SUMMARY
Primary hyperparathyroidism (PHPT) is a common cause of bone loss that is modeled by continuous PTH (cPTH) infusion. Here we show that the inflammatory cytokine IL-17A is upregulated by PHPT in humans and cPTH in mice. In humans IL-17A is normalized by parathyroidectomy. In mice treatment with anti-IL-17A antibody and silencing of IL-17A receptor IL-17RA prevent cPTH induced osteocytic and osteoblastic RANKL production and bone loss. Mechanistically, cPTH stimulates conventional T cell production of TNFα (TNF), which increases the differentiation of IL-17A producing Th17 cells via TNF receptor 1 (TNFR1) signaling in CD4+ cells. Moreover, cPTH enhances the sensitivity of naïve CD4+ cells to TNF via GαS/cAMP/Ca++ signaling. Accordingly, conditional deletion of GαS in CD4+ cells and treatment with the calcium channel blocker diltiazem prevents Th17 cell expansion and blocks cPTH induced bone loss. Neutralization of IL-17A and calcium channel blockers may thus represent novel therapeutic strategies for hyperparathyroidism.
Keywords: PTH, Hyperparathyroidism, T cells, Th17 cells, IL-17, IL-17R, IL-17 antibody, bone
INTRODUCTION
Parathyroid hormone (PTH) is a major regulator of calcium metabolism and bone homeostasis. PHPT, a condition characterized by chronic overproduction of PTH, is a common cause of osteoporosis (Parisien et al., 2001; Potts, 1998; Silverberg et al., 1989) that is modeled in animals by continuous PTH (cPTH) infusion. Both PHPT and cPTH treatment stimulate bone resorption and, to a lesser extent, bone formation (Qin et al., 2004) causing cortical bone loss and often trabecular bone loss (Iida-Klein et al., 2005; Potts, 1998).
The effects of cPTH on bone result from its binding to the PTH/PTH-related protein (PTHrP) receptor (PPR or PTHR1), which is expressed on bone marrow (BM) stromal cells, osteoblasts and osteocytes (Calvi et al., 2001; Saini et al., 2013), but also T cells (Terauchi et al., 2009) and macrophages (Cho et al., 2014). Early consensus held that the catabolic effect of cPTH is mediated by altered production of RANKL and OPG by stromal cells and osteoblasts (Ma et al., 2001). Osteocytes have now emerged as essential targets of PTH, as osteocyte-produced RANKL is pivotal for cPTH induced bone loss (Ben-awadh et al., 2014; Saini et al., 2013; Xiong et al., 2014). cPTH fails to induce bone loss in T cell null mice and mice with conditional deletion of PPR in T cells (Gao et al., 2008; Tawfeek et al., 2010), thus revealing that T cells contribute to the mechanism of action of PTH in bone. PPR activation in T cells stimulates TNF production by BM conventional CD4+ and CD8+ cells (Tawfeek et al., 2010) and cPTH fails to induce bone loss in mice specifically lacking TNF production by T cells (Tawfeek et al., 2010). TNF upregulates CD40 expression in SCs, allowing T cell expressed CD40L to regulate the responsiveness of SCs to cPTH (Gao et al., 2008). However, TNF is likely to contribute to the bone catabolic activity of cPTH via additional effects. Moreover, the mechanisms by which cPTH regulates T cell function, the specific population of T cells that mediate the activity of cPTH, and the role of T cells in the activity of PTH in humans remain to be determined.
Th17 cells are an osteoclastogenic population of CD4+ cells (Miossec et al., 2009; Sato et al., 2006) defined by the capacity to produce IL-17A and other minor members of the IL-17 family of cytokines (Basu et al., 2013). Th17 cells reside in the BM (Kappel et al., 2009) and play a pathogenic role in inflammatory conditions such as psoriasis, rheumatoid arthritis, multiple sclerosis and Crohn’s disease (Martinez et al., 2008; Miossec et al., 2009). Moreover, Th17 cells contribute to the bone wasting caused by estrogen deficiency in mice (DeSelm et al., 2012; Tyagi et al., 2012) and humans (Molnar et al., 2014; Zhang et al., 2014). Th17 cells potently induce osteoclastogenesis by secreting IL-17A, RANKL, TNF, IL-1 and IL-6, along with low levels of IFNγ (Jovanovic et al., 1998; Komatsu and Takayanagi, 2012; Waisman, 2011). IL-17A stimulates the release of RANKL by all osteoblastic cells including osteocytes (Komatsu and Takayanagi, 2012; Kotake et al., 1999; Sato et al., 2006) and potentiates the osteoclastogenic activity of RANKL by upregulating RANK (Adamopoulos et al., 2010).
cPTH stimulates bone and immune cells to release growth factors and cytokines. Among them are TGFβ, IL-6, and TNF (Koh et al., 2011; Lowik et al., 1989; Tawfeek et al., 2010), factors that direct the differentiation of naïve CD4+ cells into Th17 cells (Basu et al., 2013; Bettelli et al., 2006; Nakae et al., 2007; Sugita et al., 2012). Therefore, it is plausible that cPTH may induce Th17 cell differentiation in the BM, and that IL-17A produced by BM Th17 cells may act as an upstream cytokine that plays a pivotal role in the bone loss induced by cPTH and PHPT.
This study was designed to determine the effects of PHPT and cPTH on the production of IL-17A in humans and mice and to investigate the contribution of IL-17A to the bone loss induced by cPTH in mice. We report that PHPT increases peripheral blood cell expression of IL-17A mRNA, which is normalized by parathyroidectomy. We also show that in mice, cPTH promotes Th17 cell differentiation via TNFR1 signaling in T cells. In addition, cPTH enhances the sensitivity of naïve CD4+ cells to TNF via the GαS/cAMP/Ca++ signaling pathway. Attesting to relevance of IL-17A and PTH signaling in T cells, silencing of IL-17RA or GαS and treatment with neutralizing anti IL-17A antibody or the calcium channel blocker diltiazem prevent cPTH induced bone loss.
RESULTS AND DISCUSSION
Increased production of IL-17A in humans affected by PHPT
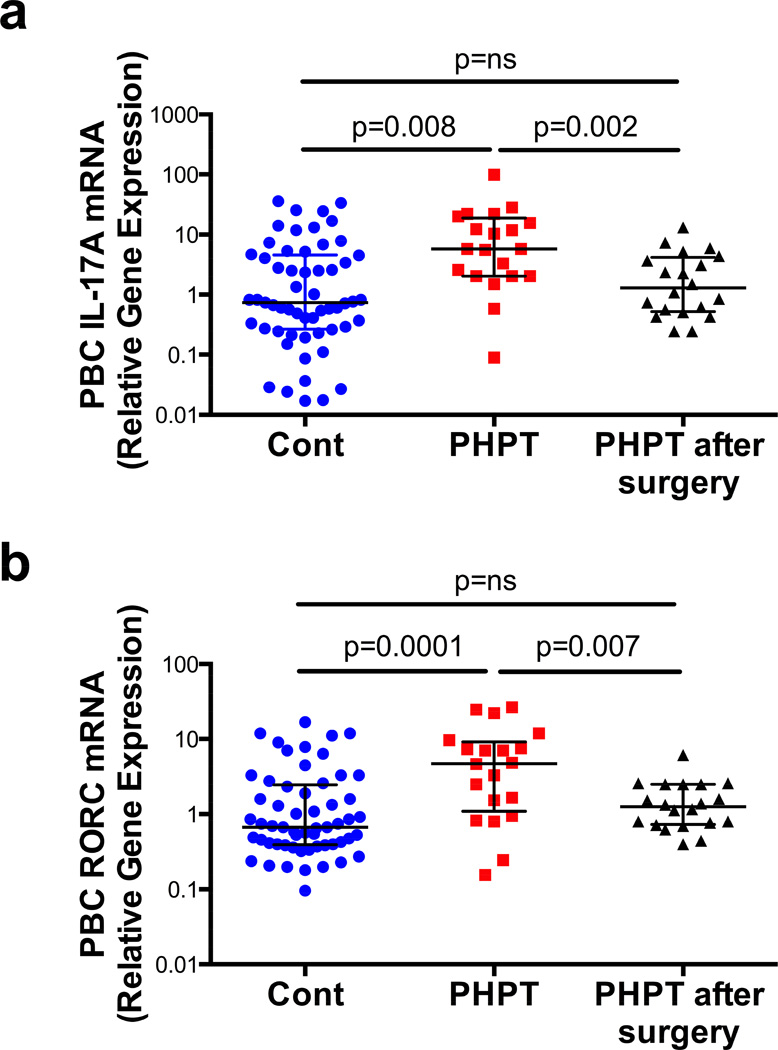
To investigate the effects of PHPT on the production of cytokines, unfractionated peripheral blood nucleated cells were obtained from 57 healthy controls and 20 subjects affected by PHPT of similar gender, age and years since menopause. The demographic characteristics of the study population and the serum levels of calcium, phosphorous, PTH and 25-hydroxy Vitamin D are shown in table 1. In PHPT patient’s blood samples were obtained before surgery and 1 month after successful resolution of PHPT by parathyroidectomy. Overproduction of IL-17A has been documented in inflammatory conditions associated with local and systemic bone loss such as psoriasis, rheumatoid arthritis and Crohn’s disease (Martinez et al., 2008; Miossec et al., 2009) but the effects of PHPT on IL-17A are unknown. Analysis by real time RT-PCR revealed that the mRNA levels of IL-17A in unfractionated peripheral blood nucleated cells were ~ 3 fold higher in PHPT patients than in healthy controls (fig.1a). Moreover, surgical restoration of normal parathyroid function was associated with the normalization of IL-17A levels, as demonstrated by the finding at 1 month after parathyroidectomy of similar IL-17A mRNA levels in healthy subjects and former PHPT patients. Furthermore, the mRNA levels of the IL-17-inducing transcription factor RORC were ~ 3 fold higher in PHPT patients before surgery than in healthy controls (fig.1b) and parathyroidectomy was followed by a decrease in RORC mRNA levels. As a result, levels of RORC mRNA in healthy controls and PHPT patients after surgery were not significantly different. Whether PHPT increases levels of IL-17A protein in the peripheral circulation and in the BM remains to be determined.
Table 1.
Demographic and clinical data of healthy controls and PHPT patients before and after surgery. Data are shown as Mean ± SEM for normally distributed variables (serum P and demographic data) and median with interquartile range for non-normally distributed variables (serum Ca, PTH, and 25OH Vitamin D). Values in squared parenthesis denote normal range.
| Healthy Controls |
PHPT before surgery |
PHPT after surgery |
|
|---|---|---|---|
| Study Participants (n) | 57 | 20 | 20 |
| Age (years) | 60.1 ± 2.3 | 57.3 ± 3.4a | 57.3 ± 3.4a |
| Males (n) | 25 | 4 | 4 |
| Males Age (years) | 66.5 ± 3.6 | 67.5 ± 7.2a | 67.5 ± 7.2a |
| Females (n) | 32 | 16 | 16 |
| Female Age (years) | 56.7 ± 2.9 | 54.5 ± 3.7a | 54.5 ± 3.7a |
|
Postmenopausal females (n) |
23 | 8a | 8a |
| Years since menopause | 15.5 ± 2.3 | 15.7 ± 3.5a | 15.7 ± 3.5a |
|
Serum Ca (mg/dL) [8.8–10.4 mg/dL] |
9.4 (9.1–9.7) | 10.8 (10.5–12.0)b | 8.8 (8.7–9.1)d,b |
|
Serum P (mg/dL) [2.5–4.5 mg/dL] |
3.3 ±0.07 | 2.7 ± 0.3c | 3.5 ± 0.4e,a |
|
PTH (pg/mL) [10–65 pg/mL] |
53 (32–63) | 102 (79–161)b | 56.5 (41.8–75.8)d,a |
|
25OH vitamin D [30–100 ng/mL] |
19.6 (5.7–27.2) | 17.8 (15.1–22.4)a | 23.6 (21.9–30.4)f,a |
p values:
ns,
<0.0001 and
0.005 compared to Healthy Controls;
<0.0001,
<0.05 and
<0.01 compared to PHPT.
Figure 1. Primary hyperparathyroidism increases IL-17A and RORC mRNA levels in humans.
Levels (Median ± interquartile range) of IL-17A and RORC mRNAs in healthy controls (n = 57) and subjects with PHPT before (n = 20) and after parathyroidectomy (n = 20). Data were analyzed by Mann Whitney (healthy controls vs. PHPT before surgery and healthy controls vs. PHPT after surgery) and Wilcoxon matched pairs signed rank tests (PHPT vs. PHPT after surgery) as the data were not normally distributed according to the Shapiro-Wilk normality test.
In the entire study population PTH levels were directly correlated with mRNA levels of IL-17A (r=0.38, p<0.005) and RORC (r=0.27, p<0.05). Moreover, disease status (healthy or PHPT) and age were independent predictors of IL-17A and RORC mRNA levels, whereas gender was not (tables S1,S2). These differences in IL-17A and RORC levels between healthy controls and PHPT patients remained significant even after adjustment for age and gender by a multiple regression model.
These findings suggest that increased IL-17A gene expression in PHPT patients is due to increased levels of circulating PTH. However, we cannot exclude the possibility that IL-17A might be regulated by factors modified by PTH, such as serum levels of calcium, phosphate and calcitriol. A previous cross-sectional study in patients on dialysis with hyperparathyroidism secondary to end-stage renal disease revealed a direct correlation between phosphate levels and frequency of peripheral blood Th17 cells (Lang et al., 2014). By contrast, in our study IL-17A levels were increased in PHPT patients who have decreased phosphate levels. Moreover, we found no correlation between IL-17A and RORC expression and serum phosphate levels.
PHPT was also associated with increased mRNA levels of TNF and IL-23, which were normalized by parathyroidectomy. By contrast all groups had similar mRNA levels of IFNγ and IL-4 suggesting that PHPT does not expand Th1 cells, Th2 cells (fig. S1).
Treatment with cPTH expands Th17 cells and IL-17A production in the mouse
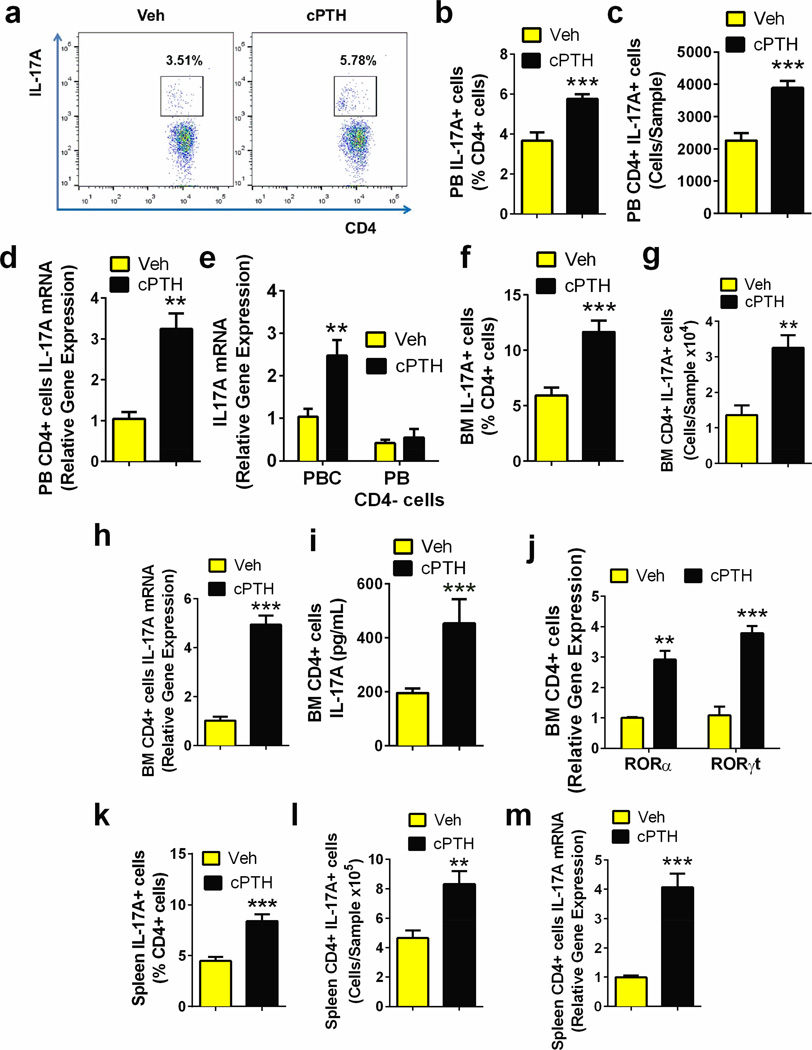
To investigate the effect of PTH in mice, human PTH 1–34 was continuously infused at the rate of 80 µg/kg/day for 2 weeks, a treatment modality referred to hereafter as cPTH. Analysis by flow cytometry of unfractionated peripheral blood nucleated cells harvested at sacrifice revealed that cPTH increased the relative and absolute frequency of peripheral blood Th17+ cells (fig.2a–c). Moreover, cPTH increased IL-17A mRNA levels in purified peripheral blood CD4+ cells (fig. 2d) and unfractionated peripheral blood nucleated cells, but not in CD4+ cell-depleted peripheral blood nucleated cells (fig.2e), indicating that CD4+ cells represent the major source of IL-17 mRNA in peripheral blood cells.
Figure 2. cPTH treatment expands Th17 cells and increases L-17A production.
a-c. Frequency of IL-17A producing Th17 cells in unfractionated peripheral blood (PB) cells (PBC). Panel a shows representative flow cytometric dot plots from 1 mouse per group. Panel b shows the relative frequency of CD4+IL-17A+ PBC. Data are expressed as % of CD4+ cells. Panel c shows the absolute number of CD4+IL-17A+ PBCs per sample. d. IL-17A mRNA levels in PB CD4+ cells. e. IL-17A mRNA levels in unfractionated PBC and CD4+ cell-depleted PBC. f. Relative frequency of Th17 cells in the BM. g. Absolute number of BM Th17 cells per sample. h. IL-17A mRNA levels in BM CD4+ cells. i. IL-17A protein levels in BM CD4+ cells. j. mRNA levels of the Th17 cells-inducing transcription factors RORα and RORγt in BM CD4+ cells. k. Relative frequency of Th17 cells in the spleen. l. Absolute number of Th17 cells in the spleen m. IL-17A mRNA levels in spleen CD4+ cells. Data in panels b-m are shown as mean ± SEM. n = 8 mice per group in all panels. All data passed the Shapiro-Wilk normality test and were analyzed by unpaired t-tests **=p<0.01 and ***=p<0.001 compared to the corresponding vehicle group.
Mirroring its activity in peripheral blood, cPTH increased by ~2 fold the relative and absolute frequency of Th17 cells in the BM (fig.2f,g), by ~5 fold the levels of IL-17A mRNA in purified BM CD4+ cells (fig. 2h), and by 2 fold the levels of IL-17A protein in the culture media of BM CD4+ cells (fig.2i). Moreover, cPTH increased the expression of the Th17-inducing transcription factors RORα and RORγt in BM CD4+ T cells (fig.2j). cPTH treatment was also associated with a ~2 fold increase in the relative and absolute frequency of splenic Th17 cells (fig.2k,l) and a ~4 fold increase in the levels of IL-17A mRNA in purified splenic CD4+ cells (fig. 2m). By contrast, cPTH did not increase the relative and absolute frequency of IFNγ+CD4+ cells, IL-4+CD4+ cells, and FoxP3+CD4+ cells in the peripheral blood, BM and spleen (fig. S2), indicating that cPTH does not expand murine Th1 cells, Th2 cells and regulatory T cells.
Although IL-17A is mostly produced by Th17 cells, γδ T cells, innate lymphoid cells, NK cells, NKT cells, neutrophils and eosinophils also produce IL-17 (Korn et al., 2009; Lockhart et al., 2006; Sutton et al., 2009). Our data do not support a role for γδ T cell produced IL-17A in the bone catabolic activity of cPTH because TCRβ−/− mice, a strain lacking αβ but not γδ T cells are completely protected against cPTH induced bone loss (Gao et al., 2008).
Neutralization of IL-17A or silencing of IL-17RA block cPTH induced bone loss
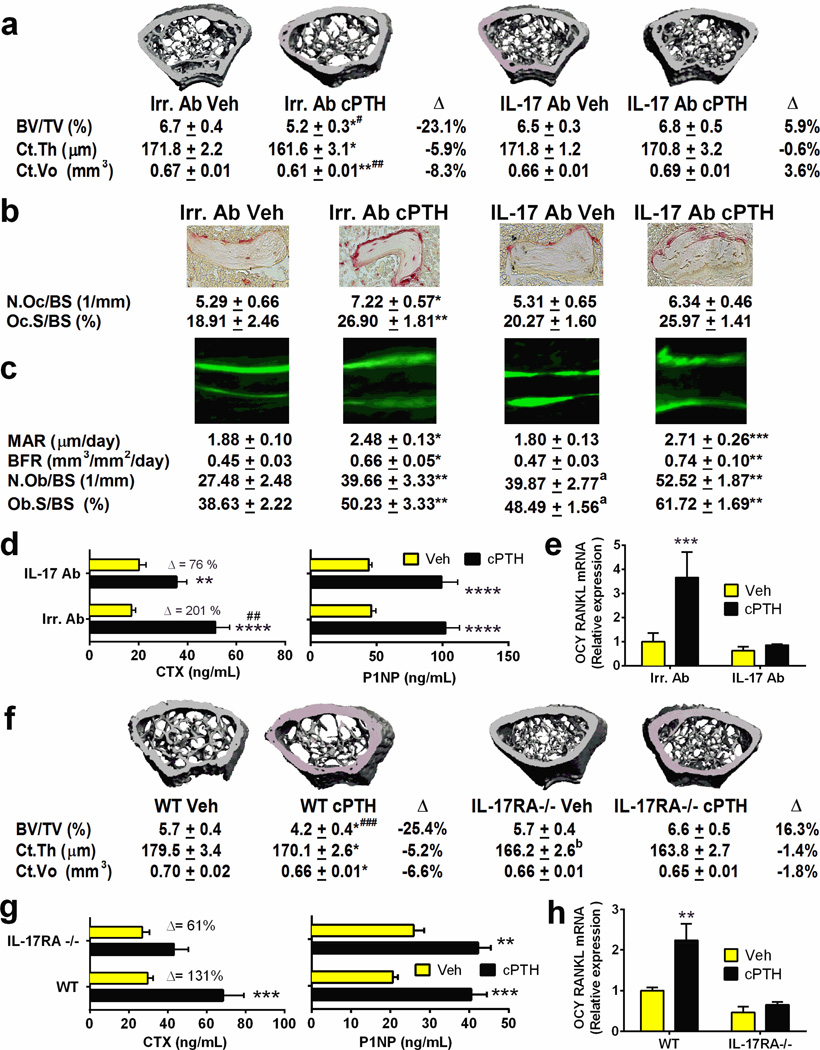
To investigate whether IL-17A contributes to the bone catabolic activity of cPTH, WT mice were treated with vehicle or cPTH and either a neutralizing antibody directed against murine IL-17A (IL-17A Ab) or isotype matched irrelevant Ab (Irr. Ab), at the dose of 2.5 mg/kg, twice per week for 2 weeks. To assess the differential effects of cPTH on cortical and trabecular bone, micro-computed tomography (µCT) was utilized to analyze femurs harvested at sacrifice. cPTH induced significant loss of cortical thickness (Ct.Th) and volume (Ct.Vo) and trabecular bone volume (BV/TV) in mice treated with Irr. Ab, but not in those treated with IL-17A Ab (fig.3a). Analysis of indices of trabecular structure revealed that cPTH had opposite effects in trabecular number (Tb.N) in mice treated with Irr. Ab and those treated with IL-17A Ab. A similar trend was observed for trabecular space (Tb.Sp). By contrast, cPTH induced decreased trabecular thickness (Tb.Th) in all mice (fig. S3a–c).
Figure 3. Silencing of IL-17A or IL-17A signaling prevents the bone catabolic effect of cPTH.
Effects (mean ± SEM) of cPTH on bone volume, structure and turnover in mice treated with IL-17A Ab or lacking IL-1RA. a. In vitro measurements of cortical and trabecular bone indices of volume and structure by µCT scanning in WT mice treated with vehicle or cPTH and Irrelevant Ab (Irr. Ab) or anti IL-17A Ab. n= 12 mice per group. Representative 3-dimensional µCT reconstructions of the femurs are shown above the data. b. Histomorphometric indices of bone resorption (obtained in the first 10 of the 12 mice per group enrolled in the study). The images show TRAP stained sections of the distal femur used to compute the number of OCs per mm bone surface (N.Oc/BS) and the percentage of bone surface covered by OCs (Oc.S/BS),which are indices of bone resorption. Original magnification × 40. c. Dynamic indices of bone formation. The images show calcein double-fluorescence labeling used to compute mineral apposition rate (MAR) and bone formation rate (BFR), which are indices of bone formation. Original magnification × 20. The number of OBs per mm bone surface (N.Ob/BS) and the percentage of bone surface covered by OBs (Ob.S/BS), which are static indices of formation, were measured on trichrome-stained sections. d. Serum levels of CTX and P1NP. n= 12 mice per group. e. mRNA levels of RANKL in purified osteocytes (OCYs). n= 5 mice per group. f. µCT measurements of cortical and trabecular bone volume and structure in samples from WT and IL-17RA−/− mice. n = 14 mice per group. The images are 3-dimensional reconstructions of the femurs. g. Serum levels of CTX and P1NP. n = 14 mice per group. h. mRNA levels of RANKL in purified OCYs from WT and IL-17RA−/− mice. n= 5 mice per group. All data passed the Shapiro-Wilk normality test and were analyzed by 2-Way ANOVA. * = p < 0.05, ** = p<0.01 and *** = p<0.001 compared to the corresponding vehicle treated group. # = p<0.05, and ## = p<0.01 compared to IL-17 Ab cPTH. ### = p<0.001 compared to IL-17RA−/− cPTH. a= p<0.05 compared to Irr. Ab Veh. b= p<0.05 compared to WT Veh.
Analysis of femoral cancellous bone by histomorphometry revealed that neutralization of IL-17A blunts the bone catabolic activity of cPTH by decreasing bone resorption. In fact, cPTH treatment increased two indices of bone resorption, the number of OCs per bone surface (N.Oc/BS) and the percentage of surfaces covered by OCs (Oc.S/BS), in mice treated with Irr. Ab but not in those treated with IL-17Ab (fig.3b). Analysis of dynamic indices of bone formation revealed that cPTH increased mineral apposition rate (MAR) and bone formation rate (BFR) in mice treated with Irr. Ab and in those treated with IL-17A Ab (fig.3c). Two static indices of bone formation, the number of OBs per bone surface (N.Ob/BS) and the percentage of surfaces covered by OBs (Ob.S/BS) increased significantly in response to treatment with cPTH in both mice treated with Irr. Ab and those treated with IL-17A Ab (fig.3c). These indices were unexpectedly higher in the vehicle/IL-17A Ab group as compared to the vehicle/Irr. Ab group.. Confirmation that neutralization of IL-17A blunts the bone catabolic activity of cPTH by decreasing bone resorption was provided by measurements of serum levels of C-terminal telopeptide of collagen (CTX), a marker of bone resorption, and total procollagen type 1 N-terminal propeptide (P1NP), a marker of bone formation. These assays revealed that treatment with IL-17A Ab significantly blunted the increase in serum CTX levels induced by cPTH, while it did not diminish the cPTH induced increase in P1NP levels (fig.3d).
The blockade of bone resorption induced by IL-17 Ab was of greater magnitude when estimated by serum levels of CTX than when measured by bone histomorphometry. The likely explanation for this phenomenon is that CTX levels reflect both cortical and trabecular bone resorption while histomorphometric indices were calculated only in the trabecular compartment. This is relevant because cortical bone accounts for most of the total bone mass and cPTH affects primarily the cortical compartment of the skeleton.
In summary, the data demonstrate neutralization of IL-17A blunts the capacity of cPTH to stimulate bone resorption without affecting bone formation. These changes in bone turnover prevented bone loss but did not cause a significant increase in bone volume presumably because of the short duration of the IL-17Ab treatment.
Osteocytes and the pool of RANKL produced by osteocytes are crucial for the activity of cPTH. In fact, not only does silencing of PPR expression in osteocytes blunts the bone catabolic activity of cPTH (Saini et al., 2013), but increased production of RANKL by osteocytes is now known to play a pivotal role in cPTH induced bone loss (Ben-awadh et al., 2014; Saini et al., 2013; Xiong et al., 2014). Reports from our laboratory have led to the hypothesis that T cells are also an important target of PTH (Pacifici, 2013), primarily because silencing of PPR in T cells protects against cPTH induced bone loss (Tawfeek et al., 2010). The fact that silencing of PPR signaling in T cells and osteocytes induces similar bone sparing effects is in keeping with a “serial circuit” regulatory model, where signals from one population affect the response to cPTH of the other. Since T cells and osteocytes have limited physical contacts, the cross talk between these populations is likely mediated by a soluble factor. IL-17A is a likely candidate because it is a potent inducer of RANKL in organ cultures containing osteoblasts and osteocytes (Nakashima et al., 2000). In support of this hypothesis we found IL-17A Ab to completely block the increase in osteocytic RANKL mRNA levels induced by cPTH (fig.3e). Further attesting to causal role of IL-17A, we found that treatment with IL-17A Ab completely blocked the capacity of cPTH treatment to increase the expression of RANKL, TNF, IL-1β and IL-6 mRNAs in purified osteoblasts and the increase in the expression of TNF mRNA in purified BM T cells (fig. S4a). These data indicate that IL-17A may mediate the bone catabolic activity of cPTH by upregulating the production of RANKL by osteocytes and osteoblasts.
IL-17A binds to the heterodimeric receptor IL-17RA/IL-17RC known as IL-17RA (Iwakura et al., 2011; Zepp et al., 2011). IL-17A signaling is silenced in IL-17RA−/− mice (Ye et al., 2001). We made use of IL-17RA−/− mice to further investigate whether IL-17A contributes to the bone catabolic activity of cPTH. 16 week-old IL-17RA−/− mice and WT littermates were treated with vehicle or cPTH for 2 weeks. In vitro µCT analysis of femurs harvested at sacrifice revealed that cPTH induced significant losses of Ct.Th, Ct.Vo and BV/TV in WT mice but not in IL-17RA−/−mice (fig.3f). For reasons that remain undetermined, Ct.Th was lower in vehicle treated IL-17RA−/− mice as compared to vehicle treated WT mice. Parameters of trabecular structure (Tb.N, Tb.Th, and Tb.Sp) were altered by cPTH in WT but not in IL-17RA−/− mice (fig. S3d–f).
Serum CTX levels were increased by cPTH in WT but not in IL-17RA−/− mice (fig.3g). By contrast serum P1NP levels were increased by cPTH in WT and IL-17RA−/− mice (fig.3g). Osteocytic RANKL mRNA levels were increased by cPTH in WT but not in IL-17RA−/− mice (fig.3h). Finally, cPTH treatment increased the osteoblastic expression of RANKL, TNF, IL-1β and IL-6 mRNAs and the T cell expression of TNF mRNA in in WT but not IL-17RA−/− mice (fig. S4b). These findings demonstrate that silencing of IL-17RA prevents the loss of cortical and trabecular bone, and the increase in bone resorption and the osteoblastic/osteocytic RANKL production induced by cPTH. Importantly, the data confirm that IL-17A acts as an upstream cytokine that drives bone loss by increasing the sensitivity of osteoblasts and osteocytes to cPTH, thus enabling these lineages to release RANKL when stimulated by cPTH. Parallel deletions of PPR in T cells, osteocytes and osteoblasts as well as conditional deletion of IL-17RA in osteocytes and osteoblasts will be necessary to conclusively confirm this hypothesis.
cPTH increases the differentiation of Th17 cells via TNF and GαS signaling
IL-6 and TGFβ initiate Th17 differentiation (Basu et al., 2013; Martinez et al., 2008) while TNF, IL-1, and IL-23 amplify it (Veldhoen et al., 2006). The relevance of TNF for Th17 cell expansion has emerged by investigations on the effects of TNF blockers, which have demonstrated that TNF contributes to the expansion of Th17 cells in inflammatory conditions in humans and rodents (Nakae et al., 2007; Sugita et al., 2012). Accumulation of cAMP in CD4+ cells and the resulting Ca2+ influx further promote Th17 cell differentiation and activity (Li et al., 2012). Treatment with cPTH induces the production of IL-6 and TGFβ by bone and immune cells (Koh et al., 2011; Lowik et al., 1989) and the release of TNF by BM CD4+ and CD8+ cells (Tawfeek et al., 2010). PTH binding to PPR activates the G protein-coupled receptor subunit GαS, leading to the generation of cAMP (Datta and Abou-Samra, 2009). Therefore, several mechanisms could account for the capacity of cPTH to expand Th17 cells.
We utilized BM cells, which are essential for the regulation of bone homeostasis, and cells from the spleen, an organ not known to regulate bone remodeling to determine the mechanism by which cPTH expands Th17 cells. BrdU incorporation studies revealed that cPTH did not increase the proliferation of Th17 cells in the BM and the spleen (fig. S5a,b). IL-17A–eGFP mice were utilized to investigate the differentiation of naïve CD4+ cells into Th17 cells. IL-17A–eGFP reporter mice possess an IRES-eGFP sequence after the stop codon of the IL17A gene so that eGFP expression is limited to IL-17A expressing cells, allowing Th17 cells to be detected by measuring eGFP by flow cytometry. Splenic naïve CD4+ cells (CD4+CD44loCD62LhieGFP-cells) were FACS sorted from IL-17A–eGFP mice and transferred into congenic T cell deficient TCRβ−/− mice. Recipient mice were treated with vehicle or cPTH for 2 weeks starting 2 weeks after the T cell transfer, and newly produced Th17 cells (CD4+eGFP+ cells) counted. Since TCRβ−/− mice were reconstituted with eGFP- cells, the number of eGFP+ cells in host mice at sacrifice provides a direct quantification of the differentiation of naïve CD4+ cells into Th17 cells. cPTH treated mice had a higher number eGFP+ cells than controls in the BM and the spleen (fig. S5c,d) demonstrating that cPTH increases Th17 cell differentiation in the BM and the spleen.
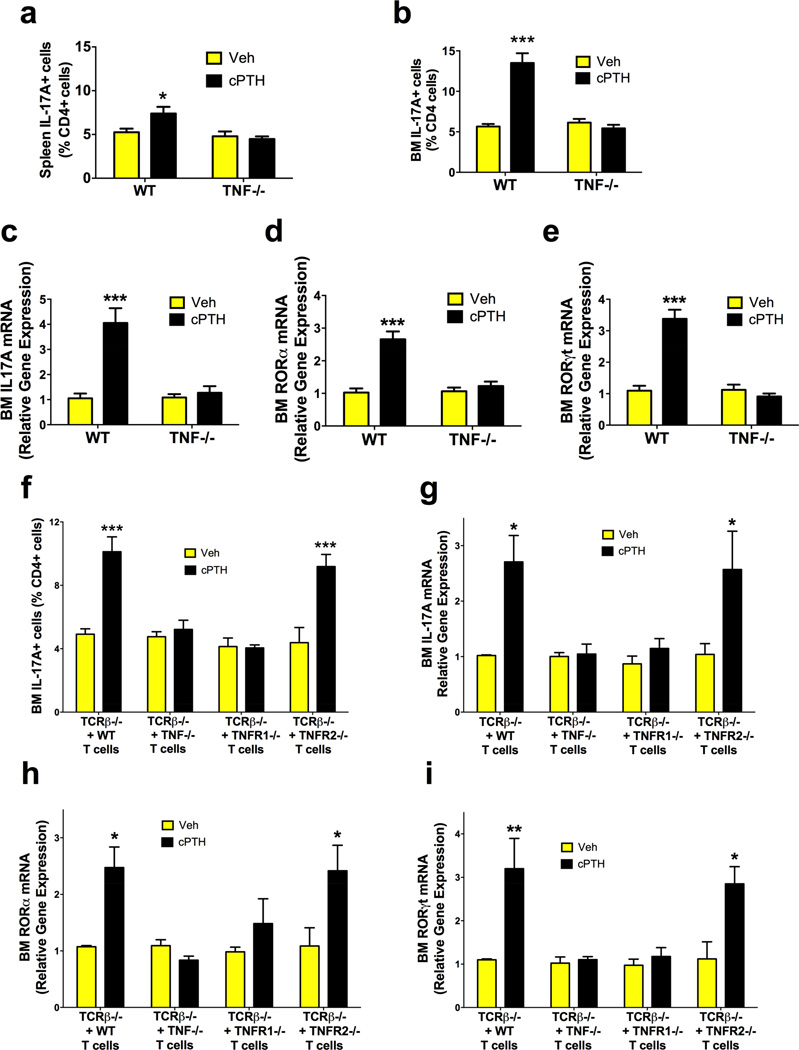
TNF expands Th17 cells and mediates the catabolic activity of cPTH (Chen et al., 2011; Nakae et al., 2007; Sugita et al., 2012). Therefore, we investigated the overall contribution of TNF and the specific role of T cell produced TNF in the expansion of Th17 cells induced by cPTH. WT and TNF−/− mice were treated with cPTH for 2 weeks and the relative frequency of BM Th17 cells determined. cPTH increased the frequency of Th17 cells in the spleen and the BM in WT mice, but not in TNF−/− mice (fig.4a,b). Analysis of purified BM CD4+ cells revealed that cPTH increased IL-17A mRNA levels (fig.4c) and the expression of RORα and RORγt (fig. 4d,e) in BM CD4+ cells from WT but not in those from TNF−/− mice. Thus, cPTH induces Th17 cells via TNF. Next, splenic T cells from WT and TNF−/− mice were transferred into TCRβ−/−mice. Recipient mice were treated with vehicle or cPTH for 2 weeks starting 2 weeks after the T cell transfer. cPTH increased the number of Th17 cells (fig.4f), IL-17A mRNA levels in CD4+ cells (fig.4g) and the expression of RORα and RORγt in CD4+ cells (fig.4h,i) in the BM of host mice with WT T cells but not in those with TNF−/− T cells. These findings demonstrate that the production of TNF by T cells is required for cPTH to expand Th17 cells.
Figure 4. cPTH expands Th17 cells through TNF and TNFR1 signaling.
a-b. Relative frequency of Th17 cells in the spleen and BM of WT and TNF−/− mice. c. IL-17A mRNA levels in BM CD4+ cells of WT and TNF−/− mice. d,e. ROR α and ROR γ t mRNA levels in BM CD4+ cells of WT and TNF−/− mice. f. Relative frequency of Th17 cells in the BM of TCRβ−/−mice previously subjected to adoptive transfer of WT T cells, TNF−/− T cells, TNFR1−/− T cells, or TNFR2−/− T cells. g. IL-17A mRNA levels in BM CD4+ cells of TCRβ−/− mice previously subjected to adoptive transfer of WT T cells, TNF−/− T cells, TNFR1−/− T cells, or TNFR2−/− T cells. h,i. Levels of RORα and RORγt mRNA in BM CD4+ cells of TCRβ−/− mice previously subjected to adoptive transfer of WT T cells, TNF−/− T cells, TNFR1−/− T cells, or TNFR2−/− T cells. Data are expressed as the mean ± SEM. n = 8 mice per group. All data passed the Shapiro-Wilk normality test and were analyzed by 2-Way ANOVA.*=p<0.05, **=p<0.01 and ***=p<0.001 compared to the corresponding vehicle group.
To determine whether TNF directly targets Th17 precursors, splenic CD4+ cells from TNFR1−/− and TNFR2−/− mice were transferred into TCRβ−/− mice. Host mice were treated with vehicle or cPTH for 2 weeks starting 2 weeks after the T cell transfer. cPTH expanded BM Th17 cells in mice with TNFR2−/− T cells but not in those with TNFR1−/− T cells (fig. 4f). Moreover, cPTH increased IL-17A mRNA levels in BM CD4+ cells (fig.4g) and the BM CD4+ cell expression of RORα and RORγt (fig.4h,i) in mice with TNFR2−/− T cells but not in those with TNFR1−/− T cells. These findings demonstrate that cPTH expands the pool of BM Th17 cells through direct TNFR1 signaling in T cells.
In addition to TNF, several cytokines are known to promote Th17 cell expansion. Among them are the T cell produced factor IL-21 and the macrophage/dendritic cell produced cytokine IL-23. We found that cPTH treatment increased the mRNA levels of IL-21, and IL-23R in BM and spleen CD4+ cells from WT but not TNF−/− mice (fig. S6a,b). Moreover, cPTH increased the mRNA levels of IL-23 in BM CD11c+ cells from WT but not TNF−/− mice (fig. S6c). These findings suggest that IL-21 and IL-23 may contribute to expansion of Th17 cells induced by cPTH. However, cPTH upregulates these factors via TNF.
In summary, our human and murine data indicate that TNF acts directly by upregulating the Th17 inducing factors IL-21 and IL-23. However, our findings do no exclude the possibility that PTH mediated stimulation of IL-6 and TGFβ production may contribute to the expansion of Th17 cells induced by cPTH.
Conditional deletion of the G protein-coupled receptor subunit GαS in T cells impairs the generation of Th17 cells (Li et al., 2012). Since PTH binding to PPR activates GαS (Datta and Abou-Samra, 2009), cPTH could further upregulate Th17 differentiation by activating Gα S in naïve CD4+ T cells. To investigate the role of G α S we generated GαSΔCD4,8 mice by crossing C57BL6 GαS fl/fl mice with C57BL6 CD4-Cre mice, as previously described (Li et al., 2012). The targeted genetic deletion of GαS with CD4-Cre occurs at the CD4+CD8+ double positive stage of T cell development (Li et al., 2012). Consequently, both CD4+ and CD8+ T cells from GαSΔCD4,8 mice lack G α S expression. GαSΔCD4,8, Gαs fl/fl, and WT mice have similar numbers of CD4+ and CD8+ cells, and similar percentages of effector, memory, and naïve CD4+ and CD8+ cells (Li et al., 2012).
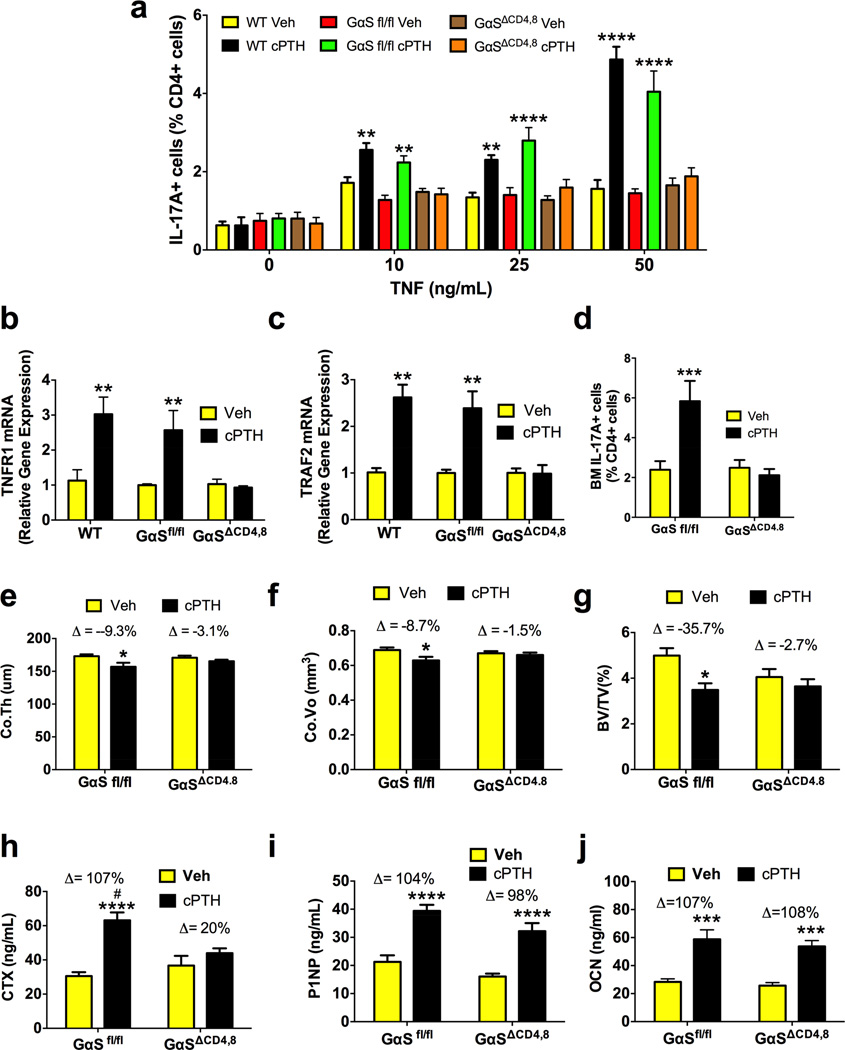
One mechanism by which activation of GαS in CD4+ cells could promote Th17 cell differentiation is increased sensitivity to TNF. To investigate this hypothesis, naïve splenic CD4+ cells from vehicle and cPTH treated WT, GαS fl/fl and GαSΔCD4,8 mice were cultured in vitro in anti-CD3 Ab and anti-CD28 coated wells for 3 days in the presence of TNF at 10–50 ng/ml to induce the conversion of CD4+ cells into Th17 cells. Cultures of CD4+ cells from cPTH treated WT and G α S fl/fl mice yielded a higher number of Th17 cells as compared to those from vehicle treated mice (fig.5a). By contrast cultures of CD4+ cells from vehicle and cPTH treated GαSΔCD4,8 mice yielded similar numbers of Th17 cells, demonstrating that cPTH increases the sensitivity of nascent Th17 cells to TNF via GαS signaling in CD4+ cells. Mechanistic studies revealed that treatment with cPTH for 2 weeks increased the mRNA levels of TNFR1 and the TNFR1 activated signaling molecule TRAF2 (Qin et al., 2012) in BM CD4+ cells from of WT and Gαs fl/fl mice but not in those from GαSΔCD4,8 mice (fig.5b,c) indicating that activation of G α S signaling by cPTH increases the sensitivity of nascent Th17 cells to TNF by upregulating TNFR1 expression and TNFR1 signaling.
Figure 5. cPTH expands Th17 cells, causes bone loss and stimulates bone resorption through activation of G α S in naïve CD4+ cells.
a. cPTH increases the sensitivity to TNF of naïve CD4+ cells from WT and G α S fl/fl mice but not of those from G α SΔCD4,8 mice. Naïve CD4+ cells were sorted from vehicle and cPTH treated mice and cultured with TNF (10–50 ng/ml) to induce their differentiation into Th17 cells. b. TNFR1 mRNA levels in BM CD4+ cells. c. TRAF2 mRNA levels in BM CD4+ cells d. Frequency of BM Th17 cells. e-g. mCT indices of bone volume and structure. h-j. Serum levels of CTX, P1NP and osteocalcin (OCN). Data are shown as mean ± SEM. n = 5 mice per group for panels b-c. n = 16 G α S fl/fl mice per group and 21 G α SΔCD4,8 mice per group for panels d-j. All data passed the Shapiro-Wilk normality test and were analyzed by 2-Way ANOVA. *=p<0.05, **=p<0.01, ***=p<0.001 and ****=p<0.0001 compared to the corresponding vehicle group. # = p<0.05 compared to the G α SΔCD4,8 cPTH group.
Treatment of GαSΔCD4,8 and Gas fl/fl control mice with cPTH for 2 weeks increased the frequency of BM Th17 cells (fig.5d), and induced significant losses of Ct.Th, Ct.Vo, and BV/TV (fig.5e–g), and Tb.Th (fig. S7a) in GαS fl/fl mice but not GαSΔCD4,8 mice. Unexpectedly, cPTH did not affect Tb.Sp and Tb.N in all mice (fig. S7b,c). cPTH also increased serum CTX levels in GαS fl/fl but not GαSΔCD4,8 mice (fig. 5h). Serum P1NP and osteocalcin (OCN) levels were increased by cPTH in GαS fl/fl and GαSΔCD4,8 mice (fig. 5i,j). These findings demonstrate that silencing of Gαs in T cells prevents the expansion of Th17 cells, the loss of cortical and trabecular bone, and the increase in bone resorption induced by cPTH.
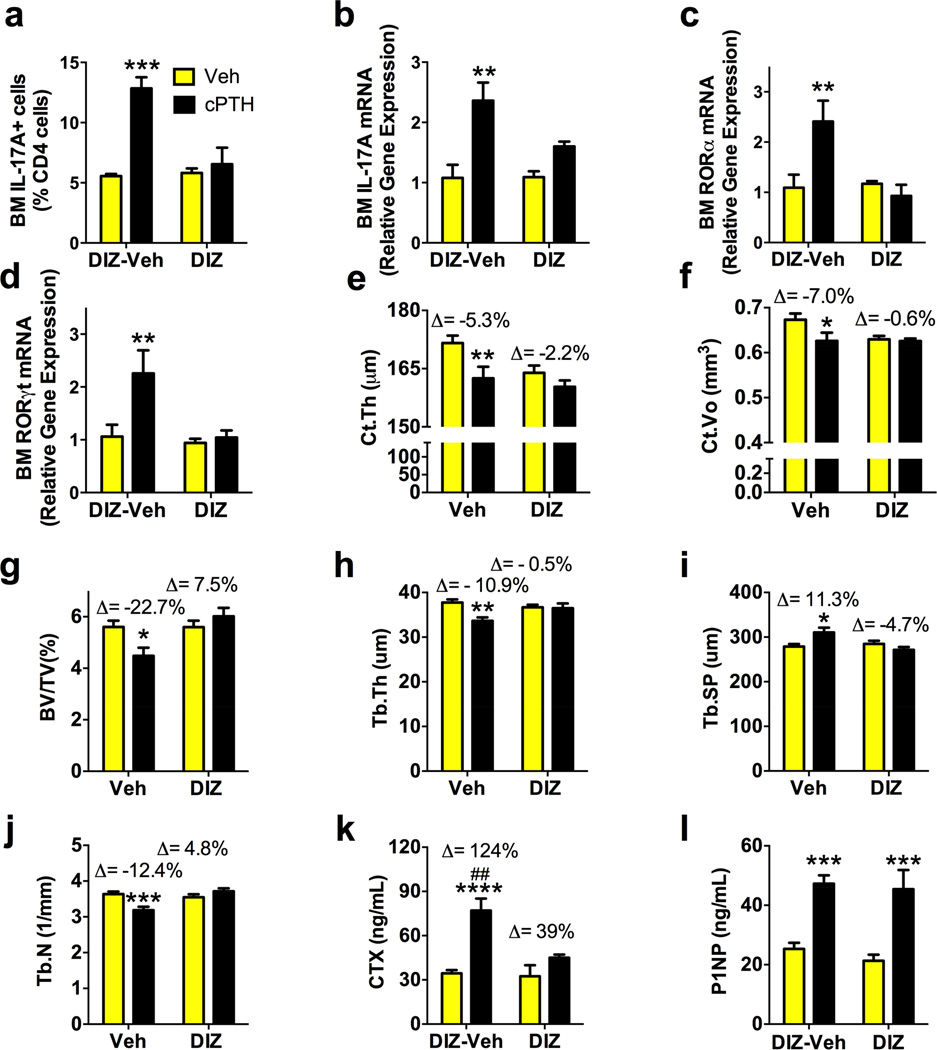
Signaling events downstream of GαS include cAMP generation (Li et al., 2012) and activation of L-type calcium channels (Hell, 2010). The latter contributes to Th17 cell differentiation (Oh-hora, 2009). Accordingly, in vitro treatment with the L-type calcium channel blocker diltiazem blunts the differentiation of CD4+ cells into Th17 cells (Li et al., 2012). We fed mice with or without diltiazem in their drinking water (Mieth et al., 2013; Semsarian et al., 2002) and infused them with vehicle or cPTH. Diltiazem blocked the increase in the number of BM Th17 cells (fig.6a), the BM mRNA levels of IL-17A (fig.6b), and the BM CD4+ cell expression of RORα and RORγt (fig.6c,d) induced by cPTH. Moreover, diltiazem completely blocked the decrease in Ct.Vo, Ct.Th and BV/TV induced by cPTH (fig.6e–g) and altered the response to cPTH of parameters of trabecular structure (fig.6h–j). Diltiazem blocked the increase in serum CTX levels but not the increase in serum P1NP induced by cPTH (fig.6k,l). These data demonstrate that diltiazem prevents the loss of cortical and trabecular bone induced by cPTH by blunting bone resorption. The finding that in vivo treatment with diltiazem blocks Th17 cell expansion and prevents cPTH induced bone loss may suggest a potential therapeutic role for L-type calcium channel blockers in the treatment of hyperparathyroidism, even though the available data do not exclude the possibility of additional effects of diltiazem on immune cells that may contribute to its bone sparing activity.
Figure 6. The L-type calcium channel blocker diltiazem (DIZ) prevents the effects of cPTH.
a relative frequency of BM Th17 cells, b IL-17A mRNA levels in BM CD4+ cells c-d expression of RORα and RORγt mRNA in BM CD4+ cells, e-j µCT indices of bone volume and structure. k,l. Serum levels of CTX and P1NP. Data are shown as mean ± SEM. n = 12 mice per group. All data passed the Shapiro-Wilk normality test and were analyzed by 2-Way ANOVA. *=p<0.05, **=p<0.01, ***=p<0.001 and ****=p<0.0001 compared to the corresponding vehicle group. ## = p<0.01 compared to the DIZcPTH group.
A striking and unexpected finding of this investigation is that neutralization of IL-17A and deletion of IL-17RA block the capacity of cPTH to increase the production of RANKL by osteocytes and osteoblasts. These findings and the published literature suggest that T cells, osteoblasts and osteocytes are all required for the high levels of PTH characteristic of PHPT to induce bone loss. By contrast, osteocytes, but not T cells and IL-17A, are required for physiologic levels of endogenous PTH to regulate bone remodeling. In fact, mice lacking PPR signaling in osteocytes have high baseline bone volume (Saini et al., 2013), while IL-17RA null mice and those lacking PPR signaling in T cells (Bedi et al., 2012; Tawfeek et al., 2010) have a normal bone volume.
Direct clinical applications of the current study arise because L-type calcium channel blocker are available, while anti-human IL-17A Abs and IL-17 receptor Abs are under investigation as therapeutic agents in psoriasis and spondyloarthropathy (Leonardi et al., 2012; Martin et al., 2013; Mease et al., 2014; Yeremenko et al., 2014). Therefore our findings demonstrate a novel role for IL-17A in the mechanism of action of cPTH and provide a proof of principle for the use of L-type calcium channel blocker and IL-17A Ab in the treatment of primary hyperparathyroidism.
EXPERIMENTAL PROCEDURES
Human Study population
All human studies were approved by the Ethical Committee of the A.O.U. Città della Salute e della Scienza - A.O. Ordine Mauriziano - A.S.L. TO1, Turin Italy and informed consent was obtained from all participants. The study population was recruited from the patients of A.O.U. Città della Salute e della Scienza, Turin Italy and healthy volunteers. The study population included 20 patients (16 women and 4 men) affected by primary hyperparathyroidism (PHPT) and 57 healthy subjects (25 males and 32 females) comparable for age and years since menopause. The demographic characteristics of the study population are shown in table 1. The diagnosis of PHPT was established based on the finding of elevated circulating levels of calcium and PTH in at least 2 instances and the presence of normal renal function. PHPT patients were subjected to parathyroidectomy and restoration of normal parathyroid function was demonstrated by the finding of normal serum PTH levels 1 month after surgery. Inclusion and exclusion criteria are provided in supplemental experimental procedures.
Measurements of IL-17A, TNFα, IL-23, IL-4, IFNγ and RORC mRNAs in human samples
Red cells were lysed in all peripheral blood samples and total nucleated cells collected and dissolved in TRIzol reagent (Ambion, Huntingdon, UK) and frozen at −80°C until RNA extraction. RNA was isolated using chloroform extraction, and subsequent isopropanol precipitation according to the manufacturer’s protocol. 1 µg of RNA was reverse transcribed to cDNA using a High Capacity cDNA Reverse Transcription Kit (Applied-Biosystems). Real time RT-PCR was performed with IQ SYBR Green Supermix (BIORAD). Relative IL-17A, RORC, IFNγ, IL-4, IL-23, and TNFα gene expression was determined using the 2−ΔΔCT method with normalization to β-Actin. The primers used are listed as supplemental information.
Animals
All the animal procedures were approved by the Institutional Animal Care and Use Committee of Emory University. Female C57BL6 WT, TNF−/−, TNFR1−/−, TNFR2−/−, TCRβ−/− and IL17A–GFP knock-in (IL17atm1Bcgen/J) mice were purchased from Jackson Laboratories (Bar Harbor, ME). C57BL6 IL-17RA−/− were provided by Amgen, Inc. C57BL6 GαS fl/fl mice were provided by Dr. Lee Weinstein (NIH). C57BL/6 CD4-Cre mice were purchased from Taconic. GαSΔCD4,8 mice were generated by crossing C57BL6 GαS fl/fl mice with C57BL6 CD4-Cre mice. All mice were maintained under specific pathogen free conditions and fed sterilized food and autoclaved water ad libitum.
In vivo cPTH treatment
80 µg/kg/day of hPTH1–34 (Bachem California Inc., Torrance, CA) or vehicle were infused for 2 weeks in 16 weeks old female mice by implanting ALZET osmotic pump model-1002 (DURECT corporation Cupertino, CA) with a delivery rate of 0.24 ml/hr, as previously described (Gao et al., 2008; Tawfeek et al., 2010).
IL-17A Ab treatment
16 week-old WT mice were infused with vehicle or PTH for 2 weeks. These mice were also injected with mouse IL-17A neutralizing antibody (IL-17A Ab) (R&D Systems, MAB421) or isotype matched irrelevant Ab (Irr.Ig) at 2.5 mg/kg, twice per week.
Diltiazem treatment
16 week-old WT mice were infused with vehicle or PTH for 2 weeks. These mice received regular water or 100mg/kg body weight/day Diltiazem (Enzo life Science, Inc. Farmingdale, NY) with the drinking water.
T cell transfers
Splenic naïve CD4+ cells (CD4+CD44loCD62Lhi) from IL-17A–eGFP- mice were FACS sorted. WT, TNF−/−, TNFR1−/− and TNFR2−/− spleen T cells were purified by negative immunoselection using MACS Pan T cell isolation kit (Miltenyi Biotech, Auburn, CA). These cells were injected (5 × 106 cells per mouse) IV into TCRβ−/− recipient mice 2 weeks before treatment. Successful T cell engraftment was confirmed by flow cytometry of the spleens of the recipient mice harvested at sacrifice.
BrdU incorporation
Mice were injected IP with 1 mg of BrdU solution 48 hours before sacrifice. The detection of BrdU incorporation for proliferating cells was performed by using BrdU Flow Kit (BD Biosciences, San Diego, CA) and analyzed by FACS. The percentage of IL-17A+ BrdU+ cells was quantified by gating IL-17A+ CD4+ T cells in CD3+ cells.
In vivo and in vitro Th17 cell differentiation and IL-17A ELISA
Sorted splenic naïve (CD62LhiCD44low) CD4+ T cells from IL17A–GFP knock-in mice (IL17atm1Bcgen/J mice) were injected IV into 14 weeks old TCRβ−/− mice (2×106 per mouse). Host mice were treated with vehicle or cPTH for 2 weeks starting 2 weeks after the adoptive transfer of T cells. Mice were then sacrificed and spleen and BM cells were harvested and incubated with phorbol 12-myristate 13-acetate (50ng/ml, Sigma) and ionomycin (1µg/ml, Sigma) in the presence of GolgiStop (1 µg/ml, Biolegend) for 4 hours. The cells were further stained with surface marker and analyzed for GFP expression. For in vitro studies, naïve CD4+ T cells from mice treated with vehicle or cPTH for 2 weeks were activated with plate bound anti-CD3 (2µg/ml) and anti-CD28 (2µg/ml) in the presence of TNF (10–50 ng/ml) for 3 days. Cells were then harvested for FACS analysis of CD4+IL-17A+ cells.
IL-17A ELISA
CD4+ T cells were purified using CD4-specific MACS Microbeads (Miltenyi Biotec) following the manufacturer’s instructions. Cells were cultured for 48 hours with 1 µg/ml anti-CD28 (Biolegend, San Diego, CA) on anti-CD3–coated plates. Supernatants were collected and assayed for IL-17 by ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s directions.
µCT measurements
µCT scanning and analysis was performed as reported previously ((Tawfeek et al., 2010; Terauchi et al., 2009)) using a Scanco µCT-40 scanner. Additional information as supplemental information.
Quantitative bone histomorphometry
The measurements, terminology and units used for histomorphometric analysis, were those recommended by the Nomenclature Committee of the American Society of Bone and Mineral Research (Dempster et al., 2013). Non-consecutive longitudinal sections of the femur were prepared and analyzed as described previously (Robinson et al., 2015). Additional information is provided as supplemental information.
Osteoblast and dendritic cell purification
BM cells were collected at sacrifice and OBs were purified as previously described (Bedi et al., 2012; Gao et al., 2008). Additional information is provided as supplemental information.
RNA isolation from enriched osteocytes
The distal end of a tibia was cut off and BM cells were removed by centrifuging at 12000 rpm for 2 minutes. The surfaces of the bone shafts were scraped l to remove the periosteum and the bone was cut into a few small pieces. Bone pieces were then digested with 1 ml of Hank’s solution containing 0.1 % bovine serum albumin, 1 mM CaCl2, and 1 mg/ml of collagenase (type I:II, ratio 1:3) in a 12-well-plate. A total of 6 digestions for 15 minutes were performed at 37°C on a rocking platform at 90 oscillations per minute to remove the cells on the bone surface. After the final digestion, bone pieces were washed with PBS and frozen in liquid nitrogen. For RNA isolation, bone pieces were transferred into a tube of 1 mL TRIzol reagent with 5 stainless steel beads and the sample was spun in a refrigerated bullet blender centrifuge at 12000 rpm for 10 minutes. The supernatant was transferred into a new tube, and RNA extracted.
Markers of bone turnover
Serum CTX, P1NP and Osteocalcin were measured by rodent specific ELISA assays (Immunodiagnostic Systems, Scottsdale, AZ).
Flow cytometry and cell sorting
For surface staining, cells were stained with anti-mouse CD3, CD4, CD62L, and CD44 antibodies (Biolegend, San Diego, CA). For intracellular staining, cells were incubated with phorbol 12-myristate 13-acetate (50ng/ml, Sigma) and ionomycin (1µg/ml, Sigma) in the presence of GolgiStop (1 µg/ml, Biolegend) at 37°C for 4 hours. Cells were then stained with anti-mouse CD3 and CD4 antibodies followed by intracellular staining with anti-mouse IL-17A, IFNγ, IL-4 and Foxp3 antibodies (Biolegend). Cells were then subjected to FACS analysis on an LSRII (BD Biosciences, Franklin Lakes, NJ) and analyzed using FlowJo software (TreeStar).
Real-time RT-PCR and murine primers
The expression levels of murine IL-17A, I RORα, RORγt, IL-21, IL-23, IL-23R, TNFR1, TRAF2, IL-1β, IL-6, RANKL and TNFα mRNAs levels were quantified by real-time RT-PCR. Murine RNAs levels were measured in BM and spleen CD4+ cells, as well as unfractionated nucleated peripheral blood cells. All the primers used were designed by Primer Express Express® Software v2.0 (PE Biosystems). Changes in relative gene expression between vehicle and cPTH groups were calculated using the 2−ΔΔCT method with normalization to 18S rRNA. The primers used are provided in supplemental experimental procedures.
Statistical Analysis
Human cytokines and RORC mRNA levels and serum Ca, PTH, and 25OH Vitamin D levels were analyzed by Mann Whitney (healthy controls vs. PHPT before surgery and healthy controls vs. PHPT after surgery) and Wilcoxon matched pairs signed rank tests (PHPT vs. PHPT after surgery), as the data were not normally distributed according to the Shapiro-Wilk normality test. Serum levels of phosphorous and the demographic data were analyzed by one-way ANOVA, as these data were normally distributed. To evaluate the effects of age and gender on IL-17A and RORC levels, multivariate general linear regression models (GLMs) on logarithmic scale were fitted for age, gender and IL-17A or RORC mRNA levels at baseline and their interactions. The relationship between serum PTH levels and IL-17A and RORC mRNA levels were further analyzed by nonparametric Spearman correlations.
Murine data were normally distributed according to the Shapiro-Wilk normality test and analyzed by unpaired t-tests or two-way analysis-of-variance as appropriate. Additional information is provided as supplemental information.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Prof. L. Richiardi (University of Turin, Italy) for his assistance with the statistical analysis of the human data, Dr. Weinstein (NIH) for providing the Gas fl/fl mice and to Amgen, Inc for providing the IL-17RA−/− mice. This study was supported by grants from the National Institutes of Health (AR54625, DK007298 and RR028009). JYL was supported by a grant from the National Institutes of Health (AR061453). MNW was supported in part, by a grant from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development (5I01BX000105) and by NIH grants R01AR059364 and R01AG040013.
Nonstandard abbreviations used
- BM
Bone marrow
- cPTH
Continuous PTH
- OBs
Osteoblasts
- OCYs
Osteocytes
- PTH
Parathyroid hormone
- PHPT
Primary Hyperparathyroidism
- PPR
PTH/PTHrP receptor
- SCs
Stromal Cells
- TNF
Tumor necrosis factor α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS: PDA, FS, IB and GCI designed the human studies, performed the human research, and analyzed the human data. RP, JYL and MNW designed the animal study/protocols. JYL, JR, LDW, CV, TL, AMT, MY, MR, and JA performed the animal research and analyzed the data. RP wrote the manuscript and was the principle investigator.
DISCLOSURES
The authors state that they have no conflicts of interest.
REFERENCES
- Adamopoulos IE, Chao CC, Geissler R, Laface D, Blumenschein W, Iwakura Y, McClanahan T, Bowman EP. Interleukin-17A upregulates receptor activator of NF-kappaB on osteoclast precursors. Arthritis Res Ther. 2010;12:R29. doi: 10.1186/ar2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Hatton RD, Weaver CT. The Th17 family: flexibility follows function. Immunol Rev. 2013;252:89–103. doi: 10.1111/imr.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi B, Li JY, Tawfeek H, Baek KH, Adams J, Vangara SS, Chang MK, Kneissel M, Weitzmann MN, Pacifici R. Silencing of parathyroid hormone (PTH) receptor 1 in T cells blunts the bone anabolic activity of PTH. Proc Natl Acad Sci U S A. 2012;109:E725–E733. doi: 10.1073/pnas.1120735109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-awadh AN, Delgado-Calle J, Tu X, Kuhlenschmidt K, Allen MR, Plotkin LI, Bellido T. Parathyroid hormone receptor signaling induces bone resorption in the adult skeleton by directly regulating the RANKL gene in osteocytes. Endocrinology. 2014;155:2797–2809. doi: 10.1210/en.2014-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Calvi LM, Sims NA, Hunzelman JL, Knight MC, Giovannetti A, Saxton JM, Kronenberg HM, Baron R, Schipani E. Activated parathyroid hormone/parathyroid hormone-related protein receptor in osteoblastic cells differentially affects cortical and trabecular bone. J Clin Invest. 2001;107:277–286. doi: 10.1172/JCI11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DY, Chen YM, Chen HH, Hsieh CW, Lin CC, Lan JL. Increasing levels of circulating Th17 cells and interleukin-17 in rheumatoid arthritis patients with an inadequate response to anti-TNF-alpha therapy. Arthritis Res Ther. 2011;13:R126. doi: 10.1186/ar3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SW, Soki FN, Koh AJ, Eber MR, Entezami P, Park SI, van Rooijen N, McCauley LK. Osteal macrophages support physiologic skeletal remodeling and anabolic actions of parathyroid hormone in bone. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1315153111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta NS, Abou-Samra AB. PTH and PTHrP signaling in osteoblasts. Cell Signal. 2009;21:1245–1254. doi: 10.1016/j.cellsig.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28:2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSelm CJ, Takahata Y, Warren J, Chappel JC, Khan T, Li X, Liu C, Choi Y, Kim YF, Zou W, et al. IL-17 mediates estrogen-deficient osteoporosis in an Act1-dependent manner. Journal of cellular biochemistry. 2012;113:2895–2902. doi: 10.1002/jcb.24165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Wu X, Terauchi M, Li JY, Grassi F, Galley S, Yang X, Weitzmann MN, Pacifici R. T cells potentiate PTH-induced cortical bone loss through CD40L signaling. Cell metabolism. 2008;8:132–145. doi: 10.1016/j.cmet.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell JW. Beta-adrenergic regulation of the L-type Ca2+ channel Ca(V)1.2 by PKA rekindles excitement. Science signaling. 2010;3:pe33. doi: 10.1126/scisignal.3141pe33. [DOI] [PubMed] [Google Scholar]
- Iida-Klein A, Lu SS, Kapadia R, Burkhart M, Moreno A, Dempster DW, Lindsay R. Short-term continuous infusion of human parathyroid hormone 1–34 fragment is catabolic with decreased trabecular connectivity density accompanied by hypercalcemia in C57BL/J6 mice. J Endocrinol. 2005;186:549–557. doi: 10.1677/joe.1.06270. [DOI] [PubMed] [Google Scholar]
- Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F, Pelletier JP. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol. 1998;160:3513–3521. [PubMed] [Google Scholar]
- Kappel LW, Goldberg GL, King CG, Suh DY, Smith OM, Ligh C, Holland AM, Grubin J, Mark NM, Liu C, et al. IL-17 contributes to CD4-mediated graft-versus-host disease. Blood. 2009;113:945–952. doi: 10.1182/blood-2008-08-172155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh AJ, Novince CM, Li X, Wang T, Taichman RS, McCauley LK. An irradiation-altered bone marrow microenvironment impacts anabolic actions of PTH. Endocrinology. 2011;152:4525–4536. doi: 10.1210/en.2011-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu N, Takayanagi H. Autoimmune arthritis: the interface between the immune system and joints. Advances in immunology. 2012;115:45–71. doi: 10.1016/B978-0-12-394299-9.00002-3. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annual review of immunology. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CL, Wang MH, Hung KY, Hsu SH, Chiang CK, Lu KC. Correlation of interleukin-17-producing effector memory T cells and CD4+CD25+Foxp3 regulatory T cells with the phosphate levels in chronic hemodialysis patients. TheScientificWorldJournal. 2014;2014:593170. doi: 10.1155/2014/593170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, Braun D, Banerjee S. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190–1199. doi: 10.1056/NEJMoa1109997. [DOI] [PubMed] [Google Scholar]
- Li X, Murray F, Koide N, Goldstone J, Dann SM, Chen J, Bertin S, Fu G, Weinstein LS, Chen M, et al. Divergent requirement for Galphas and cAMP in the differentiation and inflammatory profile of distinct mouse Th subsets. J Clin Invest. 2012;122:963–973. doi: 10.1172/JCI59097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- Lowik CWGM, van der Pluijm G, Bloys H, Hoekman K, Bijvoet OL, Aarden LA, Papapoulos SE. Parathyroid hormone (PTH) and PTH-like protein (PLP) stimulate IL-6 production by osteogenic cells: a possible role of interleukin-6 in osteoclastogensis. Biochem.Biophys.Res.Commun. 1989;162:1546–1552. doi: 10.1016/0006-291x(89)90851-6. [DOI] [PubMed] [Google Scholar]
- Ma YL, Cain RL, Halladay DL, Yang X, Zeng Q, Miles RR, Chandrasekhar S, Martin TJ, Onyia JE. Catabolic effects of continuous human PTH (1--38) in vivo is associated with sustained stimulation of RANKL and inhibition of osteoprotegerin and gene-associated bone formation. Endocrinology. 2001;142:4047–4054. doi: 10.1210/endo.142.9.8356. [DOI] [PubMed] [Google Scholar]
- Martin DA, Churchill M, Flores-Suarez L, Cardiel MH, Wallace D, Martin R, Phillips K, Kaine JL, Dong H, Salinger D, et al. A phase Ib multiple ascending dose study evaluating safety, pharmacokinetics, and early clinical response of brodalumab, a human anti-IL-17R antibody, in methotrexate-resistant rheumatoid arthritis. Arthritis Res Ther. 2013;15:R164. doi: 10.1186/ar4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez GJ, Nurieva RI, Yang XO, Dong C. Regulation and function of proinflammatory TH17 cells. Ann N Y Acad Sci. 2008;1143:188–211. doi: 10.1196/annals.1443.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mease PJ, Genovese MC, Greenwald MW, Ritchlin CT, Beaulieu AD, Deodhar A, Newmark R, Feng J, Erondu N, Nirula A. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med. 2014;370:2295–2306. doi: 10.1056/NEJMoa1315231. [DOI] [PubMed] [Google Scholar]
- Mieth A, Revermann M, Babelova A, Weigert A, Schermuly RT, Brandes RP. L-type calcium channel inhibitor diltiazem prevents aneurysm formation by blood pressure-independent anti-inflammatory effects. Hypertension. 2013;62:1098–1104. doi: 10.1161/HYPERTENSIONAHA.113.01986. [DOI] [PubMed] [Google Scholar]
- Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- Molnar I, Bohaty I, Somogyine-Vari E. IL-17A–mediated sRANK ligand elevation involved in postmenopausal osteoporosis. Osteoporos Int. 2014;25:783–786. doi: 10.1007/s00198-013-2548-6. [DOI] [PubMed] [Google Scholar]
- Nakae S, Suto H, Berry GJ, Galli SJ. Mast cell-derived TNF can promote Th17 cell-dependent neutrophil recruitment in ovalbumin-challenged OTII mice. Blood. 2007;109:3640–3648. doi: 10.1182/blood-2006-09-046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima T, Kobayashi Y, Yamasaki S, Kawakami A, Eguchi K, Sasaki H, Sakai H. Protein expression and functional difference of membrane-bound and soluble receptor activator of NF-kappaB ligand: modulation of the expression by osteotropic factors and cytokines. Biochem Biophys Res Commun. 2000;275:768–775. doi: 10.1006/bbrc.2000.3379. [DOI] [PubMed] [Google Scholar]
- Oh-hora M. Calcium signaling in the development and function of T-lineage cells. Immunol Rev. 2009;231:210–224. doi: 10.1111/j.1600-065X.2009.00819.x. [DOI] [PubMed] [Google Scholar]
- Pacifici R. Role of T cells in the modulation of PTH action: physiological and clinical significance. Endocrine. 2013;44:576–582. doi: 10.1007/s12020-013-9960-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisien M, Dempster DW, Shane E, Bilezikian JP. The parathyroids. Basic and clinical concepts. San Diego: Academic Press; 2001. Histomorphometric analysis of bone in primary hyperparathyroidism; pp. 423–436. [Google Scholar]
- Potts J. Primary hyperparathyroidism. In: Avioli LV, Krane S, editors. Metabolic Bone Diseases. San Diego: Academic Press; 1998. pp. 411–442. [Google Scholar]
- Qin J, Shang L, Ping AS, Li J, Li XJ, Yu H, Magdalou J, Chen LB, Wang H. TNF/TNFR signal transduction pathway-mediated anti-apoptosis and anti-inflammatory effects of sodium ferulate on IL-1beta-induced rat osteoarthritis chondrocytes in vitro. Arthritis Res Ther. 2012;14:R242. doi: 10.1186/ar4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Raggatt LJ, Partridge NC. Parathyroid hormone: a double-edged sword for bone metabolism. Trends Endocrinol Metab. 2004;15:60–65. doi: 10.1016/j.tem.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Robinson JW, Li JY, Walker LD, Tyagi AM, Reott MA, Yu M, Adams J, Weitzmann MN, Pacifici R. T cell-expressed CD40L potentiates the bone anabolic activity of intermittent PTH treatment. J Bone Miner Res. 2015;30:695–705. doi: 10.1002/jbmr.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini V, Marengi DA, Barry KJ, Fulzele KS, Heiden E, Liu X, Dedic C, Maeda A, Lotinun S, Baron R, et al. Parathyroid hormone (PTH)/PTH-related peptide type 1 receptor (PPR) signaling in osteocytes regulates anabolic and catabolic skeletal responses to PTH. The Journal of biological chemistry. 2013;288:20122–20134. doi: 10.1074/jbc.M112.441360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semsarian C, Ahmad I, Giewat M, Georgakopoulos D, Schmitt JP, McConnell BK, Reiken S, Mende U, Marks AR, Kass DA, et al. The L-type calcium channel inhibitor diltiazem prevents cardiomyopathy in a mouse model. J Clin Invest. 2002;109:1013–1020. doi: 10.1172/JCI14677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg SJ, Shane E, de la Cruz L, Dempster DW, Feldman F, Seldin D, Jacobs TP, Siris ES, Cafferty M, Parisien MV, et al. Skeletal disease in primary hyperparathyroidism. J Bone Miner Res. 1989;4:283–291. doi: 10.1002/jbmr.5650040302. [DOI] [PubMed] [Google Scholar]
- Sugita S, Kawazoe Y, Imai A, Yamada Y, Horie S, Mochizuki M. Inhibition of Th17 differentiation by anti-TNF-alpha therapy in uveitis patients with Behcet's disease. Arthritis Res Ther. 2012;14:R99. doi: 10.1186/ar3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Tawfeek H, Bedi B, Li JY, Adams J, Kobayashi T, Weitzmann MN, Kronenberg HM, Pacifici R. Disruption of PTH Receptor 1 in T Cells Protects against PTH-Induced Bone Loss. PLoS ONE. 2010;5:e12290. doi: 10.1371/journal.pone.0012290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terauchi M, Li JY, Bedi B, Baek KH, Tawfeek H, Galley S, Gilbert L, Nanes MS, Zayzafoon M, Guldberg R, et al. T lymphocytes amplify the anabolic activity of parathyroid hormone through Wnt10b signaling. Cell metabolism. 2009;10:229–240. doi: 10.1016/j.cmet.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi AM, Srivastava K, Mansoori MN, Trivedi R, Chattopadhyay N, Singh D. Estrogen deficiency induces the differentiation of IL-17 secreting Th17 cells: a new candidate in the pathogenesis of osteoporosis. PLoS ONE. 2012;7:e44552. doi: 10.1371/journal.pone.0044552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Waisman A. T helper cell populations: as flexible as the skin? European journal of immunology. 2011;41:2539–2543. doi: 10.1002/eji.201141938. [DOI] [PubMed] [Google Scholar]
- Xiong J, Piemontese M, Thostenson JD, Weinstein RS, Manolagas SC, O'Brien CA. Osteocyte-derived RANKL is a critical mediator of the increased bone resorption caused by dietary calcium deficiency. Bone. 2014;66C:146–154. doi: 10.1016/j.bone.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeremenko N, Paramarta JE, Baeten D. The interleukin-23/interleukin-17 immune axis as a promising new target in the treatment of spondyloarthritis. Current opinion in rheumatology. 2014;26:361–370. doi: 10.1097/BOR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- Zepp J, Wu L, Li X. IL-17 receptor signaling and T helper 17-mediated autoimmune demyelinating disease. Trends in immunology. 2011;32:232–239. doi: 10.1016/j.it.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Fu Q, Ren Z, Wang Y, Wang C, Shen T, Wang G, Wu L. Changes of serum cytokines-related Th1/Th2/Th17 concentration in patients with postmenopausal osteoporosis. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2014:1–8. doi: 10.3109/09513590.2014.975683. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.