Abstract
Objective
We aimed to review gastric dysmotility in critically ill children: (a) its pathophysiology, with a focus on critical care diseases and therapies that affect gastric motility, (b) diagnostic methodologies, and (c) current and future potential therapies.
Data Sources
Eligible studies were identified from PubMed and MEDLINE.
Study Selection
Literature search included the following key terms, “gastric emptying”, “gastric motility/ dysmotility”, “gastrointestinal motility/ dysmotility”, “nutrition intolerance”, and “gastric residual volume”. Studies since 1995 were identified and reviewed for inclusion by the authors.
Data Extraction & Data Synthesis
We present a review of the current literature related to the physiology, pathophysiology, diagnostic methodologies and available therapies for gastric dysmotility in the critically ill child with a focus on gastric emptying (GE). Delayed GE, a common presentation of gastric dysmotility, is present in up to 50% of critically ill children. It is associated with the potential for aspiration, ventilator- associated pneumonia, inadequate delivery of enteral nutrition and may affect the efficacy of enteral medications, all of which may be result in poor patient outcomes. Gastric motility is affected by critical illness and its associated therapies. Currently available diagnostic tools to identify GE at the bedside have not been systematically studied and applied in this cohort. Gastric residual volume measurement, used as an indirect marker of delayed GE in pediatric intensive care units around the world, may be inaccurate.
Conclusion
Gastric dysmotility is common in critically ill children, and impacts patient safety and outcomes. However, it is poorly understood, inadequately defined, and current therapies are limited and based on scant evidence. Understanding gastric motility, and developing accurate bedside measures and novel therapies for GE are highly desirable and need to be further investigated.
Keywords: dysmotility, gastric emptying, children, critical care, pediatric intensive care unit, nutrition
Introduction
Gastric dysmotility is common in critically ill children. It is defined as the functional impairment of the stomach's capacity to move content forward by abnormally slow and/or uncoordinated activity of the gastric and antroduodenal musculature. The most common manifestation of gastric dysmotility in this cohort is delayed gastric emptying (GE), which is defined as prolongation in the time required to empty the stomach's contents. Gastric dysmotility may be a consequence of critical illness and the therapies provided in this setting. In critically ill children the prevalence of delayed GE has been estimated to be 50%.(1) However, current methods of identifying delayed GE in this population are inaccurate, therefore the true rate of delayed GE in the pediatric intensive care unit (PICU) is unknown. Enteral nutrition (EN) intolerance is a common manifestation of delayed GE and is diagnosed by a variety of nonstandard clinical assessments that are not evidence-based. EN intolerance is the most common barrier to delivering optimal EN, and it is reported to be present in 43% to 57% of critically ill children.(2, 3) In addition, delayed GE has other clinical implications in the PICU, including increased risk for gastroesophageal reflux, potential for aspiration of gastric contents, and reduced efficacy of enteral medications.
A basic understanding of the physiology of gastric motility and its alteration during critical illness is essential for optimal bedside care in the PICU. In this review, we have presented the pathophysiology, diagnosis and management of gastric dysmotility with a focus on GE in critically ill children. Studies published in the English language from 1995 that included the following key terms, “gastric emptying”, “gastric motility/ dysmotility”, “gastrointestinal motility/ dysmotility”, “nutrition intolerance”, and “gastric residual volume”, were identified using PubMed and Medline, and reviewed for inclusion by the authors.
Clinical Relevance
Gastric dysmotility has important implications for the critically ill child. Gastric dysmotility may be associated with gastroesophageal reflux(4), which might increase the risk for aspiration of gastric contents and subsequent ventilator-associated pneumonia (VAP) in mechanically ventilated patients.(5) In a prospectively studied cohort of critically ill neonates and children, EN was associated with an increased risk for developing VAP.(6) Concerns for delayed GE lead to deferred initiation and interruptions of EN resulting in suboptimal nutrient delivery. In a prospective study of critically ill mechanically ventilated children, only a third of this cohort received 66.6% of prescribed daily EN by day 7 of PICU admission.(7) Inadequate delivery of EN has been associated with poor clinical outcomes such as worsening of underlying malnutrition, longer mechanical ventilation days, longer PICU stay, multi-organ dysfunction and increased mortality.(8-10) Lack of EN may also be associated with bacterial overgrowth, altered gut integrity, and, hence, risk for bacterial translocation and systemic infection.(11, 12) A sound approach to detecting and managing gastric dysmotility could facilitate safe EN delivery in critically ill children, particularly via the physiologic and preferred gastric route.
Normal Physiology of Gastric Motility
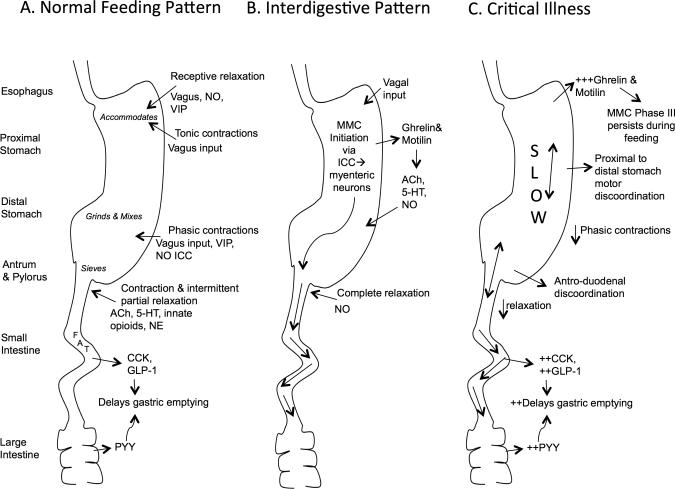
Gastrointestinal (GI) motility is a highly coordinated activity. (Figure 1A) The stomach plays a critical role in GI physiology acting as both a reservoir for food and regulating forward flow. Upon receipt of food the stomach relaxes proximally, known as receptive relaxation, to accommodate the food bolus. Tonic contractions transfer proximal stomach contents into the distal stomach. Phasic contractions in the distal stomach result in retropulsion of contents, promoting mixing and grinding. Passage of contents into the small intestine is regulated by the stomach's tonic and phasic contractions and the pylorus, which relaxes and contracts under the control of parasympathetic mediators. Small intestinal content is moved forward by periodic muscular contractions known as slow waves and spikes.
Figure 1.
Schematic of Gastrointestinal Motility during Normal Feeding Pattern (A), Interdigestive Pattern (B) and Critical Illness (C). This figure depicts the physiology of gastrointestinal motility with a focus on the stomach. It summarizes the intrinsic responses of the enteric nervous system, smooth muscle and interstitial cells of Cajal and extrinsic responses of the autonomic and somatic nervous system, and hormonal and immune systems during gastrointestinal motility. NO- nitric oxide; VIP- vasoactive intestinal polypeptide; ICC- Interstitial Cells of Cajal; Ach- Acetylcholine; 5-HT- serotonin; NE- norepinephrine; CCK- cholecystokinin; GLP-1- glucagon-like peptide 1; PYY- peptide-YY; MMC- migrating motor complex;
The interdigestive, or fasting, pattern of GI motility is dominated by the three phases of the migratory motor complex (MMC) initiated in the stomach. (Figure 1B) (13-15) Phase I lasts 45-60 minutes and exhibits little activity; Phase II consists of irregular contractile activity lasting 30-45 minutes; and Phase III exhibits regular propulsive activity lasting 5-15 minutes, sweeping remaining food and indigestible products through the GI tract. The MMC is regulated by the enteric nervous system, motilin, ghrelin, interstitial cells of Cajal and vagal input. A meal disrupts the MMC, replacing it with the distinct feeding pattern previously described. (Figure 1A)
Motility in the GI tract is controlled by a variety of mechanisms.(13-15) (Figure 1) The somatic, autonomic and enteric nervous systems provide neurologic control. The primary excitatory neurotransmitters are acetylcholine (ACh) and Substance P, promoting contraction. The primary inhibitory neurotransmitters are nitric oxide (NO), vasoactive intestinal peptide (VIP) and adenosine triphosphate (ATP), promoting relaxation.(15) The GI tract is also highly regulated by hormones released from the gut that serve as a feedback mechanism detailed in Figure 1 and Table 1. These hormones are released from different segments of the GI tract and have their primary effect on GE.(16-19)
Table 1.
Hormonal Responses during Normal Gastric Motility and during Dysmotility in the Critically Ill Patient
| Hormone | Source | Site of Action & Effect | In Critical Illness |
|---|---|---|---|
| Motilin | Stomach during interdigestive phase | Stomach- stimulated emptying Stomach & Small intestine-promotes Phase III of the MMC | -Elevated levels during nutrition 1 -Small intestine stimulation1 |
| Ghrelin | Stomach during interdigestive phase | Stomach- stimulated emptying, increases resting tone Stomach & Small intestine-promotes Phase III of the MMC | - Elevated levels in general and in feed intolerant patients 2,3 |
| Cholecystokinin (CCK) | Small intestine during feeding | Stomach-relaxation, delays emptying Antrum- inhibits motor activity Pylorus- increases contraction Small intestine- increases transit time |
-Elevated levels during fasting and nutrition4 -Inverse relationship with gastric emptying 4 |
| Glucagon-like peptide-1 (GLP-1) | Distal small intestine and large intestine during feeding | Stomach- delays emptying | -Elevated levels during fasting and postprandial and in feed intolerant patients5 -May slow gastric emptying if originally normal6 |
| Peptide-YY (PYY) | Entire intestine, predominantly colon and rectum during feeding |
Stomach- delays emptying Small intestine- increases transit time |
-Elevated levels during fasting and nutrition4, 7 -Inverse relationship with gastric emptying4, 7 |
| Amylin | Pancreas | Stomach- delays emptying | - Elevated levels in feed-intolerant pediatric patients and preterm neonates 8, 9 |
Nguyen, N.Q., et al., Abnormalities in plasma motilin response to small intestinal nutrient stimulation in critically ill patients. Gastroenterology, 2010. 138: p. S405
Koch, A., et al., Regulation and prognostic relevance of serum ghrelin concentrations in critical illness and sepsis. Crit Care, 2010. 14(3): p. R94.
Crona, D. and R. MacLaren, Gastrointestinal hormone concentrations associated with gastric feeding in critically ill patients. JPEN J Parenter Enteral Nutr, 2012. 36(2): p. 189-96.
Nguyen, N.Q., et al., The relationship between gastric emptying, plasma cholecystokinin, and peptide YY in critically ill patients. Crit Care, 2007. 11(6): p. R132.
Summers, M.J., et al., Endogenous amylin and glucagon-like peptide-1 concentrations are not associated with gastric emptying in critical illness. Acta Anaesthesiol Scand, 2014. 58(2): p. 235-42.
Deane, A.M., et al., Effects of exogenous glucagon-like peptide-1 on gastric emptying and glucose absorption in the critically ill: relationship to glycemia. Crit Care Med, 2010. 38(5): p. 1261-9.
Nguyen, N.Q., et al., Fasting and nutrient-stimulated plasma peptide-YY levels are elevated in critical illness and associated with feed intolerance: an observational, controlled study. Crit Care, 2006. 10(6): p. R175
Mayer, A.P., et al., Amylin is associated with delayed gastric emptying in critically ill children. Intensive Care Med, 2002. 28(3): p. 336-40.
Kairamkonda, V.R., et al., Amylin peptide is increased in preterm neonates with feed intolerance. Arch Dis Child Fetal Neonatal Ed, 2008. 93(4): p. F265-70.
Pathophysiology of Gastric Dysmotility
In critical illness, GI motility is affected negatively. (Figure 1C) The proximal and distal stomach activity is uncoordinated and the MMC activity persists during feeding.(20) Reduced antral motility and pyloric relaxation results in retention of stomach contents.(21) The MMC is initiated in the small intestine instead of the stomach and decreased Phase II activity and retrograde Phase III activity result in slowed small intestine transit.(22) In addition, changes in hormonal responses account for alterations in GI motility. Table 1 describes these changes.
Effect of Drugs on Gastric Motility
A majority of PICUs utilize a combination of an opioid and a benzodiazepine as part of their sedation strategy.(23) Opiates affect GI motility by altering neurotransmitter release and blocking excitatory neurons that innervate smooth muscle.(24) Morphine has been found to alter the MMC cycle, promote a sustained interdigestive motility pattern in the stomach and delay GE in a dose-dependent manner.(25, 26) Morphine and midazolam in the adult ICU has been shown to delay GE when compared to propofol.(27) In mechanically ventilated children, use of opioids was associated with significantly decreased gastric EN delivery, a possible clinical manifestation of delayed GE.(28) Benzodiazepines in comparison to chloral hydrate did not change motility patterns in children undergoing esophageal manometry in an outpatient setting.(29) Propofol at low doses in healthy volunteers does not affect GE. In critically ill patients, proposal delayed GE and gastric meal retention less than morphine and midazolam (35,38). The alpha-adrenoreceptor agonists, dexmedetomidine and clonidine, have been found to delay GE and decrease antral contractions, respectively, in healthy volunteers.(31, 32) Their effect in PICU patients has not been studied.
Paralytics or vasoactive agents are often seen as barriers to EN due to their perceived effects on GI motility. However, there is limited and equivocal evidence to support the negative effects of these drugs on GI motility. In mechanically ventilated adults paralytic agents were not associated with delayed GE.(33) In mechanically ventilated children paralytic agents were associated with lower gastric EN delivery and higher gastric residual volumes (GRV) when compared to children not receiving paralytic agents; GE was not directly measured.(28) In mechanically ventilated adults, dopamine has been associated with delayed GE and prolonged small bowel transit time by inhibiting Phase III activity and decreasing antral contractions, whereas norepinephrine and/or epinephrine has not been associated with delayed GE.(22, 27, 34) A retrospective analysis of EN tolerance in critically ill adults while receiving vasoactive agents, reported gastric EN tolerance in 75% of subjects.(35) In a single center study, a majority of mechanically ventilated children on vasoactive medications were reported to tolerate EN without adverse events. The prevalence of perceived intolerance to EN in this study was 29%.(36) The effect of drugs such as opiates, sedatives, paralytics and vasoactive agents, on GE in the PICU needs to be further examined.
Effect of Mechanical Ventilation on Gastric Motility
Manometric studies in critically ill patients requiring mechanical ventilator support have demonstrated decreased gastric motility and diminished activity in the duodenum where MMC activity was initiated.(22, 25) The mechanism by which positive pressure ventilation affects gastric motility is poorly understood and likely multifactorial. Reduced gastric blood flow in healthy volunteers and neonates on continuous positive pressure has been reported.(37, 38) Activation of the renin-angiotensin axis and inflammatory cascade by positive pressure ventilation may also lead to decreased splanchnic circulation and affect gut motility.(39-41) Decreased splanchnic circulation may result in poor intestinal perfusion, which has been associated with mucosal injury, impaired mucosal barriers and altered motility. However, studies in mechanically ventilated adults with acute respiratory distress syndrome did not report an association between positive pressure ventilation and changes in splanchnic flow.(42-44) In addition, clinical studies in mechanically ventilated children have reported successful EN delivery without adverse effects.(10, 28) The use of promotility agents to address perceived delayed GE has been reported in 8.5% and 19.9% of mechanically ventilated children in recent studies.(7, 28) Studies directly measuring GE in mechanically ventilated children are lacking. The effect of mechanical ventilation on gastric dysmotility should be further explored, particularly after accounting for other adjunct therapies that may independently affect motility.
Effect of Inflammation and Disease on Gastric Motility
Critical illness associated with hemodynamic instability, multiple organ failure, multi-trauma, or shock has been implicated as a high-risk factor for GI dysmotility.(12, 45, 46) In mechanically ventilated critically ill adults, delayed GE was associated with diagnosis at admission and occurred more frequently in patients with head injuries, burns, multi-system trauma and sepsis.(47) In patients with head injury delayed GE has been reported in up to 80% of the cohort.(48) A biphasic response with early rapid GE followed by late prolonged emptying, similar to the effects of vagotomy or pyloroplasty, has been described in this cohort.(49) In addition, raised intracranial pressure has been associated with inhibition of Phase III activity, and decreased antroduodenal contractions.(22, 49) In children with head injuries, gastric EN delivery was found to be low when compared to children with other PICU diagnoses.(28) This study also identified poor gastric EN delivery to be associated with severity of disease, including multiple organ failure, irreversible septic shock, and poor cardiac function at admission (36). In animal studies, GI motility in the setting of stress has been shown to be associated with corticotropin releasing factor(50), upregulation in inflammatory signaling proteins(51-54), and an increase in leukocyte infiltration (55). Understanding GE in the context of specific diseases would identify specific at risk cohorts allowing for targeted management strategies.
Effect of Glycemic Control and Nutrition on Gastric Motility
Although the relationship between GE and blood glucose in the critically ill has been debated, hyperglycemia has been associated with delayed GE in critically ill adults.(56, 57) Fluid overload, and potassium and magnesium derangements have been associated with slowing of GE in post-operative patients.(58-60) ICU specific studies assessing the role of fluid overload and other electrolyte abnormalities on GE have not been published. Deferred EN initiation has been associated with delayed GE.(46) Total parenteral nutrition (PN) in volunteers was associated with altered gut motility and morphology, and post-pyloric EN was suggested as an important strategy for restoring and possibly preventing intestinal morphologic changes.(61) In neonates, gastric trophic feeding combined with PN was associated with improved whole gut motility as compared to PN alone.(62) Continuous versus bolus EN has not been associated with changes in GE in healthy volunteers, but in preterm infants continuous gastric EN was associated with lower GRV.(63, 64) The stomach is the physiologic route for nutrition, but post-pyloric nutrition is considered in some critically ill patients. The results of randomized controlled studies evaluating the effect of post-pyloric versus gastric route on GRV and emesis in adults have been equivocal.(65, 66) In critically ill children, post-pyloric nutrition was associated with lower GRV but no difference in other markers of EN intolerance, when compared to gastric nutrition.(67) Post-pyloric nutrition, however, was associated with delayed GE and increased antro-duodenal tone when measured directly in critically ill adults receiving same rate of nutrition as healthy volunteers.(21)
Diagnosing Delayed Gastric Emptying
Gastric emptying is evaluated by a variety of clinical assessments and techniques, most of which have not been validated nor are easily applied in the PICU population.
Physical exam findings are commonly used as indirect measures for GI dysmotility, but may be inaccurate. The correlation between bowel sounds and GE in the ICU is poor, and bowel sounds have been detected in patients with delayed GE.(68) Surveys have shown that most bedside PICU providers use GRV measurements and feeding algorithms include GRV measurement as a measure of EN intolerance to guide bedside nutrition practices.(7, 69) The accuracy of GRV measurement to predict delayed GE or EN intolerance has not been studied in critically ill children. Furthermore, GRV measurement is complicated by a lack of standardization in the GRV threshold to define EN intolerance and in measurement techniques that are affected by patient posture and the feeding tube properties.(7, 70, 71) GRV measurements are inaccurate surrogates of GE in critically ill adults, and a higher GRV was not correlated with the frequency of VAP in a recent adult study.(68, 72, 73) Studies aimed at developing accurate bedside measures of delayed GE in critically ill children are necessary.
Scintigraphy (SCT) is the gold standard for the measurement of GE. However, SCT is impractical in the ICU and therefore other techniques have been used in adult research studies. Table 2 describes in further details SCT and the following diagnostic methods. Cutaneous electrogastrography (EGG), manometry and the wireless motility capsule measure contractility. EGG records myoelectrical activity from the abdominal surface, whereas manometry and the wireless capsule measure contractility by direct intraluminal measurement of pressure changes in the GI tract.(74) The paracetamol absorption test and the 13C-octanoic acid test measure GE indirectly by measuring the rate at which metabolites of each of these agents are detected in the blood and exhaled breaths, respectively. Ultrasound measurements of the stomach size and antrum cross-sectional area have been used to assess GE.(75, 76) Stereotypic changes in GI hormone, as described in Table 1, during delayed GE may serve as biomarkers of GE. Further research is needed on the efficacy of these diagnostic methodologies in critically ill children and on novel strategies for bedside measurement of GE.
Table 2.
Methods to Measure Gastric Emptying in the Critically Ill Patient
| DIAGNOSTIC METHOD | APPLICABILITY IN THE PICU | |
|---|---|---|
| Scintigraphy (SCT) | -GOLD-STANDARD -Measures by gamma ray the rate at which a radioisotope-labeled meal is emptied from the stomach |
- Requires transport of a patient out of the unit or a portable gamma ray |
| DIRECT MEASUREMENTS | ||
| Cutaneous electrogastrogaphy (EGG) | -Electrodes on the abdominal surface record gastric myelectrical activity -Lacks pediatric standards1 -Does not correlate with SCT in healthy children, not tested in ICU populations2 |
-Non-invasive -Abdominal wall edema may limit the study |
| Manometry | -Measures pressure changes directly in any portion of the GI tract -Pressure changes reflect GI contractions and motility and can report directionality of motility -Applied in adult ICU studies successfully |
-Invasive -Requires expertise for catheter placement -Prolonged study, usually 24h |
| Wireless motility capsule | -Measures pressure changes directly in any portion of the GI tract -Pressure changes reflect GI contractions and motility and can report directionality of motility -Applied in adult ICU studies successfully3, 4 |
-Invasive -Potential limitation in pill deployment in small NG tubes |
| INDIRECT MEASUREMENTS | ||
| Paracetamol Absorption Test (PAT) | -Paracetamol is not absorned in the stomach -Rate of rise and peak of paracetamol concentration in the blood reflects gastric emptying of the paracetamol and small intestinal absorption -Correlates well with SCT in adult ICUs5,6 -Applied in critically ill pediatric population7 |
-Non-invasive -Cannot be used in patients with liver dysfunction -May be confounded by routine use of paracetamol in the ICU |
| 13C-octanoic acid | -13C-labeled CO2 meal in absorbed and metabolized in the liver and 13C-labeled CO2 is exhaled -Rate of 13C-labeled CO2 detected in an exhaled breath reflects gastric emptying -Correlates well with SCT in adult ICUs8, 9 -Applied in neonates10 |
-Non-invasive -Cannot be used in patients with liver dysfunction -Difficult to trap exhaled breath in non-sedated or non-intubated pediatric patients |
| Ultrasound | -Measures stomach size or the cross-sectional area of the antrum over time after a meal -GE is reflected by the time for reduction in the stomach size and in the cross-sectional area -Studied in critically ill adults 11 and neonates12 |
-Non-invasive -Requires expertise -US windows may be limited by abdominal wall edema or bowel edema |
Levy J, Harris J, Chen J, Sapoznikov D, Riley B, De La Nuez W, et al. Electrogastrographic norms in children: toward the development of standard methods, reproducible results, and reliable normative data. J Pediatr Gastroenterol Nutr. 2001 ;33:455-461.
Barbar M, Steffen R, Wyllie R, Goske M. Electrogastrography versus gastric emptying scintigraphy in children with symptoms suggestive of gastric motility disorders. J Pediatr Gastroenterol Nutr. 2000;30:193-197.
Rauch S, Krueger K, Turan A, You J, Roewer N, Sessler DI. Use of wireless motility capsule to determine gastric emptying and small intestinal transit times in critically ill trauma patients. J Crit Care. 2012;27:534 e7-12.
Rauch S, Krueger K, Turan A, Roewer N, & Sessler DI. Clinical experience in the placement of a novel motility capsule by using a capsule delivery device in critical care patients. Endoscopy 2014; Epub
Willems M, Quartero AO, Numans ME. How useful is paracetamol absorption as a marker of gastric emptying? A systematic literature study. Dig Dis Sci. 2001;46:2256-2262.
Tarling MM, Toner CC, Withington PS, Baxter MK, Whelpton R, Goldhill DR. A model of gastric emptying using paracetamol absorption in intensive care patients. Intensive Care Med. 1997;23:256-260.
Mayer AP, Durward A, Turner C, Skellett S, Dalton N, Tibby SM, et al. Amylin is associated with delayed gastric emptying in critically ill children. Intensive Care Med. 2002;28:336-340.
Ritz MA, Fraser R, Edwards N, Di Matteo AC, Chapman M, Butler R, et al. Delayed gastric emptying in ventilated critically ill patients: measurement by 13 C-octanoic acid breath test. Crit Care Med. 2001;29:1744-1749.
Nguyen NQ, Bryant LK, Burgstad CM, Chapman M, Deane A, Bellon M, et al. Gastric emptying measurement of liquid nutrients using the (13)C-octanoate breath test in critically ill patients: a comparison with scintigraphy. Intensive Care Med. 2013;39:1238-1246.
Van Den Driessche M, Peeters K, Marien P, Ghoos Y, Devlieger H, Veereman-Wauters G. Gastric emptying in formula-fed and breast-fed infants measured with the 13C-octanoic acid breath test. J Pediatr Gastroenterol Nutr. 1999;29:46-51.
Hamada SR, Garcon P, Ronot M, Kerever S, Paugam-Burtz C, Mantz J. Ultrasound assessment of gastric volume in critically ill patients. Intensive care medicine. 2014;40:965-72.
Perrella SL, Hepworth AR, Simmer KN, Geddes DT. Validation of ultrasound methods to monitor gastric volume changes in preterm infants. J Pediatr Gastroenterol Nutr. 2013;57:741-9.
Management of Delayed Gastric Emptying
Treating delayed GE is important to promote effective delivery of EN and medications. Preventive measures may reduce the occurrence of dysmotility such as avoiding or limiting medications that impair motility. Therapies to address dysmotility may be considered, when preventive measures are insufficient.
Promotility agents, used in up to 19.9% of critically ill children, are often the first line of therapy for delayed GE in the PICU.(7) Table 3 describes the mechanism of action of the most commonly studied promotility agents in the ICU. In critically ill children the most common promotility agents in use are erythromycin and metoclopramide (7) but only erythromycin has been studied for its efficacy in preterm infants.(77) Novel potential promotility agents target the hormonal feedback axis of the GI tract. CCK receptor antagonists have been shown to stimulate intestinal propulsion.(45) Ghrelin, an experimental therapy, increases gastric resting tone.(78)
Table 3.
Promotility Agents for Delayed Gastric Emptying in Critically Ill Children
| Promotility Agent | Mechanism of Action | Effect | In Critical Illness/ Comments & Dosages where applicable* |
|---|---|---|---|
| Erythromycin | Motilin receptor agonist | Stimulate gastric phasic and antral contractions, & antroduodenal coordination | -Single agent therapy improves gastric motility, reduced gastric residual volume and improves EN tolerance1, 2 -Improved feeding intolerance in preterm infants i3 Children: -Lactobionate, initital 1-3mg/kg dose IV infused over 60 minutes followed by oral dosing 2.5-5 mg/lkg/dose PO, four times a day |
| Cisapride | Serotonin receptor agonists | Promote initiation of peristaltic reflex, stimulate enteric neurons → increase motility throughout the entire GI tract | -increased gastric emptying in ICU adult4 -increased risk for cardiotoxicity, no longer used Not FDA approved, no recommended dosing |
| Tegaserod, Renzapride | Serotonin receptor agonists | Same as above | -Primarily used in outpatient for irritable bowel syndrome with symptomatic relief 5 -Case reports of improved gastric emptying in ICU6 -Tegaserod safer drug profile, Renzapride unknown cardiotoxicity risk Not FDA approved, no recommended dosing |
| Cyproheptadine | Serotonin receptor agonists | Same as above | -No ICU evidence -Outpatient pediatric study showed improved dyspeptic symptoms7 Off-label use Children PO 0.25 mg/kg/day in 2-3 divided doses |
| Metoclopramide | Dopamine antagonist-centrally and peripherally | Stimulate gastric emptying and antral and small intestinal contractions; improve antroduodenal coordination | -Single agent therapy improves gastric emptying and reduces GRV1 -Not studied in pediatrics -Risk for tardive dyskinesia Off-label use Children PO 0.1mg/kg four times a day |
| Domperidone | Dopamine antagonist-peripherally | Same as above | -decreased risk of tardive dyskinesia because of mainly peripheral receptor action -Offered for compassionate use only 8 Not FDA approved, no recommended dosing |
These are recommended doses. Evidence for the use of promotility medications in critically ill children is scant, Please refer to your institutional formulary for dosing recommendations, and rely on clinical experience and institutional experience with the use of these agents. PO- per oral; FDA- Federal Drug Administration
MacLaren, R., et al., Erythromycin vs metoclopramide for facilitating gastric emptying and tolerance to intragastric nutrition in critically ill patients. JPEN J Parenter Enteral Nutr, 2008. 32(4): p. 412-9.
Nguyen, N.Q., et al., Erythromycin is more effective than metoclopramide in the treatment of feed intolerance in critical illness. Crit Care Med, 2007. 35(2): p. 483-9.
Ng, P.C., Use of oral erythromycin for the treatment of gastrointestinal dysmotility in preterm infants. Neonatology, 2009. 95(2): p. 97-104.
Heyland, D.K., et al., Cisapride improves gastric emptying in mechanically ventilated, critically ill patients. A randomized, double-blind trial. Am J Respir Crit Care Med, 1996. 154(6 Pt 1): p. 1678-83
Galligan, J.J. and S. Vanner, Basic and clinical pharmacology of new motility promoting agents. Neurogastroenterol Motil, 2005. 17(5): p. 643-53
Banh, H.L., et al., The use of tegaserod in critically ill patients with impaired gastric motility. Clin Pharmacol Ther, 2005. 77(6): p. 583-6.
Rodriguez, L., J. Diaz, and S. Nurko, Safety and efficacy of cyproheptadine for treating dyspeptic symptoms in children. J Pediatr, 2013. 163(1): p. 261-7.
Reddymasu, S.C., I. Soykan, and R.W. McCallum, Domperidone: review of pharmacology and clinical applications in gastroenterology. Am J Gastroenterol, 2007. 102(9): p. 2036-45.
Non-pharmaceutical alternatives have been considered. Abdominal massage was associated with increased gastric motility in preterm infants.(79) EN itself may reduce GI dysmotility due to the role of luminal nutrients on gut function.(12, 80) Strategies to deliver EN should be prioritized. Early EN provided within 48 hours of admission has been associated with improved tolerance and advancement of EN.(28, 81) Post-pyloric nutrition might have a role in critically ill children, where gastric EN is contraindicated. In a study of mechanically ventilated children, post-pyloric nutrition was associated with lower GRVs and improved EN delivery when compared to gastric nutrition. However, the frequency of aspiration and other markers of EN intolerance were similar between the two groups. (67) The benefits of post-pyloric nutrition have not been demonstrated in clinical trials, and post-pyloric tube placement requires expertise. In circumstances when EN cannot be advanced, trophic EN, small nonnutritive amounts of EN, has been shown to provide similar benefits to early EN.(82-84) The use of nutrition algorithms in PICUs has been associated with improved EN tolerance and delivery, although most recommendations included in protocols are based on minimal research evidence.(7, 85-87) Novel strategies based on an understanding of pathophysiology of GI motility and sound study design should be explored to manage gastric dysmotility.
Conclusion
Gastric dysmotility in critically ill children impacts safe and optimal EN and drug delivery. Gastric dysmotility is triggered and exacerbated by common illnesses and therapies in the PICU. Understanding gastric dysmotility and developing accurate bedside measures of GE in this cohort may improve EN and drug delivery and help prevent complications such as aspiration of gastric contents. Gastric dysmotility has been scarcely studied in critically ill children, yet it is a rich area for research that can lead to optimal nutrition practices and has the potential for improving patient safety and outcomes.
Acknowledgements
We would like to thank Kathleen Gura, PharmD. All authors met full authorship criteria.
Non-Standard Abbreviations
- EN
enteral nutrition
- GE
gastric emptying
- GI
gastrointestinal
- PICU
Pediatric Intensive Care Unit
Footnotes
Study Location- not applicable
Financial Support- None
References
- 1.Mayer AP, Durward A, Turner C, Skellett S, Dalton N, Tibby SM, et al. Amylin is associated with delayed gastric emptying in critically ill children. Intensive Care Med. 2002;28:336–340. doi: 10.1007/s00134-002-1224-7. [DOI] [PubMed] [Google Scholar]
- 2.Meyer R, Harrison S, Sargent S, Ramnarayan P, Habibi P, Labadarios D. The impact of enteral feeding protocols on nutritional support in critically ill children. J Hum Nutr Diet. 2009;22:428–436. doi: 10.1111/j.1365-277X.2009.00994.x. [DOI] [PubMed] [Google Scholar]
- 3.Mehta NM, McAleer D, Hamilton S, Naples E, Leavitt K, Mitchell P, et al. Challenges to optimal enteral nutrition in a multidisciplinary pediatric intensive care unit. JPEN J Parenter Enteral Nutr. 2010;34:38–45. doi: 10.1177/0148607109348065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waseem S, Islam S, Kahn G, Moshiree B, Talley NJ. Spectrum of gastroparesis in children. J Pediatr Gastroenterol Nutr. 2012;55:166–172. doi: 10.1097/MPG.0b013e31824cf06e. [DOI] [PubMed] [Google Scholar]
- 5.Inglis TJ, Sherratt MJ, Sproat LJ, Gibson JS, Hawkey PM. Gastroduodenal dysfunction and bacterial colonisation of the ventilated lung. Lancet. 1993;341:911–913. doi: 10.1016/0140-6736(93)91208-4. [DOI] [PubMed] [Google Scholar]
- 6.Srinivasan R, Asselin J, Gildengorin G, Wiener-Kronish J, Flori HR. A prospective study of ventilator-associated pneumonia in children. Pediatrics. 2009;123:1108–1115. doi: 10.1542/peds.2008-1211. [DOI] [PubMed] [Google Scholar]
- 7.Martinez EE, Bechard LJ, Mehta NM. Nutrition algorithms and bedside nutrient delivery practices in pediatric intensive care units: an international multicenter cohort study. Nutr Clin Pract. 2014;29:360–367. doi: 10.1177/0884533614530762. [DOI] [PubMed] [Google Scholar]
- 8.Hulst JM, van Goudoever JB, Zimmermann LJ, Hop WC, Albers MJ, Tibboel D, et al. The effect of cumulative energy and protein deficiency on anthropometric parameters in a pediatric ICU population. Clin Nutr. 2004;23:1381–1389. doi: 10.1016/j.clnu.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Briassoulis G, Zavras N, Hatzis T. Malnutrition, nutritional indices, and early enteral feeding in critically ill children. Nutrition. 2001;17:548–557. doi: 10.1016/s0899-9007(01)00578-0. [DOI] [PubMed] [Google Scholar]
- 10.Mehta NM, Bechard LJ, Cahill N, Wang M, Day A, Duggan CP, et al. Nutritional practices and their relationship to clinical outcomes in critically ill children--an international multicenter cohort study*. Critical Care Med. 2012;40:2204–2211. doi: 10.1097/CCM.0b013e31824e18a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ritz MA, Fraser R, Tam W, Dent J. Impacts and patterns of disturbed gastrointestinal function in critically ill patients. Am J Gastroenterol. 2000;95:3044–3052. doi: 10.1111/j.1572-0241.2000.03176.x. [DOI] [PubMed] [Google Scholar]
- 12.Windsor AC, Kanwar S, Li AG, Barnes E, Guthrie JA, Spark JI, et al. Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis. Gut. 1998;42:431–435. doi: 10.1136/gut.42.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barrett KE. Gastric Motility. In: Thomas MWCM, editor. Gastrointestinal Physiology. Second edition. McGraw Hill Education; United States of America: 2014. [Google Scholar]
- 14.Barrett KE. Intestinal Motility. In: Thomas MWCM, editor. Gastrointestinal Physiology. Second edition. McGraw Hill Education; United States of America: 2014. [Google Scholar]
- 15.Thapar NF,C, DiLorenzo C. Introduction to Gut Motility and Sensitivity. In: Faure CD,C, N Thapar, editors. Pediatric Neurogastroenterology: Gastrointestinal Motility and Functional Disorders in Children. Humana Press; New York: 2012. [Google Scholar]
- 16.Ohno T, Mochiki E, Kuwano H. The roles of motilin and ghrelin in gastrointestinal motility. Int J Pept. 2010 doi: 10.1155/2010/820794. Published online Feb 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beglinger C. Effect of cholecystokinin on gastric motility in humans. Ann N Y Acad Sci. 1994;713:219–225. doi: 10.1111/j.1749-6632.1994.tb44068.x. [DOI] [PubMed] [Google Scholar]
- 18.Lin HC, Chey WY, Zhao X. Release of distal gut peptide Y Y (PYY) by fat in proximal gut depends on CCK. Peptides. 2000;21:1561–1563. doi: 10.1016/s0196-9781(00)00312-0. [DOI] [PubMed] [Google Scholar]
- 19.Deane AM, Nguyen NQ, Stevens JE, Fraser RJ, Holloway RH, Besanko LK, et al. Endogenous glucagon-like peptide-1 slows gastric emptying in healthy subjects, attenuating postprandial glycemia. J Clin Endocrinol Metab. 2010;95:215–221. doi: 10.1210/jc.2009-1503. [DOI] [PubMed] [Google Scholar]
- 20.Chapman MJ, Nguyen NQ, Deane AM. Gastrointestinal dysmotility: clinical consequences and management of the critically ill patient. Gastroenterol Clin North Am. 2011;40:725–739. doi: 10.1016/j.gtc.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Chapman M, Fraser R, Vozzo R, Bryant L, Tam W, Nguyen N, et al. Antro-pyloro383 duodenal motor responses to gastric and duodenal nutrient in critically ill patients. Gut. 2005;54:1384–1390. doi: 10.1136/gut.2005.065672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dive A, Moulart M, Jonard P, Jamart J, Mahieu P. Gastroduodenal motility in mechanically ventilated critically ill patients: a manometric study. Crit Care Med. 1994;22:441–447. doi: 10.1097/00003246-199403000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Kudchadkar SR, Yaster M, Punjabi NM. Sedation, sleep promotion, and delirium screening practices in the care of mechanically ventilated children: a wake-up call for the pediatric critical care community*. Crit Care Med. 2014;42:1592–1600. doi: 10.1097/CCM.0000000000000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeHaven-Hudkins DL, DeHaven RN, Little PJ, Techner LM. The involvement of the mu392 opioid receptor in gastrointestinal pathophysiology: therapeutic opportunities for antagonism at this receptor. Pharmacol Ther. 2008;117:162–187. doi: 10.1016/j.pharmthera.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Bosscha K, Nieuwenhuijs VB, Vos A, Samsom M, Roelofs JM, Akkermans LM. Gastrointestinal motility and gastric tube feeding in mechanically ventilated patients. Crit Care Med. 1998;26:1510–1517. doi: 10.1097/00003246-199809000-00017. [DOI] [PubMed] [Google Scholar]
- 26.Heyland DK, Tougas G, King D, Cook DJ. Impaired gastric emptying in mechanically ventilated, critically ill patients. Intensive Care Med. 1996;22:1339–1344. doi: 10.1007/BF01709548. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen NQ, Chapman MJ, Fraser RJ, Bryant LK, Burgstad C, Ching K, et al. The effects of sedation on gastric emptying and intra-gastric meal distribution in critical illness. Intensive Care Med. 2008;34:454–460. doi: 10.1007/s00134-007-0942-2. [DOI] [PubMed] [Google Scholar]
- 28.Briassoulis GC, Zavras NJ, Hatzis MT. Effectiveness and safety of a protocol for promotion of early intragastric feeding in critically ill children. Ped Crit Care Med. 2001;2:113–121. doi: 10.1097/00130478-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Fung KP, Math MV, Ho CO, Yap KM. Midazolam as a sedative in esophageal manometry: a study of the effect on esophageal motility. J Pediatr Gastroenterol Nutr. 1992;15:85–88. doi: 10.1097/00005176-199207000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Hammas B, Hvarfner A, Thorn SE, Wattwil M. Propofol sedation and gastric emptying in volunteers. Acta Anaesthesiol Scand. 1998;42:102–105. doi: 10.1111/j.1399-6576.1998.tb05088.x. [DOI] [PubMed] [Google Scholar]
- 31.Iirola T, Vilo S, Aantaa R, Wendelin-Saarenhovi M, Neuvonen PJ, Scheinin M, et al. Dexmedetomidine inhibits gastric emptying and oro-caecal transit in healthy volunteers. Br J Anaesth. 2011;106:522–527. doi: 10.1093/bja/aer004. [DOI] [PubMed] [Google Scholar]
- 32.Gregersen H, Kraglund K, Rittig S, Tottrup A. The effect of a new selective alpha 2- adrenoreceptor antagonist, idazoxan, and the agonist, clonidine, on fasting antroduodenal motility in healthy volunteers. Aliment Pharmacol Ther. 1989;3:435–443. doi: 10.1111/j.1365-2036.1989.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 33.Tamion F, Hamelin K, Duflo A, Girault C, Richard JC, Bonmarchand G. Gastric emptying in mechanically ventilated critically ill patients: effect of neuromuscular blocking agent. Intensive Care Med. 2003;29:1717–1722. doi: 10.1007/s00134-003-1898-5. [DOI] [PubMed] [Google Scholar]
- 34.Dive A, Foret F, Jamart J, Bulpa P, Installe E. Effect of dopamine on gastrointestinal motility during critical illness. Intensive Care Med. 2000;26:901–907. doi: 10.1007/s001340051279. [DOI] [PubMed] [Google Scholar]
- 35.Mancl EE, Muzevich KM. Tolerability and safety of enteral nutrition in critically ill patients receiving intravenous vasopressor therapy. JPEN J Parenter Enteral Nutr. 2013;37:641–651. doi: 10.1177/0148607112470460. [DOI] [PubMed] [Google Scholar]
- 36.King W, Petrillo T, Pettignano R. Enteral nutrition and cardiovascular medications in the pediatric intensive care unit. JPEN J Parenter Enteral Nutr. 2004;28:334–338. doi: 10.1177/0148607104028005334. [DOI] [PubMed] [Google Scholar]
- 37.Fournell A, Schwarte LA, Kindgen-Milles D, Muller E, Scheeren TW. Assessment of microvascular oxygen saturation in gastric mucosa in volunteers breathing continuous positive airway pressure. Crit Care Med. 2003;31:1705–1710. doi: 10.1097/01.CCM.0000063281.47070.53. [DOI] [PubMed] [Google Scholar]
- 38.Havranek T, Madramootoo C, Carver JD. Nasal continuous positive airway pressure affects pre- and postprandial intestinal blood flow velocity in preterm infants. J Perinatol. 2007;27:704–708. doi: 10.1038/sj.jp.7211808. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka S, Sagawa S, Miki K, Claybaugh JR, Shiraki K. Changes in muscle sympathetic nerve activity and renal function during positive-pressure breathing in humans. Am J Physiol. 1994;266:R1220–1228. doi: 10.1152/ajpregu.1994.266.4.R1220. [DOI] [PubMed] [Google Scholar]
- 40.Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999;282:54–61. doi: 10.1001/jama.282.1.54. [DOI] [PubMed] [Google Scholar]
- 41.Mutlu GM, Mutlu EA, Factor P. GI complications in patients receiving mechanical ventilation. Chest. 2001;119:1222–1241. doi: 10.1378/chest.119.4.1222. [DOI] [PubMed] [Google Scholar]
- 42.Bruhn A, Hernandez G, Bugedo G, Castillo L. Effects of positive end-expiratory pressure on gastric mucosal perfusion in acute respiratory distress syndrome. Crit Care. 2004;8:R306–311. doi: 10.1186/cc2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarkar S, Bhattacharya P, Kumar I, Mandal KS. Changes of splanchnic perfusion after applying positive end expiratory pressure in patients with acute respiratory distress syndrome. Indian J Crit Care Med. 2009;13:12–16. doi: 10.4103/0972-5229.53109. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Kiefer P, Nunes S, Kosonen P, Takala J. Effect of positive end-expiratory pressure on splanchnic perfusion in acute lung injury. Intensive Care Med. 2000;26:376–383. doi: 10.1007/s001340051170. [DOI] [PubMed] [Google Scholar]
- 45.Deane A, Chapman MJ, Fraser RJ, Bryant LK, Burgstad C, Nguyen NQ. Mechanisms underlying feed intolerance in the critically ill: implications for treatment. World J Gastroenterol. 2007;13:3909–3917. doi: 10.3748/wjg.v13.i29.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fruhwald S, Holzer P, Metzler H. Intestinal motility disturbances in intensive care patients pathogenesis and clinical impact. Intensive Care Med. 2007;33:36–44. doi: 10.1007/s00134-006-0452-7. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen NQ, Ng MP, Chapman M, Fraser RJ, Holloway RH. The impact of admission diagnosis on gastric emptying in critically ill patients. Crit Care. 2007;11:R16. doi: 10.1186/cc5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kao CH, ChangLai SP, Chieng PU, Yen TC. Gastric emptying in head-injured patients. Am J Gastroenterol. 1998;93:1108–1112. doi: 10.1111/j.1572-0241.1998.00338.x. [DOI] [PubMed] [Google Scholar]
- 49.Ott L, Young B, Phillips R, McClain C, Adams L, Dempsey R, et al. Altered gastric emptying in the head-injured patient: relationship to feeding intolerance. J Neurosurg. 1991;74:738–742. doi: 10.3171/jns.1991.74.5.0738. [DOI] [PubMed] [Google Scholar]
- 50.Tache Y, Monnikes H, Bonaz B, Rivier J. Role of CRF in stress-related alterations of gastric and colonic motor function. Ann N Y Acad Sci. 1993;697:233–243. doi: 10.1111/j.1749-6632.1993.tb49936.x. [DOI] [PubMed] [Google Scholar]
- 51.Kalff JC, Buchholz BM, Eskandari MK, Hierholzer C, Schraut WH, Simmons RL, et al. Biphasic response to gut manipulation and temporal correlation of cellular infiltrates and muscle dysfunction in rat. Surgery. 1999;126:498–509. [PubMed] [Google Scholar]
- 52.Coimbra CR, Plourde V. Abdominal surgery-induced inhibition of gastric emptying is mediated in part by interleukin-1 beta. Am J Physiol. 1996;270:R556–560. doi: 10.1152/ajpregu.1996.270.3.R556. [DOI] [PubMed] [Google Scholar]
- 53.Schwarz NT, Kalff JC, Turler A, Engel BM, Watkins SC, Billiar TR, et al. Prostanoid production via COX-2 as a causative mechanism of rodent postoperative ileus. Gastroenterology. 2001;121:1354–1371. doi: 10.1053/gast.2001.29605. [DOI] [PubMed] [Google Scholar]
- 54.Kalff JC, Schraut WH, Billiar TR, Simmons RL, Bauer AJ. Role of inducible nitric oxide synthase in postoperative intestinal smooth muscle dysfunction in rodents. Gastroenterology. 2000;118:316–327. doi: 10.1016/s0016-5085(00)70214-9. [DOI] [PubMed] [Google Scholar]
- 55.Kalff JC, Carlos TM, Schraut WH, Billiar TR, Simmons RL, Bauer AJ. Surgically induced leukocytic infiltrates within the rat intestinal muscularis mediate postoperative ileus. Gastroenterology. 1999;117:378–387. doi: 10.1053/gast.1999.0029900378. [DOI] [PubMed] [Google Scholar]
- 56.Plummer MP, Jones KL, Cousins CE, Trahair LG, Meier JJ, Chapman MJ, et al. Hyperglycemia Potentiates the Slowing of Gastric Emptying Induced by Exogenous GLP-1. Diabetes care. 2015 Mar 17; doi: 10.2337/dc14-3091. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 57.Chapman MJ, Fraser RJ, Matthews G, Russo A, Bellon M, Besanko LK, et al. Glucose absorption and gastric emptying in critical illness. Crit Care. 2009;13:R140. doi: 10.1186/cc8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lobo DN, Bostock KA, Neal KR, Perkins AC, Rowlands BJ, Allison SP. Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial. Lancet. 2002;359:1812–1818. doi: 10.1016/S0140-6736(02)08711-1. [DOI] [PubMed] [Google Scholar]
- 59.Lowman RM. The potassium depletion states and postoperative ileus. The role of the potassium ion. Radiology. 1971;98:691–694. doi: 10.1148/98.3.691. [DOI] [PubMed] [Google Scholar]
- 60.Golzarian J, Scott HW, Jr., Richards WO. Hypermagnesemia-induced paralytic ileus. Dig Dis Sci. 1994;39:1138–1142. doi: 10.1007/BF02087570. [DOI] [PubMed] [Google Scholar]
- 61.Buchman AL, Moukarzel AA, Bhuta S, Belle M, Ament ME, Eckhert CD, et al. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. J Parenter Enteral Nutr. 1995;19:453–460. doi: 10.1177/0148607195019006453. [DOI] [PubMed] [Google Scholar]
- 62.McClure RJ, Newell SJ. Randomised controlled trial of trophic feeding and gut motility. Arch Dis Child Fetal Neonatal Ed. 1999;80:F54–8. doi: 10.1136/fn.80.1.f54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bowling TE, Cliff B, Wright JW, Blackshaw PE, Perkins AC, Lobo DN. The effects of bolus and continuous nasogastric feeding on gastro-oesophageal reflux and gastric emptying in healthy volunteers: a randomised three-way crossover pilot study. Clin Nutr. 2008;27:608–613. doi: 10.1016/j.clnu.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 64.de Ville K, Knapp E, Al-Tawil Y, Berseth CL. Slow infusion feedings enhance duodenal motor responses and gastric emptying in preterm infants. Am J Clin Nutr. 1998;68:103–108. doi: 10.1093/ajcn/68.1.103. [DOI] [PubMed] [Google Scholar]
- 65.Montejo JC, Grau T, Acosta J, Ruiz-Santana S, Planas M, Garcia-De-Lorenzo A, et al. Multicenter, prospective, randomized, single-blind study comparing the efficacy and gastrointestinal complications of early jejunal feeding with early gastric feeding in critically ill patients. Criti Care Med. 2002;30:796–800. doi: 10.1097/00003246-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 66.Davies AR, Morrison SS, Bailey MJ, Bellomo R, Cooper DJ, Doig GS, et al. A multicenter, randomized controlled trial comparing early nasojejunal with nasogastric nutrition in critical illness. Crit Care Med. 2012;40:2342–2348. doi: 10.1097/CCM.0b013e318255d87e. [DOI] [PubMed] [Google Scholar]
- 67.Meert KL, Daphtary KM, Metheny NA. Gastric vs small-bowel feeding in critically ill children receiving mechanical ventilation: a randomized controlled trial. Chest. 2004;126:872–878. doi: 10.1378/chest.126.3.872. [DOI] [PubMed] [Google Scholar]
- 68.Tarling MM, Toner CC, Withington PS, Baxter MK, Whelpton R, Goldhill DR. A model of gastric emptying using paracetamol absorption in intensive care patients. Intensive Care Med. 1997;23:256–260. doi: 10.1007/s001340050325. [DOI] [PubMed] [Google Scholar]
- 69.Tume L, Latten L, Darbyshire A. An evaluation of enteral feeding practices in critically ill children. Nurs Crit Care. 2010;15:291–299. doi: 10.1111/j.1478-5153.2010.00420.x. [DOI] [PubMed] [Google Scholar]
- 70.Metheny NA, Stewart J, Nuetzel G, Oliver D, Clouse RE. Effect of feeding-tube properties on residual volume measurements in tube-fed patients. JPEN J Parenter Enteral Nutr. 2005;29:192–197. doi: 10.1177/0148607105029003192. [DOI] [PubMed] [Google Scholar]
- 71.Bartlett Ellis RJ, Fuehne J. Examination of Accuracy in the Assessment of Gastric Residual Volume: A Simulated, Controlled Study. JPEN Journal of parenteral and enteral nutrition. 2014 doi: 10.1177/0148607114524230. [DOI] [PubMed] [Google Scholar]
- 72.McClave SA, Snider HL, Lowen CC, McLaughlin AJ, Greene LM, McCombs RJ, et al. Use of residual volume as a marker for enteral feeding intolerance: prospective blinded comparison with physical examination and radiographic findings. JPEN J Parenter Enteral Nutr. 1992;16:99–105. doi: 10.1177/014860719201600299. [DOI] [PubMed] [Google Scholar]
- 73.Reignier J, Mercier E, Le Gouge A, Boulain T, Desachy A, Bellec F, et al. Effect of not monitoring residual gastric volume on risk of ventilator-associated pneumonia in adults receiving mechanical ventilation and early enteral feeding: a randomized controlled trial. JAMA. 2013;309:249–256. doi: 10.1001/jama.2012.196377. [DOI] [PubMed] [Google Scholar]
- 74.iezzo G, Russo F, Indrio F. Electrogastrography in adults and children: the strength, pitfalls, and clinical significance of the cutaneous recording of the gastric electrical activity. Biomed Res Int. 2013:14. doi: 10.1155/2013/282757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perrella SL, Hepworth AR, Simmer KN, Geddes DT. Validation of ultrasound methods to monitor gastric volume changes in preterm infants. J Pediatr Gastroenter Nutr. 2013;57:741–749. doi: 10.1097/MPG.0b013e3182a938d7. [DOI] [PubMed] [Google Scholar]
- 76.Hamada SR, Garcon P, Ronot M, Kerever S, Paugam-Burtz C, Mantz J. Ultrasound assessment of gastric volume in critically ill patients. Intensive Care Med. 2014;40:965–972. doi: 10.1007/s00134-014-3320-x. [DOI] [PubMed] [Google Scholar]
- 77.Ng PC. Use of oral erythromycin for the treatment of gastrointestinal dysmotility in preterm infants. Neonatology. 2009;95:97–104. doi: 10.1159/000153093. [DOI] [PubMed] [Google Scholar]
- 78.Shin A, Camilleri M, Busciglio I, Burton D, Stoner E, Noonan P, et al. Randomized controlled phase Ib study of ghrelin agonist, RM-131, in type 2 diabetic women with delayed gastric emptying: pharmacokinetics and pharmacodynamics. Diabetes Care. 2013;36:41–48. doi: 10.2337/dc12-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Diego MA, Field T, Hernandez-Reif M, Deeds O, Ascencio A, Begert G. Preterm infant massage elicits consistent increases in vagal activity and gastric motility that are associated with greater weight gain. Acta Paediatr. 2007;96:1588–1591. doi: 10.1111/j.1651-2227.2007.00476.x. [DOI] [PubMed] [Google Scholar]
- 80.Kang W, Kudsk KA. Is there evidence that the gut contributes to mucosal immunity in humans? JPEN J Parenter Enteral Nutr. 2007;31:246–258. doi: 10.1177/0148607107031003246. [DOI] [PubMed] [Google Scholar]
- 81.Chellis MJ, Sanders SV, Webster H, Dean JM, Jackson D. Early enteral feeding in the pediatric intensive care unit. JPEN J Parenter Enteral Nutr. 1996;20:71–73. doi: 10.1177/014860719602000171. [DOI] [PubMed] [Google Scholar]
- 82.Burrin DG, Stoll B, Jiang R, Chang X, Hartmann B, Holst JJ, et al. Minimal enteral nutrient requirements for intestinal growth in neonatal piglets: how much is enough? Am J Clin Nutr. 2000;71:1603–1610. doi: 10.1093/ajcn/71.6.1603. [DOI] [PubMed] [Google Scholar]
- 83.Hernandez G, Velasco N, Wainstein C, Castillo L, Bugedo G, Maiz A, et al. Gut mucosal atrophy after a short enteral fasting period in critically ill patients. J Crit Care. 1999;14:73–77. doi: 10.1016/s0883-9441(99)90017-5. [DOI] [PubMed] [Google Scholar]
- 84.Pironi L, Paganelli GM, Miglioli M, Biasco G, Santucci R, Ruggeri E, et al. Morphologic and cytoproliferative patterns of duodenal mucosa in two patients after long-term totalparenteral nutrition: changes with oral refeeding and relation to intestinal resection. JPEN J Parenter Enteral Nutr. 1994;18:351–354. doi: 10.1177/014860719401800413. [DOI] [PubMed] [Google Scholar]
- 85.Hamilton S, McAleer DM, Ariagno K, Barrett M, Stenquist N, Duggan CP, et al. A stepwise enteral nutrition algorithm for critically ill children helps achieve nutrient delivery goals*. Pediatr Crit Care Med. 2014;15:583–589. doi: 10.1097/PCC.0000000000000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Petrillo-Albarano T, Pettignano R, Asfaw M, Easley K. Use of a feeding protocol to improve nutritional support through early, aggressive, enteral nutrition in the pediatric intensive care unit. Pediatr Crit Care Med. 2006;7:340–344. doi: 10.1097/01.PCC.0000225371.10446.8F. [DOI] [PubMed] [Google Scholar]
- 87.Briassoulis G, Tsorva A, Zavras N, Hatzis T. Influence of an aggressive early enteral nutrition protocol on nitrogen balance in critically ill children. J Nutr Biochem. 2002;13:560. doi: 10.1016/s0955-2863(02)00200-0. [DOI] [PubMed] [Google Scholar]