Abstract
Purpose
The primary purpose of this study was to determine prospective associations of accelerometer-assessed physical activity intensity and sedentary time with health-related quality of life (HRQOL) indicators among breast cancer survivors.
Methods
Breast cancer survivors (n=358) wore an Actigraph accelerometer for 7 days at baseline to assess different activity intensities (light, lifestyle, moderate-to-vigorous) and sedentary behavior. 6 months later, survivors completed on-line questionnaires that assessed HRQOL indicators (disease-specific HRQOL, fatigue, depression and anxiety) and relevant covariates. Relationships between activity and sedentary behavior quartiles and HRQOL indicator scores were examined using generalized liner models with Bonferronni multiple comparison adjustment.
Results
After adjustment for covariates and sedentary time, each increasing lifestyle activity quartile was associated with reduced fatigue duration (p-trend =0.03). Each increasing baseline moderate-to-vigorous physical activity (MVPA) quartile was significantly associated with higher physical well-being, Functional Assessment of Cancer Therapy-Breast FACT-B total and trial outcomes index scores, fewer breast cancer specific concerns and lower fatigue interference, and these differences were statistically and clinically significant between survivors in quartile 1 (Q1) and Q4. After controlling for covariates and MVPA, relationships between sedentary time and HRQOL were mostly null with the exception of lower fatigue duration.
Conclusions
Objectively measured MVPA was positively associated with many HRQOL indicators. Lifestyle activity was only inversely associated with fatigue duration while sedentary time was positively associated with fatigue duration. Future research is warranted to explore these relationships further.
Keywords: health-related quality, fatigue, anxiety, depression, physical activity, sedentary time, breast cancer survivors
Introduction
There are approximately 3 million breast cancer survivors in the U.S. with this number expected to increase to 4 million by 2020.1 Breast cancer treatment is associated with a myriad of deleterious negative side effects that result in compromised health-related quality of life (HQOL).2 Survivors have an increased risk of early mortality, comorbid conditions3 and second primary cancers.4 Increased moderate-to-vigorous physical activity [(MVPA); i.e. ≥3.0 metabolic equivalents (METs); brisk walking, jogging, biking] has been consistently associated with fewer negative treatment-related side effects, higher QOL, longer survival and reduced recurrence and mortality.5, 6 Additionally, emerging evidence indicates increased sedentary behavior (i.e. ≤1.5 METs; any waking activity in a sitting or reclining posture) may be associated with poorer HRQOL7, 8 and body composition9 and increased mortality10 and higher light intensity and lifestyle activity (i.e. 1.6 to <3 METs; light walking, household chores, easy gardening) may be associated with reductions in functional decline11 and improved QOL12 among cancer survivors, independent of MVPA. Furthermore, increased sedentary behavior is also associated with adverse health outcomes (i.e. diabetes, cancer, premature mortality) in the general populaiton.13
Despite these relationships, breast cancer survivors demonstrate decreases in MVPA that persist post-treatment.14 Self-report data indicate up to 70% do not meet MVPA recommendations (i.e. 150 minutes/week).15-17 Objective data indicate survivors spend <2% of waking time in MVPA.9, 18 In contrast, breast cancer survivors spend about 2/3 of waking time in sedentary behaviors.9, 18, 19 Thus, a paradox of substantial benefits, yet lack of participation represents a significant challenge in survivorship research. Understanding how sedentary behavior (high volume behavior) and lower intensity activity (potentially easier alternative to incorporate into daily life) influence HRQOL could provide greater insight into the activity dosage necessary for health benefits in survivors.
Existing literature examining activity and sedentary behavior and patient-reported outcomes among breast cancer survivors has several limitations including: a) use of self-report measures of activity and sedentary behavior; b) failure to examine light intensity activity and c) cross-sectional study designs. Thus, much of the existing evidence is likely subject to measurement error from self-report measures which may bias results and lead to incorrect inferences about these behaviors. Accelerometers provide valid and reliable objective measures of activity20, 21 and sedentary behavior.22 Activity counts from accelerometers can be used to derive the amount of time spent in different intensities of activities (e.g. light, lifestyle, moderte, vigorous) and sedentary behavior. Prospective objectively-measured activity and sedentary behavior using accelerometers enables more accurate, precise, and reliable assessment of the wide spectrum of daily movement and sedentary time. It can also provide greater insight into relationship directionality and potential activity dosage needed to achieve benefits.
Several recent papers have identified understanding relationships between specific physical activity types/intensitities and specific outcomes 23--25 and potential associations between sedentary behavior and patient reported outcomes 25, 26 as important research priorities in cancer survivorship. We sought to fill these gaps and address prior studies' limitations by prospectively examining relationships between objectively-measured activity intensities and sedentary behavior and HRQOL indicators among breast cancer survivors. We hypothesized higher physical activity duration of any intensity and less sedentary time would be significantly associated with improved HRQOL.
Methods
Participants
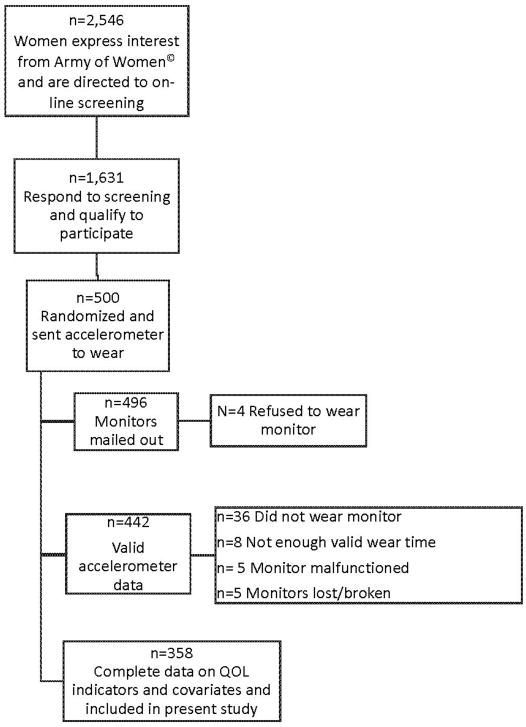
The present study consists of a subset of breast cancer survivors who participated in a larger 6-month prospective on-line questionnaire study. Full study details are provided elsewhere.27 Briefly, survivors were recruited from the Army of Women© to participate in a study on QOL. Inclusionary criteria included: age ≥18 years, prior breast cancer history, English-speaking and access to the Internet. Women (n=500) from the original study were randomized to wear an accelerometer. Only those who had ≥3 valid days of accelerometer data (n=442) and complete data on QOL indicators and covariates (n=358) were included in the present analyses. See Figure 1 for details on participant flow through the project.
Figure 1.
Participant flow through study.
Procedures
Survivors randomized to receive accelerometers were sent accelerometer packets via mail at baseline. The accelerometer packet contained the accelerometer, a log to record when the monitor was worn each day, and a self-addressed stamped envelope to return the accelerometer to study investigators. All participants were sent reminders to return the accelerometer at the end of the 7 day period. Reminders were continued until the monitor was received. At 6 months, participants answered on-line questionnaires pertaining to HRQOL indicators. All participants were sent a maximum of three reminders to complete questionnaires.
Measures
Demographics
Survivors self-reported age, education, height and weight. Body mass index (BMI) was estimated using the standard kg/m2 equation.
Health and cancer history
Survivors self-reported information regarding their breast cancer (i.e. disease stage, time since diagnosis, treatment type, recurrence). Women were also asked to report whether they had been diagnosed (yes or no) with 18 other chronic conditions (i.e. diabetes, hypertension, hyperlipidemia). The number of chronic conditions reported was summed to obtain a total comorbidity score.
Physical activity and sedentary behavior
Participants were instructed to wear an Actigraph accelerometer (Model GT1M, Health One Technology, Fort Walton Beach, FL) on the hip for 7 consecutive days during all waking hours, except when bathing or swimming. Activity data were collected in one-minute intervals (epochs). Non-wear time was defined as intervals of ≥60 consecutive minutes of zero counts, with allowance for up to 2 minutes of observations of <100 counts/min within the non-wear interval.28 A day of accelerometer wear was considered valid if it registered ≥10 hours of wear time. Each minute of wear time was classified according to intensity (counts/min) using commonly accepted activity count cut-points27, 28 as follows: sedentary (<100), light (100-759), lifestyle (760-2019), and MVPA (≥ 2020). For each valid day, the number of wear time minutes classified as sedentary, light, lifestyle, and MVPA were taken as estimates of time spent in these activities on that day. The number of minutes with intensity counts ≥100 was taken as an estimate of “total” time spent active. Raw counts from the accelerometer were summed over wear minutes to obtain “total valid counts” for the reporting day. The number of minutes in each category was divided by wear time to estimate proportions of the day spent in the respective behavior. Daily estimates of average minutes and proportion of time spent sedentary and in each classified activity were averaged across all valid days per participant to estimate mean daily minutes and proportion of time. All values controlled for wear time.
HRQOL Indicators
Functional Assessment of Cancer Therapy- Breast (FACT-B).29, 30
The FACT-B assessed physical, social, emotional and functional well-being and breast cancer specific concerns. Participants were asked to indicate how true each statement was for them over the last 7 days from 0 (not at all) to 4 (very much). Subscale scores were calculated by multiplying the sum of each subscale's items by the number of subscale items and dividing by the number of items answered. Higher scores indicate better HRQOL.
The Hospital Anxiety and Depression Scale.31
This scale assessed the frequency of depressive states (7 items) and anxiety (7 items) over the past week from 0 (not at all) to 3 (most of the time). Positively worded items were reverse scored. Higher scores indicate greater symptomology.
Fatigue Symptom Inventory.32, 33
This measure assessed fatigue severity, duration, and its perceived interference. Higher scores are indicative of greater fatigue severity, duration of interference.
Data Analysis
Generalized linear models were used to examine relationships between average daily accelerometer-estimated sedentary behavior quartiles, total, MVPA, light and lifestyle intensity activity quartiles at baseline and HRQOL indicators (FACT-B, fatigue, depression and anxiety) at 6 months. Initial models (Model 1) controlled for age (continuous) and time since treatment (continuous). Model 2 adjusted for disease stage, treatment category (surgery, radiation therapy, chemotherapy, hormone therapy), body mass index, education, income and number of chronic conditions. Next, accelerometer-estimated daily average total sedentary time and MVPA were mutually adjusted for to test for independence (Model 3). Linear trends were examined using the median of each sedentary behavior or physical activity quartile as a continuous variable. The minimally important difference (MID), the smallest difference which individuals and health care providers perceive as beneficial and would mandate a change in disease management was also calculated for all statistically significant differences in Q1 v. Q4.34
Given women who were included in the present analyses did not differ from those who were excluded by current age, time since treatment, stage, treatment, BMI, education, income, chronic conditions or HRQOL indicator scores, we assumed data were missing at random. Bonferroni's multiple comparisons test was used to correct for potential error as a result of multiple comparisons. All analyses were conducted using IBM SPSS Statistics version 19.0 [39].
Results
Participants
Sample demographic and medical characteristics are shown in Table 1. The mean age was 56.4 years (SD=9.0). The majority of women were White (97.2%), highly educated (68.4% ≥college degree) and higher income (79.6% annual household income ≥$40,000). Mean time since diagnosis was 81.7 months (SD=67.7; 6.8 years). About half (51.6%) were ≥5 years since diagnosis. All women underwent surgery. The majority (68.4%) were diagnosed with early stage (I or II) disease and received radiation therapy and/or chemotherapy (84.7%). A small proportion (10.0%) had a cancer recurrence. These women did not significantly differ from those without a history of recurrence on any of the activity measures or HRQOL indicators so we elected to include them in the present analyses. Almost half were menopausal at diagnosis (44.7%) and were overweight/obese (46.5%). Over two-thirds (72.3%) had ≥1 co-occurring chronic condition.
Table 1. Breast Cancer Survivors Demographic and Disease Characteristics (n=358).
| Variable | Mean (SD) |
|---|---|
| Age | 56.4(9.0) |
| Race/Ethnicity | |
| Non-white | 2.8% |
| Hispanic | 1.7% |
| ≥College Degree | 68.4% |
| Annual Income ≥ $40,000 | 79.6% |
| Time Since Diagnosis(months) | 81.7(67.7) |
| <5 years | 48.0% |
| 5 to <10 years | 30.7% |
| ≥10 years | 20.9% |
| Stage of Disease(%) | |
| 0 | 19.0% |
| I/II | 68.4% |
| III/IV | 12.6% |
| Experienced Menopause Prior to Diagnosis(%) | 44.7% |
| Treatment(%) | |
| Surgery/Radiation/Chemotherapy | 39.7% |
| Surgery/Radiation | 28.2% |
| Surgery/Chemotherapy | 16.8% |
| Surgery Only | 15.4% |
| Recurrence(%) | 10.6% |
| Body Mass Index(kg/m2) | 26.1(5.3) |
| <25 | 53.5% |
| 25 to <30 | 24.8% |
| ≥30 | 21.7% |
| Comorbidities(%) | 1.7(1.6) |
| None | 27.7% |
| 1-2 | 46.6% |
| ≥3 | 26.0% |
On average, women wore the accelerometer for 843.5 (SD=67.1) minutes/day and had 6.8 (SD= 1.0) valid days of wear time (see Table 2). Survivors spent approximately 65.8% of their day engaged in sedentary behavior. When considering total accumulated MVPA minutes, 43.3% of survivors were achieving ≥150 minutes of MVPA per week.
Table 2. Descriptive Statistics for Activity and Sedentary Time at Baseline and 6 month HRQOL Indicators (n=358).
| Variable | Mean(SD) | Possible Score Range | MID34, 47-49 |
|---|---|---|---|
| Baseline Activity/Sedentary Time (mins/day) | |||
| Valid Days | 6.8(1.0) | -- | -- |
| Accelerometer Wear Time | 843.5(67.1) | -- | -- |
| Sedentary Time | 553.4(69.8) | -- | -- |
| Total Physical Activity | 289.3(72.6) | -- | -- |
| Light | 202.9(48.7) | -- | -- |
| Lifestyle | 64.3(28.7) | -- | -- |
| Moderate | 20.9(18.2) | -- | -- |
| Vigorous | 1.5(4.7) | -- | -- |
| Moderate and Vigorous | 22.5(19.6) | -- | -- |
| 6 Month HRQOL Indicators | |||
| Depression | 3.9(3.9) | 0-21 | 1.4 |
| Anxiety | 4.4(3.2) | 0-21 | 1.3 |
| FACT-B | |||
| Physical Well-being | 24.2(4.2) | 0-28 | 2-3 |
| Functional Well-being | 22.7(4.7) | 0-28 | 2-3 |
| Emotional Well-being | 20.2(3.6) | 0-24 | 2 |
| Social Well-being | 21.9(5.9) | 0-28 | 2-3 |
| Breast Cancer-specific Concerns | 26.6(5.6) | 0-36 | 2-3 |
| Total Score | 115.7(18.4) | 0-144 | 7-8 |
| Trial Outcome Index | 73.6(12.2) | 0-92 | 5-6 |
| Fatigue | |||
| Severity | 2.9(2.1) | 0-10 | 0.6 |
| Interference | 1.6(2.0) | 0-10 | 0.5 SD |
| Duration | 2.9(2.2) | 0-7 | 0.5 SD |
Note: FACT-B= Functional Assessment of Cancer Therapy-Breast; MID= Minimally Important Difference; Higher scores on depression, anxiety and fatigue measures are less desirable (i.e. indicative of more symptomology) while higher scores on the FACT-B subscales are more desirable (i.e. indicative of better quality of life).
Physical Activity and HRQOL Indicators
On average, women registered 289.3 (SD=72.6) minutes per day in any intensity of activity. The majority of these minutes were light intensity (M=202.9, SD=48.7) followed by lifestyle intensity (M= 64.3, SD=28.7), and MVPA (M=20.9, SD=18.2; See Table 2). Relationships between each activity intensity and HRQOL indicators are presented in Table 3. After adjustment for covariates, greater total and light activity quartile at baseline was significantly associated with fatigue duration (p-trend=0.02 for both) at 6 months. Results were no longer significant when controlling for sedentary time. Baseline lifestyle activity was not associated with any HRQOL indicators. After controlling for sedentary time, each increasing lifestyle activity quartile was associated with reduced fatigue duration (p-trend=0.03).
Table 3. Daily Time Spent in Different Physical Activity Intensities at Baseline and 6m HRQOL Indicators.
| Type of Physical Activity (mins) | Q1 | Q2 | Q3 | Q4 | p for trend | ||
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||
| Total | (<240.1) n=89 | (240.2-284.1) n=90 | (284.2-331.6) n=89 | (≥331.7) n=90 | |||
| Depression | 4.3(3.9) | 4.1(3.6) | 3.3(3.4) | 4.1(4.6) | 0.31 | 0.86 | 0.82 |
| Anxiety | 4.1(2.8) | 4.6(3.5) | 4.0(2.7) | 5.1(3.8) | 0.15 | 0.05 | 0.12 |
| FACT-B | |||||||
| PWB | 23.4(4.6) | 24.1(4.5) | 24.7(3.7) | 24.8(3.9) | 0.03 | 0.16 | 0.60 |
| FWB | 22.1(5.3) | 22.0(4.6) | 23.1(4.7) | 23.7(4.1) | 0.02 | 0.06 | 0.13 |
| EWB | 20.6(3.5) | 19.2(3.9) | 20.9(2.7) | 20.3(3.9) | 0.76 | 0.75 | 0.57 |
| SWB | 21.1(6.2) | 21.3(6.7) | 22.7(5.3) | 22.3(5.4) | 0.13 | 0.24 | 0.21 |
| BCS | 25.9(5.9) | 26.3(5.1) | 27.3(5.6) | 27.0(5.9) | 0.10 | 0.48 | 0.78 |
| Total FACT-B | 113.1(20.1) | 113.0(18.2) | 118.7(16.0) | 118.1(18.8) | 0.03 | 0.17 | 0.36 |
| TOI | 71.4(13.1) | 72.4(11.9) | 75.0(11.3) | 75.5(12.1) | 0.01 | 0.12 | 0.36 |
| Fatigue | |||||||
| Severity | 3.2(2.0) | 3.0 (2.2) | 2.7 (1.9) | 2.5 (2.1) | 0.01 | 0.07 | 0.31 |
| Interference | 1.9(2.1) | 1.8 (2.0) | 1.4 (1.7) | 1.4 (2.0) | 0.05 | 0.24 | 0.78 |
| Duration | 3.3(2.1) | 3.1 (2.3) | 2.6 (2.0) | 2.5 (2.1) | 0.004 | 0.02 | 0.31 |
| Light Intensity | (<168.3) n=88 | (168.4-199.9) n=91 | (200.0-234.7) n=90 | (≥234.8) n=89 | |||
| Depression | 4.4(3.8) | 3.9(3.7) | 3.8(4.2) | 3.7(3.9) | 0.13 | 0.12 | 0.17 |
| Anxiety | 4.1(2.8) | 4.5(3.2) | 4.4(3.6) | 4.7(3.3) | 0.44 | 0.38 | 0.46 |
| HRQOL | |||||||
| PWB | 23.8(4.3) | 24.1(4.4) | 24.4(4.9) | 24.6(3.0) | 0.12 | 0.13 | 0.22 |
| FWB | 22.1(4.6) | 22.5(5.4) | 23.2(4.6) | 23.2(4.2) | 0.17 | 0.18 | 0.18 |
| EWB | 20.4(3.4) | 19.9(3.7) | 20.2(3.7) | 20.4(3.5) | 0.99 | 0.92 | 0.85 |
| SWB | 21.2(6.1) | 21.5(6.9) | 23.0(4.9) | 21.9(5.5) | 0.09 | 0.10 | 0.12 |
| BCS | 26.1(5.3) | 26.2(6.5) | 27.2(5.0) | 27.1(5.6) | 0.04 | 0.06 | 0.09 |
| Total FACT-B | 113.5(18.6) | 114.2(20.0) | 118.0(18.2) | 117.1(16.6) | 0.06 | 0.07 | 0.10 |
| TOI | 71.9(12.1) | 72.8(13.8) | 74.8(12.2) | 74.8(10.4) | 0.04 | 0.05 | 0.08 |
| Fatigue | |||||||
| Severity | 3.2(2.0) | 3.0(2.1) | 2.5(2.0) | 2.8(2.0) | 0.06 | 0.07 | 0.10 |
| Interference | 1.9(2.0) | 1.6(1.9) | 1.6(2.2) | 1.5(1.8) | 0.10 | 0.08 | 0.14 |
| Duration | 3.4(2.1) | 3.0(2.1) | 2.5(2.2) | 2.7(2.2) | 0.02 | 0.02 | 0.05 |
| Lifestyle Intensity | (<44.9) n=89 | (45.0-59.9) n=90 | (60.0-79.4) n=89 | (≥79.5) n=90 | |||
| Depression | 3.8(3.6) | 4.2(3.7) | 3.9 (4.0) | 3.9(4.3) | 0.47 | 0.22 | 0.15 |
| Anxiety | 4.3(3.1) | 4.3(3.1) | 4.3 (3.0) | 4.8(3.7) | 0.70 | 0.57 | 0.72 |
| HRQOL | |||||||
| PWB | 24.2(3.7) | 23.8(4.7) | 24.3 (4.1) | 24.7(4.1) | 0.43 | 0.19 | 0.07 |
| FWB | 22.4(5.1) | 22.2(5.2) | 22.9 (4.1) | 23.4(4.4) | 0.80 | 0.97 | 0.96 |
| EWB | 20.4(3.3) | 20.1(4.0) | 20.0 (3.6) | 20.5(3.5) | 0.69 | 0.91 | 0.94 |
| SWB | 22.3(6.1) | 21.7(5.5) | 21.6 (6.4) | 22.0(5.8) | 0.16 | 0.09 | 0.11 |
| BCS | 27.0(5.1) | 26.0(5.5) | 26.7 (5.8) | 26.8(6.1) | 0.12 | 0.07 | 0.05 |
| Total FACT-B | 116.2(18.0) | 113.8(18.7) | 115.5 (18.0) | 117.3(19.0) | 0.06 | 0.16 | 0.12 |
| TOI | 73.6(11.5) | 72.0(12.5) | 73.9 (12.9) | 74.9(12.9) | 0.37 | 0.19 | 0.12 |
| Fatigue | |||||||
| Severity | 3.0(2.0) | 2.9(2.0) | 3.1 (2.2) | 2.5(2.0) | 0.80 | 0.47 | 0.28 |
| Interference | 1.7(1.9) | 1.7(1.9) | 1.7 (2.0) | 1.5(2.0) | 0.41 | 0.14 | 0.06 |
| Duration | 3.1(2.0) | 2.8(2.2) | 3.0 (2.4) | 2.7(2.1) | 0.34 | 0.15 | 0.03 |
| MVPA | (<7.8) n=89 | (7.9-17.9) n=88 | (18.0-32.9) n=90 | (≥33.0) n=91 | |||
| Depression | 4.1(3.6) | 3.8(3.8) | 4.3(4.2) | 3.5(3.9) | 0.06 | 0.18 | 0.28 |
| Anxiety | 4.5(3.2) | 3.7(3.1) | 4.9(3.4) | 4.5(3.1) | 0.87 | 0.59 | 0.55 |
| HRQOL | |||||||
| PWB | 23.2(4.5) | 24.8(3.4) | 23.2(5.2) | 25.6(2.7) | 0.001 | 0.004 | 0.01 |
| FWB | 22.3(4.5) | 22.5(5.3) | 22.2(4.8) | 23.8(4.2) | 0.06 | 0.11 | 0.13 |
| EWB | 20.1(3.4) | 20.5(3.8) | 19.9(3.6) | 20.4(3.5) | 0.77 | 0.99 | 0.86 |
| SWB | 21.8(5.8) | 21.5(6.2) | 21.4(6.5) | 22.8(5.2) | 0.05 | 0.06 | 0.08 |
| BCS | 25.3(5.9) | 27.0(5.2) | 26.2(6.4) | 28.0(4.7) | <0.001 | 0.002 | 0.01 |
| Total FACT-B | 112.9(18.4) | 116.4(18.1) | 112.9(20.3) | 120.6(15.9) | 0.002 | 0.01 | 0.02 |
| TOI | 70.9(11.9) | 74.4(11.6) | 71.7(14.2) | 77.4(9.9) | <0.001 | 0.002 | 0.01 |
| Fatigue | |||||||
| Severity | 3.3(2.1) | 2.6(2.0) | 3.2(2.4) | 2.5(1.6) | 0.03 | 0.10 | 0.18 |
| Interference | 2.1(2.1) | 1.3(1.7) | 1.9(2.2) | 1.3(1.7) | 0.01 | 0.02 | 0.04 |
| Duration | 3.3(2.4) | 2.5(2.1) | 3.2(2.2) | 2.6(1.9) | 0.06 | 0.14 | 0.27 |
Note: All mean values represent observed, unadjusted means. Mean values in bold indicate differences met the MID criteria. Model 1: adjusted for age and time since diagnosis; Model 2: adjusted for age, time since diagnosis, treatment type, disease stage, education, number of comorbidities; Model 3: adjusted for all variables in Model 2 and sedentary time. All PA intensity models control for the other PA intensities examined. FACT-B=Functional Assessment of Cancer Therapy-Breast; PWB=Physical Well-being; FWB=Functional Well-being; EWB=Emotional Well-being; SWB=Social Well-being; BCS=Breast Cancer Specific Concerns; TOI=Trial Outcome Index
Each increasing baseline MVPA quartile was statistically significantly associated with higher physical well-being, total FACT-B and TOI scores, fewer breast cancer specific concerns and lower fatigue interference (p-trend<0.05). Relationships remained largely unchanged when controlling for covariates and sedentary time. Survivors in the highest MVPA quartile reported statistically significantly better scores on these measures at 6 months than those in the lowest quartile. All differences exceeded the MID threshold.
Survivors who met public health recommendations for MVPA reported statistically significantly better physical well-being (24.8 v. 23.8, p=0.03), FACT-B total scores (118.0 v. 114.0, p=0.04) and TOI score (75.5 v. 72.1, p=0.01) and fewer breast cancer specific concerns (27.5 v 26.0, p=0.01). None of these differences met MID criteria.
Sedentary Time and HRQOL Indicators
On average, participants spent 9.2 hours (M=553.4 minutes; SD=72.6) per day sedentary or 65.8% of their time. Relationships between sedentary time and HRQOL indicators are presented in Table 4. After adjustment for covariates, baseline sedentary time was significantly associated with lower physical well-being and increased fatigue duration at 6 months (p-trend<0.05). Only the relationship between sedentary time and fatigue duration held when controlling for MVPA (p-trend=0.03) and met MID criteria.
Table 4. Baseline Sedentary Time Quartile and 6m HRQOL Indicators.
| Quartile 1 (≤514.3) n=89 | Quartile 2 (514.4 to 557.5) n=90 | Quartile 3 (557.6 to 596.7) n=90 | Quartile 4 (≥ 596.8) n=89 | p for trend | |||
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||
| Total Sedentary Time (mins) | |||||||
| Depression | 4.1 (3.9) | 3.7 (3.5) | 3.6 (3.8) | 4.4 (4.4) | 0.51 | 0.44 | 0.54 |
| Anxiety | 4.7 (3.0) | 4.5 (3.4) | 4.5 (3.4) | 4.3 (3.5) | 0.44 | 0.45 | 0.55 |
| FACT-B | |||||||
| PWB | 24.7 (3.4) | 24.6 (3.4) | 24.4 (4.4) | 23.3 (5.1) | 0.06 | 0.04 | 0.12 |
| FWB | 23.1 (4.3) | 23.0 (4.1) | 22.4 (5.2) | 22.4 (5.2) | 0.31 | 0.29 | 0.47 |
| EWB | 20.2 (3.1) | 20.6 (3.3) | 20.1 (3.6) | 20.0 (4.2) | 0.56 | 0.57 | 0.56 |
| SWB | 22.0 (5.1) | 22.3 (5.3) | 21.6 (6.1) | 21.6 (7.1) | 0.61 | 0.57 | 0.65 |
| BCS | 26.4 (5.6) | 27.6 (5.3) | 26.2 (5.6) | 26.3 (6.0) | 0.67 | 0.66 | 0.96 |
| Total FACT-B | 116.4 (16.5) | 118.1 (16.4) | 114.6 (18.6) | 113.7 (21.7) | 0.27 | 0.25 | 0.43 |
| TOI | 74.2 (11.3) | 75.2 (11.0) | 72.9 (12.6) | 72.1 (13.8) | 0.22 | 0.19 | 0.43 |
| Fatigue | |||||||
| Severity | 2.7 (1.9) | 2.8 (2.0) | 2.9 (2.2) | 3.1 (2.1) | 0.17 | 0.13 | 0.31 |
| Interference | 1.5 (1.8) | 1.5 (1.7) | 1.6 (2.0) | 1.9 (2.2) | 0.13 | 0.09 | 0.20 |
| Duration | 2.5 (1.8) | 2.8 (2.1) | 2.9 (2.3) | 3.3 (2.3) | 0.01 | 0.01 | 0.03 |
Note: All mean values represent observed, unadjusted means. Model 1: adjusted for age and time since diagnosis; Model 2: adjusted for age, time since diagnosis, treatment type, disease stage, education, number of comorbidities; Model 3: adjusted for all variables in Model 2 and MVPA; FACT-B=Functional Assessment of Cancer Therapy-Breast; PWB=Physical well-being; FWB=Functional Well-being; EWB=Emotional Well-being; SWB=Social Well-being; BCS=Breast Cancer Specific Concerns; TOI=Trial Outcome Index
Discussion
The purpose of this study was to prospectively examine associations between objectively-measured physical activity of various intensities and sedentary time and HRQOL indicators among breast cancer survivors. To the best of our knowledge, this is one of the first studies to examine these relationships prospectively. After controlling for covariates and sedentary time, greater baseline MVPA quartile was statistically significantly associated with higher physical well-being, total FACT-B and TOI scores, fewer breast cancer specific concerns and lower fatigue interference. Lower intensity activity and sedentary time results were mostly null. However, increased lifestyle activity was associated with decreased fatigue duration while increased sedentary time was associated with greater fatigue duration when controlling for covariates and sedentary time and MVPA, respectively. Differences between Q1 and Q4 for all statistically significant relationships exceeded MID thresholds.34
Consistent with previous research, MVPA was positively associated with many HRQOL indicators. Our quartile analyses support a dose-response relationship and indicate, compared to those in the lowest MVPA quartile at baseline, survivors in the highest quartile (≥33.0 minutes/day) had clinically meaningful higher HRQOL at 6 months. FACT-B scores for MVPA Q4 versus Q1 were 6.6% higher at 6 months. This is consistent with other post-treatment studies35 and emerging evidence suggesting higher MVPA doses may elicit greater benefits during treatment.36 Future longitudinal and intervention research is warranted to explore relationships between different MVPA doses, HRQOL indicators and other outcomes in breast cancer survivors to develop a better understanding of specific activity doses needed for specific outcomes at different times along the survivorship continuum.23, 25
Contrary to our hypotheses, associations between light and lifestyle intensity activity and sedentary time and HRQOL indicators were less consistent and mostly null. Investigators have only recently begun to examine these behaviors in relation to health outcomes in cancers survivors. While sedentary time among breast cancer survivors is generally high,18 and survivors may spend more time sedentary and less time in lower intensity activities than similar, healthy individuals,19 associations with health and disease outcomes are still relatively unknown.25, 26, 37 Although studies have demonstrated an inverse relationship between lower intensity activities and fatigue and depression38 and physical functioning,11 no studies have prospectively examined objectively-measured light intensity activities. Additionally, only two cross-sectional8, 9 studies have used objective sedentary time measures. Both reported null findings. While we have extended existing work by prospectively examining objectively-measured light intensity activity and sedentary time, our findings were mostly non-significant. This may be attributed to our sample being relatively healthy and indicate these behaviors may have a ceiling effect. Thus, more pronounced benefits may be exhibited in less-healthy subgroups (i.e. older, overweight, functionally limited, metastatic disease). It is also possible that, after cancer treatment, MVPA has stronger effects than sedentary time or light intensity activities on the biopsychosocial pathways influencing physical and mental health including insulin,39 sex hormones,40 inflammation,41 adiposity, 40 psychosocial factors (e.g. self-efficacy, self-esteem, anxiety)42-44 and neurotransmitters (e.g. BDNF).45
Study results should be interpreted in the context of its limitations. First, we are unable to determine causal direction because we did not have HRQOL measures prior to activity and sedentary time assessment. Hence, we cannot rule out the possibility that HRQOL, fatigue, depression and anxiety influence physical activity or sedentary behavior. Further, our timeframe of 6 months was somewhat arbitrary. Future studies should evaluate how changes in different activity intensities and sedentary time influence HRQOL indicators over time with assessments pre-treatment and at multiple post-treatment time points. Second, because accelerometers were used, stationary standing was possibly included as sedentary time. Furthermore, we lack data on sedentary time context (i.e. reading v. television). Therefore, the true volume of time spent sitting or sitting in specific contexts may adversely influence HRQOL indicators in breast cancer survivors. Future research should explore relationships between HRQOL indicators and other health and disease outcomes in survivors using more precise, sensitive objective devices (e.g. ActivPals) and considering sedentary behavior context. Finally, the sample was mostly White, high income and highly educated and ∼50% were more than 5 years post-diagnosis; thus, it is important to confirm these findings in other, more demographically diverse samples at various times since diagnosis.
Our study has several strengths. To the best of our knowledge, this is the first prospective study to examine relationships between objectively-measured physical activity intensity and sedentary time with HRQOL indicators in breast cancer survivors. Using objective activity and sedentary behavior measures reduces the risk of measurement error and misclassification. Additionally, there was adequate variability in activity and sedentary time in this sample to examine these exposures, and the study sample included a wide range of disease and treatment characteristics, suggesting findings could be relevant to many breast cancer survivors. Finally, although we did not use a standard comorbidity index (e.g. Charlson Comorbidity Index),46 adjustment for many chronic conditions included in these indices did not affect our multivariate estimates. Thus, additional residual confounding due to other diseases is likely to be minor.
In conclusion, objectively-measured MVPA is prospectively associated with higher physical well-being, total FACT-B and TOI scores, fewer breast cancer specific concerns and lower fatigue interference at 6 months. Additionally, lifestyle activity was associated with reduced fatigue duration. Increased sedentary time was only associated with greater fatigue duration. These findings provide further support for a dose-response relationship between MVPA and health outcomes in breast cancer survivors. Future prospective and intervention research is warranted to explore relationships between different activity and sedentary time dosages and health outcomes in breast cancer survivors to refine exercise prescriptions and design more effective, targeted interventions.
Acknowledgments
Funding Source: NIA Grant #F31AG034025 (SP) and #AG020118 (EM); Shahid and Ann Carlson Khan endowed professorship (EM).
Footnotes
The authors have no conflicts of interest to report.
Note: All data were collected at the University of Illinois at Urbana Champaign.
References
- 1.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–7.1. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 2.Hewitt M, Greenfield S, Stovall E, editors. Institute of Medicine. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, D.C.: National Academies Press; 2005. [Google Scholar]
- 3.Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: age, health, and disability. J Gerontol A Biol Sci Med Sci. 2003;58:M82–M91. doi: 10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- 4.Meadows AT, Friedman DL, Neglia JP, et al. Second neoplasms in survivors of childhood cancer: findings from the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27:2356–2362. doi: 10.1200/JCO.2008.21.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2011;28:753–765. doi: 10.1007/s12032-010-9536-x. [DOI] [PubMed] [Google Scholar]
- 6.Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4:87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 7.George SM, Alfano CM, Groves J, et al. Objectively measured sedentary time is related to quality of life among cancer survivors. PLoS ONE. 2014;9:e87937. doi: 10.1371/journal.pone.0087937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George SM, Alfano CM, Smith AW, et al. Sedentary behavior, health-related quality of life, and fatigue among breast cancer survivors. J Phys Activ Health. 2013;10:350–8. doi: 10.1123/jpah.10.3.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lynch BM, Dunstan DW, Healy GN, Winkler E, Eakin E, Owen N. Objectively measured physical activity and sedentary time of breast cancer survivors, and associations with adiposity: findings from NHANES (2003–2006) Cancer Causes Control. 2010;21:283–288. doi: 10.1007/s10552-009-9460-6. [DOI] [PubMed] [Google Scholar]
- 10.George SM, Smith AW, Alfano CM, et al. The association between television watching time and all-cause mortality after breast cancer. J Cancer Surviv. 2013;7:247–252. doi: 10.1007/s11764-013-0265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blair CK, Morey MC, Desmond RA, et al. Light-intensity activity attenuates functional decline in older cancer survivors. Med Sci Sports Exer. 2014;46:1375–83. doi: 10.1249/MSS.0000000000000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thraen-Borowski KM, Trentham-Dietz A, Edwards DF, Koltyn KF, Colbert LH. Dose–response relationships between physical activity, social participation, and health-related quality of life in colorectal cancer survivors. J Cancer Surviv. 2013;7:369–378. doi: 10.1007/s11764-013-0277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biswas A, Oh PI, Faulkner GE, et al. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: A systematic review and meta-analysis. Ann Intern Med. 2015;162:123–132. doi: 10.7326/M14-1651. [DOI] [PubMed] [Google Scholar]
- 14.Devoogdt N, Van Kampen M, Geraerts I, et al. Physical activity levels after treatment for breast cancer: one-year follow-up. Breast Cancer Res Treat. 2010;123:417–425. doi: 10.1007/s10549-010-0997-6. [DOI] [PubMed] [Google Scholar]
- 15.Bellizzi KM, Rowland JH, Jeffery DD, McNeel T. Health behaviors of cancer survivors: examining opportunities for cancer control intervention. J Clin Oncol. 2005;23:8884–8893. doi: 10.1200/JCO.2005.02.2343. [DOI] [PubMed] [Google Scholar]
- 16.Courneya KS, Katzmarzyk PT, Bacon E. Physical activity and obesity in Canadian cancer survivors. Cancer. 2008;112:2475–2482. doi: 10.1002/cncr.23455. [DOI] [PubMed] [Google Scholar]
- 17.Blanchard CM, Courneya KS, Stein K. Cancer survivors' adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society's SCS-II. J Clin Oncol. 2008;26:2198–2204. doi: 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]
- 18.Sabiston CM, Brunet J, Vallance JK, Meterissian S. Prospective examination of objectively-assessed physical activity and sedentary time after breast cancer treatment: Sitting on the crest of the teachable moment. Cancer Epidemiol Biomarkers Prev. 2014;23:1324–30. doi: 10.1158/1055-9965.EPI-13-1179. [DOI] [PubMed] [Google Scholar]
- 19.Phillips SM, Dodd KW, Steeves J, McClain JJ, Alfano CM, McAuley E. Physical activity and sedentary behavior in breast cancer survivors: New insights into activity patterns and potential intervention targets. doi: 10.1016/j.ygyno.2015.05.026. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassett DR. Validity of four motion sensors in measuring moderate intensity physical activity. Med Sci Sports Exerc. 2000;32:S471–80. doi: 10.1097/00005768-200009001-00006. [DOI] [PubMed] [Google Scholar]
- 21.Tudor-Locke C, Ainsworth B, Thompson R, Matthews C. Comparison of pedometer and accelerometer measures of free-living physical activity. Med Scie Sports Exerc. 2002;34:2045–51. doi: 10.1097/00005768-200212000-00027. [DOI] [PubMed] [Google Scholar]
- 22.Matthews CE, Chen KY, Freedson PS, et al. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008;167:875–881. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips SM, Alfano CM, Perna FM, Glasgow RE. Accelerating translation of physical activity and cancer survivorship research into practice: recommendations for a more integrated and collaborative approach. Cancer Epidemiol Biomarkers Prev. 2014;23:687–699. doi: 10.1158/1055-9965.EPI-13-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buffart L, Galvão D, Brug J, Chinapaw M, Newton R. Evidence-based physical activity guidelines for cancer survivors: Current guidelines, knowledge gaps and future research directions. Cancer Treat Rev. 2014;40:327–340. doi: 10.1016/j.ctrv.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Courneya KS, Rogers LQ, Campbell KL, Vallance JK, Friedenreich CM. Top 10 research questions related to physical activity and cancer survivorship. Res Q Exerc Sport. 2015:1–10. doi: 10.1080/02701367.2015.991265. [DOI] [PubMed] [Google Scholar]
- 26.Lynch BM, Dunstan DW, Vallance JK, Owen N. Don't take cancer sitting down. Cancer. 2013;119:1928–1935. doi: 10.1002/cncr.28028. [DOI] [PubMed] [Google Scholar]
- 27.Phillips SM, McAuley E. Social cognitive influences on physical activity participation in long-term breast cancer survivors. Psychooncology. 2013;22:783–91. doi: 10.1002/pon.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–8. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 29.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 30.Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol. 1997;15:974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 31.Zigmond AS, Snaith R. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 32.Hann DM, Denniston MM, Baker F. Measurement of fatigue in cancer patients: further validation of the Fatigue Symptom Inventory. Qual Life Res. 2000;9:847–854. doi: 10.1023/a:1008900413113. [DOI] [PubMed] [Google Scholar]
- 33.Hann D, Jacobsen P, Azzarello L, et al. Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Qual Life Res. 1998;7:301–310. doi: 10.1023/a:1024929829627. [DOI] [PubMed] [Google Scholar]
- 34.Yost KJ, Eton DT, Garcia SF, Cella D. Minimally important differences were estimated for six Patient-Reported Outcomes Measurement Information System-Cancer scales in advanced-stage cancer patients. J Clin Epidemiol. 2011;64:507–516. doi: 10.1016/j.jclinepi.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vallance JK, Boyle T, Courneya KS, Lynch BM. Associations of objectively assessed physical activity and sedentary time with health-related quality of life among colon cancer survivors. Cancer. 2014;120:2919–26. doi: 10.1002/cncr.28779. [DOI] [PubMed] [Google Scholar]
- 36.Courneya KS, McKenzie DC, Mackey JR, et al. Effects of exercise dose and type during breast cancer chemotherapy: multicenter randomized trial. JNCI. 2013;105:1821–1832. doi: 10.1093/jnci/djt297. [DOI] [PubMed] [Google Scholar]
- 37.Brenner DR, Neilson HK, Courneya KS, Friedenreich CM. Physical activity after breast cancer: effect on survival and patient-reported outcomes. Current Breast Cancer Reports. 2014:1–12. [Google Scholar]
- 38.Rogers LQ, Markwell SJ, Courneya KS, McAuley E, Verhulst S. Physical activity type and intensity among rural breast cancer survivors: patterns and associations with fatigue and depressive symptoms. J Cancer Surviv. 2011;5:54–61. doi: 10.1007/s11764-010-0160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Werner H, Bruchim I. The insulin-like growth factor-I receptor as an oncogene. Arch Physiol Biochem. 2009;115:58–71. doi: 10.1080/13813450902783106. [DOI] [PubMed] [Google Scholar]
- 40.McTiernan A. Mechanisms linking physical activity with cancer. Nat Rev Cancer. 2008;8:205–211. doi: 10.1038/nrc2325. [DOI] [PubMed] [Google Scholar]
- 41.Petersen AMW, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 42.Phillips SM, McAuley E. Physical activity and quality of life in breast cancer survivors: the role of self-efficacy and health status. Psychooncol. 2014;23:27–34. doi: 10.1002/pon.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phillips SM, McAuley E. Physical activity and fatigue in breast cancer survivors: a panel model examining the role of self-efficacy and depression. Cancer Epidemiol Biomarkers Prev. 2013;22:773–781. doi: 10.1158/1055-9965.EPI-12-0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McAuley E, White SM, Rogers LQ, Motl RW, Courneya KS. Physical activity and fatigue in breast cancer and multiple sclerosis: psychosocial mechanisms. Psychosom Med. 2009;72:88–96. doi: 10.1097/PSY.0b013e3181c68157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erickson KI, Miller DL, Roecklein KA. The aging hippocampus interactions between exercise, depression, and BDNF. Neuroscientist. 2012;18:82–97. doi: 10.1177/1073858410397054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 47.Donovan KA, Jacobsen PB, Small BJ, Munster PN, Andrykowski MA. Identifying clinically meaningful fatigue with the Fatigue Symptom Inventory. J Pain Symptom Manage. 2008;36:480–487. doi: 10.1016/j.jpainsymman.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific quality of life questionnaire. J Clin Epidemiol. 1994;47:81–87. doi: 10.1016/0895-4356(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 49.Puhan MA, Frey M, Büchi S, Schünemann HJ. The minimal important difference of the hospital anxiety and depression scale in patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes. 2008;6:46. doi: 10.1186/1477-7525-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]