n = 13) are shown in Table 1. Broad variability of clinical factors associated with risk of DSP was observed between groups. These included age (p<0.001), diabetes duration (p<0.001) and HbA1c (p<0.001), all of which were highest in the group with both diabetes and DSP. Furthermore, broad variability was also observed in objective measures of DSP severity, such as sural nerve amplitude, which ranged from 17.3±7.7 μv in the non-diabetes controls to 2.3±1.8 μv in the group with both diabetes and DSP (p<0.001), showing significant nerve function impairment in the diabetes with DSP group. Collectively, these findings indicate a broad spectrum of DSP severity across the study group. Similarly, broad variability was also observed in the IVCCM parameters. CNFLmanual was 16.2±3.96 mm/mm2 in non-diabetes controls, and 16.9±3.79 mm/mm2 in participants with type 1 diabetes without DSP, but substantially lower at 12.0±4.23 mm/mm2 for diabetes particpants with DSP. Similar patterns were observed for CNFDManual and CNBDManual, though differences between groups did not reach statistical significance for the CNBDManual parameter. However, we noted differences in the mean values between the manual, semi-automated and fully-automated values. Specifically, for all variables, the mean values of CNFL, CNFD and CNBD determined by the semi-automated and fully-automated protocols appeared to be substantially lower than the manual measurements.
Table 1. Baseline clinical and biochemical characteristics of the 46 participants according to diabetes and DSP status.
| Non-diabetes controls (n = 20)†† | Participants with type 1 Diabetes without DSP (n = 13) | Participants with type 1 Diabetes with DSP (n = 13) | P value | |
|---|---|---|---|---|
| Clinical Characteristics | ||||
| Male sex, n (%)* | 5 (25%) | 11 (85%) | 8 (61%) | 0.009 |
| Age (years) | 41.3±17.3 | 30.3±13.7 | 56.2±8.7 | <0.001 |
| Duration of Type 1 diabetes (years) | - | 10.7±6.2 | 34.8±13.0 | <0.001 |
| Current/past smoker† | 6(30%) | 0(0%) | 2(15%) | 0.070 |
| Systolic Blood Pressure (mmHg) | 127±14 | 122±15 | 140±20 | 0.021 |
| Diastolic Blood Pressure (mmHg) | 79±10 | 70±10 | 73±6 | 0.025 |
| HbA1c (%) | 5.5±0.4 | 7.5±1.3 | 8.5±2.2 | <0.001 |
| Total Cholesterol (mmol/L) | 5.5±0.9 | 4.7±0.8 | 3.9±0.8 | <0.001 |
| Nerve Conduction Data | ||||
| Sural Nerve Amplitude Potential (μV) | 17.3±7.7 | 14.3±5.1 | 2.3±1.8 | <0.001 |
| Sural Nerve Conduction Velocity (m/s) | 52.7±4.3 | 47.9±2.8 | 40.0±4.9 | <0.001 |
| Peroneal Nerve Amplitude Potential (mV) | 7.1±1.9 | 7.4±3.3 | 1.6±1.7 | <0.001 |
| Peroneal Nerve Conduction Velocity (m/s) | 48.4±2.7 | 44.9±2.3 | 33.4±6.8 | <0.001 |
| Peroneal Nerve F-Wave (ms) | 46.4±3.0 | 50.7±4.6 | 68.6±10.1 | <0.001 |
| IVCCM Parameters | ||||
| CNFLManual (mm/mm2) | 16.2±3.96 | 16.9±3.79 | 12.0±4.23 | 0.006 |
| CNFLSemi-Automated (mm/mm2) | 10.2±3.38 | 9.74±3.56 | 7.48±2.15 | 0.053 |
| CNFLFully-Automated (mm/mm2) | 12.53±2.87 | 12.2±3.19 | 9.58±2.19 | 0.013 |
| CNFDManual (fibres/mm2) | 40.3±9.34 | 40.6±9.46 | 30.3±10.54 | 0.011 |
| CNFDSemi-Automated (fibres/mm2) | 22.77±12.47 | 20.94±11.37 | 11.54±6.60 | 0.017 |
| CNFDFully-Automated (fibres/mm2) | 28.06±8.55 | 27.77±8.49 | 17.52±8.13 | 0.002 |
| CNBDManual (branches/mm2) | 31.39±10.24 | 28.21±11.67 | 22.22±12.21 | 0.083 |
| CNBDSemi-Automated (branches/mm2) | 17.22±16.01 | 8.97±9.22 | 7.69±9.50 | 0.073 |
| CNBDFully-Automated (branches/mm2) | 24.99±12.16 | 19.23±16.76 | 13.25±13.13 | 0.068 |
One way ANOVA unless otherwise specified
*Chi-square test
† Fisher’s exact test
†† Values presented in column 1 (other than for Semi-Automated and Fully-Automated CCM parameters) have been presented in a previous publication and are shown here for comparison with the diabetes subgroups.
IVCCM, in-vivo corneal confocal microscopy; CNFL, corneal nerve fibre length; CNFD, corneal nerve fibre density; CNBD, corneal nerve branch density.
For the primary objective of determining reproducibility in all participants, we present the results of the inter- and intra-observer reliability analysis in Table 2. The first row of data in this table shows the inter-observer ICC for CNFLManual, CNFLSemi-Automated and CNFLFully-Automated and the p-values for their comparison. Specifically, the respective values were 0.73, 0.75 and 0.78, and none of the three-way comparisons between these ICC differed significantly. The second row of data in this table reports the intra-observer ICC for CNFLManual, CNFLSemi-Automated and CNFLFully-Automated, which were 0.72, 0.73 and 0.84 respectively. The intra-observer reproducibility of CNFLFully-Automated was larger than that of CNFLManual (p = 0.021) and CNFLSemi-Automated (p = 0.039).
Table 2. Intra Class Correlation coefficients for all participants (n = 46).
| Observer reliability measure | The manual protocol ICC and 95%CI* | The semi-automated protocol ICC and 95%CI | Comparison of semi-auto to manual P-Value | The fully automated protocol ICC and 95%CI | Comparison of fully auto to manual P-Value | Comparison of fully auto to semi-auto P-Value | |
|---|---|---|---|---|---|---|---|
| CNFL† | Inter | 0.73(0.55,0.84) | 0.75(0.58,0.86) | 0.39 | 0.78(0.34,0.91) | 0.40 | 0.46 |
| Intra | 0.72(0.50,0.85) | 0.73(0.56,0.84) | 0.43 | 0.84(0.73,0.91) | 0.021 | 0.039 | |
| CNFD | Inter | 0.61(0.39,0.76) | 0.68(0.48,0.81) | 0.68 | 0.50(0.17,0.71) | 0.83 | 0.95 |
| Intra | 0.57(0.33,0.73) | 0.60(0.39,0.76) | 0.37 | 0.63(0.41,0.78) | 0.27 | 0.38 | |
| CNBD | Inter | 0.32(-0.07,0.61) | 0.40(0.13,0.61) | 0.28 | 0.50(0.23,0.69) | 0.093 | 0.23 |
| Intra | 0.61(0.40,0.77) | 0.31(0.02,0.55) | 0.99 | 0.43(0.16,0.64) | 0.95 | 0.18 |
ICC: Intra-class correlation coefficient (2, 1). Inter refers to inter-observer reproducibility, intra to intra-observer reproducibility. The manual protocol included manual image selection and manual image analysis. The semi-automated protocol included manual image selection and automated analysis. The fully-automated protocol included automated image selection and automated analysis. CNFL, corneal nerve fibre length. CNFD, corneal nerve fibre density. CNBD, corneal nerve branch density.
* Manual protocol ICC have been presented in a previous publication and are shown here for comparison.
† For the 11-member sub-population with CNFL below 12.3mm/mm2, the inter-observer ICC for CNFLManual, CNFLSemi-Automated, and CNFLFully-Automated were 0.54(-0.09,0.85), 0.51(-0.04,0.83), and 0.73(0.25,0.92), respectively. P-values for comparison of the ICC of CNFLSemi-Automated to CNFLManual, CNFLFully-Automated to CNFLManual, and CNFLFully-Automated to CNFLSemi-Automated were 0.56, 0.17, and 0.15, respectively. The corresponding intra-observer ICC for CNFLManual, CNFLSemi-Automated, and CNFLFully-Automated were 0.74(0.26,0.92), 0.68(0.20,0.90), and 0.86(0.55,0.96), respectively. The p-values for the corresponding comparisons were 0.64, 0.16, and 0.19.
The third and fourth rows of the table display the inter- and intra-observer ICC of CNFDManual CNFDSemi-Automated and CNFDFully-Automated. All CNFD ICC fell in the ranges of good or moderate with no significant differences between protocols. The fifth and sixth rows display the inter- and intra-observer ICC of CNBDManual, CNBDSemi-Automated and CNBDFully-Automated. These ranged between good and fair and again no significant differences between protocols were observed.
Stratified analyses by diabetes-status are presented in Tables 3 and 4. These revealed that the ICC measured were similar between the non-diabetes controls and the participants with T1DM. When we sub-divided the type 1 diabetes participants into those with and without DSP, similar ICC for CNFL were observed (described in Table 4 Legend). Additionally, reproducibility was studied in the sub-population with CNFL below 12.3mm/mm2; 11(24%) of the entire study population had CNFL in this range. For this subgroup, the inter-observer ICC for CNFLManual, CNFLSemi-Automated, and CNFLFully-Automated was 0.54(-0.09,0.85), 0.51(-0.04,0.83), and 0.73(0.25,0.92), respectively, while the intra-observer ICC was 0.74(0.26,0.92), 0.68(0.20,0.90), and 0.86(0.55,0.96) (also summarized in Table 2, Legend). In this subgroup of individuals with the lowest CNFL values, inter- and intra-observer ICC were moderate and good, respectively, for both CNFLManual and CNFLSemi-Automated, and all lower than that observed for the ICC in the entire study population. However, ICC for CNFLFully-Automated were in keeping with the levels observed in the entire study population. None of the ICC values for each of CNFLManual, CNFLSemi-Automated, and CNFLFully-Automated differed significantly (p values shown in Table 2, Legend).
Table 3. Intra Class Correlation coefficients for all non-diabetes controls (n = 20).
| Observer reliability measure | The manual protocol ICC and 95%CI* | The semi-automated protocol ICC and 95%CI | Comparison of semi-auto to manual P-Value | The fully automated protocol ICC and 95%CI | Comparison of fully auto to manual P-Value | Comparison of fully auto to semi-auto P-Value | |
|---|---|---|---|---|---|---|---|
| CNFL | Inter | 0.68(0.35,0.86) | 0.73(0.43,0.88) | 0.34 | 0.75(0.25,0.91) | 0.38 | 0.50 |
| Intra | 0.72(0.39,0.88) | 0.77 (0.50,0.90) | 0.32 | 0.81(0.59,0.92) | 0.16 | 0.31 | |
| CNFD | Inter | 0.39(-0.04,0.70) | 0.72(0.42,0.88) | 0.018 | 0.52(0.09,0.78) | 0.27 | 0.92 |
| Intra | 0.51(0.10,0.78) | 0.69(0.36,0.87) | 0.11 | 0.72(0.39,0.88) | 0.084 | 0.41 | |
| CNBD | Inter | 0.35(-0.11,0.71) | 0.44(0.04,0.73) | 0.31 | 0.50(0.08,0.77) | 0.23 | 0.39 |
| Intra | 0.58(0.21,0.81) | 0.58(0.08,0.75) | 0.74 | 0.49(0.09,0.76) | 0.70 | 0.47 |
ICC: Intra-class correlation coefficient (2, 1). Inter refers to inter-observer reproducibility, intra to intra-observer reproducibility. The manual protocol included manual image selection and manual image analysis. The semi-automated protocol included manual image selection and automated analysis. The fully-automated protocol included automated image selection and automated analysis. CNFL, corneal nerve fibre length. CNFD, corneal nerve fibre density. CNBD, corneal nerve branch density.
* Manual protocol ICC have been presented in a previous publication and are shown here for comparison.
Table 4. Intra Class Correlation coefficients for all T1DM participants (n = 26).
| Observer reliability measure | The manual protocol ICC and 95%CI* | The semi-automated protocol ICC and 95%CI | Comparison of semi-auto to manual P-Value | The fully automated protocol ICC and 95%CI | Comparison of fully auto to manual P-Value | Comparison of fully auto to semi-auto P-Value | |
|---|---|---|---|---|---|---|---|
| CNFL† | Inter | 0.75(0.51,0.88) | 0.75(0.53,0.88) | 0.52 | 0.79(0.32,0.92) | 0.44 | 0.44 |
| Intra | 0.72(0.44,0.87) | 0.68(0.40,0.84) | 0.66 | 0.84(0.68,0.92) | 0.054 | 0.023 | |
| CNFD | Inter | 0.72(0.46,0.86) | 0.58(0.27,0.79) | 0.88 | 0.42(0.05,0.69) | 0.99 | 0.42 |
| Intra | 0.59(0.27,0.79) | 0.41(0.05,0.68) | 0.89 | 0.53(0.21,0.76) | 0.53 | 0.21 | |
| CNBD | Inter | 0.22(-0.09,0.52) | 0.19(-0.22,0.54) | 0.56 | 0.45(0.10,0.70) | 0.091 | 0.068 |
| Intra | 0.61(0.29,0.80) | 0.16(-0.21,0.51) | >0.99 | 0.34(-0.06,0.64) | 0.34 | 0.18 |
ICC: Intra-class correlation coefficient (2, 1). Inter refers to inter-observer reproducibility, intra to intra-observer reproducibility. The manual protocol included manual image selection and manual image analysis. The semi-automated protocol included manual image selection and automated analysis. The fully-automated protocol included automated image selection and automated analysis. CNFL, corneal nerve fibre length. CNFD, corneal nerve fibre density. CNBD, corneal nerve branch density.
* Manual protocol ICC have been presented in a previous publication and are shown here for comparison.
† For the 13-member sub-population without DSP, inter-observer ICC for CNFLFully-Automated was 0.78(0.30,0.93) and intra-observer ICC for CNFLFully-Automated was 0.84(0.55,0.95). For the 13-member sub-population with DSP, the inter-observer ICC for CNFLFully-Automated was 0.65(0.05,0.89) and intra-observer ICC for CNFLFully-Automated was 0.72(0.31,0.90). Comparisons of these ICC were not statistically significant.
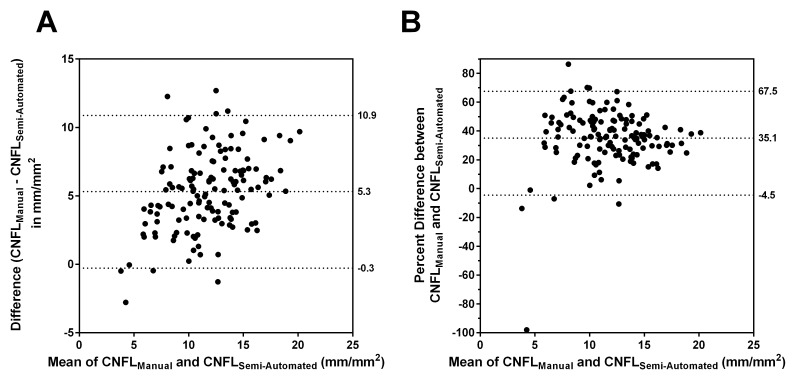
To explore the underestimation of the semi-automated and fully-automated IVCCM measures observed in the final section of Table 1, we determined the correlation and agreement between these protocols as part of a secondary objective. Fig 1 presents the Bland-Altman plots of agreement between CNFLManual and CNFLSemi-Automated. While the two protocols correlated well (Pearson r = 0.72), there was a marked lack of agreement. Fig 1 Panel A plots the difference between CNFLManual and CNFLSemi-Automated against the average of these variables, with reference lines, from top to bottom, denoting the 97.5th percentile, the mean, and the 2.5th percentile of the differences. On average, the semi-automated protocol underestimated the manual protocol by 5.3 [95% CI (-0.3, 10.9)] mm/mm2. Fig 1 Panel A also shows that the magnitude of underestimation increased as the mean of CNFLManual and CNFLSemi-Automated increased. Fig 1 Panel B shows the same Bland-Altman plot but with the difference between CNFLManul and CNFLSemi-Automated expressed as a percentage of CNFLManual. This shows that on average, CNFLSemi-Automated underestimated CNFLManual by 35.1 [95% CI (-4.5, 67.5)] %.
Fig 1. Agreement between CNFLManual and CNFLSemi-Automated.
In Panels A and B, the reference lines, from top to bottom, denote the 97.5th percentile, the mean, and the 2.5th percentile for the indicated differences.
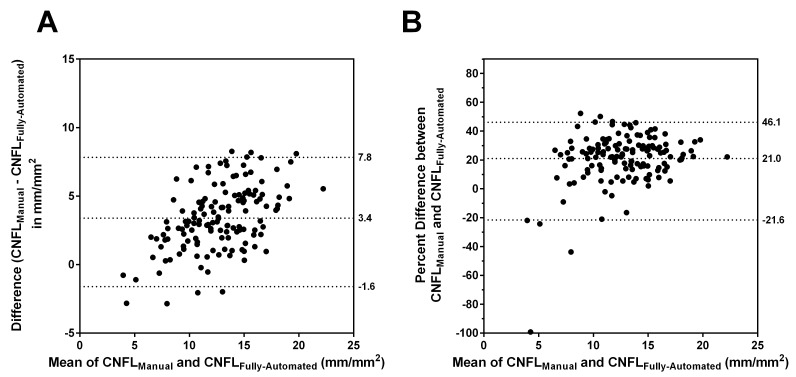
Bland-Altman plots of agreement between CNFLManual and CNFLFully-Automated are shown in Fig 2. There was excellent correlation between the protocols (Pearson r = 0.82), but Fig 2 Panel A shows that on average, CNFLFully-Automated underestimated CNFLManual by 3.4 [95% CI (-1.6, 7.8)] mm/mm2. Fig 2 Panel B shows that this was equivalent to a 21 [95% CI (-21.6, 46.1)] % underestimation.
Fig 2. Agreement between CNFLManual and CNFLFully-Automated.
In Panels A and B, the reference lines, from top to bottom, denote the 97.5th percentile, the mean, and the 2.5th percentile for the indicated differences.
Discussion
We studied, on three independent examinations, 46 participants representing a broad spectrum of nerve injury to determine the inter- and intra- observer reproducibility of a fully- and semi-automated protocol of IVCCM image analysis and compared them to the reference manual protocol. Though our main objective of this analysis was to show that the automated approach yields a level of reproducibility no worse than the previously studied manual approach, we did observe three key findings that add to the understanding of IVCCM for diabetic neuropathy. First, we report that the levels of inter- and intra- observer reproducibility were generally similar and good (ICC in the range of 0.6 to 0.8) between manual and automated image selection protocols, noting a superior reproducibility of the CNFLFully-Automated approach that was limited to intra-observer reproducibility. Second, we confirmed that the poorer reproducibility of the other IVCCM parameters–CNFD and CNBD–are not overcome by automated analysis and remain insufficient in comparison to CNFL. Finally, in our secondary analysis of agreement, we observed systematic underestimation of IVCCM parameters as measured by the fully- and semi-automated protocols compared to the reference manual protocol.
The finding of similar reproducibility of IVCCM parameters between automated and manual protocols is of fundamental importance for the translation of this technology as a measure of neuropathy to clinical trials and clinical practice. Specifically, the feasibility of the manual approach is limited by major demands on technician time for image analysis, and this limitation is overcome by the automated protocols. Though several studies have demonstrated that the reproducibility of performing repeat manual analysis of the same IVCCM image, described as “image-level reproducibility”, is very good in that ICC generally exceed 0.8, [22, 36, 37] such investigative test methodology does not sufficiently represent the true clinical reliability of the procedure. The more sound “study-level reproducibility” involves the analysis of images obtained from repeated patient IVCCM examinations and is a more clinically relevant measure of reproducibility as it accounts for the variation inherent in conducting multiple examinations. Such study-level reproducibility was later reported for the manual analysis technique, which revealed ICC levels in the good to very good range (exceeding levels of 0.7) [22, 37, 38]; quantification of nerves in the inferior whorl region of the cornea has also shown good study-level reproducibility. [39] However, to date, only the image-level reproducibility for automated image analysis has been reported (ICC level 1.0 indicating perfect reproducibility). [29] The current analysis represents the first confirmation of acceptable study-level reproducibility obtained from automated image analysis protocols using an image repository created through meticulous re-examination of participants by blinded examiners. The ICC that we report represent an unbiased estimate of the clinical reproducibility of automated IVCCM procedures. We thus confirm that the clinically-relevant inter- and intra-observer reliability of CNFL is preserved using automated approaches compared to the reference standard manual examination technique.
In contrast to these very good levels of reproducibility for CNFL, we were able to confirm that automated image analysis did not overcome the previously-described limitations in the inter- and intra-observer reproducibility of CNFD and CNBD. [22, 36, 37] We had previously hypothesized that the distinction between nerve fibres and nerve branches is not consistently clear in the examination of separate images—specifically, two crossing fibres might be interpreted as a single branching fibre but subsequently interpreted as two fibres without branches. For this reason, we had concluded that the reproducibility of these measures was inherently impaired for manual analysis protocols. [22] Though the automated image analysis protocols may remove image-level decision bias, the current results demonstrate that such bias is not reconciled by automation when applied in the context of the more clinically-relevant study-level analysis of independent clinical examinations. Inherently, CNFL is less susceptible to this measurement bias as it represents an integrated measure of the length of all nerve fibres and branches in the microscope field. As reproducibility is a key characteristic in the hierarchical model for studying diagnostic performance, [40] the current analysis confirmed the inherent advantage of CNFL over the other parameters and supports it as the preferred candidate IVCCM parameter.
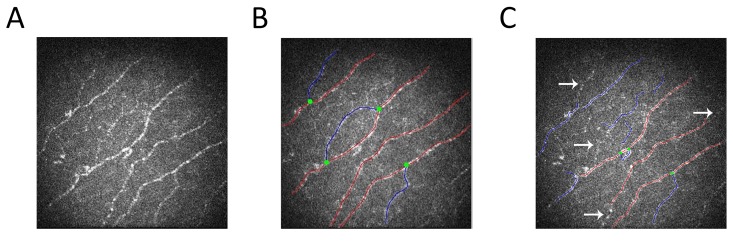
Though not the primary objective of this analysis, we explored correlation and agreement for CNFL between the three image analysis protocols. Although the correlation between the manual and fully-automated protocols were excellent (Pearson correlation coefficient 0.82), we report a substantial measurement bias. Specifically, the fully-automated approach was associated with average underestimation of 3.4 mm/mm2, which corresponded to 21% underestimation. An even greater degree of measurement bias has previously been reported with an earlier version of the automated software. [28] We observed that the underestimation of CNFL by both automated protocols arose as a result of an inability of the automated software to detect the fainter, lower contrast nerve fibres against background structures (see Fig 3 as a representative example). As the automated protocols offered overwhelming practical advantages over manual techniques in terms of time and resources required for image analysis, focused research is urgently required to determine the diagnostic thresholds for detecting DSP that are unique to the efficient automated protocols.
Fig 3. Illustration of Underestimation Measurement Bias by the Automated Image Analysis Protocol.
A) IVCCM image obtained using the Rostock corneal module of the Heidelberg Tomograph III using the 300 μm field of view lens B) Image from panel A traced for parameter quantification using CCMetrics, the software used in the manual protocol of IVCCM image analysis. Red lines indicate fibres, blue lines indicate branches and green dots indicate branching points. C) Image from Panel A post-analysis by ACCMetrics, the software used in automated IVCCM image analysis, showing which nerves were captured by the automated quantification process. The arrows were included to indicate nerves that were not quantified by ACCMetrics despite being traced for quantification using the manual protocol. This inability of ACCMetrics to detect fainter nerve fibres is representative of the entire dataset and explains the measurement (underestimation) bias inherent in using automated IVCCM image analysis protocols.
Although this study of clinically-relevant reproducibility had major methodological advantages over previous investigative test research for IVCCM, we acknowledge potential limitations. First, though we previously found excellent agreement between lens type, [41] we used a 300 μm lens that produced 0.3 x 0.3 mm2 field of view images, while other investigators have more commonly used a 400 μm lens that produces a 0.4 x 0.4 mm2 field of view. Second, there are minor variations in the protocol for image acquisition as compared to other study groups in that we used the “volume scan” mode [22, 23, 42] rather than the “section mode” [9, 29, 36, 38, 43, 44] for image acquisition and we implemented a protocol that used a single image per eye rather than multiple. [45] Third, we did not systematically determine analysis time in this study to further substantiate time- and resource-savings. Finally, our results may not be generalizable to patients with type 2 diabetes.
Automating the process of IVCCM image analysis has the potential to overcome an important barrier to the clinical implementation of this neuropathy biomarker–its resource and time consuming nature. Confirming the reproducibility of the semi- and fully-automated protocols was an important step towards verifying its appropriateness for clinical and investigative use. The measurement bias between the automated and the manual approaches requires verification in a larger cohort. Furthermore it is imperative to establish the diagnostic thresholds for the identification of diabetic neuropathy and its future risk that are specific to the automated protocols.
Supporting Information
This file includes all relevant data to support the results of the reproducibility analysis.
(XLSX)
Acknowledgments
We wish to acknowledge the generous support of Randy and Jenny Frisch and The Harvey and Annice Frisch Family Fund. An abstract based on this work was presented at the 75th Scientific Sessions of the American Diabetes Association. An abstract was also presented at the 25th Annual Meeting of the Diabetic Neuropathy Study Group of the European Association for the Study of Diabetes. All authors reviewed the manuscript for scholarly content and accuracy. BAP is the guarantor of this manuscript and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of data analysis.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The study was funded by the JDRF Operating Grant No. 17-2008-715, website: http://www.jdrf.ca/ (Juvenile Diabetes Research Foundation). The authors wish to acknowledge the generous support of Randy and Jenny Frisch and The Harvey and Annice Frisch Family Fund. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956–62. Epub 2005/03/29. doi: 28/4/956 [pii]. . [DOI] [PubMed] [Google Scholar]
- 2. Ziegler D, Luft D. Clinical trials for drugs against diabetic neuropathy: can we combine scientific needs with clinical practicalities? Int Rev Neurobiol. 2002;50:431–63. Epub 2002/08/30. . [DOI] [PubMed] [Google Scholar]
- 3. England JD, Gronseth GS, Franklin G, Miller RG, Asbury AK, Carter GT, et al. Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2005;64(2):199–207. Epub 2005/01/26. doi: 64/2/199 [pii] 10.1212/01.WNL.0000149522.32823.EA . [DOI] [PubMed] [Google Scholar]
- 4. Halpern EM, Lovblom LE, Orlov S, Ahmed A, Bril V, Perkins BA. The impact of common variation in the definition of diabetic sensorimotor polyneuropathy on the validity of corneal in vivo confocal microscopy in patients with type 1 diabetes: a brief report. Journal of diabetes and its complications. 2013;27(3):240–2. Epub 2012/12/26. 10.1016/j.jdiacomp.2012.10.011 . [DOI] [PubMed] [Google Scholar]
- 5. Smith AG, Singleton JR. Impaired glucose tolerance and neuropathy. Neurologist. 2008;14(1):23–9. Epub 2008/01/16. 10.1097/NRL.0b013e31815a3956 00127893-200801000-00006 [pii]. . [DOI] [PubMed] [Google Scholar]
- 6. Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology. 2003;60(1):108–11. Epub 2003/01/15. . [DOI] [PubMed] [Google Scholar]
- 7. Breiner A, Lovblom LE, Perkins BA, Bril V. Does the prevailing hypothesis that small-fiber dysfunction precedes large-fiber dysfunction apply to type 1 diabetic patients? Diabetes Care. 2014;37(5):1418–24. Epub 2014/02/28. 10.2337/dc13-2005 . [DOI] [PubMed] [Google Scholar]
- 8. England JD, Gronseth GS, Franklin G, Carter GT, Kinsella LJ, Cohen JA, et al. Practice Parameter: evaluation of distal symmetric polyneuropathy: role of autonomic testing, nerve biopsy, and skin biopsy (an evidence-based review). Report of the American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, and American Academy of Physical Medicine and Rehabilitation. Neurology. 2009;72(2):177–84. Epub 2008/12/06. 10.1212/01.wnl.0000336345.70511.0f . [DOI] [PubMed] [Google Scholar]
- 9. Malik RA, Kallinikos P, Abbott CA, van Schie CH, Morgan P, Efron N, et al. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. 2003;46(5):683–8. Epub 2003/05/10. 10.1007/s00125-003-1086-8 . [DOI] [PubMed] [Google Scholar]
- 10. Hossain P, Sachdev A, Malik RA. Early detection of diabetic peripheral neuropathy with corneal confocal microscopy. Lancet. 2005;366(9494):1340–3. Epub 2005/10/18. doi: S0140-6736(05)67546-0 [pii] 10.1016/S0140-6736(05)67546-0 . [DOI] [PubMed] [Google Scholar]
- 11. Ruggeri A, Scarpa F, Grisan E. Analysis of corneal images for the recognition of nerve structures. Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. 2006;1:4739–42. Epub 2007/10/20. 10.1109/iembs.2006.259805 . [DOI] [PubMed] [Google Scholar]
- 12. Quattrini C, Tavakoli M, Jeziorska M, Kallinikos P, Tesfaye S, Finnigan J, et al. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes. 2007;56(8):2148–54. Epub 2007/05/22. doi: db07-0285 [pii] 10.2337/db07-0285 . [DOI] [PubMed] [Google Scholar]
- 13. Tavakoli M, Quattrini C, Abbott C, Kallinikos P, Marshall A, Finnigan J, et al. Corneal confocal microscopy: a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care. 2010;33(8):1792–7. Epub 2010/05/04. doi: dc10-0253 [pii] 10.2337/dc10-0253 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen X, Graham J, Dabbah MA, Petropoulos IN, Ponirakis G, Asghar O, et al. Small nerve fiber quantification in the diagnosis of diabetic sensorimotor polyneuropathy: comparing corneal confocal microscopy with intraepidermal nerve fiber density. Diabetes Care. 2015;38(6):1138–44. Epub 2015/03/22. 10.2337/dc14-2422 ; PubMed Central PMCID: PMCPmc4439535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dehghani C, Pritchard N, Edwards K, Vagenas D, Russell AW, Malik RA, et al. Natural history of corneal nerve morphology in mild neuropathy associated with type 1 diabetes: development of a potential measure of diabetic peripheral neuropathy. Investigative ophthalmology & visual science. 2014;55(12):7982–90. Epub 2014/11/20. 10.1167/iovs.14-15605 . [DOI] [PubMed] [Google Scholar]
- 16. Dehghani C, Pritchard N, Edwards K, Vagenas D, Russell AW, Malik RA, et al. Morphometric stability of the corneal subbasal nerve plexus in healthy individuals: a 3-year longitudinal study using corneal confocal microscopy. Investigative ophthalmology & visual science. 2014;55(5):3195–9. Epub 2014/04/26. 10.1167/iovs.14-13959 . [DOI] [PubMed] [Google Scholar]
- 17. Tavakoli M, Petropoulos IN, Malik RA. Assessing corneal nerve structure and function in diabetic neuropathy. Clinical & experimental optometry. 2012;95(3):338–47. Epub 2012/05/19. 10.1111/j.1444-0938.2012.00743.x . [DOI] [PubMed] [Google Scholar]
- 18. Edwards K, Pritchard N, Vagenas D, Russell A, Malik RA, Efron N. Utility of corneal confocal microscopy for assessing mild diabetic neuropathy: baseline findings of the LANDMark study. Clinical & experimental optometry. 2012;95(3):348–54. Epub 2012/05/01. 10.1111/j.1444-0938.2012.00740.x . [DOI] [PubMed] [Google Scholar]
- 19. Tavakoli M, Hossain P, Malik RA. Clinical applications of corneal confocal microscopy. Clinical ophthalmology (Auckland, NZ). 2008;2(2):435–45. Epub 2008/06/01. ; PubMed Central PMCID: PMCPmc2693976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jiang MS, Yuan Y, Gu ZX, Zhuang SL. Corneal confocal microscopy for assessment of diabetic peripheral neuropathy: a meta-analysis. The British journal of ophthalmology. 2015. Epub 2015/02/14. 10.1136/bjophthalmol-2014-306038 . [DOI] [PubMed] [Google Scholar]
- 21. Dabbah MA, Graham J, Petropoulos IN, Tavakoli M, Malik RA. Automatic analysis of diabetic peripheral neuropathy using multi-scale quantitative morphology of nerve fibres in corneal confocal microscopy imaging. Med Image Anal. 2011;15(5):738–47. Epub 2011/07/02. doi: S1361-8415(11)00080-6 [pii] 10.1016/j.media.2011.05.016 . [DOI] [PubMed] [Google Scholar]
- 22. Hertz P, Bril V, Orszag A, Ahmed A, Ng E, Nwe P, et al. Reproducibility of in vivo corneal confocal microscopy as a novel screening test for early diabetic sensorimotor polyneuropathy. Diabet Med. 2011;28(10):1253–60. Epub 2011/03/26. 10.1111/j.1464-5491.2011.03299.x . [DOI] [PubMed] [Google Scholar]
- 23. Ahmed A, Bril V, Orszag A, Paulson J, Yeung E, Ngo M, et al. Detection of diabetic sensorimotor polyneuropathy by corneal confocal microscopy in type 1 diabetes: a concurrent validity study. Diabetes Care. 2012;35(4):821–8. Epub 2012/02/11. 10.2337/dc11-1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pritchard N, Edwards K, Russell AW, Perkins BA, Malik RA, Efron N. Corneal confocal microscopy predicts 4-year incident peripheral neuropathy in type 1 diabetes. Diabetes Care. 2015;38(4):671–5. Epub 2015/01/13. 10.2337/dc14-2114 . [DOI] [PubMed] [Google Scholar]
- 25. Oh SJ. Normal values for common nerve conduction tests Clinical Electromyography: Nerve Conduction Studies. 3rd ed. Baltimore: Lippincott Williams & Wilkins; 2002. p. 86–106. [Google Scholar]
- 26. Perkins BA, Ngo M, Bril V. Symmetry of nerve conduction studies in different stages of diabetic polyneuropathy. Muscle & nerve. 2002;25(2):212–7. Epub 2002/03/01. . [DOI] [PubMed] [Google Scholar]
- 27. Tavakoli M, Malik RA. Corneal confocal microscopy: a novel non-invasive technique to quantify small fibre pathology in peripheral neuropathies. J Vis Exp. 2011;47(47):2194–3001. Epub 2011/01/21. doi: 2194 [pii] 10.3791/2194 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dabbah MA, Graham J, Petropoulos I, Tavakoli M, Malik RA. Dual-model automatic detection of nerve-fibres in corneal confocal microscopy images. Med Image Comput Comput Assist Interv. 2010;13(Pt 1):300–7. Epub 2010/10/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Petropoulos IN, Alam U, Fadavi H, Marshall A, Asghar O, Dabbah MA, et al. Rapid automated diagnosis of diabetic peripheral neuropathy with in vivo corneal confocal microscopy. Investigative ophthalmology & visual science. 2014;55(4):2071–8. Epub 2014/02/27. 10.1167/iovs.13-13787 ; PubMed Central PMCID: PMCPmc3979234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–8. Epub 1979/03/01. . [DOI] [PubMed] [Google Scholar]
- 31. Oremus M, Oremus C, Hall GB, McKinnon MC. Inter-rater and test-retest reliability of quality assessments by novice student raters using the Jadad and Newcastle-Ottawa Scales. BMJ open. 2012;2(4). Epub 2012/08/03. 10.1136/bmjopen-2012-001368 ; PubMed Central PMCID: PMCPmc4400798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McGraw KO, Wong SP. Forming Inferences About Some Intraclass Correlation Coefficients. Psychological Methods. 1(1):30–46. 10.1037//1082-989X.1.1.30 [DOI] [Google Scholar]
- 33. Wu T, Ahmed A, Bril V, Orszag A, Ng E, Nwe P, et al. Variables associated with corneal confocal microscopy parameters in healthy volunteers: implications for diabetic neuropathy screening. Diabet Med. 2012;29(9):e297–303. Epub 2012/04/24. 10.1111/j.1464-5491.2012.03678.x . [DOI] [PubMed] [Google Scholar]
- 34. Tavakoli M, Ferdousi M, Petropoulos IN, Morris J, Pritchard N, Zhivov A, et al. Normative values for corneal nerve morphology assessed using corneal confocal microscopy: a multinational normative data set. Diabetes Care. 2015;38(5):838–43. Epub 2015/01/31. 10.2337/dc14-2311 ; PubMed Central PMCID: PMCPmc4407754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10. Epub 1986/02/08. . [PubMed] [Google Scholar]
- 36. Efron N, Edwards K, Roper N, Pritchard N, Sampson GP, Shahidi AM, et al. Repeatability of measuring corneal subbasal nerve fiber length in individuals with type 2 diabetes. Eye Contact Lens. 2010;36(5):245–8. Epub 2010/08/21. 10.1097/ICL.0b013e3181eea915 . [DOI] [PubMed] [Google Scholar]
- 37. Kim G, Singleton JR, Mifflin MD, Digre KB, Porzio MT, Smith AG. Assessing the Reproducibility of Quantitative In Vivo Confocal Microscopy of Corneal Nerves in Different Corneal Locations. Cornea. 2013;32(10):1331–8. Epub 2013/08/27. 10.1097/ICO.0b013e31829dd7f8 . [DOI] [PubMed] [Google Scholar]
- 38. Petropoulos IN, Manzoor T, Morgan P, Fadavi H, Asghar O, Alam U, et al. Repeatability of in vivo corneal confocal microscopy to quantify corneal nerve morphology. Cornea. 2013;32(5):e83–9. Epub 2012/11/23. 10.1097/ICO.0b013e3182749419 . [DOI] [PubMed] [Google Scholar]
- 39. Petropoulos IN, Ferdousi M, Marshall A, Alam U, Ponirakis G, Azmi S, et al. The Inferior Whorl For Detecting Diabetic Peripheral Neuropathy Using Corneal Confocal Microscopy. Investigative ophthalmology & visual science. 2015;56(4):2498–504. Epub 2015/03/19. 10.1167/iovs.14-15919 ; PubMed Central PMCID: PMCPmc4408884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. England JD, Gronseth GS, Franklin G, Carter GT, Kinsella LJ, Cohen JA, et al. Practice Parameter: evaluation of distal symmetric polyneuropathy: role of laboratory and genetic testing (an evidence-based review). Report of the American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, and American Academy of Physical Medicine and Rehabilitation. Neurology. 2009;72(2):185–92. Epub 2008/12/06. 10.1212/01.wnl.0000336370.51010.a1 . [DOI] [PubMed] [Google Scholar]
- 41. Hume DA, Lovblom LE, Ahmed A, Yeung E, Orszag A, Shin TM, et al. Higher magnification lenses versus conventional lenses for evaluation of diabetic neuropathy by corneal in vivo confocal microscopy. Diabetes research and clinical practice. 2012;97(2):e37–40. Epub 2012/05/15. 10.1016/j.diabres.2012.04.010 . [DOI] [PubMed] [Google Scholar]
- 42. Sivaskandarajah GA, Halpern EM, Lovblom LE, Weisman A, Orlov S, Bril V, et al. Structure-function relationship between corneal nerves and conventional small-fiber tests in type 1 diabetes. Diabetes Care. 2013;36(9):2748–55. Epub 2013/04/13. 10.2337/dc12-2075 ; PubMed Central PMCID: PMCPmc3747893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Petropoulos IN, Green P, Chan AW, Alam U, Fadavi H, Marshall A, et al. Corneal confocal microscopy detects neuropathy in patients with type 1 diabetes without retinopathy or microalbuminuria. PloS one. 2015;10(4):e0123517 Epub 2015/04/09. 10.1371/journal.pone.0123517 ; PubMed Central PMCID: PMCPmc4390357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dehghani C, Pritchard N, Edwards K, Russell AW, Malik RA, Efron N. Fully automated, semiautomated, and manual morphometric analysis of corneal subbasal nerve plexus in individuals with and without diabetes. Cornea. 2014;33(7):696–702. Epub 2014/06/03. 10.1097/ico.0000000000000152 . [DOI] [PubMed] [Google Scholar]
- 45. Vagenas D, Pritchard N, Edwards K, Shahidi AM, Sampson GP, Russell AW, et al. Optimal image sample size for corneal nerve morphometry. Optometry and vision science: official publication of the American Academy of Optometry. 2012;89(5):812–7. Epub 2012/03/13. 10.1097/OPX.0b013e31824ee8c9 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file includes all relevant data to support the results of the reproducibility analysis.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.